Abstract
Gene regulation arises out of dynamic competition between nucleosomes, transcription factors and other chromatin proteins for binding genomic DNA. The timescales of nucleosome assembly and factor binding with DNA determine the outcomes of this competition at any given locus. Here we review how these properties of chromatin proteins and the interplay of dynamics of different factors are critical for gene regulation. We discuss how molecular structures of large chromatin-associated complexes, kinetic measurements and high resolution mapping of protein-DNA complexes in vivo set the boundary conditions for chromatin dynamics, leading to models of how the steady state behaviors of regulatory elements arise.
Keywords: chromatin, nucleosome, RNAPII, transcription factors, chromatin remodelers, time
1. INTRODUCTION
The basic unit of chromatin is the nucleosome, where DNA is wrapped around an octamer of conserved histone proteins. Strings of nucleosome particles and linker histones compact the genome in the nucleus. These proteins must be modulated to allow access by DNA-binding proteins at functional locations in the genome. Atomic resolution structures for the key complexes that compose chromatin and those that navigate DNA make it apparent that these two kinds of complexes will clash (Figure 1). Additionally, since progressing transcriptional and replicative polymerases melt DNA in front and reanneal it behind them, these processes drive torsional changes that alter factor binding and chromatin packaging. Thus, a principal requirement for genome function in eukaryotes is to manipulate the antagonism between packaging, exposure, and torsion of DNA. This entails cycles of assembly and disassembly of both chromatin and of chromatin machines in living cells, and our understanding of these dynamics is informed by high-resolution structures, methods that map in vivo chromatin dynamics, and detailed modeling of assembly pathways. In this review, we focus particularly on the spatial and temporal restrictions that nucleosome dynamics imposes on genome function and regulation.
Figure 1. Chromatin proteins to scale.
Protein complexes sketched from 3D structures are drawn, and the segment of DNA that each protects is indicated. From left to right: nucleosome (wrapping 150 bp), nucleosome with chromatin remodeler (1) (120 bp footprint), transcription factor (~20 bp footprint), RNAPII and Mediator (2) (80 bp footprint), and a replisome (3) (60 bp footprint). A nucleosome depleted region (NDR) is usually found at promoters of active genes. The wrapping of DNA around a nucleosome causes the DNA to be negatively supercoiled, indicated by black “-” sign below the nucleosomes. The movement of remodelers, RNAPII, and replisome propagates positive supercoiling in front of these complexes and negative supercoiling behind them, indicated by red, green, and gray “+” and “-” signs for supercoiling and arrows for direction of movement of these complexes (4). Lengths of DNA are not drawn to scale as some structures like nucleosome wrap DNA.
2. CHROMATIN PROTEIN DYNAMICS
Binding of RNA polymerases, transcription factors (TF), and chromatin remodeling enzymes to DNA are all dynamic. They bind and dissociate continually, and mechanistic understanding of chromatin function in living cells must take these dynamics into account. Studies of dynamics of the transcriptional machinery in living cells have uncovered five surprises: first, transcription factor binding at regulatory elements is fast, with residence times of seconds (5). While this is affected by the concentrations of transcription factors in cells and their specific binding kinetics, most transcriptional regulatory elements are only rarely engaged with trans-acting protein (6). Second, most genes are rarely transcribed in cells. In budding yeast for example, about 170 genes account for half of the polyadenylated mRNA transcripts in a cell (calculated using 4-thiouracil labeled RNA-seq data from (7)), and the average gene is transcribed on the order of once every few minutes to hours (8). This is especially true of genes for determinative transcription factors which are expressed at extremely low levels, in spite of their requirement to be reliably expressed to maintain cell fates. Third, long-range enhancer-promoter contacts are rare and short-lived (9). Enhancer function is conceptualized as acting by stabilizing large protein complexes at promoters, but it remains unclear how rare transient contacts between enhancers and promoters affect transcriptional output. Fourth, most binding events of chromatin proteins fail to be productive. For example, only 10% of RNA polymerase II (RNAPII) molecules that load at a promoter successfully initiate transcription, and only 10% of those successfully convert to the elongating form and transcribe a gene (10). Finally, transcription sputters, where active promoters release sporadic bursts of multiple initiating RNAPII (referred to as ‘burst size’), and these bursts only occur intermittently (‘burst frequency’) (11). While some of these behaviors are inherent to factor binding to DNA, their dynamics are exacerbated by chromatin packaging. Thus, functional mechanisms must accommodate the sporadic nature of factor binding events, and integrate them with nucleosome dynamics within living cells.
3. DYNAMICS OF NUCLEOSOME POSITIONING
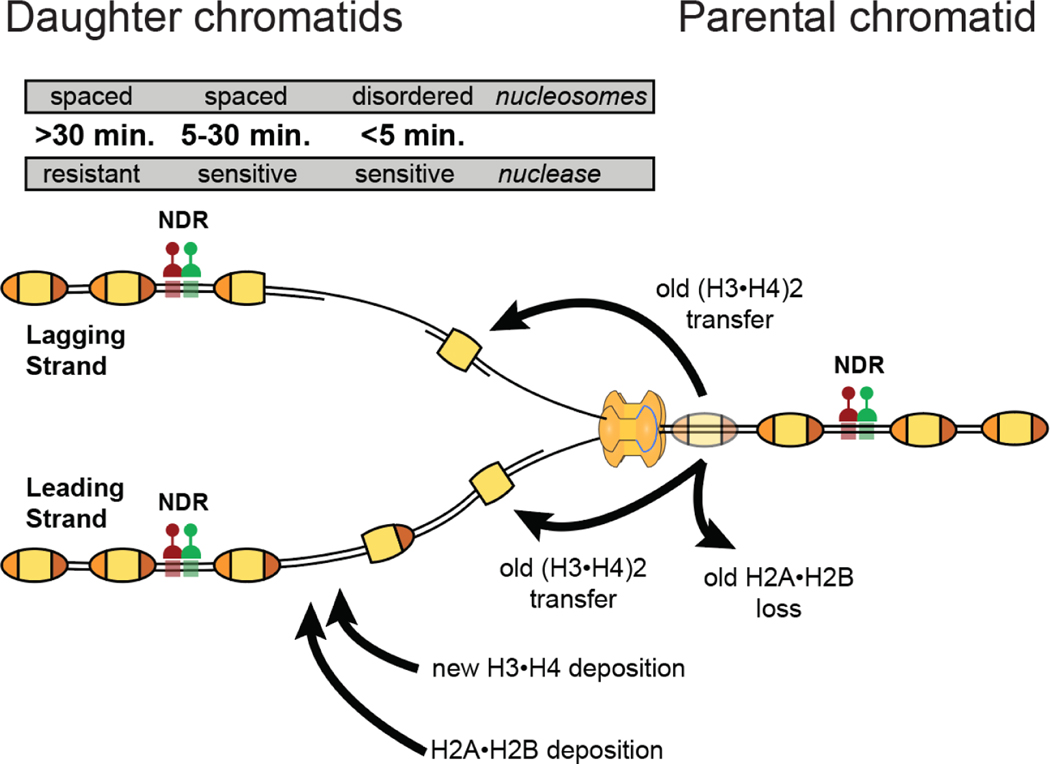
The bulk genome in most eukaryotes is intergenic sequence, and thus relatively quiescent (12). But once every cell cycle chromatin is completely disrupted as the DNA double helix is denatured and fed through the narrow pores of the replication machinery. Doubling of DNA necessitates doubling the histones and chromatin proteins that package that DNA (13), and therefore chromatin duplication is coupled to DNA replication to efficiently rebuild chromatin in the wake of replication forks (Figure 2). Rebuilding chromatin is accomplished by the transfer of old histones from the parental chromatid to the two daughter chromatids, and full packaging of daughter chromatids is completed by the deposition of new histones (14).
Figure 2. Chromatin duplication after DNA replication.
In this schematic of chromatin replication, the replication fork is shown moving to the right; thus, the parental chromatid is to the right of the fork and newly replicated daughter chromatids are to the left of the fork. Newly replicated chromatids remain nuclease sensitive up to 30 minutes post-replication (15) and the deposited nucleosomes get ordered by remodelers 5–30 minutes post-replication (16) as seen in the map of time post-replication (shown above the daughter chromatids). Similarly, transcription factors (TFs) start rebinding at most sites ~30 minutes post-replication in metazoans, creating nucleosome depleted regions (NDRs) (17).
Classic work established the patterns and rates of rebuilding chromatin behind the replication fork. These patterns are a direct consequence of the structure of the nucleosome. The nucleosome contains a tetramer subunit of (H3•H4)2 histones sandwiched between two H2A•H2B dimers, with ~146 bp of DNA in 1.65 left-handed wraps around this octamer (18). Arginine residues from histones form hydrogen bonds with the DNA phosphate backbone to act as sprockets, which together with electrostatic interactions between the basic surface of the histone octamer and acidic DNA stabilize the wrapping of nucleosomal DNA (Figure 3). Together, the first H2A•H2B dimer organizes ~30 bp of DNA, the tetramer ~60 bp, and the second H2A•H2B dimer organizes another 30 bp (two additional contacts between DNA and each H3 histone organizes another 13 bp at the entry and exit sites of the nucleosome). As a replication fork approaches a parental nucleosome and denatures the DNA, a wave of positive supercoiling in front of the fork destabilizes the nucleosome by initiating unwrapping of the negatively supercoiled DNA around the histone octamer to ease fork progression. At first a H2A•H2B dimer is released, then the (H3•H4)2 tetramer and the second dimer dissociate. The tetramer is captured by histone chaperones that accompany the replication machinery, including a histone-binding domain of the MCM helicase (19, 20) and subunits of DNA polymerase epsilon (21) for transfer to daughter strands, while H2A•H2B dimers are released to the nucleoplasm (22). These two modes for nucleosome subunits is referred to as distributive segregation of (H3•H4)2 tetramers and dispersive segregation of H2A•H2B dimers. Behind the replication fork, dimers are added to transferred parental tetramers to complete nucleosomes. This is only sufficient to partially package each daughter chromatid, and new nucleosomes are assembled in the gaps by the deposition of H3•H4 histones by the CAF1 chaperone (23). These new nucleosomes are completed by the addition of H2A•H2B dimers (Figure 2).
Figure 3. DNA wrapping and the subunit structure of the nucleosome.
The top is a sketch of the length of DNA protected by a histone octamer with the arginine-phosphate contacts (sprockets, (24)). Asymmetric unwrapping by processive helicases and polymerases would lead to progressive loss of the arginine contacts (25)(middle). (H3•H4)2 tetramers are minimal units deposited on newly replicated DNA (26, 27), engaging the central arginine sprockets (bottom).
Transfer of histones and new histone deposition occurs on the order of seconds (28, 29), so that only ~250 bp of DNA is depleted for histones behind a replication fork. However, hundreds of kb of chromatin behind a replication fork remain nuclease-sensitive for up to 30 minutes after fork passage (30), during which nucleosomes jostle for more stable positioning and other chromatin proteins including H1 histones are added to further package the daughter chromatids (Figure 2). While these rates are for newly replicated chromatin, similar rates for nucleosome assembly, repositioning, and for adding chromatin packaging proteins should presumably apply after transcription and chromatin remodeling (Figure 4).
Figure 4. The timescales of chromatin dynamics.
(top) Time in seconds plotted on a log scale, annotated with chromatin processes that occur at various timescales. (bottom) Schematic of the dynamics of chromatin proteins on DNA with length of arrows depicting relative rates. Nucleosome assembly is efficient due to chaperones and remodelers. Nucleosome disassembly is fast at regulatory sites to facilitate transcription factor binding compared to non-regulatory sites. Rates of RNA polymerase successfully forming PICs and elongating are much lower relative to the off rates based on observations that only a small fraction of polymerases successfully initiate and elongate (10). TXC - transcription complex; PIC - pre-initiation complex.
Genome-wide profiling of chromatin maturation now provides more detailed pictures of the variation in chromatin duplication across a genome. The rates of re-establishing chromatin structure after DNA replication vary at genomic locations, and are linked to the functional properties of these sites. The transcriptional starts of genes, enhancers, and other regulatory elements are usually small segments dispersed through the vast expanse of bulk chromatin. For example, active promoters span ~200–300 bp of exposed DNA, referred to as a nucleosome-depleted region (NDR), where transcription factors and interacting proteins bind. The NDRs of active promoters are maintained by regulating the position and stability of local nucleosomes (31). But, since chromatin is completely disrupted by DNA replication, promoter nucleosome positioning and NDRs must be re-established in the wake of the replication fork.
The size of a regulatory element affects its propensity to nucleosomal packaging: elements <200 bp in length cannot be wrapped into a nucleosome, thus short promoter regions can be cleared by positioning their flanking nucleosomes. A single transcription factor bound at a promoter can be sufficient to position these flanking nucleosomes and thereby maintain an NDR (32). In contrast, regulatory elements >200 bp are long enough to wrap a nucleosome, and therefore require activities to clear that sequence. This is accomplished by multiple DNA-binding proteins and the activity of chromatin remodelers (33).
In budding yeast cells, re-establishment of promoter NDRs occurs within minutes, and is driven by the rapid re-binding of ubiquitous transcription factors like Abf1 and Reb1 behind the replication fork (34). In metazoans, the dynamics of re-establishing chromatin structure at many regulatory elements and at the promoters for house-keeping genes is similarly rapid (17). These elements also use transcription factor binding to quickly re-establish their chromatin structure at rates faster than that seen for bulk chromatin.
In contrast, the re-establishment of the chromatin structure of developmental promoters and enhancers is slow (17, 35), and is obscured by poorly positioned nucleosomes for more than an hour after replication. This delay implies that transcription factors take a substantial amount of time to re-bind these elements after replication. This delay may allow for fine-tuning of gene expression. Furthermore, promoters that rely on tissue-specific transcription factors to position flanking nucleosomes will effectively limit NDR formation to cells expressing those transcription factors.
Changes in chromatin structure are most dramatic immediately after DNA replication. While comparing chromatin at different stages of the cell cycle gives the impression that nucleosomes are static outside of S phase, all nucleosomes are thermally dynamic, with DNA continually releasing and rebinding from the histone octamer. These dynamics are more extreme near regulatory elements and are crucial for maintaining the precise features of regulatory chromatin structure (36). Three mechanisms modulate nucleosome positioning in vivo, each again is the consequences of the structure of the nucleosome. First, some DNA sequences bend poorly around histone octamers, and can intrinsically destabilize nucleosomes (37). However, most natural eukaryotic sequences do not intrinsically position nucleosomes, and overall this is a minor contribution in genomes. Second, opportunistic binding of sequence-specific factors can trap transiently exposed DNA, and block wrapping of DNA into a nucleosome (38). Finally, translational sliding by chromatin remodeling enzymes can expose DNA and drive nucleosomes to specific positions on DNA (38). Counterintuitively, the most well-defined features in genomes result from the most dynamic nucleosomes.
4. SUBNUCLEOSOMAL DYNAMICS
Nucleosome dynamics also have structural consequences for the histone octamer itself. When DNA is peeled off a nucleosome, contacts with an H2A•H2B dimer of the octamer are broken (39). Eviction of this dimer leaves a histone hexasome on DNA. Such partial nucleosomes are present around active promoters and regulatory elements and contribute to the overall accessibility of DNA at these elements (25). Indeed, the genome of budding yeast, which is much more active than metazoan genomes, is predominantly packaged with subnucleosomal particles, providing an overall heightened genome accessibility (40, 41). Further peeling of the DNA past the H2A•H2B dimer into the tetramer will destabilize the entire octamer, leading to eviction. Thus sites such as NDRs with high nucleosome mobility are also sites of high histone eviction (36, 42, 43).
Subnucleosomal dynamics also allows the specialization of chromatin regions with alternative histones. All eukaryotic genomes encode variant histones in addition to the core histone repertoire. Delivering variants to specific sites in genomes relies on eviction of old histones at dynamic chromatin regions. For example, the metazoan H3.3 histone variant is deposited to fill gaps in chromatin after nucleosomes have been evicted at active promoters and regulatory elements (44–46). Localized deposition of the H3.3 variant is enhanced by specialized histone chaperones and deposition factors that further refine its genomic distribution (47). Similarly, enrichment of H2AZ variants in chromatin is driven by nucleosome destabilization, histone eviction, and recruitment of variant-specific chaperones and deposition complexes (48). The incorporation of H2AZ variant histones also alters RNA polymerase kinetics at promoters (49) and during elongation through nucleosomes (48), possibly by modulating DNA exposure dynamics.
5. ATP-DEPENDENT CHROMATIN REMODELERS DRIVE NUCLEOSOME DYNAMICS
Given the importance of DNA exposure rates for transcription factor binding and RNA polymerase elongation, cells devote machinery to modulating nucleosome dynamics. Multiple SNF2-type ATP-dependent chromatin remodelers bind and mobilize nucleosomes (50). Structural studies of remodelers engaged with nucleosomes show that these motors break DNA-histone contacts, peel DNA off of histone octamers, and propagate a wave of distortion around a nucleosome to move it to a new position (51). Remodelers are targeted to sites of chromatin accessibility (52) and act to either “push” or “pull” flanking nucleosomes (53). The timescales of remodeler action have been interrogated by acute chemical inhibition of the SWI/SNF remodeler Brg1 in mammalian cells (36, 43). Promoters are invaded by nucleosomes and lose transcription factor binding within 10 minutes of Brg1 inhibition and regain their chromatin structure within minutes of washing out the Brg1 inhibitor. Thus, the precise positioning of promoter chromatin architecture is achieved by increasing the dynamics of nucleosomes, such that NDRs and spacing are maintained. Chromatin remodelers may also alter the arrangement of nucleosomes. For example, the ISWI-, CHD-, and INO80-family remodeler enzymes have protein domains that act as rulers to fix the linker DNA length between nucleosomes, thereby altering the spacing in arrays (54). The functional outcomes of remodeling depend on what DNA sequences are exposed and what additional DNA-binding proteins bind at remodeled regulatory elements. For example, in Drosophila, the ubiquitous transcription factor GAF interacts both with Brahma and ISWI chromatin remodelers (55–58), which clear nucleosomes from regulatory elements. This potentiates the binding of additional transcription factors at some elements, and at others the binding of silencing factors.
6. TRANSCRIPTION FACTOR BINDING TO DYNAMIC NUCLEOSOMES
The dynamics of nucleosome positioning and subnucleosomal structures is critical for transcription factor binding in vivo. Wrapping double-helical DNA around the histone octamer means that translational positions of a histone octamer are coupled to the rotational positioning of sequences around the nucleosome (59). Thus, translational movement of a nucleosome both exposes previously wrapped DNA at the edge and rotates the DNA on the histone octamer; this rotation also exposes sequences that were facing the histone octamer every 5 bp. Both DNA exposure at nucleosome edges and rotating sequences on the surface of the nucleosome modulate factor binding.
Due to the wrapping of DNA on histone surface, only stretches of ~5 bp DNA of a given strand are exposed to the aqueous environment (59). The majority of transcription factors bind an extended DNA motif and so bind exposed DNA adjacent to the nucleosome (60). While nucleosomes will limit binding by these factors, in vivo nucleosomes occupy an ensemble of positions (61, 62). Thus, in some cells a DNA sequence may be accessible for binding while in other cells it is occluded. Transient site exposure by peeling also reduces the nucleosomal barrier to factor binding (63). Similarly, rotational dynamics of nucleosomal DNA allow binding by nucleosome-binding transcription factors (64, 65). Not only do nucleosome dynamics promote transcription factor binding, but factor binding also alters nucleosome dynamics. Once a transcription factor binds exposed DNA it prevents re-wrapping of the DNA around a histone octamer (63), and this a major contributor to maintaining NDRs at active regulatory elements.
Transcription factor binding can also drive dramatic changes in nucleosome structure. A small number of transcription factors bind short motifs on wrapped nucleosomal DNA and are thus modulated by the rotational positioning of motifs on the nucleosome surface. Nucleosome-binding factors such as Sox2 distort the DNA they are bound to, destabilizing a nucleosome (66, 67). However, nucleosomal binding is transient as destabilizing the nucleosome exposes motifs for direct factor binding. Indeed, multiple nucleosome-binding factors appear to first bind in a nucleosomal mode and then switch to binding in an exposed DNA mode, apparently as more extended motifs are exposed (68).
How nucleosome dynamics and chromatin protein dynamics are integrated in living cells is now being revealed by single molecule footprinting (6, 69–72). Single molecule footprinting of chromatin in vivo reports factor binding and nucleosome positions across a DNA fiber, and these studies have made surprising observations. First, nucleosome positions and spacing are heterogeneous between chromatids in different cells, implying constant widespread chromatin remodeling (71). These dynamics provide transcription factors the opportunity to bind transiently exposed DNA (38). Thus, nucleosomal binding by transcription factors may rarely be needed, as TFs can exploit transient DNA exposure in a constantly shifting nucleosome landscape. Second, factor binding sites spend a substantial fraction of time neither bound by a transcription factor, nor occluded by nucleosomes but in an empty state with no factor and no nucleosome (6). This predicts short binding times for TFs, which is corroborated by single molecule imaging studies (73). Third, many detectable transcription factor binding events are actually co-binding events of more than one factor at a regulatory element (6, 70, 72). Factor co-binding may efficiently displace nucleosomes and may be one function of juxtaposing multiple factor binding sites within cis regulatory elements (74, 75). Together these single molecule descriptions suggest constant TF and nucleosome dynamics.
7. NUCLEOSOME DYNAMICS DURING TRANSCRIPTION
What about transcriptional elongation? Just like during replication, a polymerase that denatures and copies a DNA strand displaces histone octamers as it progresses. The eviction of histones in transcribed gene bodies is minor: only ~5% of that in regulatory elements (44, 76). This is in part because elements such as promoters are undergoing continuous remodeling, while nucleosomes in gene bodies are only momentarily disrupted upon occasional transcription. Additionally, proteins with histone chaperone activity accompany RNAPII, and these serve to transfer histones from in front of RNAPII back onto DNA in the wake of the polymerase (77). This transfer is assisted by topological changes driven by RNAPII progression. Denaturing the DNA strands propagates a bow wave of positive supercoiling ahead of the polymerase, promoting the disassembly of nucleosomes, while in the wake of RNAPII negative supercoiling promotes rewrapping histone octamers into nucleosomes (78).
8. CHROMATIN DYNAMICS BEYOND THE NUCLEOSOME
While the largest inhibitory effects on DNA factor binding come from histones occluding DNA sequence, chromatin must be further compacted to fit into the nucleus. The higher-order organization of chromatin in the nucleus has been detailed at increasing resolution both by imaging and by sequencing-based methods. Nucleosome arrays are partitioned into loops that are anchored by chromatin complexes including boundary factors and cohesins, and these loops are loosely associated into active and inactive spatial compartments within the nucleus (79, 80). These higher-order structures form and dissolve, and recent experiments indicate that both loops and compartments reflect transient and relatively infrequent contacts between chromatin regions (81–83). How might large-scale transient interactions have effects on gene regulation? Some aspects of higher-order organization may limit or promote nucleosome dynamics. For example, steric effects between nucleosomes in an array will limit translational repositioning and thereby reduce exposure of sequences buried in a nucleosome. Steric effects should be most prominent in compacted nucleosome arrays, and thus heterochromatin and inactive compartments may have relatively weak effects that accumulate over arrays. Alternatively, the promoter-enhancer loops may appear transient as they might be events preceding gene activation and are lost as a consequence of transcription (84).
Packaging DNA into the confines of a cell or a virion is a problem that confronts all forms of DNA-based life. In eukaryotes, DNA packaging is only partially solved by nucleosomes, which achieve a packing ratio of 6:1, whereas the mitotic chromosome packing ratio is ~8,000:1. Topoisomerases are ancient machines for packaging, having evolved prior to the emergence of the eukaryotic nucleus. Therefore, genome packaging must be distinct from nucleosome-based gene regulation. Each nucleosome accounts for one negative superhelical turn, and so complete removal or assembly of a nucleosome requires a single net swiveling (by topoisomerase type I) or DNA pass-through (by topoisomerase type II) event to locally relieve torsion and prevent supercoiling-driven displacement at a distance. Although the transient relief of torsion by topoisomerases is essential for normal gene expression, the possibility that modulation of torsion has also evolved roles in gene regulation (85–88) is an attractive area of future research.
Acknowledgements
This work was supported by the RNA Bioscience Initiative, University of Colorado School of Medicine and NIH grants R35GM133434 (S.R.). S.R. is a Pew-Stewart Scholar for Cancer Research, supported by the Pew Charitable Trusts and the Alexander and Margaret Stewart Trust. S.H. is a Howard Hughes Medical Institute Investigator.
References
- 1.Leschziner AE, Saha A, Wittmeyer J, Zhang Y, Bustamante C, et al. 2007. Conformational flexibility in the chromatin remodeler RSC observed by electron microscopy and the orthogonal tilt reconstruction method. Proc. Natl. Acad. Sci. U. S. A 104(12):4913–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rengachari S, Schilbach S, Aibara S, Dienemann C, Cramer P. 2021. Structure of the human Mediator-RNA polymerase II pre-initiation complex. Nature. 594(7861):129–33 [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Shi Y, Georgescu RE, Yuan Z, Chait BT, et al. 2015. The architecture of a eukaryotic replisome. Nat. Struct. Mol. Biol 22(12):976–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giaever GN, Wang JC. 1988. Supercoiling of intracellular DNA can occur in eukaryotic cells. Cell. 55(5):849–56 [DOI] [PubMed] [Google Scholar]
- 5.Lu F, Lionnet T. 2021. Transcription Factor Dynamics. Cold Spring Harb. Perspect. Biol, p. a040949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao S, Ahmad K, Ramachandran S. 2021. Cooperative binding between distant transcription factors is a hallmark of active enhancers. Mol. Cell 81(8):1651–1665.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeberg MA, Han T, Moresco JJ, Kong A, Yang Y-C, et al. 2013. Pervasive and dynamic protein binding sites of the mRNA transcriptome in Saccharomyces cerevisiae. Genome Biol. 14(2):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelechano V, Chávez S, Pérez-Ortín JE. 2010. A complete set of nascent transcription rates for yeast genes. PloS One. 5(11):e15442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao JY, Hafner A, Boettiger AN. 2021. How subtle changes in 3D structure can create large changes in transcription. eLife. 10:e64320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steurer B, Janssens RC, Geverts B, Geijer ME, Wienholz F, et al. 2018. Live-cell analysis of endogenous GFP-RPB1 uncovers rapid turnover of initiating and promoter-paused RNA Polymerase II. Proc. Natl. Acad. Sci. U. S. A 115(19):E4368–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez A, Golding I. 2013. Genetic Determinants and Cellular Constraints in Noisy Gene Expression. Science. 342(6163):1188–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libbrecht MW, Rodriguez OL, Weng Z, Bilmes JA, Hoffman MM, Noble WS. 2019. A unified encyclopedia of human functional DNA elements through fully automated annotation of 164 human cell types. Genome Biol. 20(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Günesdogan U, Jäckle H, Herzig A. 2014. Histone supply regulates S phase timing and cell cycle progression. eLife. 3:e02443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alabert C, Barth TK, Reverón-Gómez N, Sidoli S, Schmidt A, et al. 2015. Two distinct modes for propagation of histone PTMs across the cell cycle. Genes Dev. 29(6):585–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cusick ME, Lee KS, DePamphilis ML, Wassarman PM. 1983. Structure of chromatin at deoxyribonucleic acid replication forks: nuclease hypersensitivity results from both prenucleosomal deoxyribonucleic acid and an immature chromatin structure. Biochemistry. 22(16):3873–84 [DOI] [PubMed] [Google Scholar]
- 16.Yadav T, Whitehouse I. 2016. Replication-Coupled Nucleosome Assembly and Positioning by ATP-Dependent Chromatin-Remodeling Enzymes. Cell Rep. 15(4):715–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran S, Henikoff S. 2016. Transcriptional Regulators Compete with Nucleosomes Post-replication. Cell. 165(3):580–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 389(6648):251–60 [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Strømme CB, Saredi G, Hödl M, Strandsby A, et al. 2015. A unique binding mode enables MCM2 to chaperone histones H3-H4 at replication forks. Nat. Struct. Mol. Biol 22(8):618–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petryk N, Dalby M, Wenger A, Stromme CB, Strandsby A, et al. 2018. MCM2 promotes symmetric inheritance of modified histones during DNA replication. Science. 361(6409):1389–92 [DOI] [PubMed] [Google Scholar]
- 21.Yu C, Gan H, Serra-Cardona A, Zhang L, Gan S, et al. 2018. A mechanism for preventing asymmetric histone segregation onto replicating DNA strands. Science. 361(6409):1386–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson V 1990. In vivo studies on the dynamics of histone-DNA interaction: evidence for nucleosome dissolution during replication and transcription and a low level of dissolution independent of both. Biochemistry. 29(3):719–31 [DOI] [PubMed] [Google Scholar]
- 23.Stillman B 1986. Chromatin assembly during SV40 DNA replication in vitro. Cell. 45(4):555–65 [DOI] [PubMed] [Google Scholar]
- 24.Hodges AJ, Gallegos IJ, Laughery MF, Meas R, Tran L, Wyrick JJ. 2015. Histone Sprocket Arginine Residues Are Important for Gene Expression, DNA Repair, and Cell Viability in Saccharomyces cerevisiae. Genetics. 200(3):795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramachandran S, Ahmad K, Henikoff S. 2017. Transcription and Remodeling Produce Asymmetrically Unwrapped Nucleosomal Intermediates. Mol. Cell 68(6):1038–1053.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu WH, Roemer SC, Zhou Y, Shen Z-J, Dennehey BK, et al. 2016. The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones. eLife. 5:e18023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattiroli F, Gu Y, Yadav T, Balsbaugh JL, Harris MR, et al. 2017. DNA-mediated association of two histone-bound complexes of yeast Chromatin Assembly Factor-1 (CAF-1) drives tetrasome assembly in the wake of DNA replication. eLife. 6:e22799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nucleosome positioning at the replication fork. 2001. EMBO J. 20(24):7294–7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirier MG, Oh E, Tims HS, Widom J. 2009. Dynamics and function of compact nucleosome arrays. Nat. Struct. Mol. Biol 16(9):938–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seale RL. 1978. Nucleosomes associated with newly replicated DNA have an altered conformation. Proc. Natl. Acad. Sci. U. S. A 75(6):2717–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krietenstein N, Wal M, Watanabe S, Park B, Peterson CL, et al. 2016. Genomic Nucleosome Organization Reconstituted with Pure Proteins. Cell. 167(3):709–721.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polach KJ, Widom J. 1995. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J. Mol. Biol 254(2):130–49 [DOI] [PubMed] [Google Scholar]
- 33.Kubik S, O’Duibhir E, de Jonge WJ, Mattarocci S, Albert B, et al. 2018. Sequence-Directed Action of RSC Remodeler and General Regulatory Factors Modulates +1 Nucleosome Position to Facilitate Transcription. Mol. Cell 71(1):89–102.e5 [DOI] [PubMed] [Google Scholar]
- 34.Vasseur P, Tonazzini S, Ziane R, Camasses A, Rando OJ, Radman-Livaja M. 2016. Dynamics of Nucleosome Positioning Maturation following Genomic Replication. Cell Rep. 16(10):2651–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart-Morgan KR, Reverón-Gómez N, Groth A. 2019. Transcription Restart Establishes Chromatin Accessibility after DNA Replication. Mol. Cell 75(2):284–297.e6 [DOI] [PubMed] [Google Scholar]
- 36.Iurlaro M, Stadler MB, Masoni F, Jagani Z, Galli GG, Schübeler D. 2021. Mammalian SWI/SNF continuously restores local accessibility to chromatin. Nat. Genet, pp. 1–9 [DOI] [PubMed] [Google Scholar]
- 37.Struhl K, Segal E. 2013. Determinants of nucleosome positioning. Nat. Struct. Mol. Biol 20(3):267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polach KJ, Widom J. 1996. A model for the cooperative binding of eukaryotic regulatory proteins to nucleosomal target sites. J. Mol. Biol 258(5):800–812 [DOI] [PubMed] [Google Scholar]
- 39.Bilokapic S, Strauss M, Halic M. 2018. Histone octamer rearranges to adapt to DNA unwrapping. Nat. Struct. Mol. Biol 25(1):101–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan ZY, Cai S, Noble AJ, Chen JK, Shi J, Gan L. 2021. Heterogeneous non-canonical nucleosomes predominate in yeast cells in situ. bioRxiv. 2021.04.04.438362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee HS, Bataille AR, Zhang L, Pugh BF. 2014. Subnucleosomal structures and nucleosome asymmetry across a genome. Cell. 159(6):1377–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deal RB, Henikoff JG, Henikoff S. 2010. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science. 328(5982):1161–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schick S, Grosche S, Kohl KE, Drpic D, Jaeger MG, et al. 2021. Acute BAF perturbation causes immediate changes in chromatin accessibility. Nat. Genet 53(3):269–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deaton AM, Gómez-Rodríguez M, Mieczkowski J, Tolstorukov MY, Kundu S, et al. 2016. Enhancer regions show high histone H3.3 turnover that changes during differentiation. eLife. 5:e15316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray-Gallet D, Woolfe A, Vassias I, Pellentz C, Lacoste N, et al. 2011. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol. Cell 44(6):928–41 [DOI] [PubMed] [Google Scholar]
- 46.Ahmad K, Henikoff S. 2002. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9(6):1191–1200 [DOI] [PubMed] [Google Scholar]
- 47.Grover P, Asa JS, Campos EI. 2018. H3-H4 Histone Chaperone Pathways. Annu. Rev. Genet 52:109–30 [DOI] [PubMed] [Google Scholar]
- 48.Weber CM, Ramachandran S, Henikoff S. 2014. Nucleosomes are context-specific, H2A.Z-modulated barriers to RNA polymerase. Mol. Cell 53(5):819–30 [DOI] [PubMed] [Google Scholar]
- 49.Mylonas C, Lee C, Auld AL, Cisse II, Boyer LA. 2021. A dual role for H2A.Z.1 in modulating the dynamics of RNA polymerase II initiation and elongation. Nat. Struct. Mol. Biol 28(5):435–42 [DOI] [PubMed] [Google Scholar]
- 50.Narlikar GJ, Sundaramoorthy R, Owen-Hughes T. 2013. Mechanisms and Functions of ATP-Dependent Chromatin-Remodeling Enzymes. Cell. 154(3):490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGinty RK, Tan S. 2021. Principles of nucleosome recognition by chromatin factors and enzymes. Curr. Opin. Struct. Biol 71:16–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brahma S, Henikoff S. 2020. Epigenome Regulation by Dynamic Nucleosome Unwrapping. Trends Biochem. Sci 45(1):13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kubik S, Bruzzone MJ, Challal D, Dreos R, Mattarocci S, et al. 2019. Opposing chromatin remodelers control transcription initiation frequency and start site selection. Nat. Struct. Mol. Biol 26(8):744–54 [DOI] [PubMed] [Google Scholar]
- 54.Oberbeckmann E, Niebauer V, Watanabe S, Farnung L, Moldt M, et al. 2021. Ruler elements in chromatin remodelers set nucleosome array spacing and phasing. Nat. Commun 12(1):3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lomaev D, Mikhailova A, Erokhin M, Shaposhnikov AV, Moresco JJ, et al. 2017. The GAGA factor regulatory network: Identification of GAGA factor associated proteins. PloS One. 12(3):e0173602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakayama T, Shimojima T, Hirose S. 2012. The PBAP remodeling complex is required for histone H3.3 replacement at chromatin boundaries and for boundary functions. Dev. Camb. Engl 139(24):4582–90 [DOI] [PubMed] [Google Scholar]
- 57.Xiao H, Sandaltzopoulos R, Wang H-M, Hamiche A, Ranallo R, et al. 2001. Dual Functions of Largest NURF Subunit NURF301 in Nucleosome Sliding and Transcription Factor Interactions. Mol. Cell 8(3):531–43 [DOI] [PubMed] [Google Scholar]
- 58.Judd J, Duarte FM, Lis JT. 2020. Pioneer-like factor GAF cooperates with PBAP (SWI/SNF) and NURF (ISWI) to regulate transcription. Genes Dev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolffe AP. 2014. Chromatin: Structure and Function. Saint Louis: Elsevier Science [Google Scholar]
- 60.Zhu F, Farnung L, Kaasinen E, Sahu B, Yin Y, et al. 2018. The interaction landscape between transcription factors and the nucleosome. Nature. 562(7725):76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brogaard K, Xi L, Wang J-P, Widom J. 2012. A map of nucleosome positions in yeast at base-pair resolution. Nature. 486(7404):496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chereji RV, Ramachandran S, Bryson TD, Henikoff S. 2018. Precise genome-wide mapping of single nucleosomes and linkers in vivo. Genome Biol. 19(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li G, Widom J. 2004. Nucleosomes facilitate their own invasion. Nat. Struct. Mol. Biol 11(8):763–69 [DOI] [PubMed] [Google Scholar]
- 64.Cui F, Zhurkin VB. 2014. Rotational positioning of nucleosomes facilitates selective binding of p53 to response elements associated with cell cycle arrest. Nucleic Acids Res. 42(2):836–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Wrange O. 1995. Accessibility of a glucocorticoid response element in a nucleosome depends on its rotational positioning. Mol. Cell. Biol 15(8):4375–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dodonova SO, Zhu F, Dienemann C, Taipale J, Cramer P. 2020. Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Nature. 580(7805):669–72 [DOI] [PubMed] [Google Scholar]
- 67.Michael AK, Grand RS, Isbel L, Cavadini S, Kozicka Z, et al. 2020. Mechanisms of OCT4-SOX2 motif readout on nucleosomes. Science [DOI] [PubMed] [Google Scholar]
- 68.Meers MP, Janssens DH, Henikoff S. 2019. Pioneer Factor-Nucleosome Binding Events during Differentiation Are Motif Encoded. Mol. Cell 75(3):562–575.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krebs AR, Imanci D, Hoerner L, Gaidatzis D, Burger L, Schübeler D. 2017. Genome-wide Single-Molecule Footprinting Reveals High RNA Polymerase II Turnover at Paused Promoters. Mol. Cell 67(3):411–422.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sönmezer C, Kleinendorst R, Imanci D, Barzaghi G, Villacorta L, et al. 2021. Molecular Co-occupancy Identifies Transcription Factor Binding Cooperativity In Vivo. Mol. Cell 81(2):255–267.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdulhay NJ, McNally CP, Hsieh LJ, Kasinathan S, Keith A, et al. 2020. Massively multiplex single-molecule oligonucleosome footprinting. eLife. 9:e59404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stergachis AB, Debo BM, Haugen E, Churchman LS, Stamatoyannopoulos JA. 2020. Single-molecule regulatory architectures captured by chromatin fiber sequencing. Science. 368(6498):1449–54 [DOI] [PubMed] [Google Scholar]
- 73.Voss TC, Hager GL. 2014. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat. Rev. Genet 15(2):69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moyle-Heyrman G, Tims HS, Widom J. 2011. Structural Constraints in Collaborative Competition of Transcription Factors against the Nucleosome. J. Mol. Biol 412(4):634–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mirny LA. 2010. Nucleosome-mediated cooperativity between transcription factors. Proc. Natl. Acad. Sci 107(52):22534–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraushaar DC, Jin W, Maunakea A, Abraham B, Ha M, Zhao K. 2013. Genome-wide incorporation dynamics reveal distinct categories of turnover for the histone variant H3.3. Genome Biol. 14(10):R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeronimo C, Poitras C, Robert F. 2019. Histone Recycling by FACT and Spt6 during Transcription Prevents the Scrambling of Histone Modifications. Cell Rep. 28(5):1206–1218.e8 [DOI] [PubMed] [Google Scholar]
- 78.Teves SS, Henikoff S. 2014. Transcription-generated torsional stress destabilizes nucleosomes. Nat. Struct. Mol. Biol 21(1):88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M Ragoczy T, et al. 2009. Comprehensive Mapping of Long-Range Interactions Reveals Folding Principles of the Human Genome. Science. 326(5950):289–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao SSP, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, et al. 2014. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell. 159(7):1665–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bintu B, Mateo LJ, Su J-H, Sinnott-Armstrong NA, Parker M, et al. 2018. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science. 362(6413): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardozo Gizzi AM, Cattoni DI, Nollmann M. 2020. TADs or no TADS: Lessons From Single-cell Imaging of Chromosome Architecture. J. Mol. Biol 432(3):682–93 [DOI] [PubMed] [Google Scholar]
- 83.Hansen AS, Cattoglio C, Darzacq X, Tjian R. 2018. Recent evidence that TADs and chromatin loops are dynamic structures. Nucl. Austin Tex 9(1):20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winick-Ng W, Kukalev A, Harabula I, Redondo LZ, Szabo D, et al. 2020. Cell-type specialization in the brain is encoded by specific long-range chromatin topologies [Google Scholar]
- 85.Li L, Williams P, Ren W, Wang MY, Gao Z, et al. 2021. YY1 interacts with guanine quadruplexes to regulate DNA looping and gene expression. Nat. Chem. Biol 17(2):161–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyu J, Shao R, Elsässer SJ. 2021. Genome-wide mapping of G-quadruplex structures with CUT&Tag. . 2021.04.25.441312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spiegel J, Cuesta SM, Adhikari S, Hänsel-Hertsch R, Tannahill D, Balasubramanian S. 2021. G-quadruplexes are transcription factor binding hubs in human chromatin. Genome Biol. 22(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu J, Kouzine F, Nie Z, Chung H-J, Elisha-Feil Z, et al. 2006. The FUSE/FBP/FIR/TFIIH system is a molecular machine programming a pulse of c-myc expression. EMBO J. 25(10):2119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]