Abstract
Background
Herein, a newly synthesised mixed ligand artemisinin/zinc (Art/Zn) is chemically characterised and examined against SARS-CoV-2.
Methods
The synthesised complex was thoroughly characterised using various spectroscopic methods (FT-IR, UV and XRD). Its surface morphology and chemical purity were investigated using transmission electron microscopy (TEM), scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) analysis. The synthesised Art/Zn complex was tested for its inhibitory effects against SARS-CoV-2 using inhibitory concentration 50 (IC50) and cytotoxicity concentration 50 (CC50).
Results
The results reveal that the Art/Zn complex exhibits a moderate in vitro inhibitory effects against SARS-CoV-2, with a CC50 index of 213.6 μg/ml and an IC50 index of 66.79 μg/ml. Notably, it exhibits the inhibitory effect (IC50 = 66.79 μg/ml) at a very low concentration without any observable cytotoxic effects on host cells (CC50 = 213.6 μg/ml). Its mode of action against SARS-CoV-2 involves inhibiting the viral replication. The predicted target classes that Art/Zn may affect include kinases, which can regulate and inhibit the viral replication and binding to the angiotensin-converting enzyme-2 (ACE2) receptor and the main protease inhibitor (MPro), thereby inhibiting the activity of SARS-CoV-2 and proved by the molecular dynamics simulation.
Conclusion
We recommend using the Art/Zn complex owing to its moderate inhibitory and antiviral effects against the SARS-CoV-2 with a low cytotoxic effect on host (Vero E6) cells. We suggest conducting further prospective studies to investigate the biological effects of Art/Zn in animal models at different concentrations for testing its clinical efficacy and safety in inhibiting SARS-CoV-2 activities.
Keywords: SARS-CoV-2, Artemisinin, Zinc metal, Antiviral activity, Cytotoxicity
Graphical abstract
1. Introduction
Diseases such as obstructive pulmonary inflammation and even pulmonary cancer can cause severe health and chronic respiratory conditions [1,2]. Pulmonary inflammation can be either acute or chronic [3]; it is a cellular response triggered by foreign agents in the lungs. Most pulmonary diseases are mainly associated with the inflammation and involve many inflammatory cells such as the macrophages and the lymphocytes.
Natural products may offer many alternative therapeutic potentials for respiratory diseases; however, they may also have side effects as several compounds may exhibit activity against the respiratory diseases such as asthma and COPD. Plant-derived natural products and their mechanism of action for treating respiratory complications have been extensively studied [[4], [5], [6], [7]].
Natural products and active compounds have a great source of medicinal efficiency, and the most modern medicines are either plant-based natural remedies or their variants [8].
Artemisinin (Art) is a trioxane lactone with presence of a peroxide bridge [9]. It is an active compound derived from Artemisia annua L.; this plant has been used for treating fevers for thousands of years [10]. The elevating resistance of Plasmodium falciparum to anti-malarial drugs in the 1950s led the scientists worldwide to search for better therapeutics against severe symptoms of malaria.
A. annua L. discovered in traditional Chinese medicines exhibits an inhibitory effect on P. falciparum [11], and its active compounds demonstrated potent anti-malarial properties [12]. Youyou Tu who received the 2015 Nobel Prize in Medicine for his great contributions in proving the therapeutic efficacy of A. annua L.’s active compounds against malaria [13].
COVID-19 was member of the Coronaries (CoV) family, this virus has a dangerous effect on the human beings, and it was proved as a global health pandemic by the World Health Organization (WHO) [14]. Corona viruses are well known for their high ability to induce sever symptoms, with mostly acute respiratory syndrome as (SARS-CoV). Though there are other coronavirus infections, they have very mild to moderate infection such as (hCoVsOC43 and 229E), which are responsible for the common cold [15].
SARS-CoV-2 virus mainly combines (4) proteins: the most distinguished part is the spike, then the membrane, followed by both the envelope and the nucleocapsid proteins [16]. Spike proteins of SARS-CoV-2 have an essential and critical role in the cellular attachment of viruses and entry and act as a main target for the antibodies and improvement of vaccines [17,18]. The spike (S1) domain of SARS-CoV-2 binds directly to the cellular receptors (ACE2) [19,20]. SARS-CoV can enter the cells quickly via “SARS-SRBD” main interactions with the cellular receptors (ACE2) and (MPro) [21,22].
Molecular docking was carried out to obtain orientation and conformations for different mixed ligands at different cellular binding sites. This test was carried out to evaluate the interactions between SARS-CoV-2 and the cellular receptors with the significant active phytocompounds of Artemisinin annua L. All calculations for docking programs were the best conformation and orientation of active compounds of Artemisia annua L. for high inhibition of SARS-CoV-2 [23,24].
Zinc (Zn) is an important essential metal that play a crucial role in the human immunity. It is vital for cellular protection and for eliminating viruses from host cells. Zn affects various cellular regulators and may exhibit inhibitory effects, including antibacterial and anticancer effect, and may be used for healing wound healings [25].
The current study aimed to evaluate the inhibitory effect of the novel metal complex “Art/Zn”, against SARS-CoV-2 virus by elucidating its chemical and spectroscopic structure and evaluating its cytotoxicity.
2. Materials and methods
2.1. Ethical approval
The inhibitory and the cytotoxic effects of the novel metal complex (Art/Zn) against SARS-CoV-2 were evaluated at the Centre of Scientific Excellence for Influenza Viruses at the National Research Centre, Giza, Egypt, with the accession, ethics and registration numbers being EPI-ISL-430820, hCoV-19/Egypt/NRC-03/2020 and SAMN14814607, respectively.
2.2. Chemicals and instrumental analyses
Artemisinin (Art) (Fig. 1) and Zinc chloride (ZnCl2) were supplied by Sigma-Aldrich, USA and used without purification.
Fig. 1.

Chemical structure of Art.
The analysis tools are listed in Table .1.
Table 1.
Tools and synthesis analysis.
| Analysis instrument | Models |
|---|---|
| Elemental analyses | Perkin Elmer |
| Conductance | Jenway conductivity meter (4010) |
| FTIR spectra | FTIR Spectrophotometer (Bruker) |
| Electronic spectra | UV2 (Spectrophotometer)Unicam |
| TEM | JEOL (100s) microscopy |
| Magnetic measurements | Sherwood magnetic balance using the Gouy method |
| SEM | Quanta FEG 250 equipment |
| X-ray diffraction patterns were recorded on | X 'Pert PRO PANanalytical X-ray powder diffraction, target copper with secondary monochromatic. |
2.3. Synthesis of artemisinin/zinc complex
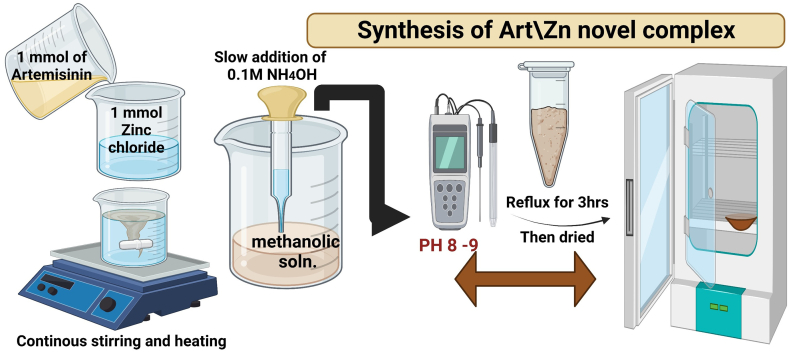
The Art/Zn novel complex was prepared by reacting methanolic solution of ZnCl2 (1 mmol, 0.137 g) with a 20 ml Art methanolic solution (1 mmol, 0.283 g). The pH was adjusted from 8–9 by using a 0.1M NH4OH solution. The mixture was refluxed for 3 hr, filtered, solid-product was washed using a mixture of methanol and dist.H2O and then, dried by using a sterilized desiccator (Fig. 2).
Fig 2.
Synthesis of Artemisinin/zinc complex.
2.4. MTT cytotoxicity assay (CC50)
To evaluate the half maximal cytotoxic concentration (CC50), a stock solution of the tested sample of “Art/Zn” was prepared in 10% DMSO that was diluted with dd.H2O and then addition to the working solutions with DMEM. The cytotoxicity of Art/Zn sample was tested by using the 3-(4,5-dimethylthiazol -2-yl)-2, 5-diphenyltetrazolium bromide (MTT) in (VERO-E6) cells. The (VERO-E6) cells were seeded in 96 well plates via concentration of (100 μl/well) and incubated at 37 °C, for 24 h in CO2. After 24 h. Seeded cells were treated with different serial concentrations of novel Art/Zn complex in triplicates. After 24 h, the supernatants were discarded immediately, and the cell monolayer was washed with sterile saline phosphate buffer (PBS)1× for 3 times, MTT solution was added immediately to the target well and then incubation for 4 h at 37 °C. The collected formazan crystals formed were highly dissolved with ∼ 200 μl of isopropanol mixed with 0.04 M HCL. The absorbance was measured at wavelengths (540–620 nm) using a well plate reader. The % of cytotoxicity compared to the untreated (VERO-E6) cells was determined with the following equation [26].
| % cytotoxicity = ((Absorbance without treatment – Absorbance with treatment) × 100)/(Absorbance without treatment). |
2.5. Inhibitory concentration 50 (IC50) determination
In 96-well tissue culture plates, Vero-E6 cells were distributed and then incubated for 24 h at 37 °C incubator with 5% CO2 condition. The cell monolayer was washed with fresh PBS 1× and then subjected to the virus adsorption (hCoV-19/Egypt/NRC-03/2020 for 1 h at the room temperature. The cells' monolayers were then overlaid with 100 μl of DMEM containing varying concentrations of Art/Zn. Following incubation for 3 days (72 h), the Vero-E6 cells were immediately fixed with about 100 μl of 4% paraformaldehyde for only 20 min and then stained with crystal violet stain in concentration (0.1%) for 1/4 h at room temperature. The crystal violet dye was then subsequently dissolved by using the absolute methanol/well and then, the optical density was measured at 570 nm by using a multi plate reader. The IC50 of Art/Zn novel complex is required to decline the virus-induced cytopathic effect (CPE) by 50%, relative to the SARS-CoV-2 virus control [27].
2.6. Mechanism of virus inhibition
The potential mechanism of the SARS-CoV-2 strain, " hCoV-19/Egypt/NRC-03/2020" (accession number on GSAID: EPI_ISL_430820) virus inhibitory effect by the synthesised complex that was examined at nearly three levels.
2.6.1. Viral replication action
The viral assay was conducted in a sterile six-well plate, with Vero E6 cells being immediately cultivated at (105 cells/ml) for only 24 h at 37 °C. The virus was greatly diluted to obtain 103 PFU/well, then applied directly to the cells, and incubated for only 1 h at 37 °C. Unabsorbed viral particles were removed by washing the cells for 3 times in a free medium. The sample was applied at different serial concentrations, and after 1 h of direct exposure, 3 ml of a DMEM medium only supplemented with 2% agarose was added to the cellular monolayer. Well plates were left to become solid and incubate at 37 °C until the viral plaques clearly appeared. Cellular monolayer was fixed in a 10% freshly prepared formalin solution for only 2 h and then deeply stained with the crystal violet stain. Control well plates were included where the cells were immediately incubated with the SARS-CoV-2 virus, the formed plaques were accurately counted and the percentage (%) of reduction in the formed plaques that was compared to the control well plates [28].
| Inhibition percentage= (Plaques in viral control-Plaques in teste/Plaques in viral control) *100 |
2.6.2. Viral adsorption action
VeroE6 cells were immediately cultivated in a six-well plate (105 cells/ml) for only 24 h at 37 °C. The sample was applied via various concentrations in a 200-μl medium without any supplementation and co-incubated with the cells for only 2 h at 4 °C. The unabsorbed sample was removed via washing the cells for three times in a supplement-free medium, and then, the virus was greatly diluted to obtain 103 PFU/well and co-incubated with the pre-treated cells for 1 h. Afterwards, 3-ml DMEM medium supplemented with 2% agarose was added. Well plates were then left to become more solid, then incubated at 37 °C to allow the viral plaques’ formation, fixed and deeply stained to calculate the % reduction in the plaque formation as compared to that for control well plates where untreated cells were infected with the SARS-CoV-2 virus [29].
2.6.3. Virucidal action
The assay was conducted in a sterile six-well plate, with E6-Vero cells being cells that were cultivated at concentration (105 cells/ml) for 24 h at 37 °C. A 200-μl DMEM free medium that contain 103 PFU of the SARS-CoV-2 virus was added to various concentrations of Art/Zn novel complex. After 1hr of incubation, the mixture was diluted 10-fold using the serum-free medium, which still allowed the virus to grow on cells but left almost no extract. Then, 100 μl of each dilution was added to the cell monolayer. After about 1hr contact time, a DMEM over layer was added to the cell monolayer. Well plates were kept to solidify, then incubated at 37 °C to allow the viral plaques' formation. They were then immediately fixed and deeply stained to calculate the % reduction in the plaque formation as compared to that of control well plates where the E6-Vero cells were infected with a SARS-CoV-2 virus that was not pre-treated with the Art/Zn novel sample [30].
2.7. Molecular docking studies
Here in the study, analyzed the pharmacophoric characteristics of SARS-CoV-2 with ACE2 receptor, co-crystallized inhibitor (Art/Zn) to synthesize novel complexes by using the ligand-based design approach [31]. we synthesizing a novel formula Art/Zn that can bind with ACE2 receptors that bind SARS-CoV-2.
We used Swiss dock tool and referenced study [32,33] in predicting the docking and affinity of binding, total fitness, and estimated ΔG. Generalized molecular mechanics During MD simulations of complexes, the Born surface area (MM-GBSA) prime module was utilized to quantify the docked complex's (Gbind) binding free energy. The Gbind was then calculated by using the OPLS 2005 force field, VSGB solvent model, and rotamer methods. The MD trajectory frames were picked at the start and end of simulation intervals following the MD run. This equation was used to compute the Gbind value.
where, dGbind means binding free energy, Gcomplex means free energy of the complex, Gprotein means free energy of the target protein, and Gligand means free energy of the ligand. The MMGBSA method is used to assess the binding energy of the ligands to the receptors [34]. The influence of additional non-bonded interaction energies was evaluated.
2.8. Physicochemical and pharmacokinetic properties of novel complex (Art/zn)
The pharmacokinetics and physicochemical investigation is the outstanding step in identifying properties of the novel Art/Zn complex. We used tool of Swiss MD for evaluation of the physicochemical and pharmacokinetic parameters of the synthesised Art/Zn novel complex [35].
2.9. Docking of the synthesised novel complex (Art/zn) to SARS-CoV-2:(ACE2 and Mpro) receptors
The molecular docking step was performed by using protocol of molecular docking that was utilized as recommended previously to investigate the RMSD, scores and L-torsion [36,37].
2.10. Molecular dynamics (MD) simulations
The Schrödinger package was used to perform the MD simulations [38]. Additionally, the MM-GBSA energies Art/Zn novel complex was measured [39].
2.11. MM-GBSA calculations
The thermal-mmgbsa by Schrodinger was used to perform the MM-GBSA binding energies [34]. Also, estimation of the Covalent-binding, Coulomb, Generalized Born electrostatic solvation, Hydrogen-bonding, Lipophilic and van der Waals energies.
3. RESULTS
3.1. Physical measurements data
ZnCl2 reacted with Art based on the following equation: ZnCl2+ Art = [Zn (Art)2(Cl)2(H2O)2]. H2O. The structure for novel zinc complex [Zn (Art)2(Cl)2(H2O)2]. H2O was in (Fig. 3). The molar reaction ratio is 1:2 for Zn: Art. The new zinc complex was soluble in DMSO and stable in the air. The molar conductivity value (Λm) was 27 Ω−1 cm2.mol−1, confirming that Art/Zn complex has non-electrolytic nature [40].
Fig. 3.

Structure of (Art/Zn) novel complex.
3.2. Infrared spectra
For Art free ligand, strong, broad bands appeared at 3750 and 3691 cm−1 assigned stretching vibrations of ν(O–H) [41] due to the formation of H-bond between hydrogen atom and oxygen atom for the ring. The stretching vibration for carbonyl group ν(C O) for free Art appeared at 1735,1690 cm−1 [42], while for Art/Zn complex [Zn (Art)2(Cl)2(H2O)2]. H2O, occur shifting for the (C O) group ranged at 1650-1719 cm−1. Confirming that zinc metal ion can chelate via the oxygen atom of the C O group. For Art free ligand and [Zn (Art)2(Cl)2(H2O)2]. H2O. In the complex aliphatic ν(C–H) Stretching vibration motions are observed at 2855-2971 cm−1.
For free ligand Art, the vibration band for ether group ν(COC) is observed at 1263 cm−1and there is no shift occurring for this band in the complex which confirms that (COC) does not participate in the chelation process (Fig. 4A), for [Zn (Art)2(Cl)2(H2O)2]. H2O there are new bands appeared at 615–660 cm−1, referring to the stretching vibration motion of ν(Zinc−O) as shown in (Fig. 4B) [43,44].
Fig. 4.
IR of (A) Art, (B) Art/Zn complex.
3.3. UV–Vis spectra and magnetic data
The UV–Vis spectrum of the free ligand (Art) has about 2 absorption bands observed at 295 and 354 nm assigned to π→π* and n→π* transitions [45]. The electronic absorption spectra for [Zn (Art)2(Cl)2(H2O)2]. H2O complex has 2 absorption bands at 299 nm and 360 nm due to π→π* and n→π* electronic transitions (Fig.S1) (Table .2) and is considered diamagnetic. Due to the formation of Art/zinc complex caused by the conjugated system by chelation, that increases wavelength value (bathochromic shift) [46]. For novel Art/Zn complex other bands are observed at 420 nm, 445 nm, and 460 nm. These bands are due to the charge transfer bands (M→L) of the metal-ligand. The magnetic moment for Zn (III) is convenient with the octahedral field.
Table 2.
Magnetic moments and electronic spectra of either free Art or its ligand with Zn [Zn (Art)2(Cl)2(H2O)2]. H2O complex.
| Sample | Electronic bands/nm |
Magnetic moment |
Geometry |
|
|---|---|---|---|---|
| π–π* | n–π* | |||
| Art | 295 | 354 | – | – |
| [Zn (Art)2(Cl)2(H2O)2]. H2O | 299 | 360 | diamagnetic | Octahedral |
3.4. X-ray diffraction analysis (XRD)
(XRD) “X-ray powder diffraction pattern” for [Zn (Art)2(Cl)2(H2O)2]. H2O complex at value of (2θ) 4–80° to study the nano-formula [Zn (Art)2(Cl)2(H2O)2]. H2O complex. XRD for [Zn (Art)2(Cl)2(H2O)2]. H2O is in Fig. 5. According to the x-ray diffraction pattern a broad peak appeared at 2θ = 23°, suggesting that Art/Zn complex has an amorphous structure (Fig. 5) [47].
Fig. 5.
XRD of Art/Zn novel complex.
3.5. Scanning electron microscopy (SEM) and energy-dispersive X-ray analysis (EDX)
Scanning electron microscopy (SEM) was used to evaluate the physical and microscopic characteristics of Art as shown in (Fig. 6A) and Art/Zn [Zn (Art)2(Cl)2(H2O)2]·H2O as in (Fig. 6B). An SEM image of [Zn (Art)2(Cl)2(H2O)2]·H2O reveal a small particle's size with nano-features. The surface morphology of the Art/Zn (II) complex was analyzed, which showed small particles with a high tendency for agglomeration and with different shapes.
Fig. 6.
SEM of (A) Art, (B) Art/Zn complex.
3.6. Transmission electron microscopy (TEM)
The transmission electron microscopy images of Art as shown in (Fig. 7A) and Art/Zn [Zn (Art)2(Cl)2(H2O)2]·H2O mixed ligand are shown in (Fig. 7B). The uniform matrix of [Zn (Art)2(Cl)2(H2O)2]·H2O is visible in the following image, confirming that [Zn (Art)2(Cl)2(H2O)2]·H2O has a material phase with a homogeneous character. Spherical black spots are observed for the Art/Zn complex within a particle size range of 31.99–48.13 nm.
Fig. 7.
TEM of (A) Art, (B) Art/Zn complex.
3.7. Cytotoxic activities of novel metal complex of (Art/zn) against virus (SARS-CoV-2)
ART/Zn exhibited a moderate inhibitory effect with antiviral properties against SARS-CoV-2 at a very low concentration (213.6 μg/ml). The current results showed that 66.79 g/ml of the Art/Zn complex substantially inhibited the cellular viability of “VERO-E6” cells (Fig. 8).
Fig. 8.

Cytotoxicity concentration 50 (CC50) and inhibitory concentration 50 (IC50) values for (Art/Zn) metal complex formula revealing its moderate activity against SARS-CoV-2. IC50 was calculated by the best line drawn between log concentration and viral inhibition % (triplicate for each concentration) to evaluate the antiviral activity against SARS-CoV-2 [hCoV-19/Egypt/NRC-03/2020 (Accession Number on GSAID: EPI_ISL_430820)] using Vero E6 cells. *Graph of the cytotoxicity concentration 50 (CC50) of Vero E6 cells. *Graph of the inhibitory concentration 50 (IC50): antiviral activity against SARS-CoV-2.
3.8. “Art/zn” mode of action against SARS-CoV-2 virus
The synthesised complex exhibited a cytotoxicity of 213.6 mg/ml on Vero E6 cells, the highest mode of action and replication mechanism as the antiviral activity; therefore, the Art/Zn formula affected the protease enzyme, inhibiting its function during the viral replication cycle. It had more than 80% inhibition on the propagation of the corona virus at a specific concentration of 0.25 mg/ml, which decreased gradually with the decreasing concentration to provide 57.6% inhibition at 0.0312 mg/ml (Table .3).
Table 3.
Mode of mechanism of action of Art/Zn against SARS-CoV-2.
| Mode of action | Conc. mg/ml | Virus Control (PFU/ml) | Viral Titer Post-Treatment (PFU/ml) | Viral Inhibition (%) |
|---|---|---|---|---|
| Virucidal | 0.25 | 0.5 * 105 | 0.3 * 105 | 25% |
| 0.125 | 0.6 * 105 | 0 | ||
| 0.0625 | 0.6 * 105 | 0 | ||
| 0.0312 | 1.0 * 105 | 0 | ||
| Replication | 0.25 | 3.1 * 105 | 0.3 * 105 | 80.5% |
| 0.125 | 0.4 * 105 | 77.7% | ||
| 0.0625 | 0.4 * 105 | 77.8% | ||
| 0.0312 | 1.0 * 105 | 57.6% | ||
| Adsorption | 0.25 | 1.5 * 105 | 1.0 * 105 | 23.1% |
| 0.125 | 2.2 * 105 | 0 | ||
| 0.0625 | 2.5 * 105 | 0 | ||
| 0.0312 | 2.8 * 105 | 0 |
3.9. Prediction of physicochemical characteristics and pharmacokinetic characteristics
The pharmacokinetic and physicochemical properties of the synthesised formula were described using SwissADME [32], as shown in Table .4. In terms of physicochemical properties, the synthesised formula (Art/Zn) is water-soluble, which may reduce concerns regarding drug formulations as it should be in a solution form at the absorption site [33].
Table 4.
Physicochemical studies of the complex formula (Art/Zn) target prediction.
| (Investigated formula) Art/Zn | ||
|---|---|---|
| Molecular properties | Formula | C30H50O13Cl2Zn |
| Molar Refractivity | 70.38 | |
| TPSA | 53.99 A2 | |
| Log P 0/w (WLOGP) | 2.39 | |
| Log P 0/w (MLOGP) | 2.62 | |
| Consensus Log P 0/w | 2.51 | |
| Water solubility | Soluble | |
| Num. Hydrogen bond acceptors | 5 | |
| Pharmacokinetics parameters | GI absorption | High |
| BBB permeant | Yes | |
| P-gp substrate | No | |
| CYP1A2 inhibitor | Yes | |
| CYP2C19 inhibitor | No | |
| CYP2C9 inhibitor | No | |
| CYP2D6 inhibitor | No | |
| CYP3A4 inhibitor | No | |
| Log KP (Skin permeation) | −5.96 cm/s | |
| Drug/Lead Likeness | Drug likeness (Lipiniski) | Yes |
| Bioavailaibilty score | 0.55 | |
| Medicinal Chemistry | Lead Likeness | Yes |
| Synthetic accessibility | 6.13 | |
Moreover, the ADME results indicate that the synthesised formula exhibits high GIT absorption. Thus, the oral route could be the most suitable route for this formula if administered in its current form. No synthesised compound crosses. Additionally, this synthesised formula is not a specific substrate for P-glycoprotein (P-gp); therefore, it may not be suitable for efflux mechanism. The target classes for Art/Zn were primarily kinase at 33%, as shown in (Fig.S2). Kinase are enzymes that attach a phosphate group to proteins and are extensively used for signal transmission and complex cellular process regulation. Molecule phosphorylation can enhance or inhibit their activity and modulate their ability to interact with other molecules. A phosphatase is an enzyme that removes a phosphate group from a protein. Together, these two enzyme families act to modulate proteins activities in a cell, often in response to external stimuli, supporting our findings and Art/Zn's activity, which may bind to kinase and regulate interaction with other molecules, potentially inhibiting SARS-CoV-2.
Additionally, the Art/Zn complex exhibits less inhibiting activity for most common metabolising enzymes in the liver (e.g., CYP3A4, CYP2C9, CYP2C19, and CYP2D6). The Art/Zn formula may be used as a lead compound.
3.10. Molecular docking studies
In the initial phase of the docking process, the programme was set up by redocking the formula (Art/Zn) against angiotensin-converting enzyme-2 (ACE2) and this (ACE2) receptor may generate a protective effects in the COVID-19 patients by decline of the severe respiratory symptoms. and Mpro target main protease for SARS-CoV-2 virus and it is the key enzyme of coronaviruses and has a key role in mediation of the viral replication and the viral transcription, as shown in (Fig. 9 A, B, C and D), (Fig. 10 A, B and C), respectively. By analysing the docking results presented in Table 5, Table 6, Table 7 and Fig. 11, Fig. 12 for our synthesised complex against ACE2 pockets and Mpro of SARS-CoV and considering the pharmacokinetic properties discussed earlier, we can conclude the following: the redocked cocrystallized formula formed hydrogen bonds with TYR, ASN, GLU, MET, SER, HSD, ASP, GLN, LYS, GLY, ARG and TRP incase of ACE2 receptor and LYS, MET, GLY,VAL, ASP, LEU, ALA, SER, ASN, THR, PHE and PRO with a very high activity and the Art/Zn complex showed a binding interaction energy ranging from −5.78 to −8.05 kcal/mol for ACE2 receptor (Table .5) and ranging from −6.21 to-8.15 (Table .6).
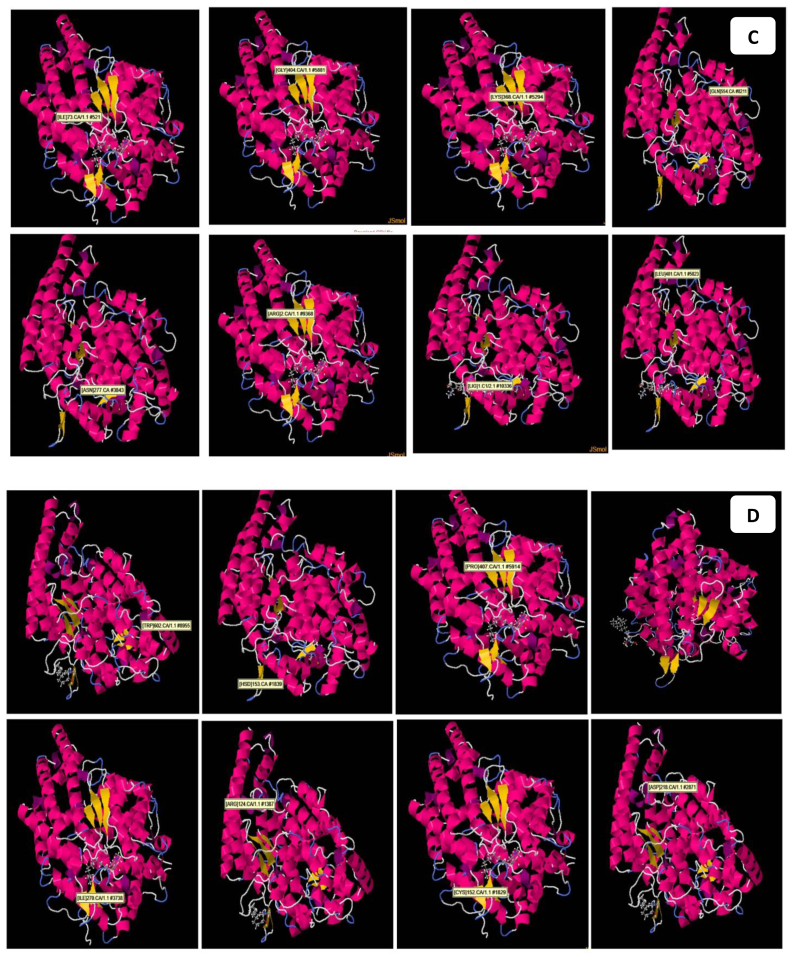
Fig. 9.
3D pictures of the synthesised compound and scores of the binding interaction, the amino acids, and bonds of the synthesised Art/Zn inside the ACE2 receptor of SARS-CoV-2 (V: View) 4 views (frontal view, side view, dorsal view and ventral view), the positioning and the interactions at the SARS-CoV-2 ACE2 receptor, with the ligand formula (Art/Zn). (A) Co-crystallized formula formed H-bonds with (TYR520, ASN586,Glu 557, MET299, LEU 427 and ILE367). (B) Co-crystallized formula formed H-bonds with (TYR520, SER284, PRO407, ILG-1H6, LIG1.C19,ASP453, LIG1C9 and HSD543). (C) Co-crystallized formula formed H-bonds with (ILE73,Gly404,Lys368, GLN55, ASN277, ARG, LIG1C and LEU401). (D) Co-crystallized formula formed H-bonds with (TRP602, HSD153, PRO407, ILE270, ARG124, CYS152, ASP218).
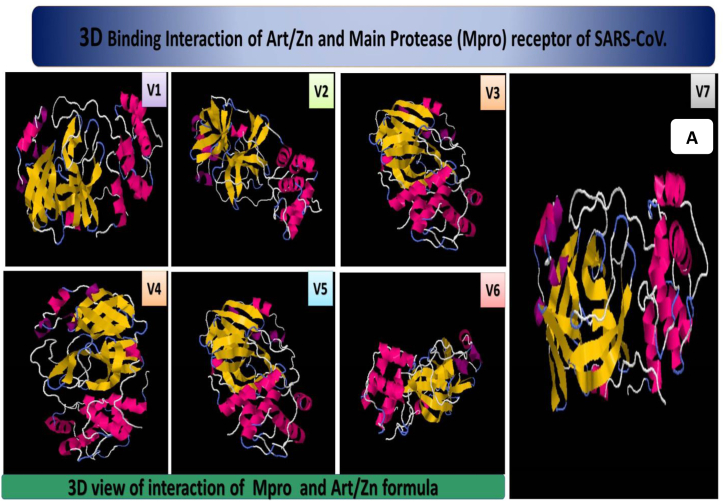
Fig. 10.
3D picture of the synthesised compound and scores of interaction, amino acids, and bonds of the synthesised Art/Zn inside the Mpro receptor of SARS-CoV-2 (V: View) (frontal view, dorsal view, horizontal view, ventral view and side views), the main protease active site of SARS-CoV-2, the positioning and the interactions and at the SARS-CoV-2Mpro receptor, with the ligand formula (Art/Zn). (A) Co-crystallized formula formed H-bonds with different seven views. (B) Co-crystallized formula formed H-bonds with SER254,ASN51.CA, PHE291,VAL261,Gly146, PHE134,THR98 and PRO132. (C) Co-crystallized formula formed H-bonds with LYS102, MET235, Gly146,Val303, ASP176, LEU177, ALA255 and SER81.
Table 5.
FullFitness (Kcal/mol) and estimated ΔG energies (kcal/mol) for binding Art/Zn, Angiotensin-converting enzyme-2 (ACE 2) receptor of SARS-CoV-2 virus.
| Cluster | FullFitness (Kcal/mol) | Estimated ΔG (Kcal/mol) |
|---|---|---|
| 0 | −2314.45 | −7.99 |
| 1 | −2308.07 | −7.35 |
| 2 | −2306.10 | −7.12 |
| 3 | −2306.73 | −7.30 |
| 4 | −2306.10 | −7.70 |
| 5 | −2305.76 | −7.27 |
| 6 | −2305.68 | −7.00 |
| 7 | −2305.97 | −6.68 |
| 8 | −2303.97 | −6.81 |
| 9 | −2304.61 | −7.19 |
| 10 | −2303.92 | −7.63 |
| 11 | −2303.77 | −7.52 |
| 12 | −2303.75 | −7.24 |
| 13 | −2303.28 | −7.08 |
| 14 | −2302.61 | −6.34 |
| 15 | −2301.62 | −6.46 |
| 16 | −2302.17 | −6.44 |
| 17 | −2302.07 | −7.49 |
| 18 | 2301.89 | −6.18 |
| 19 | −2301.78 | −6.65 |
| 20 | −2301.33 | −7.06 |
| 21 | −2301.62 | −6.59 |
| 22 | −2301.54 | −8.05 |
| 23 | −2301.12 | −6.37 |
| 24 | −2301.16 | −6.64 |
| 25 | −2301.08 | −6.19 |
| 26 | −2300.42 | −6.53 |
| 27 | −2300.12 | −6.60 |
| 28 | −2293.49 | −6.89 |
| 29 | −2299.55 | −6.86 |
| 30 | −2299.17 | −5.87 |
| 31 | −2296.88 | −7.21 |
Table 6.
FullFitness (Kcal/mol) and estimated ΔG energies (kcal/mol) for binding Art/Zn, Main Protease (Mpro) receptor of SARS-CoV.
| Cluster | FullFitness (Kcal/mol) | Estimated ΔG (Kcal/mol) |
|---|---|---|
| 0 | −915.27 | −8.15 |
| 1 | −911.46 | −7.59 |
| 2 | −911.38 | −7.73 |
| 3 | −911.17 | −7.50 |
| 4 | −908.14 | −7.88 |
| 5 | -910.62 | −7.43 |
| 6 | -903.72 | −7.00 |
| 7 | -897.22 | −6.55 |
| 8 | −904.36 | −7.37 |
| 9 | −898.16 | −6.59 |
| 10 | −885.01 | −7.26 |
| 11 | −906.04 | −7.22 |
| 12 | -906.31 | −7.18 |
| 13 | −905.78 | −6.91 |
| 14 | −888.12 | −6.41 |
| 15 | −903.50 | −7.05 |
| 16 | −906.78 | −7.35 |
| 17 | −907.71 | −7.66 |
| 18 | −890.56 | −6.61 |
| 19 | −893.05 | −6.05 |
| 20 | −901.11 | −7.09 |
| 21 | −906.37 | −7.00 |
| 22 | −905.10 | −7.16 |
| 23 | −907.27 | −7.80 |
| 24 | −906.90 | −7.06 |
| 25 | −907.17 | −7.26 |
| 26 | −906.95 | −7.03 |
| 27 | −906.94 | −7.36 |
| 28 | −906.78 | −6.91 |
| 29 | −901.68 | −6.50 |
| 30 | −898.71 | −6.39 |
| 31 | −897.49 | −6.23 |
| 32 | −882.82 | −5.63 |
| 33 | −905.72 | −6.79 |
| 34 | −905.67 | −7.25 |
| 35 | −904.28 | −6.66 |
| 36 | −902.93 | −6.98 |
| 37 | −898.20 | −6.32 |
| 38 | −901.46 | −6.54 |
| 39 | 900.89 | −7.37 |
| 40 | 884.64 | −6.26 |
| 41 | −896.06 | −6.85 |
| 42 | −896.02 | −7.31 |
| 43 | −895.94 | −6.21 |
| 44 | −889.95 | −6.78 |
| 45 | −874.59 | −6.16 |
Table 7.
MM-GBSA energies (kcal/mol) for Art/Zn complex with receptors ACE2 and MPr.o.
| Complex (Art/Zn) | ΔG binding | Coulomb | Covalent | H-bond | Lipo | Bind packing | Solv_GB | VdW | St. Dev |
|---|---|---|---|---|---|---|---|---|---|
| ACE2 | −56.817 | −18.840 | 5.396 | −1.232 | −33.825 | −2.20 | 27.692 | −36.008 | 6.657 |
| Mpro | −54.799 | −12.939 | 3.698 | −0.463 | −30.985 | −2.04 | 18.953 | −33.063 | 11.924 |
ΔG: Binding Energy; H-bond: Hydrogen bond; Lipo: lipophilic energy; Solv_GB: generalized born electrostatic solvation energy; VdW: Van der Waals energy; St. Dev: standard deviation.
Fig. 11.
Histogram for the tested ligand (Art/Zn) interactions with the SARS-CoV-2 receptors during the simulation (100 ns) for (A) ACE2, (B) MPro receptors.
Fig. 12.
Heat map for SARS-CoV-2 receptors and Art/Zn (Simulation 100 ns) for (A) ACE, (B) MPro.
3.11. Molecular dynamics simulation (MD)
Molecular dynamics simulations were carried out for Art/Zn at 100 ns using Desmond, a Package of Schrödinger.The initial stage of ligand-potential complex for MD simulation were obtained. Molecular Docking Studies provide a prediction of ligand binding status in static conditions. Since MD is a static view of the binding pose of molecule in the active sites of the protein, Molecular dynamics (MD) simulation tend to simulate the atom movements with time by applying the “Newton's classical equation” of the motion. Simulations were carried out to predict the binding status of Art/Zn novel complex in the normal physiological environment. The protein–ligand complex was pre-processed by using “Maestro” Protein Preparation Wizard. Solvent Model was selected as TIP3P (Transferable Intermolecular Interaction Potential 3 Points) with an orthorhombic box. In the simulation, OPLS_2005 force field was used. To mimic the normal physiological environment via addition of NaCl (0.15 M). For complete simulation, NPT ensemble was performed.The trajectories were saved after every 10 ps for analysis, and the simulations' stability was assessed via calculation of (RMSD) root mean square deviation of both the receptor and Art/Zn over specific time.
3.12. Histogram and heat map analyses
Histograms for the SARS-CoV ligand-protein of Art/Zn complex with ACE2 during (100 ns) (100 ns) (the simulation time) are described in (Fig. 10). Regarding Art/Zn complex with ACE2, LYS 562 contributed about 97%, besides GLN 101 contributed about 90% followed by TYR 196, GLU 208, TYR 202, ASP 206 and GLY 205 contributed as follows respectively (48,44,40,38 and 35%) of the interactions as H-bonding; However, LYS 94, GLU 564 and LEU391 formed mainly the hydrophobic interactions. Additonally, LYS 562, TYR 196 and ASP 206 were the key members contributing to the H2O-bridges, with no observation of ionic bonds. Obviously, LYS 562 was the most amino acid participating in the interactions via H-bonds (Figs. 11 and 12 (A)).
Additionally, for the SARS-CoV ligand-protein of Art/Zn complex with Mpro during the specific simulation time are described in Fig. 10. Concerning the Art/Zn novel complex with MPro, GLY 109 contributed about 93%, besides ILE 106 contributed about 59% followed by VAL 105 and ASP 295 contributed as follows respectively (30 and 16%) of the interactions as H-bonding; Meanwhile, PHE 294, ILE 200 and PRO 293 formed mainly the hydrophobic interactions. Additonally, GLY 109, VAL 104 and ASP 295 were the main members particpating to the water-bridges, with no ionic bonds were recorded.Obviously, GLY109 was the most participating amino acids in the interactions through H-bonds (Fig. 11, Fig. 12).
3.13. Ligand properties
Ligand features, including RMSD, radius of gyration (rGyr), solvent accessible surface area (SASA), the intramolecular hydrogen bond (intraHB), molecular surface area (MolSA), polar surface area (PSA), all are showed in Fig. 13. Other ligands' properties are documented in the root mean square deviation (RMSD).
Fig. 13.
Ligand properties during simulation (100 ns) for receptors (A) ACE2, (B) MPro.
The RMSD and rGyr for Art/Zn complex with ACE2 were all observed to be within the range of (0.8–2.4) and (3.3–4.2) Å, respectively. Also, intraHB with a lot of bands were observed at the range from (1.1–2) during the specific time of simulation. MolSA range was within (312–345.5 Å2). Additionally, the SASA was within the (48–200 Å2) Moreover, its PSA range was between 139 and 198 Å2 (Fig. 13A).
The RMSD and rGyr for Art/Zn complex with MPro were noticed to be within the range of (0.5–2.4) and (3.4–4.25) Å, respectively. Also, intraHB with bands were observed rang tõ1 during the specific time of simulation. Meanwhile, MolSA range was within (318–350 Å2). Additionally, the SASA was within the (180–360 Å2) Moreover, its PSA range was between 150 and 195 Å2 (Fig. 13B).
The ligand characteristics showed both fluctuation and torsion at the start of the simulation (Fig. 15 A, B) before reaching the equilibrium point, indicating the stability of Art/Zn complex to the active sites of the main protease active site of SARS-CoV-2.
Fig. 15.
Ligand torsion profile with (A) ACE2 and (B) MPro.
3.14. RMSD analysis
The RMSD values of Cα atoms were assessed for novel complex “Art/Zn” to keep an eye on the effect of this novel complex on the conformational stability of ACE2 and Mpro receptors during the simulations. The results were plotted against the simulations time (Fig. 14A and B). The fluctuation of the proteins was within acceptable range with RMSD values of less than 4.00 A, showing the stability of the protein conformation. To compare the degree of deviation for the complexed Art/zn with receptor structure related to its native form, RMSD was studied. This helps to deeply investigate the system's overall stability (Fig. 8).
Fig. 14.
The RMSD of the Art/Zn complex for the SARS-CoV receptor (A) ACE2 and (B) MPro and of RMSD of Cα atoms of the complexes against simulation (100 ns).
3.15. MM-GBSA study
Table 7 showed that complex Art/Zn with ACE2 has the highest (ΔG) MMGBSA of −56.817 kcal/mol, followed by MPro with MMGBSA binding energy of −54.799, respectively which is confirmation for mechanism of action presented as inhibitory agent against SARS-CoV-2 and considered as Mpro inhibitors.
ART with ACE2 showed H-bond energy ranged between −1.23 and −0.463 with MPro. Lipophilic energy to ACE2 and MPro.Contrary, Art/Zn showed the covalent binding energy with receptor ACE2 (5.39) as compared to MPro (3.698). VdW for Art/Zn with ACE2 showed −36.008 and showed −33.063 with MPro.
ΔG Binding is governed by interactions which are considered as non-bonded such as the following: Coulomb, Packing, Hbond, Lipo, and vdW (Table .7). The average ΔG Binding was mainly influenced by the vdW, Lipo, and Coulomb energies with all types of interactions.The Gbind, SolvGB and Gbind Covalent energies, on the other hand, made the smallest contributions to the final average binding energies. Additionally, complexes showed potent stable hydrogen bonds (Table .8) with the residues of the amino acids and their Hbond interaction values.
Table 8.
The 3D view of binding interactions between the tested Art/Zn and ACE2 binding pocket and Mpro main protease inhibitor within the SARS-CoV-2.
| Complex | 3D interaction | 3D protein positioning |
|---|---|---|
| ART/Zn binding with (ACE2) | fx1 | fx2 |
| ART/Zn binding with (Mpro) | fx3 | fx4 |
4. Discussion
The COVID-19 pandemic has motivated humanity to resort to alternative medicine remedies. Therefore, we aimed to merge the active compounds of medicinal plants and chemical compounds via metal complexation to elevate the efficacy of these chemical compounds and decline their cytotoxicity owing to the chemical composition and pharmacological action of natural sources and the presence of several biologically active substances in them that can relieve the symptoms of diseases but after the alleviation of any cytotoxicity.
The effect of current antiviral drugs against COVID-19 has weakened owing to the increment of the resistance of the virus to the drugs [40,48]. Thus, the development of new, potent and effective anti-SARS-CoV-2 agents is essential.
This study revealed that Art/Zn has a moderated inhibitory effect against SARS-CoV-2 activity and confirmed its safety with low cytotoxicity; this finding is consistent with that of El-Megharbel et al. [40], who revealed that zinc oxide (ZnO) nanoparticles (NPs) exhibit an inhibitory effect against the virus SARS-CoV-2 but with the presence of cytotoxicity, proving that ZnO NPs can play a crucial and vital role in enhancing the inhibitory effect against SARS-CoV-2, which is crucial accomplishment against SARS-CoV-2 and thus the obtained findings confirmed that complexation between Art and Zn alleviate the cytotoxicity and enhance antiviral capacities of the novel complex.
Moreover, the docking results of Rolta et al. [14] revealed that medicinal plants exhibit a good affinity for binding with the S domain (1) protein of SARS-CoV-2 and with the ACE2 receptor as compared to that of other drugs such as chloroquine. Art showed previously the best binding affinity (−10.5 kcal/mol) with SARS-CoV-2 S glycoprotein. This molecular docking study confirmed our current finding, revealing that our Art/Zn complex afforded a moderate antiviral activity against SARS-CoV-2 virus.
We clarified that Art/Zn can interact with the SARS-CoV-2 ectodomain “S1” with “Art” binding affinity of −10.3 kcal/mol and exhibits a weak hydrogen bond with (THR 778, SER 730). Thus, it can act as an antiviral agent against SARS-CoV-2 beside the role of Zn with Art, which strengthens these actions and weakens the hydrogen bond.
The synthesised Art/Zn complex confirmed the finding of Nigam et al. [49], who reported that Art species are rich sources of different types of biologically active compounds that are accountable for various pharmacological activities, which may act potent against viruses and foreign organisms.
The confirmed potency of the synthesised complex (Art/Zn) against the SARS-CoV-2 for inhibiting its severe effects on the lungs, in according with Zhang et al. [50] who revealed the potent effect of Art derivatives against tumours and malignant cancers such as lung cancer, has been widely recognised [[51], [52], [53]]. Many studies have revealed that Art derivatives induce the invasion of cancer cells [[54], [55], [56]]. Additionally, Art has been previously reported to have potential anti-tumour effect.
Another study that provides confidence on the moderate inhibitory effect of our synthesised complex against the SARS-CoV-2 is that by Xiong and Huang. [57], who confirmed that Art exhibits defensive effects on hepatic lipid metabolism disorders, thereby making it suitable for alleviation of any symptoms of the SARS-CoV-2.
The obtained data confirmed the moderate inhibitory effect of the synthesised Art/Zn complex against the activity of SARS-CoV-2 with high safety approved by low cytotoxicity. This cytotoxicity can be expected to be alleviated because of the merging of natural active compounds with metals, thereby elevating the inhibitory effects against SARS-CoV-2.
SARS-COV-2 is one of the most important coronaviruses and has been the cause for most of the emerging pandemic of pathogens [58]. SARS-CoV-2 is known to have a large RNA genome, it encodes few proteins [59]. Among these proteins, Mpro, a main protease, which intercedes the maturation cleavage process of polyproteins during the viral replication [60]. The Mpro with its 2 promoters, containing the 3 domains and composed of residues 8–101 and 102–184.The Mpro is a protein across all the coronaviruses. The desired inhibitors for SARS-CoV-2 should have high binding energy especially with the target examined receptors, with increment of binding affinity and the current results are in concurrent with these observed findings. Prospective studies are recommended to prove this novel complex efficacy or after addition of a new ligand.
5. Conclusion
Art/Zn was chemically characterised using spectroscopic methods. It exhibits a moderate inhibitory effect against SARS-CoV-2 with an IC50 of 66.79 μg/ml and can trigger excessive production of the free radicals, potentially causing the oxidative stress in cells infected with SARS-CoV-2 with considerable damage to the viral cell membranes with proving results with molecular dynamics (MD) simulation with ACE2 and MPro receptors and results confirmed binding affinity with the synthesised complex Art/Zn. Thus, we recommend conducting further prospective studies with new Art-based complexes that may enhance the inhibitory effect against the SARS-CoV-2 while maintaining its low cytotoxicity.
Author contribution statement
Fawziah Al-Salmi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Samy El-Megharbel: Reham Zakaria: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
The data that has been used is confidential.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The researchers would like to acknowledge the Deanship of scientific research at Taif University and extend their deep thanks to Zagazig University for providing facilities and labs for performing the current experimental work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17177.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Rahman M., Bibi S., Rahaman S., Rahman F., Islam F., Khan M.S., Hasan M.M., Parvez A., Hossain A., Maeesa S.K., Islam R., Najda A., Al-Malky H.S., Mohamed H.R.H., Al-Gwaiz H.I.M., Awaji A.A., Germoush M.O., Kensara O.A., Abdel-Daim M.M., Saeed M., Kamal M.A. Natural therapeutics and nutraceuticals for lung diseases: traditional significance, phytochemistry, and pharmacology. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.113041. [DOI] [PubMed] [Google Scholar]
- 2.Ito K., Ito M., Elliott W.M., Cosio B., Caramori G., Kon O.M., et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 2005;352(19):1967–1976. doi: 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- 3.Prasher P., Sharma M., Mehta M., Paudel K.R., Satija S., Chellappan D.K., et al. Plants derived therapeutic strategies targeting chronic respiratory diseases: chemical and immunological perspective. Chem. Biol. Interact. 2020 doi: 10.1016/j.cbi.2020.109125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy D.M., O'Byrne P.M. Recent advances in the pathophysiology of asthma. Chest. 2010;137(6):1417–1426. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 5.Santana F.P.R., Pinheiro N.M., Mernak M.I.B., Righetti R.F., Martins M.A., Lago J.H., et al. Evidences of herbal medicine-derived natural products effects in inflammatory lung diseases. Mediat. Inflamm. 2016:2016. doi: 10.1155/2016/2348968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta M., Sharma N., Vyas M., Khurana N., Maurya P.K., Singh H., et al. Interactions with the macrophages: an emerging targeted approach using novel drug delivery systems in respiratory diseases. Chem. Biol. Interact. 2019;304:10–19. doi: 10.1016/j.cbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Dua K., Malyla V., Singhvi G., Wadhwa R., Krishna R.V., Shukla S.D., et al. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: an emerging need for novel drug delivery systems. Chem. Biol. Interact. 2019;299:168–178. doi: 10.1016/j.cbi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Chellappan D.K., Yee L.W., Xuan K.Y., Kunalan K., Rou L.C., Jean L.S., et al. Targeting neutrophils using novel drug delivery systems in chronic respiratory diseases. Drug Dev. Res. 2020;81(4):419–436. doi: 10.1002/ddr.21648. [DOI] [PubMed] [Google Scholar]
- 9.Bosch B.J., Vander Z.R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrov D.S. The secret life of ACE2 as a receptor for the SARS virus. Cell. 2003;115:652–653. doi: 10.1016/S0092-8674(03)00976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem Bioph Res Co. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Diego M.L., Nieto-Torres J.L., Jiménez-Guardeño J.M., Regla-Nava J.A., Alvarez E., Oliveros J.C., et al. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolta R., Salaria D., Sharma P., Sharma B., Kumar V., Rathi B., Verma M., Sourirajan A., Baumler D.J., Dev K. Phytocompounds of rheum emodi, thymus serpyllum, and Artemisia annua inhibit spike protein of SARS-CoV-2 binding to ACE2 receptor: in silico approach. Current Pharmacology Reports. 2021:1–15. doi: 10.1007/s40495-021-00259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y., Jiang S., Du L. Prospects for a MERS-CoV spike vaccine. Expert Rev. Vaccines. 2018;17:677–686. doi: 10.1080/14760584.2018.1506702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV—a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2019;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Wong G., Lu G., Yan J., Gao G.F. MERS-CoV spike protein: targets for vaccines and therapeutics. Antivir. Res. 2016;133:165–177. doi: 10.1016/j.antiviral.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babcock G.J., Esshaki D.J., Thomas W.D., Ambrosino D.M. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J. Virol. 2004;78:4552–4560. doi: 10.1128/JVI.78.9.4552-4560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong S.K., Li W., Moore M.J., Choe H., Farzan M.A. 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin- converting enzyme 2. Int J Biol Chem Sci. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 22.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trott O., Olson A.J. AutoDockVina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S., Sharma P.P., Shankar U., Kumar D., Joshi S.K., Pena L., et al. Discovery of new hydroxyethylamine analogs against 3CLpro protein target of SARS-CoV-2: molecular docking, molecular dynamics simulation and structure-activity relationship studies. J. Chem. Inf. Model. 2020;60:5754–5770. doi: 10.1021/acs.jcim.0c00326. [DOI] [PubMed] [Google Scholar]
- 25.El-Megharbel S.M., Alsawat M., Al-Salmi F.A., Hamza R.Z. Utilizing of (zinc oxide nano-spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—”ZnO nanoparticles have antiviral activity against (SARS-CoV-2)”. Coatings. 2021;11:388. [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Mostafa A., Kandeil A., Elshaier A.M.M., Kutkat Y., Moatasim O., Rashad Y., Shehata A.A., Gomaa M., Mahrous M.R., Mahmoud N., GabAllah S.H., Abbas M., Taweel H., Kayed A.E., Kamel A.E., Sayes M.N., Mahmoud M.E., El-Shesheny D.B., Kayali R., Ali M.A. FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals. 2020;13:443. doi: 10.3390/ph13120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo Y.C., Lin L.C., Tsai W.J., Chou C.J., Kung S.H., Ho Y.H. Samarangenin B from limonium sinense suppresses herpes simplex virus type 1 replication in Vero cells by regulation of viral macromolecular synthesis. Antimicrob. Agents Chemother. 2002;46(9):2854–2864. doi: 10.1128/AAC.46.9.2854-2864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J., Zhan B., Yao X., Gao Y., Shong J. Antiviral activity of tannin from the pericarp of Punica granatum L. against genital Herpes virus in vitro. China J. Chin. Mater. Med. 1995;20:556–558. [PubMed] [Google Scholar]
- 30.Schuhmacher A., Reichling J., Schnitzler P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine. 2003;10(6–7):504–510. doi: 10.1078/094471103322331467. [DOI] [PubMed] [Google Scholar]
- 31.Elagawany M., Elmaaty A.A., Mostafa A., Abo Shama N.M., Santali E.Y., Elgendy B., Al-Karmalawy A.A. Ligand-based design, synthesis, computational insights, and in vitro studies of novel N-(5-Nitrothiazol-2-yl)-carboxamido derivatives as potent inhibitors of SARS-CoV-2 main protease. J. Enzym. Inhib. Med. Chem. 2022;37(1):2112–2132. doi: 10.1080/14756366.2022.2105322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.For technical information about the prediction algorithm, Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29:3073–3079. doi: 10.1093/bioinformatics/btt540. [DOI] [PubMed] [Google Scholar]
- 33.Abo Elmaaty A., Eldehna W.M., Khattab M., Kutkat O., Alnajjar R., El-Taweel A.N., Al-Rashood S.T., Abourehab M.A.S., Binjubair F.A., Saleh M.A., et al. Anticoagulants as potential SARS-CoV-2 Mpro inhibitors for COVID-19 patients: in vitro, molecular docking, molecular dynamics, DFT, and SAR studies. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godschalk F., Genheden S., Söderhjelma P., Ryde U. Comparison of MM/GBSA calculations based on explicit and implicit solvent simulations. Phys. Chem. Chem. Phys. 2013;15:7731–7739. doi: 10.1039/c3cp00116d. [DOI] [PubMed] [Google Scholar]
- 35.Daina A., Michielin O., Zoete V. Swiss ADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717–42719. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Naggar A.M., Hassan A.M.A., Elkaeed E.B., Alesawy M.S., Al-Karmalawy A.A. Design, synthesis, and SAR studies of novel 4-methoxyphenyl pyrazole and pyrimidine derivatives as potential dual tyrosine kinase inhibitors targeting both EGFR and VEGFR-2. Bioorg. Chem. 2022;123 doi: 10.1016/j.bioorg.2022.105770. [DOI] [PubMed] [Google Scholar]
- 37.Ghanem A., Al-Karmalawy A.A., Abd El-Maksoud A.I., Hanafy S.M., Emara H.A., Saleh R.M., Elshal M.F. Rumex vesicarius L. extract improves the efficacy of doxorubicin in triple-negative breast cancer through inhibiting Bcl2, mTOR, JNK1 and augmenting p21 expression. Inform. Med. Unlocked. 2022;29 [Google Scholar]
- 38.Elebeedy D., Elkhatib W.F., Kandeil A., Ghanem A., Kutkat O., Alnajjar R., Saleh M.A., Abd El Maksoud A.I., Badawya I., Al-Karmalawy A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021;11:29267–29286. doi: 10.1039/d1ra05268c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Demerdash A., Al-Karmalawy A.A., Abdel-Aziz T.M., Elhady S.S., Darwish K.M., Hassan A.H.E. Investigating the structure–activity relationship of marine natural polyketides as promising SARS-CoV-2 main protease inhibitors. RSC Adv. 2021;11:31339–31363. doi: 10.1039/d1ra05817g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Megharbel S.M., Hamza R.Z. Synthesis, spectroscopic characterizations, conductometric titration and investigation of potent antioxidant activities of gallic acid complexes with Ca (II), Cu (II), Zn(III), Cr(III) and Se (IV) metal ions. J. Mol. Liq. 2022;(358) [Google Scholar]
- 41.Nakamoto K. fourth ed. Wiley; New York: 1986. Infrared and Raman Spectra of Inorganic and Coordination Compounds. [Google Scholar]
- 42.Nakamoto K. second ed. Wiley Interscience, John Wiley & Sons; New York, NY, USA: 1970. Infrared Spectra of Inorganic and Coordination Compounds. [Google Scholar]
- 43.Bellamy L.J. Chapman and Hall; London, UK: 1975. The Infrared Spectra of Complex Molecules. [Google Scholar]
- 44.Sanna D., Ugone V., Pisano L., Serra M., Micera G., Garribba E. Behavior of the potential antitumor V(IV)O complexes formed by flavonoid ligands. 1. Coordination modes and geometry in solution and at the physiological pH. J. Inorg. Biochem. 2015;153:167–177. doi: 10.1016/j.jinorgbio.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Sachan N., Chandra P., Yadav M. Simple and validated UV-spectrophotometric method for the estimation of Levofloxacin in bulk and pharmaceutical dosage forms. Int. J. Pharm. Pharmaceut. Sci. 2012;4:383. [Google Scholar]
- 46.Jeevitha D., Sadasivam K., Praveena R., Jayaprakasam R. DFT study of glycosyl group reactivity in quercetin derivatives. J. Mol. Struct. 2016;1120:15–24. [Google Scholar]
- 47.Lever A.B.P. second ed. 1984. Electronic Spectra of Dn Ions Inorganic Electronic Spectroscopy. [Google Scholar]
- 48.Hamza R.Z., Al-Talhi T., Gobouri A.A., Alsanie W.F., El-Megharbel S.M. Are favipiravir and acyclovir with IgG injections supplemented with vitamin D “suggested therapeutic option” can fight against COVID-19. Adv. Anim. Vet. Sci. 2021;9(4):549–554. [Google Scholar]
- 49.Nigam M., Atanassova M., Mishra A.P. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019;14(7) [Google Scholar]
- 50.Zhang Q., Yi H., Yao H., Lu L., He G., Wu M., Zheng C., Li Y., Chen S., Li L., Yu H., Li G., Tao X., Fu S., Deng X. Artemisinin derivatives inhibit non-small cell lung cancer cells through induction of ROS-dependent apoptosis/ferroptosis. J. Cancer. 2021;12(13):4075–4085. doi: 10.7150/jca.57054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong Y., Liu Y., Zheng H., Zheng L., Liu W., Wu J., et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget. 2016;7:31413–31428. doi: 10.18632/oncotarget.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang J., Geng G., Yu X., Liu H., Gao J., An H., et al. Repurposing the anti-malarial drug dihydroartemisinin suppresses metastasis of non-small-cell lung cancer via inhibiting NF-κB/GLUT1 axis. Oncotarget. 2016;7:87271–87283. doi: 10.18632/oncotarget.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L., Qian H., Sha M., Luan Z., Lin M., Yuan D., et al. Downregulation of HOTAIR expression mediated anti-metastatic effect of artesunate on cervical cancer by inhibiting COX-2 expression. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong Y.K., Xu C., Kalesh K.A., He Y., Lin Q., Wong W.S.F., et al. Artemisinin as an anticancer drug: recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017;37:1492–1517. doi: 10.1002/med.21446. [DOI] [PubMed] [Google Scholar]
- 55.Tan M., Rong Y., Su Q., Chen Y. Artesunate induces apoptosis via inhibition of STAT3 in THP-1 cells. Leuk. Res. 2017;62:98–103. doi: 10.1016/j.leukres.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Eruslanov E., Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 57.Xiong Y., Huang J. Anti-malarial drug: the emerging role of artemisinin and its derivatives in liver disease treatment. Chin. Med. 2021;16:80. doi: 10.1186/s13020-021-00489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mengist H.M., Dilnessa T., Jin T. Structural basis of potential inhibitors targeting SARS-CoV-2 main protease. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.622898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dömling A., Gao L. Chemistry and biology of SARS-CoV-2. Chem. 2020;6(6):1283–1295. doi: 10.1016/j.chempr.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81(4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.