Abstract
The Notch pathway is an ancient, evolutionary conserved intercellular signaling mechanism that is involved in cell fate specification and proper embryonic development. The Jagged2 gene, which encodes a ligand for the Notch family of receptors, is expressed from the earliest stages of odontogenesis in epithelial cells that will later generate the enamel-producing ameloblasts. Homozygous Jagged2 mutant mice exhibit abnormal tooth morphology and impaired enamel deposition. Enamel composition and structure in mammals are tightly linked to the enamel organ that represents an evolutionary unit formed by distinct dental epithelial cell types. The physical cooperativity between Notch ligands and receptors suggests that Jagged2 deletion could alter the expression profile of Notch receptors, thus modifying the whole Notch signaling cascade in cells within the enamel organ. Indeed, both Notch1 and Notch2 expression are severely disturbed in the enamel organ of Jagged2 mutant teeth. It appears that the deregulation of the Notch signaling cascade reverts the evolutionary path generating dental structures more reminiscent of the enameloid of fishes rather than of mammalian enamel. Loss of interactions between Notch and Jagged proteins may initiate the suppression of complementary dental epithelial cell fates acquired during evolution. We propose that the increased number of Notch homologues in metazoa enabled incipient sister cell types to form and maintain distinctive cell fates within organs and tissues along evolution.
Keywords: Notch signaling, Tooth development, Enamel, Cell commitment, Enamel organ, Ameloblasts, Evolution, Jagged
Introduction
The Notch pathway is an evolutionarily conserved signaling mechanism that enables adjacent cells to adopt different fates [1–5]. In Drosophila, the Notch gene encodes a transmembrane receptor with a large extracellular domain carrying multiple epidermal growth factor (EGF)-like repeats and a cytoplasmic domain required for signal transduction. The Notch receptor interacts with membrane-bound ligands encoded by the Delta and Serrate genes that in their extracellular domain contain the DSL domain (Delta, Serrate, Lag-2). The DSL domain is required for interaction of ligands with the Notch receptor [6, 7]
In vertebrates, four genes encoding Notch receptors (Notch1, Notch2, Notch3, and Notch4) and five genes encoding ligands for the Notch receptors (Jagged1, Jagged2, Delta-like1, Delta-like3, and Delta-like4) have been identified [5, 8, 9]. The signal induced by ligand binding is transmitted at the intracellular part of the receptor in a process involving proteolysis and interactions with cytoplasmic and nuclear proteins [1, 10–17]. Signals exchanged between neighboring cells through the Notch receptors influence cell fate determination, differentiation, proliferation and apoptotic events at all stages of development, thus controlling organ formation and morphogenesis [8, 9, 17–20]. The increasing number of Notch homologues in vertebrates, together with the absence of Notch genes in non-metazoans, suggests a role for the Notch signaling pathway in the establishment of complex body plans [21, 22]. Notch malfunction has been shown to disrupt aspects of neurogenesis, somite formation, angiogenesis, kidney and lymphoid development [9, 16, 23–30]. In humans, mutations in the Notch1, Notch3 and Jagged1 genes are associated, respectively, with a neoplasia (a T-cell acute lymphoblastic leukemia/lymphoma), a late onset neurological disease known as CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) and a complex inherited disorder known as Alagille syndrome (affecting mainly the liver, heart, vertebrae, eye and face) [9, 31–34].
Several studies have demonstrated that Notch signaling is involved in tooth morphology and enamel matrix deposition [35–42]. Teeth are organs that arise from progressive reciprocal inductive interactions between the stomodeum epithelium and the underlying neural crest-derived mesenchyme that transform the tooth primordia into complex mineralized structures with various cell types, among which the epithelial-derived ameloblasts that synthesize and secrete the organic components of the enamel [43–45]. Initiation of odontogenesis is visualized as local epithelial thickenings of the oral epithelium, at the sites of the future teeth [45–47]. Thereafter, the thickened epithelium grows and forms the dental bud and cap structures that mark the onset of the tooth morphology. Subsequent epithelial folding and growth gives rise to a bell structure where cytodifferentiation events start. In mammals, four clearly distinct dental epithelial cell layers (i.e., inner enamel epithelium, stratum intermedium, stellate reticulum and outer enamel epithelium) are present at this stage that are important for amelogenesis. However, to date, there is not much information concerning the role of the Notch signaling pathway in gradual cell fate determination and differentiation of these dental epithelial cell lineages.
Since the core Notch pathway requires two adjacent cells in direct contact with each other, we examined if Jagged2 deletion affects Notch1 and Notch2 expression in the epithelium of developing mouse teeth. We have recently shown that epithelial deletion of Jagged1 deregulated the expression of both Notch1 and Notch2 in dental epithelium [48]. Based on our recent findings and the results obtained here we suggest a hypothetical model involving molecules of the Notch signaling pathway in dental epithelial cell morphotype and function. The proposed model could unravel a general, functional correlation between the evolution of the discrete expression of Notch receptors and its ligands and the evolution of specialized dental cell types.
Materials and methods
Notch receptors and ligands in evolution
To obtain an overview of the Notch receptors and ligands in evolution and their conservation among vertebrates, we screened available protein sequences from representative species of five main classes: fishes (Zebrafish—Danio rerio), amphibians (Western Clawed Frog—Xenopus tropicalis), reptiles (Common Wall Lizard—Podarcis muralis), birds (Chicken—Gallus gallus), and mammalians (Human—Homo sapiens). We then aligned all receptors and ligands sequences via ClustalW [49].
Animals and tissue preparation
All mice (C57Bl/6) were maintained and handled according to the Swiss Animal Welfare Law and in compliance with the regulations of the Cantonal Veterinary office, Zurich (License 11/2014). Mouse embryos from embryonic day 12.5 (E12.5) to E18.5 were used for in situ hybridization. Jag2DDSL mutant mice have been described previously [50, 51]. E12.5–E18.5 wild type, Jagged2+/− and Jagged2−/− mouse embryos were obtained by intercrossing Jag2DDSL/+ mice. Embryonic age was determined according to the appearance of the vaginal plug (day 0) and confirmed by morphological criteria. Pregnant females were sacrificed by cervical dislocation and the embryos were removed in Dulbecco’s phosphate-buffered saline (PBS). Dissected heads were fixed in 4% paraformaldehyde (PFA) for 24 h at 4 °C and prepared for sectioning.
Probes and in situ hybridization
Digoxigenin- and fluorescein-labeled (Boehringer Manhnheim) antisense riboprobes for Jagged2, Notch1 and Notch2 were used [40, 51]. In situ hybridization on cryosections of E12.5–E18.5 embryos were performed as previously described [39, 40, 52]. Double in situ was performed using first the fluorescein probe, followed by the digoxigenin one.
Results
Overview of Notch receptors and ligands in vertebrates
Alignement of representative protein sequences of all Notch receptors and ligands in species of five main classes (fishes, amphibians, reptiles, birds, mammalians) showed that Notch1, Notch2 and Notch3 were broadly identified in these classes. Notch orthologues in different classes showed higher sequence identity than paralogues within the same class (Fig. 1A). Notch4 was identified only in mammalians, and represented a clear side branch. Notch ligands clustered separately in the families of Jagged and Delta-like (Dll) ligands (Fig. 1B). Similarly to the Notch receptors, Notch ligands showed higher identity between orthologues, with Jagged1, Jagged2, Delta-like1 (Dll1), Delta-like3 (Dll3), Delta-like4 (Dll4) forming each separate branches.
Fig. 1.
Overview of Notch receptors and ligands among vertebrates. A Circular phylogram (guide tree) obtained from the alignment of protein sequences (ClustalW) of Notch receptors from five selected vertebrate species. B Circular phylogram (guide tree) obtained from the alignment of protein sequences (ClustalW) of Notch ligands from five selected vertebrate species. Notice that connections indicate sequence similarities as determined from multiple alignment (5 iterations) and do not necessarily imply phylogenetic relationships. Fish: Danio rerio (Zebrafish); Amphibian: Xenopus tropicalis (Western Clawed Frog); Reptile: Podarcis muralis (Common Wall Lizard); Bird: Gallus gallus (Chicken); Mammalian: Homo sapiens (Human)
Notch1, Notch2 and Jagged2 expression during embryonic tooth development
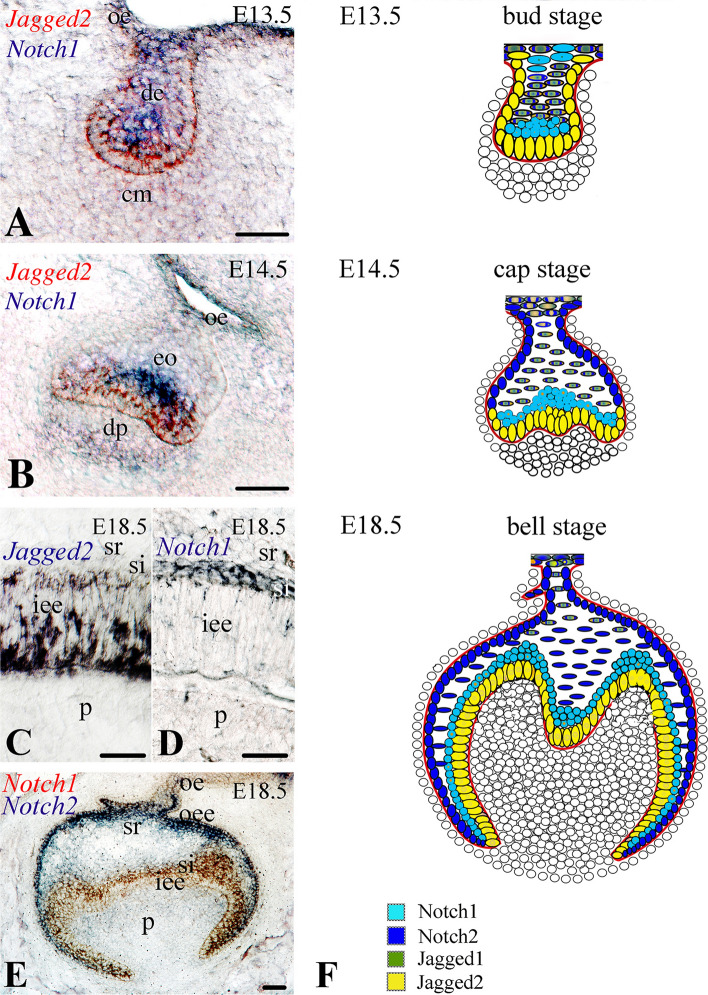
To be able to interpret the role of the Notch signaling pathway in tooth evolution linked to the cell diversity of the enamel organ we first determined the expression pattern of Notch1, Notch2 and Jagged2 in development from sections of E12.5–E18.5 mouse teeth. At E12.5–13.5 dental epithelium (bud stage), Jagged2 expression was observed in epithelial cells in contact with the condensed mesenchyme (Fig. 2A, red color), while Notch1 and Notch2 transcripts were detected in distinct epithelial territories adjacent to the Jagged2-expressing cells (Fig. 2A, blue color; Fig. 3A, B). During the cap stage (E14.5–E15.5), Jagged2 transcripts were detected in dental epithelial cells contacting the dental papilla mesenchyme (Fig. 2B, red color), whereas Notch1 labeling was restricted to cells overlying the Jagged2-expressing cells (Fig. 2B, blue color). At the bell stage (E16.5–E18.5), Jagged2 expression was observed in cells of the inner enamel epithelium (Fig. 2C), while expression of Notch1 was found in a differentiated cell layer behind, as the stratum intermedium (Fig. 2D; Fig. 2E, red color; Fig. 4A, E, I) and that of Notch2 in middle cells of the stellate reticulum and as well as in outer enamel epithelium (Fig. 2E, blue color; Fig. 4C, G, K).
Fig. 2.
Comparative analysis of the expression patterns of Jagged2, Notch1 and Notch2 in dental epithelium during embryonic tooth development. In situ hybridization on frontal cryosections of E13.5–E18.5 mouse embryos (A–E). A At E13.5 (bud stage), Jagged2 transcripts (red color) are detected in dental epithelial cells in contact with the condensed mesenchyme (cm), while Notch1 mRNA (blue color) is observed in the neighboring to the Jagged2-expressing cells. B At E14.5 (cap stage), Jagged2 transcripts (red color) are found in dental epithelial cells contacting the dental papilla mesenchyme (dp), whereas Notch1 transcripts (blue color) are restricted to cells overlying the Jagged2-expressing cells. C, D At E18.5 (bell stage), Jagged2 expression is restricted in cells of the inner enamel epithelium (iee) (C), while expression of Notch1 is delimited to cells of the overlying stratum intermedium layer (si) (D). E At the bell stage (E18.5), Notch1 transcripts (red color) are detected in cells of the stratum intermedium, while Notch2 mRNA (blue color) is observed in cells of the stellate reticulum (sr). F Schematic representation of the expression patterns of Notch1, Notch2, Jagged1 and Jagged2 in dental epithelium of E13.5–E18.5 molar tooth germs. de dental epithelium, oe oral epithelium, oee outer enamel epithelium; p dental pulp. Scale bars A, B, E = 100 μm; C, D = 25 μm
Fig. 3.
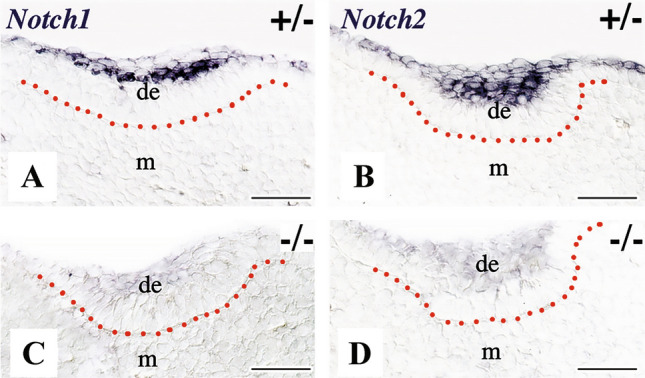
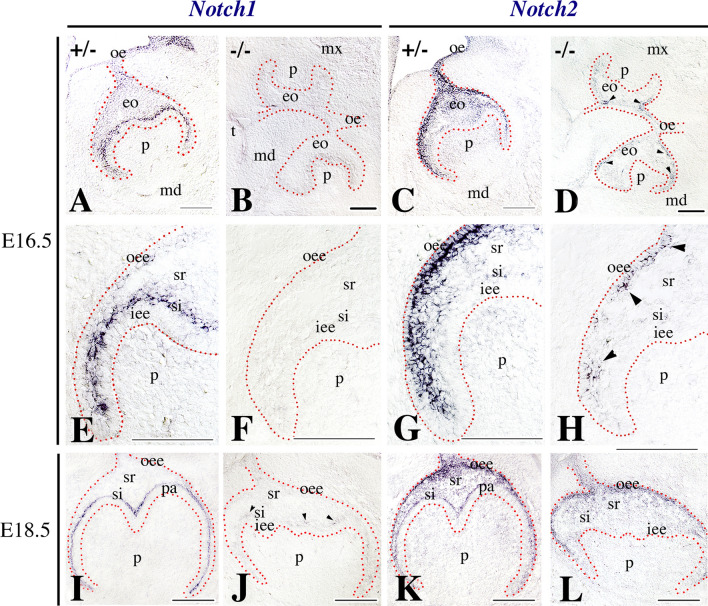
Expression of the Notch1 and Notch2 genes in the developing tooth areas of E12.5 Jagged2 heterozygous (+/−) and homozygous (−/−) mouse embryos. Genotypes are indicated in each panel. In situ hybridization on frontal cryosections. Red dotted lines represent the borders between the dental epithelium (de) and mesenchyme (m). Notch1 (A) and Notch2 (B) are expressed in distinct cell populations of the dental epithelium in heterozygous embryos. Notch1 (C) and Notch2 (D) genes are not detected in dental tissues of homozygous embryos. Scale bars 100 μm
Fig. 4.
Comparison of the expression patterns of the Notch1 and Notch2 genes in developing teeth of E16.5 and E18.5 Jagged2 heterozygous (+/−) and homozygous (−/−) mouse embryos. Genotypes are indicated in each panel. In situ hybridization on frontal cryosections. Red dotted lines represent the borders between the enamel organ (eo) and the surrounding mesenchyme. Arrowheads indicate Notch1 or Notch2 expressing cells in Jagged2−/− embryos. Distinct expression patterns of Notch1 (A, E, I) and Notch2 (C, G, K) in the dental epithelium of E16.5 and E18.5 Jagged2 ± embryos. Higher magnifications show that Notch1 is expressed in stratum intermedium (si) (E), while Notch2 is expressed in cells of the outer enamel epithelium (oee) and stellate reticulum (sr) (G). Notch1 (B, F, J) and Notch2 (D, H, L) are downregulated in the dental epithelium of E16.5 and E18.5 Jagged2−/− embryos. Also, note the fusions between the maxillary (mx) and mandibular processes (md) (B, D) in homozygous embryos. Higher magnifications show that Notch1 is not detected in stratum intermedium (F), while Notch2 expression is greatly reduced in cells of the outer enamel epithelium and stellate reticulum (H) in dental tissues of E16.5 Jagged2−/− embryos. iee inner enamel epithelium, oe oral epithelium, p dental papilla mesenchyme, pa preameloblasts, t tongue. Scale bars 200 μm
Downregulation of Notch1 and Notch2 expression in Jagged2−/− teeth
To analyze the effects of Jagged2 deletion in other molecules of the Notch signaling pathway, we examined the expression of Notch1 and Notch2 in teeth of E12.5–E18.5 Jagged2 deficient embryos. At E12.5 (early bud stage), the expression of both Notch1 (Fig. 3C) and Notch2 (Fig. 3D) was severely downregulated in the dental epithelium of homozygous mutant embryos. Downregulation of these two genes, but to a lesser extent for Notch2 when compared to Notch1, persisted at more advanced developmental stages. Very few, if not at all, Notch1 transcripts were detected in cells of the stratum intermedium of E16.5 (Fig. 4B, F) and E18.5 (Fig. 4J) Jagged2−/− mouse embryo teeth. Similarly, Notch2 expression was greatly reduced in cells of the outer enamel epithelium and stellate reticulum of E16.5 (Fig. 4D, H) and E18.5 (Fig. 4L) Jagged2−/− teeth.
Discussion
Evolutionary processes have contributed to the extensive diversification of cell types in animals. Cell homology in an increased number of new cell types that appeared during animal evolution could be due to inheritance from a common precursor [53, 54]. Notch signaling is an ancient, evolutionarily conserved signaling pathway that allows distinctive cell types with defined functions to be delineated and compared within and between species [1]. Evolutionary changes in the genome coding for molecules of the Notch pathway from the simplest to the most complex organisms could have permitted the sprouting of distinct sister cell types and ensure their independent evolution by regulating cell-type specific traits. The Notch gene has been initially identified in Drosophila melanogaster [55]. Insects, Ciona species, sea urchin and amphioxus carry only one Notch copy [21, 22]. The two Notch copies in Caenorhabditis elegans (C. elegans), resulted from an independent duplication event within its linage [56], differ from the Notch copies from other taxa. Four Notch paralogues (i.e., Notch1, Notch2, Notch3, Notch4) have been found in invertebrates and vertebrates [9]. It is believed that Notch1, Notch2 and Notch3 have originated by two duplication events in vertebrates prior to the divergence of mammals, birds, reptiles, amphibians and teleost [21, 22] (Figs. 1 and 5). Notch2 has emerged from Notch1, possibly at the first round of duplication events in vertebrates, whereas Notch2 duplication led to the appearance of Notch3. The exclusive presence of a second Notch1 copy in fishes might be due to an independent duplication event after the differentiation of tetrapoda and teleost fish [21, 57]. The Notch4 gene has been identified only in mammals and its origin is still under debate [22]. Notch evolution in birds and reptiles is still unclear: Notch3 has not yet identified in birds, while both Notch3 and Notch4 have not detected in reptiles [21, 22]. A more thorough sequencing of avian and reptile genomes could elucidate the Notch evolutionary gap between teleost fish and mammals. Albeit this lack of information, it is well-established that Notch genes are highly conserved throughout metazoans [21, 22]. There is still no evidence of the existence of Notch genes in any group besides metazoan phyla, suggesting that Notch appeared as a necessity for complex cellular communication and organization.
Fig. 5.

Evolutionary scenario of Notch duplication events. Commonly accepted tree of the taxa was extracted from NCBI taxonomy browser [21]. Spots indicate duplication events in the Notch family. Red spot: two duplication events prior to the differentiation of Teleostei and Tetrapoda. Dark blue spots: independent recent duplication events, one for Notch1 in Teleostei and one for Notch in nematode. Light blue: possible independent duplication event that gave rise to Notch4 in mammalian lineage. Alternatively, Notch4 could have been present already before the differentiation of Teleostei and Tetrapoda but lost along all lineages except Mammalia. Figure adapted from [21]
The canonical Notch signaling pathway mediates interactions between two neighboring cells, one of which is the signaling cell and the other is the receiving cell, via the physical interaction of the ligand with the Notch receptor at the cell surface [8]. It is well-established that the fine regulation of the Notch pathway is efficient for the activation of distinct downstream mechanisms in both developmental and evolution processes. Therefore, Notch is essential for the formation of complex and exquisite tissues that require often the cooperation of different cell types with discrete functions. By directing cell fates toward proliferation, differentiation, self-renewal, or cell death, Notch signaling is also involved in the assemblage of distinct cell populations that will accomplish the refined, ordinated and complex mechanisms for the generation of a unique tissue. For example, duplicated Notch paralogues expressed in the cerebral cortex resulted in progenitors’ clonal expansion and improved neurogenesis [58, 59]. Deletion of the partially duplicated NOTCH paralogues (NOTCH2NL) in the human cortex induced microcephaly, while their duplication caused megacephaly [58]. These findings suggest that appropriate Notch signaling supplementation in higher vertebrates might contribute to the evolution of specific tissues. The numerous and distinct roles of Notch signaling in vertebrates are facilitated by different combinations of ligands and receptors [60, 61], interactions through additional signaling molecules [62, 63] or addition of novel genes [58, 59]. These events determine the predominant role of Notch signaling in the evolution of tissues and organs [64–67].
The evolution of teeth could also depend on Notch signaling for the generation of new dental cell types from the already existing primitive dental cell types, thus allowing the formation of more complex dental structures such as the tooth enamel. Indeed, tooth morphology shows an astounding heterogeneity among vertebrates [68–73]. While all teeth display the same basic organization [74, 75], their positioning, shapes, and mineral composition vary considerably [68, 76–82]. Cartilaginous and bony fishes are characterized by either homodont or heterodont dentitions (i.e., no or little morphological variability within the same dentition) that are continuously renewed (polyphydonts) [70, 73, 74, 83–87]. The single teeth can have nevertheless highly complex morphologies, and their positioning and orientation within the jaw is thought to confer a certain level of functional specialization [88]. Reptiles and amphibians possess relatively simple teeth, which are often continuously replaced [72, 89, 90]. Mammals display more complex dental structures and generally exhibit a reduced tooth turnover [68, 89]. At the level of mineralization, the teeth of fishes are covered by enameloid, a highly mineralized hard tissue that contains collagenous and non-collagenous proteins [83, 91–95]. In contrast to fishes, teeth of reptiles, amphibians and mammals are covered by proper enamel [74]. Enamel does not contain collagenous proteins, and it is characterized by a higher degree of mineralization and a more complex structure when compared to enameloid of fishes [92]. Although tooth enamel in reptiles and amphibians is, with some exceptions [96], structurally simple and aprismatic [72, 74, 89, 97, 98], enamel in mammalian teeth is prismatic and characterized by the presence of organized bundles of hydroxyapatite crystals that confer it exceptional hardness and resistance to stresses [89, 99–101].
It was hypothesized that the reduction of tooth turnover in primordial mammals triggered the need for more durable teeth, leading to the formation of accurate and solid new enamel structures [89]. The complexity of enamel correlates with the specialization of the dental epithelium. In the mammalian dental epithelium, also called enamel organ, four distinct cell types have been identified based on histological analysis, gene expression analysis, functional characterization, and modern imaging techniques [102]. A similar organization of the dental epithelium was observed in other enamel-producing taxa, such as reptiles, where three to four dental epithelial layers were described [72, 103, 104]. In fishes, however, only two dental epithelial cell types are present [105].
Previous studies in mammals have demonstrated that Notch signaling is essential for tooth development, morphology and tooth-specific mineral matrices deposition [36, 37, 39, 40, 42, 48, 51, 106]. Notch signaling defines the four dental epithelial cell lineages through the temporo-spatial differential expression pattern of the various Notch receptors and ligands during odontogenesis [41] (Fig. 2). However, it remains unclear how Notch signaling contributes to the establishment of distinct enamel or enamel-like structures in different species. Enamel formation represents a very sophisticated cellular process, as it requires a tightly controlled sequence of cell proliferation, differentiation, extracellular matrix secretion and re-absorbance, and crystal mineralization [100, 107]. This process needs to be tightly regulated spatially and temporally, as even minor changes can lead to functionally relevant alterations of the fine enamel structure [42, 101]. In mammals, all four dental epithelial cell types of the enamel organ are indispensable for the formation of a properly structured and mineralized enamel [42]. Among these, ameloblasts are the most characterized and directly responsible for the secretion and maturation of the enamel matrix [41, 100]. The role of the other three dental epithelial cell types (i.e., stratum intermedium, stellate reticulum and outer enamel epithelium) is not yet well-studied or understood. In lower vertebrates, such as different fish taxa (e.g., Teleosts), the enamel structure is less refined and organized than in mammals, which is indicative of a simpler and less precise mechanism for the formation of enamel. This procedure is carried out by a single epithelial cell type and requires mesenchymal-derived odontoblasts to co-participate in the processes of both organic matrices secretion and minerals deposition [74, 108]. Enameloid formation by a single cell type may represent a phylogenetically early stage in the differentiated capability of the evolving ameloblasts [105, 109]. We can assume that a primitive dental epithelial cell type, forming a set of cells within the enamel organ, has changed during evolution and gave rise to additional, closely related cell types. It is indeed well accepted that the number of cell types has changed during animal evolution [110]. Basal metazoans have relatively few cell types, indicating that there was a large expansion of cell type diversity before the bilaterian ancestor [111]. This increase of cell types was accompanied by the shift from few, multifunctional cells, towards multiple, specialized sister cells. These new cells can exert precise functions previously performed by a primitive single cell, or acquire completely new functions [112]. In many cases, this segregation and divergence is driven by gene duplication [112], by expression of novel genes, or by co-option of already existing genes for new cellular functions [110, 112]. By these means, sister cells can synergistically lead to the formation of extremely complex tissues that could not be generated by single multifunctional cells. We propose that the Notch signaling pathway, and in particular the differential expression of its ligands and receptors, could be a key determinant of cell specification and functional segregation in the evolution of teeth, and most probably in other organs and tissues (Fig. 6). Notably, Notch could also exert its biological functions via non-canonical signaling, as it does during neurogenesis and myogenesis [113]. However, there is no yet evidence of involvement of the non-canonical Notch signaling during odontogenesis and amelogenesis.
Fig. 6.

A model showing the generation of the enamel organ composed by different cell types in teeth. In fishes, only one specialized epithelial cell type, the ameloblast (am), can be distinguished in the tooth germ. In mice, oral epithelial (oe) cells in close contact with the mesenchyme (m; yellow color) give rise to two cell types, the inner enamel epithelial (iee) cells and outer enamel epithelial (ooe) cells, while the rest of the epithelial cells give rise to cells of the stratum intermedium (si) and stellate reticulum (sr). All these cell populations compose the enamel organ, which is an evolutionary unit essential for elaborating the extremely refined enamel structure in mammalian teeth. Physical interactions between all these cell types (green arrows) through the Notch signaling machinery are necessary for proper amelogenesis. In fishes, amelogenesis relies exclusively to ameloblasts (am), having as consequence the formation of a less elaborated structure called enameloid. de dental epithelium, E embryonic day
Studies in fishes have shown that members of the Notch signaling pathway are actually expressed during tooth development [105]. In cichlid fishes, Notch1 and Jagged2 expression are associated with the successional lamina (i.e., the structure responsible for tooth renewal, and hence new tooth buds), while during the maturation and secretion stages they are co-expressed in ameloblasts and the adjacent epithelial cells [105]. It is noteworthy that in the teeth of fishes the expression domains of Notch1 and Jagged2 are largely overlapping [105], and they are thus not obviously distinct and demarcated as in the teeth of mice, where the expression of Notch ligands and receptors clearly defines the four cell types of the enamel organ (Fig. 2F) [41]. No studies described the expression of Notch ligands and receptors in other taxa such as reptiles or amphibians. In mice, mutations or inhibition of Notch signaling affects teeth and most specifically the formation and structure of enamel [36, 37, 42, 48, 51]. Constitutive deletion of Jagged2 is perinatally lethal in mice, and affects dental epithelial progenitor cells ability to form ameloblasts, leading to the development of teeth with abnormal morphology and lacking enamel [51]. Previous studies have shown that the postnatal inhibition of Notch signaling leads to alterations in cell–cell contacts at the ameloblasts-stratum intermedium interface, without major direct effects on ameloblasts [36]. Nevertheless, this disturbance eventually results in enamel defects [36]. However, we have shown recently that the epithelial deletion of Adam10, a membrane-bound metalloproteinase regulating Notch signaling, causes the loss of the stratum intermedium layer and the disorganization of ameloblasts that triggers deficient enamel formation [42]. Furthermore, deletion of the Jagged1 ligand in dental epithelium dysregulates the expression of genes involved in the Notch pathway (e.g., Notch1, Notch2, Hes5), as well as of enamel-specific genes (e.g., Amelx, Enam) [48]. Moreover, deletion of Jagged1 in the dental epithelium of transgenic mice leads to tooth crown shape modifications convergent to those observed along Muridae evolution [48]. Analogous mechanisms have been observed in humans. Mutations in TSPEAR lead to enamel defects via down-regulation of NOTCH signaling in human patients [114]. Similarly, mutations in AMELX, which cause severe enamel defects in humans, are associated with aberrant overexpression of NOTCH1 in ameloblasts [115]. Therefore, Notch signaling deregulation within the enamel organ, which can be seen as an evolutionary unit, do not allow sister cell lines to express distinct molecular programs that maintain their cellular specificity, resulting in defective enamel formation (Fig. 7) [42]. In fishes, pharmacological inhibition of Notch signaling impairs tooth renewal [105], while to date no studies investigated its roles in fish dental epithelium differentiation and enameloid formation. Molecules of the Notch pathway control the dental cell-type specificity and mediate their distinct responses to common signals [48]. On a broader scale, Notch signaling is the central hub of a molecular network that determines cell fate choice throughout animal development, homeostasis, and regeneration via lateral inhibition [8, 18, 116–119]. A flat hierarchy of gene regulation [18, 118] upon Notch signaling deletion could thus revert the evolutionary path, impairing the specialization of the cells that contribute to amelogenesis and thus generating structures resembling more enameloid of fishes than enamel of mammals [42]. Hence, loss of interactions between Notch and Jagged/Delta-like proteins within the enamel organ may either shift the behavior of cell types or initiate the suppression of complementary dental epithelial cell fates.
Fig. 7.
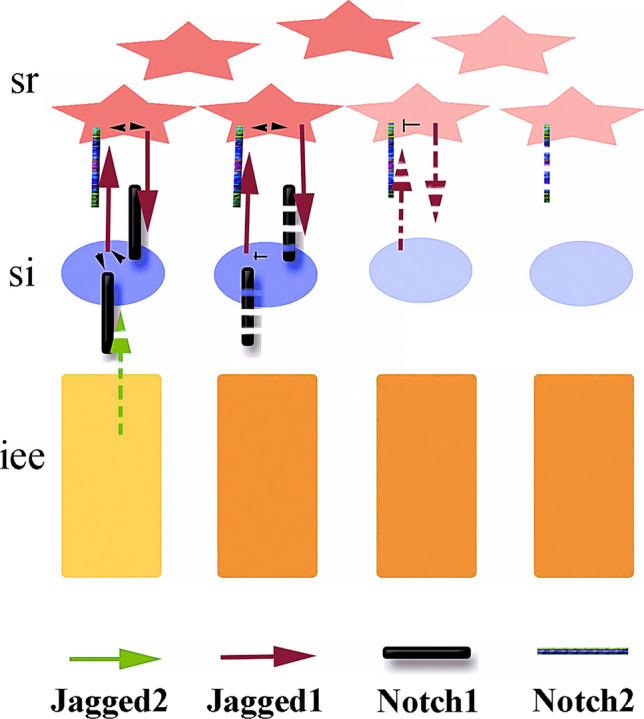
Hypothetical model of Notch signaling action in the successive cell layers of the enamel organ during mouse odontogenesis. The defined expression pattern of the Notch ligands Jagged1 and Jagged2 (arrows) and the Notch receptors Notch1 and Notch2 (bars) in different cell types of the enamel organ with discrete and complementary functions contribute to the formation of the highly refined and well-structured mammalian enamel. Deletion of Jagged2 (green spaced dotted arrow) in inner enamel epithelium (iee) cells results in Notch1 down-regulation (thin spaced dotted bar) in stratum intermedium (si) cells, followed by Jagged1 (dark red spaced dotted arrows) down-regulation in stratum intermedium and of Jagged1 and Notch2 (thin spaced dotted bar) in stellate reticulum (sr) cells, according to the Notch specific lateral inhibition mode of action. Loss of interactions between Notch and Jagged proteins may either shift the behavior of these cells or initiate loss of their identity, thus returning back the evolutionary path by impairing the specialization of the cells that contribute to mammalian amelogenesis. As a consequence, amelogenesis will be carried out by only one single cell type, thus generating structures resembling more enameloid of fishes than enamel of mammals
The expansion of the functions of the Notch signaling pathway in the generation of highly specialized cell types could be due not only to the duplications of the genes coding for its ligands and receptors, but also to the refinement of their expression domains. Many loci involved in the patterning and growth of the musculoskeletal system and dental apparatus in vertebrates are controlled by complex cis-regulatory systems, as these systems permit highly compartmentalized and fine-tuned control of gene expression in specific cellular and tissue-specific contexts [120–128]. Indeed, it is becoming clear that changes in gene expression patterns play a pivotal role in the evolution of complex morphological traits [48, 129–131]. These changes are more often due to mutations in cis-regulatory sequences, rather than coding sequences, the latter of which can pleiotropically alter the expression domains of key signaling molecules [129]. Members of the Notch pathway should also be subject to this type of fine-tuned tissue-specific control and future functional genomics study on developing teeth and their cell populations will likely reveal this to be the case. These leads us to the suggestion that the concomitant duplication of Notch ligands and receptors, and their progressively more defined expression domains via the evolution of associated complex cis-regulatory systems could be the driving force of the generation of highly specialized cell types during the evolution of teeth [129]. The proposed correlation between Notch receptors and ligands, and the generation and maintenance of distinct dental cell types, could represent a general mechanism underlying the evolution of specialized cell types in metazoa.
Acknowledgements
The authors would like to thank the staff of the Institute of Oral Biology (University of Zurich) for inspiring discussions and suggestions. We thank Professor Thomas Gridley (Maine Medical Center Research Institute, Tufts University, USA) for the generation and generous gift of the Jagged−/− mice.
Author contributions
TAM: conceived the topic, realized the experiments, designed the figures, wrote the initial draft and the final manuscript, and perform the editing. PP, TDC, MMS: critical reviewing, writing and editing of the final manuscript.
Funding
Open access funding provided by University of Zurich. This work was financially supported by institutional funds from the University of Zurich (UZH).
Data availability
This is not applicable to the present article.
Declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
All mice (C57Bl/6) were maintained and handled according to the Swiss Animal Welfare Law and in compliance with the regulations of the Cantonal Veterinary office, Zurich (License 11/2014).
Consent to participate
N/A.
Consent for publication
All authors have read the manuscript and agreed to give their consent for the publication of information in the Journal of Cellular and Molecular Life Sciences.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 2.Gridley T. Notch signaling in vertebrate development and disease. Mol Cell Neurosci. 1997;9:103–108. doi: 10.1006/mcne.1997.0610. [DOI] [PubMed] [Google Scholar]
- 3.Robey E. Notch in vertebrates. Curr Opin Genet Dev. 1997;7:551–557. doi: 10.1016/S0959-437X(97)80085-8. [DOI] [PubMed] [Google Scholar]
- 4.Weinmaster G. The ins and outs of notch signaling. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- 5.Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126:2135–2140. doi: 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–2924. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- 7.Muskavitch MA. Delta-notch signaling and Drosophila cell fate choice. Dev Biol. 1994;166:415–430. doi: 10.1006/dbio.1994.1326. [DOI] [PubMed] [Google Scholar]
- 8.Ho DM, Artavanis-Tsakonas S. The Notch-mediated proliferation circuitry. Curr Top Dev Biol. 2016;116:17–33. doi: 10.1016/bs.ctdb.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97:1235–1294. doi: 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- 10.Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. doi: 10.1016/S0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 11.Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/S0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- 12.Fortini ME, Artavanis-Tsakonas S. The suppressor of hairless protein participates in notch receptor signaling. Cell. 1994;79:273–282. doi: 10.1016/0092-8674(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 13.Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 14.Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci USA. 1996;93:1683–1688. doi: 10.1073/pnas.93.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuno K, Diederich RJ, Go MJ, Blaumueller CM, Artavanis-Tsakonas S. Deltex acts as a positive regulator of Notch signaling through interactions with the Notch ankyrin repeats. Development. 1995;121:2633–2644. doi: 10.1242/dev.121.8.2633. [DOI] [PubMed] [Google Scholar]
- 16.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 17.Kovall RA, Gebelein B, Sprinzak D, Kopan R. The canonical Notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell. 2017;41:228–241. doi: 10.1016/j.devcel.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guruharsha KG, Kankel MW, Artavanis-Tsakonas S. The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet. 2012;13:654–666. doi: 10.1038/nrg3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Sato C, Cerletti M, Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr Top Dev Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 21.Theodosiou A, Arhondakis S, Baumann M, Kossida S. Evolutionary scenarios of Notch proteins. Mol Biol Evol. 2009;26:1631–1640. doi: 10.1093/molbev/msp075. [DOI] [PubMed] [Google Scholar]
- 22.Vlachakis D, Papageorgiou L, Papadaki A, Georga M, Kossida S, Eliopoulos E. An updated evolutionary study of the Notch family reveals a new ancient origin and novel invariable motifs as potential pharmacological targets. PeerJ. 2020;8:e10334. doi: 10.7717/peerj.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de La Coste A, Freitas AA. Notch signaling: distinct ligands induce specific signals during lymphocyte development and maturation. Immunol Lett. 2006;102:1–9. doi: 10.1016/j.imlet.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 27.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. doi: 10.1101/gad.14.11.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 30.Ho DM, Artavanis-Tsakonas S, Louvi A. The Notch pathway in CNS homeostasis and neurodegeneration. Wiley Interdiscip Rev Dev Biol. 2020;9:e358. doi: 10.1002/wdev.358. [DOI] [PubMed] [Google Scholar]
- 31.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-B. [DOI] [PubMed] [Google Scholar]
- 32.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 34.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 35.Felszeghy S, Suomalainen M, Thesleff I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing mouse incisor. Differentiation. 2010;80:241–248. doi: 10.1016/j.diff.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Jheon AH, Prochazkova M, Meng B, Wen T, Lim YJ, Naveau A, et al. Inhibition of Notch signaling during mouse incisor renewal leads to enamel defects. J Bone Miner Res. 2016;31:152–162. doi: 10.1002/jbmr.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsiadis TA, Regaudiat L, Gridley T. Role of the Notch signalling pathway in tooth morphogenesis. Arch Oral Biol. 2005;50:137–140. doi: 10.1016/j.archoralbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Mucchielli ML, Mitsiadis TA. Correlation of asymmetric Notch2 expression and mouse incisor rotation. Mech Dev. 2000;91:379–382. doi: 10.1016/S0925-4773(99)00293-2. [DOI] [PubMed] [Google Scholar]
- 39.Mitsiadis TA, Hirsinger E, Lendahl U, Goridis C. Delta-notch signaling in odontogenesis: correlation with cytodifferentiation and evidence for feedback regulation. Dev Biol. 1998;204:420–431. doi: 10.1006/dbio.1998.9092. [DOI] [PubMed] [Google Scholar]
- 40.Mitsiadis TA, Lardelli M, Lendahl U, Thesleff I. Expression of Notch 1, 2 and 3 is regulated by epithelial-mesenchymal interactions and retinoic acid in the developing mouse tooth and associated with determination of ameloblast cell fate. J Cell Biol. 1995;130:407–418. doi: 10.1083/jcb.130.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitsiadis TA, Graf D. Cell fate determination during tooth development and regeneration. Birth Defects Res C Embryo Today. 2009;87:199–211. doi: 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- 42.Mitsiadis TA, Jimenez-Rojo L, Balic A, Weber S, Saftig P, Pagella P. Adam10-dependent Notch signaling establishes dental epithelial cell boundaries required for enamel formation. iScience. 2022;25:105154. doi: 10.1016/j.isci.2022.105154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitsiadis TA, Luder HU. Genetic basis for tooth malformations: from mice to men and back again. Clin Genet. 2011;80:319–329. doi: 10.1111/j.1399-0004.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 44.Balic A, Thesleff I. Tissue interactions regulating tooth development and renewal. Curr Top Dev Biol. 2015;115:157–186. doi: 10.1016/bs.ctdb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Bluteau G, Luder HU, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1–9. doi: 10.22203/eCM.v016a01. [DOI] [PubMed] [Google Scholar]
- 46.Ruch JV, Lesot H, Karcher-Djuricic V, Meyer JM, Mark M. Epithelial-mesenchymal interactions in tooth germs: mechanisms of differentiation. J Biol Buccale. 1983;11:173–193. [PubMed] [Google Scholar]
- 47.Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18:75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 48.Mitsiadis TA, Pagella P, Gomes Rodrigues H, Tsouknidas A, Ramenzoni LL, Radtke F, et al. Notch signaling pathway in tooth shape variations throughout evolution. Cells. 2023;12:761. doi: 10.3390/cells12050761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 50.Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, et al. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitsiadis TA, Graf D, Luder H, Gridley T, Bluteau G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development. 2010;137:3025–3035. doi: 10.1242/dev.049528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson DI, Theeuwes MJ, Farber EM. Nerve growth factor increases the mitogenicity of certain growth factors for cultured human keratinocytes: a comparison with epidermal growth factor. Exp Dermatol. 1994;3:239–245. doi: 10.1111/j.1600-0625.1994.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 53.Sebe-Pedros A, Chomsky E, Pang K, Lara-Astiaso D, Gaiti F, Mukamel Z, et al. Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nat Ecol Evol. 2018;2:1176–1188. doi: 10.1038/s41559-018-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Callier V. Inner workings: understanding the evolution of cell types to explain the roots of animal diversity. Proc Natl Acad Sci USA. 2020;117:5547–5549. doi: 10.1073/pnas.2002403117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Artavanis-Tsakonas S, Muskavitch MA, Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc Natl Acad Sci USA. 1983;80:1977–1981. doi: 10.1073/pnas.80.7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maine EM, Lissemore JL, Starmer WT. A phylogenetic analysis of vertebrate and invertebrate Notch-related genes. Mol Phylogenet Evol. 1995;4:139–149. doi: 10.1006/mpev.1995.1014. [DOI] [PubMed] [Google Scholar]
- 57.Westin J, Lardelli M. Three novel Notch genes in zebrafish: implications for vertebrate Notch gene evolution and function. Dev Genes Evol. 1997;207:51–63. doi: 10.1007/s004270050091. [DOI] [PubMed] [Google Scholar]
- 58.Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, et al. Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell. 2018;173(1356–1369):e1322. doi: 10.1016/j.cell.2018.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki IK, Gacquer D, Van Heurck R, Kumar D, Wojno M, Bilheu A, et al. Human-specific NOTCH2NL genes expand cortical neurogenesis through delta/notch regulation. Cell. 2018;173(1370–1384):e1316. doi: 10.1016/j.cell.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mase S, Shitamukai A, Wu Q, Morimoto M, Gridley T, Matsuzaki F. Notch1 and Notch2 collaboratively maintain radial glial cells in mouse neurogenesis. Neurosci Res. 2021;170:122–132. doi: 10.1016/j.neures.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Nelson BR, Hodge RD, Bedogni F, Hevner RF. Dynamic interactions between intermediate neurogenic progenitors and radial glia in embryonic mouse neocortex: potential role in Dll1-Notch signaling. J Neurosci. 2013;33:9122–9139. doi: 10.1523/JNEUROSCI.0791-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cardenas A, Villalba A, de Juan RC, Pico E, Kyrousi C, Tzika AC, et al. Evolution of cortical neurogenesis in amniotes controlled by robo signaling levels. Cell. 2018;174(590–606):e521. doi: 10.1016/j.cell.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardenas A, Borrell V. Molecular and cellular evolution of corticogenesis in amniotes. Cell Mol Life Sci. 2020;77:1435–1460. doi: 10.1007/s00018-019-03315-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nian FS, Hou PS. Evolving roles of notch signaling in cortical development. Front Neurosci. 2022;16:844410. doi: 10.3389/fnins.2022.844410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borrell V, Reillo I. Emerging roles of neural stem cells in cerebral cortex development and evolution. Dev Neurobiol. 2012;72:955–971. doi: 10.1002/dneu.22013. [DOI] [PubMed] [Google Scholar]
- 67.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 68.Tucker AS, Fraser GJ. Evolution and developmental diversity of tooth regeneration. Semin Cell Dev Biol. 2014;25–26:71–80. doi: 10.1016/j.semcdb.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 69.Meredith RW, Zhang G, Gilbert MT, Jarvis ED, Springer MS. Evidence for a single loss of mineralized teeth in the common avian ancestor. Science. 2014;346:1254390. doi: 10.1126/science.1254390. [DOI] [PubMed] [Google Scholar]
- 70.Guinot G, Adnet S, Shimada K, Shimada K, Underwood CJ, Siversson M, et al. On the need of providing tooth morphology in descriptions of extant elasmobranch species. Zootaxa. 2018;4461:118–126. doi: 10.11646/zootaxa.4461.1.8. [DOI] [PubMed] [Google Scholar]
- 71.Mello W, Brito PM. Contributions to the tooth morphology in early embryos of three species of hammerhead sharks (Elasmobranchii: Sphyrnidae) and their evolutionary implications. C R Biol. 2013;336:466–471. doi: 10.1016/j.crvi.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 72.Landova Sulcova M, Zahradnicek O, Dumkova J, Dosedelova H, Krivanek J, Hampl M, et al. Developmental mechanisms driving complex tooth shape in reptiles. Dev Dyn. 2020;249:441–464. doi: 10.1002/dvdy.138. [DOI] [PubMed] [Google Scholar]
- 73.Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J Oral Sci. 1998;106(Suppl 1):437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 74.Slavkin HC, Diekwisch T. Evolution in tooth developmental biology: of morphology and molecules. Anat Rec. 1996;245:131–150. doi: 10.1002/(SICI)1097-0185(199606)245:2<131::AID-AR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 75.Zanolli C, Kaifu Y, Pan L, Xing S, Mijares AS, Kullmer O, et al. Further analyses of the structural organization of Homo luzonensis teeth: Evolutionary implications. J Hum Evol. 2022;163:103124. doi: 10.1016/j.jhevol.2021.103124. [DOI] [PubMed] [Google Scholar]
- 76.Mohring S, Cieplik F, Hiller KA, Ebensberger H, Ferstl G, Hermens J, et al. Elemental compositions of enamel or dentin in human and bovine teeth differ from murine teeth. Materials (Basel) 2023;16:1514. doi: 10.3390/ma16041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa BM, Iwamoto AS, Puppin-Rontani RM, Pascon FM. Comparative analysis of root dentin morphology and structure of human versus bovine primary teeth. Microsc Microanal. 2015;21:689–694. doi: 10.1017/S1431927615000434. [DOI] [PubMed] [Google Scholar]
- 78.Botella H, Blom H, Dorka M, Ahlberg PE, Janvier P. Jaws and teeth of the earliest bony fishes. Nature. 2007;448:583–586. doi: 10.1038/nature05989. [DOI] [PubMed] [Google Scholar]
- 79.Velasco-Hogan A, Huang W, Serrano C, Kisailus D, Meyers MA. Tooth structure, mechanical properties, and diet specialization of Piranha and Pacu (Serrasalmidae): a comparative study. Acta Biomater. 2021;134:531–545. doi: 10.1016/j.actbio.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 80.Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 81.Renvoise E, Evans AR, Jebrane A, Labruere C, Laffont R, Montuire S. Evolution of mammal tooth patterns: new insights from a developmental prediction model. Evolution. 2009;63:1327–1340. doi: 10.1111/j.1558-5646.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- 82.Kavanagh KD, Evans AR, Jernvall J. Predicting evolutionary patterns of mammalian teeth from development. Nature. 2007;449:427–432. doi: 10.1038/nature06153. [DOI] [PubMed] [Google Scholar]
- 83.Wilmers J, Waldron M, Bargmann S. Hierarchical microstructure of tooth enameloid in two lamniform shark species, Carcharias taurus and Isurus oxyrinchus. Nanomaterials (Basel) 2021;11:969. doi: 10.3390/nano11040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seidel R, Blumer M, Pechriggl EJ, Lyons K, Hall BK, Fratzl P, et al. Calcified cartilage or bone? Collagens in the tessellated endoskeletons of cartilaginous fish (sharks and rays) J Struct Biol. 2017;200:54–71. doi: 10.1016/j.jsb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Geerinckx T, Huysseune A, Boone M, Claeys M, Couvreur M, De Kegel B, et al. Soft dentin results in unique flexible teeth in scraping catfishes. Physiol Biochem Zool. 2012;85:481–490. doi: 10.1086/667532. [DOI] [PubMed] [Google Scholar]
- 86.Kemp A. Ultrastructure of developing tooth plates in the Australian lungfish, Neoceratodus forsteri (Osteichthyes: Dipnoi) Tissue Cell. 2003;35:401–426. doi: 10.1016/S0040-8166(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 87.Underwood CJ, Johanson Z, Welten M, Metscher B, Rasch LJ, Fraser GJ, et al. Development and evolution of dentition pattern and tooth order in the skates and rays (batoidea; chondrichthyes) PLoS ONE. 2015;10:e0122553. doi: 10.1371/journal.pone.0122553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hulsey CD, Cohen KE, Johanson Z, Karagic N, Meyer A, Miller CT, et al. Grand challenges in comparative tooth biology. Integr Comp Biol. 2020;60:563–580. doi: 10.1093/icb/icaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alazem O, Abramyan J. Reptile enamel matrix proteins: Selection, divergence, and functional constraint. J Exp Zool B Mol Dev Evol. 2019;332:136–148. doi: 10.1002/jez.b.22857. [DOI] [PubMed] [Google Scholar]
- 90.Zahradnicek O, Buchtova M, Dosedelova H, Tucker AS. The development of complex tooth shape in reptiles. Front Physiol. 2014;5:74. doi: 10.3389/fphys.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prostak K, Skobe Z. Ultrastructure of the dental epithelium and odontoblasts during enameloid matrix deposition in cichlid teeth. J Morphol. 1986;187:159–172. doi: 10.1002/jmor.1051870204. [DOI] [PubMed] [Google Scholar]
- 92.Enax J, Janus AM, Raabe D, Epple M, Fabritius HO. Ultrastructural organization and micromechanical properties of shark tooth enameloid. Acta Biomater. 2014;10:3959–3968. doi: 10.1016/j.actbio.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 93.Kawasaki K, Keating JN, Nakatomi M, Welten M, Mikami M, Sasagawa I, et al. Coevolution of enamel, ganoin, enameloid, and their matrix SCPP genes in osteichthyans. iScience. 2021;24:102023. doi: 10.1016/j.isci.2020.102023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillis JA, Donoghue PC. The homology and phylogeny of chondrichthyan tooth enameloid. J Morphol. 2007;268:33–49. doi: 10.1002/jmor.10501. [DOI] [PubMed] [Google Scholar]
- 95.Sasagawa I, Ishiyama M, Yokosuka H, Mikami M, Oka S, Shimokawa H, et al. Immunolocalization of enamel matrix protein-like proteins in the tooth enameloid of spotted gar, Lepisosteus oculatus, an actinopterygian bony fish. Connect Tissue Res. 2019;60:291–303. doi: 10.1080/03008207.2018.1506446. [DOI] [PubMed] [Google Scholar]
- 96.Cooper JS, Poole DFG. The dentition and dental tissues of the agamid lizard, Uromastyx. J Zool. 1973;169:85–100. doi: 10.1111/j.1469-7998.1973.tb04654.x. [DOI] [Google Scholar]
- 97.Suarez CA, You HL, Suarez MB, Li DQ, Trieschmann JB. Stable isotopes reveal rapid enamel elongation (Amelogenesis) rates for the early cretaceous iguanodontian dinosaur Lanzhousaurus magnidens. Sci Rep. 2017;7:15319. doi: 10.1038/s41598-017-15653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enax J, Fabritius HO, Rack A, Prymak O, Raabe D, Epple M. Characterization of crocodile teeth: correlation of composition, microstructure, and hardness. J Struct Biol. 2013;184:155–163. doi: 10.1016/j.jsb.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Diekwisch TGH, Jin T, Wang X, Ito Y, Schmidt M, Druzinsky R, et al. Amelogenin evolution and tetrapod enamel structure. Front Oral Biol. 2009;13:74–79. doi: 10.1159/000242395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bartlett JD. Dental enamel development: proteinases and their enamel matrix substrates. ISRN Dent. 2013;2013:684607. doi: 10.1155/2013/684607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cantu C, Pagella P, Shajiei TD, Zimmerli D, Valenta T, Hausmann G, et al. A cytoplasmic role of Wnt/beta-catenin transcriptional cofactors Bcl9, Bcl9l, and Pygopus in tooth enamel formation. Sci Signal. 2017;10:eaah4598. doi: 10.1126/scisignal.aah4598. [DOI] [PubMed] [Google Scholar]
- 102.Liu H, Yan X, Pandya M, Luan X, Diekwisch TG. Daughters of the enamel organ: development, fate, and function of the stratum intermedium, stellate reticulum, and outer enamel epithelium. Stem Cells Dev. 2016;25:1580–1590. doi: 10.1089/scd.2016.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zahradnicek O, Horacek I, Tucker AS. Tooth development in a model reptile: functional and null generation teeth in the gecko Paroedura picta. J Anat. 2012;221:195–208. doi: 10.1111/j.1469-7580.2012.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richman JM, Handrigan GR. Reptilian tooth development. Genesis. 2011;49:247–260. doi: 10.1002/dvg.20721. [DOI] [PubMed] [Google Scholar]
- 105.Fraser GJ, Bloomquist RF, Streelman JT. Common developmental pathways link tooth shape to regeneration. Dev Biol. 2013;377:399–414. doi: 10.1016/j.ydbio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitsiadis TA, Henrique D, Thesleff I, Lendahl U. Mouse Serrate-1 (Jagged-1): expression in the developing tooth is regulated by epithelial-mesenchymal interactions and fibroblast growth factor-4. Development. 1997;124:1473–1483. doi: 10.1242/dev.124.8.1473. [DOI] [PubMed] [Google Scholar]
- 107.Zheng L, Seon YJ, Mourao MA, Schnell S, Kim D, Harada H, et al. Circadian rhythms regulate amelogenesis. Bone. 2013;55:158–165. doi: 10.1016/j.bone.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith MM, Fraser GJ, Mitsiadis TA. Dental lamina as source of odontogenic stem cells: evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zool B Mol Dev Evol. 2009;312B:260–280. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- 109.Smith MM, Johanson Z, Butts T, Ericsson R, Modrell M, Tulenko FJ, et al. Making teeth to order: conserved genes reveal an ancient molecular pattern in paddlefish (Actinopterygii) Proc Biol Sci. 2015;282:20142700. doi: 10.1098/rspb.2014.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, et al. The origin and evolution of cell types. Nat Rev Genet. 2016;17:744–757. doi: 10.1038/nrg.2016.127. [DOI] [PubMed] [Google Scholar]
- 111.Valentine JW, Collins AG, Meyer CP. Morphological complexity increase in metazoans. Paleobiology. 1994;20:131–142. doi: 10.1017/S0094837300012641. [DOI] [Google Scholar]
- 112.Arendt D. The evolution of cell types in animals: emerging principles from molecular studies. Nat Rev Genet. 2008;9:868–882. doi: 10.1038/nrg2416. [DOI] [PubMed] [Google Scholar]
- 113.Andersen P, Uosaki H, Shenje LT, Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 2012;22:257–265. doi: 10.1016/j.tcb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peled A, Sarig O, Samuelov L, Bertolini M, Ziv L, Weissglas-Volkov D, et al. Mutations in TSPEAR, encoding a regulator of Notch signaling, affect tooth and hair follicle morphogenesis. PLoS Genet. 2016;12:e1006369. doi: 10.1371/journal.pgen.1006369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen X, Li Y, Alawi F, Bouchard JR, Kulkarni AB, Gibson CW. An amelogenin mutation leads to disruption of the odontogenic apparatus and aberrant expression of Notch1. J Oral Pathol Med. 2011;40:235–242. doi: 10.1111/j.1600-0714.2010.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gazave E, Lapebie P, Richards GS, Brunet F, Ereskovsky AV, Degnan BM, et al. Origin and evolution of the Notch signalling pathway: an overview from eukaryotic genomes. BMC Evol Biol. 2009;9:249. doi: 10.1186/1471-2148-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guruharsha KG, Hori K, Obar RA, Artavanis-Tsakonas S. Proteomic analysis of the Notch interactome. Methods Mol Biol. 2014;1187:181–192. doi: 10.1007/978-1-4939-1139-4_14. [DOI] [PubMed] [Google Scholar]
- 118.Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, et al. Architecture of the human interactome defines protein communities and disease networks. Nature. 2017;545:505–509. doi: 10.1038/nature22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rhee DY, Cho DY, Zhai B, Slattery M, Ma L, Mintseris J, et al. Transcription factor networks in Drosophila melanogaster. Cell Rep. 2014;8:2031–2043. doi: 10.1016/j.celrep.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chandler KJ, Chandler RL, Mortlock DP. Identification of an ancient Bmp4 mesoderm enhancer located 46 kb from the promoter. Dev Biol. 2009;327:590–602. doi: 10.1016/j.ydbio.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang S, Chandler RL, Fritz DT, Mortlock DP, Rogers MB. Repressive BMP2 gene regulatory elements near the BMP2 promoter. Biochem Biophys Res Commun. 2010;392:124–128. doi: 10.1016/j.bbrc.2009.12.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen H, Capellini TD, Schoor M, Mortlock DP, Reddi AH, Kingsley DM. Heads, shoulders, elbows, knees, and toes: modular Gdf5 enhancers control different joints in the vertebrate skeleton. PLoS Genet. 2016;12:e1006454. doi: 10.1371/journal.pgen.1006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dathe K, Kjaer KW, Brehm A, Meinecke P, Nurnberg P, Neto JC, et al. Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am J Hum Genet. 2009;84:483–492. doi: 10.1016/j.ajhg.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.DiLeone RJ, Russell LB, Kingsley DM. An extensive 3' regulatory region controls expression of Bmp5 in specific anatomical structures of the mouse embryo. Genetics. 1998;148:401–408. doi: 10.1093/genetics/148.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Indjeian VB, Kingman GA, Jones FC, Guenther CA, Grimwood J, Schmutz J, et al. Evolving new skeletal traits by cis-regulatory changes in bone morphogenetic proteins. Cell. 2016;164:45–56. doi: 10.1016/j.cell.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guenther C, Pantalena-Filho L, Kingsley DM. Shaping skeletal growth by modular regulatory elements in the Bmp5 gene. PLoS Genet. 2008;4:e1000308. doi: 10.1371/journal.pgen.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Portnoy ME, McDermott KJ, Antonellis A, Margulies EH, Prasad AB, Program NCS, et al. Detection of potential GDF6 regulatory elements by multispecies sequence comparisons and identification of a skeletal joint enhancer. Genomics. 2005;86:295–305. doi: 10.1016/j.ygeno.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 128.Sugiura T. Cloning and functional characterization of the 5'-flanking region of the human bone morphogenetic protein-2 gene. Biochem J. 1999;338(Pt 2):433–440. doi: 10.1042/bj3380433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Romero IG, Ruvinsky I, Gilad Y. Comparative studies of gene expression and the evolution of gene regulation. Nat Rev Genet. 2012;13:505–516. doi: 10.1038/nrg3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Capellini TD, Chen H, Cao J, Doxey AC, Kiapour AM, Schoor M, et al. Ancient selection for derived alleles at a GDF5 enhancer influencing human growth and osteoarthritis risk. Nat Genet. 2017;49:1202–1210. doi: 10.1038/ng.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chan YF, Marks ME, Jones FC, Villarreal G, Jr, Shapiro MD, Brady SD, et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327:302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is not applicable to the present article.