Abstract
Background
Management of thyroid dysfunction has a direct effect on the quality of life and studies have recognized that hypothyroidism has become a public health challenge. Although conventional medicine is widely used, its long-term side effects are elucidated. This study aims to conduct a randomized controlled trial (RCT) through tele-mode to assess the effectiveness of the newly developed and validated “Scientific Yoga Module” as a telehealth concept for improving the quality of life in patients with hypothyroidism along with management of other symptoms as compared to the standard of care.
Method
This is a single-blinded, two-arm, parallel-group RCT in which at least a total of 120 primary hypothyroid subjects both male and female between the age group 18 to 60 will be recruited from the database of Swami Vivekananda Yoga Anusandhana Samsthana (SVYASA). Participants will be randomly divided into a yoga intervention group (n = 60) and a waitlist control group (n = 60) as per the inclusion and exclusion criteria of the study. A tele-yoga intervention for six months will be administered and pre-interim-post data will be recorded for both groups. This protocol is designed to study the effect of Scientific Yoga Module intervention on primary assessments of SF-36 scale [health-related quality of life (HRQOL) that includes physical, mental, emotional, and social states] along with secondary assessments on the biochemical test of thyroid profile-{Triiodothyronine (T3), Thyroxine (T4), Thyroid Stimulating Hormones (TSH)}, Body Mass Index (BMI), Blood Pressure (BP), Fatigue Assessment Scale (FAS), Perceived Stress Scale (PSS), Gita Inventory of personality scale (GIP).
Conclusion
To the best of our knowledge, this tele-yoga RCT for hypothyroidism will be the first clinical trial to analyze the effectiveness of a “Scientific Yoga Module” imparted through tele-mode.
Keywords: Hypothyroidism, Scientific yoga module, Tele-yoga, Protocol
1. Introduction
As a leading endocrine disorder, hypothyroidism remains one of the most common health conditions affecting up to 5% of the general population, with a further estimated 5% being undiagnosed [1]. Hypothyroidism manifests when the thyroid gland is unable to generate or secrete enough thyroxine [2]. Because thyroxine is an essential hormone for regulating heart rate, digestion, physical growth, brain development and functioning, an insufficient supply to cells could lead to the manifestation of life-threatening complications [3].
The typical course of therapy for people with hypothyroidism is replacing their thyroid hormone with levothyroxine {LT4} or alternatively using a combination with levothyroxine preparations [4]. While the treatment plan for primary hypothyroidism has been one of the biggest "success stories" in medicine, a significant portion of patients treated with levothyroxine have persistent complaints such as lack of energy, fatigue, cognitive problems, musculoskeletal pain, weight gain, constipation, etc., despite achieving the biochemical therapy targets [5]. In fact, the treatment goals of TSH {≤5 mIU/l} itself have been associated with many pathological issues [6] such as poorer cognitive function, anxiety, depression, and impaired quality of life scores, that could also underlie the unmet needs of patients with hypothyroidism [7].
Yoga is an ancient holistic health system that integrates the mind, body, and spirit and is identified as a form of complementary and alternative medicine for developing and maintaining good mental and physical health [8]. Yoga therapy is being increasingly used in experimental and clinical studies [9] as they demonstrate the positive effect in preventing and managing a variety of endocrinal diseases without any side effects [10]. Stress conditions may cause excess cortisol secretion that can alter thyroid axis function by influencing sympathetic activity affecting the immune response [11] wherein yoga practices bring about relaxation to the mind-body system [12].
Preliminary findings of a pilot study with “general yoga” conducted on 22 female participants having hypothyroidism resulted in a moderate reduction in TSH levels [13]. Given the plausible role of stress physiology in hypothyroidism [14] and the documented effectiveness of yoga in stress regulation, we hypothesized that developing a “Scientific Yoga Module” specific to hypothyroidism may bring significant symptomatic improvement in patients only on standard treatment with Levothyroxine {LT4}, in particular, in their quality of life as compared to “general yoga” [15].
Hence, we present this study protocol that proposes “Tele-yoga” which is a virtual online mode of yoga teaching and learning methodology that has been approved by the Ministry of Ayurveda, Yoga, Naturopathy, Unani, Siddha, and Homoeopathy as a substitute for in-person yoga during the COVID-19 pandemic and will be useful even after the pandemic [16]. Telehealth is an approaching concept to facilitate health care across the world. Moderate yoga therapy practices imparted through tele-mode have been found to be not only feasible and effective but also cost-effective and suitable for the adult population [17].
1.1. Hypothesis
Administration of the “Scientific Yoga module” via tele-mode on hypothyroid patients may have a significant improvement in the primary variable of HRQOL and secondary variables of T3, T4, TSH, BMI, BP, FAS, PSS, and GIP.
2. Methodology
2.1. Design and development of the tele-yoga module
A Scientific Yoga Module {SYM} specific to hypothyroidism was developed by systematically reviewing the literature in the field of yoga science along with receiving inputs from yoga therapists and researchers as presented in (Table 1). The module was then validated for its construct, sequence, and duration of each practice by 40 subject matter experts. A Content Validity Ratio {CVR} was calculated using Lawshe's formula [18] and finally, 24 precise practices were selected having significant CVR {cut-off value: 0.29}. A feasibility test was conducted through online mode using a sample size from a previous study of 15 participants [19] in a community set up under the guidance of SVYASA for people suffering from hypothyroidism for a period of two weeks for 1 h daily. All participants completed a pre-tested survey questionnaire [20] on their experiences of the tele-yoga module with regard to the efficacy of the module, construct, teaching expertise, and satisfaction [20]. The feasibility study confirms the efficacy and acceptability of the SYM suitable for adults having hypothyroidism.
Table 1.
Yoga module for hypothyroidism.
| NO. | YOGA PRACTICE | TIME | CVR | BENEFITS | POSTURES |
|---|---|---|---|---|---|
| STANDING ASANAS (8 MINUTES) | |||||
| 1. | GRIEVA SHAKTI (Neck movements: Front-back-side bending, twisting & rotation) | 2 min | 0.85 | Strengthens the neck and activates the thyroid gland |  |
| 2. | ARDHA-CHAKRASANA (Half Wheel Posture) | 2 min | 0.95 | Works on throat and neck region, promotes a healthy metabolic function by stimulating the thyroid & adrenal gland for uptake of thyroid hormones by the cells of the body. | 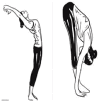 |
| 3. | PADAHASTASAN (Hand to Toe Posture) | 2 min | 0.50 | ||
| 4. | SEQUENTIAL (Ardha-chakrasana & Padahastasana) | 2 min | 0.42 | ||
| SITTING ASANAS (8 MINUTES) | |||||
| 5. | Ushtrasana (Camel Posture) | 2 min | 1.00 | Works on Vishuddhi Chakra. Activates thyroid and parathyroid glands on the neck, strengthens the shoulder and thigh muscles | 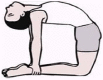 |
| 6. | Sahankasana (Rabbit Posture) | 2 min | 0.55 | Improves blood flow towards neck and head region and provides relaxation. | 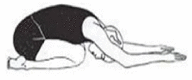 |
| 7. | Ardha-Matsendrasana (Half Fish Posture) | 4 min | 0.45 | A self-complementary deep twisting posture helps to activate thyroid gland, parathyroid gland & Adrenal gland for proper secretion of hormones T3, T4, Calcitonin and Cortisol. |  |
| PRONE POSTURE (8 MINUTES) | |||||
| 8. | Bhujangasana (Cobra Posture) | 2 min | 0.90 | Massages thyroid & adrenals glands and helps in overcoming lethargy as it has a direct effect on the solar plexus at the naval and on throat chakras |  |
| 9. | Dhanurasana (Bow Posture) | 2 min | 0.80 | Stretches thyroid gland and compels it to produce the required amount of thyroid hormone for regulating metabolism. |  |
| 10. | SEQUENTIAL (Parvatasana & Bhujangasana Stretch) | 2 min | 0.50 | Tones the spinal nerves and balances the nervous system balancing Central and Autonomic nervous system) |  |
| 11. | Makrasana (Crocodile Posture) | 2 min | 0.35 | Complete Relaxation in prone position | |
| SUPINE POSTURE (10 MINUTES) | |||||
| 12. | Vipareeta Karni Sarvangasana (Supported Inverted posture) | 2 min | 0.90 | By exerting pressure on the thyroid gland, it helps in improving blood circulation and nourishes the thyroid gland. | 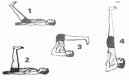 |
| 13. | Halasana (Plough Posture) | 2 min | 0.70 | By squeezing the thyroid gland, the stagnant secretion nourishes gets released. | 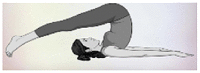 |
| 14. | SEQUENTIAL (Pashchimotanasana & Halasana) | 4 min | 0.50 | These supine postures provide great stretch and squeeze to thyroid and adrenal glands for proper secretion of thyroid hormone and cortisol to combat stress |  |
| 15. | Matsyasana (Fish Posture) | 2 min | 0.90 | By stretching the front of the neck fully and placing the crown on the floor, it activates pituitary and thyroid glands |  |
| SURYANAMASKAR (12 MINUTES) | |||||
| 16. | Surya Namaskar (Sun Salutation) | 11 min | 0.88 | A sequence of gracefully linked Asanas that are synchronized with breath enhances metabolic efficiency and enables overall health and wellbeing. |  |
| 17. | Shavasana (Corpse Position) | 1 min | 0.90 | A relaxation that harmonizes energies of the mind-body system. | 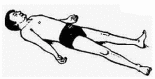 |
| MUDRAS-BANDAS- PRANAYAMA (12 MINUTES) | |||||
| 18. | Simha Mudra (Lion Gesture) | 2 min | 0.95 | Activates Vishuddhi Chakra and improves thyroid health |  |
| 19. | Jalandhara Banda (Throat Locking) | 2 min | 0.70 | It awakens the inner energy centres, especially the Vishuddhi chakra and regulates thyroid function |  |
| 20. | Ujjaii Pranayama (Victorious Breath) | 2 min | 0.80 | Activates thyroid gland and regulates secretion of Thyroid Stimulating Hormones | 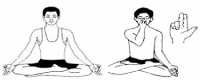 |
| 21. | Brahmri Pranayama (Bumble Bee Breath) | 2 min | 0.50 | Calms the mind and has significant benefits for the overall endocrine system. |  |
| 22. | Nadi Shodhana Pranayama (Balancing of Breath) | 2 min | 0.65 | Activates the Nadi system (psychic channels), brings better balance to the nervous system, and helps combat stress |  |
| 23. | A-Kara with Shanka Mudra (Conch Bell with A-kara) | 2 min | 0.45 | A-Kara sound from the throat by adopting shanka-mudra activates Vishuddhi chakra and helps to regularize thyroid hormone secretion |  |
| 24. | Shavasana (Corpse Position) | 2 min | 0.55 | Brings complete calmness and mind-body relaxation |  |
2.2. RCT protocol guidelines
This protocol study follows “SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials” [21] and CONSORT 2017 Statement [22] for Randomized Trials of Nonpharmacologic Treatments as well as its extension [23]. The Institutional ethics committee approves the study and is prospectively registered in the Clinical Trial Registry of India {CTRI/2022/03/041047}. This RCT protocol proposes to compare the effect of SYM administration for six months between yoga intervention group and waitlist control group. Data will be recorded at pre-{day 0 ± 7 days as time-point 1}, Interim-{day 90 ± 7 days as time-point 2}, and post-{day 180 ± 7 days as time-point 3} on primary outcome measure of SF-36 {HRQOL} along with secondary outcome measures that include thyroid profile {T3, T4, TSH}, BMI, BP, FAS, PSS, and GIP for both yoga intervention and waitlist control groups.
2.3. Study setting through tele-yoga
The study will be performed through a tele-mode of teaching and learning method. An Introductory session will be organized through a virtual platform to elaborate on the pathophysiology of the condition by an expert along with introducing a step-by-step computer application program for easy access to online mode. To date, there are no tele-yoga modes of studies conducted using a scientific yoga module specific to hypothyroidism. Tele-Yoga initiatives are not only beneficial for spreading yoga to a larger number of individuals in society but also one can practice at the convenience of their time and place. Not only it is cost-effective but also recorded videos may be useful as a motivation to practice yoga regularly.
2.4. Participants recruitment process
A total number of 120 individuals identified from the clinical and primary care laboratory database of SVYASA having biochemical features consistent with primary hypothyroidism will be recruited through invitation. Subjects clinically diagnosed with primary hypothyroidism with elevated TSH [24] will be eligible for participation in the study. The Trial flow chart plan as per Consolidated Standards of Reporting Trials {CONSORT} is presented in {Fig. 1}. The primary investigator will screen the eligibility of the potential participants who will be requested to fill out a screening and registration form. Eligible subjects providing consent for participation in the trial will be recruited as per the consolidated standard of reporting. The participants would never be asked to alter or cease their ongoing thyroid medication, however, in case of any changes in their medication regime, they will be instructed to notify the principal investigator of any such changes right away so that it may be taken into account when interpreting the final results.
i) Inclusion Criteria – (a) Age 18–60 years; (b) both genders; (c) with controlled co-morbidity (viz. Diabetes Mellitus, Hypertension, BMI≤40); (d) on a stable dose of levothyroxine for at least six months before enrolment into the trial; (e) people having not practiced yoga in the last 3 months; (f) people having access to the Internet; (g) people having a smartphone, tablet, laptop or television.
ii) Exclusion Criteria- (a) Thyroid Malignancy; (b) secondary hypothyroidism (c) people with other auto-immune disorders (viz. Lupus, multiple sclerosis, etc.); (d) people with a severe psychiatric disorder (schizophrenia, mania, dissociative disorder, etc.); (e) individuals on medications that could potentially impair thyroid function e.g. lithium, history of significant head or neck trauma/surgery/radiotherapy; (f) Pregnant women or those who plan to conceive within the trial period; (g) people having undergone recent surgery in the last 6 months; (h) subjects with visual and hearing disabilities; (i) those planning to move out of the region in which the trial is being conducted within the next 2 years; (j) recent hospitalization for major illness {within 4 weeks}.
Withdrawal criteria
Fig. 1.
CONSORT (consolidated standards of reporting trials). Flow chart plan.
Participants will be withdrawn from the study if - the attendance falls below 70% of total sessions {i.e., participating in fewer than 100 of the 144 scheduled treatment sessions}; participant's decision to withdraw from the study at any time for any reason; and incidence of any unexpected adverse event. They will also be withdrawn in case of development of overt biochemical hypothyroidism {TSH >20 mU/L and/or fT4 below the reference range}. Upon confirmation of the same, they will be referred to the general practitioner for usual care.
2.5. Sample size calculation
The estimation of sample size based on effect size, feasibility, precision about the mean, and variance using G-Power software [25] was computed from the previous study [26]. With an effect size of 0.8 and significance fixed at 0.05, the power of the study at 0.95, and a 25% dropout rate, a total sample size of 120 subjects {Yoga = 60; Control = 60} was computed for the current research.
2.6. Randomization and allocation concealment
A total of 120 hypothyroid patients will be randomized into two groups with an allocation ratio of 1:1 by an independent statistician, who would have no role in the current research, through a computerized random number sequence method using www.randomizer.org [27]. An opaque sealed envelope with a sequential number for each participant will be preserved by an individual who will not be a part of the study in order to maintain privacy. After receiving consent from the eligible participants, the clinical researcher will receive an envelope containing sequence numbers for group allocation from the independent researcher. Given the nature of the intervention, blinding the subjects is not possible in this trial, though, two data researchers in charge of the collection, preservation, and analysis of data will be blinded. The experimental group will receive a 60-min yoga module intervention through tele-yoga for 6 days a week, whereas the waitlist control group will continue with their daily routine activity. Post completion of the trial, the waitlist control group will also be offered the module practice via tele-mode.
2.7. Yoga intervention group
The yoga intervention group will receive training for the practice of SYM via tele-mode by a trained and certified yoga therapist with over five years of experience. The Microsoft Office 365 application “Microsoft Teams”, which is a widely used program for online yoga sessions will be used for conducting the tele-yoga RCT. Recorded video of the complete yoga module and picture-based practices will also be given to all the participants after a minimum of 7 online supervised sessions as per AYUSH 2020 guidelines, to compensate for missed sessions due to poor internet connectivity or other personal reasons. Additionally, for educational purposes, the participants will be given a document through mail explaining the disease, its causes, diagnosis, prognosis, and details of the yoga module, and its health benefits. A yoga diary is to be maintained by the patients to record the number of times SYM is practiced watching the recorded video to compensate for missed sessions due to poor internet connectivity or other personal reasons and will be supervised regularly. Further, Yoga Performance Assessment Scale (YPA) will be used to measure the overall yoga performance of each yoga practice on a scale of 0–4 (0 cannot practice at all and 4 can practice with ease) [28]. The attendance sheet will be downloaded from the computer application log along with monitoring the dropouts during the course of the trial. Further, a separate attendance sheet will be maintained for those who practiced using recorded video.
2.8. Control waitlist group
Participants in the control waitlist group will be under observation and will be asked not to initiate any yoga practice regimen during the trial period. They will be advised to follow their regular routine and report any drastic change in their symptoms like weight gain, depression episodes, etc., on a fortnightly basis. Data will be collected at three-time points for both primary and secondary measures. Post-completion of the trial, they will be offered SYM practices.
2.9. Safety of the intervention
The module was developed with moderate intensity and comprises of a combination of yoga postures, breathing practices, meditation, and relaxation techniques keeping in mind the needs of the hypothyroid subjects and the tele-yoga mode for imparting and learning of the practices that will be well set out to avoid any stress or injury to the patients. An Introductory session detailing the SYM practices will be organized along with elaborating on the pathophysiology of the condition by the experts at SVYASA, Bengaluru.
Additionally, a one-week (6 sessions) Introductory program will be organized to -
-
(1)
Acquaint the subjects with the therapists for mutual understanding for imparting and learning through virtual mode;
-
(2)
To familiarize the subjects with the Scientific Yoga Module practices with step-by-step guidance;
-
(3)
To handhold the patients to use “Microsoft Team Application online mode” for simple and easy access.
As per tele-yoga guidelines, there will be therapists supervising the sessions in a ratio of 1:5 (i.e., 1 therapist for every 5 subjects for monitoring the correctness of the practices). All participants will be asked to fill yoga intervention satisfactory questionnaire relating to their satisfaction with the practices and any adverse events encountered during the intervention period. As this is a first-of-its-kind tele-yoga trial for hypothyroidism, the yoga therapists will be trained repeatedly to ensure the standard, effectiveness, and safe operation through online mode.
2.10. Adverse events
Any adverse event that occurs during the online yoga sessions will be recorded and in case of any serious adverse event, the clinical trial shall be interrupted immediately and effective treatment measures shall be taken.
2.11. Outcome measures
2.11.1. Primary
-
(i)
SF 36 {HRQOL}: HRQOL with 36 item questions covers the physical, psychological, emotional, and social aspects of an individual, and is a validated measure with high test-retest reliability and Cronbach alpha coefficients [29].
2.11.2. Secondary
-
(i)
Blood sample collection: Blood samples will be collected to determine the amount of hormones produced by thyroid gland as per standardized tests for T3, T4, and TSH levels [30].
-
(ii)
Body Mass Index {BMI}: Obesity measurement is a vital component to investigate whether there is an association between TSH within the normal range and BMI [31].
-
(iii)
Blood Pressure {BP}: To access the correlation between BP and TSH [32].
-
(iv)
Fatigue Assessment Scale {FAS}: To understand the fatigue levels and represents a potentially valuable assessment instrument with promising internal consistency reliability and validity [33].
-
(v)
Perceived Stress Scale {PSS}: A classic stress assessment instrument that helps to understand how different situations affect an individual's feelings and perceived stress [34].
-
(vi)
Gita Inventory of Personality {GIP}: A 10-item validated questionnaire that would assist in ascertaining the prominent personality, that explains Sattva as tranquillity or poise, Rajas as aggression or passion, and Tamas as Ignorance or laziness as per tri-guna theory [35].
2.12. Data collection process
A Google form for all the clinical scales that include HRQOL, FAS, PSS, and GIP will be shared online and participants will be guided through the process of filling out the questionnaire along with signing the informed consent form. The blood samples along with measurement of height, weight, and blood pressure will be collected Offline from the homes of the patients by a reputed lab using standard instruments. The data analysts will receive the results of the questionnaire and blood test for safekeeping till the end of the study for maintaining privacy and further conduct analysis once the study concludes.
2.13. Statistical analysis
Data of this clinical trial will be analyzed using SPSS Version {IBM, Chicago Inc, Version 21} along with checking the normality of the data using Shapiro Wilk Test through estimation using the skewness statistics and normal probability plot. Based on the normality will select the appropriate parametric or non-parametric test. Independent sample t-test or Wilcoxon rank-sum test would be used for comparison between groups. Descriptive statistics will be used to outline the socio-demographic details {age, gender, medical history, dosage of drugs, morbidity, and health status} of the participants at the baseline. While interpreting the results, the multivariate analysis will be conducted to control the confounding factor viz change or cease of ongoing thyroid medication. The primary outcome for changes in SF-36 scores will be analyzed using the mixed model for repeated measures (MMRM}. In two groups, all the statistical tests will be performed with bilateral tests, and P < 0.05 indicates the difference is considered statistically significant. The intention-to-treat principle will be applied in all the analyses.
3. Conclusion
To the best of our knowledge, this prospective study is the first attempt to test the effectiveness of SYM intervention through an online platform to help promote the well-being of patients with hypothyroidism. The findings of this study would aid in establishing the beneficial aspects of yoga-based intervention for hypothyroidism treatment along with the standard of care. This study would also provide an indication of the feasibility of tele-yoga, in the management of hypothyroidism, particularly for quality-of-life outcomes. Telehealth has definitely emerged as a potential tool for transforming mental and physical health care in the era of the COVID-19 pandemic. This becomes more pertinent with respect to a reduction in in-person clinic visits hence, a telehealth-based approach would aid in tackling the commonly noted barriers to participation in center-based programs such as adverse weather events, and facilitate the integration of holistic interventions into plans of care for hypothyroidism.
The findings of this study would be particularly important given the long-term induced side effects of treatment such as acute myocardial infarction, angina pectoris, change in thyroid hormone requirements, or hypo responsiveness to hormonal therapy. In this regard, yoga intervention could be effective in dose reductions in levothyroxine administration to maintain euthyroidism and thereby aid in reducing drug-related side effects as well. Further, a recent yoga study on obesity and metabolic comorbidities indicates that a specific yoga module intervention has more chances of improving metabolic functions as compared to regular yoga practices [36].
This RCT protocol paper provides healthcare practitioners with information that could be beneficial using mind-body practices as an adjunct therapy and may be promising for both prevention and treatment of hypothyroidism. Based on the evidence from this study we aim to establish that using a Scientific Yoga Module is a simple and effective adjunct treatment for hypothyroid patients. This tele-yoga program will set an example to bring together the general population from all over India and other nations suffering from hypothyroidism as a convenient and low-cost self-help tool for long-term practices to lead a healthy lifestyle.
Author credit statement
Savithri Nilkantham: Conceptualization, design of the study, writing original draft, editing and finalising the draft, Vijaya Majumdar: Writing original draft, reviewing, editing and finalising the draft, Amit Singh: Conceptualization, design of the study, reviewing the draft, All authors agree to be accountable for all aspects of the work and gave final approval for the version to be published.
Declaration of competing interest
The authors of the original manuscript titled “Integrated Yoga Module for Hypothyroidism: A Study Protocol for Tele-yoga RCT” which is being submitted in the journal “Contemporary Clinical Trials Communications” hereby declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The study is approved by Institutional Ethics Committee SVYASA (RES/IEC-SVYASA/222/2022) and is prospectively registered in the Clinical Trial Registry of India (CTRI/2022/03/041047).
Contributor Information
Savithri Nilkantham, Email: savithri.nilkantham@gmail.com.
Vijaya Majumdar, Email: vijaya.majumdar@svyasa.edu.in.
Amit Singh, Email: dramits90@gmail.com.
References
- 1.Chiovato L., Magri F., Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv. Ther. 2019;36:47–58. doi: 10.1007/s12325-019-01080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giorda C.B., Carna’ P., Romeo F., Costa G., Tartaglino B., Gnavi R. Prevalence, incidence, and associated co-morbidities of treated hypothyroidism. An update from a European population. Eur. Soc. Endocrinol. 2017;175:533–542. doi: 10.1530/EJE-16-0559. [DOI] [PubMed] [Google Scholar]
- 3.Taylor Peter N., et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018;14(5):301–316. doi: 10.1038/nrendo.2018.18. [DOI] [PubMed] [Google Scholar]
- 4.Hegedüs L., Bianco A.C., Jonklaas J., Pearce S.H., Weetman A.P., Perros P. Primary hypothyroidism and quality of life. Nat. Rev. Endocrinol. 2022;18(4):230–242. doi: 10.1038/s41574-021-00625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiersinga W.M. Therapy of endocrine disease: T4 + T3 combination therapy: is there a true effect? Eur. J. Endocrinol. 2017;177(6):R287–R296. doi: 10.1530/EJE-17-0645. [DOI] [PubMed] [Google Scholar]
- 6.Jack Baskin, et al. Am. Assoc. Clin. Endocrinol. Med. Guidelines Clin. Pract. Eval. Treatment of Hyperthyroidism and Hypothyroidism. 2006;8(6):457–469. [Google Scholar]
- 7.al Quran T., et al. Quality of life among patients on levothyroxine: a cross-sectional study. Ann. Med. Surg. Dec. 2020;60:182–187. doi: 10.1016/j.amsu.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterfield N., Schultz T., Rasmussen P., Proeve M. Yoga and mindfulness for anxiety and depression and the role of mental health professionals: a literature review. J. Ment. Health Train Educ. Pract. 2017;12(1):44–54. doi: 10.1108/JMHTEP-01-2016-0002. [DOI] [Google Scholar]
- 9.Riley K.E., Park C.L. How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry. Health Psychol. Rev. 2015;9(3):379–396. doi: 10.1080/17437199.2014.981778. [DOI] [PubMed] [Google Scholar]
- 10.da Silva T.L., Ravindran L.N., Ravindran A.V. Yoga in the treatment of mood and anxiety disorders: a review. Asian J. Psychiatr. 2009;2(1):6–16. doi: 10.1016/j.ajp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Cremaschi G.A., Gorelik G., Klecha A.J., Lysionek A.E., Genaro A.M. Chronic stress influences the immune system through the thyroid axis. Life Sci. 2000;67(26):3171–3179. doi: 10.1016/S0024-3205(00)00909-7. [DOI] [PubMed] [Google Scholar]
- 12.Gura S.T. Yoga for stress reduction and injury prevention at work. Work. 2002;19(1):3–7. [PubMed] [Google Scholar]
- 13.Nilakanthan S., Metri K., Raghuram N., Hongasandra N. Effect of 6 months intense Yoga practice on lipid profile, thyroxine medication and serum TSH level in women suffering from hypothyroidism: a pilot study. J. Compl. Integr. Med. Jan. 2016;13(2) doi: 10.1515/jcim-2014-0079. [DOI] [PubMed] [Google Scholar]
- 14.Helmreich D.L., Tylee D. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm. Behav. Aug. 2011;60(3):284–291. doi: 10.1016/j.yhbeh.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonklaas J., et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. Dec. 2014;24(12):1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AYUSH, “Advisory on Tele-Yoga Services . July 2020. Central Council for Research in Yoga & Naturopathy (CCRYN) [Google Scholar]
- 17.Jagannathan A., Bhide S., Varambally S., Chandra P., Gangadhar B. Tele-yoga therapy for common mental health disorders: need for assessment tool and guidelines. Int. J. Yoga. 2021;14(1):83. doi: 10.4103/ijoy.ijoy_99_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawshe C.H. A quantitative approach to content validity. Person. Psychol. 1975;28(4):563–575. doi: 10.1111/j.1744-6570.1975.tb01393.x. [DOI] [Google Scholar]
- 19.Bhargav H., et al. Development, validation, and feasibility testing of a yoga module for opioid use disorder. Adv. Mind Body Med. 2021;35(3):20–30. [PubMed] [Google Scholar]
- 20.Singh J., et al. Designing, validation, and the feasibility of a yoga module for patients with ankylosing spondylitis. J. Ayurveda Integr. Med. Jan. 2022;13(1) doi: 10.1016/j.jaim.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan A.-W., et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. Jan. 2013;158(3) doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutron I., Altman D.G., Moher D., Schulz K.F. 2017. RESEARCH and REPORTING METHODS CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments : A 2017 Update and a CONSORT Extension for. [DOI] [PubMed] [Google Scholar]
- 23.Arunachalam M.A., Halwai A., Arunachalam C. Indian J Med Ethics; 2021. National Guidelines for Ethics Committees Reviewing Biomedical & Health Research during COVID-19 Pandemic: an Analysis; pp. 1–12. VI, no. 1. [DOI] [PubMed] [Google Scholar]
- 24.Jadav P.M., Pradhan R. Establishment of reference range for thyroid hormones (T3, T4 & TSH) in adult female population at clinical biochemistry laboratory of GCS medical college & hospital- A descriptive study. Int. J. Clin. Biochem. Res. 2020;5(3):449–452. doi: 10.18231/2394-6377.2018.0095. [DOI] [Google Scholar]
- 25.Kang H. Sample size determination and power analysis using the G*Power software. J Educ Eval Health Prof. 2021;18:1–12. doi: 10.3352/JEEHP.2021.18.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer H., Thoms M.S., Anheyer D., Lauche R., Dobos G. Yoga in women with abdominal obesity - a randomized controlled trial. Dtsch Arztebl Int. 2016;113(39):645–652. doi: 10.3238/arztebl.2016.0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doig, Gordon S., Simpson, Fiona “Randomization and allocation concealment: a practical guide for researchers” J. Crit. Care, vol.20, no.2, pp. 187-191, doi: 10.1016/j.jcrc.2005.04.005. [DOI] [PubMed]
- 28.Bhat S., Varambally S., Karmani S., Govindaraj R., Gangadhar B.N. Designing and validation of a yoga-based intervention for obsessive compulsive disorder. Int. Rev. Psychiatr. May 03, 2016;28(3):327–333. doi: 10.3109/09540261.2016.1170001. Taylor and Francis Ltd. [DOI] [PubMed] [Google Scholar]
- 29.Garratt A.M., Ruta D.A., Abdalla M.I., Buckingham J.K., Russell I.T. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? Br. Med. J. 1993;306(6890):1440–1444. doi: 10.1136/bmj.306.6890.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu Y., Kawashiri S.Y., Noguchi Y., Nagata Y., Maeda T., Hayashida N. Normal range of anti-thyroid peroxidase antibody (TPO-Ab) and atherosclerosis among eu-thyroid population: a cross-sectional study. Medicine. 2020;99(38) doi: 10.1097/MD.0000000000022214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyrnes A., Jorde R., Sundsfjord J. Serum TSH is positively associated with BMI. Int. J. Obes. 2006;30(1):100–105. doi: 10.1038/sj.ijo.0803112. [DOI] [PubMed] [Google Scholar]
- 32.Åsvold B.O., Bjøro T., Nilsen T.I.L., Vatten L.J. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J. Clin. Endocrinol. Metab. 2007;92(3):841–845. doi: 10.1210/jc.2006-2208. [DOI] [PubMed] [Google Scholar]
- 33.Michielsen H.J., De Vries J., Van Heck G.L. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J. Psychosom. Res. 2003;54(4):345–352. doi: 10.1016/S0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S. Perceived stress scale (PSS) The Soc. Psychol. Health. 1988;24:386–396. doi: 10.1007/978-3-030-39903-0_773. [DOI] [Google Scholar]
- 35.Das R.C. Standardization of the Gita inventory of personality. J. Indian Psychol. 1991;9(1–2):47–54. [Google Scholar]
- 36.Chobe M.P., Nanjundaiah R.M., Chobe S. Designing and validation of a yoga-based module for obesity with metabolic comorbidities. J. Compl. Integr. Med. Mar. 2021;18(1):159–163. doi: 10.1515/jcim-2019-0249. [DOI] [PubMed] [Google Scholar]