Abstract
A novel PANI@CS solid-phase dispersive extractant combined with ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) was developed for the first time, which was used for high-throughput, multi-component, real-time online rapid pretreatment and quantitative classification of 16 mycotoxins from five different medicinal parts of 13 genuine traditional Chinese medicines (TCMs). Ultra performance liquid chromatography combined with triple quadrupole mass spectrometry was used for separation and ESI detection. An internal standard isotope matching calibration was used for quantification purposes to compensate for matrix effects. The limits of detection (LOD) of 16 mycotoxins ranged from 0.1 to 6.0 μg/kg. The linear coefficients (R2) were ≥0.996 in the linear range from 10.0 to 200 μg/L. The recoveries of the 16 mycotoxins ranged from 90.1% to 105.8%, and the relative standard deviations (RSDs) ranged from 1.3% to 4.1%. Thirteen TCMs from five representative medicinal parts were selected and tested under the best sample preparation procedure and chromatographic analysis conditions. The results showed that the method could improve the sensitivity and accuracy of the sample analysis, improve the selectivity and reproducibility of the decolorization and purification of TCMs, which is suitable for the practical application of mycotoxin in trace analysis. This method can also provide a new idea for accurate, efficient, rapid and multi-component online detection of mycotoxins for quality and safety control of TCMs.
Keywords: Genuine traditional Chinese medicine, Mycotoxins, PANI@CS extraction, Medicinal parts, UPLC-MS/MS
Graphical abstract
1. Introduction
Traditional Chinese medicines (TCMs) resources are extremely rich in China, the TCMs culture has a long history, and the curative effect of TCMs is particularly unique. TCMs and its preparations are widely used to treat a variety of diseases [1,2]. High-quality genuine TCMs is a valuable heritage verified and recognized by production and clinical practice of traditional and modern Chinese medicine. Many achievements have been made in the development of TCMs, new drugs and resources, as well as the evaluation and safety of modern quality. It is also a hot spot in the quality, health and safety of modern TCMs [3,4]. According to the statistics of the World Health Organization (WHO), about 4 billion people in the world at present are using TCMs to prevent and treat diseases, and the “TCM outpatient fever” is in the ascendant at home and abroad. But so far, the export trade of TCMs has not occupied the leading position in the international pharmaceutical market. As TCMs have entered the international market, more attention is paid internationally to the production process, quality standards, safety performance and other factors. Especially in recent years, the existence of exogenous harmful substances such as foodborne mycotoxins (FMs) [5,6], which affect the safety of TCMs, have caused international doubts about the safety, effectiveness and quality control of TCMs. Mycotoxins are secondary metabolites produced by fungal organisms [7,8], they have a wide range of sources and have three toxic effects. The WTO designates aflatoxin B1 (AFB1) as a class I carcinogen [[9], [10], [11], [12], [13]]. The European Union in the Commission Regulation (EC) No. 1881/2006 has established the maximum residue limits (MRLs) of four AFs (AFB1, AFG1, AFB2 and AFG2) for 4 μg/kg. The quality detection and control methods of TCMs are also constantly updated in China. The general principles of the fourth part of the newly revised Pharmacopoeia of the people's Republic of China (2020 Edition) contain and establish the detection methods and general technologies of eleven common mycotoxins, mainly aflatoxins. The general rule requires that the common determination method of common mycotoxins is ultra high performance liquid chromatography tandem mass spectrometry [14,15]. However, in the production, harvest, storage, treatment and processing of TCMs, due to the limited external environment and production process, it is inevitable to introduce exogenous mycotoxins into the treatment process [16].
The quality standards and safety risks of TCMs must comply with international norms whenever go abroad. The commonly used pretreatment methods in the laboratory are organic solvent microwave ultrasonic extraction [[17], [18], [19]], solid-phase extraction [20,21], and dispersed solid-phase extraction [[22], [23], [24]]. The commercial solid phase extraction column is expensive, and the detection target is specific and relatively single. Although the simple solid-phase extraction pretreatment method saves time and effort, it is urgent to develop new composite materials and other methods that are generally suitable for the quantitative detection of mycotoxins in grass-roots inspection and detection institutions [25,26]. Carbon sphere (CS) is a good medium carrier of the carbon material family [27], which is suitable for the polymerization reaction medium further coating nanoparticle materials, and can not only be used to prepare porous core-shell structure composites [28], but can also be used as a template for molecular imprinting adsorption [29,30]. Modified polyaniline (PANI) material surface contains a large number of oxygen-containing groups, which is widely used in the adsorption of pigments, organic substances and other chemical impurities [[31], [32], [33], [34]].
In this study, a novel PANI@CS solid-phase dispersion extractant was established, which can be used for the pretreatment of mycotoxin samples in five medicinal parts of 13 genuine TCMs. The method has a low detection limit and high recovery. It can be applied to the detection of mycotoxins in TCMs with high universality and low matrix effect, which can provide a new idea for the accuracy, efficient, rapid and multi-component online determination of the quality and safety hazard factors of TCMs.
2. Materials and methods
2.1. Chemicals and reagents
Sixteen mycotoxin standards were purchased from Romer Labs (purity ≥98%). The components were zearalenone (ZEN, 100 μg/mL), nivalenol (NIV, 100 μg/mL), deoxynivalenol (DON, 100 μg/mL), 3-acetyl deoxynivalenol (3-Ac-DON, 100 μg/mL), 15-acetyl deoxynivalenol (15-Ac-DON, 100 μg/mL), ochratoxin A (OTA, 100 μg/mL), T-2 toxin (T-2, 100 μg/mL), HT-2 toxin (HT-2, 100 μg/mL), fumonisin B1 (FB1, 50 μg/mL), fumonisin B2 (FB2, 50 μg/mL), fumonisin B3 (FB3, 50 μg/mL), aflatoxin B1 (AFB1, 5.0 μg/mL), aflatoxin B2 (AFB2, 5.0 μg/mL), aflatoxin G1 (AFG1, 5.0 μg/mL), aflatoxin G2 (AFG2, 5.0 μg/mL), and sterigmatocystin (ST, 10 μg/mL). The molecular structures of 16 mycotoxins are shown in Fig. 1. Seven mycotoxin isotope internal standards (ISs) were purchased from Romer Labs (purity ≥98%), The components were 13C15–NIV (25 μg/mL), 13C15-DON (25 μg/mL), 13C18-ZEN (25 μg/mL), 13C34-FB1 (25 μg/mL), 13C24-T-2 (25 μg/mL), 13C22-HT-2 (25 μg/mL), 13C20-OTA (10 μg/mL), isotope internal standards were used as internal standards.
Fig. 1.
Molecular structural formula of 16 mycotoxins.
Acetonitrile, acetic acid, citric acid, and ammonium acetate were purchased from Merck Reagents (Darmstadt, Germany). Sodium chloride, sodium sulfate, ammonium sulfate, sodium citrate, styrene, ammonium persulfate, aniline, perchloric acid, phosphoric acid, ammonia and N,N-dimethylformamide were purchased from Sinopharm Chemical Reagent (Shanghai, China). Acetonitrile and acetic acid are HPLC grade, and the other solvents and chemicals are analytical grade. The solid reagents were dried at 500 °C for 4 h before use. Ultrapure water was produced using the Milli-Q™ Advantage A10 purification water system (Madrid, Spain).
2.2. Instruments and equipments
API 3200 triple quadrupole Ultra Performance liquid chromatography-tandem mass spectrometer was purchased from AB SCIEX with an electrospray ionization source. UFLCXR ultra-fast liquid chromatograph was purchased from Shimadzu Company, Japan. Vacuum dryer was purchased from Shanghai Jinghong Instrument Co., Ltd. (DHG-9147A). High speed crusher was purchased from Tianjin Taist Instrument Co., Ltd. (FW100). High-speed centrifuges were purchased from Thermo Field (X1R). Thermostatic ultrasound instrument was purchased from Kunshan, Shanghai (KS501). Vortex mixer was purchased from iKa (Germany). High speed pulverizer was purchased from RETSCH (GM200), Germany. High-resolution scanning electron microscopy was purchased from Hitachi (S-4800).
2.3. UPLC conditions
Electron spray ionization (ESI): positive ion mode (ESI+): column temperature was 30–40 °C, temperature of the sample plate was 15 °C, mobile phase was 0.1% formic acid (A) -acetonitrile (B), flow rate was 0.4 mL/min, injection volume was 10 μL. Gradient elution was used for the separation of the target mycotoxins. The gradient elution procedure of ESI+ positive ion mode was as follows: mobile phase A/B (0.1% formic acid/acetonitrile), 0–0.8 min: A/B = 90/10%; 0.8–2.5 min: A/B = 72%/28%; 2.5–11.5 min: A/B = 32%/68%; 11.5–13.0 min: A/B = 0%/100%; 13.0–15.0 min: A/B = 90/10%. 11 kinds of components were measured.
Negative ion mode (ESI−): column temperature was 30–40 °C, temperature of the injection plate was 15 °C, mobile phase was 0.1% ammonia (A) -acetonitrile (B), flow rate was 0.4 mL/min, injection volume was 10 μL. Liquid chromatography was performed by gradient elution injection. Gradient elution procedure was as follows: mobile phase A/B (0.1% ammonia/acetonitrile), 0–0.5 min: A/B = 95%/5%; 0.5–1.0 min: A/B = 80%/20%; 1.0–3.0 min: A/B = 30%/70%; 3.0–4.0 min: A/B = 5%/95%; 4.0–6.0 min: A/B = 95%/5%. 5 kinds of components were measured.
The ion source mode (ESI+/ESI−) was monitored by liquid chromatograph mass spectrometer (LC-MS) with an Agilent Poroshell 120 EC C18 column (3.0 mm × 100 mm, 2.7 μm). Before batch analysis of the sample sets, three shots of the blank solvent (acetonitrile) were injected to ensure that the system was free of contaminants or interference peaks.
2.4. Mass spectrometry
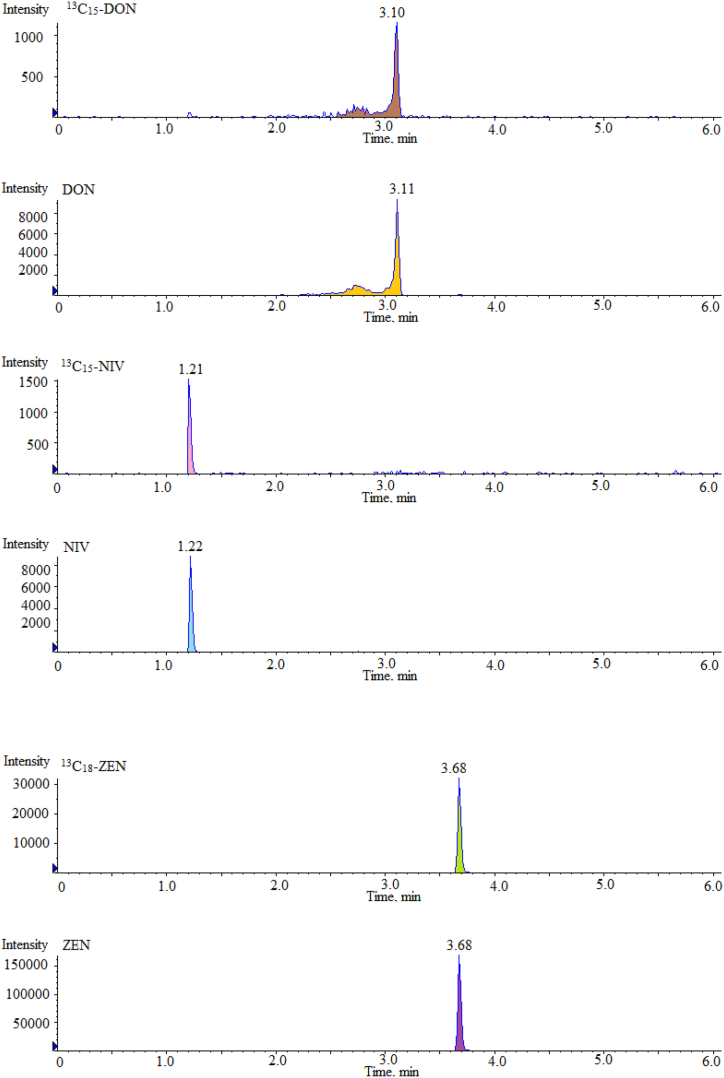
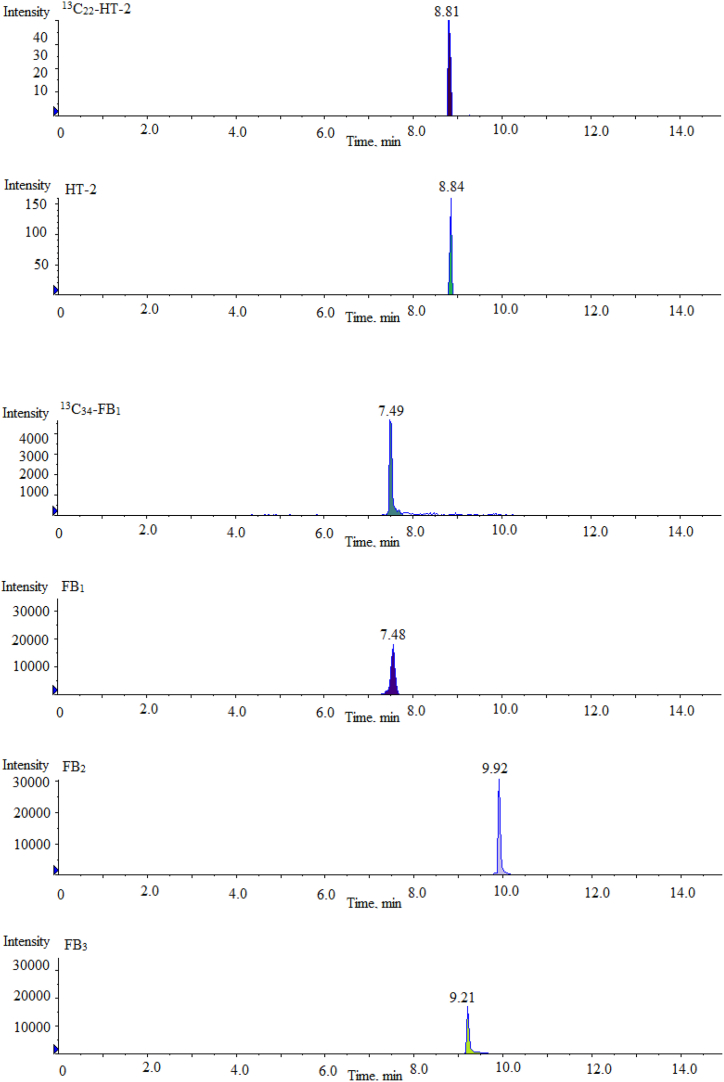
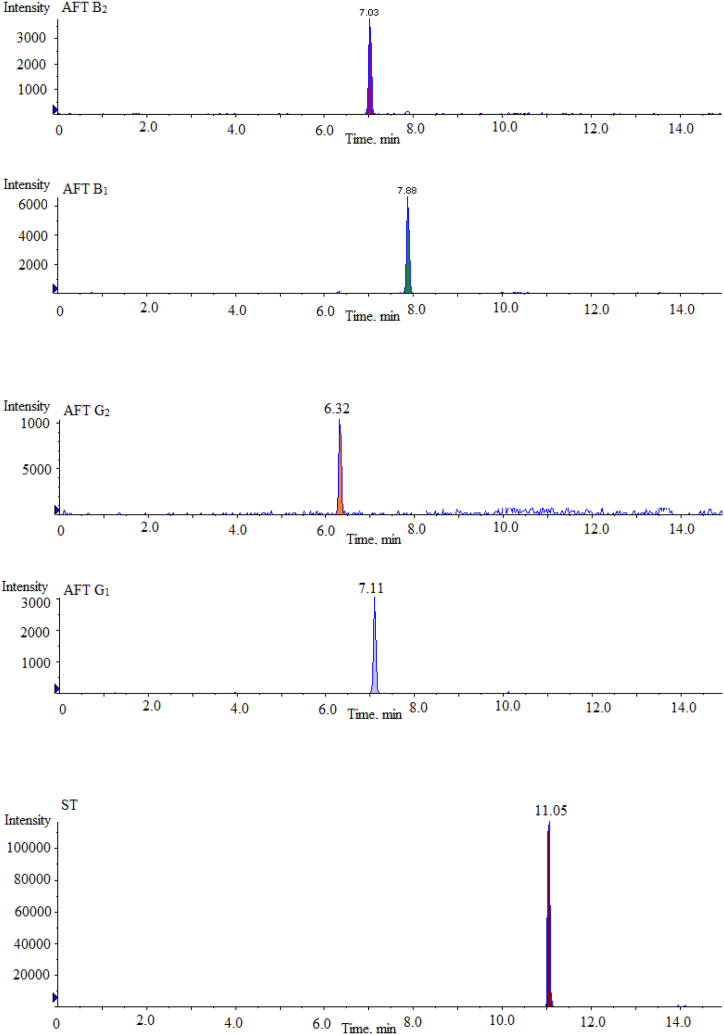
Ion source was electrospray ion source, mass spectrometry scanning mode was multiple reaction monitoring mode (MRM), cone hole voltage was 3.0 kV, heating temperature was 500 °C, ion source temperature was 150 °C, desolvent gas was 800 L/H. The MRM chromatograms of 16 mycotoxin mixed standards and their ISs under different ion source modes are shown in Fig. 2. The mass spectrometry conditions of 16 mycotoxins and their ISs in ESI+ and ESI− modes are shown in Table 1.
Fig. 2.
Multi reaction monitoring chromatograms of 16 mycotoxin mixed standards and their isotope internal standards under different ion source modes.
Table 1.
Mass spectrometry parameters of 16 mycotoxins and their isotope internal standards (ISs) in positive and negative ionization modes.
| Mycotoxin | Full Name | Abbreviationa | Ionization Mode | Time (min) | precursor ion (m/z) | product ion (m/z) | Collision energy (V) |
|---|---|---|---|---|---|---|---|
| 1 | Ochratoxin A | OTA | ESI + | 10.67 | 404.0 | 239.0\358.1 | −36\-37 |
| 2 | T-2 toxin | T-2 | ESI + | 10.43 | 484.2 | 305.2\185.2 | −32\-30 |
| 3 | HT-2 toxin | HT-2 | ESI + | 8.84 | 425.3 | 263.2\245.4 | −36\-35 |
| 4 | Fumonisin B1 | FB1 | ESI + | 7.48 | 722.4 | 334.3\352.3 | −37\-38 |
| 5 | Fumonisin B2 | FB2 | ESI + | 9.92 | 706.3 | 336.4\318.2 | −36\-38 |
| 6 | Fumonisin B3 | FB3 | ESI + | 9.21 | 706.2 | 336.3\354.4 | −35\-37 |
| 7 | Aflatoxin B1 | AFB1 | ESI + | 7.88 | 313.1 | 279.1\285.1 | −37\-36 |
| 8 | Aflatoxin B2 | AFB2 | ESI + | 7.03 | 313.1 | 231.3\241.0 | −38\-40 |
| 9 | Aflatoxin G1 | AFG1 | ESI + | 7.11 | 315.1 | 263.1\287.1 | −37\-36 |
| 10 | Aflatoxin G2 | AFG2 | ESI + | 6.32 | 315.1 | 233.2\243.1 | −39\-37 |
| 11 | Sterigmatocystin | ST | ESI + | 11.05 | 325.1 | 281.0\310.1 | −28\-35 |
| 12 | 13C20H18ClNO6 (ISs) | 13C20-OTA | ESI + | 10.66 | 424.1 | 250.1 | −26\-37 |
| 13 | 13C24H34O9 (ISs) | 13C24-T-2 | ESI + | 10.43 | 508.3 | 322.0 | −33\-30 |
| 14 | 13C22H32O8 (ISs) | 13C22-HT-2 | ESI + | 8.81 | 464.0 | 278.3 | −32\-35 |
| 15 | 13C34H59NO15 (ISs) | 13C34-FB1 | ESI + | 7.49 | 756.4 | 356.2 | −29\-38 |
| 16 | Zearalenone | ZEN | ESI - | 3.68 | 317.1 | 130.9\174.9 | 26\31 |
| 17 | Nivalenol | NIV | ESI - | 1.22 | 311.1 | 281.3\191.2 | 32\28 |
| 18 | Deoxynivalenol | DON | ESI - | 3.11 | 295.1 | 265.0\138.0 | 21\27 |
| 19 | 3-acetyl deoxynivalenol | 3-Ac-DON | ESI - | 3.74 | 337.3 | 173.1\307.1 | 30\26 |
| 20 | 15-acetyl deoxynivalenol | 15-Ac-DON | ESI - | 3.68 | 337.1 | 150.0\189.0 | 31\37 |
| 21 | 13C15H20O7 (ISs) | 13C15–NIV | ESI - | 1.21 | 326.3 | 295.3 | 30\28 |
| 22 | 13C15H20O6 (ISs) | 13C15-DON | ESI - | 3.10 | 310.1 | 279.1 | 22\27 |
| 23 | 13C18H22O5 (ISs) | 13C18-ZEN | ESI - | 3.68 | 335.2 | 321.3 | 27\31 |
aAbbreviations: OTA: Ochratoxin A; T-2: T-2 toxin; HT-2: HT-2 toxin; FB1: Fumonisin B1; FB2: Fumonisin B2; FB3: Fumonisin B3; AFB1: Aflatoxin B1; AFB2: Aflatoxin B2; AFG1: Aflatoxin G1; AFG2: Aflatoxin G2; ST: Sterigmatocystin; ZEN: Zearalenone; NIV: Nivalenol; DON: Deoxynivalenol; 3-Ac-DON: 3-acetyl deoxynivalenol; 15-Ac-DON: 15-acetyl deoxynivalenol.
2.5. Preparation of standard solutions
ESI− mixed standard stock solutions (0.5 μg/mL): 25 μL of DON, ZEN, NIV, 3-Ac-DON and 15-Ac-DON were added into acetonitrile and fixed to 5 mL. ESI+ mixed standard stock solutions (0.5 μg/mL): 25 μL of T-2, HT-2, 50 μL of FB1, FB2, FB3, 25 μL of OTA, 250 μL of ST, 500 μL of AFB1, AFB2, AFG1, and AFG2 were added into acetonitrile and fixed to 5 mL. The resulting solutions were stored at −20 °C in the dark. Acetonitrile was used as the dilution solvent, and the concentration range of 10.0–200 μg/L working solutions were prepared by step-by-step dilution of 0.5 μg/mL mixed standard stock solution.
Mixed internal standard solution (0.5 μg/mL): 200 μL of 13C15–NIV, 13C15-DON, 13C18-ZEN, 13C34-FB1, 13C24-T-2, 13C22-HT-2 and 500 μL of 13C20-OTA were added into acetonitrile and fixed to 10 mL. The resulting solutions were stored at −20 °C in the dark.
Blank matrix matching working solution: TCMs without target components were selected as the matrix in the experiment, blank matrix solution was prepared according to the sample pretreatment method, mixed standard solution was added into the matrix of TCMs, and the matrix effect experiment was carried out.
2.6. Samples selection
13 kinds of medicinal materials and their classification are: Roots and rhizomes (n = 5): Ophiopogon japonicus (Sichuan medicine), Paris polyphylla (Yunyao), Hangzhou White Peony (Zhejiang medicine), Licorice (Guanyao), Angelica (northwest medicine). Fruits and seeds (n = 3): Gallnut (Guiyao), Coix seed (Northern medicine), Medlar (South China medicine). Flowers (n = 2): Saffron (Tibetan medicine), Honeysuckle (Huaiyao). Skins (n = 2): Tangerine peel (Guangyao), Eucommia ulmoides (Guiyao). Bacteria and algae (n = 1): Poria Cocos (Yunyao).
2.7. Sample pretreatment
2.7.1. Purification materials
Synthesis of carbon spheres: 4–8 g glucose was dissolved in 50 mL water and placed in a 100 mL reaction kettle under temperature control at 160–180 °C for 5–8 h, then cooled to 80 °C for 3 h. The black liquid was repeatedly centrifuged and washed with water and ethanol, and centrifuged at 3500 rpm for 10 min.
Modified carbon spheres: 2–5 g carbon spheres were placed in 50 mL of 5% acrylic acid, dispersed by ultrasound for 10 min, bathed in water at 60 °C for 2 h, and dried at room temperature for 5 h.
Polyaniline@Carbon spheres: 5 g modified carbon spheres and 25 mL 1.0 mol/L perchloric acid were added to a 250 mL flask and dispersed evenly by low temperature ultrasound. 1.0 mol/L purified aniline was added and stirred in an ice bath at 400 rpm for 20 min. 2.0 mol/L ammonium persulfate initiator was added drop by drop, and the stirring was continued in the ice bath for 12 h. After the reaction was completed, the products were washed by repeated centrifugation with absolute ethanol and water successively, and centrifuged at 5000 rpm for 10 min. The product was oven-dried at 80 °C for 12 h.
2.7.2. Sample extraction
Samples were collected and dried at about 200 g for 4–6 h using a drying oven. After drying, the samples were crushed by high-speed pulverizer. The powder was sieved through 40 mesh sieve, divided by quartering method, then labeled, and stored in sealed sample bottles at −20 °C. 2.0 g homogenized sample TCMs was accurately weighed, 10 mL of 1% citric acid -acetonitrile/water extraction solvent was added, and the samples were mixed by vortex for 2 min, soaked at room temperature for 20 min, extracted by shaking for 20 min, and extracted by constant temperature ultrasound at 50 °C for 20 min. Add proper amount of 0.1 mol/L phosphate buffer to adjust pH 5.5–6.0.
2.7.3. Sample purification
2.0 g of extraction salting agent containing 0.4 g of sodium chloride, 1.0 g of sodium sulfate and 0.6 g of ammonium sulfate were added into the extraction tube, the solution was centrifuged at 5000 rpm for 10 min, extracted and transferred to the upper organic phase. 2 mL of 13C-labeled mycotoxin ISs solution was accurately removed from the organic phase, and 0.20 g of PANI@CS adsorbent purifier was added to the extraction tube, which was vortex for 2 min to fully disperse and adsorb pigments and impurities, and centrifuged at 5000 rpm for 10 min. Finally, the solution was passed through a 0.22 μm nylon filter membrane and was ready for injection.
3. Results and discussion
3.1. Validation of methodology
3.1.1. Standard curve and detection limit
In order to reduce the influence of matrix effect on the quantitative results, isotope dilution internal standard method was used to ensure the accuracy of the analytical results. The matrix effect was investigated by calculating the ratio of peak area of 16 compounds in blank matrix additive solution to the corresponding compounds in pure solvent standard solution at the same concentration. The isotope internal standard method was used for quantitative analysis. The response abundance ratio (peak area ratio) between the object to be measured and the internal object was used for linear regression. The results showed that the linear relationship was good, the correlation coefficient R was ≥0.996, the detection limit (LOD, S/N > 3) of each component was in the range of 0.1–6.0 μg/kg, and the quantification limit (LOQ, S/N > 10) was in the range of 0.33–20 μg/kg. Table 2 represents the matrix correction curve, correlation coefficient, detection limit and quantification limit of 16 mycotoxins in negative ion mode and positive ion mode, respectively.
Table 2.
Matrix correction curve, correlation coefficient, detection limit and quantitative limit of 16 mycotoxins under different ion modes.
| Mycotoxin | Full Name | Abbreviationa | Molecular formula | Molecular weight | Ionization Mode | Correction curve | R2 | LOD (μg/kg) | LOQ (μg/kg) | Matrix Effect (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ochratoxin A | OTA | C20H18ClNO6 | 403.81 | ESI + | Y = 0.83X+0.201 | 0.9969 | 0.5 | 1.6 | 95 |
| 2 | T-2 toxin | T-2 | C24H34O9 | 466.53 | ESI + | Y = 1.26X-0.153 | 0.9993 | 2.0 | 6.7 | 92 |
| 3 | HT-2 toxin | HT-2 | C22H32O8 | 424.48 | ESI + | Y = 0.165X+0.0312 | 0.9967 | 1.5 | 5.0 | 93 |
| 4 | Fumonisin B1 | FB1 | C34H59NO15 | 721.83 | ESI + | Y = 0.0342X+0.105 | 0.9978 | 3.0 | 10 | 84 |
| 5 | Fumonisin B2 | FB2 | C34H59NO14 | 705.83 | ESI + | Y = 0.144X+0.0742 | 0.9979 | 3.0 | 10 | 85 |
| 6 | Fumonisin B3 | FB3 | C34H59NO14 | 705.83 | ESI + | Y = 0.112X+0.0351 | 0.9974 | 6.0 | 20 | 84 |
| 7 | Aflatoxin B1 | AFB1 | C17H12O6 | 312.27 | ESI + | Y = 2.64X-0.504 | 0.9971 | 0.1 | 0.33 | 88 |
| 8 | Aflatoxin B2 | AFB2 | C17H14O6 | 314.29 | ESI + | Y = 2.37X-0.641 | 0.9989 | 0.1 | 0.33 | 87 |
| 9 | Aflatoxin G1 | AFG1 | C17H12O7 | 328.27 | ESI + | Y = 1.47X-0.454 | 0.9978 | 0.2 | 0.67 | 86 |
| 10 | Aflatoxin G2 | AFG2 | C17H14O7 | 330.29 | ESI + | Y = 3.51X-0.306 | 0.9964 | 1.0 | 3.3 | 88 |
| 11 | Sterigmatocystin | ST | C18H12O6 | 324.28 | ESI + | Y = 0.0479X+0.0114 | 0.9998 | 5.0 | 16 | 90 |
| 12 | Zearalenone | ZEN | C18H22O5 | 318.36 | ESI - | Y = 0.0211X+0.0381 | 0.9991 | 5.0 | 16 | 94 |
| 13 | Nivalenol | NIV | C15H20O7 | 312.32 | ESI - | Y = 0.0309X-0.0102 | 0.9974 | 6.0 | 20 | 107 |
| 14 | Deoxynivalenol | DON | C15H20O6 | 296.32 | ESI - | Y = 0.0319X-0.0269 | 0.9971 | 5.0 | 16 | 89 |
| 15 | 3-acetyl deoxynivalenol | 3-Ac-DON | C17H22O6 | 322.35 | ESI - | Y = 43.3X+20.9 | 0.9965 | 3.0 | 10 | 92 |
| 16 | 15-acetyl deoxynivalenol | 15-Ac-DON | C17H22O7 | 338.35 | ESI - | Y = 79.1X+138 | 0.9989 | 3.0 | 10 | 91 |
aAbbreviations: OTA: Ochratoxin A; T-2: T-2 toxin; HT-2: HT-2 toxin; FB1: Fumonisin B1; FB2: Fumonisin B2; FB3: Fumonisin B3; AFB1: Aflatoxin B1; AFB2: Aflatoxin B2; AFG1: Aflatoxin G1; AFG2: Aflatoxin G2; ST: Sterigmatocystin; ZEN: Zearalenone; NIV: Nivalenol; DON: Deoxynivalenol; 3-Ac-DON: 3-acetyl deoxynivalenol; 15-Ac-DON: 15-acetyl deoxynivalenol.
3.1.2. Recovery
Under optimized experimental conditions, three supplemental levels of low, medium and high were selected according to the range of standard curves, and six parallel experiments were conducted for each supplemental level. Mycotoxin components AFB1, AFB2, AFG1, AFG2 and OTA were spiked at 1.0 μg/kg, 2.0 μg/kg and 10 μg/kg. The other components were spiked at 20 μg/kg, 50 μg/kg and 200 μg/kg. The design, average recovery results and relative standard deviations of the added three concentration levels are shown in Table 3, which shows the recoveries and relative standard deviations (RSD) of 16 mycotoxins at three spiked mass concentrations of Eucommia ulmoides and Ophiopogon. The recoveries and RSD of 16 mycotoxins results showed that the recoveries ranged from 90.1% to 105.8%, and RSD ranged from 1.3% to 4.1%.
Table 3.
Average recovery and precision of the three spiked mass concentrations of 16 mycotoxins in Eucommia ulmoides and Ophiopogon japonicus samples (n = 6).
| Mycotoxin | Full Name | Abbreviationa | Ionization Mode | Eucommia ulmoides Recovery (%) (RSD,%) |
Ophiopogon japonicas Recovery (%) (RSD,%) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | ||||
| 1 | Ochratoxin A | OTA | ESI + | 93.8 (2.1) | 91.6 (3.0) | 94.4 (2.8) | 91.5 (2.4) | 90.4 (2.7) | 97.4 (1.8) |
| 2 | T-2 toxin | T-2 | ESI + | 92.9 (2.0) | 92.5 (2.1) | 98.2 (1.7) | 90.3 (3.9) | 91.1 (2.0) | 92.4 (4.0) |
| 3 | HT-2 toxin | HT-2 | ESI + | 97.6 (3.8) | 93.2 (2.6) | 98.0 (1.9) | 98.7 (3.1) | 92.8 (3.3) | 90.6 (2.1) |
| 4 | Fumonisin B1 | FB1 | ESI + | 105.8 (4.1) | 91.6 (3.3) | 97.1 (2.8) | 92.9 (2.4) | 92.6 (2.9) | 98.0 (1.4) |
| 5 | Fumonisin B2 | FB2 | ESI + | 92.6 (3.7) | 95.8 (3.6) | 93.2 (3.7) | 97.5 (3.7) | 95.5 (4.0) | 95.2 (1.3) |
| 6 | Fumonisin B3 | FB3 | ESI + | 90.9 (3.5) | 97.5 (1.6) | 93.7 (1.9) | 93.7 (2.1) | 98.1 (1.9) | 95.1 (2.8) |
| 7 | Aflatoxin B1 | AFB1 | ESI + | 94.8 (2.6) | 90.7 (3.4) | 90.6 (3.2) | 94.8 (1.9) | 96.0 (3.5) | 91.5 (2.0) |
| 8 | Aflatoxin B2 | AFB2 | ESI + | 95.3 (3.1) | 92.8 (4.1) | 95.4 (2.9) | 93.1 (2.8) | 91.5 (3.3) | 96.6 (1.7) |
| 9 | Aflatoxin G1 | AFG1 | ESI + | 92.7 (3.6) | 97.2 (2.1) | 93.7 (2.8) | 97.5 (3.8) | 97.5 (3.1) | 98.7 (1.8) |
| 10 | Aflatoxin G2 | AFG2 | ESI + | 91.8 (2.7) | 93.1 (3.7) | 94.2 (3.4) | 91.4 (2.7) | 91.6 (3.9) | 95.4 (2.2) |
| 11 | Sterigmatocystin | ST | ESI + | 97.1 (3.5) | 95.4 (2.5) | 98.2 (1.9) | 95.4 (3.5) | 99.1 (2.8) | 92.5 (3.7) |
| 12 | Zearalenone | ZEN | ESI - | 95.3 (2.4) | 94.2 (1.5) | 94.7 (1.8) | 95.5 (3.1) | 93.8 (3.5) | 91.1 (2.5) |
| 13 | Nivalenol | NIV | ESI - | 91.4 (1.5) | 92.1 (3.2) | 95.2 (1.9) | 94.2 (4.0) | 97.2 (1.9) | 93.0 (3.4) |
| 14 | Deoxynivalenol rowhead | DON | ESI - | 90.1 (1.8) | 95.4 (2.7) | 94.9 (2.8) | 97.4 (4.1) | 91.7 (2.6) | 90.7 (2.9) |
| 15 rowhead | 3-acetyl deoxynivalenol rowhead | 3-Ac-DON | ESI - | 93.7 (2.3) | 93.1 (2.2) | 91.7 (3.0) | 97.9 (2.5) | 92.3 (3.9) | 91.7 (2.5) |
| 16 rowhead | 15-acetyl deoxynivalenol rowhead | 15-Ac-DON | ESI - | 95.2 (1.7) | 96.5 (3.7) | 94.2 (3.1) | 92.5 (3.0) | 90.2 (4.1) | 92.2 (2.0) |
aAbbreviations: OTA: Ochratoxin A; T-2: T-2 toxin; HT-2: HT-2 toxin; FB1: Fumonisin B1; FB2: Fumonisin B2; FB3: Fumonisin B3; AFB1: Aflatoxin B1; AFB2: Aflatoxin B2; AFG1: Aflatoxin G1; AFG2: Aflatoxin G2; ST: Sterigmatocystin; ZEN: Zearalenone; NIV: Nivalenol; DON: Deoxynivalenol; 3-Ac-DON: 3-acetyl deoxynivalenol; 15-Ac-DON: 15-acetyl deoxynivalenol.
3.2. Material characterization
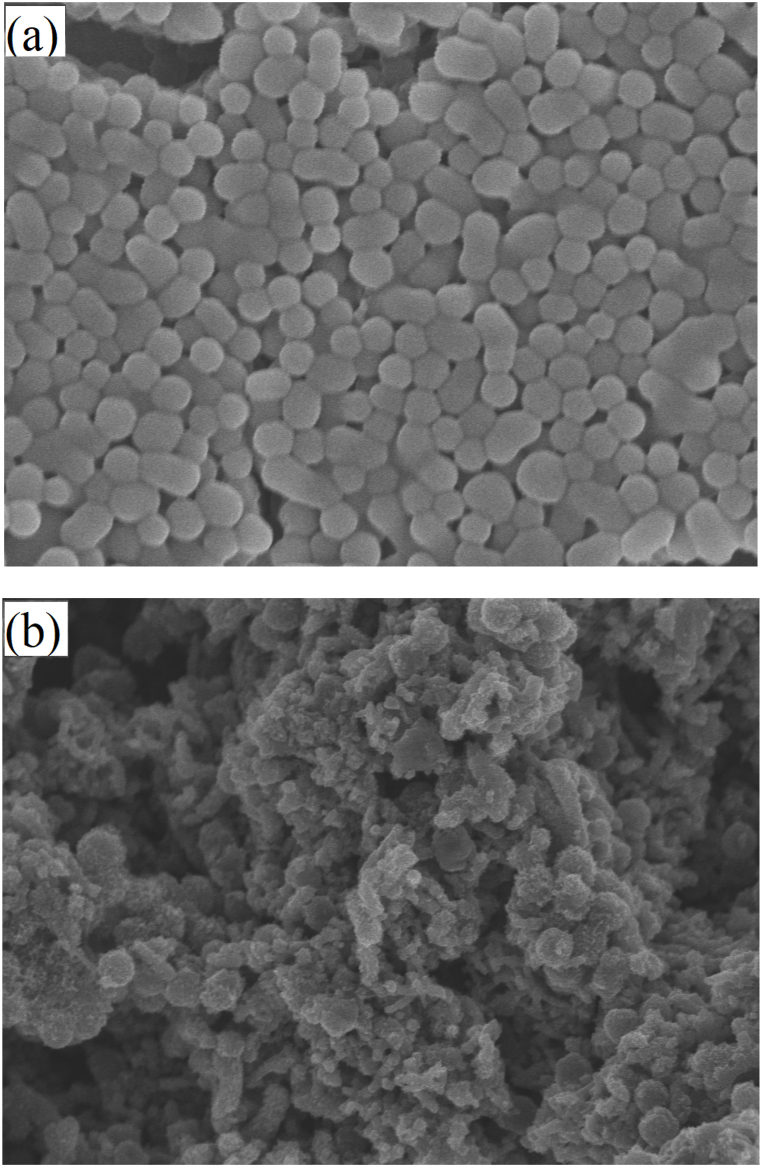
The morphology of CS and PANI@CS composites was studied by scanning electron microscopy (SEM). CS was dissolved in N, N-dimethylformamide (DMF) dispersion, the spherical particle size was uniform and well dispersed, and its particle size remained at about 400 nm. PANI@CS in DMF dispersion showed that PANI was uniformly coated on CS, forming a particle size of about 410 nm PANI@CS Composite spheres. The SEM results of CS and PANI@CS composites are shown in Fig. 3(a) and (b).
Fig. 3.
Scanning electron microscope of (a) Carbon spheres (CS) and (b) Polyaniline@carbon composite spheres (PANI@CS).
The infrared spectra of CS and PANI@CS composites are shown in Fig. 4. The characteristic peaks at 3450 cm−1, 1622 cm−1 and 1162 cm−1 were observed in the infrared spectrum of CS (Fig. 4(a)). These peaks are attributed to the stretching vibration peaks of –OH, C O and C–C–O. Fig. 4(b) shows the characteristic peaks at 1574 cm−1 and 1482 cm−1 correspond to quinone ring and benzene ring respectively, the characteristic peaks at 1251 cm−1 and 1304 cm−1 are C–N stretching vibration of aromatic amine, and the characteristic peak at 1144 cm−1 is the characteristic absorption peak of N O N bond of PANI. These results showed that polyaniline was successfully polymerized on the surface of carbon spheres.
Fig. 4.
Infrared spectra of (a) Carbon spheres (CS) and (b) Polyaniline@carbon composite spheres (PANI@CS).
3.3. Optimization of extraction solvent
In order to select and optimize the extraction solvent, four commonly used mixed solvents, acetonitrile/water (80/20, v/v), methanol/water (80/20, v/v), acetonitrile/water (60/40, v/v) and acetonitrile/water/citric acid (80/19/1, v/v), were used as the extraction solvent for TCMs. Fig. 5(a) illustrates the effect of different solvents on the extraction efficiency of the target mycotoxins. The extraction efficiency is determined by spiking appropriate amounts of mycotoxin standard solutions into TCMs and comparing the percentage recovery of the actual concentration before and after extraction. The results showed that acetonitrile/water/citric acid (80/19/1, v/v) as a mixed solvent had the highest extraction recovery, and the best extraction effect could be obtained when using this solvent. Pure water increases the permeability of acetonitrile, reduces the use of organic solvents, and improves the extraction efficiency. Among them, the extraction efficiency of several toxin compounds containing –COOCH3 acetoxy group is better, which may be because acetoxy compounds are more stable and selective than methanol in acetonitrile water system and easier to be fully extracted. Fumonisins, ochratoxin A and other toxins have carboxyl groups and strong hydrophilicity, the use of high organic phase system will lead to partial loss of recovery, so it is necessary to use aqueous solvent for extraction. Because mycotoxins such as DON, ZEN, OTA, FB1 and FB2 are sensitive to pH, appropriately reducing the pH of the extraction solution can improve the stability and increase the extraction efficiency. Therefore, in order to ensure the extraction effect of various mycotoxins, it is necessary to add an appropriate amount of 0.1 mol/L phosphate buffer to adjust the pH of 5.5–6.0. At this time, the weak acidic environment is helpful to improve the joint extraction effect of mycotoxins. Generally, acetonitrile/water/citric acid (80/19/1, v/v) was finally selected as the extraction solvent to enhance the extraction efficiency of the sample.
Fig. 5.
Comparison of extraction and purification efficiency of TCM samples with different extraction salting out agents and adsorption purification agents. (a) Extraction efficiency of four different mixed extraction solvents, acetonitrile/water (80/20, v/v), methanol/water (80/20, v/v), acetonitrile/water (60/40, v/v) and acetonitrile/water/citric acid (80/19/1, v/v). (b) Extraction efficiency of different fillers adsorption purifier, PSA, Al–N, PSA + C18 (1:1) and PANI@CS.
3.4. Optimization of cleanup
Ethylenediamine-n-propylsilane as primary secondary amine (PSA), octadecylsilane bonded silica gel (C18) and neutral alumina (Al–N) were commonly used for rapid purification fillers. According to the literature [35], PSA is an alkaline adsorbent, which has two amino groups in structure. It can remove carbohydrates, organic acids and a small amount of pigments through hydrogen bonding, but it will reduce the detection results of acidic compounds. As a non-polar adsorbent, C18 can adsorb non-polar substances in the matrix and play an effective role in removing lipids, sugars and other organic compounds in the sample, but it will also make the detection results of fat soluble substances worse, and the purification ability is limited when used alone. Al–N can adsorb electric rich compounds such as aromatic hydrocarbons and aliphatic amines, and is most stable under electrically neutral conditions.
In this study, 0.20 g PANI@CS was used for selective adsorption, and the purification effect of traditional fillers PSA, Al–N, PSA + C18 (1:1) is compared, as shown in Fig. 5(b). The results show that, PANI@CS has the best effect of adsorption purification. Because the PANI@CS adsorption purification agent has the common adsorption characteristics of Polyaniline and carbon materials, which can effectively adsorb the pigments and acidic impurities of TCMs, reduce matrix interference and improve extraction efficiency. Using PANI@CS can not only quickly realize adsorption purification, avoid using a large amount of organic solvents in the process of repeated activation and elution of solid-phase extraction column, but also prevent secondary pollution and fungal pollution in the process of vacuum concentration and transfer, reduce cost-effectiveness and save treatment time. It is worth mentioning that in this experiment, the combination of extraction salting out agent and adsorption purification agent is used for comprehensive purification of samples, and 2.0 g of extraction salting out agent containing sodium chloride, sodium sulfate and ammonium sulfate (m/m, 2:5:3) were selected for preliminary salting out. The addition of solid salting out agent can not only be used to remove excess water and protein, but also maintain the acidic extraction environment and ensure the salting out separation effect, which may be related to the synergistic salting out effect of acetonitrile and salting out agent. The extraction recoveries reach 90.7%∼97.5%, with an RSD (%) of 1.5%∼4.7%, which has a good extraction effect comparaed to Schmidt et al. (91%∼103%, 4%∼10%) [36], Lee et al. (78.7%∼106.5%, 4%∼24%) [37], and Ok et al. (86.2%∼106.6%, 3%∼15%) [38].
3.5. Optimization of liquid chromatography conditions
3.5.1. Selection of chromatographic columns
Agilent Poroshell 120 EC-C18 chromatographic column (3.0 mm × 100 mm, 2.7 μm) and Agilent Poroshell 120 SB-C18 column (3.0 mm × 100 mm, 2.7 μm) were compared under the same LC conditions, and it was found that the EC-C18 column had a better separation degree and higher column efficiency for aflatoxin, effectively avoiding peak tailing and achieving baseline separation of aflatoxin. The separation effect was significantly better than that of the SB-C18 column. Therefore, the EC-C18 chromatographic column was selected in this experiment.
3.5.2. Selection of mobile phase
The composition and ratio of the mobile phase had an impact on the chromatographic behavior, ionization efficiency, and detection sensitivity of the target compound. The experiment showed that in ESI+ mode, when acetonitrile-pure water was used as the mobile phase, the response values of aflatoxin B1 (AFB1) and fumatoxin B1 (FB1) were significantly reduced, indicating that when pure water was used as the mobile phase, the ionization efficiency was poor and the sensitivity was low. To enhance the ionization strength of each component, 0.1% formic acid was added to the aqueous solution in this experiment. The results showed that the addition of 0.1% formic acid solution was conducive to inhibiting the electrostatic interaction between mycotoxins and the chromatographic column with avoiding the phenomenon of tailing. Therefore, in this experiment, acetonitrile 0.1% formic acid solution was ultimately selected as the mobile phase in ESI+ mode. In ESI− mode, adding 0.1% ammonia water to the mobile phase aqueous phase is conducive to the ionization of compounds. Compared to acetonitrile-pure water solution, it can achieve higher detection sensitivity and better separation, especially enhancing the response value of aflatoxin, and improving the symmetry of peak shape. Therefore, acetonitrile 0.1% ammonia-water solution was selected as the mobile phase in ESI− mode in the experiment. After optimizing the mobile phase conditions, the chromatographic peaks and response intensities of the 16 toxins were ideal, and the retention time was stable.
3.6. Optimization of mass spectrometry conditions
When using ESI, without connecting to a chromatographic column, with the standard directly injected, add 100 μg/L standard solution was fully scanned in the positive ion (ESI+) and negative ion (ESI−) modes to select the appropriate ionization mode. The results showed that the five mycotoxins, ZEN, NIV, DON, 3-Ac-DON and 15-Ac-DON were highly sensitive in ESI− mode, and the other 11 mycotoxins have higher sensitivity in ESI+ mode. Therefore, this experiment selected positive ion and negative ion modes.
3.7. Matrix effect
The experiment uses stable isotope internal standard method to determine the content of the target substance, ensuring the accuracy and reliability of the analysis method. In order to correct the losses during sample purification and ionization processes, the selection of internal standard compounds mainly considers the similarity of the structure and properties between the internal standard and the target compound as much as possible. In this experiment, stable isotope [13C] internal standards of each compound were selected. Due to the matrix effect affecting ionization efficiency, signal suppression or enhancement occurs. This experiment adopts the relative response value method. The calculation formula is: Matrix effect (%) = (Sample matrix spiked response value/Standard solution response value) × 100%, with which the matrix effects of 16 mycotoxins were investigated. The results in Table 2 showed that the matrix effect of 16 mycotoxins was 84%∼107%, and the matrix effect could be ignored.
3.8. Samples analysis
Once verifying the optimization method, it was applied to investigate the occurrence of different batches of normal samples. Thirteen batches of normal genuine TCMs samples collected from different regions of China were tested. The results showed that 16 mycotoxin pollutants in the TCMs samples were not detected, which may be due to the high quality of this batch of genuine TCMs itself, the good storage conditions of this kind of common TCMs or not easy to be infected by toxic fungi. In addition, it may be caused by the high LOQ value of this method. For example, the LOQ values of NIV and DON were 20 μg/kg and 16 μg/kg, respectively. This result is lower than that of Bryła et al. (NIV 15 μg/kg, DON 30 μg/kg) [39] and Gab-Allah et al. (NIV 35.0–39.4 μg/kg, DON 42.8–46.7 μg/kg) [12,13], but much higher than that of Lee et al. (NIV 3.9–10.6 μg/kg, DON 1.3–3.5 μg/kg) [40]. The recoveries of different concentrations were tested in Eucommia ulmoides and Ophiopogon japonicus. The results showed that the recoveries were >90.1% and RSD was <4.1%, which could meet the actual test requirements. However, there are many kinds of TCMs library, and the storage risks of different medicinal parts are different. There are still some toxins that have not formulated corresponding limit standards, which still cannot rule out the potential safety risks in mycotoxins of TCMs. Therefore, new rapid and accurate quantitative testing technology is still popular in the realization of TCMs analysis.
4. Conclusion
In this study, a method based on PANI@CS combined with ultra performance liquid chromatography-tandem mass spectrometry was successfully established for the first time to analyze 13 TCMs from five different medicinal parts. The purification of PANI@CS has the advantages of wide application range and low economic cost. When measuring the actual samples with the electrospray ionization source (ESI+ and ESI−), combined with multi-reaction monitoring scanning mode (MRM), all 16 compounds could peak within 12 min, which effectively improved the selectivity of the target analytes, reduced the possibility of false positive results, and had a low detection limit and quantification limit. The reliability and innovation of the method can be verified. In general, this method has the characteristics of rapid, accurate, high recovery rate and high sensitivity. It is especially suitable for the qualitative analysis and quantitative detection of 16 mycotoxins residues in TCMs complex matrix samples. It complements the quantitative detection methods for many kinds of mycotoxins, which can be used as UPLC-MS/MS method in the trace analysis of mycotoxins in TCMs. It is worth mentioning that, according to the requirements of the current 2020 edition of Chinese Pharmacopoeia, the current focus is on aflatoxin testing, which has the problems of fewer types of testing toxins and high testing cost. In terms of the customization of standard limits, only a few TCMs have formulated the limit standards for mycotoxins, and only the limits of some varieties are specified. For example, the limit of AFB1 is 5 μg/kg, the limit of AFs is 10 μg/kg, and the limit of ZEN is 500 μg/kg. There is no comprehensive limit standard for mycotoxins in the world. This study suggests that relevant departments should continue to strengthen the safety supervision and quality control of TCMs contaminated by multiple toxigenic mycotoxins, pay particular attention to high-throughput multi-component simultaneous online rapid detection and pollution level research, and conduct targeted and scientific risk assessment of the development of mixed toxins. It will contribute to the comprehensive control of mycotoxin and the development of Chinese medicine, as well as the comprehensive guarantee of the double-effect mechanism of the quality and medication safety of TCMs industry.
Author contribution statement
Xinying Guo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Feng Chen: Contributed reagents, materials, analysis tools or data; Wrote the paper. Weibing Zhang: Conceived and designed the experiments.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Xinying Guo: Performed the experiments; Analyzed and interpreted the data; Wrote the paper. Feng Chen: Contributed reagents, materials, analysis tools or data; Wrote the paper. Weibing Zhang: Conceived and designed the experiments.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Gromek K., Drumond N., Simas P. Pharmacovigilance of herbal medicines. Int. J. Risk Saf. Med. 2015;27(2):55. doi: 10.3233/JRS-150643. [DOI] [PubMed] [Google Scholar]
- 2.Houghton P.J. In: Trease and Evans Pharmacognosy. fifteenth ed. Evans W.C., editor. W.B. Saunders; Edinburgh: 2002. Traditional plant medicines as a source of new drugs. [Google Scholar]
- 3.Liang Z.T., Zhao Z.Z. The original source of modern research on Chinese medicinal materials: bencao Texts. Alternative Complementary & Integrative Medicine. 2017;3(4):45–53. [Google Scholar]
- 4.Williamson E.M., Lorenc A., Booker A., et al. The rise of traditional Chinese medicine and its materia medica: a comparison of the frequency and safety of materials and species used in Europe and China. J. Ethnopharmacol. 2013;149(2):453–462. doi: 10.1016/j.jep.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Fang L., Zhao B., Zhang R.H., et al. Occurrence and exposure assessment of aflatoxins in Zhejiang province, China. Environ. Toxicol. Pharmacol. 2022;92 doi: 10.1016/j.etap.2022.103847. [DOI] [PubMed] [Google Scholar]
- 6.Feng X., Kong W.J., Yang M.H., et al. Latest advancement for detection methods of mycotoxins in traditional Chinese medicine. World Sci. Technol. 2012;14(5):1944–1952. [Google Scholar]
- 7.Tang Z.T., Liu F., Fang F., et al. Solid-phase extraction techniques based on nanomaterials for mycotoxin analysis: an overview for food and agricultural products. J. Separ. Sci. 2022;43 doi: 10.1002/jssc.202200067. [DOI] [PubMed] [Google Scholar]
- 8.Mamo F.T., Abate B.A., Zhen g Y.Q., Nie C.R., He M.J., Liu Y. Distribution of aspergillus Fungi and recent aflatoxin reports, health risks, and advances in developments of biological mitigation strategies in China. Toxins. 2021;13(10):678. doi: 10.3390/toxins13100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Q., Qin J.A., Fu Y.W., Luo J.Y., Lu J.H., Logrieco A.F., Yang M.H. Modified mycotoxins in foodstuffs, animal feed, and herbal medicine: a systematic review on global occurrence, transformation mechanism and analysis methods. TrAC, Trends Anal. Chem. 2020;133 [Google Scholar]
- 10.Chen L., Guo W.P., Zheng Y.Q., Zhou J.Z., Liu T.T., Chen W., Liang D.Q., Zhao M.P., Zhu Y.D., Wu Q.P., Zhang J.M. Occurrence and characterization of Fungi and mycotoxins in contaminated medicinal herbs. Toxins. 2020;12(1):30. doi: 10.3390/toxins12010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocha-Miranda F., Venncio A. Mycotoxigenic fungi in plant-based supplements and medicines. Curr. Opin. Food Sci. 2019;30:27–31. [Google Scholar]
- 12.Gab-Allah M.A., Mekete K.G., Choi K., Kim B. Occurrence of major type-B trichothecenes and deoxynivalenol-3-glucoside in cereal-based products from Korea. J. Food Compos. Anal. 2021;99 [Google Scholar]
- 13.Gab-Allah M.A., Tahoun I.F., Yamani R.N., Rend E.A., Shehata A.B. Natural occurrence of deoxynivalenol, nivalenol and deoxynivalenol-3-glucoside in cereal-derived products from Egypt. Food Control. 2022;137 [Google Scholar]
- 14.Alberto Ritieni. Ultra-high-performance liquid chromatography coupled with quadrupole orbitrap high-resolution mass spectrometry for multi-residue analysis of mycotoxins and pesticides in botanical nutraceuticals. Toxins. 2020;12(2):114. doi: 10.3390/toxins12020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K., Banerjee K. A Review: sample preparation and chromatographic technologies for detection of aflatoxins in foods. Toxins. 2020;12(9):539. doi: 10.3390/toxins12090539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y.C., Kong W.J., Luo H.L., Zhao L.H., Yang M.H. Dynamic variation of bioactive compounds and aflatoxins in contaminated Radix Astragali during extraction process. J. Sci. Food Agric. 2016;96(5):1571–1579. doi: 10.1002/jsfa.7257. [DOI] [PubMed] [Google Scholar]
- 17.Liu X.F., Ying G.Y., Sun C.N., Yang M.H., Zhang L., Zhang S.S., Xing X.Y., Li Q., Kong W.J. Development of an ultrasonication-assisted extraction based HPLC with a fluorescence method for sensitive determination of aflatoxins in highly acidic hibiscus sabdariffa. Front. Pharmacol. 2018;9:284–292. doi: 10.3389/fphar.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J., Xu J.J., Huang B.F., Cai Z.X., Ren Y.P. High-performance liquid chromatographic determination of multi-mycotoxin in cereals and bean foodstuffs using interference-removal solid-phase extraction combined with optimized dispersive liquid-liquid microextraction. J. Separ. Sci. 2017;40(10):2141–2150. doi: 10.1002/jssc.201601326. [DOI] [PubMed] [Google Scholar]
- 19.Han Z., Ren Y., Zhu J., et al. Multianalysis of 35 mycotoxins in traditional Chinese medicines by ultra-high-performance liquid chromatography–tandem mass spectrometry coupled with accelerated solvent extraction. J. Agric. Food Chem. 2012;60(33):8233–8247. doi: 10.1021/jf301928r. [DOI] [PubMed] [Google Scholar]
- 20.Zhao D.T., Gao Y.J., Zhang W.J., Bi T.C., Wang X., Ma C.X., Rong R. Development a multi-immunoaffinity column LC-MS-MS method for comprehensive investigation of mycotoxins contamination and co-occurrence in traditional Chinese medicinal materials. J. Chromatogr. B. 2021;1178 doi: 10.1016/j.jchromb.2021.122730. [DOI] [PubMed] [Google Scholar]
- 21.Dong H., Xian Y.P., Xiao K.J., Wu Y.L., Zhu L., He J.P. Development and comparison of single-step solid phase extraction and QuEChERS clean-up for the analysis of 7 mycotoxins in fruits and vegetables during storage by UHPLC-MS/MS. Food Chem. 2019;274:471–479. doi: 10.1016/j.foodchem.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 22.Tanveer Z.I., Huang Q.W., Liu L., Jiang K.Q., Nie D.X., Pan H.Y., Chen Y., Liu X.S., Luan L.J., Han Z., Wu Y.J. Reduced graphene oxide-zinc oxide nanocomposite as dispersive solid-phase extraction sorbent for simultaneous enrichment and purification of multiple mycotoxins in Coptidis rhizoma (Huanglian) and analysis by liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2020;1630 doi: 10.1016/j.chroma.2020.461515. [DOI] [PubMed] [Google Scholar]
- 23.Jiang K.Q., Huang P., Luan L.J., Fan K., Guo W.B., Zhao Z.H., Wu Y.J., Han Z. Iron (II, III) oxide/multi-walled carbon nanotube composite as solid-phase extraction sorbent followed by ultra-high performance liquid chromatography tandem mass spectrometry for simultaneous determination of zearalenone and type A trichothecenes in Salviae miltiorrhizae Radix et Rhizoma (Danshen) J. Chromatogr. A. 2017;1482:1–10. doi: 10.1016/j.chroma.2016.12.058. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S.S., Lu J.W., Wang S.M., Mao D., Miao S., Ji S. Multi-mycotoxins analysis in Pheretima using ultra-high-performance liquid chromatography tandem mass spectrometry based on a modified QuEChERS method. J. Chromatogr. B. 2016;1035:31–41. doi: 10.1016/j.jchromb.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S.P., Zhang D., Tan L.H., et al. Analysis of aflatoxins in traditional Chinese medicines: classification of analytical method on the basis of matrix variations. Sci. Rep. 2016;6(1) doi: 10.1038/srep30822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Z., Zheng Y.L., Luan L.J., et al. Analysis of ochratoxin A and ochratoxin B in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry using [13C20]-ochratoxin A as an internal standard. J. Chromatogr. A. 2010;1217(26):4365–4374. doi: 10.1016/j.chroma.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y., Zhang Y., Sha L., et al. Grafting molecularly imprinted poly (2-acrylamido-2-methylpropanesulfonic acid) onto the surface of carbon microspheres. Appl. Surf. Sci. 2012;258(17):6441–6450. [Google Scholar]
- 28.Yang L.F., Liu Y.Z., Chen X.G., et al. Preparation and characterization of 5-fluorouracil surface-imprinted thermosensitive magnetic microspheres. Monatshefte fur Chemie. 2015;51:261–267. [Google Scholar]
- 29.Qin L., Liu W., Yang Y., et al. Functional monomer screening and preparation of dibenzothiophene-imprinted polymers on the surface of carbon microsphere. Monatshefte für Chemie-Chemical Monthly. 2015;146(3):449–458. [Google Scholar]
- 30.Cai H.M., Pan J.M. A study on preferential adsorption of 2,4,5-trichlorophenol by an imprinted polymer on the surface of a (Chitosan/maghemite)@C magnetic composite. Mod. Food Sci. Technol. 2017;33(5):135–140. [Google Scholar]
- 31.Avelar Dutra F.V., Pires B.C., Nascimento T.A., Mano V., Borges K.B. Polyaniline-deposited cellulose fiber composite prepared via in situ polymerization: enhancing adsorption properties for removal of meloxicam from aqueous media. RSC Adv. 2017;7:12639–12649. [Google Scholar]
- 32.Chakraborty P., Kothari A., Nagarajan R. Highly ordered polyaniline as an efficient dye remover. Adsorpt. Sci. Technol. 2017;36:429–440. [Google Scholar]
- 33.Cho M.H. Adsorption modeling and mechanistic insight of hazardous chromium on para toluene sulfonic acid immobilized-polyaniline@CNTs nanocomposites. J. Saudi Chem. Soc. 2019;23:188–197. [Google Scholar]
- 34.Nasar A., Mashkoor F. Application of polyaniline-based adsorbents for dye removal from water and wastewater: a review. Environ. Sci. Pollut. Control Ser. 2019;26:5333–5356. doi: 10.1007/s11356-018-3990-y. [DOI] [PubMed] [Google Scholar]
- 35.Guo X.Y., Gu J., Chen F., Yang Q.H. The determination of the level, source, and risk of polycyclic aromatic hydrocarbon content in traditional Chinese medicines using a QuEChERS based extraction and HPLC-UV-FLD analysis. J. Liq. Chromatogr. Relat. Technol. 2021;443–4:210–219. [Google Scholar]
- 36.Schmidt J., Cramer B., Turner P.C., Stoltzfus R.J., Humphrey J.H., Smith L.E., Humpf H.-U. Determination of urinary mycotoxin biomarkers using a sensitive online solid phase extraction-UHPLC-MS/MS method. Toxins. 2021;13(6):418. doi: 10.3390/toxins13060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S.Y., Woo S.Y., Malachová A., Michlmayr H., Kim S.-H., Kang G.J., Chun H.S. Simple validated method for simultaneous determination of deoxynivalenol, nivalenol, and their 3-β-D-glucosides in baby formula and Korean rice wine via HPLC-UV with immunoaffinity cleanup. Food Addit. Contam. 2019;36(6):964–975. doi: 10.1080/19440049.2019.1606454. [DOI] [PubMed] [Google Scholar]
- 38.Ok H.E., Lee S.Y., Chun H.S. Occurrence and simultaneous determination of nivalenol and deoxynivalenol in rice and bran by HPLC-UV detection and immunoaffinity cleanup. Food Control. 2018;87:53–59. [Google Scholar]
- 39.Bryła M., Ksieniewicz-Woźniak E., Waśkiewicz A., Szymczyk K., Jędrzejczak R. Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in polish winter wheat. Toxins. 2018;10(2):81. doi: 10.3390/toxins10020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.Y., Woo S.Y., Tian F., Song J., Michlmayr H., Kim J.-B., Chun H.S. Occurrence of deoxynivalenol, nivalenol, and their glucosides in Korean market foods and estimation of their population exposure through food consumption. Toxins. 2020;12(2):89. doi: 10.3390/toxins12020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.