Abstract
Background
Cross-country skiers train and compete during the winter for long periods of time in subfreezing conditions, which strains the airways and provokes respiratory symptoms. This study aimed to compare the prevalence of exercise-related symptoms and prolonged cough in competitive cross-country skiers versus the general population and to investigate the association between these symptoms and asthma.
Methods
A questionnaire was sent to Finnish cross-country skiers (n=1282) and a random sample of the general population (n=1754), with response rates of 26.9% and 19.0%, respectively.
Results
Both groups were mostly asymptomatic at rest, but symptoms were increased in both groups during and after exercise. Cough was more prevalent after exercise in skiers and phlegm production was more common during and after exercise in skiers. Asthma did not provoke specific symptoms, but symptom prevalence was higher in asthmatic individuals. Skiers had a higher prevalence of cough after exercise (60.6% vs 22.8%, p<0.001) compared with controls, but controls had a higher prevalence of prolonged cough (4.1% vs 9.6%, p=0.004). In participants without asthma, cold air triggered symptoms more often in skiers than controls, while strong odours triggered symptoms more often in asthmatic controls than skiers. Chronic cough lasting more than 8 weeks was rare, reported by 4.8% of controls and 2.0% of skiers.
Conclusion
Cross-country skiers, especially those with asthma, experience a higher burden of exercise-related respiratory symptoms compared with controls. However, repeated exposure to cold air does not appear to result in long-term hypersensitivity of the cough reflex arc.
Keywords: Respiratory, Cross-country skiing, Sports medicine
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Respiratory symptoms are very common in cross-country skiers.
WHAT THIS STUDY ADDS
Skiers and controls were mostly asymptomatic at rest, but symptoms were increased in both groups during and after exercise.
Asthmatic participants had more symptoms than non-asthmatics in both skiers and controls. Skiers were more symptomatic than the controls, even when grouped by asthma status.
Even though cough after exercise was more prevalent in skiers compared with the controls, prolonged cough was more prevalent in the controls.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/ OR POLICY
High prevalence rates of respiratory symptoms should be graded with severity and burden to further evaluate their effect on training and competition.
Introduction
Cross-country skiing is a demanding Olympic winter sport that requires a high maximal oxygen uptake, anaerobic capacity and high levels of upper body power.1 Minute ventilation in elite skiers may well exceed 200 L/min, and their forced vital capacity and forced expiratory volume in one second often exceed normal values.2 During the winter season, most of the training and competition in cross-country skiing takes place in subfreezing temperatures and cold and dry air acts as an external irritant, which cools the airways and increases osmolarity of the respiratory lining fluid due to water loss. This increase in osmolarity stimulates sensory nerves and may activate inflammatory cells and smooth muscle, contributing to the provocation of symptoms.3 Respiratory symptoms are very common among cross-country skiers and may sometimes even be normal reactions to intensive exercise and the environment, thus being completely benign and physiological.
Respiratory symptoms in skiers are often reported using point prevalence, which refers to the proportion of individuals experiencing specific symptoms at a given time.4–8 Reported symptoms include cough, chest tightness, phlegm production, wheezing and shortness of breath. The first study on respiratory symptoms in cross-country skiers was by Heir and Oseid in 1994, who found that 58% out of 153 Norwegian skiers had two or more respiratory symptoms and that skiers with asthma had more symptoms than non-asthmatic skiers, except for cough.4 Sue-Chu et al reported respiratory symptoms separately for Norwegian and Swedish cross-country skiers, finding in the winter that 42% of Norwegian skiers and 64% of Swedish skiers (p<0.001) reported coughing during training. During the summer, the respective percentages were only 10% in Norwegian and 9% in Swedish skiers.5 Turmel et al found in a screening study that 50% of the tested Canadian skiers and biathletes had respiratory symptoms, but possible differences between genders were not reported.6 In recent studies by Norqvist and Eklund, female skiers had significantly more symptoms than male skiers in Sweden.7 8 Various factors, such as exposure to cold air, may contribute to the provocation of respiratory symptoms. The effect of cold air on symptoms in skiers has been reported in one study that showed cold air increased respiratory symptoms in 14% of adolescent cross-country skiers in Norway and 45% in Sweden, without providing a specific comparison point.5
Cough in winter athletes is highly prevalent during exercise and after exercise.4 5 9 10 However, it is not clear whether this causes a decrease in performance or leads to prolonged cough. Although cough as a respiratory symptom during and after exercise has been studied in skiers, no studies have investigated prolonged cough in athletes of any sport.11 12 It is uncertain whether repeated high-intensity exercise in cold air damages the airways and induces long-term cough reflex hypersensitivity. Among athletes training in cold air, cough reflex sensitivity to capsaicin remained unchanged during the season, but cough after training was observed to be more frequent up to 8 hours after training.10 This suggests that training might not change sensitivity of the cough reflex but to act as a trigger of cough.
The primary aim of the current study was to investigate the prevalence of respiratory symptoms in Finnish cross-country skiers, with a focus on prolonged cough and asthma subgroups at rest, during exercise and after exercise. Additionally, we sought to compare these findings with the general population of the same age, gender and region. We hypothesised that respiratory symptoms are more common in skiers during and after exercise, as they frequently engage in high-intensity exercise and have a higher prevalence of asthma.13 14 Both factors may contribute to an increased occurrence of acute and prolonged cough in skiers.
Methods
The present study protocol has been described in detail previously.14 In short, all Finnish cross-country skiers who had enrolled in either national championships (from 17 years of age onwards to seniors) or the largest national junior skiing competition (13–16 years of age, Hopeasompa competition) were invited to participate in this cross-sectional questionnaire survey (n=1282). The control group was collected from the Finnish Digital and Population Data Services Agency, matching the skiers who had responded regarding age, gender and region of the country in which they lived. The controls were allowed to participate in competitive sports, but none of the controls competed in cross-country skiing.14 Engaging in competitive sports was divided into four categories: team sports and sports based on required ventilation rate (high, moderate, low). Written informed consent was obtained from each respondent and from their guardians for participants under 18 years of age.
Skiers participated in the study at the beginning of the training season in May and June, while the controls participated in March and April. The participants filled out a questionnaire where different respiratory symptoms (cough, wheezing, shortness of breath, phlegm production and chest tightness) were each inquired about separately for at rest, during exercise and after exercise. The common triggers for respiratory symptoms were enquired. The participants were also asked whether they suffered from a current cough and its duration (less than a week, over a week but under 3 weeks, over 3 weeks but under 2 months, over 2 months but under 1 year, over 1 year but under 5 years, over 5 years but under 10 years or over 10 years). Cough was defined as acute current cough if it had lasted less than 3 weeks, prolonged cough if it had lasted more than 3 weeks but under 8 weeks and chronic cough lasting over 8 weeks.15
Current asthma was defined as self-reported physician-diagnosed asthma and at least one of the following criteria: currently having three asthma-related symptoms (cough, chest tightness, shortness of breath, wheezing or sputum production), active use of any asthma medication or an asthma control test score less than 25 points.16 Allergic rhinitis was defined as self-reported physician-diagnosed allergic rhinitis. Smoking was defined as never-smoking or current smoking.
Statistical analyses were performed using SPSS V.27.0 (IBM, Armonk, New York). The continuous variables were tested for normality (Kolmogorov–Smirnov). Unpaired t-test, Mann-Whitney U test and one-way analysis of variance (ANOVA) were used for the comparisons between the groups, as appropriate. A χ2 test or Fisher’s exact test was used for comparisons of the categorical variables. A p value of <0.05 was considered statistically significant.
Results
The total response rate in the current study was 19.0% (n=334) in the controls and 26.9% (n=345) in the skiers. The participants’ characteristics are presented in table 1. The controls were slightly older, and a larger proportion of the controls compared with the skiers were women. Skiers had asthma more often, and none of them was currently smoking.
Table 1.
Participants’ characteristics in the cross-country skiers and controls
| Cross-country skiers n=345 |
Controls n=334 |
P | |||
| Median/n | Q1-Q3/% | Median/n | Q1-Q3/% | ||
| Age, years | 16.5 | 14.3–21.5 | 17.0 | 15–22.5 | 0.033 |
| Body mass index, kg/m2 | 21.0 | 19.2–22.8 | 21.6 | 19.7–25.1 | <0.001 |
| Women | 204 | 58.1 | 235 | 69.5 | 0.002 |
| Engaged in competitive sports other than cross-country skiing | 223 | 63.5 | 88 | 26.0 | <0.001 |
| Team sports | 36 | 16.1 | 54 | 61.3 | <0.001 |
| High ventilation sports (cycling, running, triathlon, orienteering, aerobic gymnastics) | 195 | 87.6 | 7 | 7.9 | <0.001 |
| Moderate ventilation sports (combat sports, gymnastics, dancing) | 0 | 0 | 19 | 21.6 | <0.001 |
| Low ventilation sports (shooting, horseback riding, weightlifting) | 34 | 15.2 | 5 | 5.7 | 0.022 |
| Smoking | 0 | 0 | 20 | 5.9 | <0.001 |
| Current asthma | 91 | 25.9 | 31 | 9.2 | <0.001 |
| Physician-diagnosed allergy to pollens or animals | 113 | 32.8 | 82 | 24.6 | 0.018 |
| Self-reported allergic rhinitis | 159 | 46.1 | 122 | 36.5 | 0.011 |
P values were obtained with a Mann-Whitney U test for continuous variables and a Chi-squared test or Fisher’s test for categorical variables; statistical significance (p<0.05) is indicated in bold.
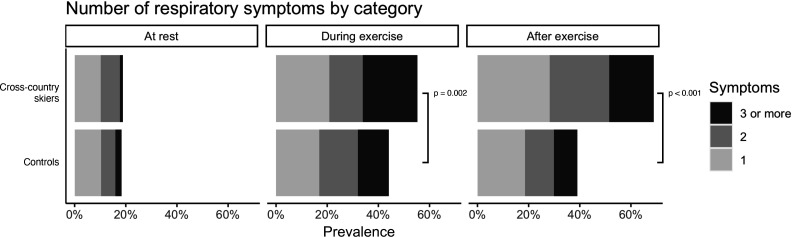
Number of different respiratory symptoms in cross-country skiers and controls at different states are presented in figure 1, and all percentages from all figures are presented in online supplemental file 1. Both groups were mostly asymptomatic at rest, but symptoms were increased in both groups during and after exercise. Skiers had more exercise-related respiratory symptoms than the controls (figure 1). Moreover, skiers tended to have more symptoms after exercise than during exercise (p<0.001).
Figure 1.
Number of different respiratory symptoms in cross-country skiers and controls at rest, during exercise and after exercise.
bmjsem-2022-001502supp001.pdf (570.8KB, pdf)
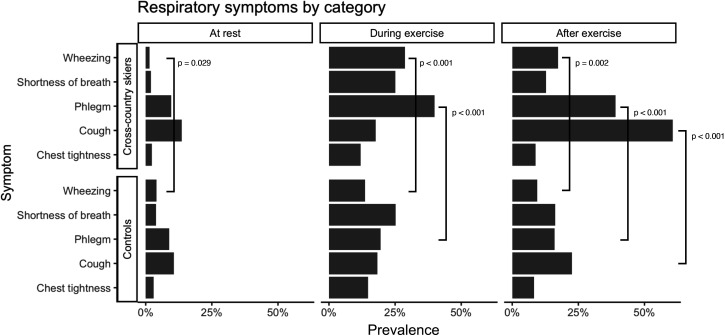
The most common respiratory symptoms in cross-country skiers were cough, phlegm production, wheezing and shortness of breath. Only chest tightness was uncommon in cross-country skiers. In controls, there were fewer symptoms overall compared with skiers and there was no clear difference in prevalence between different types of symptoms in controls themselves (figure 2). There were significant differences between the groups regarding respiratory symptoms. Cough was more prevalent after exercise in skiers. Phlegm production was more common in skiers both during and after exercise. In the controls, wheezing was more common at rest than in skiers, but during exercise and after exercise, skiers experienced wheezing significantly more (figure 2)
Figure 2.
Prevalence of different respiratory symptoms in cross-country skiers and controls at rest, during exercise and after exercise.
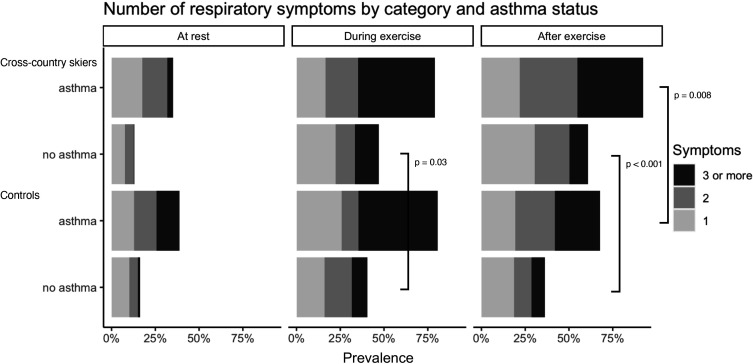
As asthma was more prevalent among skiers, the prevalence of respiratory symptoms in different physical activity categories according to asthma status is shown in figure 3. Skiers and controls with asthma reported a similar number of respiratory symptoms at rest and during exercise, but cross-country skiers with asthma had more symptoms after exercise than the asthmatic controls. In participants with no asthma, cross-country skiers had more symptoms during and after exercise compared with the controls.
Figure 3.
Number of respiratory symptoms in cross-country skiers and controls at rest, during exercise and after exercise according to the asthma status.
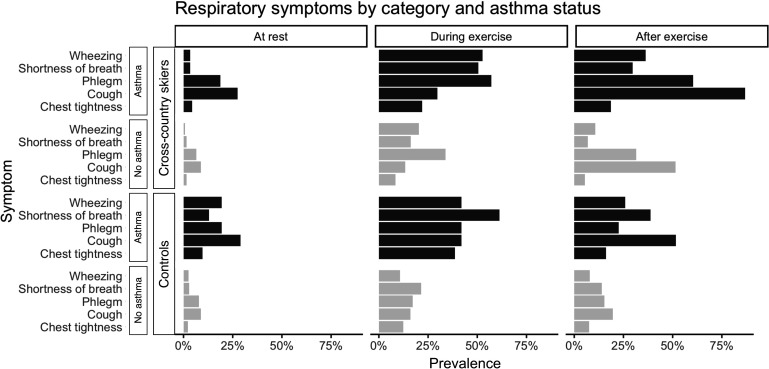
When respiratory symptoms were grouped by category and asthma status, a higher prevalence in all symptoms was found in participants with asthma. The relative proportions of different symptoms between asthmatic and non-asthmatic participants were similar (ie, asthma did not provoke specific symptoms), but the prevalence of symptoms in general was higher in participants with asthma (figure 4).
Figure 4.
Prevalence of different respiratory symptoms in cross-country skiers and controls at rest, during exercise and after exercise according to asthma status.
The most common triggers causing respiratory symptoms were dust, tobacco smoke, exhaust gases, cold air, pollen and strong odours. All triggers caused more respiratory symptoms in participants with asthma than in non-asthmatic participants. In participants without asthma, cold air triggered symptoms more often in skiers than in the controls. On the other hand, strong odours triggered symptoms more often in asthmatic controls than in asthmatic skiers. The data are presented in table 2.
Table 2.
Triggers causing respiratory symptoms
| No asthma n=567 | P between groups with no asthma | Asthma n=122 | P between groups with asthma | |||||||
| Controls n=307 |
Cross-country skiers n=260 |
Controls n=31 |
Cross-country skiers n=91 |
|||||||
| n | % | n | % | n | % | n | % | |||
| Cold air | 55 | 18.0 | 68 | 26.1 | 0.025 | 19 | 61.3 | 56 | 62.2 | 1.000 |
| Dust, tobacco smoke, exhaust gases | 100 | 32.7 | 89 | 34.4 | 0.739 | 22 | 71.0 | 55 | 60.4 | 0.404 |
| Pollen | 46 | 15.0 | 56 | 21.6 | 0.055 | 17 | 54.8 | 42 | 46.7 | 0.564 |
| Hairy animals | 21 | 6.9 | 18 | 7.0 | 1.000 | 11 | 36.7 | 18 | 19.8 | 0.103 |
| Strong odours | 51 | 16.7 | 28 | 10.7 | 0.058 | 16 | 51.6 | 23 | 25.3 | 0.013 |
| Humid, misty air | 15 | 4.9 | 10 | 3.8 | 0.693 | 3 | 9.7 | 17 | 18.7 | 0.399 |
P values were obtained with Chi-squared test; statistical significance (p<0.05) is indicated in bold.
Current cough, both acute and prolonged, was more prevalent in controls compared with skiers (table 3). When skiers and controls were pooled together, participants with prolonged coughs also reported coughs more frequently at exercise and after exercise (table 4). Furthermore, participants with prolonged cough reported cold air and strong odours as triggers for their respiratory symptoms more frequently.
Table 3.
Current cough in cross-country skiers and controls
| Controls n=332 |
Cross-country skiers n=345 |
P | |||
| n | % | n | % | ||
| Any current cough | 105 | 32.0 | 61 | 17.7 | <0.001 |
| Acute cough (<3 weeks) | 73 | 21.9 | 47 | 13.6 | 0.006 |
| Prolonged cough (≥3 weeks) | 32 | 9.6 | 14 | 4.1 | 0.004 |
| Chronic cough (>8 week) | 16 | 4.8 | 7 | 2.0 | 0.047 |
P values were obtained with Chi-squared test; statistical significance (p<0.05) is indicated in bold.
Table 4.
Characteristics of participants with and without current prolonged cough among the total population
| No current cough or cough <3 weeks n=640 |
Prolonged cough ≥ 3 weeks n=46 |
P | |||
| n | % | n | % | ||
| Cross-country skier | 337 | 52.6 | 14 | 30.4 | 0.004 |
| Women | 407 | 63.5 | 30 | 65.2 | 0.815 |
| Current asthma | 110 | 17.2 | 12 | 26.1 | 0.126 |
| Smoking | 19 | 3.0 | 1 | 2.2 | 1.000 |
| Cough at exercise | 101 | 15.8 | 22 | 47.8 | <0.001 |
| Cough after exercise | 258 | 40.2 | 31 | 67.4 | <0.001 |
| Cold air provokes respiratory symptoms | 176 | 27.5 | 22 | 47.8 | 0.003 |
| Strong odours provoke respiratory symptoms | 100 | 15.6 | 18 | 39.1 | <0.001 |
P values were obtained with χ2 test or Fisher’s test; statistical significance (p<0.05) is indicated in bold.
Discussion
In the current study that was conducted among competitive cross-country skiers, we have reported a high prevalence of respiratory symptoms during and after exercise. Skiers are more symptomatic than controls, even when the participants are grouped by asthma status. Asthmatic participants had more symptoms than non-asthmatics in both skiers and controls. Even though cough after exercise was more prevalent in skiers compared with the controls, prolonged cough (>3 weeks) and chronic cough (>8 weeks) were less prevalent in skiers than in controls.
Different respiratory symptoms may have different causes. Cough and phlegm may be triggered by irritation of the airways, which activates sensory nerves and mucous glands, while wheezing and shortness of breath may be signs of obstruction, possibly due to smooth muscle contraction or mucosal oedema.17 18 Also, dysfunctional breathing or exercise-induced laryngeal obstruction (EILO) may case dyspnoea and wheezing, mimicking asthma. Gastro-oesophageal reflux, although not extensively studied in skiers regarding cough,19 may also play a role. Furthermore, cold air hyperpnea can increase airway lining fluid osmolarity by evaporating water, activating certain sensory endings and causing coughing.20 Exercise-induced bronchoconstriction (EIB) could trigger coughing; however, bronchoconstriction is a relatively weak stimulus for cough.21
Although asthma has been reported to be highly prevalent according to earlier articles in this current population,14 22 prolonged cough was not prevalent in skiers in the current study. The mechanisms behind asthma and cough reflex hypersensitivity are at least partly different.23 High ventilation rate in cold air may trigger epithelial damage, induce inflammatory response and lead to hyper-reactivity of airway smooth muscle and asthma.24 Once hyper-reactivity has developed, EIB is thought to be caused by water loss, resulting in heat loss and hyperosmolality of the airway surface liquid during exercise, leading to activation of mast cells releasing substances contracting smooth muscle.24
The most common respiratory symptom in skiers was cough, especially after exercise. Prolonged cough for over 8 weeks was very rare in skiers (2.0%). In both groups, current cough had mainly lasted less than 3 weeks, but prolonged cough was two times as prevalent in controls as in skiers. This does not support our hypothesis that repeated exposure through high-volume endurance training causes long-term cough reflex hypersensitivity. This may be partly explained by the survey taking place outside the competition season during which skiers may be less symptomatic in the training season. A study to support this was reported by Sue-Chu et al,5 where in the winter, 42% of Norwegian skiers and 64% of Swedish skiers (p<0.001) reported coughing during training and while during the summer, the respective percentages were 10% in Norwegian and 9% in Swedish skiers. To support this, in another Norwegian study in cross-country skiers, the airways were found to be more hyperreactive to methacholine challenge during competition season in skiers.25 26
Cough after exercise and prolonged cough may be related to different mechanisms. Airway mucosa irritation due to intense ventilation in cold air is a physiological response, as suggested by the high prevalence of rhinorrhoea in healthy participants exposed to cold air.17 18 This may also trigger short-term cough after intense exercise in cold air. On the other hand, prolonged cough is often related to cough reflex hypersensitivity, which may involve altered function in peripheral sensory neurons or altered central processing of the urge to cough.27 Turmel et al discussed that cough receptors could be desensitised due to long-term exposure to cold and dry air.10 This would explain why exercise may still trigger a short-term cough right after exercise but decreases the tendency to have prolonged cough. Moreover, capsaicin, another hypertussive irritant in addition to cold air, triggers cough after a single exposure but has been suggested to decrease cough sensitivity and to treat chronic cough as repeated dosing.28 29 This similarity between capsaicin and repeated exposure to cold air further supports the idea that different mechanisms are involved in cough after exercise and prolonged cough. Exposure to a trigger like cold air or capsaicin provokes cough, but repeated long-term exposure to the same trigger may desensitise cough reflex and protect from chronic cough.
In the current study, we found that 39.1% of all participants and 35.7% (5 with prolonged cough vs 44 with no cough, p<0.035) of skiers with prolonged cough were sensitive to strong odours. This feature, known to be associated with cough reflex hypersensitivity in the general population,30 31 has not been reported in athletes previously. Furthermore, participants with asthma were more sensitive to strong odours, and this may be related to asthmatic inflammation sensitising cough reflex.32
To interpret these high prevalence rates of respiratory symptoms in skiers, it should be highlighted that cross-country skiing puts an exceptionally high strain on airways through high sustained ventilation and cold subfreezing air in the winter.1 2 Although respiratory symptoms may be a sign of respiratory illness, such as asthma, the airway response in the form of cough and increased sputum production may also be a physiological response to exercise.17 18 Although skiers are symptomatic, asthma is well controlled overall and across different performance levels.14 22 However, it is possible that skiers with respiratory health issues may have retired their athletic career and, thus, not affecting the results. Skiers may also present with different conditions affecting the airways simultaneously, such as EILO or dysfunctional breathing.33 The presence of other respiratory conditions may further confound the results, as not all symptoms are due to asthma and different respiratory conditions may exist simultaneously, as shown by Irewall et al.34
Strengths and weaknesses
In the present study, we did not measure the intensity of symptoms, only the mere presence of symptoms in different situations and the time frame presented (at rest, at exercise, after exercise) was not strictly defined. The intensity and effect of symptoms may differ, and mild symptoms may not be disturbing. Survivor bias may also affect the results because skiers with difficult symptoms may not continue a successful athletic career for long. However, respiratory symptoms in skiers may be in general mild in nature as most skiers report respiratory symptoms.
The survey times were slightly different in skiers and controls. Skiers mainly responded to the study during May/June and the controls in March/April. This may confound the results because the most intensive tree-pollen season in Finland is in May (usually from March to June). Moreover, the survey in skiers was conducted in early training season outside of competitions, so the prevalence of respiratory symptoms and current cough might have been lower at this of year. Again, as discussed earlier, truly prolonged cough over 8 weeks was rare in skiers and supports the argument that prolonged cough is less prevalent in skiers, irrespective of the time of the year.
The methods used in each skier’s diagnostic work out could not be verified, but in Finland, asthma diagnosis is most often based on objective lung function measures because of the criteria for drug reimbursement. Validation of self-reported asthma by lung function measures has been studied in a similar demographic population as the participants in the present study, finding that among Finnish university students, 18 to 25 years of age, the specificity of physician-diagnosed current asthma was 99%.35
The response rate in the current study was relatively low (19.0% (n=334) in the controls and 26.9% (n=345) in the skiers). Women responded more often, which has also been reported earlier in a similar age group investigating respiratory health.36 Low response rate is a common issue when surveying young and healthy populations, and it is particularly challenging to obtain responses from young men living in rural areas,37 38 who constituted the majority of participants in this study. Responses may be subjected to recall bias, and this type of cross-sectional study is limited in investigating time effects.
Conclusion
We conclude that cross-country skiers have more exercise-associated respiratory symptoms than controls and that the prevalence of symptoms is especially high in participants with asthma. Although exercise often provokes cough in cross-country skiers, repeated hyperpnea of cold air does not seem to lead to long-standing hypersensitivity of the cough reflex and prolonged cough. This is in contrast to the high prevalence of asthma in skiers. Thus, the results suggest that the high prevalence of symptoms does not cause a major burden to prevent a successful athletic career. In the future, research on respiratory symptoms should be graded with symptom severity and burden to further evaluate their effect on training and competition and explore whether long-time exposure to high ventilation of dry and cold air actually desensitises cough receptors and prevents prolonged cough.
Acknowledgments
The authors wish to thank Eero Hietanen and Larissa Erola from Finnish Ski Association for their help in contacting the athletes for this study.
Footnotes
Twitter: @rikhardfi
Contributors: All authors designed the study and approved the final manuscript. RM-H acted as the guarantor, collected the data, conducted the data analyses and wrote the first manuscript.
Funding: This study was financially supported by Tampere Tuberculosis Foundation and Foundation of the Finnish Anti-Tuberculosis Association, Väinö and Laina Kivi Foundation, Ida Montin Foundation, Urheiluopistosäätiö, Allergy Research Foundation and The Research Foundation of the Pulmonary Diseases.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Pirkanmaa Health Care District (R18108). Participants gave informed consent to participate in the study before taking part.
References
- 1.Sandbakk Ø, Holmberg HC. A reappraisal of success factors for Olympic cross-country skiing. Int J Sports Physiol Perform 2014;9:117–21. 10.1123/ijspp.2013-0373 [DOI] [PubMed] [Google Scholar]
- 2.Holmberg HC, Rosdahl H, Svedenhag J. Lung function, arterial saturation and oxygen uptake in elite cross country skiers: influence of exercise mode. Scand J Med Sci SPORTS 2007;17:437–44. 10.1111/j.1600-0838.2006.00592.x [DOI] [PubMed] [Google Scholar]
- 3.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced Bronchoconstriction in elite athletes. Journal of Allergy and Clinical Immunology 2008;122:225–35. 10.1016/j.jaci.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 4.Heir T, Oseid S. Self‐Reported asthma and Exercise‐Induced asthma symptoms in High‐Level competitive Cross‐Country skiers. Scand J Med Sci Sports 1994;4:128–33. 10.1111/j.1600-0838.1994.tb00415.x [DOI] [Google Scholar]
- 5.Sue-Chu M, Larsson L, Bjermer L, et al. Prevalence of asthma in young cross-country skiers in central Scandinavia: differences between Norway and Sweden. Respir Med 1996;90:99–105. 10.1016/s0954-6111(96)90206-1 [DOI] [PubMed] [Google Scholar]
- 6.Turmel J, Poirier P, Bougault V, et al. Cardiorespiratory screening in elite endurance sports athletes: the Quebec study. Phys Sportsmed 2012;40:55–65. 10.3810/psm.2012.09.1982 [DOI] [PubMed] [Google Scholar]
- 7.Norqvist J, Eriksson L, Söderström L, et al. Self-reported physician-diagnosed asthma among Swedish adolescent, adult and former elite endurance athletes. Journal of Asthma 2015;52:1046–53. 10.3109/02770903.2015.1038389 [DOI] [PubMed] [Google Scholar]
- 8.Eklund LM, Irewall T, Lindberg A, et al. Prevalence, age at Onset, and risk factors of self-reported asthma among Swedish adolescent elite cross-country skiers. Scand J Med Sci Sports 2018;28:180–6. 10.1111/sms.12879 [DOI] [PubMed] [Google Scholar]
- 9.Rundell KW, Im J, Mayers LB, et al. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med Sci Sports Exerc 2001;33:208–13. 10.1097/00005768-200102000-00006 [DOI] [PubMed] [Google Scholar]
- 10.Turmel J, Bougault V, Boulet LP. Seasonal variations of cough reflex sensitivity in elite athletes training in cold air environment. Cough 2012;8:2. 10.1186/1745-9974-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull JH, Dickinson JW, Jackson AR. Cough in exercise and athletes. Pulm Pharmacol Ther 2017;47:49–55. 10.1016/j.pupt.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Boulet L-P, Turmel J, Irwin RS, et al. Cough in the athlete: CHEST guideline and expert panel report. Chest 2017;151:441–54. 10.1016/j.chest.2016.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäki-Heikkilä R, Karjalainen J, Parkkari J, et al. Asthma in competitive cross-country skiers: A systematic review and meta-analysis. Sports Med 2020;50:1963–81. 10.1007/s40279-020-01334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mäki-Heikkilä R, Karjalainen J, Parkkari J, et al. Higher prevalence but later age at onset of asthma in Cross‐Country skiers compared with General population. Scand J Med Sci Sports 2021;31:2259–66. 10.1111/sms.14040 [DOI] [PubMed] [Google Scholar]
- 15.Irwin RS, French CL, Chang AB, et al. Classification of cough as a symptom in adults and management Algorithms. Chest 2018;153:196–209. 10.1016/j.chest.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test – A survey for assessing asthma control. J Allergy Clin Immunol 2004;113:59–65. 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Silvers WS. The skier’s nose: a model of cold-induced Rhinorrhea. Ann Allergy 1991;67:32–6. [PubMed] [Google Scholar]
- 18.Cruz AA, Togias A. Upper Airways reactions to cold air. Curr Allergy Asthma Rep 2008;8:111–7. 10.1007/s11882-008-0020-z [DOI] [PubMed] [Google Scholar]
- 19.Boulet L-P, Turmel J. Cough in exercise and athletes. Pulm Pharmacol Ther 2019;55:67–74. 10.1016/j.pupt.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Koskela HO, Nurmi HM, Purokivi MK. Cough-provocation tests with Hypertonic aerosols. ERJ Open Res 2020;6:00338-2019. 10.1183/23120541.00338-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koskela HO, Kontra KM, Purokivi MK, et al. Interpretation of cough provoked by airway challenges. Chest 2005;128:3329–35. 10.1378/chest.128.5.3329 [DOI] [PubMed] [Google Scholar]
- 22.Mäki-Heikkilä R, Karjalainen J, Parkkari J, et al. High training volume is associated with increased prevalence of non-allergic asthma in competitive cross-country skiers. BMJ Open Sport Exerc Med 2022;8:e001315. 10.1136/bmjsem-2022-001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koskela HO, Nurmi HM, Birring SS. Utility of cough provocation tests in chronic cough and respiratory diseases: A comprehensive review and introduction of new reference ranges for the capsaicin test. Allergy Asthma Immunol Res 2021;13:833–49. 10.4168/aair.2021.13.6.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson SD, Kippelen P. Exercise-induced Bronchoconstriction: pathogenesis. Curr Allergy Asthma Rep 2005;5:116–22. 10.1007/s11882-005-0084-y [DOI] [PubMed] [Google Scholar]
- 25.Heir T. Longitudinal variations in bronchial responsiveness in Cross‐Country skiers and control subjects. Scand J Med Sci Sports 1994;4:134–9. 10.1111/j.1600-0838.1994.tb00416.x [DOI] [PubMed] [Google Scholar]
- 26.Heir T, Larsen S. The influence of training intensity, airway infections and environmental conditions on seasonal variations in bronchial responsiveness in Cross‐Country skiers. Scand J Med Sci Sports 1995;5:152–9. 10.1111/j.1600-0838.1995.tb00029.x [DOI] [PubMed] [Google Scholar]
- 27.Chung KF, McGarvey L, Song W-J, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022;8. 10.1038/s41572-022-00370-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ternesten-Hasséus E, Johansson EL, Millqvist E. Cough reduction using capsaicin. Respiratory Medicine 2015;109:27–37. 10.1016/j.rmed.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 29.Slovarp L, Reynolds JE, Bozarth-Dailey E, et al. Cough desensitization treatment: A randomized, sham-controlled pilot trial for patients with refractory chronic cough. Respir Med 2022;193:106739. 10.1016/j.rmed.2022.106739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morice AH, Millqvist E, Belvisi MG, et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Respir J 2014;44:1132–48. 10.1183/09031936.00218613 [DOI] [PubMed] [Google Scholar]
- 31.Millqvist E. The airway sensory Hyperreactivity syndrome. Pulm Pharmacol Ther 2011;24:263–6. 10.1016/j.pupt.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 32.Diver S, Russell RJ, Brightling CE. Cough and eosinophilia. J Allergy Clin Immunol Pract 2019;7:1740–7. 10.1016/j.jaip.2019.04.048 [DOI] [PubMed] [Google Scholar]
- 33.Schwellnus M, Adami PE, Bougault V, et al. International Olympic Committee (IOC) consensus statement on acute respiratory illness in athletes part 2: non-infective acute respiratory illness. Br J Sports Med 2022:bjsports-2022-105567. 10.1136/bjsports-2022-105567 [DOI] [PubMed] [Google Scholar]
- 34.Irewall T, Bäcklund C, Nordang L, et al. High prevalence of exercise-induced Laryngeal obstruction in a cohort of elite cross-country skiers. Med Sci Sports Exerc 2021;53:1134–41. 10.1249/MSS.0000000000002581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilpeläinen M, Terho EO, Helenius H, et al. Validation of a new questionnaire on asthma, allergic rhinitis, and Conjunctivitis in young adults. Allergy 2001;56:377–84. 10.1034/j.1398-9995.2001.056005377.x [DOI] [PubMed] [Google Scholar]
- 36.Kotaniemi JT, Hassi J, Kataja M, et al. Does non-responder bias have a significant effect on the results in a postal questionnaire study Eur J Epidemiol 2001;17:809–17. 10.1023/a:1015615130459 [DOI] [PubMed] [Google Scholar]
- 37.Lallukka T, Pietiläinen O, Jäppinen S, et al. Factors associated with health survey response among young employees: a register-based study using Online, mailed and telephone interview data collection methods. BMC Public Health 2020;20:184. 10.1186/s12889-020-8241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrahamsen R, Svendsen MV, Henneberger PK, et al. Non-response in a cross-sectional study of respiratory health in Norway. BMJ Open 2016;6:e009912. 10.1136/bmjopen-2015-009912 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2022-001502supp001.pdf (570.8KB, pdf)
Data Availability Statement
No data are available. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.