Abstract
Objective
There is room for improvement in the knowledge of female gout, often noted at risk of gender blindness. This study aims to compare the prevalence of comorbidities in women versus men hospitalised with gout in Spain.
Methods
This is an observational, multicentre, cross-sectional study in public and private Spanish hospitals analysing the minimum basic data set from 192 037 hospitalisations in people with gout (International Classification of Diseases, Ninth Revision (ICD-9) coding) from 2005 to 2015. Age and several comorbidities (ICD-9) were compared by sex, with a subsequent stratification of comorbidities by age group. The association between each comorbidity and sex was assessed using multivariable logistic regression. A clinical decision tree algorithm was constructed to predict the sex of patients with gout based on age and comorbidities alone.
Results
Women with gout (17.4% of the sample) were significantly older than men (73.9±13.7 years vs 64.0±14.4 years, p<0.001). Obesity, dyslipidaemia, chronic kidney disease, diabetes mellitus, heart failure, dementia, urinary tract infection and concurrent rheumatic disease were more common in women. Female sex was strongly associated with increasing age, heart failure, obesity, urinary tract infection and diabetes mellitus, while male sex was associated with obstructive respiratory diseases, coronary disease and peripheral vascular disease. The decision tree algorithm built showed an accuracy of 74.4%.
Conclusions
A nationwide analysis of inpatients with gout in 2005–2015 confirms a different comorbidity profile between men and women. A different approach to female gout is needed to reduce gender blindness.
Keywords: Gout, Cardiovascular Diseases, Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
A different profile of comorbidities is often reported between women and men with gout.
WHAT THIS STUDY ADDS
This analysis of the Spanish hospital database demonstrates that women with gout are almost 10 years older than men and suffer from heart failure, obesity, diabetes mellitus, dyslipidaemia and chronic kidney disease, in some cases with differences exceeding 10 percentage points, compared with men.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our results imply women with gout should be approached with special consideration in clinical practice to address gender blindness and guarantee the best care from healthcare professionals.
Introduction
In health, there are differences between men and women that can be attributed to biological sex (related to androgen or oestrogen blood levels, among others) and gender (ie, the social conception of men and women). Whereas the former is unavoidable, the latter responds to human biases that can and must be addressed. In 1991, Bernadine Healy coined the term ‘Yentl syndrome’ to illustrate that women with symptoms of myocardial infarction were less likely to be tested and treated than men.1 This initial description rapidly extended to distinct diseases of different areas and specialties. It is our impression and that of other authors2 that gender blindness also exists in gout, a rheumatic condition based on the deposition of monosodium urate crystals in joints and surrounding structures. This deposition leads to an inflammatory state with acute local pain, but also systemic long-term consequences, such as accelerated arteriosclerosis or a risk of kidney function decline. Gout is more prevalent in men, who intrinsically have higher serum urate levels; after the oestrogen drop in menopause, the difference between men and women diminishes, although levels rarely overlap.3
Patients with gout present a myriad of comorbidities, especially cardiovascular, renal and endocrine-metabolic conditions. Many of these, such as kidney failure, are already present at diagnosis and are associated with the development of hyperuricaemia and gout.4 Moreover, the odds of developing new comorbidities increase progressively after the first gout flare, with risk higher than in the general population.5 Furthermore, gout and its associated comorbidities have increased over the last few decades.6 Recent studies have identified different phenotypes of patients with gout based on clustering of certain comorbidities.7–9 These studies also revealed that the accompanying comorbidities vary with time. For instance, stroke develops earlier in women with gout and type 2 diabetes than in men.9 Gout-related hospital admissions have risen over the last decades,10 and it is unknown whether some comorbidities play a role here.
Gout in women has received less attention in the published literature and is notably under-represented in phenotype evaluation studies and clinical trials.7 11 In addition, no particular notes about gout in women are given in the main guidelines and recommendations.12–14 Both our clinical experience and some published reports suggest that prevalence in women increases at older ages, with more accompanying conditions (higher use of diuretics, higher prevalence of chronic kidney disease and heart failure)15 16 and different clinical manifestations hampering the identification of gout (subtle, vague, often migratory joint pain, not fulfilling flare criteria, or persistent low-grade arthritis) and delaying proper management, with larger disability and higher urate levels.17 18 Two population-based studies reported a numerically higher gout-related mortality risk in women compared with men19 20; however, the impact of different comorbidity profiles still needs to be elucidated. In terms of treatment, women with gout are less likely to receive urate-lowering agents and more likely to receive corticosteroids and non-steroidal anti-inflammatory drugs.15 Thus, there is room for improvement in the knowledge of female gout.
This study aimed to assess differences in comorbidities in women versus men hospitalised with gout in Spain and specifically to identify the characteristics of women with gout. We evaluated the strength of association between each comorbidity and sex and constructed an automatic algorithm to predict the sex of patients with gout based only on age and comorbidities.
Materials and methods
Study design, population and study variables
This observational, multicentre, cross-sectional study used the minimum basic data set (MBDS), which contains relevant information (age, sex, primary and secondary diagnoses, diagnostic and therapeutic procedures) for all admissions in private and public Spanish hospitals, extracted from discharge reports. We included all adults (≥18 years) admitted to Spanish hospitals with a primary or secondary diagnosis of gout from 1 January 2005 to 31 December 2015. Gout was defined according to the International Classification of Diseases, Ninth Revision (ICD-9: 274.0, 274.00, 274.01, 274.02, 274.03, 274.1, 274.10, 274.11, 274.19, 274.8, 274.81, 274.82, 274.89 or 274.9). Data after 2015 were not analysed due to the introduction of ICD-10. The National Statistics Institute provided data, and the first report assessing trends in gout hospitalisations has been previously published.21 The present analysis focuses on age and the presence of comorbidities in hospitalised women versus men.
The primary explanatory variable was sex (male/female), as registered in the discharge reports. The outcome variables were age at admission and the following comorbidities (according to their ICD-9 coding; see online supplemental material): obesity, dyslipidaemia, chronic kidney disease, diabetes mellitus, coronary heart disease, chronic heart failure, peripheral vascular disease, arrhythmia, venous thromboembolism, cerebrovascular disease, dementia, urinary tract infection, pneumonia, sepsis, obstructive pulmonary disease, liver disease and rheumatic disease. Two comorbidities of interest in patients with gout, arterial hypertension and urinary lithiasis, were not analysed because they showed lower prevalence (26.3% and 0.5%, respectively) than expected according to previous studies in Spanish rheumatology clinics and wards, where hypertension was present in approximately 62.1%–82.5% of patients.7 22 These discrepant data suggest under-recording in the MBDS, and in fact the undercoding of arterial hypertension is common and has been described in other settings.23 24 In addition, we noted whether gout was recorded as the primary diagnosis or one of the secondary diagnoses.
rmdopen-2023-003191supp001.pdf (286.6KB, pdf)
Statistical analysis
Age was expressed as mean with SD, and qualitative variables (sex, comorbidities) as frequencies and percentages. To compare the sexes, Student’s t-test was used for quantitative variables and χ2 test for qualitative variables. To rule out age as a potential confounder, we later categorised the population into six age groups with similar size (≤70 years, 71–80 years, 81–85 years, 86–90 years, 91–95 years and >95 years) and stratified the analyses.
In order to assess the strength of the association between each comorbidity and sex, a multivariable logistic regression analysis was undertaken. To avoid the potential bias of having many more men than women in the population, we randomly selected a sample of men of the same size as that of women (n=33 384). Age and comorbidities were considered dependent variables, whereas sex was the independent variable. Results were expressed as coefficients ranging from −1 to +1, with negative coefficients showing a relation to men and positive coefficients to women, and as ORs and their corresponding 95% CIs. To determine whether comorbidity profiles were similar in younger and older patients, we fit new models after dividing the population into age groups (dichotomously, ≤60 years vs >60 years; and categorically, using the six age bands described above). Moreover, the models were stratified according to whether gout was a primary or secondary diagnosis in the MBDS. To confirm our findings, another model was then built, which also included year of admission, Spanish region of residence, type of admission and hospitalisation service as covariates.
A decision tree algorithm was constructed to predict the sex of individuals with gout, considering their age and comorbidities. These are flow chart-like tree structures that help in decision-making, where an internal node represents an attribute, the branch a decision rule and each leaf node the outcome.25 Intersections are generated based on the number of records and attributes in the data set.
For the regression models and decision tree algorithm, the accuracy was estimated as the rate of the population whose sex was correctly predicted by the model.
Google Colab (Alphabet, Mountain View, California), which allows execution of Python, was used to analyse the data using the packages matplotlib V.3.2.2, pandas V.1.3.5, scikit-learn V.1.0.2 and statsmodels V.0.12.2. Statistical significance was set at p<0.050.
Patient and public involvement
No patients were involved in formulating the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of the results. Due to study characteristics, we are not able to disseminate the results of the research to study participants, although they will be widely disseminated to the general public and the relevant gout community through local and national patient associations.
Results
Data for 192 037 hospital admissions were analysed, including 158 646 (82.6%) in men and 33 384 (17.4%) in women. The mean age was 64.0 (SD 14.4) years in men and 73.9 (SD 13.7) years in women (p<0.001). Gout was the primary diagnosis in 10 512 (5.5%) patients, with 9027 men (85.9%) and 1485 women (14.1%), and a secondary diagnosis in 181 518 (94.5%) patients, with 149 619 men (82.4%) and 31 899 women (17.6%). In addition, seven cases were classified as ‘undetermined sex’ and were excluded from the analysis.
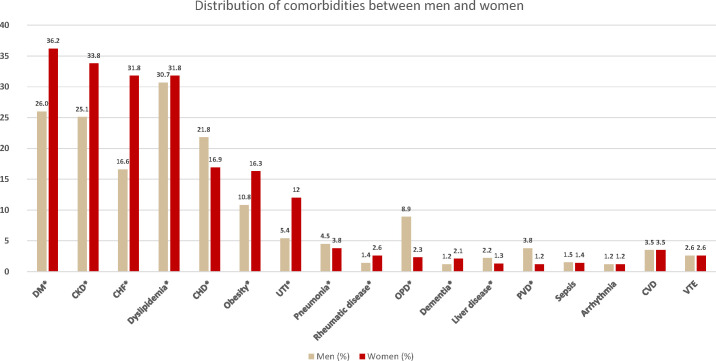
The prevalence of comorbidities according to age and sex is presented in tables 1–3. Figure 1 illustrates the global prevalence of comorbidities without stratification by age. Comorbidities that were significantly more prevalent in women were obesity (16.3% of women vs 10.8% of men), dyslipidaemia (31.8% vs 30.7%), chronic kidney disease (33.8% vs 25.1%), diabetes mellitus (36.2% vs 26.0%), heart failure (31.8% vs 16.6%), dementia (2.1% vs 1.2%), urinary tract infection (12.0% vs 5.4%) and concurrent rheumatic disease (2.6% vs 1.4%). Conversely, comorbidities predominant in men were coronary heart disease (21.8% of men vs 16.9% of women), peripheral vascular disease (3.8% vs 1.2%), pneumonia (4.5% vs 3.8%), obstructive pulmonary disease (8.9% vs 2.3%) and liver disease (2.2% vs 1.3%). No differences were observed in arrhythmia (p=0.53), venous thromboembolism (p=0.97), cerebrovascular disease (p=0.74) or sepsis (p=0.34).
Table 1.
Prevalence of obesity, dyslipidaemia, chronic kidney disease, diabetes mellitus and coronary heart disease, by sex and age group
| Comorbidity | Age group (years) (men/women, N/N) | Men | Women | P value |
| n (%) | n (%) | |||
| Obesity | Total | 17 084 (10.8) | 5444 (16.3) | <0.001 |
| <70 (28 214/1912) | 4496 (15.9) | 373 (19.5) | 0.031 | |
| 71–80 (33 673/3319) | 4623 (13.7) | 786 (23.7) | <0.001 | |
| 81–85 (20 771/3179) | 2410 (11.6) | 779 (24.6) | <0.001 | |
| 86–90 (27 521/6089) | 2800 (10.2) | 1229 (20.2) | <0.001 | |
| 91–95 (25 032/7374) | 1795 (7.2) | 1192 (16.2) | <0.001 | |
| >95 (23 407/11 521) | 960 (4.1) | 1085 (9.4) | <0.001 | |
| Dyslipidaemia | Total | 48 634 (30.7) | 10 624 (31.8) | 0.020 |
| <70 (28 214/1912) | 9126 (32.3) | 631 (33.0) | 0.57 | |
| 71–80 (33 673/3319) | 12 347 (36.7) | 1241 (37.4) | 0.42 | |
| 81–85 (20 771/3179) | 7092 (34.1) | 1219 (38.4) | 0.009 | |
| 86–90 (27 521/6089) | 8840 (32.1) | 2217 (36.4) | <0.001 | |
| 91–95 (25 032/7374) | 6866 (27.4) | 2465 (33.4) | <0.001 | |
| >95 (23 407/11 521) | 4363 (18.6) | 2851 (24.8) | <0.001 | |
| Chronic kidney disease | Total | 39 817 (25.1) | 11 291 (33.8) | <0.001 |
| <70 (28 214/1912) | 3772 (13.4) | 539 (28.2) | <0.001 | |
| 71–80 (33 673/3319) | 6381 (18.9) | 959 (28.9) | <0.001 | |
| 81–85 (20 771/3179) | 5120 (24.7) | 922 (29.0) | 0.001 | |
| 86–90 (27 521/6089) | 7850 (28.5) | 2197 (36.1) | <0.001 | |
| 91–95 (25 032/7374) | 8386 (33.5) | 2729 (37.0) | <0.001 | |
| >95 (23 407/11 521) | 8307 (35.5) | 3945 (34.2) | 0.022 | |
| Diabetes mellitus | Total | 41 185 (26.0) | 12 073 (36.2) | <0.001 |
| <70 (28 214/1912) | 4759 (16.9) | 440 (23.0) | <0.001 | |
| 71–80 (33 673/3319) | 9206 (27.3) | 1286 (38.8) | <0.001 | |
| 81–85 (20 771/3179) | 6393 (30.8) | 1314 (41.3) | <0.001 | |
| 86–90 (27 521/6089) | 8349 (30.3) | 2583 (42.4) | <0.001 | |
| 91–95 (25 032/7374) | 7108 (28.4) | 2937 (39.8) | <0.001 | |
| >95 (23 407/11 521) | 5360 (22.9) | 3507 (30.4) | <0.001 | |
| Coronary heart disease | Total | 34 507 (21.8) | 5633 (16.9) | <0.001 |
| <70 (28 214/1912) | 3764 (13.3) | 116 (6.1) | <0.001 | |
| 71–80 (33 673/3319) | 7115 (21.1) | 407 (12.3) | <0.001 | |
| 81–85 (20 771/3179) | 4715 (22.7) | 456 (14.3) | <0.001 | |
| 86–90 (27 521/6089) | 6883 (25.0) | 1067 (17.5) | <0.001 | |
| 91–95 (25 032/7374) | 6226 (24.9) | 1333 (18.1) | <0.001 | |
| >95 (23 407/11 521) | 5804 (24.8) | 2254 (19.6) | <0.001 |
Table 2.
Prevalence of heart failure, peripheral vascular disease, arrhythmia, venous thromboembolism, cerebrovascular disease and dementia, by sex and age group
| Comorbidity | Age group (years) (men/women, N/N) | Men | Women | P value |
| n (%) | n (%) | |||
| Heart failure | Total | 26 406 (16.6) | 10 631 (31.8) | <0.001 |
| <70 (28 214/1912) | 1648 (5.8) | 169 (8.8) | <0.001 | |
| 71–80 (33 673/3319) | 3505 (10.4) | 578 (17.4) | <0.001 | |
| 81–85 (20 771/3179) | 3050 (14.7) | 780 (24.5) | <0.001 | |
| 86–90 (27 521/6089) | 5414 (19.6) | 1818 (29.9) | <0.001 | |
| 91–95 (25 032/7374) | 6054 (24.2) | 2657 (36.0) | <0.001 | |
| >95 (23 407/11 521) | 6735 (28.8) | 4629 (40.2) | <0.001 | |
| Peripheral vascular disease | Total | 5980 (3.8) | 385 (1.2) | <0.001 |
| <70 (28 214/1912) | 417 (1.5) | 9 (0.5) | <0.001 | |
| 71–80 (33 673/3319) | 1206 (3.6) | 42 (1.3) | <0.001 | |
| 81–85 (20 771/3179) | 887 (4.3) | 32 (1.0) | <0.001 | |
| 86–90 (27 521/6089) | 1333 (4.8) | 76 (1.3) | <0.001 | |
| 91–95 (25 032/7374) | 1133 (4.5) | 106 (1.4) | <0.001 | |
| >95 (23 407/11 521) | 1004 (4.3) | 120 (1.0) | <0.001 | |
| Arrhythmia | Total | 1950 (1.2) | 396 (1.2) | 0.53 |
| <70 (28 214/1912) | 260 (0.9) | 6 (0.3) | 0.009 | |
| 71–80 (33 673/3319) | 429 (1.3) | 54 (1.6) | 0.10 | |
| 81–85 (20 771/3179) | 309 (1.5) | 47 (1.5) | 1.00 | |
| 86–90 (27 521/6089) | 371 (1.4) | 90 (1.5) | 0.46 | |
| 91–95 (25 032/7374) | 337 (1.4) | 89 (1.2) | 0.39 | |
| >95 (23 407/11 521) | 244 (1.0) | 110 (1.0) | 0.48 | |
| Venous thromboembolism | Total | 4087 (2.6) | 862 (2.6) | 0.97 |
| <70 (28 214/1912) | 816 (2.9) | 33 (1.7) | 0.004 | |
| 71–80 (33 673/3319) | 874 (2.6) | 76 (2.3) | 0.32 | |
| 81–85 (20 771/3179) | 549 (2.6) | 78 (2.5) | 0.57 | |
| 86–90 (27 521/6089) | 690 (2.5) | 182 (3.0) | 0.034 | |
| 91–95 (25 032/7374) | 613 (2.5) | 210 (2.9) | 0.061 | |
| >95 (23 407/11 521) | 545 (2.3) | 283 (2.5) | 0.48 | |
| Cerebrovascular disease | Total | 5620 (3.5) | 1170 (3.5) | 0.74 |
| <70 (28 214/1912) | 629 (2.2) | 36 (1.9) | 0.36 | |
| 71–80 (33 673/3319) | 1108 (3.3) | 102 (3.1) | 0.54 | |
| 81–85 (20 771/3179) | 782 (3.8) | 102 (3.2) | 0.13 | |
| 86–90 (27 521/6089) | 1079 (3.9) | 219 (3.6) | 0.26 | |
| 91–95 (25 032/7374) | 1054 (4.2) | 271 (3.7) | 0.045 | |
| >95 (23 407/11 521) | 968 (4.1) | 440 (3.8) | 0.17 | |
| Dementia | Total | 1903 (1.2) | 709 (2.1) | <0.001 |
| <70 (28 214/1912) | 15 (0.1) | 1 (0.1) | 1.00 | |
| 71–80 (33 673/3319) | 88 (0.3) | 3 (0.1) | 0.087 | |
| 81–85 (20 771/3179) | 149 (0.7) | 25 (0.8) | 0.75 | |
| 86–90 (27 521/6089) | 372 (1.4) | 87 (1.4) | 0.67 | |
| 91–95 (25 032/7374) | 460 (1.8) | 149 (2.0) | 0.33 | |
| >95 (23 407/11 521) | 819 (3.5) | 444 (3.9) | 0.10 |
Table 3.
Prevalence of urinary tract infection, pneumonia, sepsis, obstructive pulmonary disease, liver disease and concurrent rheumatic disease, by sex and age group
| Comorbidity | Age group (years) (men/women, N/N) | Men | Women | P value |
| n (%) | n (%) | |||
| Urinary tract infection | Total | 8480 (5.4) | 4017 (12.0) | <0.001 |
| <70 (28 214/1912) | 916 (3.3) | 188 (9.8) | <0.001 | |
| 71–80 (33 673/3319) | 1289 (3.8) | 343 (10.3) | <0.001 | |
| 81–85 (20 771/3179) | 1036 (5.0) | 319 (10.0) | <0.001 | |
| 86–90 (27 521/6089) | 1531 (5.6) | 658 (10.8) | <0.001 | |
| 91–95 (25 032/7374) | 1631 (6.5) | 887 (12.0) | <0.001 | |
| >95 (23 407/11 521) | 2077 (8.9) | 1622 (14.1) | <0.001 | |
| Pneumonia | Total | 7068 (4.5) | 1273 (3.8) | <0.001 |
| <70 (28 214/1912) | 607 (2.2) | 37 (1.9) | 0.58 | |
| 71–80 (33 673/3319) | 1116 (3.3) | 85 (2.6) | 0.022 | |
| 81–85 (20 771/3179) | 881 (4.2) | 103 (3.2) | 0.009 | |
| 86–90 (27 521/6089) | 1376 (5.0) | 184 (3.0) | <0.001 | |
| 91–95 (25 032/7374) | 1455 (5.8) | 319 (4.3) | 0.009 | |
| >95 (23 407/11 521) | 1633 (7.0) | 545 (4.7) | <0.001 | |
| Sepsis | Total | 2399 (1.5) | 481 (1.4) | 0.34 |
| <70 (28 214/1912) | 332 (1.2) | 33 (1.7) | 0.044 | |
| 71–80 (33 673/3319) | 461 (1.4) | 53 (1.6) | 0.32 | |
| 81–85 (20 771/3179) | 333 (1.6) | 47 (1.5) | 0.65 | |
| 86–90 (27 521/6089) | 423 (1.5) | 97 (1.6) | 0.78 | |
| 91–95 (25 032/7374) | 452 (1.8) | 105 (1.4) | 0.030 | |
| >95 (23 407/11 521) | 398 (1.7) | 146 (1.3) | 0.002 | |
| Obstructive pulmonary disease | Total | 14 080 (8.9) | 761 (2.3) | <0.001 |
| <70 (28 214/1912) | 844 (3.0) | 40 (2.1) | 0.029 | |
| 71–80 (33 673/3319) | 2379 (7.1) | 51 (1.5) | <0.001 | |
| 81–85 (20 771/3179) | 1953 (9.4) | 74 (2.3) | <0.001 | |
| 86–90 (27 521/6089) | 3144 (11.4) | 127 (2.1) | <0.001 | |
| 91–95 (25 032/7374) | 2957 (11.8) | 184 (2.5) | <0.001 | |
| >95 (23 407/11 521) | 2803 (12.0) | 285 (2.5) | <0.001 | |
| Liver disease | Total | 3547 (2.2) | 427 (1.3) | <0.001 |
| <70 (28 214/1912) | 988 (3.5) | 68 (3.6) | 0.95 | |
| 71–80 (33 673/3319) | 1029 (3.1) | 85 (2.8) | 0.12 | |
| 81–85 (20 771/3179) | 565 (2.7) | 58 (1.8) | 0.004 | |
| 86–90 (27 521/6089) | 461 (1.7) | 91 (1.5) | 0.35 | |
| 91–95 (25 032/7374) | 327 (1.3) | 66 (0.9) | 0.006 | |
| >95 (23 407/11 521) | 177 (0.8) | 59 (0.5) | 0.011 | |
| Rheumatic disease | Total | 2283 (1.4) | 874 (2.6) | <0.001 |
| <70 (28 214/1912) | 271 (1.0) | 85 (4.5) | <0.001 | |
| 71–80 (33 673/3319) | 423 (1.3) | 111 (3.3) | <0.001 | |
| 81–85 (20 771/3179) | 281 (1.4) | 96 (3.0) | <0.001 | |
| 86–90 (27 521/6089) | 451 (1.6) | 142 (2.3) | <0.001 | |
| 91–95 (25 032/7374) | 471 (1.2) | 214 (2.9) | <0.001 | |
| >95 (23 407/11 521) | 386 (1.7) | 226 (2.0) | 0.040 |
Figure 1.
Prevalence of comorbidities in men and women with gout. Prevalence is expressed as the percentage of the population with each comorbidity. *Indicates statistical significance between sex groups. CHD, coronary heart disease; CHF, chronic heart failure; CKD, chronic kidney disease; CVD, cerebrovascular disease; DM, diabetes mellitus; OPD, obstructive pulmonary disease; PVD, peripheral vascular disease; UTI, urinary tract infection; VTE, venous thromboembolism.
After stratification by age group (tables 1–3), differences in dementia disappeared. For dyslipidaemia, differences reached significance starting at 81–85 years. In the case of chronic kidney disease, there was a downward trend, as in the oldest subgroup (>95 years) the prevalence was significantly higher in men. Differences remained statistically significant for all subgroups in the rest of the analysed comorbidities.
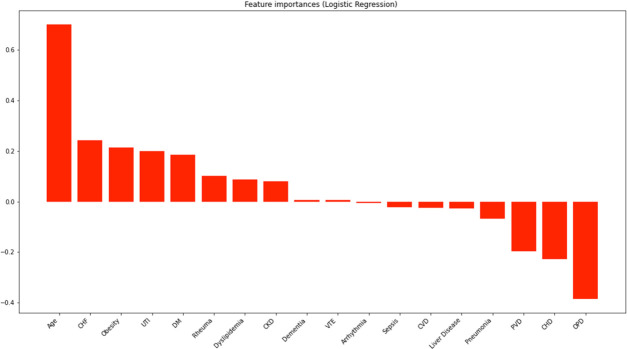
The multiple logistic regression model (figure 2) showed an accuracy of 68.5%. Comorbidities with positive coefficients (related to female sex) were age (+0.71), heart failure (+0.24), obesity (+0.21), urinary tract infection (+0.20), diabetes mellitus (+0.19), rheumatic disease (+0.10), dyslipidaemia (+0.09), chronic kidney disease (+0.08), venous thromboembolism (+0.01) and dementia (+0.01). Comorbidities with negative coefficients (related to male sex) were obstructive pulmonary disease (−0.39), coronary heart disease (−0.23), peripheral vascular disease (−0.20), pneumonia (−0.07), liver disease (−0.03), cerebrovascular disease (−0.03), sepsis (−0.02) and arrhythmia (−0.01). Table 4 presents the ORs with 95% CIs and p values; all were statistically significant. Model 2, with an accuracy score of 68.7%, delivered similar results, but dementia and venous thrombosis were no longer associated with female sex.
Figure 2.
Association between age and comorbidities with sex, where positive coefficients indicate an association with female sex and negative ones with male sex. CHD, coronary heart disease; CHF, chronic heart failure; CKD, chronic kidney disease; CVD, cerebrovascular disease; DM, diabetes mellitus; OPD, obstructive pulmonary disease; PVD, peripheral vascular disease; Rheuma, concurrent rheumatic disease; UTI, urinary tract infection; VTE, venous thromboembolism.
Table 4.
Logistic regression coefficients, OR with 95% CI, and p values for age and each comorbidity in the whole population
| Model 1* | Model 2† | |||||||
| Coefficient | OR | 95% CI | P value | Coefficient | OR | 95% CI | P value | |
| Age | +0.71 | 2.02 | 2.00 to 2.02 | <0.001 | +0.71 | 2.04 | 2.04 to 2.05 | <0.001 |
| Obesity | +0.21 | 1.24 | 1.23 to 1.24 | <0.001 | +0.21 | 1.24 | 1.23 to 1.24 | <0.001 |
| Dyslipidaemia | +0.09 | 1.09 | 1.09 to 1.09 | <0.001 | +0.08 | 1.08 | 1.08 to 1.09 | <0.001 |
| Chronic kidney disease | +0.08 | 1.08 | 1.08 to 1.09 | <0.001 | +0.06 | 1.06 | 1.05 to 1.06 | <0.001 |
| Diabetes mellitus | +0.19 | 1.20 | 1.20 to 1.21 | <0.001 | +0.18 | 1.20 | 1.20 to 1.21 | <0.001 |
| Coronary disease | −0.23 | 0.80 | 0.79 to 0.80 | <0.001 | −0.23 | 0.80 | 0.79 to 0.80 | <0.001 |
| Heart failure | +0.24 | 1.27 | 1.27 to 1.28 | <0.001 | +0.23 | 1.25 | 1.25 to 1.26 | <0.001 |
| Peripheral vascular disease | −0.20 | 0.82 | 0.82 to 0.82 | <0.001 | −0.20 | 0.82 | 0.82 to 0.82 | <0.001 |
| Arrhythmia | −0.01 | 1.00 | 0.99 to 1.00 | 0.011 | −0.01 | 0.99 | 0.99 to 0.99 | 0.011 |
| Venous thromboembolism | +0.01 | 1.01 | 1.00 to 1.01 | 0.002 | +0.01 | 1.00 | 1.00 to 1.01 | 0.137 |
| Cerebrovascular disease | −0.03 | 0.98 | 0.97 to 0.98 | <0.001 | −0.03 | 0.97 | 0.97 to 0.97 | <0.001 |
| Dementia | +0.01 | 1.01 | 1.00 to 1.01 | <0.001 | +0.001 | 1.00 | 1.00 to 1.01 | 0.180 |
| Urinary tract infection | +0.20 | 1.22 | 1.22 to 1.22 | <0.001 | +0.19 | 1.21 | 1.21 to 1.22 | <0.001 |
| Pneumonia | −0.07 | 0.93 | 0.93 to 0.94 | <0.001 | −0.08 | 0.92 | 0.92 to 0.93 | <0.001 |
| Sepsis | −0.02 | 0.98 | 0.97 to 0.98 | <0.001 | −0.03 | 0.97 | 0.97 to 0.98 | <0.001 |
| Obstructive pulmonary disease | −0.39 | 0.68 | 0.68 to 0.68 | <0.001 | −0.39 | 0.68 | 0.67 to 0.68 | <0.001 |
| Liver disease | −0.03 | 0.97 | 0.97 to 0.98 | <0.001 | −0.03 | 0.97 | 0.97 to 0.97 | <0.001 |
| Concurrent rheumatic disease | +0.10 | 1.11 | 1.10 to 1.11 | <0.001 | +0.10 | 1.11 | 1.10 to 1.11 | <0.001 |
Coefficients range from −1 to +1, with negative coefficients showing a relation to men and positive coefficients to women.
*Only includes age and comorbidities as covariates.
†Also adjusted for year of admission, Spanish region of residence, type of admission and hospitalisation service.
The logistic regression performed after dichotomising the population by age (≤60 years and >60 years) showed an accuracy of 61.6% and 62.9%, respectively (figure 3 and online supplemental table S1). Some differences were observed: for example, in younger patients, heart failure and dyslipidaemia were associated with male sex, while cerebrovascular disease and obstructive pulmonary disease were associated with female sex. The results of logistic regression for gout as a primary or secondary diagnosis and for populations divided into six age groups can be found in online supplemental tables S2 and S3.
Figure 3.
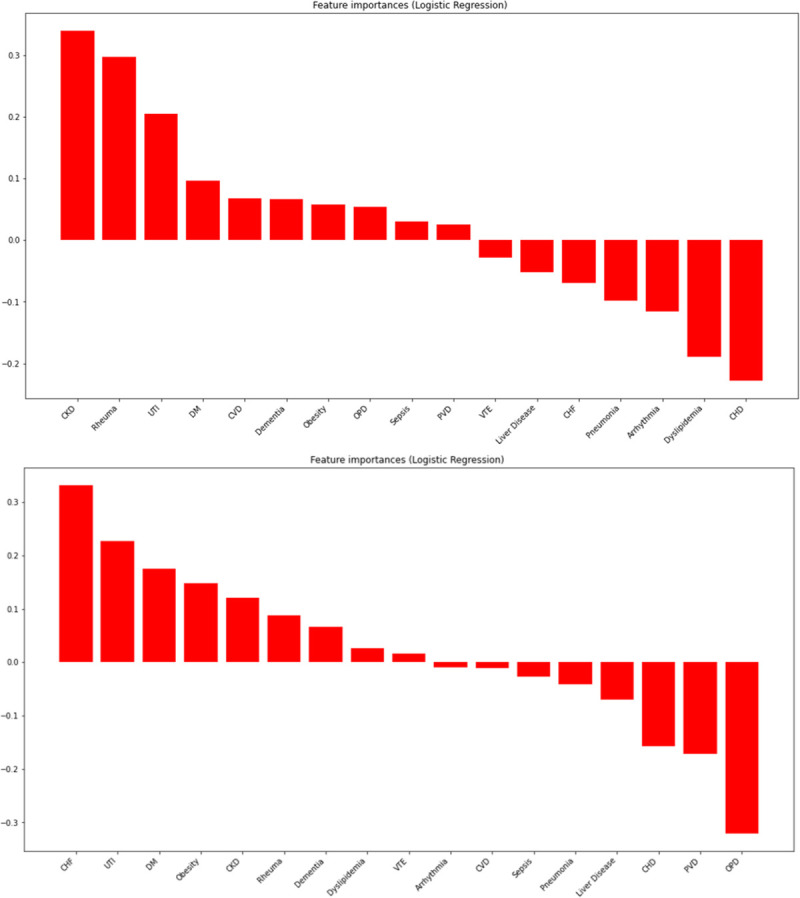
Association between sex and comorbidities, stratified by age: top image for population ≤60 years and bottom image for population >60 years. CHD, coronary heart disease; CHF, chronic heart failure; CKD, chronic kidney disease; CVD, cerebrovascular disease; DM, diabetes mellitus; OPD, obstructive pulmonary disease; PVD, peripheral vascular disease; Rheuma, concurrent rheumatic disease; UTI, urinary tract infection; VTE, venous thromboembolism.
A clinical decision tree algorithm was constructed (online supplemental figures S1–S3), obtaining an accuracy score of 74.4%. The first cut-off point was 85.5 years of age, which had the greatest discriminative value. The presence or absence of specific comorbidities led the algorithm to conclude male (orange) or female sex (blue boxes).
rmdopen-2023-003191supp002.pdf (4.7MB, pdf)
Discussion
This is the first study that used a large population of inpatients to assess comorbidities in female gout. Data from the Spanish hospital discharge database (MBDS) show that women with gout are almost 10 years older than their male counterparts and that they carry a higher risk of heart failure, obesity, diabetes mellitus, dyslipidaemia and chronic kidney disease, in some cases with differences exceeding 10 percentage points. Even after stratifying by age and adjusting for the other covariates, our results demonstrate different comorbidity profiles in women and men with gout. We were able to design a clinical decision tree capable of predicting the sex of the individuals solely using age and comorbidities, with an accuracy of almost 75%. These findings imply that age and comorbidities play a major role in women developing gout, with great relevance for clinical practice. The 25% inaccuracy of the models could be attributable to other contributors to gout, such as diet, drugs and genetics, probably with a more considerable impact on men.26
Some studies have previously addressed the differences in comorbidities between men and women with gout, but mainly as outpatients. The multicentre CACTUS study in France proved that hypertension, metabolic syndrome, heart failure and renal failure were more prevalent in women, who on average were also almost 10 years older.7 Similarly, Harrold et al17 analysed 1012 men and 262 women with gout in the USA and found that women were older and had higher rates of hypertension, diabetes, kidney failure and obesity. Our results among inpatients align with the previous ones and confirm that women with gout are older and carry a higher risk of developing certain comorbidities, mainly cardiovascular, kidney and endocrine-metabolic diseases. These differences were also present in younger subgroups, so particular attention to these conditions is needed when treating women with gout, regardless of their age.
Hospitalised women with gout in Spain were almost 10 years older than men, which might influence the comorbidity profiles. However, most results remained unaltered after age stratification, although the prevalence of dementia was no longer higher in women. In the case of chronic kidney disease, in younger subgroups its prevalence was higher in women, but with advancing age this imbalance progressively equalised and was then inverted. While the association between kidney disease and female sex was strongest in younger patients, in older women other comorbidities, such as heart failure, took the leading role. Gout in young women, especially before menopause, is unusual. Most cases are related to reduced renal excretion of urate due to renal diseases, whereas in younger men hyperuricaemia tends to be more related to genetics, diet or habits, or associated with metabolic syndrome.27 28 At later stages of life, kidney diseases are common in both men and women, weakening the association with gout.
Cardiovascular comorbidities closely derived from atherosclerosis, such as coronary heart disease and peripheral vascular disease, were associated with male sex, as occurs in the general population. The prevalence of cerebrovascular diseases was similarly distributed and later found mildly associated with men in the regression model. However, heart failure, which can have diverse origins, was strongly linked to female sex, especially at advanced ages. Heart failure, especially with preserved ejection fraction, is also known to occur more commonly in older women.29 This association is likely attributable to the greater use of diuretics in women with gout,16 as both loop agents and thiazides can increase serum urate levels by interacting with their renal transporters30; unfortunately, pharmacological therapies are not coded in the MBDS, so their role could not be assessed here. Hypertension is a major risk factor for heart failure and was also more prevalent in women with gout in other works,7 but we excluded this condition from the analysis because it was clearly under-recorded in the MBDS.
The relationship between sex and comorbidities in gout described here should directly concern general practitioners, rheumatologists and other specialists attending patients with gout. Clinicians should be aware of the role of sex as a primary, direct indicator of accompanying diseases: a woman with gout would probably be in her 70s and present obesity, kidney disease, diabetes mellitus or heart failure. On the other hand, a man with gout would be middle-aged and present with respiratory problems, coronary heart disease and peripheral vascular involvement. Therefore, sex may guide the search for comorbidities and inform tailored management of gout.
Our study has several strengths. First, the sample size (192 037 cases, with more than 30 000 women) is larger than similar studies, provides estimates with narrow CIs and uses robust multivariable models to rule out confounders. The sample was representative of the Spanish population and can be generalised to similar European populations with gout, but caution is advised if generalised to North Americans, with known differences in comorbidities.31 We used data from hospitalisations, but the overall prevalence of comorbidities is equivalent to other gout studies performed on an outpatient basis.22 The MBDS relies on ICD coding, which is extensively used for gout-related research in population-based data sets.32
Some limitations should also be noted. The cross-sectional nature of the study impedes any temporal evaluation that could label comorbidities as a cause or consequence of gout. As indicated earlier, no treatment data were available, nor was the patients’ ethnicity. We expect that most were white people of European descent, so our findings may need verification in other populations. Gout cases are included in the MBDS if they were registered in discharge records. Subsequently, some variability in the diagnosis of gout may occur, as data cover the practice of hundreds of physicians who might use different approaches, from the typical big toe inflammation in a person with hyperuricaemia to the identification of monosodium urate crystals in joint samples, the gold standard.33 Although gout is often under-recorded in discharge reports,34 the available numbers are sufficient to perform robust analyses. Some comorbidities of interest, such as hypertension and urinary lithiasis, were excluded since their prevalence was implausibly low according to our practice and other studies, suggesting under-recording. Other comorbidities evaluated in previous studies, like anaemia, cancer and psoriasis, were not analysed.9 The selected study period was until 2015, as in 2016 the diagnostic coding in MBDS was substituted by ICD-10 and discrepancies could have arisen.
Conclusion
A nationwide analysis of 11 years of hospitalisations in patients with gout confirms a different comorbidity profile between men and women. Women with gout were older and had a higher prevalence of heart failure, obesity, urinary tract infection, diabetes, dyslipidaemia, chronic kidney disease and concurrent rheumatic disease, and this difference persisted after multivariable adjustment. The association between sex, age and comorbidities is intense enough for sex prediction by an algorithm with considerable accuracy. Therefore, these results confirm the need to approach gout in women with special consideration, evaluating concurrent diseases that may impact the woman’s well-being and life expectancy, as well as the treatment choices for gout flares and urate reduction. All these should contribute to addressing gender blindness and guaranteeing the best care from healthcare professionals.
Acknowledgments
Ms Meggan Harris performed English editing of the manuscript.
Footnotes
Contributors: ER-S and MA designed the study. EDM provided data. ER-S and FB performed the data analyses. ER-S, EDM, FB and MA discussed and interpreted the results. ER-S wrote the first draft of the manuscript. ER-S, EDM, FB and MA all reviewed and approved the final version of the paper. MA is the guarantor for the present study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: EDM declares having received speaking fees from AbbVie, Novartis, Pfizer, MSD, BMS, UCB, Roche, Janssen, Sanofi and Lilly; consultancies from Novartis and Janssen; and a research grant from Roche. MA declares having received speaking fees from Menarini and an ongoing research grant from Grunenthal.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by Alicante-General Hospital Health Department Ethics Committee (ref 2022-113) and by Miguel Hernandez University Responsible Research Office (ref TFG.GME.MAC.ERS.221005). The study followed the principles of the Declaration of Helsinki. As the data were retrospective and pseudonymised, no informed consent from participants was needed.
References
- 1.Healy B. The Yentl syndrome. N Engl J Med 1991;325:274–6. 10.1056/NEJM199107253250408 [DOI] [PubMed] [Google Scholar]
- 2.Guillén AG, Te Karu L, Singh JA, et al. Gender and ethnic inequities in gout burden and management. Rheum Dis Clin North Am 2020;46:693–703. 10.1016/j.rdc.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsen WM, Dodge HJ, Valkenburg H. The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959–1960. Am J Med 1965;39:242–51. 10.1016/0002-9343(65)90048-3 [DOI] [PubMed] [Google Scholar]
- 4.Kanbay M, Solak Y, Dogan E, et al. Uric acid in hypertension and renal disease: the chicken or the egg Blood Purif 2010;30:288–95. 10.1159/000321074 [DOI] [PubMed] [Google Scholar]
- 5.Kuo C-F, Grainge MJ, Mallen C, et al. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis 2016;75:210–7. 10.1136/annrheumdis-2014-206410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elfishawi MM, Zleik N, Kvrgic Z, et al. The rising incidence of gout and the increasing burden of comorbidities: a population-based study over 20 years. J Rheumatol 2018;45:574–9. 10.3899/jrheum.170806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richette P, Clerson P, Périssin L, et al. Revisiting comorbidities in gout: a cluster analysis. Ann Rheum Dis 2015;74:142–7. 10.1136/annrheumdis-2013-203779 [DOI] [PubMed] [Google Scholar]
- 8.Bevis M, Blagojevic-Bucknall M, Mallen C, et al. Comorbidity clusters in people with gout: an observational cohort study with linked medical record review. Rheumatology 2018;57:1358–63. 10.1093/rheumatology/key096 [DOI] [PubMed] [Google Scholar]
- 9.Huang H-C, Chiang H-P, Hsu N-W, et al. Differential risk group of developing stroke among older women with Gouty arthritis: a latent transition analysis. Eur J Clin Invest 2019;49:e13090. 10.1111/eci.13090 [DOI] [PubMed] [Google Scholar]
- 10.Kiadaliri AA, Englund M. Temporal trends and regional disparity in rheumatoid arthritis and gout hospitalizations in Sweden, 1998–2015. Clin Rheumatol 2018;37:825–30. 10.1007/s10067-018-3983-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Dell JR, Brophy MT, Pillinger MH, et al. Comparative effectiveness of allopurinol and Febuxostat in gout management. NEJM Evid 2022;1. 10.1056/evidoa2100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richette P, Doherty M, Pascual E, et al. Updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017;76:29–42. 10.1136/annrheumdis-2016-209707 [DOI] [PubMed] [Google Scholar]
- 13.FitzGerald JD, Dalbeth N, Mikuls T, et al. American college of rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744–60. 10.1002/acr.24180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NICE guideline [NG219] . Gout: diagnosis and management. 2022. Available: https://www.nice.org.uk/guidance/ng219/chapter/Recommendations
- 15.Harrold LR, Yood RA, Mikuls TR, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis 2006;65:1368–72. 10.1136/ard.2006.051649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Te Kampe R, Janssen M, van Durme C, et al. Sex differences in the clinical profile among patients with gout: cross-sectional analyses of an observational study. J Rheumatol 2021;48:286–92. 10.3899/jrheum.200113 [DOI] [PubMed] [Google Scholar]
- 17.Harrold LR, Etzel CJ, Gibofsky A, et al. Sex differences in gout characteristics: tailoring care for women and men. BMC Musculoskelet Disord 2017;18:108. 10.1186/s12891-017-1465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puig JG, Michán AD, Jiménez ML. Female gout. Arch Intern Med 1991;151:726. 10.1001/archinte.1991.00400040074016 [DOI] [PubMed] [Google Scholar]
- 19.Teng GG, Ang L-W, Saag KG, et al. Mortality due to coronary heart disease and kidney disease among middle-aged and elderly men and women with gout in the Singapore Chinese health study. Ann Rheum Dis 2012;71:924–8. 10.1136/ard.2011.200523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehlin M, Sandström TZ, Jacobsson LT. Incident gout: risk of death and cause-specific mortality in Western Sweden: a prospective, controlled inception cohort study. Front Med 2022;9:802856. 10.3389/fmed.2022.802856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benavent D, Peiteado D, Martinez-Huedo MÁ, et al. Healthcare-related impact of gout in hospitalized patients in Spain. Sci Rep 2021;11:13287. 10.1038/s41598-021-92673-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrés M, Bernal JA, Sivera F, et al. Cardiovascular risk of patients with gout seen at rheumatology clinics following a structured assessment. Ann Rheum Dis 2017;76:1263–8. 10.1136/annrheumdis-2016-210357 [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Southern D, Beck CA, et al. Validity of Canadian discharge abstract data for hypertension and diabetes from 2002 to 2013. CMAJ Open 2016;4:E646–53. 10.9778/cmajo.20160128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin EA, D’Souza AG, Lee S, et al. Hypertension identification using inpatient clinical notes from electronic medical records: an explainable, data-driven algorithm study. CMAJ Open 2023;11:E131–9. 10.9778/cmajo.20210170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Che D, Liu Q, Rasheed K, et al. Decision tree and ensemble learning algorithms with their applications in Bioinformatics. Adv Exp Med Biol 2011;696:191–9. 10.1007/978-1-4419-7046-6_19 [DOI] [PubMed] [Google Scholar]
- 26.Merriman TR. An update on the genetic architecture of hyperuricemia and gout. Arthritis Res Ther 2015;17. 10.1186/s13075-015-0609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hak AE, Curhan GC, Grodstein F, et al. Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis 2010;69:1305–9. 10.1136/ard.2009.109884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumpter NA, Takei R, Cadzow M, et al. Association of gout polygenic risk score with age at disease onset and Tophaceous disease in European and Polynesian men with gout. Arthritis Rheumatol 2023;75:816–25. 10.1002/art.42393 [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res 2019;124:1598–617. 10.1161/CIRCRESAHA.119.313572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben Salem C, Slim R, Fathallah N, et al. Drug-induced Hyperuricaemia and gout. Rheumatology (Oxford) 2017;56:679–88. 10.1093/rheumatology/kew293 [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med 2012;125:679–87. 10.1016/j.amjmed.2011.09.033 [DOI] [PubMed] [Google Scholar]
- 32.Singh JA. Veterans affairs databases are accurate for gout-related health care utilization: a validation study. Arthritis Res Ther 2013;15:R224. 10.1186/ar4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richette P, Doherty M, Pascual E, et al. Updated European League against rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis 2020;79:31–8. 10.1136/annrheumdis-2019-215315 [DOI] [PubMed] [Google Scholar]
- 34.Calabuig I, Gómez-Garberí M, Andrés M. Gout is prevalent but under-registered among patients with cardiovascular events: a field study. Front Med (Lausanne) 2020;7:560. 10.3389/fmed.2020.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003191supp001.pdf (286.6KB, pdf)
rmdopen-2023-003191supp002.pdf (4.7MB, pdf)
Data Availability Statement
Data are available upon reasonable request.