Abstract
Interstitial lung disease (ILD) is a collective term representing a diverse group of pulmonary fibrotic and inflammatory conditions. Due to the diversity of ILD conditions, paucity of guidance and updates to diagnostic criteria over time, it has been challenging to precisely determine ILD incidence and prevalence. This systematic review provides a synthesis of published data at a global level and highlights gaps in the current knowledge base. Medline and Embase databases were searched systematically for studies reporting incidence and prevalence of various ILDs. Randomised controlled trials, case reports and conference abstracts were excluded. 80 studies were included, the most described subgroup was autoimmune-related ILD, and the most studied conditions were rheumatoid arthritis (RA)-associated ILD, systemic sclerosis associated (SSc) ILD and idiopathic pulmonary fibrosis (IPF). The prevalence of IPF was mostly established using healthcare datasets, whereas the prevalence of autoimmune ILD tended to be reported in smaller autoimmune cohorts. The prevalence of IPF ranged from 7 to 1650 per 100 000 persons. Prevalence of SSc ILD and RA ILD ranged from 26.1% to 88.1% and 0.6% to 63.7%, respectively. Significant heterogeneity was observed in the reported incidence of various ILD subtypes. This review demonstrates the challenges in establishing trends over time across regions and highlights a need to standardise ILD diagnostic criteria.PROSPERO registration number: CRD42020203035.
Keywords: Asbestos Induced Lung Disease, Clinical Epidemiology, Interstitial Fibrosis, Systemic disease and lungs
Introduction
Interstitial lung disease (ILD) is a collective term representing a diverse group of lung conditions characterised by the presence of non-infective infiltrates, most commonly in the pulmonary interstitium and alveoli, which in certain cases manifest as architectural distortion and irreversible fibrosis. These conditions vary in their aetiology, clinical pathways, severity and prognosis.1 Some conditions resolve completely without pharmacological intervention, whereas others, such as idiopathic pulmonary fibrosis (IPF) and non-IPF progressive fibrosing (PF) ILDs, inexorably progress to respiratory failure and premature mortality despite treatment.
Given its universally progressive nature and poor prognosis, IPF has attracted the most research attention and the current literature suggests a wide variation in disease distribution across Europe and USA. IPF prevalence varies between 0.63 and 7.6 per 100 000 persons in the USA and Europe2 3 with a sharp increase with age.
More recently, there have been several studies investigating the incidence and prevalence of non-IPF ILDs, mainly autoimmune ILDs. Most of these reviews included studies drawn from single centres. Epidemiological data for non-IPF ILDs is inconsistent which makes it challenging to fully appreciate the ILD landscape. A recent review reported the prevalence of ILD in myositis conditions ranged from 23% in America to 50% in Asia.4 Sambataro et al5 reported about 20% of primary Sjogren’s syndrome patients were diagnosed with ILD. Additionally, there have been a few studies evaluating the incidence of drug induced ILD (DILD).6–8 Guo et al9 reported ILD incidence ranged from 4.6 to 31.5 per 100 000 persons in Europe and North America. A recent study using Global Burden of Disease data indicated the global ILD incidence in the past 10 years has risen by 51% (313.2 cases in 1990 to 207.2 per 1 00 000 cases in 2019).10 These published estimates highlight a discernible variation in the ILD epidemiology across countries. It is unclear whether this is an ‘actual’ difference in the numbers across regions or whether the heterogeneity is driven by lack of guidelines and inconsistencies in ILD diagnostic pathways and standards of care. Likewise, while evidence suggests that the incidence of ILD has been rising over time,9 whether this increase reflects a true increase in the disease burden, possibly related to an ageing population or whether this is due to improvements in detection, increased availability of cross-sectional imaging or coding practices over time is unknown.
This systematic review appraises the published literature on the incidence and prevalence of various ILDs over the last 6 years. We aimed to provide a comprehensive understanding of global incidence and prevalence. Specifically, we sought to identify areas where data are robust, to better appreciate the burden of ILD conditions and to comprehend the implications on healthcare utilisation and resources. We also set out to highlight areas where there remains a need for further study.
Methods
Study registration
This protocol has been drafted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols guidelines11 and registered with the International Prospective Register of Systematic Reviews, PROSPERO (CRD42020203035). Please refer to the online supplemental material for the full study protocol.
bmjresp-2022-001291supp001.pdf (739.7KB, pdf)
Search strategy and selection criteria
A systematic search of Medline and Embase was carried out in September 2021 to identify relevant studies investigating the incidence and prevalence of various ILDs. The search criteria were developed with support of librarian (online supplemental figure E1). Due to the high volume of papers, we restricted this study period to papers published in the past 6 years. This search was limited to human studies written in English that were published between 2015 and 2021. The full search strategy and data sources included are described in online supplemental material.
Study population
Inclusion criteria included observational studies reporting the incidence and/or prevalence of individual ILDs, with study participants aged over 18 years old. Randomised controlled trials, case reports, reviews and conference abstracts were excluded. Studies which referred to DILD only were excluded because (1) there were many abstracts reporting on DILD, therefore this could be a standalone review and (2) epidemiology of DILD was a subject of a recent systematic review.12 The first author (RG) screened all records by title and abstract; to begin with, the second reviewer (AK) independently screened 10% of all records. If there was a disagreement between RG and AK, an additional 15% were screened by AK. All studies identified as eligible for full text review were reviewed by RG, with AK reviewing 50% of eligible studies. Any disagreement was resolved through discussion with other authors, including an ILD expert. Reference of included studies were searched for additional literature.
Following full text review, RG carried out data extraction for eligible studies. AK independently extracted data for 25% of studies using the same template. RG assessed the quality for all included studies, reporting incidence and/or prevalence using a modified Newcastle Ottawa Scale (NOS). There were two NOS modified scales, one each for studies reporting prevalence and/incidence. AK independently assessed the quality of 25% of included studies. If there was a discrepancy between the data extraction and/or quality assessment conducted by RG and AK, then additional 15% were extracted and/or reviewed by AK.
It was noted that for IPF, many authors adopted what they termed ‘broad’ and ‘narrow’ case definitions. For example, Raghu et al2 defined patients with International Classification of Disease, Ninth Revision (ICD-9) code 516.3 as a broadly defined case of IPF, and those who had this ICD-9 code alongside a claim for a surgical lung biopsy, transbronchial lung biopsy, or CT thorax as a narrowly defined case. We summarised the data using various reported case definitions. If multiple estimates were reported in a study, only the most recent estimate was included in this review.
There were two common themes around the reporting of prevalence. Studies drawn from the general population (reported prevalence per 100 000 persons) and studies drawn from multicentre or single centres (reported prevalence as the proportion of patients with ILD in the study cohort).
For this review, we have classified ILDs based on aetiology, grouped by conditions linked to environmental or occupational exposures, conditions typified by granulomatous inflammation, autoimmune ILDs and ILDs with no known cause (online supplemental figure E2).1
Evidence synthesis
The initial plan for this review was to conduct meta-analysis. However, due to high heterogeneity, we were unable to meta-analyse. Therefore, we have proceeded with data synthesis across the ILD subgroups.
Results
Total number of included studies
The literature search yielded a total of 12 924 studies, of which 80 were included in this review. Online supplemental figure E3 demonstrates the selection process for all studies and highlights reasons for exclusion at each stage.
Although 80 unique publications were included, some papers explored the epidemiology of more than one ILD, the total count of reported estimates is 88. Half of the included publications explored autoimmune-related ILDs (n=44/88)(online supplemental figure E4).
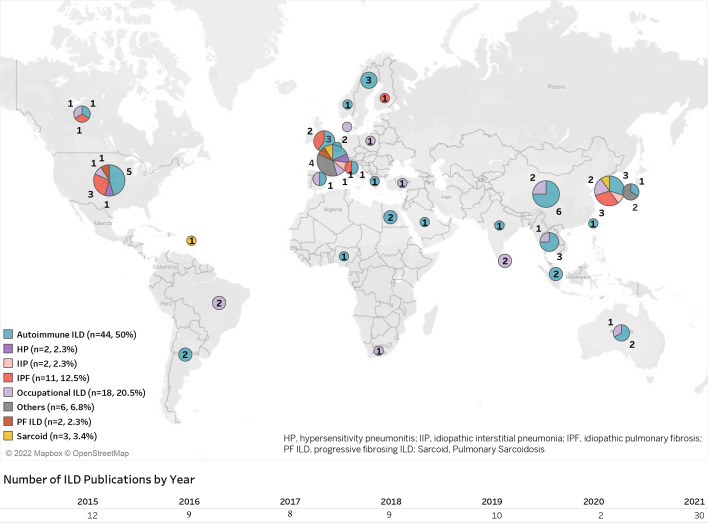
Geographically, ILD publications represented all major world regions, but were predominantly from Asia (n=30, 34.1%) and Europe (n=23, 26.1%) (figure 1).
Figure 1.
Geographical distribution of publications included.
Studies reporting prevalence
Eight studies reported the prevalence of IPF in general population. Prevalence of IPF was commonly reported applying ‘primary’, ‘broad’, ‘intermediate’ and/or ‘narrow’ case definitions. In the general population, the prevalence of IPF ranged from 7 to 1650 per 100 000 persons (table 1). When explored within various case definitions, the prevalence for ‘broad’ cases ranged from 11 (USA, 2010)2 to 1160 (USA, 2021)16; for ‘narrow’ cases, this ranged from 7 (USA, 2010)2 to 725 (USA, 2019).16 There was only one study that reported IPF prevalence of 8.6% using a multicentre study setting.19
Table 1.
Studies reporting IPF prevalence per 100 000 persons by various case definitions
| Case definitions | Prevalence estimate (per 100 000 persons) | Case descriptions | Country, author-published year |
| General/primary | 36 |
|
Italy, Harari,13 2016 |
| General/primary | 13 |
|
USA, Raghu,2 2016 |
| Overall IPF | 20 |
|
USA, Raimundo,14 2016 |
| Overall IPF | 35 |
|
South Korea, Lee,15 2016 |
| Broad | 1160 |
|
USA, Zhang,16 2021 |
| Broad | 11 |
|
USA, Raghu,2 2016 |
| Broad | 42 |
|
Canada, Hopkins,17 2016 |
| Broad | 22 |
|
Italy, Harari,13 2016 |
| Broad | 39 |
|
UK, Strongman,18 2018 |
| Narrow | 13 |
|
Italy, Harari,13 2016 |
| Narrow | 725 |
|
USA, Zhang,16 2021 |
| Narrow | 7 |
|
USA, Raghu,2 2016 |
| Narrow | 20 |
|
Canada, Hopkins,17 2016 |
ICD, International Classification of Diseases; ILD, interstitial lung disease; IPF, Idiopathic pulmonary fibrosis; SLB, surgical lung biopsy; TLB, transbronchial lung biopsy.
Twelve studies reported estimates for non-IPF ILDs in the general population (online supplemental figure E5), with most of these conducted in the USA. The prevalence of systemic sclerosis (SSc) ILD in the general population ranged from 2.3 (Canada, 2018)20 to 19 (USA, 2017)21 per 100 000 persons. The highest SSc-ILD prevalence was reported in Medicare data which included patients aged 65 years and above.21 22 For rheumatoid arthritis (RA) ILD, prevalence in an RA Medicare cohort was 2%.23
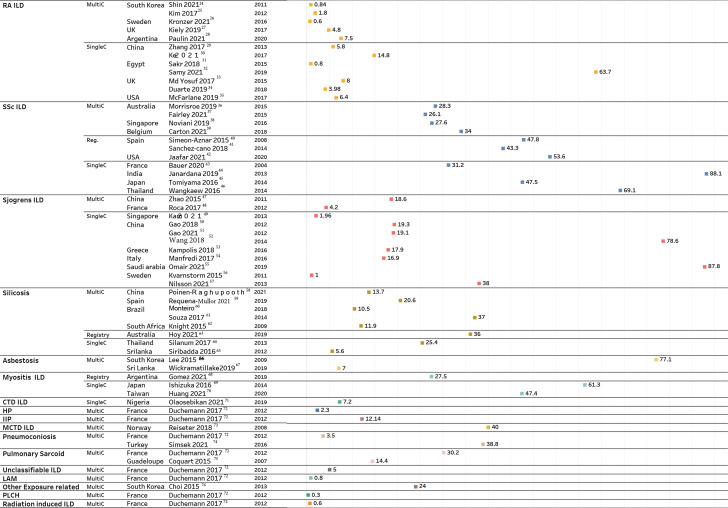
Forty-six studies reported the prevalence of autoimmune-related ILD in cohorts of patients with an autoimmune condition or occupational ILD in workers with specific exposures. These studies primarily reported prevalence as a proportion, with the denominator representing patients with an autoimmune disorder or people working at a factory with exposure to certain agents, such as silica or asbestosis (figure 2). Most of these estimates were drawn from cohorts at single or multiple tertiary centres, disease registries or a factory in the case of occupational ILD. Significant heterogeneity was noted in the reported prevalence of ILD associated with SSc, RA and Sjogren’s (figure 2). The prevalence of ILD in SSc ranged from 26.1% (Australia, 2015)36 to 88.1% (India, 2013).44 Similarly, Sjogren’s ILD ranged from 1% (Sweden, 2011)55 to 87.8% (Saudi Arabia, 2021).56 In addition to dissimilarities in the prevalence across various regions, we also observed variation within region-specific estimates. For example, the 4 studies47 50–52 which reported Sjogren’s ILD prevalence within China, estimated a 4-fold variation in magnitude (18.6% in 201147 to 78.6% in 2014).52 Likewise, for RA ILD, there was substantial variation in the reported prevalence in Egypt (0.8% vs 63.7%).31 32 Among the occupational-related ILDs (figure 2), silicosis was the most explored condition (n=8)). Among these eight studies, there was a considerable variation in the reported prevalence of silicosis. Souza et al61 reported an approximately 7-fold higher estimate of silicosis prevalence than that reported by Siribaddana et al (37% vs 5.6%, respectively).65
Figure 2.
Studies reporting non-IPF prevalence as percentage of study population. DM, dermatomyositis; HP, hypersensitivity pneumonitis; IIP, idiopathic interstitial pneumonia; ILD, interstitial lung disease; LAM, lymphangioleiomyomatosis; MCTD, mixed connective tissue disorder; multiC, multicentre; PLCH, pulmonary langerhans cell histiocytosis; PM, polymyositis; RA, rheumatoid arthritis; reg, registry; single, single centre; SSc, systemic sclerosis. Details on the study population, sample size and ILD diagnosis methods are summarised in online supplemental tables E1–E31.
Studies reporting incidence
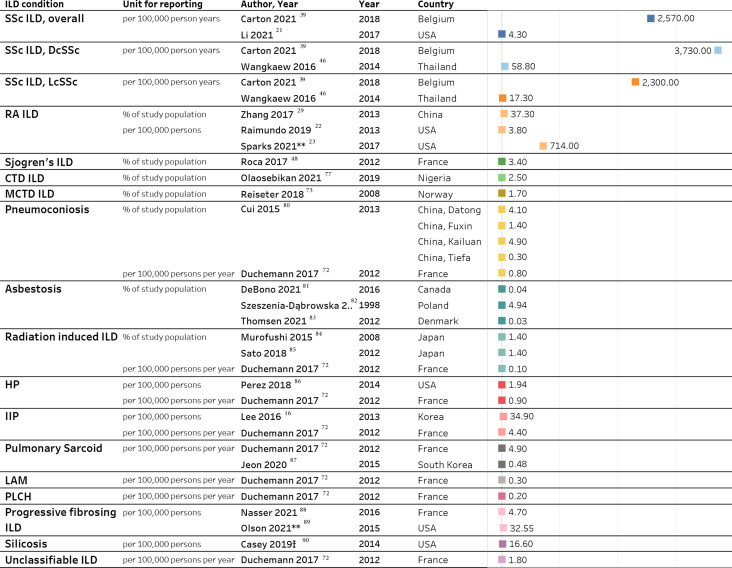
Significant discrepancies were observed in reported ILD incidence across subgroups and individual conditions, mainly due to differences in the study setting. Depending on the study setting and type of data source used, some authors reported an incidence rate (per 100 000 person-years), while others reported incidence proportion. Table 2 lists IPF incidence by case classification and country, and figure 3 provides a list of studies reporting incidence of non-IPF ILDs.
Table 2.
Published estimates of IPF incidence, stratified by various case definitions
| Incidence estimate | Case definitions | Case descriptions | Country, author, published year |
| 183.3 per 100 000 persons | NR | Self-reported | UK, Belbasis, 202177 |
| 5.8 per 100 000 py | Primary | Patients with at least one claim for idiopathic fibrosing alveolitis | USA, Raghu, 20162 |
| 5.2 (5.1–5.4) per 100 000 persons | General | Patients with at least one hospitalisation or at least one outpatient visits with IPF diagnosis | Italy, Harari, 201613 |
| 12.9 per 100 000 persons | Overall | Diagnostic codes | South Korea, Lee, 201615 |
| 35.8 per 100 000 persons | Overall | Diagnostic codes | South Korea, Lim, 201978 |
| 3.6–5.1 per 100 000 py | Broad | Primary patients with an exclusion of ICD code 515 after the last diagnosis code 516.3. | USA, Raghu, 20162 |
| 18.7 per 100 000 persons | Broad | Patients with IPF diagnosis excluding cases with a diagnosis for another ILD | Canada, Hopkins, 201617 |
| 331 per 100 000 py | Broad | Patients who had a diagnosis code for IPF | USA, Zhang, 202116 |
| 3.7 (3.6–3.9) per 100 000 persons | Broad | Patients that satisfied the general definition and had no claims with another ILD | Italy, Harari, 201613 |
| 8.7 (8.4–8.9) per 100 000 py | Broad | Included Read codes
|
UK, Strongman, 201818 |
| 2.4–2.9 per 100 000 py | Narrow | Broad cases further restricted by requiring a claim for a surgical lung biopsy, TLB or CT thorax scan | USA, Raghu, 20162 |
| 9 per 100 000 persons | Narrow | Patients with IPF diagnosis excluding cases that did not have chest CT, bronchus or lung biopsy, or bronchoscopy record | Canada, Hopkins, 201617 |
| 2.3 (2.2–2.5) per 100 000 persons | Narrow |
|
Italy, Harari, 201613 |
| 2.8 (2.7–3) per 100 000 py | Narrow | Additional Read codes to the broad definition | UK, Strongman, 201818 |
| 210 per 100 000 py | Narrow |
|
USA, Zhang, 202116 |
| 48.5 per 100 000 persons | Definition 1 | ICD codes; (bronchoalveolar lavage, BAL) or lung biopsy | South Korea, Gjonbrataj, 201579 |
| 32.2 per 100 000 persons | Definition 2 | Diagnostic ICD code and HRCT, bronchoalveolar lavage or SLB | South Korea, Gjonbrataj, 201579 |
| 16.2 per 100 000 persons | Definition 3 | ICD code | South Korea, Gjonbrataj, 201579 |
| 11.4 per 100 000 persons | Definition 4 | ICD code and HRCT, BAL or SLB; and | South Korea, Gjonbrataj, 201579 |
| 11.4 per 100 000 persons | Definition 5 | ICD code based on the 2011 international statement. | South Korea, Gjonbrataj, 201579 |
Details on the study population, sample size and ILD diagnosis methods are summarised in online supplemental tables E1–E31.
HRCT, high-resolution CT; ICD, International Classification of Disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; NOS, Newcastle Ottawa scale; NR, not reported; py, person-years; SLB, surgical lung biopsy; TLB, transbronchial lung biopsy.
Figure 3.
Studies reporting ILD incidence, grouped by ILD subgroups. ICD-9-CM, International Classification of Disease, Ninth Revision, Clinical Modification; ILD, interstitial lung disease; py, person-years; RA, rheumatoid arthritis; SSc, systemic sclerosis. Ɨ Narrow silicosis definition used: Medicare beneficiaries with any claim that included ICD-9-CM code 502, pneumoconiosis due to other silica or silicates, listed in any position during 1999–2014, with at least one inpatient, skilled nursing or home health agency claim, or at least two outpatient provider claims within 365 days of each other and cases with a chest X-ray or CT scan 30 days before or 30 days after a silicosis claim. Details on the study population, sample size and ILD diagnosis methods are summarised in online supplemental tables E1–E31.
Discussion
In this review, we synthesised the evidence for the incidence and prevalence of ILDs from studies published between 2015 and 2021. Considering the changing ILD nomenclature and the desire to reflect more current estimates, in this review, we decided to restrict the study period to past 6 years. We took this conscious effort with the aim to limit the heterogeneity across reported estimates. We evaluated 39 incidence and 78 prevalence estimates for individual ILD disorders that were distributed globally. We noted an increase in the number of studies investigating non-IPF ILDs and more specifically autoimmune ILDs in recent years. There was a 6-fold rise in the autoimmune ILDs studies, in 2021 when compared with 2015 (18 vs 3 studies, respectively). This increase in non-IPF ILD studies may be related to the emergence of antifibrotic therapies for non-IPF fibrosing lung diseases.91–93 Interestingly, the publication trend for IPF has remained unchanged.
This review revealed considerable inconsistencies in the incidence and prevalence estimated of the main ILD subgroups. The reported prevalence of IPF ranged from 7 to 1650 per 100 000 persons,2 16 an approximately 800-fold difference across case definitions, despite most studies reporting IPF prevalence in the general population. The incidence and prevalence estimates reported by Zhang et al16 were a notable outlier; this study was based on the USA veterans’ healthcare database which included mostly White patients aged over 70 years—the demographic in which IPF is most common. Aside from this study, the majority of studies reported a prevalence of IPF ranging from 7 to 42 per 100 000 persons across different case definitions.2 17
Unlike prevalence, we found considerable inconsistencies in how the incidence of IPF is reported. An important factor is the lack of uniformity in reporting units. Half of the studies reported incidence using person-years, whereas others reported per 100 000 person-years. We were, therefore, unable to compare incidence estimates in a similar fashion to prevalence. It is also important to note that changes in diagnostic guidelines for IPF over the years may have made it more challenging to accurately estimate its burden and temporal trends.94–96
For non-IPF subgroups, such as autoimmune ILDs, there were wide variations in prevalence estimates between countries and within different healthcare settings in the same country. Overall, the variation in prevalence and incidence estimates was even greater for non-IPF ILDs than IPF. This can be attributed to several factors. First, in clinical practice, it is common for the clinical presentation and serological autoantibody profiles to result in overlap syndromes. Autoimmune conditions can coexist and patients with occupational ILDs may also have autoimmune conditions. Such fluidity of diagnoses at a clinical level reflects the challenges in estimating non-IPF ILDs. Second, the denominator more frequently differs for non-IPF ILDs, resulting in lack of standardised reporting. Unlike IPF, for which there are published validated algorithms to identify ‘true’ cases in the general population.18 24 97 For non-IPF ILDs, studies relied on disease registries or were conducted at single/multispecialist clinics.
Majority of the autoimmune-related ILD estimates were in RA and SSc ILD. When assessing SSc ILD prevalence, we observed a wide range (26.1% to 88.1%)37 44 in reported estimates, but when studies were dichotomised into single-centre studies and multicentre studies, it became clear that the highest variability was contributed by single centre studies (SSc prevalence, 31.2%–88.1%).43–46 Owing to a smaller number of studies reporting incidence, we were unable to observe whether the same challenge existed.
The prevalence of silicosis ranged from 5.6%65 to 37%61 in workers exposed to silica. Occupational ILD studies were conducted at a factory, in a neighbourhood with proximity to industries, a registry or multicentre settings. Therefore, lack of generalisability and applicability of findings only to certain populations contributed largely to the wide variabilities of these reported estimates. The geographical distribution of occupational ILD papers alludes to dominance of exposure related ILDs in low-income and middle-income countries in Asia and South America (42.8% were in Asia).
While historical diagnostic classification has been founded on underlying aetiology or clinical pathways, there is now a growing emphasis on disease behaviour.98 99 Attention has focused on a subgroup of ILD patients who go on to develop a PF phenotype. IPF is the archetypal PF ILD but other ILDs such as chronic hypersensitivity pneumonitis (HP), SSc ILD can exhibit ‘IPF-like’ behaviour, including rapid decline in lung function and early mortality.100 The epidemiology of PF ILD is particularly challenging to examine as accepted guidelines on definition and diagnosis have yet to be published The reported prevalence of PF ILDs (per 100 000 persons) was 19.4 in France and 57.8 in the USA.88 89 The future direction of research will likely focus on PF ILD as a phenotype which transcends previously adhered-to diagnostic labels and is associated with poorer outcomes and increased mortality.100 101
Among the 39 studies reporting ILD incidence (online supplemental figure E6), most studies were categorised as medium risk (n=25/39, 64.1%). Two studies were categorised as high-risk primarily because of lack of information on ILD diagnosis and poor quality of reporting estimates (ie, descriptive statistics were not reported, were incomplete or did not include proper measures of dispersion).
Similarly, there were 78 prevalence assessments (online supplemental figure E7) of which approximately 18% (n=14/78) were categorised as high risk, 64.1% (n=50/78) as medium risk and 18% (n=14/76) as low risk. Most studies assessed as high risk were studies reporting autoimmune ILDs, mainly because of ILD diagnosis, single-centre studies or small sample size. Most of the studies reporting prevalence based on large healthcare datasets or disease registries were classified as low risk.
There are several strengths of this systematic review. We have provided an assessment of the incidence and prevalence of several ILD conditions globally and have grouped ILDs based on their aetiology to allow the appraisal of incidence and/prevalence at a disease level with as much granularity as possible. This review underlines the need for standardisation of diagnostic classifications for non-IPF ILDs—the narrower estimates for IPF provide the evidence that clear and consistent diagnostic guidelines are of great clinical utility. Guidelines have recently emerged for the diagnosis of HP102 103 which we envisage will further improve the epidemiological reporting of this important condition, although incorporation of guidelines into routine clinical practice and then into epidemiological estimates takes time. Cross-specialty guideline groups will undoubtedly improve standardisation of reporting for autoimmune driven ILDs.
It is possible that genetic differences between individuals from different ethnic backgrounds may play a role in the global variability in incidence and prevalence. For example, the MUC5B promoter polymorphism (rs35705950) is the dominant risk factor for IPF104 and is also a key risk factor for other ILDs such as RA.105 This gain of function polymorphism is frequent in those of European decent but almost completely absent in those of African ancestry.106 As more research is performed unravelling the complex interplay between genetics and environment in the development of ILD, it is likely that genetic variability will be found to play an important role in the global variability of ILD.
Despite the strengths, there are limitations to this systematic review. The certainty of the ILD case definition varied across studies. It was not always possible to be sure of how reliable the ascertainment method was. However, we attempted to reflect the differences in the ILD diagnostic methods in our risk of bias quality assessment. Along with the uncertainty in the diagnosis of ILD, there were different disease definitions used across studies. Therefore, in this review due to high heterogeneity, in how ILD was defined, we were unable to perform a meta-analysis. In this review, we have only included studies reporting ILD estimates in general populations, registries or populations with a specific disorder of interest. For single-centre studies reporting incidence and/or prevalence of autoimmune or exposure ILDs, the estimates were not generalisable and this has been reflected in the risk of bias quality assessment score. This review is limited to English publications only. However, due to high volume of papers found with the study period, we are confident it has a minimal effect on the overall conclusion.107
Conclusion
This review highlights the lack of uniformity in the published estimates of incidence and prevalence of ILD conditions. In addition, there is a dissimilarity in disease definitions across the studies and geographical regions. Owing to these discrepancies, we were unable to derive estimates for the global incidence and prevalence of ILD and moreover unable to confirm whether there has been a ‘true’ increase in ILD incidence over time. Revisions to diagnostic criteria have augmented the challenges of estimating incidence and prevalence of individual ILD conditions and determining the drivers for temporal trends in incidence. Improving our estimates of the burden of fibrosing lung conditions is essential for future health service planning, a need that has been heightened by the development of new antifibrotic treatments. Guidelines have recently emerged for non-IPF ILDs, we envisage this may improve the epidemiological reporting for future research. There is a fundamental need to standardise ILD diagnosis, disease definitions and reporting in order to provide the data which will drive the provision of a consistently high level of care for these patients across the globe.108
Footnotes
Twitter: @DrPeter_George
Contributors: RG, AM, PMG and JKQ developed the research question. RG, AM, PMG and JKQ developed the study protocol. RG developed the search strategy with input from AM and JKQ. RG screened the studies for inclusion, extracted the data from included studies and carried out quality assessment of the data. AK was the secondary reviewer for screening, data extraction and quality assessment. PMG supported with the understanding of various ILD diseases and their clinical pathways. All authors interpreted the review results. RG drafted the manuscript. All authors read, commented on and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: RG is a current employee of Gilead Sciences, outside the submitted work. JKQ has received grants from The Health Foundation, MRC, GSK, Bayer, BI, British Lung Foundation, IQVIA, Chiesi AZ, Insmed and Asthma UK. JKQ has received personal fees for advisory board participation or speaking fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Bayer and Insmed. PMG has received grants from the MRC, Boehringer Ingelheim and Roche Pharmaceuticals and personal fees from Boehringer Ingelheim, Roche Pharmaceuticals, Teva, Cippla, AZ and Brainomix. AK and AM have nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277–304. 10.1164/ajrccm.165.2.ats01 [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Chen S-Y, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J 2016;48:179–86. 10.1183/13993003.01653-2015 [DOI] [PubMed] [Google Scholar]
- 3.Olson AL, Brown KK, Swigris JJ. Understanding and optimizing health-related quality of life and physical functional capacity in idiopathic pulmonary fibrosis. Patient Relat Outcome Meas 2016;7:29–35. 10.2147/PROM.S74857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun K-Y, Fan Y, Wang Y-X, et al. Prevalence of interstitial lung disease in polymyositis and dermatomyositis: a meta-analysis from 2000 to 2020. Semin Arthritis Rheum 2021;51:175–91. 10.1016/j.semarthrit.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 5.Sambataro D, Sambataro G, Pignataro F, et al. Patients with interstitial lung disease secondary to autoimmune diseases: how to recognize them? Diagnostics 2020;10:208. 10.3390/diagnostics10040208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roubille C, Haraoui B. Interstitial lung diseases induced or exacerbated by DMARDS and biologic agents in rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum 2014;43:613–26. 10.1016/j.semarthrit.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 7.Deidda A, Pisanu C, Micheletto L, et al. Interstitial lung disease induced by fluoxetine: systematic review of literature and analysis of vigiaccess, eudravigilance and a national pharmacovigilance database. Pharmacol Res 2017;120:294–301. 10.1016/j.phrs.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 8.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. 10.1136/ard.2008.093690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo B, Wang L, Xia S, et al. The interstitial lung disease spectrum under a uniform diagnostic algorithm: a retrospective study of 1,945 individuals. J Thorac Dis 2020;12:3688–96. 10.21037/jtd-19-4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global Burden of Disease Collaborative Network . Global burden of disease study 2019 (GBD 2019) reference life table. Seattle, United States of America: Institute for Health Metrics and Evaluation (IHME), 2021. [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skeoch S, Weatherley N, Swift AJ, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med 2018;7:356. 10.3390/jcm7100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harari S, Madotto F, Caminati A, et al. Epidemiology of idiopathic pulmonary fibrosis in northern Italy. PLoS ONE 2016;11:e0147072. 10.1371/journal.pone.0147072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raimundo K, Chang E, Broder MS, et al. Clinical and economic burden of idiopathic pulmonary fibrosis: a retrospective cohort study. BMC Pulm Med 2016;16:2. 10.1186/s12890-015-0165-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H-E, Myong J-P, Kim H-R, et al. Incidence and prevalence of idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis 2016;20:978–84. 10.5588/ijtld.16.0003 [DOI] [PubMed] [Google Scholar]
- 16.Zhang N, Kaul B, Lee JS, et al. Epidemiology of idiopathic pulmonary fibrosis among U.S. veterans, 2010-2019. Ann Am Thorac Soc 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins RB, Burke N, Fell C, et al. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J 2016;48:187–95. 10.1183/13993003.01504-2015 [DOI] [PubMed] [Google Scholar]
- 18.Strongman H, Kausar I, Maher TM. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther 2018;35:724–36. 10.1007/s12325-018-0693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaunisto J, Kelloniemi K, Sutinen E, et al. Re-evaluation of diagnostic parameters is crucial for obtaining accurate data on idiopathic pulmonary fibrosis. BMC Pulm Med 2015;15:92. 10.1186/s12890-015-0074-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope JE, Quansah K, Hassan S, et al. Systemic sclerosis and associated interstitial lung disease in Ontario, Canada: an examination of prevalence and survival over 10 years. J Rheumatol 2021;48:1427–34. 10.3899/jrheum.201049 [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Wallace L, Patnaik P, et al. Disease frequency, patient characteristics, comorbidity outcomes and immunosuppressive therapy in systemic sclerosis and systemic sclerosis-associated interstitial lung disease: a US cohort study. Rheumatology 2021;60:1915–25. 10.1093/rheumatology/keaa547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimundo K, Solomon JJ, Olson AL, et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol 2019;46:360–9. 10.3899/jrheum.171315 [DOI] [PubMed] [Google Scholar]
- 23.Sparks JA, Jin Y, Cho S-K, et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis–associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatology 2021;60:3689–98. 10.1093/rheumatology/keaa836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin S, Park EH, Kang EH, et al. Sex differences in clinical characteristics and their influence on clinical outcomes in an observational cohort of patients with rheumatoid arthritis. Jt Bone Spine 2021;88:105124. 10.1016/j.jbspin.2020.105124 [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Cho S-K, Choi C-B, et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol Int 2017;37:1735–45. 10.1007/s00296-017-3781-7 [DOI] [PubMed] [Google Scholar]
- 26.Kronzer VL, Westerlind H, Alfredsson L, et al. Respiratory diseases as risk factors for seropositive and seronegative rheumatoid arthritis and in relation to smoking. Arthritis Rheumatol 2021;73:61–8. 10.1002/art.41491 [DOI] [PubMed] [Google Scholar]
- 27.Kiely P, Busby AD, Nikiphorou E, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERas and ERAN inception cohorts. BMJ Open 2019;9:e028466. 10.1136/bmjopen-2018-028466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulin F, Secco A, Benavidez F, et al. Lung involvement prevalence in patients with early rheumatoid arthritis without known pulmonary disease: a multicentric cross sectional study. Adv Rheumatol 2021;61:52. 10.1186/s42358-021-00209-0 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Li H, Wu N, et al. Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 2017;36:817–23. 10.1007/s10067-017-3561-5 [DOI] [PubMed] [Google Scholar]
- 30.Ke Y, Dai X, Xu D, et al. Features and outcomes of elderly rheumatoid arthritis: does the age of onset matter? A comparative study from a single center in China. Rheumatol Ther 2021;8:243–54. 10.1007/s40744-020-00267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakr BR, Elfishawi MM, ElArousy MH, et al. Rheumatoid arthritis: a single-center Egyptian experience. Immunol Invest 2018;47:293–302. 10.1080/08820139.2018.1425700 [DOI] [PubMed] [Google Scholar]
- 32.Samy N, Salah H, Hammoda RM. Rheumatoid arthritis patients with interstitial lung disease: clinical, radiological and laboratory characteristics. Egypt Rheumatol 2021;43:29–34. 10.1016/j.ejr.2020.08.004 [DOI] [Google Scholar]
- 33.Md Yusof MY, Kabia A, Darby M, et al. Effect of rituximab on the progression of rheumatoid arthritis–related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology 2017;56:1348–57. 10.1093/rheumatology/kex072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients—an overview of different types of involvement and treatment. Rheumatology 2019;58:2031–8. 10.1093/rheumatology/kez177 [DOI] [PubMed] [Google Scholar]
- 35.McFarlane IM, Zhaz SY, Bhamra MS, et al. Assessment of interstitial lung disease among black rheumatoid arthritis patients. Clin Rheumatol 2019;38:3413–24. 10.1007/s10067-019-04760-6 [DOI] [PubMed] [Google Scholar]
- 36.Morrisroe K, Hansen D, Huq M, et al. Incidence, risk factors, and outcomes of cancer in systemic sclerosis. Arthritis Care Res (Hoboken) 2020;72:1625–35. 10.1002/acr.24076 [DOI] [PubMed] [Google Scholar]
- 37.Fairley JL, Hansen D, Proudman S, et al. Clinical features of systemic sclerosis-mixed connective tissue disease and systemic sclerosis overlap syndromes. Arthritis Care Res (Hoboken) 2021;73:732–41. 10.1002/acr.24167 [DOI] [PubMed] [Google Scholar]
- 38.Noviani M, Saffari SE, Tan JL, et al. Mortality and hospitalization outcomes of interstitial lung disease and pulmonary hypertension in the Singapore systemic sclerosis cohort. Semin Arthritis Rheum 2020;50:473–9. 10.1016/j.semarthrit.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 39.Vandecasteele E, Melsens K, Vanhaecke A, et al. Incidence, prevalence and long-term progression of goh algorithm rated interstitial lung disease in systemic sclerosis in two independent cohorts in Flanders: a retrospective cohort study. Semin Arthritis Rheum 2021;51:969–76. 10.1016/j.semarthrit.2021.07.018 [DOI] [PubMed] [Google Scholar]
- 40.Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C, et al. Registry of the Spanish network for systemic sclerosis: survival, prognostic factors, and causes of death. Medicine (Baltimore) 2015;94:e1728. 10.1097/MD.0000000000001728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez-Cano D, Ortego-Centeno N, Callejas JL, et al. Interstitial lung disease in systemic sclerosis: data from the Spanish scleroderma study group. Rheumatol Int 2018;38:363–74. 10.1007/s00296-017-3916-x [DOI] [PubMed] [Google Scholar]
- 42.Jaafar S, Lescoat A, Huang S, et al. Clinical characteristics, visceral involvement, and mortality in at-risk or early diffuse systemic sclerosis: a longitudinal analysis of an observational prospective multicenter US cohort. Arthritis Res Ther 2021;23. 10.1186/s13075-021-02548-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer E, Fauny M, Tanguy M, et al. Relationship between calcifications and structural lesions on hand radiography and axial calcifications on CT-scan: a retrospective study in systemic sclerosis. Medicine (Baltimore) 2020;99:e22443. 10.1097/MD.0000000000022443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janardana R, Nair AM, Surin AK, et al. Unique clinical and autoantibody profile of a large Asian Indian cohort of scleroderma—do South Asians have a more aggressive disease? Clin Rheumatol 2019;38:3179–87. 10.1007/s10067-019-04659-2 [DOI] [PubMed] [Google Scholar]
- 45.Tomiyama F, Watanabe R, Ishii T, et al. High prevalence of acute exacerbation of interstitial lung disease in Japanese patients with systemic sclerosis. Tohoku J Exp Med 2016;239:297–305. 10.1620/tjem.239.297 [DOI] [PubMed] [Google Scholar]
- 46.Wangkaew S, Euathrongchit J, Wattanawittawas P, et al. Incidence and predictors of interstitial lung disease (ILD) in Thai patients with early systemic sclerosis: inception cohort study. Mod Rheumatol 2016;26:588–93. 10.3109/14397595.2015.1115455 [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Li Y, Wang L, et al. Primary Sjögren syndrome in Han Chinese: clinical and immunological characteristics of 483 patients. Medicine (Baltimore) 2015;94:e667. 10.1097/MD.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roca F, Dominique S, Schmidt J, et al. Interstitial lung disease in primary Sjögren’s syndrome. Autoimmun Rev 2017;16:48–54. 10.1016/j.autrev.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 49.Kam J-W, Charan N, Leong R-L, et al. Clinical features and outcomes from the Singapore Sjögren’s syndrome study. Lupus 2021;30:248–55. 10.1177/0961203320976932 [DOI] [PubMed] [Google Scholar]
- 50.Gao H, Zhang X-W, He J, et al. Prevalence, risk factors, and prognosis of interstitial lung disease in a large cohort of Chinese primary Sjögren syndrome patients: a case-control study. Medicine (Baltimore) 2018;97:e11003. 10.1097/MD.0000000000011003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao H, Sun Y, Zhang X-Y, et al. Characteristics and mortality in primary Sjögren syndrome-related interstitial lung disease. Medicine (Baltimore) 2021;100:e26777. 10.1097/MD.0000000000026777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Hou Z, Qiu M, et al. Risk factors for primary Sjögren syndrome-associated interstitial lung disease. J Thorac Dis 2018;10:2108–17. 10.21037/jtd.2018.03.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kampolis CF, Fragkioudaki S, Mavragani CP, et al. Prevalence and spectrum of symptomatic pulmonary involvement in primary Sjögren’s syndrome. Clin Exp Rheumatol 2018;36 Suppl 112:94–101. [PubMed] [Google Scholar]
- 54.Manfredi A, Sebastiani M, Cerri S, et al. Erratum to: prevalence and characterization of non-sicca onset primary Sjögren syndrome with interstitial lung involvement. Clin Rheumatol 2017;36:1931. 10.1007/s10067-017-3635-4 [DOI] [PubMed] [Google Scholar]
- 55.Omair MA, AlQahtani BS, AlHamad EH, et al. Disease phenotype and diagnostic delay in Saudi patients with primary Sjögren’s syndrome. SMJ 2021;42:405–10. 10.15537/smj.2021.42.4.20200767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kvarnström M, Ottosson V, Nordmark B, et al. Incident cases of primary Sjögren’s syndrome during a 5-year period in Stockholm county: a descriptive study of the patients and their characteristics. Scand J Rheumatol 2015;44:135–42. 10.3109/03009742.2014.931457 [DOI] [PubMed] [Google Scholar]
- 57.Nilsson AM, Aaltonen HL, Olsson P, et al. Mixed airway and pulmonary parenchymal disease in patients with primary Sjögren syndrome: a 6-year follow-up. J Rheumatol 2021;48:232–40. 10.3899/jrheum.200247 [DOI] [PubMed] [Google Scholar]
- 58.Poinen-Rughooputh S, Rughooputh MS, Guo Y, et al. Sex-related differences in the risk of silicosis among Chinese pottery workers: a cohort study. J Occup Environ Med 2021;63:74–9. 10.1097/JOM.0000000000002068 [DOI] [PubMed] [Google Scholar]
- 59.Requena-Mullor M, Alarcón-Rodríguez R, Parrón-Carreño T, et al. Association between crystalline silica dust exposure and silicosis development in artificial stone workers. Int J Environ Res Public Health 2021;18:5625. 10.3390/ijerph18115625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souza TP, van Tongeren M, Monteiro I. Respiratory health and silicosis in artisanal mine workers in Southern Brazil. Am J Ind Med 2021;64:511–8. 10.1002/ajim.23242 [DOI] [PubMed] [Google Scholar]
- 61.Souza TP, Watte G, Gusso AM, et al. Silicosis prevalence and risk factors in semi-precious stone mining in Brazil. Am J Ind Med 2017;60:529–36. 10.1002/ajim.22719 [DOI] [PubMed] [Google Scholar]
- 62.Knight D, Ehrlich R, Fielding K, et al. Trends in silicosis prevalence and the healthy worker effect among gold miners in South Africa: a prevalence study with follow up of employment status. BMC Public Health 2015;15:1258. 10.1186/s12889-015-2566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoy RF, Glass DC, Dimitriadis C, et al. Identification of early-stage silicosis through health screening of stone benchtop industry workers in Victoria, Australia. Occup Environ Med 2021;78:296–302. 10.1136/oemed-2020-106897 [DOI] [PubMed] [Google Scholar]
- 64.Silanun K, Chaiear N, Rechaipichitkul W. Prevalence of silicosis in stone carving workers being exposed to inorganic dust at sikhiu district nakhonratchasima Province, Thailand; preliminary results. J Med Assoc Thai 2017;100:598–602. [Google Scholar]
- 65.Siribaddana AD, Wickramasekera K, Palipana WM, et al. A study on silicosis among employees of a silica processing factory in the central province of Sri Lanka. Ceylon Med J 2016;61:6–10. 10.4038/cmj.v61i1.8252 [DOI] [PubMed] [Google Scholar]
- 66.Lee EK, Kim JS, Kim Y, et al. CT findings in people who were environmentally exposed to asbestos in Korea. J Korean Med Sci 2015;30:1896–901. 10.3346/jkms.2015.30.12.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wickramatillake BA, Fernando MA, Frank AL. Prevalence of asbestos-related disease among workers in Sri Lanka. Ann Glob Health 2019;85:108. 10.5334/aogh.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gómez GN, Pérez N, Braillard Poccard A, et al. Myositis-specific antibodies and clinical characteristics in patients with autoimmune inflammatory myopathies: reported by the Argentine registry of inflammatory myopathies of the Argentine society of rheumatology. Clin Rheumatol 2021;40:4473–83. 10.1007/s10067-021-05797-2 [DOI] [PubMed] [Google Scholar]
- 69.Ishizuka M, Watanabe R, Ishii T, et al. Long-term follow-up of 124 patients with polymyositis and dermatomyositis: statistical analysis of prognostic factors. Mod Rheumatol 2016;26:115–20. 10.3109/14397595.2015.1054081 [DOI] [PubMed] [Google Scholar]
- 70.Huang H-L, Lin W-C, Lin P-Y, et al. The significance of myositis autoantibodies in idiopathic inflammatory myopathy concomitant with interstitial lung disease. Neurol Sci 2021;42:2855–64. 10.1007/s10072-020-04911-7 [DOI] [PubMed] [Google Scholar]
- 71.Olaosebikan H, Adeyeye O, Akintayo R, et al. Connective tissue disease -- associated interstitial lung disease: an underreported cause of interstitial lung disease in sub-Saharan Africa. Clin Rheumatol 2021;40:3455–60. 10.1007/s10067-020-05336-5 [DOI] [PubMed] [Google Scholar]
- 72.Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of greater Paris. Eur Respir J 2017;50:1602419. 10.1183/13993003.02419-2016 [DOI] [PubMed] [Google Scholar]
- 73.Reiseter S, Gunnarsson R, Mogens Aaløkken T, et al. Progression and mortality of interstitial lung disease in mixed connective tissue disease: a long-term observational nationwide cohort study. Rheumatology 2018;57:255–62. 10.1093/rheumatology/kex077 [DOI] [PubMed] [Google Scholar]
- 74.Şimşek C, Sarı G, Üzmezoğlu BA, et al. The relationship between respiratory health and hard metal dust exposure: a cross-sectional study. Arch Environ Occup Health 2022;77:227–33. 10.1080/19338244.2020.1870911 [DOI] [PubMed] [Google Scholar]
- 75.Coquart N, Cadelis G, Tressières B, et al. Epidemiology of sarcoidosis in afro-caribbean people: a 7-year retrospective study in Guadeloupe. Int J Dermatol 2015;54:188–92. 10.1111/ijd.12633 [DOI] [PubMed] [Google Scholar]
- 76.Choi S, Won YL, Kim D, et al. Interstitial lung disorders in the indium workers of Korea: an update study for the relationship with biological exposure indices. Am J Ind Med 2015;58:61–8. 10.1002/ajim.22402 [DOI] [PubMed] [Google Scholar]
- 77.Bellou V, Belbasis L, Evangelou E. Tobacco smoking and risk for pulmonary fibrosis: a prospective cohort study from the UK Biobank. Chest 2021;160:983–93. 10.1016/j.chest.2021.04.035 [DOI] [PubMed] [Google Scholar]
- 78.Lim S-S, Lee W, Kim Y-K, et al. The cumulative incidence and trends of rare diseases in South Korea: a nationwide study of the administrative data from the National health insurance service database from 2011-2015. Orphanet J Rare Dis 2019;14:49. 10.1186/s13023-019-1032-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gjonbrataj J, Choi W-I, Bahn YE, et al. Incidence of idiopathic pulmonary fibrosis in Korea based on the 2011 ATS/ERS/JRS/ALAT statement. Int J Tuberc Lung Dis 2015;19:742–6. 10.5588/ijtld.14.0650 [DOI] [PubMed] [Google Scholar]
- 80.Cui K, Shen F, Han B, et al. Comparison of the cumulative incidence rates of coal workers’ pneumoconiosis between 1970 and 2013 among four state-owned colliery groups in China. Int J Environ Res Public Health 2015;12:7444–56. 10.3390/ijerph120707444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeBono NL, Warden H, Logar-Henderson C, et al. Incidence of mesothelioma and asbestosis by occupation in a diverse workforce. Am J Ind Med 2021;64:476–87. 10.1002/ajim.23245 [DOI] [PubMed] [Google Scholar]
- 82.Szeszenia-Dąbrowska N, Świątkowska B, Sobala W, et al. Asbestos related diseases among workers of asbestos processing plants in relation to type of production and asbestos use. Med Pr 2015;66:1–9. [PubMed] [Google Scholar]
- 83.Thomsen RW, Riis AH, Flachs EM, et al. Risk of asbestosis, mesothelioma, other lung disease or death among motor vehicle mechanics: a 45-year Danish cohort study. Thorax 2022;77:477–85. 10.1136/thoraxjnl-2020-215041 [DOI] [PubMed] [Google Scholar]
- 84.Murofushi KN, Oguchi M, Gosho M, et al. Radiation-induced bronchiolitis obliterans organizing pneumonia (BOOP) syndrome in breast cancer patients is associated with age. Radiat Oncol 2015;10:103. 10.1186/s13014-015-0393-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sato H, Ebi J, Tamaki T, et al. Incidence of organizing pneumonia after whole-breast radiotherapy for breast cancer, and risk factor analysis. J Radiat Res 2018;59:298–302. 10.1093/jrr/rry001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernández Pérez ER, Kong AM, Raimundo K, et al. Epidemiology of hypersensitivity pneumonitis among an insured population in the United States: a claims-based cohort analysis. Ann Am Thorac Soc 2018;15:460–9. 10.1513/AnnalsATS.201704-288OC [DOI] [PubMed] [Google Scholar]
- 87.Jeon MH, Kang T, Yoo SH, et al. The incidence, comorbidity and mortality of sarcoidosis in Korea, 2008-2015: a nationwide population-based study. Sarcoidosis Vasc Diffuse Lung Dis 2020;37:24–6. 10.36141/svdld.v37i1.7660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nasser M, Larrieu S, Boussel L, et al. Estimates of epidemiology, mortality and disease burden associated with progressive fibrosing interstitial lung disease in France (the progress study). Respir Res 2021;22:162. 10.1186/s12931-021-01749-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olson AL, Patnaik P, Hartmann N, et al. Prevalence and incidence of chronic fibrosing interstitial lung diseases with a progressive phenotype in the United States estimated in a large claims database analysis. Adv Ther 2021;38:4100–14. 10.1007/s12325-021-01786-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Casey ML, Mazurek JM. Silicosis prevalence and incidence among Medicare beneficiaries. Am J Ind Med 2019;62:183–91. 10.1002/ajim.22944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020;8:453–60. 10.1016/S2213-2600(20)30036-9 [DOI] [PubMed] [Google Scholar]
- 92.Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021;9:96–106. 10.1016/S2213-2600(20)30330-1 [DOI] [PubMed] [Google Scholar]
- 93.Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (relief): a double-blind, randomised, placebo-controlled, phase 2B trial. Lancet Respir Med 2021;9:476–86. 10.1016/S2213-2600(20)30554-3 [DOI] [PubMed] [Google Scholar]
- 94.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raghu G, Rochwerg B, Zhang Y, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med 2015;192:e3–19. 10.1164/rccm.201506-1063ST [DOI] [PubMed] [Google Scholar]
- 96.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 97.Ley B, Urbania T, Husson G, et al. Code-based diagnostic algorithms for idiopathic pulmonary fibrosis. Case validation and improvement. Annals ATS 2017;14:880–7. 10.1513/AnnalsATS.201610-764OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ryerson CJ, Collard HR. Update on the diagnosis and classification of ILD. Curr Opin Pulm Med 2013;19:453–9. 10.1097/MCP.0b013e328363f48d [DOI] [PubMed] [Google Scholar]
- 99.Travis WD, Costabel U, Hansell DM, et al. An official American thoracic society/European respiratory Society statement: update of the International multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.George PM, Spagnolo P, Kreuter M, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. The Lancet Respiratory Medicine 2020;8:925–34. 10.1016/S2213-2600(20)30355-6 [DOI] [PubMed] [Google Scholar]
- 101.Brown KK, Martinez FJ, Walsh SLF, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 2020;55:2000085. 10.1183/13993003.00085-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández Pérez ER, Travis WD, Lynch DA, et al. Diagnosis and evaluation of hypersensitivity pneumonitis. Chest 2021;160:e97–156. 10.1016/j.chest.2021.03.066 [DOI] [PubMed] [Google Scholar]
- 103.Fernández Pérez ER, Travis WD, Lynch DA, et al. Executive summary: diagnosis and evaluation of hypersensitivity pneumonitis. Chest 2021;160:595–615. 10.1016/j.chest.2021.03.067 [DOI] [PubMed] [Google Scholar]
- 104.Seibold MA, Wise AL, Speer MC, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011;364:1503–12. 10.1056/NEJMoa1013660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Juge P-A, Lee JS, Ebstein E, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018;379:2209–19. 10.1056/NEJMoa1801562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Genomes Project C, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nussbaumer-Streit B, Klerings I, Dobrescu AI, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol 2020;118:42–54. 10.1016/j.jclinepi.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 108.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001291supp001.pdf (739.7KB, pdf)