Abstract
Background
Management of immune-related adverse events (irAEs) is important as they cause treatment interruption or discontinuation, more often seen with combination immune checkpoint inhibitor (ICI) therapy. Here, we retrospectively evaluated the safety and effectiveness of anti-interleukin-6 receptor (anti-IL-6R) as therapy for irAEs.
Methods
We performed a retrospective multicenter study evaluating patients diagnosed with de novo irAEs or flare of pre-existing autoimmune disease following ICI and were treated with anti-IL-6R. Our objectives were to assess the improvement of irAEs as well as the overall tumor response rate (ORR) before and after anti-IL-6R treatment.
Results
We identified a total of 92 patients who received therapeutic anti-IL-6R antibodies (tocilizumab or sarilumab). Median age was 61 years, 63% were men, 69% received anti-programmed cell death protein-1 (PD-1) antibodies alone, and 26% patients were treated with the combination of anti-cytotoxic T lymphocyte antigen-4 and anti-PD-1 antibodies. Cancer types were primarily melanoma (46%), genitourinary cancer (35%), and lung cancer (8%). Indications for using anti-IL-6R antibodies included inflammatory arthritis (73%), hepatitis/cholangitis (7%), myositis/myocarditis/myasthenia gravis (5%), polymyalgia rheumatica (4%), and one patient each with autoimmune scleroderma, nephritis, colitis, pneumonitis and central nervous system vasculitis. Notably, 88% of patients had received corticosteroids, and 36% received other disease-modifying antirheumatic drugs (DMARDs) as first-line therapies, but without adequate improvement. After initiation of anti-IL-6R (as first-line or post-corticosteroids and DMARDs), 73% of patients showed resolution or change to ≤grade 1 of irAEs after a median of 2.0 months from initiation of anti-IL-6R therapy. Six patients (7%) stopped anti-IL-6R due to adverse events. Of 70 evaluable patients by RECIST (Response Evaluation Criteria in Solid Tumors) V.1.1 criteria; the ORR was 66% prior versus 66% after anti-IL-6R (95% CI, 54% to 77%), with 8% higher complete response rate. Of 34 evaluable patients with melanoma, the ORR was 56% prior and increased to 68% after anti-IL-6R (p=0.04).
Conclusion
Targeting IL-6R could be an effective approach to treat several irAE types without hindering antitumor immunity. This study supports ongoing clinical trials evaluating the safety and efficacy of tocilizumab (anti-IL-6R antibody) in combination with ICIs (NCT04940299, NCT03999749).
Keywords: immune checkpoint inhibitors, autoimmunity, cytokines
WHAT IS ALREADY KNOWN ON THIS TOPIC
Immune checkpoint inhibitors (ICIs) have been associated with immune-related adverse events (irAEs), causing significant morbidity and impacting the development of combination ICI approaches. Corticosteroids remain the standard of care therapy for irAEs; however, high doses and prolonged duration might dampen antitumor immunity and cause morbid adverse events. Thus, selective immune modulation strategies that attenuate immunotoxicity and preserve antitumor immunity are an unmet need.
WHAT THIS STUDY ADDS
This is the largest study assessing the impact of anti-interleukin-6 receptor (anti-IL-6R) therapy on irAE and antitumor immune response. Selective immunosuppression using anti-IL-6R has shown promising results in improving several irAEs, including patients with corticosteroid refractory irAEs and autoimmune disease flare-ups; however, further studies are needed to determine the efficacy of this approach in life-threatening irAEs.
Early introduction of anti-IL-6R therapy resulted in a rapid irAE improvement compared with delayed treatment as a second line of therapy. Also, comparable efficacy was achieved with subcutaneous and intravenous administrations of anti-IL-6R therapy in the largest irAE subgroup of patients with ICI-induced arthritis.
Anti-IL-6R therapy did not seem to compromise the ICI-induced tumor response.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings of this study provide a safety signal and proof of concept support testing this therapeutic strategy in prospective trials for patients who develop irAEs and even as a preventive strategy concomitantly with ICI combination therapy.
Introduction
The use of immune checkpoint inhibitors (ICIs) has expanded exponentially, due to their impressive survival benefit in a broad spectrum of cancer types. ICIs block the checkpoint proteins that downregulate T-cell activation like cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1), thereby reinvigorating the antitumor immune surveillance.1–8 However, ‘taking the breaks off’ the immune system may often result in collateral inflammatory and immune-related adverse events (irAEs) in any organ. The likelihood of these toxicities increases with combination ICI regimens, leading to treatment interruption or discontinuation as well as morbidity, mortality and burden to healthcare systems.9–13 Therefore, identification of strategies that can effectively manage the immune toxicities without blunting the tumor response to ICIs is imperative.
The current treatment guidelines for irAEs recommend corticosteroids as a first line of therapy.14–17 However, the best initial corticosteroid dose and optimal treatment duration, risk of corticosteroid toxicity, and its impact on cancer outcomes remain to be further determined.18–20 Conventional synthetic disease-modifying anti-rheumatic drugs (DMARDs) and/or certain biologics such as anti-tumor necrosis factor-alpha (anti-TNF-α), and anti-interleukin-6 receptor (anti-IL-6R) are recommended only for steroid-refractory irAEs.14–17 These recommendations were based on existing treatment strategies of autoimmune diseases and available data on the management of ICI-induced irAEs comes primarily from case reports and small series where corticosteroids showed effectiveness in treating most irAEs.21 To date, randomized controlled trials have not been published that could provide information on what treatment might be superior in minimizing the deleterious effects of ICIs without affecting the ICI-induced tumor immunity.
Currently, the use of anti-IL-6R therapy for irAEs management has been primarily reported in case reports and small series which mainly included patients with cytokine release syndrome and lacked information on tumor response to ICI prior to anti-IL-6R, making it difficult to interpret its impact on ICI tumor immunity.22–25 Indeed, IL-6 is a key cytokine for differentiating naïve CD4 T cells into T helper 17 (TH17) cells, which are implicated in the pathogenesis of several autoimmune diseases and could similarly play a role in irAEs.26 Therefore, we recently studied the role of IL-6/TH17 axis in ICI-related immunity, and through gene expression analysis, we have demonstrated its role in multiple irAEs and tumor resistance.27 Herein, we report the results from a retrospective, multicenter study that evaluated the use of anti-IL-6R for irAE management and assessed its impact on the tumor response to ICIs and overall survival (OS).
Methods
Cohort selection
After obtaining Institutional Review Board (IRB) approval, we searched The University of Texas MD Anderson (MD Anderson) databases (eg, pharmacy records, tumor registries, oncology clinics, and/or specialty clinics for diagnosis and management of irAEs) to identify patients who had received (1) a Food and Drug Administration-approved ICI therapy (ipilimumab, pembrolizumab, nivolumab, cemiplimab, atezolizumab, avelumab, durvalumab) between January 1, 2014, and March 31, 2021; and (2) an anti-IL-6R agent (tocilizumab or sarilumab) at or after initiation of ICIs. Subsequently, the patients’ medical records were thoroughly reviewed to identify the patients who had received anti-IL-6R specifically for management of ICI-induced irAEs or flares of pre-existing autoimmune diseases, and those patients were then followed-up until September 30, 2021. Patients who received anti-IL-6R for cytokine release syndrome, occurring as an isolated toxicity following ICIs, were excluded. Eligible patients were also identified at Mass General Brigham/Dana-Farber Cancer Institute, Mayo Clinic, University of Washington School of Medicine, and NYU Langone/Laura and Isaac Perlmutter Cancer Center after IRB approval was obtained.
Data collection
A universal data collection protocol was established to harmonize data collection across all centers, and data were abstracted by two independent physician-investigators. We extracted data on patient demographics; cancer type; ICI type; prior autoimmune disease if any; serological autoimmune tests including rheumatoid factor, anti-citric citrullinated peptide, antinuclear antibodies, anti-Sjogren’s Syndrome A and B, human leukocyte antigen-B27, IL-6, TNF-α, and interferon gamma when available; type of irAEs for which anti-IL-6R was administered and time until irAEs onset; first-line of therapy used for de novo irAEs and flare of pre-existing autoimmune disease if any; type, dose, time until initiation of and duration of anti-IL-6R treatment as well as concomitant immunosuppressive therapy if any; C-reactive protein (CRP) levels at onset of irAEs, at initiation of anti-IL-6R, and within 8–12 weeks of treatment; reason for anti-IL-6R discontinuation (side effects or treatment failure); median duration of follow-up, and the tumor response to ICI as per the Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1 criteria.
For patients identified from MD Anderson, three independent radiologists (MOA; NHT; KME) reviewed all the imaging since the initiation of ICIs until the last available follow-up to characterize the tumor response using RECIST V.1.1. For patients identified from other centers, tumor response to ICIs was characterized based on the treating oncologists’ notes in the electronic medical records.
Outcome assessment
Our primary objective was evaluating the effectiveness of anti-IL-6R blockade for management of ICI-related AEs in terms of (1) clinical improvement of AEs (resolution or change to ≤grade 1 of irAEs or autoimmune disease flares), as documented in the medical records by the treating oncologist and/or subspecialist. For those patients who were evaluated by autoimmune disease specialists, we also took into consideration the disease activity measures to provide an objective assessment of irAEs improvement when data were available, while remaining cognizant that these outcome measures are adapted from primary autoimmune diseases and are yet to be validated for irAEs (detailed in online supplemental table S1); (2) normalization of CRP levels within 8–12 weeks of anti-IL-6R treatment when available; and (3) other diagnostic testing as required for diagnosis and management of each irAE.
jitc-2023-006814supp001.pdf (607.6KB, pdf)
Our secondary objectives were assessing the safety of anti-IL-6R treatment in terms of (1) anti-IL-6R-related AEs; (2) determining the best overall response rate (ORR) to ICI therapy as per RECIST V.1.1. For that, we evaluated the best ORR to ICI before the initiation of, or early on anti-IL-6R therapy (within 8–12 weeks) compared with the best ORR after anti-IL-6R administration per last available follow-up for each individual patient. Subgroup analyses were performed for patients (a) who received concomitant therapy after irAE development with both anti-IL-6R and ICI agents for a minimum of 15 days, (b) who started anti-IL-6R therapy within the first 3 months of ICI initiation (excluding those who initiated therapy later during their ICI treatment course), (c) patients with melanoma, and (d) those with pre-existing autoimmune disease. Of note, patients switched to other line of cancer therapies before anti-IL-6R initiation were excluded from the tumor response analysis; and (3) OS calculated from the first ICI dose until death for any reason.
Statistical analysis
Patient demographics and baseline characteristics, irAE characteristics, IL-6 treatment characteristics (type, dose, duration, and outcome of anti-IL-6R therapy) were summarized using descriptive statistics, mean (SD) or median (IQR) for continuous variables and frequency (%) for categorical variables. We estimated the proportion of patients who achieved complete response (CR) or partial response as the best response after ICI treatment along with the 95% CI. McNemar’s test was used to test marginal homogeneity of best response before the initiation or early on anti-IL-6R therapy and after anti-IL-6R treatment. We then used the univariate and multivariable logistic regression models to estimate the correlative factors associated with irAEs improvement within 1 month of anti-IL-6R therapy as well as with achieving best ORR to ICI in anti-IL-6R treated patients. Kaplan-Meier curve was used to estimate the OS. A p value of less than 0.05 indicated a statistical significance. SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) was used for data analysis.
Results
After excluding 3 patients who received anti-IL-6R as a preventative therapy, 92 patients were included in the final analysis, 69 from MD Anderson and 23 from other sites (online supplemental figure S1).
Patient characteristics
Patient characteristics are summarized in table 1. Their median age at ICI initiation was 61 years and 58 (63%) were men. Most patients had melanoma (46%). ICIs were mainly given as a single anti-PD-1 agent in 63 patients (69%) and used in the metastatic setting in 80 (89%) (online supplemental table S2). The median duration of ICI treatment was 8.6 months (range, 0.03–53.9 months), and the median duration of follow-up since ICIs initiation was 23 months (95% CI, 20 to 27 months) and since anti-IL-6R initiation was 12 months (95% CI, 8 to 15 months). Twenty-two patients (24%) had a pre-existing autoimmune disease, predominantly rheumatoid arthritis. Serological autoimmune testing was performed for 60 patients (excluding patients with pre-existing autoimmune disease) before initiation of anti-IL-6R; at least one autoimmune biomarker was positive in 37 (62%), and elevated IL-6 level was detected in 23/32 patients (72%).
Table 1.
Patient demographics and baseline characteristics
| Patient characteristics | N=92 |
| Mean age at ICI initiation (±SD) | 60.4±12.66 |
| Sex, n (%) | |
| Female | 34 (37) |
| Male | 58 (63) |
| Cancer type, n (%) | |
| Melanoma | 42 (46) |
| Renal cell carcinoma | 19 (21) |
| Bladder | 11 (12) |
| Non-small cell lung cancer | 7 (8) |
| Breast | 2 (2) |
| Prostate | 2 (2) |
| Squamous cell carcinoma of the head and neck | 2 (2) |
| Others* | 7 (8) |
| Immune checkpoint inhibitor type, n (%) | |
| Anti-PD-1 | 63 (69) |
| Anti-CTLA-4+anti-PD-1 | 24 (26) |
| Anti-PD-L1 | 3 (3) |
| Anti-CTLA-4 | 2 (2) |
| Cancer stage, n (%)† | |
| IV | 80 (87) |
| III | 10 (11) |
| Immune checkpoint inhibitor setting, n (%)‡ | |
| Metastatic | 80 (89) |
| Adjuvant | 10 (11) |
| History of pre-existing autoimmune disease, n (%) | 22 (24) |
| Rheumatoid arthritis§ | 14 (15) |
| Psoriasis with/without psoriatic arthritis | 4 (4) |
| Undifferentiated arthritis | 2 (2) |
| Polymyalgia rheumatica | 1 (1) |
| Sjogren’s syndrome | 1 (1) |
| Positive autoimmune markers, n (%) | |
| Positive at least one autoimmune marker | 48/78 (62) |
| IL-6 | 25/37 (68) |
| TNF-α | 13/28 (46) |
| IFN-γ | 8/29 (28) |
| ANA | 13/57 (23) |
| RF | 13/58 (22) |
| Anti-CCP | 8/55 (15) |
| Anti-SSA | 2/17 (12) |
| Anti-SSB | 2/42 (5) |
| HLA-B27 | 1/31 (3) |
| Types of irAEs treated with anti-IL-6R, n (%) | |
| Arthritis (new-onset or flare-ups of pre-existing arthritis) | 67 (73) |
| Rheumatoid arthritis-like | 46 (50) |
| Inflammatory polyarthritis | 11 (12) |
| Oligoarthritis of large joints | 5 (5) |
| Spondyloarthropathy-like (SPA-like) | 2 (2) |
| Psoriatic arthritis | 2 (2) |
| Polymyalgia rheumatica with peripheral synovitis | 1 (1) |
| Cholangitis/hepatitis | 6 (7) |
| Encephalitis | 5 (5) |
| Myositis/myasthenia gravis/myocarditis | 5 (5) |
| Myositis, myasthenia gravis and myocarditis | 2 (2) |
| Myositis and myasthenia gravis | 2 (2) |
| Myositis and myocarditis | 1 (1) |
| Polymyalgia rheumatica | 4 (4) |
| Pneumonitis | 1 (1) |
| Nephritis | 1 (1) |
| Colitis | 1 (1) |
| Systemic sclerosis | 1 (1) |
| CNS vasculitis | 1 (1) |
| First-line therapy for irAEs | |
| Corticosteroids | 81 (88) |
| Corticosteroid-sparing agents¶ | 33 (36) |
| Anti-IL-6R | 8 (9) |
| Pyridostigmine | 2 (2) |
| Plasmapheresis | 2 (2) |
| Intravenous immunoglobulin | 1 (1) |
| Anti-IL-6R type, dose/frequency, route, n (%) | |
| Sarilumab, 200 mg/2 weeks, subcutaneous | 4 (4) |
| Tocilizumab, 162 mg/2 weeks, subcutaneous | 31 (34) |
| Tocilizumab, 4 mg/kg/4 weeks, intravenous | 21 (23) |
| Tocilizumab, 8 mg/kg/4 weeks, intravenous | 10 (11) |
| Tocilizumab, 162 mg/1 week, subcutaneous | 9 (10) |
| Tocilizumab, 8 mg/kg once, intravenous | 8 (9) |
| Tocilizumab, 4 mg/kg once, intravenous | 7 (8) |
| Tocilizumab, 4 mg/kg/3 weeks, intravenous | 1 (1) |
| Tocilizumab, 162 mg once, subcutaneous | 1 (1) |
Numbers are rounded to the nearest whole number.
*Others: One patient each with acute myeloid leukemia, central nervous system lymphoma, cecal cancer, cervical cancer, Merkel cell, sarcoma, adrenal cancer.
†One patient had leukemia and one CNS lymphoma.
‡Information on ICI setting was not available for two patients.
§One patient had associated Sjogren’s Syndrome.
¶Corticosteroid-sparing agents used as a first-line treatment and varied widely according to the irAEs type including methotrexate, hydroxychloroquine, sulfasalazine, azathioprine, apremilast, mycophenolate, tacrolimus, rituximab, vedolizumab, anakinra, infliximab, etanercept, and adalimumab.
ANA, antinuclear antibodies; anti-CCP, anti-citric citrullinated peptide; anti-SSA, anti-Sjogren’s Syndrome A; anti-SSB, anti-Sjogren’s Syndrome B; CNS, central nervous system; CTLA-4, cytotoxic T lymphocyte-associated protein 4; HLA-B27, human leukocyte antigen-B27; ICI, immune checkpoint inhibitor; IFN-γ, interferon gamma; IL-6, interleukin-6; IL-6RA, interleukin-6 receptor antagonist; irAE, immune-related adverse event; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; RF, rheumatoid factor; SC, subcutaneous; TNF-α, tumor necrosis factor-alpha.
The main indications for using anti-IL-6R were inflammatory arthritis in 67 patients (73%); 50 had de novo inflammatory arthritis and 17 had flare-up of pre-existing arthritis (table 1). The median CRP level at irAE onset was 37.3 mg/L (range 0.4–301 mg/L). The median time to anti-IL-6R initiation from onset of irAE for which it was administered as a treatment was 3 months (range 0.03–51.0 months). Only eight patients (9%) received anti-IL-6R as the first-line therapy, primarily for inflammatory arthritis (n=7) and encephalitis (n=1) as recommended by the treating physician. However, the remaining 84 (91%) patients received it after failure of at least one immunosuppressant; 81 (88%) received prior corticosteroids (median dose of 40 mg/daily (range 7.5–1250 mg/daily) prednisone equivalent), and 33 (36%) received DMARDs (methotrexate, hydroxychloroquine, sulfasalazine, azathioprine, apremilast, mycophenolate mofetil, tacrolimus, rituximab, vedolizumab, anakinra, infliximab, etanercept, and adalimumab) without adequate improvement. Tocilizumab was the most frequently used anti-IL-6R in 88 patients (96%). The mode of administration varied widely depending on the irAEs type; tocilizumab 162 mg was administered subcutaneously (SC) every 2 weeks in 31 patients (34%), and 4–8 mg/kg intravenously every 4 weeks in 31 patients (34%), and the median duration of treatment was 4 months (range, 0.5–59 months). Anti-IL-6R was administered alone in 15 patients (16%), and in combination with corticosteroids in 72 patients (78%), and with other corticosteroid-sparing agents in 20 patients (22%). Of note, anti-IL-6R was administered concomitantly with ICI therapy in 36 patients after irAE development for a median of 4.4 months (range, 0.5–37.1 months).
Effectiveness of anti-IL-6R therapy for management of irAEs
In the overall study sample, treatment with anti-IL-6R led to improvement of irAEs in 67 patients (73%) after a median of 2 months (range, 0.03–23.0 months), along with discontinuation of corticosteroids in 14 (17%) on initiation of therapy and corticosteroid taper in additional 49 patients (60%) to a median dose of 10 mg/daily (IQR, 5–12 mg daily) prednisone equivalent within 2 months. The median CRP level at initiation of anti-IL-6R was 55.2 mg/L (IQR, 1.2–321 mg/L) and dropped to 1 mg/L (IQR, 0.1–84 mg/L) within 10 weeks of therapy (p<0.0001) in 20 patients with both CRP measurements. Subgroup analyses of the patients who had elevated IL-6 levels (n=23) and those who had normal levels (n=7) before anti-IL-6R initiation showed similar median time to irAE improvement after therapy (1 month each). Of note, receipt of anti-CTLA-4 and anti-PD-1 combination therapy was associated with irAE improvement within 1 month of anti-IL-6R therapy (adjusted OR (aOR) 4.4, 95% CI 1.34 to 14.44, p=0.01) (table 2). Conversely, history of pre-existing autoimmune disease (aOR 0.11, 95% CI 0.01 to 1.01, p=0.05), and use of corticosteroid-sparing agents as first-line therapy for irAEs (aOR 0.17, 95% CI 0.04 to 0.76, p=0.02) were associated with a lower odds of irAEs improvement.
Table 2.
Univariate and multivariable ORs for improvement of immune-related adverse events within 1 month of anti-IL-6R therapy
| Covariate | Univariate model | Multivariable model (n=84) | ||
| OR (95% CI) | P value | aOR (95% CI) | P value | |
| Age | ||||
| ≤50 years | 1.00 (Ref) | |||
| >50 years | 1.17 (0.37 to 3.71) | 0.78 | ||
| Sex | ||||
| Male | 1.00 (Ref) | |||
| Female | 0.9 (0.31 to 2.56) | 0.84 | ||
| Cancer type | ||||
| Melanoma | 1.00 (Ref) | |||
| Bladder | 1.49 (0.27 to 8.22) | 0.65 | ||
| Breast | 1.42 (0.01 to 148.72) | 0.88 | ||
| Non-small cell lung cancer | 2.03 (0.34 to 12.14) | 0.44 | ||
| Prostate | 4.47 (0.25 to 80.00) | 0.31 | ||
| Renal cell carcinoma | 2.53 (0.71 to 9.02) | 0.15 | ||
| Squamous cell carcinoma of the head and neck | 0.89 (0.02 to 40.10) | 0.95 | ||
| Others | 2.48 (0.39 to 15.82) | 0.34 | ||
| Checkpoint inhibitor monotherapy versus combination therapy | ||||
| Monotherapy | 1.00 (Ref) | 1.00 (Ref) | ||
| Combination ICIs | 3.67 (1.29 to 10.45) | 0.02 | 4.40 (1.34 to 14.44) | 0.01 |
| Checkpoint inhibitor type | ||||
| Anti-PD-1/PD-L1 | 1.00 (Ref) | |||
| Anti-CTLA-4 based therapy (anti-CTLA-4 single agent or anti-CTLA-4 + anti-PD-1) | 2.92 (1.01 to 8.45) | 0.05 | ||
| History of prior autoimmune disease | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 0.12 (0.01 to 0.93) | 0.04 | 0.11 (0.01 to 1.01) | 0.05 |
| Positive autoimmune markers | ||||
| No | 1.00 (Ref) | |||
| Yes | 0.95 (0.31 to 2.94) | 0.93 | ||
| Type of irAEs treated with anti-IL-6R | ||||
| Arthritis | 1.00 (Ref) | |||
| Non-arthritis | 1.68 (0.54 to 5.23) | 0.37 | ||
| Corticosteroids as first-line therapy for irAEs | ||||
| No | 1.00 (Ref) | |||
| Yes | 3.52 (0.42 to 29.31) | 0.25 | ||
| Corticosteroid sparing agents as first-line therapy for irAEs | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 0.23 (0.06 to 0.85) | 0.03 | 0.17 (0.04 to 0.76) | 0.02 |
| Anti-IL-6R dose | ||||
| 162 mg | 1.00 (Ref) | |||
| 200 mg | 4.86 (0.58 to 40.55) | 0.14 | ||
| 4 mg/kg | 1.62 (0.47 to 5.55) | 0.44 | ||
| 8 mg/kg | 2.43 (0.63 to 9.34) | 0.20 | ||
| Corticosteroid use with anti-IL-6R | ||||
| No | 1.00 (Ref) | |||
| Yes | 1.22 (0.36 to 4.23) | 0.75 | ||
| Corticosteroid dose with anti-IL-6R | ||||
| None | 1.00 (Ref) | |||
| <5 mg prednisone equivalent | 3.75 (0.40 to 35.54) | 0.25 | ||
| 5–10 mg prednisone equivalent | 1.21 (0.33 to 4.50) | 0.78 | ||
| >10 mg prednisone equivalent | 0.94 (0.20 to 4.44) | 0.94 | ||
anti-IL-6R, anti-interleukin-6 receptor; aOR, adjusted OR; CTLA-4, cytotoxic T lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.
Detailed information on the effectiveness of anti-IL-6R for various organ-specific toxicities is summarized below and in table 3.
Table 3.
Effectiveness of anti-IL-6R therapy for various organ-specific toxicities
| irAE type (n) | Clinical improvement n (%) |
| Inflammatory arthritis (67) | 56 (84) |
| Hepatitis/cholangitis (6) | 4 (67) |
| Myositis with myasthenia gravis and/or myocarditis overlap (5) | 0 (0) |
| Encephalitis (5) | 1 (20) |
| Polymyalgia rheumatica (4) | 3 (75) |
| CNS vasculitis (1) | 1 (100) |
| Diffuse systemic sclerosis (1) | 1 (100) |
| Interstitial nephritis (1) | 1 (100) |
| Colitis (1) | 0 (0) |
| Pneumonitis (1) | 0 (0) |
anti-IL-6, anti-interleukin-6; CNS, central nervous system; irAE, immune-related adverse event.
Inflammatory arthritis (n=67)
Most patients were treated with tocilizumab 162 mg/SC every 1–2 weeks as approved for rheumatoid arthritis. Fifty-six (84%) experienced clinical improvement of their joint symptoms after a median of 2.4 months (figure 1) since anti-IL-6R initiation, which was given subcutaneously every 1–2 weeks in 32 (57%) patients. Forty-three patients were evaluated by rheumatologists; the median Clinical Disease Activity Index (CDAI) score at onset of anti-IL-6R was 24 indicating high disease activity and dropped to 6 after treatment indicating low disease activity. The improvement was seen in 84% (n=32/38) who were treated with SC anti-IL-6R and 83% (n=24/29) who received intravenous anti-IL-6R. The median CRP level measured for 17 patients at anti-IL-6R initiation was 49 mg/L (normal range <10 mg/L) but dropped to 1 mg/L within 10 weeks of treatment. Of note, 58 (86%) patients initially failed to respond to first-line corticosteroid therapy at a median dose of 30 mg/day, and 22 patients failed to respond to other treatments including methotrexate, hydroxychloroquine, sulfasalazine, adalimumab, etanercept, golimumab, apremilast, vedolizumab, anakinra, rituximab, and infliximab.
Figure 1.
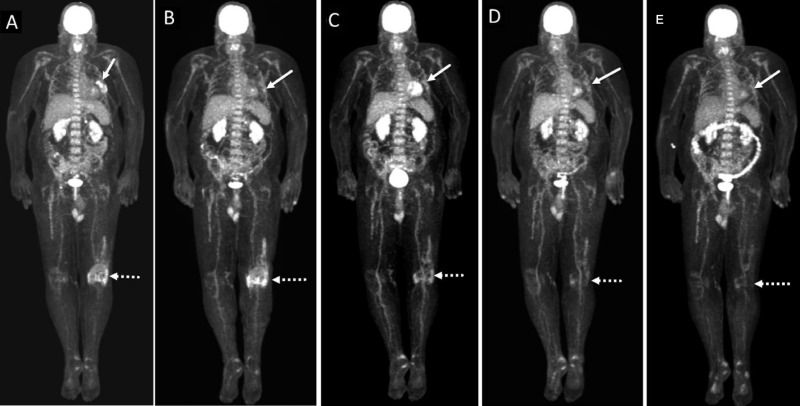
A patient with metastatic melanoma. (A) Baseline maximal intensity projection (MIP) PET images showing FDG avid left lung nodules. Note the hypermetabolic FDG uptake at the left knee joint is consistent with arthritis (dotted arrow). (B) MIP PET image at 6 months after initiation of pembrolizumab shows near complete resolution of metabolic activity of left pulmonary metastases (arrow). Note the increase in radiotracer uptake at the left knee joint (dotted arrow). (C) MIP PET image at 1 month after concomitant therapy with anti-IL-6R and pembrolizumab shows persistent near complete resolution of metabolic activity of left pulmonary metastases (arrow). Note the decrease in metabolic activity at the left knee joint (dotted arrow). (D) MIP PET image at 4 months after cessation of both anti-IL-6R and pembrolizumab shows persistent near complete resolution of metabolic activity of left pulmonary metastases. There is further decrease in metabolic activity at the left knee joint (dotted arrow). (E) MIP PET image at 7 months after cessation of ICI shows persistent near complete resolution of metabolic activity of left pulmonary metastases (arrow). The physiologic metabolic activity at the left knee joint (dotted arrow) consistent with resolution of arthritis. anti-IL-6R, anti-interleukin-6 receptor; FDG, F-fluorodeoxyglucose; ICI, immune checkpoint inhibitor; PET, positron emission tomography;
Hepatitis/cholangitis (n=6)
Five patients received intravenous tocilizumab 8 mg/kg administered once for three patients and every 4 weeks for two others. Four (67%) had improvement after a median of 12 days; the median aspartate aminotransferase level was 221 IU/L (range, 11–678) and alanine transaminase level was 174 IU/L (range, 8–315) at initiation of anti-IL-6R and dropped to 96 IU/L (range, 14–517) and 45 IU/L (range, 10–178), respectively, within 12 days (figure 2). The CRP level was measured for only one patient at anti-IL-6R initiation and was 109 mg/L but dropped to 12.4 mg/L within 10 weeks of treatment. Of note, one patient with hepatitis was treated with tocilizumab 162 mg SC every 1 week but did not improve. All six patients initially failed to respond to first-line corticosteroid therapy at a median dose of 72 mg/day, and two of them also failed to respond to mycophenolate and azathioprine.
Figure 2.
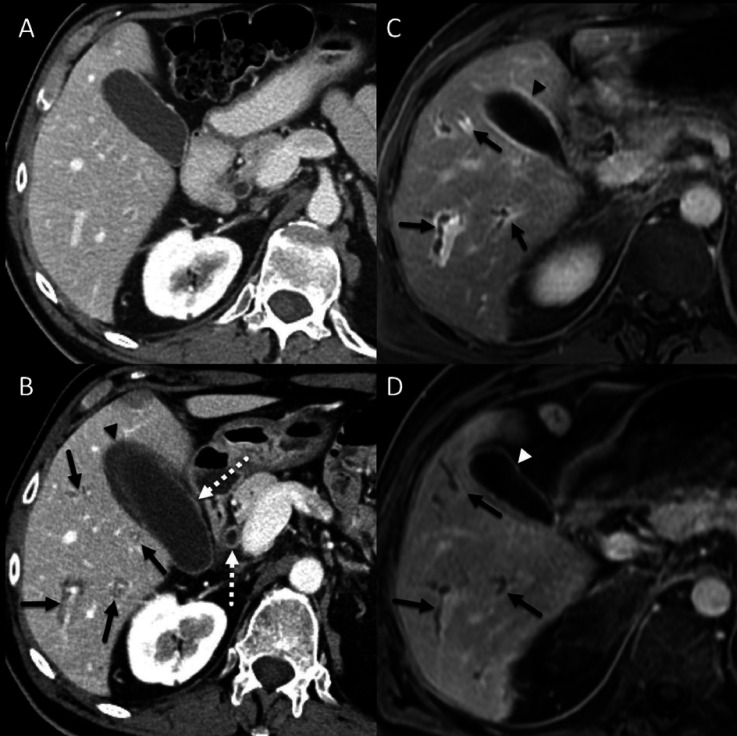
A patient with non-small cell lung cancer. (A) Baseline contrast-enhanced CT image showing unremarkable appearance of the liver. (B) Contrast-enhanced CT image at about 1 year after immune checkpoint inhibitor (ipilimumab and nivolumab) initiation showing periportal cuffing (solid arrows), thickening and avid enhancement of the common bile duct and the gallbladder wall (dotted arrows) and pericholecystic fluid (arrowhead). (C) 5-min delayed post-contrast gradient spoiled echo image showing periportal cuffing (arrows) and avid enhancement of the gallbladder wall (arrowhead), these findings are consistent with cholangitis. (D) 5-min delayed post-contrast gradient spoiled echo image 3 months after initiation of anti-IL-6R therapy showing resolution of periportal enhancement but there is persistent irregularity and mild dilatation of the intrahepatic biliary ducts (arrows). Avid enhancement of the gallbladder wall has also resolved (arrowhead). anti-IL-6R, anti-interleukin-6 receptor; ICI, immune checkpoint inhibitor.
Myositis with myasthenia gravis and/or myocarditis overlap (n=5)
Three patients with those conditions were treated with intravenous tocilizumab at 4 mg/kg (once, every 3 and 4 weeks respectively), one patient received 8 mg/kg every 4 weeks, and another patient received SC tocilizumab 162 mg every 2 weeks; duration of therapy varied widely with three patients that received a total of two doses, one received a single dose and another one received four doses of therapy, however, none had clinical improvement in spite of the improvement in chemical parameters. The median creatine kinase level was 2439 U/L (range 1050–3194) at initiation of anti-IL-6R and dropped to 98 U/L (range 41–104), median aldolase level dropped from 29 U/L (range, 26.8–33.6) to 10.8 U/L (range 5.7–19.1), and median troponin-T level dropped from 485 ng/mL (range 346–810) to 381 ng/mL (range 222–670). The CRP level was not measured for any of those patients at anti-IL-6R initiation but was measured in three patients within 10 weeks of treatment and the median CRP level was 0.38 mg/L. Of note, all five patients initially failed to respond to first-line treatment with high dose corticosteroid at a median dose of 225 mg/daily (range, 175–1250 mg/daily) prednisone or equivalent, and three of those patients also failed to respond to other treatments including plasmapheresis, pyridostigmine, mycophenolate mofetil, rituximab, intravenous immunoglobulin, tacrolimus, and infliximab that were administered as second-line treatments due to not achieving the anticipated response with corticosteroids alone.
Encephalitis (n=5)
All five patients with encephalitis were treated with intravenous tocilizumab of which four received 8 mg/kg intravenously once, leading to improvement within a day in one patient in addition to mild improvement (grade 4 to grade 3) in three others. The CRP level was measured for only one patient and was 321 mg/L before and dropped to 18 mg/L within 10 weeks of tocilizumab initiation. Tocilizumab was given for an additional patient as 4 mg/kg intravenously once, but remained intubated and never regained consciousness. Of note, two patients initially failed to respond to first-line corticosteroid therapy at a median dose of 100 mg/day.
Polymyalgia rheumatica (n=4)
Two patients were treated with tocilizumab 162 mg SC every 2 weeks, and one received 4 mg/kg intravenously every 4 weeks leading to significant improvement of their symptoms after a median of 1.4 months of therapy. Another patient received sarilumab 200 mg SC every 2 weeks for 4 months, without improvement. The CRP level was not measured for any of those patients at anti-IL-6R initiation but was measured in three within 10 weeks of treatment and the median CRP level was 3 mg/L. All four patients initially failed to respond to first-line corticosteroid therapy at a median dose 35 mg/day, including one who failed to respond to prior methotrexate.
Other irAEs (n=1 each)
One patient had ICI-induced central nervous system vasculitis and was treated with tocilizumab 162 mg SC every 2 weeks, leading to improvement after 2 months of treatment (figure 3). Similarly, one patient with diffuse systemic sclerosis improved after 5 months of tocilizumab given 162 mg SC every 2 weeks; this patient was evaluated by rheumatologists using the modified Rodnan Skin Score to quantify skin thickness. The total score was 40 before tocilizumab initiation and went down to 30 after 5 months of therapy. Another patient with acute interstitial nephritis experienced clinical improvement after one dose of intravenous tocilizumab 4 mg/kg. However, two other patients, one each with colitis and pneumonitis, were treated, respectively, with 4 mg/kg once and 8 mg/kg once but without improvement. The CRP levels were not measured in these patients before and after tocilizumab except for the patient with systemic sclerosis; CRP level was 1.5 mg/L at tocilizumab initiation and dropped to 0.4 mg/L within 10 weeks.
Figure 3.
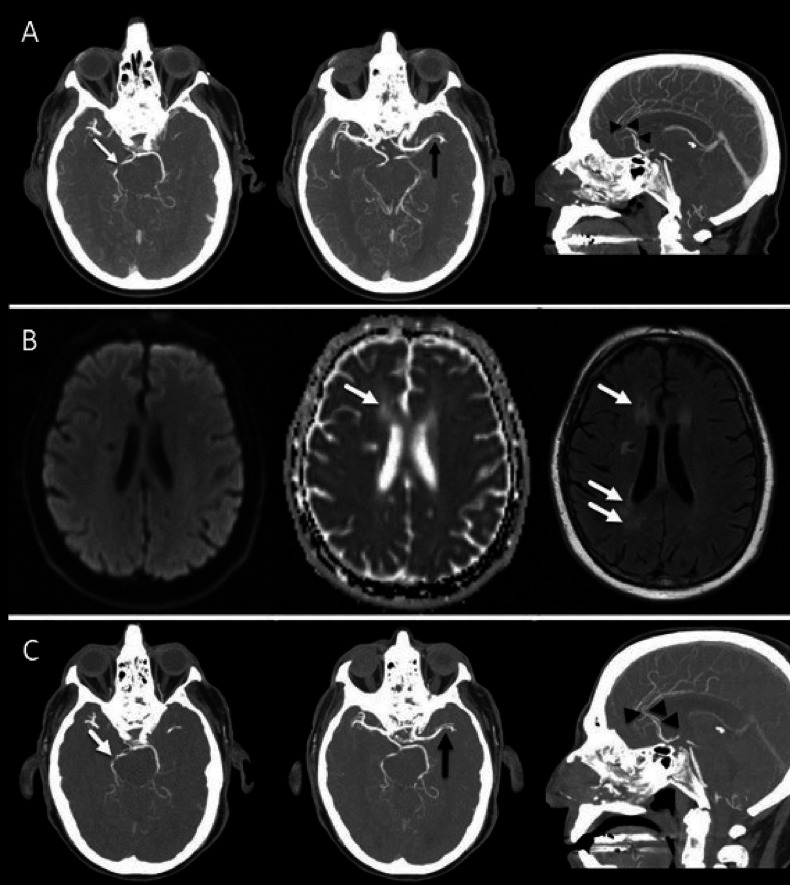
A patient with malignant melanoma. (A) Maximal intensity projection (MIP) images of concurrent cranial CT angiography at 7 months after immune checkpoint inhibitor (ipilimumab and nivolumab) initiation show multiple sites of intracranial arterial narrowing and/or occlusion involving the right posterior cerebral artery (white arrow), left middle cerebral artery and both anterior cerebral arteries (black arrowheads). This multi vascular pattern of involvement is suggestive of vasculitis. (B) Diffusion-weighted image, corresponding apparent diffusion coefficient (ADC) map, and fluid-attenuated inversion recovery (FLAIR) 3 months after initiation of anti-IL-6R therapy show normalization of ADC signal and development of high signal intensity foci on FLAIR (arrows) without corresponding diffusion restriction, in keeping with chronic infarcts. (C) MIP images of cranial CT angiography after anti-IL-6R therapy show recanalization with persistent narrowing of the right posterior cerebral artery (white arrow), persistent narrowing of the left middle cerebral artery, and recanalization of the anterior cerebral arteries (black arrowheads). anti-IL-6R, anti-interleukin-6 receptor.
Safety of anti-IL-6R therapy
Anti-IL-6R treatment-related AEs
Overall, treatment was well tolerated in 86 patients (93%). However, six patients reported adverse events that were attributed to anti-IL-6R, including abdominal pain, chills, tinnitus, fungal infection, rash, and tachycardia in one patient each, leading to treatment discontinuation. Of note, three of those patients were on concomitant anti-IL-6R and ICI therapy for a minimum of 14 days. Additionally, one patient was treated with sarilumab 200 mg SC every 2 weeks and developed neutropenia, but the patient was able to continue treatment after reducing the dose to 150 mg.
Tumor response to ICI therapy
Information on tumor response to ICI therapy was available for 70 patients before and after treatment with anti-IL-6R. Overall, the ORR was 66% (46/70) prior to anti-IL-6R initiation and remained 66% post initiation (95% CI, 55% to 77%), with 8% higher CR rate; 17% (12/70) versus 16% (11/70) had stable disease, 17% (12/70) versus 19% (13/70) had disease progression (online supplemental table S3). Notably, patients older than 50 years old had a higher ORR (aOR 3.5, 1.2–10.7, p=0.02) (table 4). Also, a longer duration of anti-IL-6R therapy was significantly associated with a higher ORR; the best ORR after initiation of anti-IL-6R therapy was 80% (24/30) among those who received ≥24 weeks of therapy compared with 54% (19/35) in patients who received anti-IL-6R for <24 weeks (aOR 3.2, 1.03–10.0, p=0.04).
Table 4.
Univariate and multivariable ORs for best overall response rate to immune checkpoint inhibitor in anti-IL-6R therapy treated patients
| Covariate | Univariate model | Multivariable model (n=70) | ||
| OR (95% CI) | P value | aOR (95% CI) | P value | |
| Age | ||||
| ≤50 years | 1.00 (Ref) | 1.00 (Ref) | ||
| >50 years | 3.48 (1.20 to 10.04) | 0.02 | 3.53 (1.17 to 10.65) | 0.02 |
| Sex | ||||
| Male | 1.00 (Ref) | |||
| Female | 1.56 (0.54 to 4.51) | 0.41 | ||
| Cancer type | ||||
| Melanoma | 1.00 (Ref) | |||
| Bladder | 1.08 (0.19 to 6.20) | 0.93 | ||
| Breast* | – | |||
| Non-small cell lung cancer | 6.36 (0.26 to 154.25) | 0.26 | ||
| Prostate | 1.57 (0.02 to 166.83) | 0.85 | ||
| Renal cell carcinoma | 0.39 (0.11 to 1.31) | 0.13 | ||
| Squamous cell carcinoma of the head and neck | 1.57 (0.02 to 166.83) | 0.85 | ||
| Others | 0.69 (0.10 to 4.66) | 0.70 | ||
| Checkpoint inhibitor monotherapy versus combination therapy | ||||
| Monotherapy | 1.00 (Ref) | |||
| Combination ICIs | 1.07 (0.39 to 2.96) | 0.89 | ||
| Checkpoint inhibitor type | ||||
| Anti-PD-1/PD-L1 | 1.00 (Ref) | |||
| Anti-CTLA-4 based therapy (anti-CTLA-4 single agent or anti-CTLA-4 + anti-PD-1) | 1.42 (0.49 to 4.13) | 0.52 | ||
| History of prior autoimmune disease | ||||
| No | 1.00 (Ref) | |||
| Yes | 1.06 (0.34 to 3.29) | 0.92 | ||
| Positive autoimmune markers | ||||
| No | 1.00 (Ref) | |||
| Yes | 0.49 (0.16 to 1.49) | 0.21 | ||
| Type of irAEs treated with anti-IL-6R | ||||
| Arthritis | 1.00 (Ref) | |||
| Non-arthritis | 0.51 (0.16 to 1.64) | 0.26 | ||
| Corticosteroids as first-line therapy for irAEs | ||||
| No | 1.00 (Ref) | |||
| Yes | 0.75 (0.13 to 4.16) | 0.74 | ||
| Corticosteroid sparing agents as first-line therapy for irAEs | ||||
| No | 1.00 (Ref) | |||
| Yes | 1.71 (0.59 to 4.92) | 0.32 | ||
| Anti-IL-6R dose | ||||
| 162 mg | 1.00 (Ref) | |||
| 200 mg | 4.2 (0.15 to 119.60) | 0.40 | ||
| 4 mg/kg | 0.43 (0.14 to 1.33) | 0.14 | ||
| 8 mg/kg | 1.4 (0.33 to 6.02) | 0.65 | ||
| Duration of anti-IL-6R treatment | ||||
| Stopped <24 weeks | 1.00 (Ref) | 1.00 (Ref) | ||
| Continued ≥24 weeks | 3.1 (1.06 to 9.10) | 0.04 | 3.21 (1.03 to 9.96) | 0.04 |
| Duration of ICI treatment | ||||
| Stopped <24 weeks | 1.000 | |||
| Continued ≥24 weeks | 0.76 (0.25 to 2.33) | 0.63 | ||
| Corticosteroid use with anti-IL-6R | ||||
| No | 1.00 (Ref) | |||
| Yes | 2.38 (0.76 to 7.43) | 0.14 | ||
| Corticosteroid dose with anti-IL-6R | ||||
| None | 1.00 (Ref) | |||
| < 5 mg prednisone equivalent | 3.24 (0.93 to 11.32) | 0.07 | ||
| 5–10 mg prednisone equivalent | 5 (0.11 to 233.72) | 0.41 | ||
| >10 mg prednisone equivalent | 1.12 (0.29 to 4.38) | 0.87 | ||
*None of the patients with breast cancer had evaluable tumor response after anti-IL-6R therapy and therefore were excluded from this analysis.
anti-IL-6R, anti-interleukin-6 receptor; aOR, adjusted OR; CTLA-4, cytotoxic T lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; irAEs, immune-related adverse events; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1.
Subgroup analyses showed that in patients who received concomitant therapy with both anti-IL-6R and ICIs for a minimum of 15 days and were evaluable for tumor response (n=30), the ORR was 50% (15/30) prior to anti-IL-6R initiation and remained 50% after concomitant therapy with both agents, with a 13% higher rate achieving CR (online supplemental table S3). In patients who initiated anti-IL-6R therapy within the first 3 months of ICI (n=12), the ORR was 58% (7/12) prior to anti-IL-6R and increased to 83% (10/12) after therapy (p=0.08). When evaluating only patients who initiated anti-IL-6R therapy within the first 3 months of ICI and continued concomitant therapy with both agents (n=6), the ORR was 83% (5/6) prior and increased to 100% (6/6) after concomitant therapy.
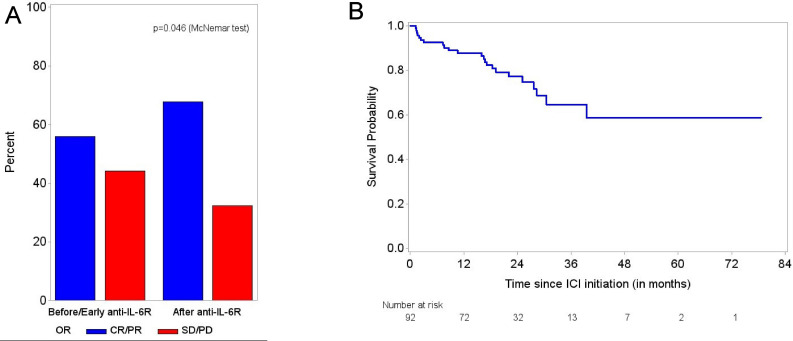
Of 34 evaluable patients with melanoma, the ORR was 56% prior to anti-IL-6R initiation and increased to 68% after treatment (p=0.046) (figure 4A). When restricting the analysis to only patients with melanoma who received concomitant therapy with both anti-IL-6R and ICI (n=15), the ORR was 33% (5/15) prior and increased to 47% (7/15) after concomitant therapy (p=0.16), with a 20% higher rate achieving CR. In four patients with melanoma who started anti-IL-6R therapy within the first 3 months of ICI onset, the ORR increased from 50% prior to 75% after anti-IL-6R therapy. Further subgroup analyses of other cancer types were not possible because of the small number of evaluable patients.
Figure 4.
(A) The best overall response rate to ICI therapy in patients with melanoma treated with anti-IL-6R. (B) Overall survival from ICI initiation: median follow-up from ICI initiation 23 months (95% CI, 20 to 27). anti-IL-6R, anti-interleukin-6 receptor; CR, complete response; ICI, immune checkpoint inhibitor; PD, progressive disease; PR, partial response; SD, stable disease.
Overall, discontinuation of ICI therapy occurred in 52 patients (56%); because of toxicity in 41 (45%), progression in 9 (10%), both progression and toxicity, and infection with COVID-19 in 1 patient each (1%). Forty patients (44%) successfully completed their ICI therapy including 9 (10%) whose treatment was temporarily held because of toxicity but resumed ICI therapy after improvement of irAEs.
Overall survival
Overall, 23 patients (25%) died during the follow-up period of 23 months (95% CI, 20 to 27 months) since ICI initiation. In only 4 (4%), death was primarily attributable to irAEs (2 patients with encephalitis, 1 pneumonitis, and 1 with multi-organ irAEs). The median OS was not reached (figure 4B).
Use of anti-IL-6R therapy in patients with pre-existing autoimmune disease
Among the 22 patients who had pre-existing autoimmune disease, the main indications for using anti-IL-6R was flare-up of pre-existing rheumatoid arthritis (n=14) and psoriatic arthritis (n=1), and de novo rheumatoid-like arthritis (n=4), psoriatic arthritis (n=1), myasthenia gravis/myositis overlap (n=1), and polymyalgia rheumatica (PMR) (n=1) following initiation of ICI (detailed in online supplemental table S4). Eighteen patients (82%) received first-line therapy for pre-existing autoimmune disease flare-up prior to anti-IL-6R; 17 (77%) received corticosteroids and 10 (45%) received DMARDs (methotrexate, hydroxychloroquine, sulfasalazine, apremilast, rituximab) without improvement. With the use of anti-IL-6R, improvement of these AEs was seen in 17 patients (77%) after a median of 3 months with discontinuation of corticosteroids in 5 patients (29%) and corticosteroid taper to a median dose of 10 mg/daily prednisone equivalent in 12 others (71%). Of 11 evaluable patients with flare or de novo arthritis, the median CDAI score was 30 at anti-IL-6R initiation and dropped to 8 within 4 months of initiation of anti-IL-6R therapy. The median CRP level at initiation of anti-IL-6R was 77.4 mg/L and dropped to 3.6 mg/L within 10 weeks of therapy. Eleven patients (50%) were able to continue ICI, and the median duration of concomitant therapy with both anti-IL-6R and ICIs was 3.4 months (range 0.6–37.1 months). Of 10 evaluable patients with melanoma and pre-existing autoimmune disease, the ORR was 50% (5/10) prior to anti-IL-6R initiation and remained 50% after treatment. There has been no AE-related mortality.
Discussion
This report is the largest retrospective analysis of ICI-treated patients with cancer who developed irAEs and were treated with anti-IL-6R agents. In this study, we observed an improvement in symptoms and severity of irAEs in three-quarters of the patients within a median of 2.0 months post anti-IL-6R therapy initiation. Importantly, two-thirds of all patients showed a durable response to ICI. Indeed, the use of anti-IL-6R improved multiple organ toxicity, including inflammatory arthritis, hepatitis/cholangitis, PMR, nephritis, systemic sclerosis, and central nervous system vasculitis. Owing to the small number of patients with encephalitis and myositis overlapping with myasthenia gravis and/or myocarditis and the variations in the anti-IL-6R treatment doses they received, we could not draw a definitive conclusion on how IL-6 blockade might impact these very severe toxicities.
Interestingly, we observed correlation between biomarker levels and clinical improvement in specific irAEs; clinical improvement of inflammatory arthritis and hepatitis/cholangitis was associated with decrease in CRP and liver enzyme levels, respectively. On the contrary, it was difficult to conclude the correlation in other irAEs due to the limited number and complex clinical evaluation. Furthermore, our results showed no correlation between elevated IL-6 levels and faster irAE resolution. Due to the variations of anti-IL-6R doses and frequencies observed in our cohort, we could not identify the ideal therapeutic regimen for each organ-specific toxicity, which remains an important question that could be answered only in a clinical trial setting. Our data have also shown that receiving ICI combination therapy predicted irAEs improvement within 1 month of anti-IL-6R therapy. This finding could be explained by our previous observation that TH17 were more induced in synovial fluid from patients with arthritis-irAE treated with the combination versus ICI monotherapy and that those patients were more resistant to treatment with corticosteroids.27 28 In contrast, the use of corticosteroid-sparing agents as first-line was associated with slower irAE improvement after anti-IL-6R therapy, raising an important question about the ideal timing for the initiation of anti-IL-6R therapy to achieve rapid resolution of irAEs. It is well known that IL-6 signaling is important in intestinal epithelial repair, and therefore it is crucial to monitor patients closely for potentially serious complications such as gastrointestinal perforation.29 In our cohort, 7% of patients experienced an adverse event that led to anti-IL-6R discontinuation but none had gastrointestinal perforation.
Although the published guidelines from several oncology societies recommend the use of corticosteroids as the first line of therapy for irAEs,14–17 it is unclear whether corticosteroids can provide therapeutic benefit without impacting antitumor immunity. In fact, previous studies have shown that the use of prednisone ≥10 mg/day at baseline led to detrimental cancer outcomes with shorter OS in patients with melanoma and non-small cell lung cancer treated with ICI.18 20 Also, earlier use of corticosteroids during the first 2 months of ICI therapy led to shorter PFS and OS compared with their use later during ICI treatment course.19 In contrast to patients receiving corticosteroids, two-thirds of the anti-IL-6R treated patients in our retrospective cohort achieved or continued to have a clinical response to ICI. We also found that the duration of anti-IL-6R therapy ≥24 weeks was significantly associated with a higher ORR. While there are preclinical data demonstrating that anti-IL-6R can contribute directly to antitumor immune response induced by ICIs,27 however, the continuous response we have seen in our cohort could be just due to prolonged ICI treatment and not necessarily impacted by anti-IL-6R therapy.
When we looked specifically at the patients who received both ICI and anti-IL-6R therapies concomitantly and those who developed early-onset irAEs and initiated anti-IL-6R within the first 3 months of ICI, we observed a trend towards a higher response rate after receiving anti-IL-6R in the overall study population and in patients with melanoma. Moreover, in a special cancer population with pre-existing autoimmune disease, we found that using anti-IL-6R agents improved adverse events among patients who did not initially improve despite corticosteroids with or without DMARDs. However, the median time to improvement was longer than patients without baseline autoimmunity, which could be partially explained by the severity and duration of disease prior to ICI initiation. More importantly, these patients were able to continue both ICI and anti-IL-6R therapy concomitantly for up to 37 months without impacting their tumor response to ICI. These data support our hypothesis that targeting IL-6R to treat irAEs could possibly be safer and preferable than corticosteroids, especially for patients with early-onset irAEs (occurring during the first 3 months of ICIs) and those with pre-existing autoimmune diseases who require prolonged immunosuppressive treatment.
It is well-known that IL-6 is an essential cytokine for the differentiation of naïve CD4+ T cells into TH17 cell lineage,26 30–32 which plays a pivotal role in the pathogenesis of several autoimmune diseases such as rheumatoid arthritis, giant cell arteritis, systemic sclerosis-associated interstitial lung diseases, and cytokine release syndrome, where the use of IL-6R inhibitors is currently approved.33 34 Specifically, in ICI-treated patients, elevated IL-6 was shown to be associated with de novo arthritis, pneumonitis, and psoriasis,35–37 and an increase in circulating levels of TH17 cells was suggested as a potential biomarker associated with irAEs.38 39 Furthermore, a small systematic literature review, that also involved a case series23 and a multicenter retrospective analysis,24 have both concluded by suggesting that tocilizumab be used to treat irAEs. In contrast, another study identified that low baseline serum levels of IL-6 was an independent risk factor for severe irAEs in patients with ipilimumab-treated melanoma, however, inflamed tissues were not evaluated in this study.40 Also, another study found 11 circulating cytokines to be significantly elevated at baseline and early during treatment with anti-PD-1 or combination ICIs in patients with melanoma and were validated as predictors of severe irAEs; IL-6 was not one among the identified cytokines.41 However, the cellular immune response induced by these cytokines was not evaluated in this study.41 A separate cytokine analysis in patients with melanoma included eight of these cytokines and failed to discern patients who developed severe irAEs from those who had ≤grade 2 irAEs, and IL-6 was not one among the cytokines identified as predictors of dermatitis, colitis, and pneumonitis irAEs.42 In these studies baseline levels of free IL-6 were measured,40–42 however, it is conceivable that measuring free IL-6 levels may be less informative here since a significant proportion of the IL-6 would be bound to its soluble receptors prior to the onset of irAEs. Indeed, IL-6 is known to have dual mechanisms of action (1) trans-signaling where IL-6 binds to soluble IL-6 receptor and the complex then promotes cellular changes that drive inflammatory events and development of autoimmune disease, and (2) cis-signaling where IL-6 binds to membrane bound IL-6 receptor; this pathway has not been associated with pathogenesis of inflammation and autoimmunity.43
While there are few reports in the literature on the use of tocilizumab for management of irAEs,23 24 about one-third of the reported patients had cytokine release syndrome, which is an already approved indication for tocilizumab. Furthermore, these studies did not describe how this treatment approach impacted the tumor response to ICI as tumor outcome data were not available for most patients.23 24 More recently, an open-label clinical study reported the use of tocilizumab in 20 patients with various cancer types who developed de novo arthritis and/or colitis following ICI (NCT03601611).25 In this study, 84% of patients achieved ≥1 grade reduction in severity of irAEs and 30% had cancer progression within 24 weeks after treatment with tocilizumab. However, the study did not provide information on the tumor response to ICI before administration of tocilizumab which makes it difficult to interpret the impact of tocilizumab on ICI tumor immunity as those patients might have been non-responders. Therefore, there is a gap in knowledge regarding the role of IL-6 in irAE pathogenesis, and the potential benefit of combining IL-6 blockade with ICIs to improve outcomes. This question can only be more accurately answered in prospective and translational studies. Based on our recent data,27 which highlight the role of the IL-6/TH17 axis in the biology of number of the ICI-induced toxicities in both human and murine studies, we mechanistically defined the role of the IL-6/TH17 axis in ICI autoimmunity and tumor immunity. In that study, gene expression analyses of inflamed, healthy, and tumor tissue biopsies along with the preclinical mechanistic experiments, suggested a role of IL-6 in ICI-related colitis and encephalitis as well as in tumor resistance to ICI therapy. Importantly, we also showed in preclinical models that targeting IL-6 could be an effective therapeutic strategy for irAEs without damping antitumor immunity.
Indeed, cytokine inhibitors have been suggested in the current guidelines for treatment of refractory irAEs and as a preventive strategy in special cancer populations with pre-existing autoimmune disease.14–17 These recommendations were adapted primarily from the treatment of autoimmune diseases, and the efficacy of individual cytokine inhibitors for various organ-specific toxicities developed due to ICI treatment is unclear.21 While the use of anti-TNF-α for treating various irAEs has been suggested in several studies,44–62 this was found to be associated with an increased risk of cardiovascular mortality52 and cancer progression63 64 and worse survival outcomes.65 Moreover, in a recent two-arm phase 1b clinical trial the upfront use of infliximab (anti-TNF-α) with ipilimumab+nivolumab (triple therapy) had a similar safety profile to ipilimumab+nivolumab, while adding certolizumab (another anti-TNF-α) was associated with an increased rate of hepatitis-irAE and a higher ORR compared with ipilimumab+nivolumab alone (NCT03293784).66 Thus, further mechanistic and prospective studies are warranted to help understand the pathogenic role of TNF-α in various irAE types and explore the basis for the differential impact of various TNF inhibitors on development of irAEs and oncologic outcomes. Similarly, the use of other cytokine inhibitors targeting IL-23,67–70 IL-17,71–75 IL-5,76 77 and IL-478 has also been reported for treatment of irAEs, but the data are extremely limited and therefore, the impact of these treatment strategies on antitumor response, progression-free and OS in ICI-treated patients still needs to be evaluated in prospective clinical trials.
In the current retrospective study, we limited our inclusion criteria to patients with irAEs (regardless of the organ affected by toxicity) treated with anti-IL-6R in order to determine its role in management of various types of irAEs. We aimed to comprehensively evaluate the safety of this treatment approach by comparing best ORR in those same patients before versus after treatment with anti-IL-6R, so we could control for potential confounding factors that could directly impact the patients’ response to ICI therapy. Although limited by its retrospective nature, the heterogeneous indications for using IL-6R blockers, small number of patients with specific irAEs, different cancer types that precluded further subgroup analyses, lack of comparator group treated with different corticosteroid-sparing agents, and potential for survival bias, this study provides a safety signal that would support the design of clinical studies that can prospectively and more accurately assess the role of IL-6 blocking in mitigating immune toxicity. Currently, our group and other investigators have initiated trials that are investigating the efficacy and safety of IL-6 blockade in patients with ICI-treated cancer (NCT04940299, NCT04375228, NCT03601611, NCT03999749, NCT03821246, NCT04524871, NCT03424005, NCT03869190).
Conclusion
Deep understanding of the immunohistopathological findings of various organ-specific toxicities as well as the similarities and differences between autoimmunity and tumor immunity is crucial, so that we can establish a personalized treatment strategy for each irAE. This study provides proof of concept supporting further evaluation of the use of IL-6R inhibitors in prospective clinical trials in combination with checkpoint inhibition. Biomarker analyses that include longitudinal collection of blood, stool (for microbiome), tumor, and inflamed tissues are important to better understand the mechanism, safety and efficacy of anti-IL-6R or other anti-cytokine therapies. Such clinical trials can provide a better understanding of how we can effectively decouple autoimmunity from antitumor immunity on cytokine(s) level, so that patients can benefit more from the efficacy of ICI therapy.
Acknowledgments
We thank Dr Sirisha Yadugiri, the Senior Technical Writer in the Department of Melanoma Medical Oncology at The University of Texas MD Anderson Cancer Center for editorial support.
Footnotes
Twitter: @OsamaRahma2
NA-W and AD contributed equally.
Presented at: This study was presented at the Annual Meeting of the Society for Immunotherapy of Cancer on November 13, 2021.
Correction notice: This article has been corrected since it was published online. Noha Abdel-Wahab has been listed as a corresponding author.
Contributors: NA-W and AD had full access to all the data in the study and take responsibility for the integrity and the accuracy of the data analysis. Study concept and design: FF, NA-W, and AD. Acquisition of data: FF, MB, AF, CMZ, VAT, MOA, NHMT, KME, EJM, JC, MD, SS, OA-S, UT, AMZ, RT, NS, and SHC. Analysis and interpretation of data: FF, NA-W, and JS. Quality appraisal: FF and NA-W. Drafting of the manuscript: FF. Critical revision of the manuscript for important intellectual content: FF, DHJ, HL, KL, JAS, OR, MD, JHT, AA, MES-A, PG, IO, JW, NA-W, and AD. Statistical analysis: FF and JS. Administrative, technical, or material support: NA-W and AD. Study supervision: NA-W and AD. All authors read and approved the final manuscript.
NA-W and AD are guarantor for the content.
Funding: NA-W has a K01 Mentored Research Scientist Development Award from the National Institute of Allergy and Infectious Diseases (K01AI163412). AD received a Wilkes Melanoma Foundation grant. The statistical analysis work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Competing interests: The authors declare that they have no competing interests. MES-A has received consulting fees in the past 12 months from Pfizer, Eli Lilly and Bristol Myers Squibb, all unrelated to this study. IO funded by NYU SPORE P50CA225450. JAS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers R01 AR077607, P30 AR070253, and P30 AR072577), the R. Bruce and Joan M. Mickey Research Scholar Fund, and the Llura Gund Award for Rheumatoid Arthritis Research and Care. JAS has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health. PG consulting for 4D Pharma, Aadi Bioscience, Astellas, Asieris Pharmaceuticals, AstraZeneca, BostonGene, Bristol Myers Squibb, CG Oncology, Dyania Health, Exelixis, Fresenius Kabi, Genentech, Gilead Sciences, Guardant Health, ImmunityBio, Infinity Pharmaceuticals, Janssen, Lucence, Merck KGaA, Mirati Therapeutics, MSD, Pfizer, PureTech, QED Therapeutics, Regeneron, Roche, Seattle Genetics, Silverback Therapeutics, Strata Oncology, UroGen Pharma; and has received institutional research funding from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm Group, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Merck KGaA, Mirati Therapeutics, MSD, Pfizer, QED Therapeutics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. IRB number: PA19–0089. Due to the study’s retrospective nature and the fact that all patients’ clinical data and results were reviewed from the charts, we obtained a waiver of informed consent.
References
- 1. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84. 10.1016/S1470-2045(15)70076-8 [DOI] [PubMed] [Google Scholar]
- 2. Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109–17. 10.1016/S0140-6736(14)60958-2 [DOI] [PubMed] [Google Scholar]
- 3. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with Cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018;379:341–51. 10.1056/NEJMoa1805131 [DOI] [PubMed] [Google Scholar]
- 4. Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti-programmed death 1 Monoclonal antibody Dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: A Nonrandomized phase 1 clinical trial. JAMA Oncol 2020;6:1766–72. 10.1001/jamaoncol.2020.4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of Durvalumab (MEDI4736), an Anti–Programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. JCO 2016;34:3119–25. 10.1200/JCO.2016.67.9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a Multicentre, single-group, open-label, phase 2 trial. Lancet Oncol 2016;17:1374–85. 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, Multicentre, phase 2 trial. Lancet 2016;387:1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with Ipilimumab in patients with metastatic Melanoma. N Engl J Med 2010;363:711–23. 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune Checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One 2016;11:e0160221. 10.1371/journal.pone.0160221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. 10.1186/s12916-015-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Osta B, Hu F, Sadek R, et al. Not all immune-Checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol 2017;119:1–12. 10.1016/j.critrevonc.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 12. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or monotherapy in untreated Melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune Checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 14. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 2021;39:4073–126. 10.1200/JCO.21.01440 [DOI] [PubMed] [Google Scholar]
- 16. Thompson JA, Schneider BJ, Brahmer J, et al. Management of Immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022;20:387–405. 10.6004/jnccn.2022.0020 [DOI] [PubMed] [Google Scholar]
- 17. Haanen J, Obeid M, Spain L, et al. Management of toxicities from Immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1217–38. 10.1016/j.annonc.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 18. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 19. Maslov DV, Tawagi K, Kc M, et al. Timing of steroid initiation and response rates to immune Checkpoint inhibitors in metastatic cancer. J Immunother Cancer 2021;9:e002261. 10.1136/jitc-2020-002261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 21. Esfahani K, Elkrief A, Calabrese C, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol 2020;17:504–15. 10.1038/s41571-020-0352-8 [DOI] [PubMed] [Google Scholar]
- 22. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25:551–7. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 23. Campochiaro C, Farina N, Tomelleri A, et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med 2021;93:87–94. 10.1016/j.ejim.2021.07.016 [DOI] [PubMed] [Google Scholar]
- 24. Dimitriou F, Hogan S, Menzies AM, et al. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer 2021;157:214–24. 10.1016/j.ejca.2021.08.031 [DOI] [PubMed] [Google Scholar]
- 25. Holmstroem RB, Nielsen OH, Jacobsen S, et al. COLAR: open-label clinical study of IL-6 blockade with Tocilizumab for the treatment of immune Checkpoint inhibitor-induced colitis and arthritis. J Immunother Cancer 2022;10:e005111. 10.1136/jitc-2022-005111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 2002;39:531–6. 10.1016/s0161-5890(02)00210-9 [DOI] [PubMed] [Google Scholar]
- 27. Hailemichael Y, Johnson DH, Abdel-Wahab N, et al. Interleukin-6 blockade Abrogates Immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022;40:509–23. 10.1016/j.ccell.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim ST, Chu Y, Misoi M, et al. Distinct molecular and immune hallmarks of inflammatory arthritis induced by immune checkpoint inhibitors for cancer therapy. Nat Commun 2022;13. 10.1038/s41467-022-29539-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rempenault C, Lukas C, Combe B, et al. Risk of diverticulitis and gastrointestinal perforation in rheumatoid arthritis treated with tocilizumab compared to rituximab or abatacept. Rheumatology 2022;61:953–62. 10.1093/rheumatology/keab438 [DOI] [PubMed] [Google Scholar]
- 30. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic Effector Th17 and regulatory T cells. Nature 2006;441:235–8. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 31. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor Rorgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–33. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 32. Miossec P, Kolls JK. Targeting IL-17 and Th17 cells in chronic inflammation. Nat Rev Drug Discov 2012;11:763–76. 10.1038/nrd3794 [DOI] [PubMed] [Google Scholar]
- 33. Administration USFaD . Drugs@FDA: FDA-approved drugs: U.S Department of health and human services. 2022. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125276s134lbl.pdf
- 34. Administration USFaD . Drugs@FDA: FDA-approved drugs: U.S Department of health and human services. 2018. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761037s001lbl.pdf
- 35. Kowalski B, Valaperti A, Bezel P, et al. Analysis of Cytokines in serum and Bronchoalveolar Lavage fluid in patients with immune-Checkpoint inhibitor-associated Pneumonitis: a cross-sectional case-control study. J Cancer Res Clin Oncol 2022;148:1711–20. 10.1007/s00432-021-03750-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanaka R, Okiyama N, Okune M, et al. Serum level of Interleukin-6 is increased in Nivolumab-associated Psoriasiform dermatitis and tumor necrosis factor-Α is a biomarker of Nivolumab Recativity. J Dermatol Sci 2017;86:71–3. 10.1016/j.jdermsci.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 37. Zhou X, Jaquith J, Li Y. Inflammatory arthritis induced by immune Checkpoint inhibitor therapy: a distinct clinical entity and immunologic phenotype arthritis. Rheumatol 2019;71. [Google Scholar]
- 38. Callahan MK, Yang A, Tandon S, et al. Evaluation of serum IL-17 levels during Ipilimumab therapy: correlation with colitis. JCO 2011;29:2505. 10.1200/jco.2011.29.15_suppl.2505 [DOI] [Google Scholar]
- 39. Fujimura T, Sato Y, Tanita K, et al. Serum levels of soluble Cd163 and Cxcl5 may be predictive markers for immune-related adverse events in patients with advanced Melanoma treated with Nivolumab: a pilot study. Oncotarget 2018;9:15542–51. 10.18632/oncotarget.24509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valpione S, Pasquali S, Campana LG, et al. Sex and Interleukin-6 are Prognostic factors for autoimmune toxicity following treatment with anti-Ctla4 blockade. J Transl Med 2018;16:94. 10.1186/s12967-018-1467-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res 2019;25:1557–63. 10.1158/1078-0432.CCR-18-2795 [DOI] [PubMed] [Google Scholar]
- 42. Tyan K, Baginska J, Brainard M, et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol Immunother 2021;70:2209–21. 10.1007/s00262-021-02855-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest 2011;121:3375–83. 10.1172/JCI57158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson DH, Zobniw CM, Trinh VA, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related Enterocolitis. J Immunother Cancer 2018;6:103. 10.1186/s40425-018-0412-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakashima K, Demura Y, Oi M, et al. Infliximab was found to be effective for treating immunosuppressive drug-resistant hepatitis due to Durvalumab. Intern Med 2020;59:3055–9. 10.2169/internalmedicine.5216-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aldrich J, Pundole X, Tummala S, et al. Inflammatory Myositis in cancer patients receiving immune Checkpoint inhibitors. Arthritis Rheumatol 2021;73:866–74. 10.1002/art.41604 [DOI] [PubMed] [Google Scholar]
- 47. Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer 2019;7. 10.1186/s40425-019-0774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin JS, Mamlouk O, Selamet U, et al. Infliximab for the treatment of patients with Checkpoint inhibitor-associated acute tubular interstitial nephritis. Oncoimmunology 2021;10:1877415. 10.1080/2162402X.2021.1877415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang S, Jordan A, Jenneman D, et al. Rapid improvement following receipt of infliximab in steroid-refractory Durvalumab-associated grade 3 pneumonitis. Cureus 2022;20. 10.7759/cureus.22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kadokawa Y, Takagi M, Yoshida T, et al. Efficacy and safety of Infliximab for steroid-resistant immune-related adverse events: A retrospective study. Mol Clin Oncol 2021;14:65. 10.3892/mco.2021.2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moriyama S, Fukata M, Tatsumoto R, et al. Refractory Constrictive Pericarditis caused by an immune Checkpoint inhibitor properly managed with Infliximab: a case report. Eur Heart J Case Rep 2021;5:ytab002. 10.1093/ehjcr/ytab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cautela J, Zeriouh S, Gaubert M, et al. Intensified immunosuppressive therapy in patients with immune checkpoint inhibitor-induced myocarditis. J Immunother Cancer 2020;8:e001887. 10.1136/jitc-2020-001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Giglio D, Berntsson H, Fred Åsa, et al. Immune checkpoint inhibitor-induced polymyositis and myasthenia gravis with fatal outcome. Case Rep Oncol 2020;13:1252–7. 10.1159/000510740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paparoupa M, Stupperich S, Goerg-Reifenberg L, et al. Successful treatment of an immune-mediated colitis induced by checkpoint inhibitor therapy in a patient with advanced melanoma. Case Rep Gastroenterol 2020;14:554–60. 10.1159/000511252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choi JS, Chen M, McQuade JL, et al. Recurrent audiovestibular dysfunction and associated neurological immune-related adverse events in a melanoma patient treated with nivolumab and ipilimumab. Head Neck 2020;42:E35–42. 10.1002/hed.26455 [DOI] [PubMed] [Google Scholar]
- 56. Vindum HH, Agnholt JS, Nielsen AWM, et al. Severe steroid refractory gastritis induced by nivolumab: a case report. World J Gastroenterol 2020;26:1971–8. 10.3748/wjg.v26.i16.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chang VA, Simpson DR, Daniels GA, et al. Infliximab for treatment-refractory transverse Myelitis following immune therapy and radiation. J Immunother Cancer 2018;6:153. 10.1186/s40425-018-0471-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alhammad RM, Dronca RS, Kottschade LA, et al. Brachial plexus neuritis associated with Anti-Programmed cell death-1 antibodies: report of 2 cases. Mayo Clin Proc Innov Qual Outcomes 2017;1:192–7. 10.1016/j.mayocpiqo.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kopecký J, Kubeček O, Geryk T, et al. Nivolumab induced encephalopathy in a man with metastatic renal cell cancer: a case report. J Med Case Rep 2018;12:262. 10.1186/s13256-018-1786-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2020;79:332–8. 10.1136/annrheumdis-2019-216109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Araujo DV, Muniz TP, Yang A, et al. Real world outcomes and hepatotoxicity of infliximab in the treatment of Steroid-Refractory immune-related adverse events. Curr Oncol 2021;28:2173–9. 10.3390/curroncol28030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019;569:428–32. 10.1038/s41586-019-1162-y [DOI] [PubMed] [Google Scholar]
- 63. Zou F, Abu-Sbeih H, Ma W, et al. Association of chronic immune-mediated diarrhea and colitis with favorable cancer response. J Natl Compr Canc Netw 2020;19:700–8. 10.6004/jnccn.2020.7647 [DOI] [PubMed] [Google Scholar]
- 64. Jacoberger-Foissac C, Blake SJ, Liu J, et al. Concomitant or delayed anti-TNF Differentially impact on immune-related adverse events and antitumor efficacy after anti-Cd40 therapy. J Immunother Cancer 2020;8:e001687. 10.1136/jitc-2020-001687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory Ipilimumab and anti-Pd1-treated patients in the Dutch Melanoma treatment Registry. Clin Cancer Res 2020;26:2268–74. 10.1158/1078-0432.CCR-19-3322 [DOI] [PubMed] [Google Scholar]
- 66. Montfort A, Filleron T, Virazels M, et al. Combining nivolumab and ipilimumab with infliximab or Certolizumab in patients with advanced melanoma: first results of a phase Ib clinical trial. Clin Cancer Res 2021;27:1037–47. 10.1158/1078-0432.CCR-20-3449 [DOI] [PubMed] [Google Scholar]
- 67. Thomas AS, Ma W, Wang Y. Ustekinumab for refractory colitis associated with immune checkpoint inhibitors. N Engl J Med 2021;384:581–3. 10.1056/NEJMc2031717 [DOI] [PubMed] [Google Scholar]
- 68. Phillips GS, Wu J, Hellmann MD, et al. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol 2019;37:2746–58. 10.1200/JCO.18.02141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fernández-Gordón Sánchez FM, Gómez-Domínguez E, Paredes Ruiz D, et al. Ustekinumab for corticodependent immune-mediated colitis by pembrolizumab, an alternative for patients with concomitant liver injury. Rev Esp Enferm Dig 2022. 10.17235/reed.2022.8618/2022 [DOI] [PubMed] [Google Scholar]
- 70. Glinos GD, Fisher WS, Morr CS, et al. Nivolumab-induced psoriasis successfully treated with risankizumab-rzaa in a patient with stage III melanoma. JAAD Case Reports 2021;11:74–7. 10.1016/j.jdcr.2021.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vinaixa Aranzazu A, Morillas-Lahuerta V, Blanco De Tord M, et al. Ixekizumab for the treatment of erythrodermic psoriasis triggered by durvalumab-tremelimumab in a cancer patient. Eur J Dermatol 2021;31:564–5. 10.1684/ejd.2021.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Monsour EP, Pothen J, Balaraman R. A novel approach to the treatment of Pembrolizumab-induced psoriasis exacerbation: A case report. Cureus 2019;11:e5824. 10.7759/cureus.5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ma VT, Lao CD, Fecher LA, et al. Successful use of secukinumab in two melanoma patients with immune checkpoint inhibitor-induced inflammatory arthropathy. Immunotherapy 2022;14:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Johnson D, Patel AB, Uemura MI, et al. Il17A blockade successfully treated Psoriasiform Dermatologic toxicity from Immunotherapy. Cancer Immunol Res 2019;7:860–5. 10.1158/2326-6066.CIR-18-0682 [DOI] [PubMed] [Google Scholar]
- 75. Esfahani K, Miller WH. Reversal of autoimmune toxicity and loss of tumor response by interleukin-17 blockade. N Engl J Med 2017;376:1989–91. 10.1056/NEJMc1703047 [DOI] [PubMed] [Google Scholar]
- 76. Sumi T, Nagahisa Y, Matsuura K, et al. Successful management of severe bronchial asthma exacerbated by Anti‐PD‐L1 treatment: A report of two cases. Respirol Case Rep 2021;9:e0868. 10.1002/rcr2.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harada M, Naoi H, Yasuda K, et al. Programmed cell Death-1 blockade in kidney carcinoma may induce eosinophilic granulomatosis with polyangiitis: a case report. BMC Pulm Med 2021;21:6. 10.1186/s12890-020-01375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Neagu N, Dianzani C, Avallone G, et al. Dupilumab ocular side effects in patients with Atopic dermatitis: a systematic review. J Eur Acad Dermatol Venereol 2022;36:820–35. 10.1111/jdv.17981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-006814supp001.pdf (607.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.