Highlights
-
•
Low-dose radiotherapy mediates immune responses, instigating tumour recession at both primary and metastatic sites, thereby exhibiting systemic, long-term anti-carcinogenic efficacy.
-
•
Low-dose radiotherapy sensitizes "cold" tumours to immune checkpoint blockade (ICB), effectively mobilizing both innate and adaptive immune responses.
-
•
Low-dose radiotherapy promotes the infiltration of immune cells without instigating toxicity, making it compatible with additional anti-carcinogenic treatments.
-
•
The implementation of radiotherapy plans with various fractionation doses may potentially contribute more significantly to the anti-carcinogenic effects.
Keywords: Low-dose radiotherapy, Biological effect, Microenvironment, Immune system, Tumor
Abstract
The history of low-dose radiotherapy (LDRT or LDR) as a treatment modality for malignant tumors dates back to the 1920s. Even with the minimal total dose administered during treatment, LDRT can result in long-lasting remission. Autocrine and paracrine signaling are widely recognized for fostering the growth and development of tumor cells. LDRT exerts systemic anti-tumor effects through various mechanisms, such as enhancing the activity of immune cells and cytokines, shifting the immune response towards an anti-tumor phenotype, influencing gene expression, and blocking crucial immunosuppressive pathways. Additionally, LDRT has been demonstrated to enhance the infiltration of activated T cells and initiate a series of inflammatory processes while modulating the tumor microenvironment. In this context, the objective of receiving radiation is not to directly kill tumor cells but to reprogram the immune system. Enhancing anti-tumor immunity may be a critical mechanism by which LDRT plays a role in cancer suppression. Therefore, this review primarily focuses on the clinical and preclinical efficacy of LDRT in combination with other anti-cancer strategies, such as the interaction between LDRT and the tumor microenvironment, and the remodeling of the immune system.
Graphical abstract
Introduction
Tumors engage with the immune system throughout their initiation and progression. The human immune system not only defends against microbial invasions but also possesses anti-tumor immune mechanisms [1]. The body can eliminate tumor cells or inhibit their proliferation through various means, such as immune surveillance. However, due to immune escape mechanisms, it remains challenging to prevent tumor initiation and progression [2]. When immune surveillance function is weakened due to external factors, tumors have more favorable conditions to develop and progress [3,4]. Low immune function is common in cancer patients, hindering the elimination of tumor cells, reducing the effectiveness of anti-tumor treatments, and weakening the ability to kill tumor cells, significantly increasing the likelihood of tumor development and metastasis [5,6]. Consequently, interventions to improve the immune function of patients diagnosed with cancer have a profound impact on patient prognosis.
The clinical application of 2.0 Gy per dose radiotherapy can enhance the body's anti-tumor immune response by promoting macrophage polarization, activating reactive oxygen species, inducing direct DNA damage, and other mechanisms, thereby surpassing the initial immunosuppressive effects [7,8]. In recent years, there has been a growing number of clinical case studies and basic experiments focused on the combination of radiotherapy and immunotherapy [9,10]. Although radiotherapy is a local treatment, it needs to be combined with systemic therapies, such as chemotherapy, targeted therapy, and immunotherapy, for patients with extensive lesions or distant metastases [11,12]. However, even moderate radiotherapy doses might be detrimental to the surrounding healthy tissues, potentially leading to immunosuppression, tumor recurrence, induction of secondary tumors, or adverse reactions that impact the treatment process [13,14]. The "linear no-threshold" theory posits that the risk of cancer linearly increases with ionizing radiation dose, suggesting that any dose of radiation can cause harm to the human body [15]. As a result, researchers have shown increased interest in low-dose radiotherapy (LDRT).Furthermore, numerous studies have demonstrated that LDRT can produce biological effects distinct from those of high and medium doses of radiation. In-depth investigations of enhancing immune function and inhibiting tumor initiation and progression have confirmed that LDRT has positive effects on various tumors and promotes the body's anti-tumor immune function [16,17]. The cellular basis for low-dose radiation-enhanced immune function primarily involves the activation of intercellular reactions in immune organs, and its molecular mechanism mainly includes the activation of multiple T cell signaling pathways [18,19]. Epidemiological and animal studies have also discovered that low-dose radiotherapy can inhibit or delay the development or metastasis of primary tumors, and the anti-tumor effect of low-dose radiotherapy has been observed in clinical practice as well [20]. Early clinical data suggest that low-dose radiation (i.e., 0.5 - 2 Gy per dose) can reprogram the tumor microenvironment (TME) to recruit large numbers of effector T cells, thereby inducing tumor vascular normalization, inflammatory microenvironment, increased T cell infiltration, and enhanced anti-tumor effects. In a study involving 35 patients with relapsed and chemotherapy-resistant non-Hodgkin lymphoma (NHL), low-dose total body irradiation (0.10–0.25 Gy) was applied. The complete response rate, 2-year progression-free survival rate, and 2-year survival rate were 29%, 32%, and 42%, respectively, with improvements in median progression-free survival time and median overall survival time [21]. Some studies have compared low-dose radiotherapy combined with immunotherapy, low-dose radiotherapy alone, and immunotherapy alone, and found that the comprehensive treatment of LDRT combined with immunotherapy plays a role in improving tumor control rates. Therefore, this paper focuses on reviewing the interaction of low-dose radiotherapy with the immune system and tumor microenvironment, as well as the effects of combined low-dose radiotherapy on the comprehensive therapeutic efficacy of malignant tumors.
Low dose radiation to reprogram the tumor microenvironment
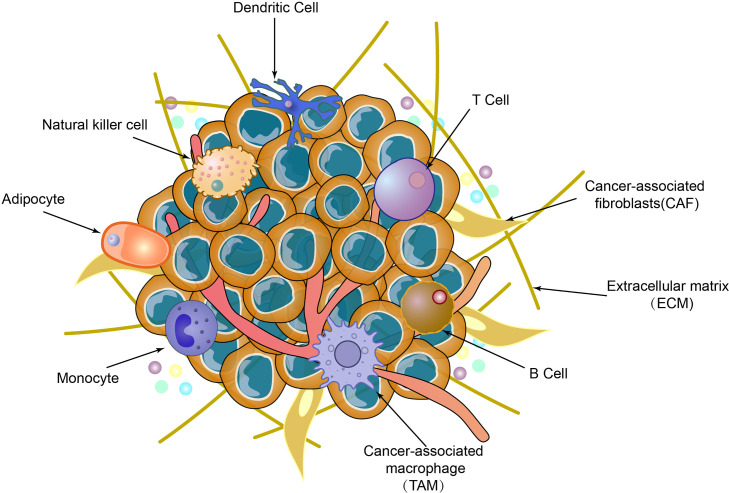
The immune system is subdivided into innate immunity (also referred to as non-specific immunity) and adaptive immunity (also known as specific immunity). Adaptive immunity is further divided into humoral immunity and cellular immunity (Fig. 1) [22]. The tumor microenvironment (TME) encompasses the cellular milieu in which tumors or tumor stem cells exist, including surrounding immune cells, blood vessels, extracellular matrix (ECM), fibroblasts, lymphocytes, bone marrow-derived inflammatory cells, signaling molecules, and numerous soluble mediators. Both innate immune cells (macrophages, mast cells, neutrophils, dendritic cells, bone marrow-derived suppressor cells, natural killer cells) and adaptive immune cells (T and B lymphocytes) are present in the TME, interacting with tumor cells through direct contact or via chemokine and cytokine signaling ([23,24]). As tumor cells engage with neighboring cells through the circulatory and lymphatic systems, tumor development is intimately associated with the TME (Fig. 2) ([25,26]). The subsequent behavior of these cells determines tumor progression and influences treatment outcomes [27]. Low-dose radiation (LDR) elicits anti-tumor immune mechanisms, including the elimination of reactive chemical intermediates, stimulation of DNA damage repair, reduction of inflammation, initiation of selective apoptosis or senescence of abnormal cells, and modulation of anti-tumor innate and adaptive immune systems [28], [29], [30].
Fig. 1.
Immune system composition.
Fig. 2.
Composition of tumor microenvironment.
The immune system primarily comprises two components: the innate and adaptive immune systems. Upon encountering antigens, the innate immune system exhibits rapid, non-specific recognition, rapidly forming the body's first line of defense. By releasing cytokines and chemokines, the innate immune system swiftly performs antigen phagocytosis and initiates adaptive immunity.The adaptive immune system primarily comprises T lymphocytes (including CD4+ helper and CD8+ cytotoxic cells) and B lymphocytes. T cells are chiefly responsible for cell-mediated immune responses, while B cells mainly facilitate antibody-mediated immune responses, establishing a secondary line of defense for the body.
When tumor cells invade the body, tumor antigens rapidly activate human immune cells, including T cells, B cells, NK cells, macrophages, and dendritic cells. Immune cells recognize and attack tumor cells through various mechanisms, enabling the tumor immune microenvironment to maintain immune equilibrium. During growth, tumor cells can evade the immune system's detection, leading to immune escape.
The first line of defense of human immunity system——innate immunity system
First, low-dose radiotherapy (LDRT) exerts its effects in the human body by promoting the proliferation of natural killer (NK) cells, macrophages, and dendritic cells (DCs), thereby facilitating intercellular reactions within immune synapses and stimulating the expression of various surface molecules and cytokines to regulate secretion.This mechanism ultimately results in the amplification of immune responses. NK cells, as innate immune effectors, act as the body's initial defense against infections and cancer. NK cells can eliminate infected or transformed cells through the secretion of pro-inflammatory cytokines and cytotoxic activities. Given that NK cells represent an early response to exogenous stress, they may exhibit functional changes following LDRT [31].
Animal experiments have demonstrated that the effects on the tumor microenvironment involve NK lymphocytes, and NK cell-rich spleen cells collected from animals exhibit enhanced anti-tumor cytotoxic function and significantly reduced induced metastasis in the lungs after low-dose ionizing radiation exposure [32]. However, in a follow-up experiment conducted by Sonn et al., no significant difference in cell viability or functional changes was observed when purified NK cells were treated with 0.2 Gy LDRT in vitro. Notably, cytotoxicity significantly increased when NK cells were stimulated with a moderate dose of IL-2 prior to irradiation. In conclusion, LDRT can cooperate with IL-2, a cytokine previously exposed to NK cells, to enhance NK cell toxicity and thus exert its effects. It is hypothesized that a synergistic effect may exist between LDRT and cytokines [33]. Yang et al. also found that LDRT could significantly enhance the expansion and cytotoxicity of NK cells in experiments focused on the activation and expansion of NK cells in adoptive cellular immunotherapy. In vitro, LDRT may directly activate NK cells and stimulate activation and amplification through the P38-MAPK pathway at doses of 75–150 mGy, leading to a significant increase in the amplification and secretion of effector proteins, such as IFN-γ, IFN-c, and TNF-α. The P38-MAPK pathway has been reported to be involved in regulating the cytotoxic function of NK cells [34,35]. Two primary molecular mechanisms underlie NK cell-mediated cytotoxicity: one based on perforin and the other on Fas. This study demonstrated that the expression of perforin and Fas ligand increased in NK cells following LDRT stimulation. In the 75 mGy group, the cytotoxic activity was higher 24 h after LDRT, suggesting that 75 mGy is the optimal LDRT dose for enhancing NK cell cytotoxic activity. Under LDRT influence, this pathway may be involved in enhancing NK cell cytotoxic activity [36]. Collectively, these fundamental studies indicate that LDRT can activate and promote the proliferation and cytotoxicity of NK cells within the immune system. Further research is needed to elucidate the primary molecular mechanisms, signaling pathways, and optimal radiation doses of NK cells in the tumor microenvironment improved by LDRT.
Macrophages are immune cells primarily responsible for antigen processing and presentation to T and B cells. B cell proliferation and differentiation into mature plasma cells that secrete specific antibodies are stimulated by them. Concurrently, macrophages encourage T lymphocyte proliferation and differentiation into helper/inducer T cells (Th) and cytotoxic T cells (CTL or Tc) [37]. Macrophages can be activated through two pathways: M1 and M2. M1 macrophages mainly activate T helper type 1 (Th1) cells to augment immune responses, while M2 macrophages, also known as tumor-associated macrophages (TAMs) [38], foster tumor cell growth, angiogenesis, invasion, and metastasis, primarily mediated by the anti-inflammatory response of helper T cell type 2 (Th2) [39]. M1 and M2 macrophages exhibit high plasticity, allowing them to transform in response to changes in the tumor microenvironment or therapeutic intervention [39]. TAMs, abundant in the tumor microenvironment, are crucial inflammatory cells that regulate tumor tissue immune function and drive tumor progression, metastasis, and drug resistance [40].
In an experiment by Klug et al., LDRT induced endothelial cell activation, promoted Th1 chemokine expression, and simultaneously inhibited angiogenesis and immunosuppression. This was accompanied by tumor growth factor production and the programming of iNOS (+) M1 macrophage differentiation via iNOS. These effects reprogrammed macrophage iNOS activity, leading to subsequent vascular normalization, further activation, T cell recruitment, and tumor rejection. This resulted in CTL recruitment and killing of solid tumors (e.g., melanoma), ultimately achieving tumor eradication. The critical role of iNOS+ macrophages in facilitating macrophage and T cell transfer under LDRT conditions, contributing to vascular normalization, T cell infiltration, and tumor eradication, was further elucidated [41].
Similarly, Nadella et al. demonstrated in a pancreatic cancer mouse model that LDRT downregulated HIF-1 in irradiated tumors and induced M2 phenotype transformation to the M1 phenotype. The M1 phenotype may alter the angiogenic signaling induced by sphingosine 1-phosphate/VEGF in tumorigenic tumor-derived endothelial cells, further highlighting the importance of iNOS+ macrophages in eNOS+ tumor endothelial cell signal transduction. Moreover, M1 macrophages can directly impact angiogenic responses in eNOS+ endothelial cells, which produce nitric oxide with vascular and physiological effects [42]. These experimental results strongly suggest that tumor endothelium-activated macrophages may enhance targeted cancer immunotherapy.
In prior experiments, it has been established that low-dose radiotherapy (LDRT) can enhance the cytotoxicity of macrophages in innate immune responses, activate the P38-MAPK pathway to improve natural killer (NK) cell activity, promote cytokine secretion by dendritic cells (DCs), augment their antigen presentation capacity, and bolster immune surveillance and anti-tumor effects, ultimately inhibiting tumor development and metastasis [36]. Subsequent research investigated the role of DCs, revealing that low-dose radiation can further induce DC activation and maturation by disrupting DNA double strands and stimulating the cGAS-STING pathway [[43], [44]]. As demonstrated by Shigematsu et al., LDRT can increase the production of IL-2, IL-12, and IFN-γ, indirectly enhancing T cell activation, promoting proliferation, inducing naïve helper T cell differentiation into Th1 cells, and amplifying immune responses [45]. Wang et al. showed that the anti-tumor effects of DC vaccines in mouse bone marrow were significantly strengthened after exposure to 0.2 Gy LDRT, improving DC-induced T cell proliferation and enhancing the cytotoxic effects of CTLs induced by LDRT-exposed DCs. LDRT-irradiated DC vaccines can increase mouse survival rates, promote CTL infiltration into tumor tissue, induce tumor cell apoptosis, and upregulate serum interferon-γ and interleukin-12 levels, providing new strategies and insights for anti-tumor therapies (Fig. 3) [46].
Fig. 3.
Alterations in NK cells, macrophages, and dendritic cells within the innate immune system when LDR targets tumor cells.
Upon initiation of low-dose radiotherapy, NK cells, DCs, and macrophages within the innate immune system begin to exert their effects.NK cells enhance non-specific anti-tumor activity in the body by producing IFN-γ, TNF-α, perforin, and granzyme. Macrophages stimulate T cell proliferation and differentiation through cytokine release (e.g., IL-12, IL-1β, and TNF-α), and M2 macrophages are driven to differentiate into M1 macrophages, which, in conjunction with other macrophages, release cytokines and enhance NK cell activity via the NF-kβ pathway to cooperatively eliminate tumor cells. DCs stimulate T cell proliferation by releasing cytokines (e.g., IL-12, IL-2, and IFN-γ) and signaling targets, thereby exerting anti-tumor effects.
The alliance of the first line of defense——adaptive immunity
Adaptive immune responses primarily encompass cellular and humoral immunity, referring to the complete process of antigen-specific T and B lymphocytes self-activating, proliferating, and differentiating into effector cells upon antigen stimulation, subsequently generating a series of biological effects [47].
T cell subsets represent the primary mode of immune response in cell-mediated immunity They can be divided into two subgroups based on cell surface differentiation antigen (CD) differences: CD4+ and CD8+ [48]. LDRT can directly enhance adaptive immune responses by amplifying T cell stimulation for antigen-driven hyperplasia and mitosis while increasing tumor cell cytotoxicity [49]. Patel et al. demonstrated that LDRT promotes the polarization of tumor M2 macrophages into anti-tumor M1 phenotypes and enhances CD4+T cell and NK cell infiltration [50]. Additionally, LDRT downregulated TGF-β inhibitory cytokines and upregulated Granzyme B, MIP1a, and CD137 in tumor-infiltrating CD4+T cells within the tumor microenvironment (TME), indicating activation and effector function, and improving the efficacy of immune checkpoint inhibitors (anti-CTLA-4/anti-PD-1) in mouse lung adenocarcinoma anti-tumor processes.
Anti-tumor therapy depends on innate immunity and adaptive activation, and its effectiveness relies on cytotoxic CD4 and CD8 T cells. LDRT has previously been shown to sufficiently increase T cells within the TME for neoadjuvant pancreatic cancer patients [41]. LDRT (0.2 Gy) systemic irradiation effectively inhibited distant metastasis of lung cancer in rats with locally implanted hepatocellular carcinoma lines, although the same dose of local irradiation did not control distant metastasis. In this study, LDRT increased IFN-γ and TNF-α gene expression and CD8+ cells in the tumor microenvironment [51]. Spary et al. demonstrated that 0.6–2.4 Gy LDRT enhanced T cell function by increasing T cell proliferation, cytokine production (IFN-γ and TNF-α), T cell receptor signaling (Akt, ERK1/2), and CD8+ cell versatility. Furthermore, the final data in this experiment suggest that greater benefits may be achieved by combining radiotherapy with T cell stimulation [52]. Similarly, Herrera et al. used low-dose radiation in a syngeneic ID8 ovarian cancer mouse model to simulate the series of changes in low T-cell infiltration observed in many human epithelial ovarian cancers. Total abdominal irradiation of 0.5–2 Gy induced proliferation of lymphocytes, monocytes, dendritic cells, and NK cells (NKG20) in the TME, while administering 1 Gy per week repeatedly resulted in continuous immune cell recruitment into ovarian tumors. The highest immune cell infiltration and the maximum CD8+ T:Treg cell ratio were induced by 1 Gy irradiation. This treatment regimen inflamed cold tumors and reprogrammed the tumor microenvironment to enhance tumor control. More importantly, T cells infiltrating immune "cold" tumors initiated IFN-I responses, immune cell activation, antigen presentation, and further T cell receptor activation [53].
B cells primarily participate in the body's humoral immune response, producing antibodies within plasma and lymphatic circulation [54]. Research on the effects of low-dose radiation on humoral immunity is limited; however, it is hypothesized that low-dose radiation may act as a unique weak antigen to stimulate the repeated polyclonal activation of B cells, thereby inducing immunoglobulin formation and enhancing humoral immune function [55]. Studies have indicated that LDRT might increase Ikaros gene protein phosphorylation by activating casein kinase 2 and Akt, consequently promoting B lymphocyte proliferation, stimulating specific cytokine secretion, and activating related cellular pathways to eliminate tumor cells [56,57]. EOM et al. investigated the potential for radiation-specific signaling of various effects induced by low-dose ionizing radiation (LDIR) in human B-lymphocyte IM-9 cells. The results suggested that protein phosphorylation patterns induced by LDRT might also contribute to diverse cell survival responses or cell maintenance functions [58]. Consequently, LDRT can trigger immune cell clustering similar to tumors, ultimately resulting in tumor cell death. Low-dose radiation may also reverse the immunosuppressive functions of various immune cell types, such as Tregs [59], immunosuppressive tumor-associated macrophages (TAMs) [42], and tolerant dendritic cells (DCs) [60], promoting T cell infiltration into tumors by fostering tumor microenvironment reprogramming, thereby providing a suitable treatment platform for immunotherapy (Fig. 4).
Fig. 4.
Changes in T and B cells within the adaptive immune system upon low-dose radiation (LDR) targeting tumor cells.
When the body receives low-dose radiation over a period, the adaptive immune system's T and B cells come into play. LDR enhances signaling target Akt and casein kinase, promoting B lymphocyte proliferation. T cells undergo rapid proliferation and differentiation into CD4+ and CD8+ T cells. LDRT stimulates T cell proliferation and differentiation through NF-κB, P38-MAPK, and JNK pathways to produce TNF-α, IL-4, and IL-2. In conjunction with IL-12, IL-10, and IFN-γ produced by helper T cell 1 (Th1), these cytokines bolster the immune system and exert anti-tumor effects.
Foundational experiments and clinical trials of low-dose radiotherapy
Low-dose radiotherapy elicits anti-tumor immune responses by processes such as the elimination of reactive chemical intermediates, stimulation of DNA damage repair, reduction of inflammation, and the selective induction of apoptosis or senescence in aberrant cells. Presently, employing low-dose radiotherapy as an immunoadjuvant to stimulate anti-tumor immune responses may offer novel hope in clinical practice. Preclinical evidence of low-dose radiotherapy combined with other anti-cancer therapies has invigorated clinical efforts, yielding promising outcomes. An increasing number of studies have discovered that low-dose radiotherapy can inhibit tumor initiation, progression, and metastasis; enhance the efficacy of chemoradiotherapy; and increase the cure rates for cancer.
Systemic low-dose radiotherapy
At present, both systemic and localized low-dose radiotherapy constitute methods of radiation treatment in cancer therapy. Low-dose whole-body irradiation (LDWBI) is a relatively novel and unconventional form of radiation treatment, involving the administration of low doses of ionizing radiation to the entire body or specific target regions, rather than the high doses employed in conventional radiotherapy. The primary objective of LDWBI is to induce anti-tumor immune responses, provoke remission in various cancers, without triggering the adverse side effects of conventional chemotherapy and radiotherapy, reduce inflammation, and stimulate the body's reparative mechanisms. LDWBI has been investigated for the treatment of various ailments, including autoimmune diseases, inflammatory disorders, and certain types of cancer. While some studies have yielded hopeful results, further research is needed to ascertain the safety, efficacy, and optimal dosing regimens of LDWBI in diverse clinical settings. A summary of current research on systemic low-dose radiotherapy is presented in Table 1.
Table 1.
Experimental study on low dose whole-body irradiation.
| Preclinical/Clinical | Cancer | treatment mode | Radiation dose Rate(mGy/h) |
Radiation dose (Gy) |
Immune cell & Functions | Refs. |
|---|---|---|---|---|---|---|
| Preclinical | Melanoma with lung metastasis | LDWBI and IL-2 | 0.05 Gy/min | 0.75 Gy/f | 1. LDWBI combined with IL-2 treatment resulted in a significant increase in the number of natural killer (NK) cells and macrophages infiltrating the metastatic site. 2. CD122+ expression and the number of NK cells and T lymphocytes in peripheral blood and spleen were significantly increased. |
[61] |
| Preclinical | Melanoma(B16F1) | LDWBI and IL-2 | 0.05 Gy/min | 0.075 Gy/f 0.75 Gy/f |
The combination of high doses of IL-2 and LDWBI (either dose) resulted in a significant reduction in tumor load and an increase in tumor invasion of natural killer cells. | [62] |
| Preclinical |
1.Melanoma(B16) 2.Lewis lung carcinoma (LLC) |
Conventional local radiotherapy + LDWBI + gene therapy by intratumor injection of a recombinant plasmid Egr-mIL-18-B7.1 (E18B) |
/ | 0.075Gy | 1.The mean survival rate was improved, the mean tumor weight was reduced, lung metastasis was reduced, the growth of capillaries in the tumor was inhibited, and the radiation dose was reduced by 2/3. 2. Natural killer (NK) and cytotoxic T lymphocyte (CTL) activities were enhanced, and interferon-γ (IFN-γ) secretion was increased. |
[63] |
| Preclinical | Lewis lung carcinoma (LLC) | Conventional local radiotherapy (2 Gy x 6)+ LDWBI+ gene therapy(intratumor injection ofpEgr-IL-18-B7) |
12.5 mGy/min | 0.075Gy | 1. Compared with radiotherapy alone, triple therapy achieved the most significant improvements in cancer control, with a 60.4% increase in mean survival time, a 70.8% reduction in mean tumor weight, a 66.9% reduction in lung metastasis, and a 64.8% reduction in intratumor angiogenesis. 2.Triple therapy stimulated NK (natural killer) and CTL (cytotoxic T lymphocyte) activity, IFN (interferon)-gamma and TNF (tumor necrosis factor)-alpha secretion, PKC (protein kinase C)-theta activation and LAMP (lysosomal associated membrane protein)−1 expression. |
[64] |
| Preclinical | Sarcoma | LDWBI alone | 12.5 mGy/min | 0.075Gy | 1. LDWBI group tumor growth slowed down and average tumor weight was significantly reduced. That means that LDWBI can significantly improve the anti-tumor ability, erythrocyte immune function and oxygen carrying capacity. 2.The expression of EPO in LDWBI group decreased with the extension of irradiation time, and the expression of VEGFR also decreased, which was the lowest at 24 h. That means that LDWBI can reduce the expression of hypoxia factors EPO and VEGFR within a certain period of time, thereby improving the hypoxia status of tumor and enhancing the radiosensitivity of tumor itself. |
[65] |
| Clinical | Prostate cancer | 1. 1st patient: LDWBI with X-rays at 0.15 Gy once a week for 30 weeks. 2. 2nd patient: LDWBI with X-rays at 0.15 Gy 3 times a week for 10 weeks + “radon sheet” placed under the bed for 6 h/night for 10 months. |
20 to 50 mGy/min | 0.15 Gy/ 0.1 Gy | 1.1st patient: reduction of PSA level from >5 to 0.085 by the 6th treatment. 2.2nd patient: reduction of PSA level from 4.8 to 0.008 with apparent disappearance of bone metastases. |
[66] |
| Preclinical | 1.Mammary carcinoma 2. Colon carcinoma |
LDWBI + H-RT (8 Gy x 3) | 24 cGy/min | 0.1Gy | 1. LDWBI (0.1 Gy) combined with H-RT (8 Gy × 3) had an inhibitory effect on primary tumor, and could effectively inhibit secondary tumor. 2. LDWBI can reduce DNA damage caused by H-RT. 3. In the H-RT+LDWBI group, the percentage of CD 86+ cells was the highest. The number of CD 3+ and CD 86+ positive cells increased significantly in secondary tumor tissues. 4. There was a significant increase in invasive CD 8 + T cells and a significant increase in IFN-γ levels in secondary tumors in the H-RT+LDWBI group. Induces a tumor-specific T cell response, which, when strong enough, leads to complete remission of distal tumors. 5. H-RT+LDWBI reverses the immunosuppressive tumor microenvironment (TME) in distant tumors by reducing immunosuppressive G-MDSC and M2 macrophages, and increases the percentage of anti-tumor eosinophilic population, inducing a systemic immune response. |
[18] |
| Preclinical | Mammary cancer | low dose tritium exposure (βInternal irradiation) |
/ | 0.01 Gy/ 0.1 Gy | 1. Mice irradiated by 0.01 Gy directly stimulated NK cells through the P38-MAPK pathway and up-regulated the expression level of NKG2D on NK cells. At the same time, the number of NK cells was increased by stimulating cytokines producing IFN-γ, suggesting that even a low dose of 10mGy could induce immune regulation by increasing the proportion of NK cells 2. The proportion of TCRb+CD8+T cells in spleen tissue of 100mGy irradiated mice increased obviously. 3. LDWBI can significantly affect immune cell functional plasticity by inducing cytokine production in NK cells and cytotoxicity in NK and T cells. |
[67] |
| Preclinical | liver cancer | LDWBI | / | 0.025 Gy/f, 0.05 Gy/f, 0.075 Gy/f, 0.1 Gy/f |
1. LDWBI has an immunostimulating effect, which can promote the immune function in the body to achieve its tumor inhibitory effect. 2. LDWBI can directly increase the expression of IFN-c and IFN-a mRNA, induce TH type 1 cytokine activation and enhance IL-12 gene transcription and protein expression, and IL-12 induces TH 1 cells to produce IFN-γ. 3. LDWBI can regulate humoral and cellular immunity in response to tumors by upregating secretion levels of IL-4 and IFN-c, thus achieving antitumor effects. The mechanism needs to be further explored. 4. As a special weak antigen, LDWBI can stimulate the repeated polyclonal activation of B cells, induce the formation of immunoglobulin, and enhance humoral immunity. 5. A large number of IgG and IgM antibodies with high affinity can be produced after LDWBI stimulation, suggesting that low ionizing radiation can improve the level of immunoglobulin in tumor patients. The results suggest that 75 mGy may be the best dose to stimulate the immune system. The mechanism may be that a dose of 75mGy of low ionizing radiation maximizes the number of immunoactive cells (e.g. T lymphocytes, B lymphocytes, NK cells), and cytokine and antibody formation can be regulated by LDWBI. |
|
| Preclinical | Breast cancer | LDWBI + H-RT(8 Gy x 3) + aPD-1 | / | 0.1 Gy/f | 1. Compared with H-RT and aPD-1 combined therapy, triple therapy with LDWBI delayed primary and secondary tumor growth, improved survival, and reduced the number of lung metastases. 2. Activated dendritic cells and CD 8 + T cell populations were increased, and infiltration of medullary suppressor cells in the secondary tumor microenvironment was reduced. |
[68] |
Localized low-dose radiotherapy
Localized low-dose radiotherapy (LLDR) is a targeted form of radiation treatment involving the administration of low doses of ionizing radiation to specific regions of the body, as opposed to the whole-body approach employed in systemic low-dose radiotherapy. The primary objective of localized low-dose radiotherapy is to provide therapeutic effects within the targeted area while minimizing exposure to surrounding healthy tissues. Low doses utilized in localized low-dose radiotherapy are believed to elicit hormetic responses, including the stimulation of beneficial effects, such as cellular repair and immune system activation, under low-dose radiation. In contrast, high doses of radiation lead to deleterious consequences, including DNA damage, cell death, and increased cancer risk. Localized low-dose radiotherapy has been employed in the treatment of various ailments, encompassing benign and malignant tumors, functioning through the modulation of immune responses, reduction of inflammation, and promotion of cellular repair processes within affected areas. A summary of current research on localized low-dose radiotherapy is presented in Table 2.
Table 2.
Experimental study on Local low dose irradiation.
| Preclinical/Clinical | Cancer | Irradiated site | Radiation dose Rate(mGy/min) |
Radiation dose (Gy) | Immune cell & Functions | Refs. |
|---|---|---|---|---|---|---|
| Preclinical | Insulinoma | pancreatic regions | / | LLDR (alone) 0.5 Gy/f |
1. LLDR increased the number of T cells in the tumor area, and the number of CD4+ FoxP3+ T cells was the highest. 2. CD8+T cells in the irradiated area after LLDR normalized tumor blood vessels and increased the expression of iNOS+. |
[41] |
| Preclinical | Lung adenocarcinoma | Left leg | / | LLDR (alone) 1 Gy/f |
1. LDWBI given alone can effectively prolong survival by controlling tumor growth. 2.LDWBI boosts antitumor immunity through T cell activation, NK cell infiltration, M1 macrophage polarization, and reduction of TGF-β cytokine. 3.The combination of LDR with anti-PD 1 and anti-CTLA-4 immunotherapeutic agents significantly enhances the antitumor efficacy of these systemic immune checkpoint inhibitors. 4.High-dose RT combined with LDWBI and immunotherapy can down-regulate TGF-β and enhance systemic antitumor response depending on CD4+ T cells and NK cells. |
[50] |
| Clinical | metastatic human papillomavirus-positive oropharyngeal squamous cell carcinoma (Abdominal metastasis) |
Whole abdomen | / | H-XRT (12.5 Gy /f) + LLDR (1.5 Gy /f) + anti-PD1 therapy (pembrolizumab) | After 6 months, the focal volume interval decreased and the affinity on PET almost completely subsided, indicating a robust response. | [50] |
| Clinical | metastatic melanoma(Splenic and pulmonary metastases) | Splenic | / | LLDR (1 Gy/f) + anti-PD1 therapy (pembrolizumab) | After 4 months, the spleen lesions were less dense and smaller in size, indicating partial remission and showing signs of response. | [50] |
| Clinical | 1.Non-Small Cell Lung Cancer 2.Melanoma 3.squamous cell carcinoma of the oropharynx |
Site of lesion | / | HD-RT (3–12.5 Gy/f) +LLDR ((0.5–2 Gy/f) | 1. HD-RT plus LLDR safely improves the lesion specific response of patients with immunoresistant solid tumors by promoting the infiltration of effector immune cells into the tumor microenvironment and enhancing the infiltration of T cells and NK cells. 2.Three of the four patients with oropharyngeal squamous cell carcinoma responded to LLDR therapy, suggesting that LD-RT may have a potential activating effect on cold tumors |
[69] |
| Clinical | metastatic vaginal melanoma |
Secondary sites (liver and right groin) |
/ | peri-urethral/vaginal tumor (HD-RT 6 Gy /f)+Liver(1 Gy/f) + Right groin (1 Gy/f)+ dual checkpoint inhibitors (ipilimumab and nivolumab) |
Triple therapy results in lasting complete remission of all lesions. | [70] |
| Preclinical | Ovarian cancer | Whole abdomen | / | LLDR (alone) 1 Gy/f |
1.Important transcriptional changes that induce inflammation, including IFNα and IFNγ responses, complement activation, IL6/JAK/STAT3 signaling, attracting T and natural killer (NK) cells, as well as cross-presenting DCs. 2. the highest infiltration of CD8+, CD4+, and CD11b+ cells and the highest CD8+: Foxp3+ cell ratio following 1 Gy. |
[53] |
| Clinical |
1.advanced meta- static prostate 2. ovarian cancer 3. gastrointestinal tract tumor |
Site of lesion | / | LLDR (alone) 0.5 Gy/f 1 Gy/f |
1. The tumor volume of three patients subjected to targeted irradiation has decreased by 37.5%, while the remaining four patients have experienced disease stability. 2.overall disease control rate is 87.5%, whereas one patient (12.5%) had confirmed disease progression. |
[53] |
The impact of radiotherapy fractionation
Radiotherapy stands as a cornerstone of cancer treatment, commonly employing a fractionated approach whereby the total required radiation dose is divided into smaller dosages or fractions ([71,72]). The most prevalent scheme is conventional fractionation, implementing minor dosages (1.8–2.0 Gy) five days a week over several weeks [73]. Hypo-fractionation and ultrafractionation represent modifications to this timetable. Hypo-fractionation utilizes larger doses (greater than 2 Gy) per fraction over a shorter duration, while ultra-fractionation employs smaller doses (less than 2 Gy) multiple times daily over an extended period. Both schemes aim to enhance therapeutic efficacy and/or mitigate side effects. While significant strides have been made in cancer treatment through hyper-fractionation and hypo-fractionation, challenges are not absent [74].
Hypo-fractionated radiotherapy
Despite the convenience provided by a shortened treatment duration, hypo-fractionation can also present challenges. Higher doses of radiation can directly annihilate cancer cells, possibly leading to an upsurge in the release of tumour antigens and pro-inflammatory cytokines, and potentially intensifying anti-tumour immune responses. However, these heightened doses can also inflict greater damage on surrounding healthy tissues and immune cells, potentially leading to an immunosuppressive milieu and an elevated risk of side effects, such as inflammation, infection, and tissue injury. This may result in lymphocytopenia, a condition characterized by reduced levels of lymphocytes in the blood, which can impede immune responses and escalate the risk of infection and disease progression. Furthermore, while radiation can stimulate the immune system by promoting the release of cancer antigens, excessive doses may paradoxically suppress immune responses.
During the rapid proliferation of tumour tissue, the newly formed tumour vessels become dysfunctional, unable to effectively deliver oxygen. This incites hypoxia within the tumour, triggering a reprogramming of the tumour cell's energy metabolism - a pivotal factor in the exacerbation of cancerous conditions. Ameliorating tumour hypoxia to enhance the tumoricidal effect is the main objective of researchers. Tong et al., irradiated subcutaneous xenograft models bearing H460 and HCC827 NSCLC cells, as well as dorsal skinfold chamber (DSWC) models. The planned doses were 0 Gy (sham surgery group), 22 Gy divided into 11 fractions (CRT group), or 12 Gy divided into one fraction (HFRT group). Following irradiation, the radiobiological effects were assessed by measuring the volume, hypoxic area, pericyte coverage, and microvascular density (MVD) of the subcutaneous xenograft model. The tumour vascular system was visualized within the DSMC model, and the expression of phosphorylated signal transducer and transcription activator (p-STAT3), hypoxia-inducible factor 1-alpha (HIF-1α), CXCL12, and VEGFA was detected. Compared to the conventional radiotherapy group, HFRT demonstrated more potent tumour growth inhibition, accompanied by a period of vascular normalization induced by HFRT, during which tumour hypoxia improved. Additionally, during this window, HFRT suppressed the signalling levels of the p-STAT3/HIF-1α pathway and the expression of downstream angiogenic factors (VEGFA and CXCL12). The residual vessels became less tortuous, and pericyte coverage of tumour vessels increased, thereby mitigating tumour hypoxia and enhancing the tumoricidal effect [75]. Nam et al. analysed 60 patients with locally recurrent nasopharyngeal carcinoma who received hypo-fractionated re-irradiation, with the goal of evaluating the treatment outcomes after moderately hypo-fractionated re-irradiation (re-RT) in patients with locally recurrent nasopharyngeal carcinoma (NPC). The results showed 2-year and 5-year overall survival rates, local failure-free survival rates, and ≥ grade 3 toxicity-free survival rates of 57.9% and 45.8%, 64.1% and 52.5%, and 54.8% and 44.9% respectively. In the multivariate analysis, poorer factors for overall survival (OS) were iT3–4 (p = 0.010) and age ≥ 53 at the time of re-irradiation (p = 0.003), poorer factors for local failure-free survival (LFFS) were rT3–4 (p = 0.022) and rN0–1 (p = 0.035), and for toxicity-free survival (TFS) they were iT3–4 (p = 0.020) and intensity-modulated radiotherapy/boost (p = 0.030). It is inferred that re-irradiation using modern radiotherapy techniques through a moderately hypo-fractionated scheme seems feasible in the treatment of patients with locally recurrent nasopharyngeal carcinoma [76]. Adlakha and colleagues administered two distinct hypo-fractionated radiotherapy regimens to 70 patients with locally advanced head and neck cancer (LAHNC), with each group of 35 individuals receiving palliative radiotherapy. Group A was treated with 30 Gy/10F over two weeks, while Group B received 20 Gy/5F within a week. This prospective investigation endeavoured to contrast two short-term hypo-fractionated radiotherapy protocols for LAHNC patients in a cancer center in the northwestern region of India. Following the conclusion of radiotherapy and subsequent follow-ups at 4–6 weeks, both cohorts displayed analogous outcomes in terms of symptom alleviation, objective response, and acute toxicity. Group B exhibited a higher incidence of grade III and above mucositis (P = 0.027). Median overall survival for Group A was recorded at 5.9 months (ranging 1–15 months) and for Group B, it was 6.1 months (ranging 1–18 months). Hypo-fractionated radiotherapy promises to effectively alleviate LAHNC symptoms and incrementally enhance median overall survival.
Hyper-fractionated radiotherapy
Hyper-fractionated radiotherapy represents a novel treatment modality involving the adjustment of the number of radiotherapy fractions and the size of fraction doses. Conventional fractionated radiotherapy generally delivers 2.0 Gy per fraction, but hyper-fractionated radiotherapy administers less than 2 Gy per fraction, typically around 1.1–1.2 Gy per fraction. Regular fractionated radiotherapy is performed once daily, whereas hyper-fractionated radiotherapy may be administered twice a day, such as once in the morning and once in the afternoon with a 12-hour interval. Under specific circumstances, such as with rapidly growing squamous cell carcinomas of the head and neck skin, conventional fractionated radiotherapy is unable to control the tumour. Employing hyper-fractionated radiotherapy, which enables two treatments per day, can effectively regulate the tumour. Hyper-fractionated radiotherapy is primarily used for certain special tumours with exceptionally rapid proliferation rates. Under normal circumstances, conventional fractionated treatment is adopted, and hyper-fractionated radiotherapy is only employed to improve patient cure rates when conventional fractionated radiotherapy is ineffective.
Wang and his team randomly distributed 98 patients into two distinct radiotherapy model groups, with 50 patients receiving conventional fractionation (CF) three-dimensional conformal radiotherapy with a total dosage of 60–68 Gy; 2 Gray per fraction; five fractions per week. A cohort of 48 patients underwent late-course accelerated hyper-fractionation (LCAF), initially with a CF treatment dosage of 40 Gy, followed by an LCAF dosage of 1.5 Gy/F; twice daily; 21–27 Gy; total dosage of 61–67 Gy. The study compared the efficacy and side effects of esophageal cancer treatments using CF and LCAF three-dimensional conformal radiotherapy. The results indicated a marginal superiority in 1, 2, 3-year local control and survival rates of esophageal cancer patients treated with LCAF as compared to CF radiotherapy. However, the LCAF group exhibited greater radiogenic side effects, specifically radiogenic esophagitis, than the CF group [77]. Subsequently, Long and his colleagues aimed to observe the efficacy and adverse reactions of alternating hyper-fractionated radiotherapy for large hepatocellular carcinoma (HCC), randomly dividing 72 large HCC cases into Group A and Group B. In Group A, liver lesions were divided into sublesions, treated with alternating hyper-fractionated radiotherapy. Radiation treatment of sublesions had a minimum interval of 6 h, with an average radiotherapy dosage of 2 Gy per fraction, once daily, five times weekly, total treatment dose of 40–50 Gy for gross tumours, and a total clinical target dose of 30–40 Gray. In contrast, lesions in Group B were not divided into multiple sublesions for IMRT treatment; rather, the same regimen was employed with 2 Gy per fraction, once daily, five times weekly, and equivalent total dosage. The research demonstrated that compared to conventional IMRT, alternating hyper-fractionated radiotherapy for large HCC can improve quality of life and prolong overall survival [78].
In recent years, as the positive effects of hyper-fractionated radiotherapy have gradually been established, research emphasis has incrementally shifted towards the effectiveness and safety post-hyper-fractionated radiotherapy. In their study, Takeda and others conducted a retrospective analysis of 26 patients who underwent re-irradiation using hyper-fractionated technology twice daily with 1.2 Gy per fraction. They investigated overall survival post-secondary radiotherapy and the incidence of late adverse reactions. The results suggested that higher-dose re-irradiation and concurrent chemotherapy significantly improved survival rates. High-dose hyper-fractionated re-irradiation combined with concurrent chemotherapy yielded favourable prognoses with low long-term severe adverse reaction rates [79]. High-grade gliomas (HGG) are fatal brain tumours, classified under malignant diseases of the central nervous system. HGG demonstrates low-dose hyper-radiosensitivity (HRS). Frosina and others utilised primary glioma-initiating cell (GIC)-β driven in situ animal models that accurately recapitulate the heterogeneity and growth patterns of patient tumours to investigate the therapeutic effect of low radiation dose on HGG. In cases with equivalent total dosages, compared to standard fractionation, beginning radiotherapy fractionation of ≤0.5 Gray twice weekly during the early stages of tumour progression [hyper-hyper-fractionation (hyper-hyper-FRT)] effectively improved radiotherapy efficacy and animal survival rates ([80,81]).
However, current research concerning the impact of fractionated radiotherapy on the immune system remains extremely limited and no definitive conclusions can yet be drawn. In actual clinical treatment, a comprehensive consideration of a patient's specific condition, the balance of efficacy and side effects, is required to devise the most optimal personalised radiotherapy plan.
Conclusion
In recent years, as global anti-tumor treatment technologies and medications have advanced, a single anti-tumor treatment is no longer sufficient to achieve desired efficacy [82], [83], [84]. LDRT, defined as doses lower than the threshold for directly killing cancer cells, can be applied broadly to irradiate all metastatic deposits and encourage immune cell infiltration without inducing toxicity. It offers the advantages of low treatment cost, minimal toxicity, good patient tolerance, and compatibility with surgical, radiotherapeutic, chemotherapeutic, and immunotherapeutic interventions.
Specifically, low-dose radiotherapy (LDRT) offers immense potential in stimulating the immune system, thereby increasing clinicians' confidence in incorporating this therapeutic approach into clinical practice. LDRT activates anti-tumor immune mechanisms, including the elimination of reactive chemical intermediates, stimulation of DNA damage repair, reduction of inflammation, initiation of selective apoptosis or senescence of abnormal cells, and regulation of anti-tumor innate and adaptive immune systems [83], [84], [85]. By modifying the tumor immune microenvironment and enhancing patients' sensitivity to immunotherapy, chemotherapy, and radiotherapy, LDRT can boost overall survival rates. As a result, LDRT may emerge as the primary choice for comprehensive anti-tumor adjuvant therapy in the future.
Recent studies demonstrate that LDRT can reprogram the tumor microenvironment (TME) [86], and sensitize cold tumors to immune checkpoint blockade (ICB), effectively mobilizing both innate and adaptive immunity [50,87,88]. LDRT regimens can promote T cell infiltration into immune "cold" tumors and trigger immune gene markers related to IFN-I response, immune cell activation, antigen presentation, T cell receptor activation, and effector-memory phenotype in responsive patients [88].
Indeed, the low-dose radiation-induced immune response generates a systemic, long-lasting anti-tumor effect and promotes tumor regression at both primary and metastatic sites. With ongoing advancements in the anti-tumor immune microenvironment within the cancer therapy field, LDRT offers more possibilities for comprehensive tumor treatment. In conclusion, we believe that the time is ripe to refocus and appropriately restore the application of low-dose radiotherapy in oncology.
CRediT authorship contribution statement
Lei Gao: Writing – original draft. Anqi Zhang: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Archilla-Ortega A., Domuro C., Martin-Liberal J., Muñoz P. Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity. J. Exp. Clin. Cancer Res. 2022;41(1):62. doi: 10.1186/s13046-022-02264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H., Li G., Xu P., Li Z. B cells and tumor immune escape. Zhong nan da xue xue bao Yi xue ban. J. Cent. South Univ. Med. Sci. 2022;47(3):358–363. doi: 10.11817/j.issn.1672-7347.2022.210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalaf K., Hana D., Chou J.T.T., Singh C., Mackiewicz A., Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.656364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z., Sun G., Sun G., Cheng Y., Wu L., Wang Q., et al. Various uses of Pd1/Pd-L1 inhibitor in oncology: opportunities and challenges. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.771335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locy H., Mey Sd, Mey Wd, Ridder M.D., Thielemans K., Maenhout S.K. Immunomodulation of the tumor microenvironment: turn foe into friend. Front. Immunol. 2018;9:2909. doi: 10.3389/fimmu.2018.02909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing R., Gao J., Cui Q., Wang Q. Strategies to improve the antitumor effect of immunotherapy for hepatocellular carcinoma. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.783236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaue D., McBride W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015;12(9):527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M., Yang M., Zhang J., Yin Y., Fan X., Zhang Y., et al. Immunogenic cell death induction by ionizing radiation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.705361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johdi N.A., Sukor N.F. Colorectal Cancer immunotherapy: options and strategies. Front. Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Chen R., Wa Y., Ding S., Yang Y., Liao J., et al. Tumor immune microenvironment and immunotherapy in brain metastasis from non-small cell lung cancer. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.829451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashrafizadeh M., Farhood B., Musa A.E., Taeb S., Rezaeyan A., Najafi M. Abscopal effect in radioimmunotherapy. Int. Immunopharmacol. 2020;85 doi: 10.1016/j.intimp.2020.106663. [DOI] [PubMed] [Google Scholar]
- 12.Mondini M., Levy A., Meziani L., Milliat F., Deutsch E. Radiotherapy-immunotherapy combinations - perspectives and challenges. Mol. Oncol. 2020;14(7):1529–1537. doi: 10.1002/1878-0261.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Yang M., Luo J., Zhou H. Radiotherapy targeting cancer stem cells "awakens" them to induce tumour relapse and metastasis in oral cancer. Int. J. Oral Sci. 2020;12(1):19. doi: 10.1038/s41368-020-00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng J., Shuryak I. Minimizing second cancer risk following radiotherapy: current perspectives. Cancer Manag. Res. 2015;7:1–11. doi: 10.2147/cmar.S47220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacks B., Meyerson G. Linear No-threshold (Lnt) Vs. hormesis: paradigms, assumptions, and mathematical conventions that bias the conclusions in favor of Lnt and against hormesis. Health Phys. 2019;116(6):807–816. doi: 10.1097/hp.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 16.Abbassi L.M., Arsène-Henry A., Amessis M., Kirova Y.M. Radiation dose to the low axilla in patients treated for early-stage breast cancer by locoregional intensity-modulated radiotherapy (Imrt) Cancer Radiother. 2022;26(3):445–449. doi: 10.1016/j.canrad.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Pei S., Chen K., Yang Y., Chen L., Zhu X. A Retrospective cohort study of low-dose intensity-modulated radiotherapy for unresectable liver metastases. J. Int. Med. Res. 2020;48(4) doi: 10.1177/0300060519892382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Zhou J., Wu M., Hu C., Yang J., Li D., et al. Low-dose total body irradiation can enhance systemic immune related response induced by hypo-fractionated radiation. Front. Immunol. 2019;10:317. doi: 10.3389/fimmu.2019.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rödel F., Frey B., Manda K., Hildebrandt G., Hehlgans S., Keilholz L., et al. Immunomodulatory properties and molecular effects in inflammatory diseases of low-dose X-irradiation. Front. Oncol. 2012;2:120. doi: 10.3389/fonc.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer-Valuck B.W., Gay H.A., Patel S., Baumann B.C., Michalski J.M. A brief review of low-dose rate (Ldr) and high-dose rate (Hdr) brachytherapy boost for high-risk prostate. Front. Oncol. 2019;9:1378. doi: 10.3389/fonc.2019.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safwat A., Bayoumy Y., El-Sharkawy N., Shaaban K., Mansour O., Kamel A. The potential palliative role and possible immune modulatory effects of low-dose total body irradiation in relapsed or chemo-resistant non-hodgkin's lymphoma. Radiother. Oncol. 2003;69(1):33–36. doi: 10.1016/s0167-8140(03)00247-0. [DOI] [PubMed] [Google Scholar]
- 22.Tomar N., De R.K. A brief outline of the immune system. Methods Mol. Biol. 2014;1184:3–12. doi: 10.1007/978-1-4939-1115-8_1. [DOI] [PubMed] [Google Scholar]
- 23.Xiao Y., Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021;221 doi: 10.1016/j.pharmthera.2020.107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Chen Z., Han J., Ma X., Zheng X., Chen J. Functional and therapeutic significance of tumor-associated macrophages in colorectal cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.781233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petitprez F., Meylan M., Reyniès Ad, Sautès-Fridman C., Fridman W.H. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front. Immunol. 2020;11:784. doi: 10.3389/fimmu.2020.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terrén I., Orrantia A., Vitallé J., Zenarruzabeitia O., Borrego F. Nk cell metabolism and tumor microenvironment. Front. Immunol. 2019;10:2278. doi: 10.3389/fimmu.2019.02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinshaw D.C., Shevde L.A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi: 10.1158/0008-5472.Can-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feinendegen L.E., Pollycove M., Neumann R.D. Low-dose cancer risk modeling must recognize up-regulation of protection. Dose Response. 2009;8(2):227–252. doi: 10.2203/dose-response.09-035.Feinendegen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott B.R. Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. J. Cell Commun. Signal. 2014;8(4):341–352. doi: 10.1007/s12079-014-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farooque A., Mathur R., Verma A., Kaul V., Bhatt A.N., Adhikari J.S., et al. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev. Anticancer Ther. 2011;11(5):791–802. doi: 10.1586/era.10.217. [DOI] [PubMed] [Google Scholar]
- 31.Cheda A., Wrembel-Wargocka J., Lisiak E., Nowosielska E.M., Marciniak M., Janiak M.K. Single Low doses of x rays inhibit the development of experimental tumor metastases and trigger the activities of nk cells in mice. Radiat. Res. 2004;161(3):335–340. doi: 10.1667/rr3123. [DOI] [PubMed] [Google Scholar]
- 32.Nowosielska E.M., Cheda A., Wrembel-Wargocka J., Janiak M.K. Effect of low doses of low-let radiation on the innate anti-tumor reactions in radioresistant and radiosensitive mice. Dose Response. 2012;10(4):500–515. doi: 10.2203/dose-response.12-018.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonn C.H., Choi J.R., Kim T.J., Yu Y.B., Kim K., Shin S.C., et al. Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by Il-2. J. Radiat. Res. 2012;53(6):823–829. doi: 10.1093/jrr/rrs037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuadrado A., Nebreda A.R. Mechanisms and functions of P38 mapk signalling. Biochem. J. 2010;429(3):403–417. doi: 10.1042/bj20100323. [DOI] [PubMed] [Google Scholar]
- 35.Wagner E.F., Nebreda A.R. Signal integration by Jnk and P38 mapk pathways in cancer development. Nat. Rev. Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 36.Yang G., Kong Q., Wang G., Jin H., Zhou L., Yu D., et al. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother. Radiopharm. 2014;29(10):428–434. doi: 10.1089/cbr.2014.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson N.R., Minutolo N.G., Gill S., Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81(5):1201–1208. doi: 10.1158/0008-5472.Can-20-2990. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J., Tang Z., Gao S., Li C., Feng Y., Zhou X. Tumor-associated macrophages: recent insights and therapies. Front. Oncol. 2020;10:188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan Y., Yu Y., Wang X., Zhang T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N., et al. Low-dose irradiation programs macrophage differentiation to an inos⁺/m1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Nadella V., Singh S., Jain A., Jain M., Vasquez K.M., Sharma A., et al. Low dose radiation primed inos + M1macrophages modulate angiogenic programming of tumor derived endothelium. Mol. Carcinog. 2018;57(11):1664–1671. doi: 10.1002/mc.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo S.R., Fuertes M.B., Corrales L., Spranger S., Furdyna M.J., Leung M.Y.K., et al. Sting-dependent cytosolic dna sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spranger S., Dai D., Horton B., Gajewski T.F. Tumor-residing batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711–723. doi: 10.1016/j.ccell.2017.04.003. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shigematsu A., Adachi Y., Koike-Kiriyama N., Suzuki Y., Iwasaki M., Koike Y., et al. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. J. Radiat. Res. 2007;48(1):51–55. doi: 10.1269/jrr.06048. [DOI] [PubMed] [Google Scholar]
- 46.Wang S., Yu H., He R., Song X., Chen S., Yu N., et al. Exposure to low-dose radiation enhanced the antitumor effect of a dendritic cell vaccine. Dose Response. 2019;17(1) doi: 10.1177/1559325819832144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vesely M.D., Kershaw M.H., Schreiber R.D., Smyth M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 48.Zander R., Schauder D., Xin G., Nguyen C., Wu X., Zajac A., et al. Cd4+ T cell help is required for the formation of a cytolytic Cd8+ T cell subset that protects against chronic infection and cancer. Immunity. 2019;51(6):1028–1042. doi: 10.1016/j.immuni.2019.10.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishton R.J., Sukumar M., Restifo N.P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26(1):94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barsoumian H.B., Ramapriyan R., Younes A.I., Caetano M.S., Menon H., Comeaux N.I., et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer. 2020;8(2) doi: 10.1136/jitc-2020-000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashimoto S., Shirato H., Hosokawa M., Nishioka T., Kuramitsu Y., Matushita K., et al. The Suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat. Res. 1999;151(6):717–724. [PubMed] [Google Scholar]
- 52.Spary L.K., Al-Taei S., Salimu J., Cook A.D., Ager A., Watson H.A., et al. Enhancement of T cell responses as a result of synergy between lower doses of radiation and T cell stimulation. J. Immunol. 2014;192(7):3101–3110. doi: 10.4049/jimmunol.1302736. Baltimore, Md: 1950. [DOI] [PubMed] [Google Scholar]
- 53.Herrera F.G., Ronet C., MOd Olza, Barras D., Crespo I., Andreatta M., et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 2022;12(1):108–133. doi: 10.1158/2159-8290.Cd-21-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franchina D.G., Grusdat M., Brenner D. B-cell metabolic remodeling and cancer. Trends Cancer. 2018;4(2):138–150. doi: 10.1016/j.trecan.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Du Y., Sun H., Lux F., Xie Y., Du L., Xu C., et al. Radiosensitization effect of aguix, a gadolinium-based nanoparticle, in nonsmall cell lung cancer. ACS Appl. Mater. Interfaces. 2020;12(51):56874–56885. doi: 10.1021/acsami.0c16548. [DOI] [PubMed] [Google Scholar]
- 56.Cho S.J., Kang H., Kim M.Y., Lee J.E., Kim S.J., Nam S.Y., et al. Site-specific phosphorylation of ikaros induced by low-dose ionizing radiation regulates cell cycle progression of B lymphoblast through Ck2 and Akt activation. Int. J. Radiat. Oncol. Biol. Phys. 2016;94(5):1207–1218. doi: 10.1016/j.ijrobp.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Ohshima Y., Kitami A., Kawano A., Tsukimoto M., Kojima S. Induction of extracellular Atp mediates increase in intracellular thioredoxin in raw264.7 cells exposed to low-dose Γ-rays. Free Radic. Biol. Med. 2011;51(6):1240–1248. doi: 10.1016/j.freeradbiomed.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Eom H.S., Park H.S., You G.E., Kim J.Y., Nam S.Y. Identification of cellular responses to low-dose radiation by the profiling of phosphorylated proteins in human B-lymphoblast Im-9 Cells. Int. J. Radiat. Biol. 2017;93(11):1207–1216. doi: 10.1080/09553002.2017.1377362. [DOI] [PubMed] [Google Scholar]
- 59.Cao M., Cabrera R., Xu Y., Liu C., Nelson D. Different radiosensitivity of Cd4(+)Cd25(+) regulatory T cells and effector T cells to low dose gamma irradiation in vitro. Int. J. Radiat. Biol. 2011;87(1):71–80. doi: 10.3109/09553002.2010.518208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Persa E., Szatmári T., Sáfrány G., Lumniczky K. In vivo irradiation of mice induces activation of dendritic cells. Int. J. Mol. Sci. 2018;19(8) doi: 10.3390/ijms19082391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Safwat A., Aggerholm N., Roitt I., Overgaard J., Hokland M. Low-dose total body irradiation augments the therapeutic effect of interleukin-2 in a mouse model for metastatic malignant melanoma. J. Exp. Ther. Oncol. 2003;3(4):161–168. doi: 10.1046/j.1359-4117.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 62.Safwat A., Aggerholm N., Roitt I., Overgaard J., Hokland M. Tumour burden and interleukin-2 dose affect the interaction between low-dose total body irradiation and interleukin 2. Eur. J. Cancer. 2004;40(9):1412–1417. doi: 10.1016/j.ejca.2004.01.037. Epub 2004/06/05. [DOI] [PubMed] [Google Scholar]
- 63.Jin S.Z., Pan X.N., Wu N., Jin G.H., Liu S.Z. Whole-body low dose irradiation promotes the efficacy of conventional radiotherapy for cancer and possible mechanisms. Dose Response. 2007;5(4):349–358. doi: 10.2203/dose-response.07-020.Jin. Epub 2008/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu N., Jin S.Z., Pan X.N., Liu S.Z. Increase in efficacy of cancer radiotherapy by combination with whole-body low dose irradiation. Int. J. Radiat. Biol. 2008;84(3):201–210. doi: 10.1080/09553000801902133. [DOI] [PubMed] [Google Scholar]
- 65.Yu H.S., Liu Z.M., Yu X.Y., Song A.Q., Liu N., Wang H. Low-dose radiation induces antitumor effects and erythrocyte system hormesis. Asian Pac. J. Cancer Prev. 2013;14(7):4121–4126. doi: 10.7314/apjcp.2013.14.7.4121. Epub 2013/09/03. [DOI] [PubMed] [Google Scholar]
- 66.Kojima S., Tsukimoto M., Shimura N., Koga H., Murata A., Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. Dose Response. 2017;15(1) doi: 10.1177/1559325817697531. 1559325817697531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan A.U.H., Blimkie M., Yang D.S., Serran M., Pack T., Wu J., et al. Effects of chronic low-dose internal radiation on immune-stimulatory responses in mice. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/ijms22147303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu S., Liao Y., Chen Y., Yang H., Hu Y., Chen Z., et al. Effect of triple therapy with low-dose total body irradiation and hypo-fractionated radiation plus anti-programmed cell death protein 1 blockade on abscopal antitumor immune responses in breast cancer. Int. Immunopharmacol. 2023;117 doi: 10.1016/j.intimp.2023.110026. Epub 2023/03/20. [DOI] [PubMed] [Google Scholar]
- 69.Patel R.R., He K., Barsoumian H.B., Chang J.Y., Tang C., Verma V., et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: results of a phase Ii trial. Radiother. Oncol. 2021;162:60–67. doi: 10.1016/j.radonc.2021.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sezen D., Patel R.R., Tang C., Onstad M., Nagarajan P., Patel S.P., et al. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol. Oncol. 2021;161(3):645–652. doi: 10.1016/j.ygyno.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Allen C., Her S., Jaffray D.A. Radiotherapy for cancer: present and future. Adv. Drug Deliv. Rev. 2017;109:1–2. doi: 10.1016/j.addr.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Minniti G., Goldsmith C., Radiotherapy B.M. Radiotherapy. Handb. Clin. Neurol. 2012;104:215–228. doi: 10.1016/b978-0-444-52138-5.00016-5. [DOI] [PubMed] [Google Scholar]
- 73.Wilkins A., Melcher A., Somaiah N. Science in focus: biological optimisation of radiotherapy fraction size in an era of immune oncology. Clin. Oncol. 2018;30(10):605–608. doi: 10.1016/j.clon.2018.07.001. (R. Coll. Radiol.) [DOI] [PubMed] [Google Scholar]
- 74.Yang Y.C., Chiang C.S. Challenges of using high-dose fractionation radiotherapy in combination therapy. Front. Oncol. 2016;6:165. doi: 10.3389/fonc.2016.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tong F., Xiong C.J., Wei C.H., Wang Y., Liang Z.W., Lu H., et al. Hypo-fractionation radiotherapy normalizes tumor vasculature in non-small cell lung cancer xenografts through the p-stat3/hif-1 alpha signaling pathway. Ther. Adv. Med. Oncol. 2020;12 doi: 10.1177/1758835920965853. 1758835920965853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Nam H., Ahn Y.C., Yang K., Oh D., Noh J.M. Re-irradiation with moderate hypo-fractionation using intensity modulated photon or proton radiation therapy in locally recurrent squamous cell carcinoma of nasopharynx. Cancer Res. Treat. 2022;54(1):96–108. doi: 10.4143/crt.2020.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adlakha P., Maheshwari G., Dhanawat A., Sinwer R., Singhal M., Jakhar S.L., et al. Comparison of two schedules of hypo-fractionated radiotherapy in locally advanced head-and-neck cancers. J. Cancer Res. Ther. 2022;18(Supplement) doi: 10.4103/jcrt.JCRT_1793_20. S151-S6. [DOI] [PubMed] [Google Scholar]
- 78.Long Z., Wang B., Tao D., Liu Y., Zhang J., Tan J., et al. Clinical research on alternating hyperfraction radiotherapy for massive hepatocellular carcinoma. Oncol. Lett. 2015;10(1):523–527. doi: 10.3892/ol.2015.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takeda K., Matsushita H., Umezawa R., Yamamoto T., Ishikawa Y., Takahashi N., et al. Hyperfractionated radiotherapy for re-irradiation of recurrent esophageal cancer. Radiat. Oncol. J. 2021;39(4):265–269. doi: 10.3857/roj.2021.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frosina G., Fontana V., Verzola D., Rosa A., Gaggero G., Garibotto G., et al. Ultra-hyper-fractionated radiotherapy for high-grade gliomas. J. Neurosci. Res. 2021;99(12):3182–3203. doi: 10.1002/jnr.24929. [DOI] [PubMed] [Google Scholar]
- 81.Frosina G. Improving control of high-grade glioma by ultra-hyper-fractionated radiotherapy. J. Neurosci. Res. 2022;100(4):933–946. doi: 10.1002/jnr.25030. [DOI] [PubMed] [Google Scholar]
- 82.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol. Cancer. 2019;18(1):128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guha P., Heatherton K.R., O'Connell K.P., IS Alexander, Katz S.C. Assessing the future of solid tumor immunotherapy. Biomedicines. 2022;10(3) doi: 10.3390/biomedicines10030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shu Y., Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer. 2020;1874(2) doi: 10.1016/j.bbcan.2020.188434. [DOI] [PubMed] [Google Scholar]
- 85.Vinod S.K., Hau E. Radiotherapy treatment for lung cancer: current status and future directions. Respirology. 2020;25(Suppl 2):61–71. doi: 10.1111/resp.13870. (Carlton, Vic) [DOI] [PubMed] [Google Scholar]
- 86.Koukourakis M.I., Giatromanolaki A. Tumor microenvironment, immune response and post-radiotherapy tumor clearance. Clin. Transl. Oncol. 2020;22(12):2196–2205. doi: 10.1007/s12094-020-02378-8. [DOI] [PubMed] [Google Scholar]
- 87.Patel R.B., Hernandez R., Carlson P., Grudzinski J., Bates A.M., Jagodinsky J.C., et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade. Sci. Transl. Med. 2021;13(602) doi: 10.1126/scitranslmed.abb3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herrera F.G., Romero P., Coukos G. Lighting up the tumor fire with low-dose irradiation. Trends Immunol. 2022;43(3):173–179. doi: 10.1016/j.it.2022.01.006. [DOI] [PubMed] [Google Scholar]