Abstract
Objectives
To explore the multifactorial causality of prolonged viral shedding time and identify different viral shedding trajectories in Omicron BA.2 variant infections.
Methods
The Kaplan-Meier method was used to estimate the survivor function, and the Cox proportional hazards model was fitted to identify factors associated with viral shedding time. Group-based trajectory model (GBTM) was used to identify different viral shedding trajectories. Ordinal logistic regression was used to identify factors that significantly impacted the trajectory membership.
Results
The overall median viral shedding time was 12 days (interquartile range [IQR]: 8–15). Viral shedding time was longer for female cases, cases who were incompletely vaccinated, cases with comorbidities, cases with severe or critical infections and cases who had not taken Paxlovid within 5 days after diagnosis. Compared to the 3 to 17-year-old group, all older groups had significantly longer viral shedding times. The GBTMs based on the N gene and the ORF1ab gene were consistent. Three viral shedding trajectories were identified and age group, comorbidities, vaccination status, disease state, Paxlovid treatment were significantly associated with the trajectory membership.
Conclusion
Increased age, comorbidities, incomplete vaccination, severe or critical infections, and delayed Paxlovid treatment were the risk factors for prolonged viral shedding time.
Keywords: Viral shedding time, SARS-CoV-2, COVID-19, Observational study
1. Introduction
The 2019 coronavirus disease (COVID-19) pandemic remains a global public health emergency. The Omicron BA.2 variant was first detected in the United States in November of 2021 and has subsequently spread worldwide [1]. A major community epidemic of the Omicron BA.2 in Shanghai began in late February 2022 [2].
Understanding the transmission dynamics of the Omicron BA.2 variant infection has significant implications for epidemic prevention and control. The long viral shedding time of some persons infected with the Omicron BA.2 variant has drawn considerable concern because this phenomenon has increased the difficulties of epidemic prevention and control [[3], [4], [5], [6]]. Confirming the significance of prolonged polymerase chain reaction (PCR) positivity and the clinical importance of various routes of viral shedding time are critical to informing guidance around transmission-based isolation precautions. However, the characteristics of the viral shedding time in Omicron BA.2 variant infections have not been well clarified. Even though there have been many studies on the viral shedding time of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the results are heterogeneous and do not specifically focus on Omicron BA.2 [7,8].
Research to identify different viral shedding trajectories of individuals with the SARS-CoV-2 infection and to identify factors that significantly impact the trajectory membership is helpful. A group-based trajectory model (GBTM) could be a feasible approach to identify the different viral shedding types. The GBTM—also known as latent class growth analysis (LCGA)—postulates a discrete distribution of the population and makes it possible to distinguish subgroups or classes of homogeneous individuals who have a similar trajectory within that population [9]. The GBTM has recently been used in medical research [10,11]. Studies have explored using the GBTM as an improved approach to describe medication adherence [12,13].
This study aimed to explore the multifactorial causality of viral shedding time in infections of the Omicron BA.2 variant, identify different viral shedding types, and identify the characteristics of each type. Based on the population from 3 mobile cabin hospitals and 2 COVID-19–designated hospitals in Shanghai, we performed a comprehensive evaluation of the viral shedding time in people infected with the Omicron BA.2 variant with different demographic and clinical features.
2. Methods
2.1. Study design and participants
We evaluated the viral shedding time of the Omicron BA.2 variant infection in Shanghai in this retrospective observational study. The study population covered 2 areas of Shanghai, including 2 mobile cabin hospitals, 1 COVID-19–designated hospital in the western area, and 2 COVID-19–designated hospitals in the eastern area. Cases infected with the Omicron BA.2 variant between February 17, 2022, and June 28, 2022, during Shanghai’s COVID-19 epidemic were confirmed with positive real-time reverse transcriptase-polymerase chain reaction (RT-PCR) testing.
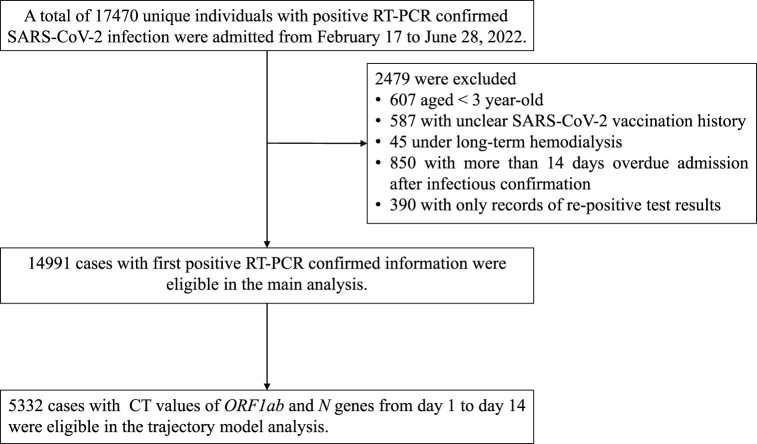
Cases were excluded if they satisfied any of the following conditions: (1) they were under the age of 3 years old, (2) they had an unclear SARS-CoV-2 vaccination history, (3) they were admitted more than 14 days after the confirmation that they were infected, (4) they were under long-term hemodialysis, and/or (5) cases had records of re-positive test results, but no corresponding records of the initial positive tests were available.
The study was approved by the Ethical Committee of Zhongshan Hospital, Fudan University (no. B2022-244R), Shanghai Pudong hospital, Fudan University (no. WZ-22), and Renji Hospital, Shanghai Jiao Tong University (no. RA-2022-601). The study was registered with the Chinese Clinical Trial Registry (no. ChiCTR2200060003). The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist was used to guide transparent reporting (Table S1).
2.2. Procedures
We defined the viral shedding time as the time from the first positive RT-PCR results to viral shedding cessation. Viral shedding cessation was considered to be the occurrence of 2 consecutive negative tests with an interval of over 24 h (a cycle threshold [CT] value large than 35 in at least the N and ORF1ab genes). Based on the timing of the infection waves in Shanghai and the clustering of selected viral genomes into BA.2 [2], the infections were assumed to be of the BA.2 variant.
Baseline information about each participant’s age, gender, vaccination status, comorbidities, clinical manifestations, and prognosis were available to study investigators, along with the results of laboratory examinations and treatments. Baseline characteristics and clinical information were acquired from the hospitals’ electronic medical records. Each participant’s vaccination history was obtained from the Shanghai Group Immunization System, which included comprehensive vaccination information of all vaccine recipients, such as the type, manufacturer, date, and doses of vaccines.
All participants were COVID-19 patients with recent infection confirmed by SARS-CoV-2 RT-PCR. We retrieved their vaccination history, and those who received the latest dose of vaccination at least 7 days before SARS-CoV-2 exposure were included in the analysis. The participants were divided into three groups based on their vaccination status: (1) incomplete vaccination (0 or 1 dose), (2) full vaccination (2 doses), and (3) booster vaccination (3 doses).
The diagnosis and clinical severity classification were made according to the Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (seventh edition) [14]. Patients categorized as asymptomatic were confirmed cases without clinical symptoms. Patients categorized as having a mild infection had mild symptoms without signs of pneumonia on chest imaging. Patients categorized as having a moderate infection had signs of pneumonia on radiologic assessment. Patients were categorized as having a severe infection if they had 1 of the following conditions: (1) a respiration rate ≥30/min, (2) SpO2 ≤ 93% in the resting state, (3) an arterial partial pressure of oxygen/fraction of inspired oxygen ≤300 mmHg, or (4) progressive clinical aggravation or chest imaging that showed lesion progression >50% within 24–48 h. Patients were categorized as having a critical infection if they presented any of the following: (1) respiratory failure requiring mechanical ventilation, (2) shock, or (3) a combination of other organ failure requiring care in the intensive care unit.
2.3. Statistical analysis
The mean ± standard deviation (SD) or median (IQR) are reported for continuous variables, and frequencies and percentages are reported for categorical variables. To identify factors associated with viral shedding time, we used the Kaplan-Meier method to calculate the median viral shedding time and the cumulative viral shedding rates over 7 and 14 days by subgroups (based on age group, gender, vaccination status, comorbidities, diagnosis, and treatments). Moreover, the log-rank method was used to compare within subgroups. We then fitted a multivariable Cox proportional hazards model to identify the risk factors for a prolonged viral shedding time. From the estimated coefficients, the hazard ratio (HR) and its 95% CI were calculated and reported.
The GBTM was used to examine heterogeneity in the CT values of N or ORF1ab gene trajectories over the first 14 days after diagnosis [9]. We fitted the model from 5 group trajectories to 2 group trajectories, and the timescale was the pharyngeal swab SARS-CoV-2 PCR test time (days). To identify the model with optimal functional forms of trajectories starting from the highest polynomial, the cubic, quadratic, and linear terms were considered and assessed according to the significance level. The statistical fit criteria were the Bayesian information criterion (BIC) value (a smaller BIC indicated a better model fit), the average posterior probability above 0.7, and a trajectory size greater than or equal to 5% of the sample [15]. Confidence in the final solution was based on the above statistical indices of fit as well as the theoretical meaningfulness and conceptual interpretability of the class structure. Once the final trajectory subgroup structure was determined, we sorted it according to the speed of recovery of each class and used ordinal logistic regression to estimate predictors of the trajectory membership. For the ordinal logistic regression analysis, the odds ratio (OR) and its 95% CI were calculated and reported.
Statistical analyses were performed using R version 4.2.2 (The R Foundation of Statistical Computing). A 2-sided P value < 0.05 was considered statistically significant.
3. Results
3.1. Study population
During the study period, 17,470 individuals with a positive RT-PCR–confirmed Omicron BA.2 variant infection were enrolled. After excluding 2479 cases due to the exclusion criteria (Fig. 1), we included 14,991 cases were eligible for the main analysis. Most cases only included the qualitative RT-PCR results (+/−), but for 5332 cases, detailed CT values of the N and ORF1ab genes were available for trajectory analyses.
Fig. 1.
Flow chart of research population screening.
The mean age of 14,991 cases was 50.0 (SD 20.7) years. The most common age group was 40–59 years (33.3%), followed by 18–39 years (29.5%). Of the cases, 24.5% had comorbidities, 30.7% had an incomplete vaccination status, 1.6% had severe or critical infections, and only a few (3.9%) had taken Paxlovid within 5 days after their diagnosis (Table 1).
Table 1.
Characteristics of SARS-CoV-2 infection individuals for the main analyses.
| Total (N = 14,991) | Median time to viral shedding, day (IQR) | 7-day cumulative viral shedding rate, % (95%CI) | 14-day cumulative viral shedding rate, % (95%CI) | |
|---|---|---|---|---|
| Characteristics | ||||
| Age group - no. (%) | ||||
| 3–17 years | 731 (4.9) | 11 (8–13) | 18.5 (15.6–21.2) | 84.4 (81.5–86.8) |
| 18–39 years | 4420 (29.5) | 11 (8–14) | 21.5 (20.2–22.7) | 81.4 (80.2–82.5) |
| 40–59 years | 4998 (33.3) | 11 (8–14) | 21.4 (20.2–22.5) | 75.7 (74.5–76.9) |
| 60–79 years | 3529 (23.5) | 13 (9–16) | 17.5 (16.2–18.7) | 61.4 (59.7–62.9) |
| ≥80 years | 1313 (8.8) | 16 (11–20) | 14.3 (12.4–16.2) | 42.0 (39.2–44.6) |
| Gender - no. (%) | ||||
| Female | 7458 (49.7) | 12 (9–15) | 18.2 (17.3–19.0) | 69.6 (68.5–70.6) |
| Male | 7533 (50.3) | 11 (8–15) | 21.3 (20.4–22.2) | 73.6 (72.5–74.5) |
| With comorbidities - no. (%) | ||||
| No | 11,315 (75.5) | 11 (8–14) | 20.6 (19.9–21.3) | 20.6 (19.9–21.3) |
| Yes | 3676 (24.5) | 14 (10–17) | 17.0 (15.8–18.2) | 17.0 (15.8–18.2) |
| Vaccination status - no. (%) | ||||
| Incomplete vaccination | 4603 (30.7) | 13 (10–17) | 15.8 (14.8–16.9) | 59.5 (58.0–60.9) |
| Full vaccination | 4430 (29.6) | 11 (8–14) | 21.7 (20.5–22.9) | 75.9 (74.6–77.2) |
| Booster vaccination | 5958 (39.7) | 11 (8–14) | 21.3 (20.2–22.3) | 77.6 (76.5–78.6) |
| Admission and treatment | ||||
| Severe or critical infection - no. (%) | ||||
| No | 14,752 (98.4) | 12 (8–15) | 19.9 (19.2–20.5) | 72.2 (71.4–72.9) |
| Yes | 239 (1.6) | 18 (13–24) | 10.5 (6.5–14.4) | 31.8 (25.5–37.7) |
| Take Paxlovid within 5 days - no. (%) | ||||
| No | 14,404 (96.1) | 12 (8–15) | 19.7 (19.0–20.3) | 71.4 (70.7–72.2) |
| Yes | 587 (3.9) | 11 (8–14) | 21.3 (17.9–24.6) | 75.5 (71.7–78.7) |
IQR, interquartile range; CI, confidence interval.
For the subgroup of 5332 cases that had CT values of the N and ORF1ab genes, the characteristics were similar to those of the main population. The mean age was 46.5 (SD 20.7) years, and most cases were younger than 60 years old. A total of 21.6% of these cases had comorbidities, 33.0% had an incomplete vaccination status, 2.0% had severe or critical infections, and only 7.8% had taken Paxlovid within 5 days after the illness was confirmed (Table S2).
3.2. Viral shedding time in infections and subgroup results
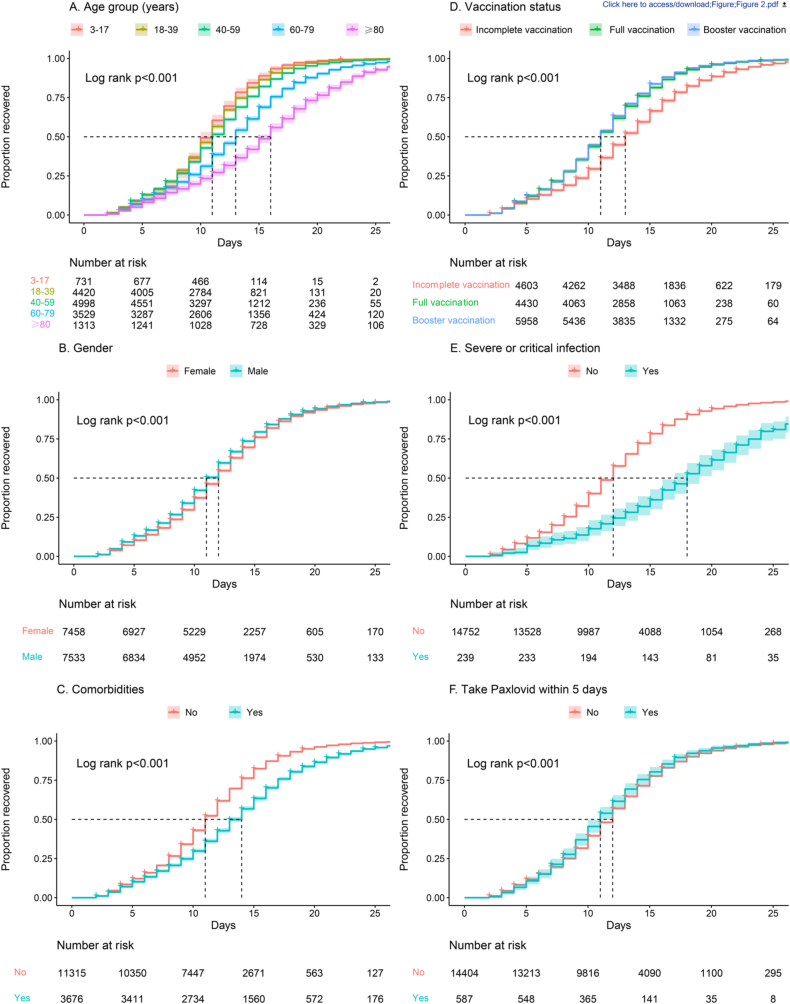
A total of 102 cases with the Omicron BA.2 variant infection died, and 14,889 cases were observed until viral shedding cessation. The descriptive and Kaplan-Meier analyses of viral shedding time are shown in Table 1 and Fig. 2. The overall median viral shedding time was 12 days (IQR 8–15). The 7-day and 14-day cumulative viral shedding rates were 17.9% (95% CI: 19.1–20.4) and 71.6% (95% CI: 70.8–72.3), respectively.
Fig. 2.
Crude Kaplan-Meier survival curves.
Among the different age groups, the median viral shedding time was significantly longest in cases older than 80 years (16 days; IQR: 11–20; P < 0.001). The median viral shedding time of the incomplete vaccination group (13 days) was longer than that of the full vaccination group (11 days) and the booster vaccination group (11 days), with 15.8% (95% CI: 14.8–16.9) 7-day and 59.5% (95% CI: 58.0–60.9) 14-day cumulative viral shedding rates. Further comparison showed that full and booster vaccination significantly shortened the viral shedding time compared to the incomplete vaccination group (P < 0.001).
Cases who were male had shorter viral shedding time (11 days; IQR: 8–15; P < 0.001). Cases who had taken Paxlovid within 5 days after diagnosis also had shorter viral shedding time (11 days; IQR: 8–14; P = 0.010). In contrast, cases had a significant longer viral shedding time (P < 0.001) if they had a severe or critical infection (18 days; IQR 13–24) or comorbidities (14 days; IQR 10–17).
3.3. Risk factors of viral shedding time
We explored risk factors for viral shedding time (Figure S1). Viral shedding time was shorter for male cases (adjusted HR = 1.099, 95% CI: 1.064–1.135). Compared to the incomplete vaccination group, the full vaccination group (adjusted HR = 1.203, 95% CI: 1.151–1.258) and the booster vaccination group (adjusted HR = 1.284, 95% CI: 1.229–1.341) had significantly shorter viral shedding times. The group that had taken Paxlovid within 5 days after their diagnosis also had shorter viral shedding times (adjusted HR = 1.410, 95% CI: 1.296–1.534). Compared to the 3 to 17-year-old group, all older groups (18–39, 40–59, 60–79, and ≥80 years) had significantly longer viral shedding times. Cases with comorbidities (adjusted HR = 0.867, 95% CI: 0.828–0.907) and severe or critical infections (adjusted HR = 0.521, 95% CI: 0.451–0.603) also had longer viral shedding times.
In addition, we compared the vaccine types among the vaccinated subgroups, as detailed in Table S3 and Figure S2. Of the total 10,388 cases that received vaccination, the majority were given inactivated vaccine, while only 74 cases received Adv vaccine, and 32 cases received mRNA vaccine. Our findings indicate that there was no statistically significant difference in viral shedding times between the Adv vaccine group and the inactivated vaccine group (adjusted HR = 1.034, 95% CI: 0.821–1.302) or between the mRNA vaccine group and the inactivated vaccine group (adjusted HR = 0.908, 95% CI: 0.642–1.285).
3.4. N gene or ORF1ab gene trajectories
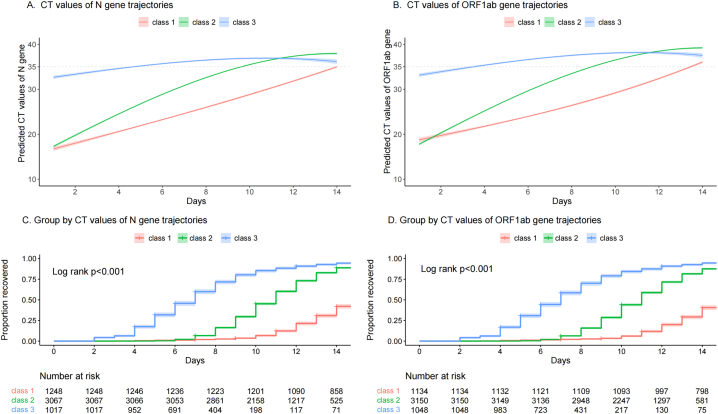
Based on 5332 cases with CT values of the N gene, 3 viral shedding trajectories were identified (Fig. 3A, C). They were the slow recovery group (class 1: 23.4% of the cases), the moderate recovery group (class 2: 57.5% of the cases), and the quick recovery group (class 3: 19.1% of the cases). The median viral shedding times of class 1, class 2, and class 3 trajectories were 15 days (IQR 13–17), 11 days (IQR 9–13), and 7 days (IQR 5–9), respectively. Of the quick recovery group, the 7-day and 14-day viral shedding rates were 60.0% (95% CI: 56.8–62.8) and 94.5% (95% CI: 92.9–95.7), respectively. However, the 14-day viral shedding rate of the slow recovery group (class 1) was still less than 50% (42.1%; 95% CI: 39.3–44.8).
Fig. 3.
Trajectories of CT values of N gene or ORF1ab gene and Kaplan-Meier survival curves (Class 1, slow recovery group; Class 2, moderate recovery group; Class 3, quick recovery group. CT, cycle threshold.).
Table 2 shows the results of the ordinal logistic regression analysis to identify factors of trajectory membership for CT values of the N gene. The full vaccination group (OR = 1.684, 95% CI: 1.457–1.948), the booster vaccination group (OR = 1.777, 95% CI: 1.546–2.043), and the group that had taken Paxlovid within 5 days after diagnosis (OR = 1.505, 95% CI: 1.234–1.836) recovered faster compared to the incomplete vaccination group. In contrast, compared to the 3 to 17-year-old group, all older groups (18–39, 40–59, 60–79, and ≥80 years) recovered more slowly. Cases with comorbidities (OR = 0.829, 95% CI: 0.705–0.974) and severe or critical infections (OR = 0.499, 95% CI: 0.327–0.756) also were predicted to belong to the 3 ordinal recovery trajectories. The factor of sex did not impact the trajectory membership (P = 0.054).
Table 2.
Results of ordinal logistic regression analysis to estimate factors that significantly impact the N gene trajectory membership.
|
N gene trajectoriesa |
OR (95%CI) | P value | |||
|---|---|---|---|---|---|
| Class 1 (N = 1248) | Class 2 (N = 3067) | Class 3 (N = 1017) | |||
| Age group (years) | |||||
| 3–17 | 49 (3.9) | 236 (7.7) | 86 (8.5) | Ref. | |
| 18–39 | 254 (20.4) | 1192 (38.9) | 378 (37.2) | 0.732 (0.587–0.913) | 0.006 |
| 40–59 | 397 (31.8) | 1042 (34.0) | 305 (30.0) | 0.500 (0.398–0.629) | <0.001 |
| 60–79 | 389 (31.2) | 488 (15.9) | 171 (16.8) | 0.369 (0.287–0.473) | <0.001 |
| ≥80 | 159 (12.7) | 109 (3.6) | 77 (7.6) | 0.433 (0.310–0.603) | <0.001 |
| Gender | |||||
| Female | 609 (48.8) | 1567 (51.1) | 535 (52.6) | Ref. | |
| Male | 639 (51.2) | 1500 (48.9) | 482 (47.4) | 0.901 (0.810–1.002) | 0.054 |
| Comorbidities | |||||
| No | 809 (64.8) | 2559 (83.4) | 813 (79.9) | Ref. | |
| Yes | 439 (35.2) | 508 (16.6) | 204 (20.1) | 0.829 (0.705–0.974) | 0.023 |
| Vaccination status | |||||
| Incomplete | 611 (49.0) | 850 (27.7) | 299 (29.4) | Ref. | |
| Full | 274 (22.0) | 856 (27.9) | 320 (31.5) | 1.684 (1.457–1.948) | <0.001 |
| Booster | 363 (29.1) | 1361 (44.4) | 398 (39.1) | 1.777 (1.546–2.043) | <0.001 |
| Severe or critical infection | |||||
| No | 1189 (95.3) | 3035 (99.0) | 1003 (98.6) | Ref. | |
| Yes | 59 (4.7) | 32 (1.0) | 14 (1.4) | 0.499 (0.327–0.756) | 0.001 |
| Take Paxlovid within 5 days | |||||
| No | 1170 (93.8) | 2804 (91.4) | 941 (92.5) | Ref. | |
| Yes | 78 (6.2) | 263 (8.6) | 76 (7.5) | 1.505 (1.234–1.836) | <0.001 |
OR, odds ratio; CI, confidence interval.
Class 1, slow recovery group; Class 2, moderate recovery group; Class 3, quick recovery group.
Based on the 5332 cases with CT values of the ORF1ab gene, 3 viral shedding trajectories were also identified (Fig. 3B, D). These trajectories were similar to the 3 viral shedding trajectories based on CT values of the N gene. The factors that significantly impacted the ORF1ab gene trajectory membership were consistent with the N gene trajectories (Table S4).
The details of the dynamic changes of CT values of the N and ORF1ab genes from 1 to 14 days after diagnosis are shown in Figure S3 and Figure S4. Table S5 shows the descriptive results of CT values of the N and ORF1ab genes on different study days.
4. Discussion
Viral shedding time is important for determining hospital discharge, discontinuation of quarantine, and the effect of antiviral treatment for COVID-19. During an Omicron BA 2.2–predominant wave of COVID-19 in Shanghai, China, from February 17, 2022, to June 28, 2022, we used detailed, individual-level data from mobile cabin hospitals and COVID-19–designated hospitals to evaluate the characteristics and influencing factors of virus shedding time. We also identified 3 viral shedding trajectories and the factors that significantly impacted the trajectory membership. Various factors have been associated with increased shedding duration, including increased age, male sex, the severity of illness, and the use of corticosteroids [[16], [17], [18]]. In this study, we also found that increased age and severity of illness were associated with an increased viral shedding time. Moreover, cases with comorbidities, with incomplete vaccination status and those who delayed Paxlovid treatment also had prolonged viral shedding.
Our study demonstrated that patients with comorbidities had prolonged viral shedding of 3 days compared to cases without comorbidities. Furthermore, inactivated SARS-CoV-2 vaccines are widely available in Shanghai. Several previous studies confirmed that 2 doses and a booster of inactivated vaccine provided protection against severe cases and death in all ages [19,20]. In this study, we found that 2 doses and a booster of inactivated vaccination shortened the virus shedding time, which provided new evidence for evaluating vaccine effectiveness.
The viral shedding time is also an important parameter for evaluating the effect of antiviral treatment for infectious diseases. It was reported that treatment with Paxlovid in the first 5 days since the onset of COVID-19 symptoms was associated with a markedly reduced risk of progression to severe COVID-19 or mortality, regardless of vaccination status for SARS-CoV-2 [[21], [22], [23], [24], [25]]. Our study found that cases who received Paxlovid treatment within the first 5 days of their first positive PCR results had a shorter virus shedding time. This finding was consistent with the clinical outcomes (e.g., low viral burden, disease progression, or mortality) and supports the conclusions of other studies on antiviral treatment indicating that antiviral treatment should be administered earlier [26,27].
We ultimately identified 3 viral shedding trajectories, which may help characterizing the heterogeneity of viral shedding types among individuals with SARS- CoV-2 infection. Through the ordinal logistic analyses, we explored the factors that significantly impacted N or ORF1ab gene trajectory membership and further confirmed the results of the main analysis. The factor of sex did not significantly impact the trajectory membership. More research identifying different viral shedding trajectories of individuals with SARS- CoV-2 infection is needed for different populations.
This study had several limitations. Firstly, the virus shedding time was calculated based on the time of the RT-PCR test results. However, a case’s first positive test time might have been later than the actual infectious time, especially for asymptomatic infections. As most cases were from COVID-19–designated hospitals and the proportion of the high-risk population was relatively high, the virus shedding time might not represent the general infected population. Secondly, the infections were assumed to be of the BA.2 variant based on the timing of the infection waves in Shanghai, without precise sequencing. Although there should theoretically be no other variant strains in Shanghai during during the Omicron variant BA.2 surge in 2022, this assumption may still be not rigorous enough. Furthermore, only 35.57% of cases (5332/14,991) in the main analyses were used for the trajectory analyses, which may result in a certain heterogeneity on the baseline in the population of the main analyses and the trajectory analyses.
In conclusion, understanding viral shedding time is critical to optimizing treatment options and preventing transmission of this disease. Prolonged viral load shedding also provides an opportunity for virus mutation and transmission. Our study found several risk factors for prolonged virus shedding time, including increased age, comorbidities, incomplete vaccination status, a more severe disease state, and delayed Paxlovid treatment. More evidence is needed to confirm whether females have a longer viral shedding time than do males.
Author contribution statement
Zhenzhen Lu: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Zhongshu Kuang: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Binzhe Li: Analyzed and interpreted the data.
Zhenju Song: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Lihong Huang: Conceived and designed the experiments; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e17173.
Contributor Information
Zhenju Song, Email: song.zhenju@zs-hospital.sh.cn.
Lihong Huang, Email: huang.lihong@zs-hospital.sh.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organization . vol. 2. 2022. https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2 (Statement on Omicron Sublineage BA). Accessed 1 June 2023. [Google Scholar]
- 2.Zhang X., Zhang W., Chen S. Shanghai’s life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399:2011–2012. doi: 10.1016/S0140-6736(22)00838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L., Kang X., Zhang B., Zheng S., Wang, Miao H., et al. A special case of COVID-19 with long duration of viral shedding for 49 days. medRxiv. 2020 doi: 10.1101/2020.03.22.20040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J., Deng D., Wu N., et al. Persistent viral RNA positivity during the recovery period of a patient with SARS-CoV-2 infection. J. Med. Virol. 2020;92:1681–1683. doi: 10.1002/jmv.25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Zheng X., Shen X., et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 in patients with COVID-19. Emerg. Microb. Infect. 2020;9:2571–2577. doi: 10.1080/22221751.2020.1852058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing Y., Ni W., Wu Q., et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi D., Wu W., Wang Q., et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-center 28-day study. J. Infect. Dis. 2020;222:910–918. doi: 10.1093/infdis/jiaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y., Zheng F., Sun D., et al. Epidemiology and clinical course of COVID-19 in Shanghai, China. Emerg. Microb. Infect. 2020;9:1537–1545. doi: 10.1080/22221751.2020.1787103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagin D.S. Group-based trajectory modeling: an overview. Ann. Nutr. Metab. 2014;65:205–210. doi: 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- 10.Feldman B., Shen J., Chen C., et al. Perceived health after adult traumatic brain injury: a group-based trajectory modeling (GBTM) analysis. Brain Inj. 2020;34:741–750. doi: 10.1080/02699052.2020.1753111. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z., Zheng J., Liu X., et al. Assessing potassium levels in critically ill patients with heart failure: application of a group-based trajectory model. ESC. Heart Fail. 2022 doi: 10.1002/ehf2.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhazami M., Pontinha V.M., Patterson J.A., et al. Medication adherence trajectories: a systematic literature review. J. Manag. Care. Spec. Pharm. 2020;26:1138–1152. doi: 10.18553/jmcp.2020.26.9.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park K.H., Tickle L., Cutler H. Identifying temporal patterns of adherence to antidepressants, bisphosphonates and statins, and associated patient factors. SSM. Popul. Health. 2022;17 doi: 10.1016/j.ssmph.2021.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.China NHCotPsRo . China NHCotPsRo; 2022. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (9th Version)http://www.nhc.gov.cn/cms-search/downFiles/ef09aa4070244620b010951b088b8a27.pdf [Google Scholar]
- 15.Nylund K.L., Asparouhov T., Muthén B.O. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct. Equ. Model. 2007;14:535–569. doi: 10.1080/10705510701575396. [DOI] [Google Scholar]
- 16.Meiring S., Tempia S., Bhiman J.N., et al. Prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at high viral loads among hospitalized immunocompromised persons living with human immunodeficiency virus (HIV), South Africa. Clin. Infect. Dis. 2022;75:e144–e156. doi: 10.1093/cid/ciac077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu K., Chen Y., Yuan J., et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin. Infect. Dis. 2020;71:799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMenamin M.E., Nealon J., Lin Y., et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect. Dis. 2022;22:1435–1443. doi: 10.1016/s1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z., Xu S., Liu J., et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022;20:400. doi: 10.1186/s12916-022-02606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piscoya A., Ng-Sueng L.F., Parra Del Riego A., et al. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najjar-Debbiny R., Gronich N., Weber G., et al. Effectiveness of Paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin. Infect. Dis. 2022;76:e342–e349. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen W., Chen C., Tang J., et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann. Med. 2022;54:516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong C.K.H., Au I.C.H., Lau K.T.K., et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect. Dis. 2022;22:1681–1693. doi: 10.1016/s1473-3099(22)00507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Y., Ai J., Lin N., et al. An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants. Emerg. Microb. Infect. 2022;11:1518–1523. doi: 10.1080/22221751.2022.2078230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.