Abstract
The BarA/UvrY two-component signal transduction system mediates adaptive responses of Escherichia coli to changes in growth stage. At late exponential growth phase, the BarA sensor kinase autophosphorylates and transphosphorylates UvrY, which activates transcription of the CsrB and CsrC noncoding RNAs. CsrB and CsrC, in turn, sequester and antagonize the RNA binding protein CsrA, which posttranscriptionally regulates translation and/or stability of its target mRNAs. Here, we provide evidence that during stationary phase of growth, the HflKC complex recruits BarA to the poles of the cells and silences its kinase activity. Moreover, we show that during the exponential phase of growth, CsrA inhibits hflK and hflC expression, thereby enabling BarA activation upon encountering its stimulus. Thus, in addition to temporal control of BarA activity, spatial regulation is demonstrated.
Keywords: Escherichia coli, two component system, BarA, HflKC complex, spatiotemporal regulation
The BarA (bacterial adaptive response) protein, a membrane-bound tripartite sensor kinase (1, 2), and the UvrY protein, a typical response regulator of the FixJ family (3), constitute a two-component signaling system in Escherichia coli that mediates adaptative responses by modulating the Csr global regulatory system (4). BarA senses and responds to the presence of the protonated form of short-chain carboxylic acids such as formate and acetate (5, 6, 7), at the late exponential phase of growth, leading to its autophosphorylation and transphosphorylation of the cognate response regulator UvrY (3, 8, 9). UvrY-P, in turn, activates transcription of the CsrB and CsrC noncoding regulatory RNAs (4, 10, 11, 12) that possess repeated sequence elements enabling them to interact with multiple copies of the RNA-binding protein CsrA and thereby antagonize its regulatory functions, by preventing the interaction of CsrA with its mRNA targets (13, 14).
CsrA, an RNA binding protein, posttranscriptionally coordinates gene expression by interacting with its target RNAs at sites characterized by a conserved GGA sequence, typically located within 5′ untranslated mRNA leaders, and positively or negatively regulates RNA stability, translation, or transcription elongation (15, 16, 17, 18, 19, 20). In this way, CsrA activates exponential phase processes while represses several stationary phase functions (21, 22). CsrA is widely distributed among eubacteria (11, 23, 24) and regulates expression of genes for virulence factors (25, 26, 27), quorum sensing (28, 29), motility (30, 31), carbon metabolism (32, 33), biofilm formation (34, 35, 36), cyclic di-GMP synthesis (37), iron homeostasis (38) and peptide uptake (17).
In this study, we provide evidence that during stationary phase of growth, the HflKC complex recruits BarA to the poles of the cells, leading to the inhibition of BarA-dependent gene expression. Moreover, we show that CsrA negatively affects HflK and HflC expression during the exponential phase of growth. This allows BarA activation in the presence of its stimulus, at the transition from exponential to stationary phase of growth. Our findings are incorporated into a complex model for the BarA/UvrY-CsrA/B/C circuitry to include, in addition to temporal control, a spatial regulation of the BarA activity.
Results
BarA interacts with HflK and HflC
It has been previously reported that activation of the BarA sensor kinase, which normally takes place at the transition from the exponential to stationary phase of growth (4), does not occur in a CsrA mutant strain (39). The effect of CsrA on BarA was suggested to be indirect, and therefore, it was proposed that in addition to acetate, which acts as its physiological stimulus for BarA (5, 6), other factors are involved in the control of the activity of BarA (39). To identify such factors, a pull-down experiment using BarA as the bait was performed. Briefly, a barA mutant strain (IFC5035) harboring plasmid pMX559, which carries a His6-tagged BarA version under the arabinose promoter, was grown to an absorbance at 600 nm (A600) of ∼1.0, and 0.13 mM of arabinose was added to induce BarA expression. After an hour of induction, the protein crosslinker formaldehyde was added for 10 min. As a control, the same procedure was pursued for the barA mutant strain but without the His6-BarA-expressing plasmid. Subsequently, cells were harvested, lysed by French Press, BarA was purified under denaturing conditions by nickel affinity chromatography, the crosslinking was reversed, and proteins that copurified with BarA were identified by LC-MS/MS analyses (Table S1). After eliminating the proteins that appeared in the control experiments and the cytosolic proteins, HflK emerged as the highest hit (Table S1). HflK, which is encoded on the hfq-hflXKC operon, is an inner membrane protein that forms part of the HflKC complex. This complex interacts with and regulates the ATP-dependent protease FtsH (40, 41).
To assure that HflK indeed interacts with BarA in vivo, the bacterial adenylate cyclase-based two hybrid system (42) was employed. For this purpose, the T25 or the T18 subunit, corresponding to amino acids 1 to 224 or 225 to 399 of the adenylate cyclase (CyaA), respectively, was fused to the N terminus of BarA, HflK, HflC, and ArcA (see Experimental procedures) to generate plasmids pT25BarA, pT25HflK, pT18HflK, pT25ArcA, pT18ArcA, and pT18HflC (Table S2). Interaction between two hybrid proteins leads to reconstitution of the catalytic domain of the adenylate cyclase resulting in restoration of cAMP production in E. coli cya mutants and thereby activation of, among others, the lactose operon. The activation of this operon can be detected on selective agar plates or using β-galactosidase assays. It is relevant to mention that bacterial adenylate cyclase–based two hybrid system has been shown to detect interactions between not only cytoplasmic proteins but also between membrane-associated proteins (43). E. coli BTH101, a cya mutant, was cotransformed with pairs of the recombinant plasmids of interest, and bacteria were plated on lysogeny broth (LB) + 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) plates (Fig. 1A). It was observed that when BTH101 was cotransformed with the pT25BarA/pT18, pT25/pT18HflK, pT25/pT18HflC, pT25BarA/pT18ArcA, pT25ArcA/pT18HflK, or pT25ArcA/pT18HflC vectors yielded colorless colonies. On the other hand, when BTH101 was cotransformed with pT25HflK/pT18HflC, which serves as positive control, or with pT25BarA/pT18HflK and pT25BarA/pT18HflC yielded blue colonies. This indicates heterodimerization of the chimeric proteins, resulting in efficient functional complementation of the adenylate cyclase fragments.
Figure 1.
BarA interacts with HflK and HflC. Strain BTH101 (cya−) was cotransformed with recombinant plasmids expressing BarA, HflK, HflC, or ArcA hybrid proteins fused to either the T25 or the T18 domain of adenylate cyclase. A, bacterial cultures were spotted on a X-gal and IPTG containing agar plates, and transcriptional activation of the lac operon, indicative of protein interaction, was visualized by the formation of blue colonies. B, transformants were grown in LB medium supplemented with 0.5 mM IPTG to an A600 of 0.6, and β-galactosidase activity was measured. The average from three independent experiments is presented, and standard deviations (error bars) are indicated. LB, lysogeny broth; X-gal, 5-bromo-4-chloro-3-indolyl-β-D-galactoside.
The efficiency of functional complementation between T25 and T18 domains was then quantified by measuring β-galactosidase activity (Fig. 1B) of liquid cultures. It was observed that the β-galactosidase activity in cells harboring pT25HflK/pT18HflC, pT25BarA/pT18HflK, and pT25BarA/pT18HflC was significantly higher than in cells harboring pT25BarA/pT18, pT25/pT18HflK, pT25/pT18HflC, pT25BarA/pT18ArcA, pT25ArcA/pT18HflK, or pT25ArcA/pT18HflC (Fig. 1B). It can therefore be concluded that BarA does interact with the HflKC complex in vivo.
BarA colocalizes with the HflKC complex in vivo
To provide further evidence for the interaction of BarA with the HflKC complex, a strain (IFC5043 pMX560) harboring the BarA-Yfp and HflK-mCherry hybrid proteins was constructed, and the localization/colocalization of the proteins was monitored. It has to be mentioned that a plasmid-born arabinose-inducible BarA-Yfp (pMX560) was used, because the chromosomal BarA-Yfp hybrid was not detectable, most likely due to its low expression. On the other hand, the HflK-mCherry fusion was introduced into the wildtype hflK chromosomal locus, and therefore, its expression relies on the native promoter.
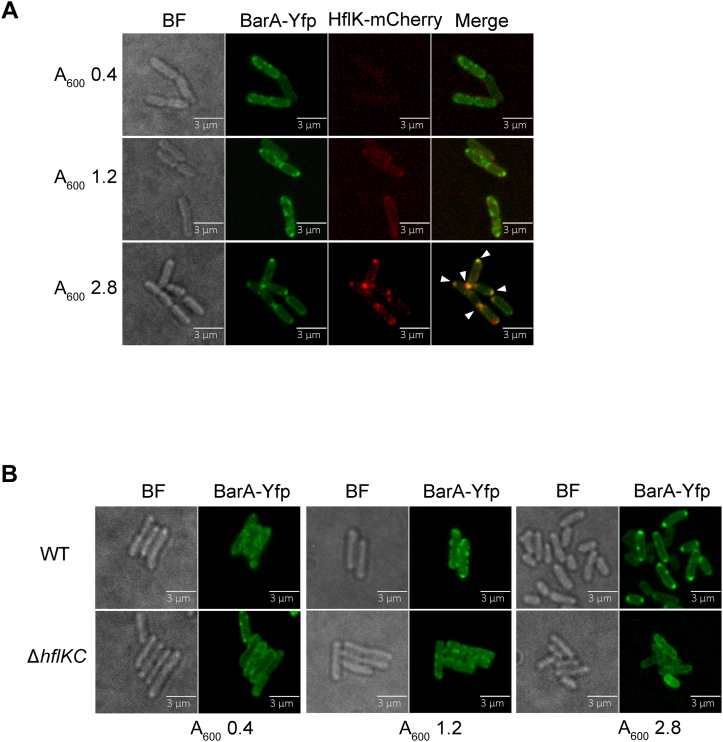
Fluorescence microscopy of live cells revealed that the intensity of fluorescence of HflK-mCherry was very low in exponential growing cells and significantly higher in cells from the stationary phase of growth (Fig. 2A), suggesting that the expression of hflK is growth stage dependent. Also, HflK-mCherry was found to localize in discrete foci on cell poles or on the septum region of the cells, as previously reported for its protein complex partner HflC (44, 45). On the other hand, BarA-Yfp was found to form discrete foci that were scattered on the cytosolic membrane of exponential growing cells (A600 0.4) but were progressively recruited to the cell poles as the cell density increased (A600 1.2) (Figs. 2A, and S1). Finally, during the stationary phase of growth (A600 2.8), BarA-Yfp was found to almost exclusively localize on the cell poles, colocalizing with HflK (Figs. 2A and S1). As expected, a similar topological distribution was observed when the colocalization of HflC-mCherry and BarA-Yfp was analyzed (Fig. S2). Next, we asked whether the HflKC complex is required for the recruitment of BarA to the cell poles at the stationary phase of growth. To this end, the in vivo localization of the BarA-Yfp hybrid protein in an hflK hflC double mutant (IFC5047) and its isogenic wildtype strain (CF7789) was compared. It was noticed that the BarA-Yfp hybrid protein, in contrast to the polar localization in the wildtype strain, was dispersed throughout the membrane in the hflK hflC mutant strain (Fig. 2B), indicating that the HflKC complex is required for proper BarA localization. It, thus, appears that the HflKC complex, when present, interacts with BarA and restrains its localization to the poles of the cells.
Figure 2.
HflK is required for the recruitment of BarA to the cell poles at the stationary phase of growth.A, representative fluorescence images of E. coli live cells expressing BarA-mEyfp (colored in green) and HflK-mCherry (colored in red) translational fusions (strain IFC5043 harboring pMX560 plasmid), harvested during the exponential growth phase (A600 of 0.4), the early stationary phase (A600 of 1.2), or during the stationary phase (A600 of 2.8). Left panels show the bright field (BF) imaging of cells; right panels show the merged fluorescence signals, which appear yellow where the two fluorescence signals overlapped. Triangles indicate colocalized foci. B, effect of the in-frame deletion of hflKC in the BarA-mEyfp localization. Representative bright field (BF) and fluorescence images of CF7789 (WT) and IFC5047 (ΔhflKC::Kanr) cells, expressing BarA-mEyfp (colored in green) from plasmid pMX560, harvested at exponential growth phase (A600 of 0.4, left panels), early stationary phase (A600 of 1.2, middle panels) or at stationary phase (A600 of 2.8, right panels).
CsrA binds to the hflK mRNA and inhibits its translation
To confirm that hflK expression is growth stage dependent, as suggested above (Fig. 2A), the amount of HflK was monitored throughout the growth curve of IFC5021, carrying a chromosomal HflK-HA protein fusion (Fig. 3A). Indeed, the amount of HflK was almost undetectable during exponential growth and increased significantly at the early stages of the stationary phase of growth (Fig. 3A). Because the expression of the hfq-hflXKC operon is not known to be transcriptionally activated during stationary phase of growth, the nucleotide sequence in the vicinity of the hflK start codon was inspected for possible regulatory motifs. Various possible CsrA binding sites (hflk 1–5), characterized by the GGA sequence (46, 47), located at −65, −32, −11, +7, and +27 relative to the start codon of HflK (Fig. 3C) were found. Comparison of the GGA sequences and flanking regions with the reported consensus (Fig. S3) revealed good match of hflk 2–4 to consensus sequences. However, folding prediction of the mRNA region spanning nucleotides −86 to +48 relative to the start codon of the hflK mRNA (Fig. S4) places hflk 2 in a base-paired region, which should prevent CsrA interaction. To test whether CsrA is indeed involved in the growth-dependent regulation of hflK expression, the amount of HflK in a wildtype (IFC5021) and an isogenic csrA::kan mutant (IFC5054) strains, harboring a chromosomal HflK-HA fusion, was compared by Western blotting, using specific HA antibodies. It was found that the amount of HflK was significantly higher in the csrA mutant strain than in the wildtype strain (Fig. 3B). The level of HflK protein was re-established at wildtype levels when the csrA mutant strain was complemented with a csrA-expressing plasmid (pMX544) (Fig. 3B), indicating that CsrA inhibits, directly or indirectly, hflK expression. Similar results were obtained for an HflC-HA hybrid (Fig. 3B).
Figure 3.
CsrA modulates the expression of the HflKC complex.A, levels of HflK-HA protein (46.8 KDa) as determined by Western blot analysis. A culture of strain IFC5021 (hflK::ha) was grown in LB medium, cells were harvested by centrifugation throughout the growth curve (A600 of 0.3–3.0), and HflK-HA content was determined by Western blot using monoclonal antibodies against the HA epitope. DnaK (69.1 kDa), detected using DnaK polyclonal antibodies, was used as a loading control. B, (upper panels) levels of HflK-HA protein (46.8 KDa) in IFC5021 (hflK::ha) and IFC5054 (ΔcsrA hflK::ha) strains and in IFC5054 (ΔcsrA hflK::ha) complemented with the csrA-expressing plasmid pMX544 (indicated as pCsrA), as determined by Western blot analyses using monoclonal antibodies against HA; (lower panels). Levels of HflC-HA protein (38.9 KDa) in the IFC5019 (hflC::ha) and IFC5055 (ΔcsrA hflC::ha) strains and in IFC5055 (ΔcsrA hflC::ha) complemented with the csrA-expressing plasmid pMX544 (indicated as pCsrA), as determined by Western blot analyses using monoclonal antibodies against HA. DnaK (69.1 kDa), detected using DnaK polyclonal antibodies, was used as a loading control. Cultures of the indicated strains were grown in LB medium, and at an A600 of 0.5, cells were harvested for Western blot analysis. Experiments were repeated three times in their entirety with essentially identical results. C, nucleotide sequence of the hflK RNA (134 nt) used in EMSA’s comprised of RNA extending from −86 to +48 nt with respect to the start codon. The GGA sequences are underlined, and the start codon is given in lowercase letters. The Shine–Dalgarno (SD) sequence is boxed. D, gel shift analysis of CsrA binding to mRNA leader of hflK. 5′ end-labeled RNA (0.5 nM) was incubated with the concentration of CsrA indicated at the bottom of each lane. Kd value of the CsrA interaction with the hflK transcript is shown. E, RNA competition assay. Labeled hflK leader RNA (0.5 nM) was incubated with CsrA ± specific (hflK) or nonspecific (phoB) competitor RNA. Positions of free (F) and bound (B) RNA are indicated. LB, lysogeny broth.
Finally, the question whether CsrA interacts directly with the mRNA region spanning nucleotide −86 to +48 (134 nt) relative to the start codon of the hflK mRNA (Fig. 3D) was addressed, by electrophoretic mobility shift assays. It was observed that the intensity of a band with slower migration began to increase at ∼35 nM CsrA, which is indicative of CsrA-hflK RNA complex formation. This complex became more prominent as the CsrA concentration increased further, at the expense of free hflK. A linear regression analysis of the data for this interaction yielded an apparent dissociation constant (Kd) of 55 nM (Fig. S5). Also, it was noted that the shifted band migrated progressively slower with increasing amounts of CsrA, suggesting that multiple CsrA proteins may bind to the hflK RNA. Finally, unlabeled hflK RNA was able to compete for the formation of CsrA complexes with the labeled hflK RNA (Fig. 3E), while unlabeled phoB RNA, which does not bind to CsrA (48), did not compete with the hflK RNA. It can therefore be concluded that CsrA directly binds to hflK mRNA and inhibits hflK expression (Fig. 3B). Because the GGA site at −11 overlaps the Shine–Dalgarno sequence of this mRNA (Figs. 3C and S4), CsrA binding is predicted to inhibit ribosome loading and hflK translation, the predominant inhibitory mode of CsrA (22). However, we did not further investigate the CsrA-dependent inhibitory mechanism.
The HflKC complex silences the BarA kinase activity
We then asked whether the activity of BarA is affected in an hflK, hflC, or a double hflKC mutant. To this end, the expression of the csrB-lacZ reporter, which depends directly on the activity of the BarA/UvrY TCS, was monitored in a wildtype strain (KSB837) and the isogenic ΔhflK (IFC5051), ΔhflC (IFC5052), and ΔhflKC (IFC5053) mutant strains (see Experimental procedures). It was found that, during exponential growth, reporter expression was slightly higher in the ΔhflK mutant strain, and approximately 3- and 4-fold higher in the ΔhflC and ΔhflKC mutant strains, respectively (Fig. 4A). Reporter expression was restored to almost wildtype levels when these mutant strains were complemented with the corresponding HflK (pT25HflK), HflC (pT25HflC), and HflKC (pMX561) expressing plasmids (Fig. 4B). It can therefore be concluded that the HflKC complex negatively affects the BarA/UvrY signaling system. The effect of HflKC was most likely exerted through BarA, because no activation of csrB-lacZ expression was obtained in an hflK hflC barA triple mutant (IFC5058) (Fig. 4C). To examine whether the HflKC complex affects other two-component sensor kinases, the activity of ArcB, which is activated during anoxic growth conditions (49, 50), in an ΔhflKC mutant (IFC5057) and the corresponding isogenic wildtype strain (ECL5003) was tested, by monitoring the expression of the activatable cyd-lacZ reporter (49). No difference in reporter expression was noted between the wildtype and the hflKC mutant strains under aerobic or anoxic growth conditions (Fig. 4D), indicating that the HflKC complex specifically affects the BarA sensor kinase.
Figure 4.
The HflKC complex negatively affects the BarA kinase activity.A, cells of the csrB-lacZ transcriptional fusion-carrying strains KSB837 (WT) (blue, filled circles), IFC5051 (ΔhflK) (red, filled squares), IFC5052 (ΔhflC) (green, filled up-pointing triangle), and IFC5053 (ΔhflKC) (violet, filled down-pointing triangle) were harvested at various times throughout growth and assayed for β-galactosidase activity. The β-galactosidase activity is presented as a function of growth density (A600). Data represent the averages from three independent experiments, and standard deviations (error bars) are indicated. B, strains KSB837 (WT), IFC5051 (ΔhflK), IFC5052 (ΔhflC), IFC5053 (ΔhflKC), IFC5051 (ΔhflK) carrying plasmid pT25HflK (pHflK), IFC5052 (ΔhflC) carrying plasmid pT25HflC (pHflC), and IFC5053 (ΔhflKC) carrying plasmid pMX561 (indicated as pHflKC and expressing hflK and hflC) were grown in LB medium to an A600 of ∼0.6 (exponential phase) and β-galactosidase activity was measured. The average from three independent experiments is presented, and standard deviations (error bars) are indicated. C, strains KSB837 (WT), IFC5053 (ΔhflKC), IFC5035 (ΔbarA), and IFC5058 (ΔhflKC ΔbarA) were grown in LB medium to an A600 of 0.6 (exponential phase) or 2.5 (stationary phase), and β-galactosidase activity was measured. The average from three independent experiments is presented, and standard deviations (error bars) are indicated. D, strains ECL5003 (cyd-lacZ) and its isogenic IFC5057 (ΔhflKC::Kanrcyd-lacZ), carry the ArcA-P−activatable cydA-lacZ reporter, were grown aerobically (nonstimulatory conditions) or anaerobically (stimulatory conditions) in LB medium to an A600 of 0.6, and β-galactosidase activity was measured. The average from three independent experiments is presented, and standard deviations (error bars) are indicated. E, determination of stability of the BarA protein, after inhibition of protein synthesis with tetracycline, in a wildtype strain (CF7789) and in a hflKC mutant strain (IFC5047). (Top) Western blot analysis using BarA polyclonal antibodies. (Bottom) Semilogarithmic plot of BarA protein decay. F, β-galactosidase activities of KSB837 (WT), IFC5053 (ΔhflKC), IFC5010 (ΔcsrA), and IFC5056 (ΔhflKC ΔcsrA) cells grown in LB medium to an A600 of 0.6 (exponential phase) or 2.5 (stationary phase). The average and standard deviations from three independent experiments are presented. LB, lysogeny broth.
As mentioned earlier, FtsH, an ATP-dependent metalloprotease, whose activity is modulated by the HflKC complex, degrades a number of soluble and inner membrane proteins (51). However, the fact that the HflKC complex antagonizes the FtsH proteolytic activity (41) in combination with our result demonstrating a BarA gain-of-function phenotype in the hflKC mutant excludes the possibility that the observed effect is due to the degradation of BarA. At any rate, this possibility was explored by comparing the half-life of BarA in an ΔhflKC mutant (IFC5047) and its wildtype isogenic strain (CF7789). No significant difference in the stability of BarA in these two strains was found (Fig. 4E). This is in agreement with a previous finding that no difference in the amount of BarA was found between a wildtype strain and a csrA mutant where expression of hflK and hflC are not repressed (39). Therefore, it can be concluded that the HflKC complex exerts a direct effect on the BarA kinase activity.
The facts that the HflKC complex binds to BarA (Figs. 1 and 2) and inhibits its kinase activity, as judged by csrB-lacZ expression (Fig. 4A) and that CsrA inhibits hflK expression (Fig. 3), prompted us to ask whether deletion of hflK and hflC in a csrA mutant could lead to activation of BarA. No activation of csrB-lacZ was detected in a csrA hflK hflC triple mutant (IFC5056) (Fig. 4F), suggesting that additional factors are involved in the regulation of the BarA kinase activity.
HflKC acts in the stationary phase of growth
The result demonstrating that the HflKC complex inhibits the activity of BarA during exponential growth (Fig. 4A) appears to be inconsistent with the time of activation of HflK and HflC expression, which takes place during stationary phase of growth (Figs. 2A, S1, and 3, A and B). A possible explanation could be that BarA inhibition by the HflKC complex takes place during the stationary phase of growth and is inherited by the exponentially growing cells when cell growth is resumed. Subsequently, as the cells grow, the existing HflKC complex becomes undetectable, via its proteolysis or gradual dilution, permitting the stimulus-dependent activation of BarA at late exponential growth phase. To test this hypothesis, the hflKC mutant and its isogenic wildtype strain were grown to the stationary phase of growth, the cultures were shifted to exponential growth by diluting them to an A600 of 0.05, and the csrB-lacZ expression was followed (Fig. 5). It was found that the β-galactosidase activity in the wildtype decreased with time until the culture reached an A600 of ∼1.0, indicating that BarA remains silent during this period of time (Fig. 5A). On the other hand, the β-galactosidase activity in the hflKC mutant decreased significantly slower (Fig. 5), indicating that BarA retains some of its kinase activity. It can therefore be concluded that the HflKC complex binds to the BarA sensor kinase and inhibits its kinase activity during the stationary phase of growth. It should be noted that the hflKC mutant grows slightly slower than the wildtype strain (Figs. 5 and S6A), raising the possibility that the hflKC mutant could have a fitness defect. To test this, a competition between the wildtype strain (KSB837, Ampr) and the isogenic ΔhflKC mutant strain (IFC5047, Kanr) was pursued. Equal number of KSB837 and IFC5047 cells, corresponding to an A600 of 0.01 each, were inoculated into LB medium, and the number of wildtype cells (ampicillin resistant-CFUs/ml) and mutant cells (kanamycin resistant-CFUs/ml) was monitored throughout the growth curve. Under this condition, the wildtype strain outcompeted the hflKC mutant strain, becoming the predominant species in the culture as early as 100 min postincubation and reaching almost 75% of the total bacteria after 8 h (Fig. S6B). This result highlights the significance of the BarA/UvrY-Csr-HflKC regulatory loop in maintaining bacterial fitness.
Figure 5.
The BarA inhibition by the HflKC complex takes place at the stationary phase of growth. Cultures of strain KSB837 (WT) and its isogenic IFC5053 (ΔhflKC), both carrying the UvrY-P activatable csrB-lacZ reporter, were grown to the stationary phase of growth, shifted to exponential growth by diluted with fresh LB medium to an A600 of 0.05, and β-galactosidase activity and cell growth (insert) were followed for 240 min. LB, lysogeny broth.
Discussion
We previously reported that the global translational regulator CsrA is required for BarA to activate as a histidine kinase even in the presence of the physiological BarA stimulus (39) and speculated that other factors could participate in the regulation of the activity of the BarA/UvrY TCS. Here, we provide evidence that, during stationary phase of growth, the HflK and HflC proteins, whose expression was found to be regulated by CsrA, bind and recruit BarA to the cell poles, leading to its inactivation. The HflK and HflC proteins have been shown to form a heteromultimer that interacts with and negatively modulates the FtsH protease, which degrades a group of short-lived or misfolded membrane proteins (41, 52, 53). However, the inhibitory effect of the HflKC complex on BarA appears to be independent of the FtsH protease activity, because the BarA stability was not affected in a hflK hflC mutant strain. Nonetheless, it cannot be ruled out that FtsH influences the stability of other downstream regulatory components. It is of interest to mention that both the HflK and HflC proteins contain a functional SPFH domain (for Stomatin/Prohibitin/Flotillin/HflK/C), which is found in proteins that are invariant components of lipid rafts or membrane microdomains in eukaryotic and prokaryotic cells (54, 55). SPFH-containing proteins are thought to be involved in protein recruitment to the lipid rafts (54, 56) and have been found to be spatially and functionally associated with signaling pathways in bacteria (55). Indeed, HflK and HflC were recently identified in lipid raft-like membrane microdomains in E. coli (45). It is therefore tempting to speculate that the HflKC-dependent BarA recruitment to polar membrane microdomains provides the spatial context for modulating the BarA activity. This hypothesis is consistent with the fact that other protein factors, along the HflKC complex, appear to be involved in the regulation of the BarA kinase activity, since BarA is not active in a csrA hflK hflC triple mutant strain.
Nevertheless, the following physiological model (Fig. 6) can be put forward: (A) during exponential growth, BarA remains silent due to the absence of the physiological stimulus (5, 6), and CsrA exerts its regulatory functions, including inhibition of hflK and hflC expression; (B) the stimulus-dependent activation of BarA occurs at the transition to stationary phase (5, 6), leading to phosphorylation of UvrY, which initiates transcription of csrB/C sRNAs (3, 4); (C) when CsrB/C accumulate, they bind to and inhibit the regulatory functions of CsrA (13); (D) the resulting inactivation of CsrA posttranscriptionally derepress hflK and hflC expression. The accumulated HflK/C binds to BarA, recruiting it to the cell poles and causing its inactivation late in the stationary phase.
Figure 6.
Physiological model for the BarA/UvrY TCS regulation. During exponential growth (A), BarA activity (black) remains low due to the absence of the physiological stimulus and acts as a UvrY-P phosphatase, CsrB and CsrC are not transcribed, and CsrA (red) remains free and active, repressing the hflK and hflC translation (blue). At the transition from exponential to stationary phase of growth (B), the acetate concentration increases, and the BarA/UvrY TCS becomes active. C, the phosphorylated form of UvrY (UvrY-P) activates CsrB expression (green), which sequesters and inactivates CsrA (red). D, CsrA inactivation triggers HflK and HflC expression (blue), which localize in the cell poles and recruits BarA, leading to its inactivation. When cell growth resumes (A), the existing HflKC complex becomes gradually diluted, permitting the stimulus-dependent activation of BarA at late exponential growth phase.
The global regulatory role of the Csr system includes the activation of numerous genes and enzymes required for growth, such as glycolytic genes (47). Inhibition of the BarA-UvrY phosphorylation cascade by HflKC and the resulting inhibition of CsrB/C transcription will increase the availability of free CsrA in the late stationary phase of growth and should poise CsrA for rapid posttranscriptional activation of growth-supporting genes when nutrition becomes available and growth resumes. Moreover, such a feedback-loop mechanism could serve to relieve the energy burden during stationary phase of growth, since neither the BarA/UvrY phosphorylation cascade is active nor the CsrB and CsrC sRNAs are transcribed.
In summary, our results provide evidence for a novel cellular function of the HflKC complex and provide further insights into the BarA/UvrY-CsrA regulatory circuitry of E. coli. Several two component regulatory systems have been found to rely on auxiliary proteins to modulate the activity of the HK (57). On the other hand, dynamic localization patterns of bacterial sensors have been previously described (58, 59). Yet, this work reveals a regulatory mechanism that involves temporal and spatial sensor kinase dynamics controlled by an auxiliary protein complex in E. coli, reminiscent of the Caulobacter crescentus CcKA histidine kinase regulatory mechanism (59, 60).
Experimental procedures
Bacterial strains, plasmids, and culture conditions
Bacterial strains and plasmids used in this work are listed in Table S2. To construct strains IFC5043 (hflK:mCherry-Cmr) and IFC5044 (hflC:mCherry-Cmr), the hflK and hflC genes, respectively, were fused in-frame with the mCherry gene by using the lambda Red recombinase system (61, 62). Briefly, PCR-amplified DNA fragments, using primers pFluor-hflK-Fw and pKD-hflK-Rv or pFluor-hflC-Fw and pKD-hflC-Rv (the sequence of all oligonucleotides used in PCR amplification reactions are shown in Table S3) and plasmid pMXFL2 (44) as the template, were used to transform cells of the pKD46 carrying CF7789 E. coli strain, and recombinants were selected by growth on chloramphenicol-agar plates. Similarly, strains IFC5045 (ΔhflK::Kanr), IFC5046 (ΔhflC::Kanr), and IFC5047 (ΔhflKC::Kanr) were generated by lambda red recombinase-facilitated homologous recombination of PCR-amplified products using primers pair hflK70-Fw/hflK70-Rv and plasmid pKD13 (61) as template, or hflC70-Fw/hflC70-Rv and plasmid pKD13 (61) as template, or hflK70-Fw/hflC-pKD-Rv and pKD4 (61) as template, respectively. Then, the FRT-flanked Kanr cassette was removed from strains IFC5045, IFC5046, and IFC5047 using the Flp recombinase encoded by the temperature-sensitive plasmid pCP20 (63), obtaining strains IFC5048 (ΔhflK), IFC5059 (ΔhflC), and IFC5050 (ΔhflKC), respectively. The csrB-lacZ-Apr allele was transferred from KSB837 (CF7789 csrB-lacZ) into strains IFC5048 (ΔhflK), IFC5049 (ΔhflC), and IFC5050 (ΔhflKC), by P1vir transduction to obtain strains IFC5051 (ΔhflK csrB-lacZ), IFC5021 (ΔhflC csrB-lacZ), and IFC5053 (ΔhflKC csrB-lacZ), respectively. The barA::Kanr allele was transferred from strain IFC5035 (6) to IFC5053 (ΔhflKC csrB-lacZ) to obtain IFC5058 (ΔhflKC ΔbarA::Kanr csrB-lacZ). Strains IFC5054 (csrA::Kanr hflK:ha-Cmr), IFC5055 (csrA::Kanr hflC:ha-Cmr), and IFC5056 (ΔhflKC csrA::Kanr csrB-lacZ) were constructed by transfer of the csrA::Kanr allele from strain TR1-5CF7789 (4) into strain IFC5021 (hflK:ha-Cmr) (45), IFC5019 (hflC:ha-Cmr) (44), or IFC5053 (ΔhflKC csrB-lacZ), respectively, by P1vir transduction. Similarly, strain IFC5057 (ΔhflKC::Kanr cyd-lacZ) was constructed by P1vir transduction of the ΔhflKC::Kanr allele from strain IFC5047 (ΔhflKC::Kanr) into strain ECL5003 (cyd-lacZ) (64).
To construct plasmid pMX559, expressing barA under the control of the L-arabinose-inducible promoter ara, a 1.5 Kb DNA fragment containing the ara promoter and the ArcB521–778 coding sequence, obtained from plasmid pMX020 (65) by NruI and HindIII digestion, was cloned into the same restriction sites of plasmid pACT3 (66), to obtain pACT3-araP-arcB521–778. Subsequently, the barA open reading frame was isolated as a 2.8 Kb NdeI-HindIII fragment from the plasmid pUC18-barA (6) and inserted between the NdeI and HindIII sites of the above construct, resulting in the plasmid pMX559. To construct plasmid pUC19-barA-mEYFP, the mEYFP coding sequence was amplified by PCR using the primers Yfpcf1SacI and YfPcr1HindIII and plasmid pYFPC-4 (67) as the template. The PCR product was digested with SacI-HindIII and cloned between the SacI-HindIII sites of plasmid pUC19 (68), resulting in the plasmid pUC19-mEYFP. Then, the barA coding sequence was PCR amplified using the primer pair barA-NdeI-Fw/barAr1SacI and plasmid pUC18-barA as the template, and the purified PCR product was digested with NdeI and SacI and cloned into the same restriction sites of pUC19-mEYFP to obtain pUC19-barA-mEYFP. To construct plasmid pMX560, which carries a barA-mEyfp fusion under the control of the L-arabinose-inducible promoter ara, a 3.5 Kb NdeI-HindIII–restricted DNA fragment, obtained from plasmid pUC19-barA-mEYFP and containing the barA-mEyfp fusion, was used to replace the arcB551–778 coding sequence in plasmid pMX020 (65), resulting in the plasmid pMX560. To construct the low copy plasmid pMX561, expressing hflK and hflC under the control of the native hfq-hflXKC-operon promoter, a 755 bp DNA fragment was amplified by PCR using primers hfqP-Fw-Hind and hfqP-Rv and the chromosomal DNA of strain CF7789 as a template and cloned as a HindIII-BamHI-restricted DNA fragment into the same restriction sites of plasmid pEXT21 (66). The resulting plasmid was opened by digestion with NdeI and EcoRI and used to clone a DNA fragment, containing the contiguous hflK and hflC genes, which was PCR amplified by using the primer pair hflK-ORF-Fw/hflC-Rv and the chromosomal DNA of strain CF7789 as a template and subsequently digested with NdeI and EcoRI restriction enzymes, obtaining plasmid pMX561. To construct plasmids pT25BarA and pT18BarA, containing T25 and T18 N-terminal tagged BarA, respectively, the DNA sequence encoding the full-length barA was PCR-amplified by using primers DH-ACBarA-Fw and DH-ACBarA-Rv, and the chromosomal DNA of strain CF7789 as a template. The PCR product was digested with BamHI and EcoRI and cloned into the same restriction sites of plasmid pKT25 or pUT18 C to obtain pT25BarA and pT18BarA, respectively. Similarly, DNA fragments containing the hflK, hflC, or arcA coding sequence, amplified by PCR using primers DH-ACHflK-Fw and DH-ACHflK-Rv, DH-ACHflC-Fw and DH-ACHflC-Rv, or DH-ACArcA-Fw and DH-ACArcA-Rv, respectively, were cloned into the BamHI-EcoRI restriction sites of plasmid pKT25 or pUT18C to construct pT25HflK, pT25HflC, pT25ArcA, pT18HflK, pT18HflC, and pT18ArcA.
Bacteria were routinely cultured at 37 °C in LB medium. When required, the LB medium was buffered at pH 7.0 with 100 mM 3-(N-morpholino)propanesulfonic acid (Mops) and/or supplemented with kanamycin (50 μg/ml), ampicillin (100 μg/ml), or chloramphenicol (20 μg/ml). LB agar medium was prepared by addition of 1.5% (w/v) agar. For the qualitative detection of β-galactosidase activity, LB agar was supplemented with X-gal (40 μg/ml).
In vivo protein crosslinking, purification of protein complexes, and mass spectrometry
In vivo formaldehyde crosslinking was carried out as previously described (69), with slight modifications. Briefly, cells of strain IFC5035 (ΔbarA::Kanr) harboring plasmid pMX559 or pACT3 (66), as a control, were grown in 500 ml of LB medium, supplemented with kanamycin and chloramphenicol, in a rotary shaker at 37 °C, and at an A600 of 1.0, the expression of the His6-tagged BarA was induced by the addition of 0.13 mM L-arabinose. After 1 h of induction, formaldehyde was added to the cultures at a final concentration of 1% and cells were incubated for further 10 min at 37 °C. The formaldehyde was inactivated by the addition of ice-cold glycine in PBS at a final concentration of 0.125 mM, cells were incubated at 4 °C for 15 min, harvested by centrifugation at 4000g for 10 min, and the cell pellet was resuspended in 10 ml of lysis buffer (100 mM NaH2PO4, 10 mM Tris/Cl, pH 8.0, 8 M urea). The protein complexes were purified under denaturing conditions by Ni-NTA–agarose affinity chromatography, according to protocol N°17 of the QIAexpressionist manual (5th edition, Qiagen). Protein fractions were analyzed by SDS–PAGE and Coomassie blue stained to determine the visibility of large protein complexes and dialyzed against storage buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 0.1% Triton X-100). The crosslinking of the purified complexes was reversed by adding 2% SDS and heating at 95 °C for 20 min. Then, proteins were precipitated with trichloroacetic acid, pelleted by centrifugation, washed with cold acetone, and dried at room temperature for 20 min. Precipitated proteins (100 μg) were solubilized in 10 μl of water, reduced by with 2.5 μl of reduction buffer (45 mM DTT, 100 mM ammonium bicarbonate) for 30 min at 37 °C, and alkylated by adding 2.5 μl of alkylation buffer (100 mM iodoacetamide, 100 mM ammonium bicarbonate) for 20 min at 24 °C in dark. The peptide mixture was subjected to trypsin digestion and separated by LC-MS/MS, as previously described (45).
Bacterial two-hybrid assay
Protein interactions between BarA and HflK or HflC were tested by a bacterial adenylate cyclase two-hybrid assay (42). Briefly, recombinant plasmids encoding proteins of interest fused to the T25 or T18 domain of adenylate cyclase were cotransformed into E. coli BTH101 (cya−) cells (Euromedex). Five single colonies from each transformant was grown in LB, supplemented with kanamycin and ampicillin, overnight at 30 °C. Then, cells were spot plated on LB agar supplemented with X-gal, 0.5 mM isopropyl-1-thio-β-D-galactopyranoside and antibiotics, or diluted into fresh LB medium with the same antibiotics and 0.5 mM isopropyl-1-thio-β-D-galactopyranoside, and grown at 30 °C to an A600 of 0.6 for the quantitative determination of β-galactosidase activity.
Fluorescence microscopy
E. coli cells carrying either hflK-mCherry, HflK-mCherry, or barA-mEyfp were grown in LB medium at 37 °C and, at the indicated A600, 2 μl aliquots of the cell cultures were collected and immobilized on glass slides previously covered with freshly made M9 medium 1% agarose pads (70). The barA-mEyfp expression was slightly induced by adding 80 μM l-arabinose 20 min before samples were taken, and cells were immobilized. Cells were examined using a Leica DM6000B upright fluorescence microscope equipped with an oil-immersion objective lens microscope (100x/1.47). Yfp fluorescence was monitored using a K3 filter (excitation, BP 470–490 nm filter; DM510 nm; emission LP515 nm filter), and mCherry fluorescence was monitored using a N3 filter (excitation, BP 546–512 nm filter; DM564 nm; emission BL 600/40 nm filter). Fluorescence images were analyzed with ImageJ software (71) and subjected to background subtraction using a rolling ball radius of 20 pixels, and mCherry or Yfp fluorescence signals were colored in red or green, respectively.
Electrophoretic gel mobility shift assays for CsrA-RNA binding
Binding of CsrA to RNAs was determined by EMSA with recombinant CsrA-His6, purified as described previously (20), and RNA synthesized in vitro with MEGAshortscript Kit (Ambion). The template DNA for in vitro transcription of sRNAs was generated by PCR from MG1655 genomic DNA, using primers hflK Fwd T7 and hflK Rev T7. In vitro-transcribed sRNAs were gel-purified, treated with Antarctic phosphatase (NEB), and radiolabeled at the 5′ end using [γ-32P] ATP and T4 polynucleotide kinase. Binding reactions contained 0.5 nM labeled RNA, 10 mM MgCl2, 100 mM KCl, 32.5 ng total yeast RNA, 20 mM DTT, 7.5% glycerol, 4 U SUPERasin (Ambion), and various concentrations of CsrA (0–400 nM) and incubated at 37 °C for 30 min. Reaction mixtures were separated on native polyacrylamide gels using 1X TBE as the electrophoresis buffer, gels dried and imaged using a PMI phosphorimager (Bio-Rad). The signals of free (F) and shifted/bound RNA-Protein (B) complex were quantified with the help of Quantity One software (Bio-Rad). A linear regression analysis of the data was performed to calculate the apparent equilibrium binding constant (Kd) for the RNA-protein interaction (Fig. S5). Data presented in the Fig. S5 are averages of two independent experiments.
Determination of β-galactosidase activity
E. coli cells carrying the UvrY-P–activatable csrB-lacZ reporter were grown aerobically in LB adjusted to pH 7.0 and buffered with 100 mM Mops, at 37 °C. Cells carrying the ArcA-P–activatable cydA-lacZ operon fusion were grown aerobically in 10 ml of LB medium containing 100 mM Mops (pH 7.4) in 100-ml baffled flasks at 37 °C with shaking (300 rpm) or were grown anaerobically in a screw-capped tube filled with medium up to the rim at 37 °C and stirred by a magnet. Samples were withdrawn at mid-exponential growth (A600 of 0.6), and β-galactosidase activity was assayed and expressed in Miller units as described previously (72).
Western blot analysis and protein stability determination
Cultures for Western blot analyses were grown aerobically at 37 °C and harvested by centrifugation at the indicated A600. Cultures for BarA half-life determination were grown aerobically in LB medium at 37 °C, at an A600 of 2,0, 20 μg/ml tetracycline were added, samples were withdrawn, and cells were harvested by centrifugation at the indicated times. For the BarA, HflK-HA, HflC-HA, and DnaK identification and semiquantification by immunoblotting, the cell pellets were resuspended in an appropriate volume of 2X Laemmli sample buffer and boiled for 5 min. Aliquots of 10 μl were separated by SDS-PAGE (10% polyacrylamide gel), and the proteins were transferred to a Hybond-ECL filter (Amersham Biosciences). The filter was equilibrated in TTBS buffer (25 mM Tris, 150 mM NaCl, and 0.05% Tween 20) for 10 min and incubated in blocking buffer (1% milk in TTBS) for 1 h at room temperature. BarA polyclonal antibodies, raised against His6-BarA198–918 (39), monoclonal antibodies against HA (Sigma Aldrich), or monoclonal antibodies against DnaK (Enzo Life Sciences) were added at a dilution of 1:10,000 and incubated for 1 h at room temperature. The bound antibody was detected by using anti-rabbit IgG antibody or anti-mouse IgG antibody conjugated to horseradish peroxidase (1:10,000 dilution) and the Immobilon Western detection system (Millipore). Protein bands were quantified using ImageJ software (71).
Data availability
All data are contained in the article and in the Supporting information.
Supporting information
This article contains supporting information (4, 6, 10, 39, 42, 44, 45, 46, 47, 61, 64, 65, 66, 67, 68).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful to Claudia Rodriguez Rangel for technical assistance; to Ruth Rincón Heredia, of the Unidad de Imagenología of the Instituto de Fisiología Celular, for her valuable support in epifluorescence imaging; and the Unidad de Biología Molecular of the Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, for oligonucleotide synthesis and sequencing.
Author contributions
F. U. C., D. G., and T. R. conceptualization; F. U. C. and D. G. methodology; F. U. C., M. I. C., A. P., and A. F. A. investigation; D. G. and T. R. supervision; F. U. C., A. F. A., and D. G. writing–original draft; D. G. and T. R. writing–review & editing; A. F. A. and D. G. funding acquisition.
Funding and additional information
This work was supported by grants IN208721 (A. F. A.) and IN207921 (D.G.) from the Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México (UNAM), and 140614 (A. F. A.) and 514856 (D. G.) from the Consejo Nacional de Ciencia y Tecnología (CONACyT) and by the National Institutes of Health (GM059969, T. R.). The funders had no role in study design, data collection, analysis and interpretation, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Chris Whitfield
Supporting information
References
- 1.Ishige K., Nagasawa S., Tokishita S., Mizuno T. A novel device of bacterial signal transducers. EMBO J. 1994;13:5195–5202. doi: 10.1002/j.1460-2075.1994.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagasawa S., Tokishita S., Aiba H., Mizuno T. A novel sensor-regulator protein that belongs to the homologous family of signal-transduction proteins involved in adaptive responses in Escherichia coli. Mol. Microbiol. 1992;6:799–807. doi: 10.1111/j.1365-2958.1992.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 3.Pernestig A.K., Melefors O., Georgellis D. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J. Biol. Chem. 2001;276:225–231. doi: 10.1074/jbc.M001550200. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K., Wang X., Weilbacher T., Pernestig A.K., Melefors O., Georgellis D., et al. Regulatory circuitry of the CsrA/CsrB and BarA/UvrY systems of Escherichia coli. J. Bacteriol. 2002;184:5130–5140. doi: 10.1128/JB.184.18.5130-5140.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez R.G., Alvarez A.F., Romeo T., Georgellis D. The physiological stimulus for the BarA sensor kinase. J. Bacteriol. 2010;192:2009–2012. doi: 10.1128/JB.01685-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez A.F., Rodríguez C., González-Chávez R., Georgellis D. The Escherichia coli two-component signal sensor BarA binds protonated acetate via a conserved hydrophobic-binding pocket. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondragón V., Franco B., Jonas K., Suzuki K., Romeo T., Melefors O., et al. pH-dependent activation of the BarA-UvrY two-component system in Escherichia coli. J. Bacteriol. 2006;188:8303–8306. doi: 10.1128/JB.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vázquez-Ciros O.J., Alvarez A.F., Georgellis D. Identification of Z nucleotides as an ancient signal for two-component system activation in bacteria. Proc. Natl. Acad. Sci. U. S. A. 2020;117:33530–33539. doi: 10.1073/pnas.2006209117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita-Kikuta E., Kinoshita E., Eguchi Y., Koike T. Validation of cis and trans modes in multistep phosphotransfer signaling of bacterial tripartite sensor kinases by using Phos-Tag SDS-PAGE. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gudapaty S., Suzuki K., Wang X., Babitzke P., Romeo T. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherichia coli. J. Bacteriol. 2001;183:6017–6027. doi: 10.1128/JB.183.20.6017-6027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zere T.R., Vakulskas C.A., Leng Y., Pannuri A., Potts A.H., Dias R., et al. Genomic targets and features of BarA-UvrY (-SirA) signal transduction systems. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pannuri A., Vakulskas C.A., Zere T., McGibbon L.C., Edwards A.N., Georgellis D., et al. Circuitry linking the catabolite repression and Csr global regulatory systems of Escherichia coli. J. Bacteriol. 2016;198:3000–3015. doi: 10.1128/JB.00454-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M.Y., Gui G., Wei B., Preston J.F., Oakford L., Yüksel U., et al. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 1997;272:17502–17510. doi: 10.1074/jbc.272.28.17502. [DOI] [PubMed] [Google Scholar]
- 14.Weilbacher T., Suzuki K., Dubey A.K., Wang X., Gudapaty S., Morozov I., et al. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol. Microbiol. 2003;48:657–670. doi: 10.1046/j.1365-2958.2003.03459.x. [DOI] [PubMed] [Google Scholar]
- 15.Romeo T., Vakulskas C.A., Babitzke P. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ. Microbiol. 2013;15:313–324. doi: 10.1111/j.1462-2920.2012.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueroa-Bossi N., Schwartz A., Guillemardet B., D’Heygère F., Bossi L., Boudvillain M. RNA remodeling by bacterial global regulator CsrA promotes Rho-dependent transcription termination. Genes Dev. 2014;28:1239–1251. doi: 10.1101/gad.240192.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubey A.K., Baker C.S., Suzuki K., Jones A.D., Pandit P., Romeo T., et al. CsrA regulates translation of the Escherichia coli carbon starvation gene, cstA, by blocking ribosome access to the cstA transcript. J. Bacteriol. 2003;185:4450–4460. doi: 10.1128/JB.185.15.4450-4460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu M.Y., Romeo T. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J. Bacteriol. 1997;179:4639–4642. doi: 10.1128/jb.179.14.4639-4642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert M., Lapouge K., Duss O., Oberstrass F.C., Jelesarov I., Haas D., et al. Molecular basis of messenger RNA recognition by the specific bacterial repressing clamp RsmA/CsrA. Nat. Struct. Mol. Biol. 2007;14:807–813. doi: 10.1038/nsmb1285. [DOI] [PubMed] [Google Scholar]
- 20.Lai Y.J., Yakhnin H., Pannuri A., Pourciau C., Babitzke P., Romeo T. CsrA regulation via binding to the base-pairing small RNA Spot 42. Mol. Microbiol. 2022;117:32–53. doi: 10.1111/mmi.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babitzke P., Romeo T. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 2007;10:156–163. doi: 10.1016/j.mib.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Pourciau C., Lai Y.J., Gorelik M., Babitzke P., Romeo T. Diverse mechanisms and circuitry for global regulation by the RNA-binding protein CsrA. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.601352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White D., Hart M.E., Romeo T. Phylogenetic distribution of the global regulatory gene csrA among eubacteria. Gene. 1996;182:221–223. doi: 10.1016/s0378-1119(96)00547-1. [DOI] [PubMed] [Google Scholar]
- 24.Mercante J., Suzuki K., Cheng X., Babitzke P., Romeo T. Comprehensive alanine-scanning mutagenesis of Escherichia coli CsrA defines two subdomains of critical functional importance. J. Biol. Chem. 2006;281:31832–31842. doi: 10.1074/jbc.M606057200. [DOI] [PubMed] [Google Scholar]
- 25.Fortune D.R., Suyemoto M., Altier C. Identification of CsrC and characterization of its role in epithelial cell invasion in Salmonella enterica serovar Typhimurium. Infect. Immun. 2006;74:331–339. doi: 10.1128/IAI.74.1.331-339.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatt S., Edwards A.N., Nguyen H.T., Merlin D., Romeo T., Kalman D. The RNA binding protein CsrA is a pleiotropic regulator of the locus of enterocyte effacement pathogenicity island of enteropathogenic Escherichia coli. Infect. Immun. 2009;77:3552–3568. doi: 10.1128/IAI.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vakulskas C.A., Potts A.H., Babitzke P., Ahmer B.M.M., Romeo T. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol. Mol. Biol. Rev. 2015;79:193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Y., Chatterjee A., Liu Y., Dumenyo C.K., Chatterjee A.K. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-L-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 1995;177:5108–5115. doi: 10.1128/jb.177.17.5108-5115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenz D.H., Miller M.B., Zhu J., Kulkarni R.V., Bassler B.L. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol. Microbiol. 2005;58:1186–1202. doi: 10.1111/j.1365-2958.2005.04902.x. [DOI] [PubMed] [Google Scholar]
- 30.Wei B.L., Brun-Zinkernagel A.M., Simecka J.W., Pruss B.M., Babitzke P., Romeo T. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 2001;40:245–256. doi: 10.1046/j.1365-2958.2001.02380.x. [DOI] [PubMed] [Google Scholar]
- 31.Yakhnin H., Pandit P., Petty T.J., Baker C.S., Romeo T., Babitzke P. CsrA of Bacillus subtilis regulates translation initiation of the gene encoding the flagellin protein (hag) by blocking ribosome binding. Mol. Microbiol. 2007;64:1605–1620. doi: 10.1111/j.1365-2958.2007.05765.x. [DOI] [PubMed] [Google Scholar]
- 32.Romeo T., Gong M., Liu M.Y., Brun-Zinkernagel A.M. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 1993;175:4744–4755. doi: 10.1128/jb.175.15.4744-4755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabnis N.A., Yang H., Romeo T. Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA. J. Biol. Chem. 1995;270:29096–29104. doi: 10.1074/jbc.270.49.29096. [DOI] [PubMed] [Google Scholar]
- 34.Jackson D.W., Suzuki K., Oakford L., Simecka J.W., Hart M.E., Romeo T. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 2002;184:290–301. doi: 10.1128/JB.184.1.290-301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Dubey A.K., Suzuki K., Baker C.S., Babitzke P., Romeo T. CsrA post-transcriptionally represses pgaABCD, responsible for synthesis of a biofilm polysaccharide adhesin of Escherichia coli. Mol. Microbiol. 2005;56:1648–1663. doi: 10.1111/j.1365-2958.2005.04648.x. [DOI] [PubMed] [Google Scholar]
- 36.Pannuri A., Yakhnin H., Vakulskas C.A., Edwards A.N., Babitzke P., Romeo T. Translational repression of NhaR, a novel pathway for multi-tier regulation of biofilm circuitry by CsrA. J. Bacteriol. 2012;194:79–89. doi: 10.1128/JB.06209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonas K., Edwards A.N., Simm R., Romeo T., Romling U., Melefors O. The RNA binding protein CsrA controls cyclic di-GMP metabolism by directly regulating the expression of GGDEF proteins. Mol. Microbiol. 2008;70:236–257. doi: 10.1111/j.1365-2958.2008.06411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pourciau C., Pannuri A., Potts A., Yakhnin H., Babitzke P., Romeo T. Regulation of iron storage by CsrA supports exponential growth of Escherichia coli. mBio. 2019;10 doi: 10.1128/mBio.01034-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camacho M.I., Alvarez A.F., Gonzalez Chavez R., Romeo T., Merino E., Georgellis D. Effects of the global regulator CsrA on the BarA/UvrY two-component signaling system. J. Bacteriol. 2015;197:983–991. doi: 10.1128/JB.02325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banuett F., Herskowitz I. Identification of polypeptides encoded by an Escherichia coli locus (hflA) that governs the lysis-lysogeny decision of bacteriophage lambda. J. Bacteriol. 1987;169:4076–4085. doi: 10.1128/jb.169.9.4076-4085.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kihara A., Akiyama Y., Ito K. A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J. 1996;15:6122–6131. [PMC free article] [PubMed] [Google Scholar]
- 42.Karimova G., Pidoux J., Ullmann A., Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehla J., Caufield J.H., Sakhawalkar N., Uetz P. A Comparison of two-hybrid approaches for detecting protein–protein interactions. Methods Enzymol. 2017;586:333–358. doi: 10.1016/bs.mie.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzmán-Flores J.E., Alvarez A.F., Poggio S., Gavilanes-Ruiz M., Georgellis D. Isolation of detergent-resistant membranes (DRMs) from Escherichia coli. Anal. Biochem. 2017;518:1–8. doi: 10.1016/j.ab.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Guzmán-Flores J.E., Steinemann-Hernández L., González de la Vara L.E., Gavilanes-Ruiz M., Romeo T., Alvarez A.F., et al. Proteomic analysis of Escherichia coli detergent-resistant membranes (DRM) PLoS One. 2019;14 doi: 10.1371/journal.pone.0223794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubey A.K., Baker C.S., Romeo T., Babitzke P. RNA sequence and secondary structure participate in high-affinity CsrA–RNA interaction. RNA. 2005;11:1579. doi: 10.1261/rna.2990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potts A.H., Vakulskas C.A., Pannuri A., Yakhnin H., Babitzke P., Romeo T. Global role of the bacterial post-transcriptional regulator CsrA revealed by integrated transcriptomics. Nat. Commun. 2017;8:1596. doi: 10.1038/s41467-017-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonas K., Edwards A.N., Ahmad I., Romeo T., Römling U., Melefors O. Complex regulatory network encompassing the Csr, c-di-GMP and motility systems of Salmonella Typhimurium. Environ. Microbiol. 2010;12:524–540. doi: 10.1111/j.1462-2920.2009.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch A.S., Lin E.C. Transcriptional control mediated by the ArcA two-component response regulator protein of Escherichia coli: characterization of DNA binding at target promoters. J. Bacteriol. 1996;178:6238–6249. doi: 10.1128/jb.178.21.6238-6249.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malpica R., Sandoval G.R., Rodriguez C., Franco B., Georgellis D. Signaling by the arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid. Redox Signal. 2006;8:781–795. doi: 10.1089/ars.2006.8.781. [DOI] [PubMed] [Google Scholar]
- 51.Ito K., Akiyama Y. Cellular functions, mechanism of action, and regulation of FtsH protease. Annu. Rev. Microbiol. 2005;59:211–231. doi: 10.1146/annurev.micro.59.030804.121316. [DOI] [PubMed] [Google Scholar]
- 52.Kihara A., Akiyama Y., Ito K. Host regulation of lysogenic decision in bacteriophage λ: transmembrane modulation of FtsH (HflB), the cII degrading protease, by HflKC (HflA) Proc. Natl. Acad. Sci. U. S. A. 1997;94:5544–5549. doi: 10.1073/pnas.94.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma C., Wang C., Luo D., Yan L., Yang W., Li N., et al. (2022) Structural insights into the membrane microdomain organization by SPFH family proteins. Cell Res. 2021;322:176–189. doi: 10.1038/s41422-021-00598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Browman D.T., Hoegg M.B., Robbins S.M. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17:394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 55.López D., Kolter R. Functional microdomains in bacterial membranes. Genes Dev. 2010;24:1893–1902. doi: 10.1101/gad.1945010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langhorst M.F., Reuter A., Stuermer C.A.O. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell. Mol. Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buelow D.R., Raivio T.L. Three (and more) component regulatory systems - auxiliary regulators of bacterial histidine kinases. Mol. Microbiol. 2010;75:547–566. doi: 10.1111/j.1365-2958.2009.06982.x. [DOI] [PubMed] [Google Scholar]
- 58.Fu B., Sengupta K., Genova L.A., Santiago A.G., Jung W., Krzemiński Ł., et al. Metal-induced sensor mobilization turns on affinity to activate regulator for metal detoxification in live bacteria. Proc. Natl. Acad. Sci. U. S. A. 2020;117:13248–13255. doi: 10.1073/pnas.1919816117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angelastro P.S., Sliusarenko O., Jacobs-Wagner C. Polar localization of the CckA histidine kinase and cell cycle periodicity of the essential master regulator CtrA in Caulobacter crescentus. J. Bacteriol. 2010;192:539–552. doi: 10.1128/JB.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsokos C.G., Perchuk B.S., Laub M.T. A dynamic complex of signaling proteins uses polar localization to regulate cell-fate asymmetry in Caulobacter crescentus. Dev. Cell. 2011;20:329–341. doi: 10.1016/j.devcel.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uzzau S., Figueroa-Bossi N., Rubino S., Bossi L. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cherepanov P.P., Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- 64.Kwon O., Georgellis D., Lynch A.S., Boyd D., Lin E.C. The ArcB sensor kinase of Escherichia coli: genetic exploration of the transmembrane region. J. Bacteriol. 2000;182:2960–2966. doi: 10.1128/jb.182.10.2960-2966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peña-Sandoval G.R., Kwon O., Georgellis D. Requirement of the receiver and phosphotransfer domains of ArcB for efficient dephosphorylation of phosphorylated ArcA in vivo. J. Bacteriol. 2005;187:3267–3272. doi: 10.1128/JB.187.9.3267-3272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dykxhoorn D.M., St Pierre R., Linn T. A set of compatible tac promoter expression vectors. Gene. 1996;177:133–136. doi: 10.1016/0378-1119(96)00289-2. [DOI] [PubMed] [Google Scholar]
- 67.Thanbichler M., Iniesta A.A., Shapiro L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 2007;35:e137. doi: 10.1093/nar/gkm818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983;26:101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- 69.Klockenbusch C., Kast J. Optimization of formaldehyde cross-linking for protein interaction analysis of non-tagged integrin β 1. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/927585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sambrook J., Russell D.W. 3rd Ed. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 2001. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 71.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller J.H. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. β-Galactosidase assay; pp. 352–355. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained in the article and in the Supporting information.