Abstract
A late-onset Pompe disease patient developed high sustained antibody titers (HSAT) of ≥51,200 after 11+ years on alglucosidase alfa and previous tolerance. There was a corresponding worsening of motor function and rise in urinary glucose tetrasaccharide (Glc4). Following immunomodulation therapy, HSAT were eliminated with improved clinical outcomes and biomarker trends. This report highlights the importance of continued surveillance of antibody titers and biomarkers, the negative impact of HSAT, and improved outcomes with immunomodulation therapy.
Keywords: Pompe disease, Anti-drug antibody titers, Enzyme replacement therapy, Immune modulation therapy, Urine glucose tetrasaccharide (Glc4)
1. Introduction
Pompe disease (OMIM #232300), also referred to as glycogen storage disease type II, is an autosomal recessive disorder caused by a deficiency of the lysosomal enzyme acid α-glucosidase (GAA, EC 3.2.1.20). The condition is characterized by progressive myopathy and respiratory disease. The presence or absence of cardiomyopathy in the first year of life defines two broad phenotypes: infantile Pompe disease (IPD) and late-onset Pompe disease (LOPD). Patients with IPD present with cardiomyopathy while those with the late-onset form present with progressive limb-girdle and respiratory muscle weakness without cardiomyopathy. Enzyme replacement therapy (ERT) with recombinant human acid α-glucosidase (rhGAA), alglucosidase alfa, became available in 2006 with a recommended dose of 20 mg/kg every other week. In the past several years, higher doses of alglucosidase alfa have been proven to be well tolerated and can lead to improved clinical outcomes in some Pompe disease patients [[1], [2], [3]].
Even on treatment, improvement of clinical symptoms is impacted by factors such as cross-reactive immunologic material (CRIM) status, presence of high sustained antibody titers (HSAT; defined as titers of ≥12,800), stage of disease at start of treatment, and extent of muscle fiber damage [4,5]. The development of anti-drug antibody (ADA), the negative impact of ADA, and the success of immunomodulation therapy in IPD have been well recognized [[5], [6], [7], [8], [9]]. In contrast, there is no clear consensus on the challenges of immunogenicity in LOPD. Some studies have shown that LOPD patients who developed HSAT had deterioration in clinical status, whereas others have proposed that ADA does not affect the efficacy of ERT in LOPD patients [[10], [11], [12], [13], [14]].
2. Case report
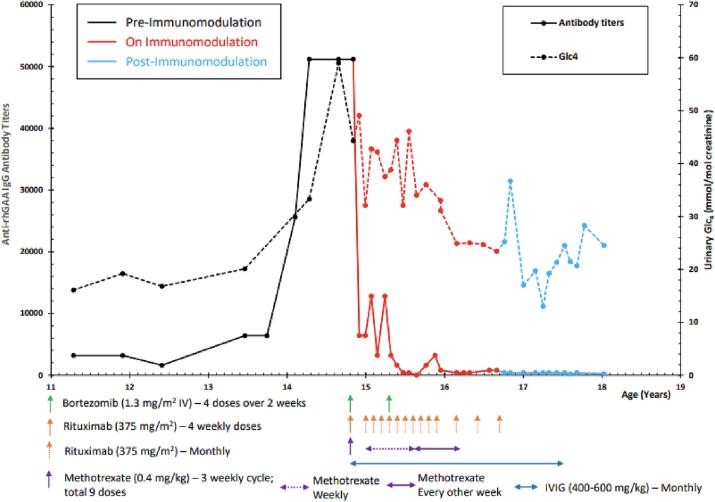
An adolescent patient with LOPD developed HSAT and clinical decline after 11+ years of alglucosidase alfa treatment. The diagnosis of Pompe disease was confirmed at age 9 months by muscle biopsy, enzyme analysis, and GAA sequence analysis, revealing compound heterozygosity for c.525delT and c.-32-13T >G, predictive of LOPD. There was absence of cardiomyopathy in the first year of life. At age 20 months, the patient began ERT with alglucosidase alfa at 20 mg/kg IV every other week (EOW) due to worsening muscle weakness and motor delay. Treatment was well tolerated with no infusion-associated reactions (IARs). Anti-rhGAA IgG antibody titers collected intermittently were negative or very low <1:800. At age 30 months, the patient began taking a few independent steps with a broad-based gait and exaggerated lumbar lordosis. At age 40 months, the ERT dose was increased to 40 mg/kg IV EOW. Significant clinical improvement and decline in urinary glucotetrasaccharide (Glc4) levels were observed without change in ADA titers. At 10 years of age, ERT was adjusted to 40 mg/kg IV weekly due to persistence of muscle weakness. There were no IARs and the patient experienced improvements in ambulation and endurance. Anti-rhGAA antibody titers remained low at <3200 (Fig. 1).
Fig. 1.
Anti-rhGAA IgG antibody level, urinary Glc4 levels and timing of immunomodulation therapy.
At 12.4 years of age, the patient underwent spinal fusion with rod insertion due to significant scoliosis impairing quality of life. The patient experienced a CSF leak post-operatively but recovered after bed rest and had no change in clinical status. At age 13.8 years, the patient experienced a steady rise of ADA titers from 6400 to 51,200 with a corresponding rise in urinary Glc4 levels (Fig. 1). Despite HSAT, the patient continued to receive alglucosidase alfa at 40 mg/kg weekly without infusion-associated reactions. A small increase in CK levels from 1400 U/L to 1700 U/L was also observed around the time of HSAT development. At age 14.7 years, the family reported a clinical plateau progressing to a clinical decline. The patient experienced a plateau in weight gain and gastrointestinal symptoms of intermittent diarrhea and feeling of bladder and bowel urgency after eating. Worsening gait and balance, decreased endurance, and increased weakness were observed in clinic. A six minute walk test (6MWT) obtained at 14.7 years noted a very unsteady gait with shoulders and arms flailing and a fall at 1 min and 45 s into the test. Prior 6MWT at age 14.1 years was completed with gait typical of LOPD patients but without stopping or falls. While no significant change in total distance walked was recorded on the two 6MWTs; the patient reported a decline in walking ability in daily life and a deterioration in his ability to climb stairs (Table 1).
Table 1.
FVCs and PT outcomes.
| Age (years) | FVC % Predicted | 6MWT (Meters) | 6MWT % predicted | GSGC (total) | QMFT | GMFM (Standing) % | GMFM (Walk/Run/Jump) % |
|---|---|---|---|---|---|---|---|
| 8.1 | 368.5 | 49.5 | 12 | 84.62 | 87.5 | ||
| 9.6 | 401.2 | 54.7 | 10 | 89.74 | 81.94 | ||
| 10.2 | 458.0 | 59.7 | 10 | 87.18 | 94.44 | ||
| 12.1 | 87 | 502.9 | 68.2 | ||||
| 12.4 | 87 | ||||||
| 13.7 | 74 | ||||||
| 13.9 | 77 | 511.8 | 62.6 | ||||
| 14.1 | 511.0 | 64.1 | 14 | 41 | |||
| 14.2 | 74 | ||||||
| 14.7 | 526.1 | 64.1 | |||||
| 15.3 | 512.6 | 63.1 | 16 | 44 | 84.62 | 91.67 | |
| 15.8 | 61 | ||||||
| 17.1 | 480.0 | 59.2 | |||||
| 17.5 | 57 | ||||||
| 18.6 | 65 | 508.0 | 62.3 | 13 | 47 | 89.7 | 95.8 |
Table 1 footnotes: pre-immunomodulation: <14.9 years, on immunomodulation: 14.9–17.4 years, post-immunomodulation: >17.4 years. Functional capacity for walking was measured using the Six Minute Walk Test (6MWT) conducted during clinical visits in accordance with American Thoracic Society guidelines [16]. The percent of normal distance walked was calculated using published formulas [17,18]. The Gait, Stairs, Gowers, Chair Test (GSGC) was used to measure motor function as previously described [19,20]; scores range from 4 to 27 with a score of 4 indicating normal function. The Quick Motor Function Test (QMFT) has scores ranging from 0 to 64 (score of 64 indicates normal function) and was developed to measure motor function in patients with Pompe disease [21]. The Gross Motor Function Measure (GMFM-88) is a test for motor skills, with scores ranging from 0 to 100%, with 100% indicating normal function [22].
At age 15.3 years, the family reported that the patient's gait was wider and that quadriceps strength was declining. On tests of motor abilities, the patient had stable to very slightly decreased performance. On the Gait, Stairs, Gower, Chair (GSGC) scale, total score worsened from 14 to 16 due to decreased performance on stair climb assessment. A small decline in performance on the Standing and Walking/Running/Jumping dimensions of the Gross Motor Function Measure (GMFM) from 87.18% to 84.62% and 94.44% to 91.67%, respectively, was observed. There was slight decline in the 6MWT distance from 526.1 m at age 14.7 years to 512.6 m at age 15.3 years and in the percent predicted from 64.1% to 63.1% (Table 1 and Supplementary Figs. 1 and 2).
Pulmonary function tests (PFTs) noted a steady decline of FVC from 87% predicted at 12.4 years to 77% predicted at 13.9 years, 74% predicted at 14.2 years, and 61% predicted at 15.8 years (Table 1). Despite the decline in FVC values, the patient reported no clinical symptoms and denied any difficulty in respiration with physical activity.
Immunomodulation therapy was initiated at age 14.9 years with bortezomib, rituximab, methotrexate, and IVIG based on a protocol previously published by Banugaria et al. (2013) (Fig. 1) [7]. Bortezomib was added to the immune modulation protocol due to its effectiveness in eliminating long lived plasma cells [7]. The patient continued weekly rhGAA at 40 mg/kg. Laboratory monitoring included CBC with differential, comprehensive metabolic profile, creatine kinase isoenzymes, lymphocyte subpopulation counts (CD3, CD4, CD8, CD19), immunoglobulin panel (IgG, IgA, IgM, IgE), urinary Glc4, and anti-rhGAA IgG antibody titers.
With the implementation of immune tolerance induction (ITI), the patient had a slow decrease of anti-rhGAA IgG antibody titer level to 3200, with fluctuation of levels between 12,800 and 3200 (Fig. 1). Given the lack of a sustained low ADA titer and minimal decline in urine Glc4 levels, a second course of bortezomib was administered with maintenance of rituximab, methotrexate, and IVIG. Prior experience of HSAT in patients with Pompe disease and mucopolysaccharidosis (MPS) II who underwent immunomodulation therapy suggested that an intensified regimen is needed to eliminate the long lived plasma cells secreting antibodies to therapeutic proteins [15]. At 8 weeks following the second course of bortezomib (age 15.6 years), the patient's ADA titers decreased to pre-HSAT levels, and have remained below 800 (Fig. 1). Since ITI treatment week 64 (age 16.1 years), urine Glc4 levels have declined to levels observed prior to development of HSAT (Fig. 1). Rituximab was administered at reduced intervals until week 94 (age 16.8 years). Methotrexate was administered weekly on day of ERT until treatment week 35 (age 15.6 years), and then every other week until week 70 (age 16.2 years). Immunoglobulin therapy was administered at reduced intervals until week 142 (age 17.4 years), when complete recovery of lymphocyte subpopulation counts, specifically CD19, was observed. The patient continued to maintain adequate humoral response to routine vaccines. No long-term side effects related to immunomodulation were observed. Based on low ADA titers in absence of immunosuppressive agents, a complete reconstitution of B cells, and adequate humoral response to vaccines, the patient was considered immune tolerant to alglucosidase alfa.
With elimination of HSAT, the patient reported that gait and endurance had returned to baseline or possibly slightly better than prior to HSAT, and that strength overall had improved. Gastrointestinal symptoms had resolved with noted weight gain. The patient had slightly improved measurement on several motor function tests, including the GSGC and GMFM-88 (Table 1). On the QMFT, the patient's score improved from 44 to 47 points, largely due to ability to lift his head off of the mat in supine and ascend stairs without a rail. On the GMFM, standing dimension improved from 84.6% to 89.7%, and Walking/Running/Jumping dimension improved from 91.7 to 95.8% (Table 1, Supplementary Figs. 1 and 2). On 6MWTs performed at 17.1 years and 18.6 years, total distance improved from 480 m to 508 m, with percent predicted increasing from 59.2% to 62.3%. On PFT, FVC continued to decline from 74% predicted at 14.2 years to 57% predicted at 17.5 years, then increased to 65% predicted at 18.7 years.
3. Discussion and conclusion
We report on the case of a patient with LOPD who developed HSAT after 11+ years on ERT. This patient had previously never mounted a significant ADA response and was considered immune tolerant to alglucosidase alfa. His ADA titers remained low with 3+ years of treatment on rhGAA dose of 40 mg/kg weekly. Immunomodulation with bortezomib, rituximab, methotrexate, and IVIG was successful in eliminating HSAT and inducing tolerance to alglucosidase alfa. Clinical improvement was noted with restitution of endurance and gait to pre-HSAT levels, and improvements in objective measures such as GSGC, GMFM, QMFT, and PFTs. Reduction in urinary Glc4 biomarker levels was observed with successful reduction of drug antibody titers. Our LOPD patient required a prolonged duration of immunomodulation treatment similar to what has been noted for other LOPD patients and reported on an MPS II patient with HSAT to idursulfase who had been on ERT for several years prior to ITI [15]. Thus it appears that patients who develop HSAT after long-term ERT may require a longer duration of immunomodulation for successful elimination of high titer antibodies than those treated early in the course of therapy [15].
This case illustrates the importance of continued surveillance of anti-rhGAA IgG antibodies and monitoring of biomarkers for Pompe disease patients on rhGAA treatment. In recent years, high-dose of ERT and long-term exposure have been suggested as pathways for immune tolerance in patients with LOPD [10,11]. The long duration and higher dose of ERT did not tolerize our patient, emphasizing the need for continued regular antibody titer and biomarker monitoring. High sustained antibody titers can impact the efficacy of ERT in LOPD patients. Our patient experienced decline in motor function with rising titer levels, with subtle changes in 6MWT, GSGC, and GMFM scores. Quantitative motor assessments at regular intervals may be needed to better capture the subtle changes in clinical progression of LOPD patients.
The factors leading to development of an immune-mediated response to alglucosidase alfa remain unclear and require further investigation. The only significant occurrence in the patient's medical history was scoliosis repair approximately 12 months prior to the rise of ADA titers. The patient otherwise remained healthy with no history of frequent infections and no history suggestive of an autoimmune process. Further studies are needed to better understand the immunogenicity in LOPD and assess the factors that may lead to a break in immune tolerance. Pompe disease patients who develop HSAT can have successful reduction of anti-drug IgG titers with immunomodulation therapy and remain on high dose rhGAA for continued clinical benefit.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2023.100981.
Appendix A. Supplementary data
Supplementary material
Data availability
The data that has been used is confidential.
References
- 1.Case L.E., Bjartmer C., Morgan C., et al. Safety and efficacy of alternative alglucosidase alfa regimens in Pompe disease. Neuromuscul. Disord. 2015;25:321–332. doi: 10.1016/j.nmd.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 2.van Gelder C.M., Poelman E., Plug I., et al. Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe disease: an open label single-center study. J. Inherit. Metab. Dis. 2016;39:383–390. doi: 10.1007/s10545-015-9912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A.A., Case L.E., Herbet M., et al. Higher dosing of alglucosidase alfa improves outcomes in children with Pompe disease: a clinical study and review of the literature. Genet. Med. 2020 Jan 6 doi: 10.1038/s41436-019-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katzin L.W., Amato A.A. Pompe disease: a review of the current diagnosis and treatment recommendations in the era of enzyme replacement therapy. J. Clin. Neuromuscul. Dis. 2008;9:421–431. doi: 10.1097/CND.0b013e318176dbe4. [DOI] [PubMed] [Google Scholar]
- 5.Kishnani P.S., Goldenberg P.C., DeArmey S.L., et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messinger Y.H., Mendelsohn N.J., Rhead W., et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet. Med. 2012;14:135–142. doi: 10.1038/gim.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banugaria S.G., Prater S.N., McGann J.K., et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet. Med. 2013;15:123–131. doi: 10.1038/gim.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazi Z.B., Desai A.K., Berrier K.L., et al. Sustained immune tolerance induction in enzyme replacement therapy-treated CRIM-negative patients with infantile Pompe disease. JCI Insight. 2017;2(14):e.94328. doi: 10.1172/jci.insight.94328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai A.K., Li C., Rosenberg A.S., Kishnani P.S. Immunological challenges and approaches to immunomodulation in Pompe disease: a literature review. Ann. Transl. Med. 2019 Jul;7(13):285. doi: 10.21037/atm.2019.05.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masat E., Laforet P., DeAntonio M., et al. Long-term exposure to Myozyme results in decrease of anti-drug antibodies in late-onset Pompe disease patients. Sci. Rep. 2016;6:36182. doi: 10.1038/srep36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kooten H.A., Ditters I.A.M., Hoogeveen-Westerveld M., et al. Antibodies against recombinant human alpha-glucosidase do not seem to affect clinical outcome in childhood onset Pompe disease. Orphanet. J. Rare Dis. 2022;17:31. doi: 10.1186/s13023-022-02175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries J.M., Kuperus K., Hoogeveen-Westervel M., et al. Pompe disease in adulthood: effects of antibody formation on enzyme replacement therapy. Genet. Med. 2017;19(1):90–97. doi: 10.1038/gim.2016.70. [DOI] [PubMed] [Google Scholar]
- 13.de Vries J.M., van der Beek N.A., Kroos M.A., et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol. Genet. Metab. 2010;101(4):338–345. doi: 10.1016/j.ymgme.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Patel T.T., Banugaria S.G., Case L.E., Wenninger S., Schoser B., Kishnani P.S. The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol. Genet. Metab. 2012;106(3):301–309. doi: 10.1016/j.ymgme.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Kim K.H., Messinger Y.H., Burton B.K. Successful reduction of high-sustained anti-idursulfase antibody titers by immune modulation therapy in a patient with severe mucopolysaccharidosis type II. Mol. Genet. Metab. Rep. 2015;2:20–24. doi: 10.1016/j.ymgmr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ATS Committee on proficiency standards for clinical pulmonary function laboratories. ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002 Jul 1;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. Erratum in: Am. J. Respir. Crit. Care Med. 2016 May 15;193(10):1185. PMID: 12091180. [DOI] [PubMed] [Google Scholar]
- 17.Li A.M., Yin J., Au J.T., So H.K., Tsang T., Wong E., Fok T.F., Ng P.C. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am. J. Respir. Crit. Care Med. 2007 Jul 15;176(2):174–180. doi: 10.1164/rccm.200607-883OC. Epub 2007 Apr 26. PMID: 17463419. [DOI] [PubMed] [Google Scholar]
- 18.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998 Nov;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. Erratum in: Am. J. Respir. Crit. Care Med. 2020 Feb 1;201(3):393. PMID: 9817683. [DOI] [PubMed] [Google Scholar]
- 19.Khan A.A., Boggs T., Bowling M., Austin S., Stefanescu M., Case L., Kishnani P.S. Whole-body magnetic resonance imaging in late-onset Pompe disease: clinical utility and correlation with functional measures. J. Inherit. Metab. Dis. 2020 May;43(3):549–557. doi: 10.1002/jimd.12190. Epub 2019 Nov 26. PMID: 31710733. [DOI] [PubMed] [Google Scholar]
- 20.Angelini C., Semplicini C., Ravaglia S., Moggio M., Comi G.P., Musumeci O., Pegoraro E., Tonin P., Filosto M., Servidei S., Morandi L., Crescimanno G., Marrosu G., Siciliano G., Mongini T., Toscano A., Italian Group on GSDII New motor outcome function measures in evaluation of late-onset Pompe disease before and after enzyme replacement therapy. Muscle Nerve. 2012 Jun;45(6):831–834. doi: 10.1002/mus.23340. (PMID: 22581536) [DOI] [PubMed] [Google Scholar]
- 21.van Capelle C.I., van der Beek N.A., de Vries J.M., van Doorn P.A., Duivenvoorden H.J., Leshner R.T., Hagemans M.L., van der Ploeg A.T. The quick motor function test: a new tool to rate clinical severity and motor function in Pompe patients. J. Inherit. Metab. Dis. 2012 Mar;35(2):317–323. doi: 10.1007/s10545-011-9388-3. Epub 2011 Sep 13. PMID: 21912959; PMCID: PMC3278629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell D.J., Rosenbaum P.L., Cadman D.T., Gowland C., Hardy S., Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev. Med. Child Neurol. 1989 Jun;31(3):341–352. doi: 10.1111/j.1469-8749.1989.tb04003.x. (PMID: 2753238) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that has been used is confidential.