Abstract
Objective
To determine whether a “test for Helicobacter pylori and treat” strategy improves symptoms in patients with uninvestigated dyspepsia in primary care.
Design
Randomised placebo controlled trial.
Setting
36 family practices in Canada.
Participants
294 patients positive for H pylori (13C- urea breath test) with symptoms of dyspepsia of at least moderate severity in the preceding month.
Intervention
Participants were randomised to twice daily treatment for 7 days with omeprazole 20 mg, metronidazole 500 mg, and clarithromycin 250 mg or omeprazole 20 mg, placebo metronidazole, and placebo clarithromycin. Patients were then managed by their family physicians according to their usual care.
Main outcome measures
Treatment success defined as no symptoms or minimal symptoms of dyspepsia at the end of one year. Societal healthcare costs collected prospectively for a secondary evaluation of actual mean costs.
Results
In the intention to treat population (n=294), eradication treatment was significantly more effective than placebo in achieving treatment success (50% v 36%; P=0.02; absolute risk reduction=14%; number needed to treat=7, 95% confidence interval 4 to 63). Eradication treatment cured H pylori infection in 80% of evaluable patients. Treatment success at one year was greater in patients negative for H pylori than in those positive for H pylori (54% v 39%; P=0.02). Eradication treatment reduced mean annual cost by $C53 (−86 to 180) per patient.
Conclusions
A “test for H pylori with 13C-urea breath test and eradicate” strategy shows significant symptomatic benefit at 12 months in the management of primary care patients with uninvestigated dyspepsia.
What is already known on this topic
Dyspepsia is a common problem in primary health care, although controversy exists about its definition
Studies of H pylori eradication in patients with uninvestigated dyspepsia have shown reduced need for endoscopy and thus significant cost savings compared with a strategy of prompt endoscopy
The “test for H pylori and treat” strategy has been recommended for uninvestigated dyspepsia, but there have been no randomised controlled trials showing improvement in symptoms
What this study adds
When given eradication treatment in primary care, H pylori positive patients with uninvestigated dyspepsia show improvement in overall dyspepsia symptoms at 12 months
This supports the “test for H pylori and treat” strategy
Introduction
Dyspepsia is a common condition that affects up to 40% of the general population and has adverse effects on quality of life.1 In Canada, 7% of visits to family practitioners are for dyspepsia.2 Most patients presenting with upper gastrointestinal symptoms in primary care are uninvestigated, and the cause of the symptoms is usually unknown. The differential diagnoses include functional dyspepsia, peptic ulcer disease, gastro-oesophageal reflux disease, and (rarely) gastric cancer. Family practitioners are comfortable treating patients without an initial diagnosis, prescribing up to 2.5 courses of empirical drug treatment before referring the patient for investigations.2 In most (up to 60%) of these patients, results of investigations are normal and the diagnosis is functional dyspepsia.3 Whether treatment to eradicate Helicobacter pylori in functional (that is, investigated) dyspepsia is beneficial has been controversial; positive and negative trials have been reported.4,5
A suggested strategy for managing uninvestigated dyspepsia is to screen patients aged under 50 without alarm symptoms with a non-invasive test for H pylori and to treat patients with positive results with drugs to eradicate H pylori.6 As this recommendation is not based on evidence from randomised controlled trials, we undertook a study to determine whether a non-invasive H pylori “test and treat” strategy in primary care for adult patients of any age with uninvestigated dyspepsia would result in improvement or cure of dyspepsia over one year.
Methods
This was a double blind placebo controlled parallel group multicentre randomised trial, performed in 36 family practitioner centres across Canada between September 1997 and April 1999. Local ethics committees approved the study protocol, and each participant gave written informed consent.
Selection of patients
Patients were eligible if they were aged 18 years or over with uninvestigated symptoms of dyspepsia for at least the previous three months. We defined dyspepsia as a symptom complex of epigastric pain or discomfort thought to originate in the upper gastrointestinal tract and including any of the following additional symptoms: heartburn, acid regurgitation, excessive burping or belching, increased abdominal bloating, nausea, feeling of abnormal or slow digestion, or early satiety.7,8 Patients with only heartburn, regurgitation, or both were considered to have a diagnosis of gastro-oesophageal reflux disease and were excluded. We also excluded patients investigated by upper gastrointestinal endoscopy, barium study, or both less than six months before randomisation or on more than two separate occasions within the preceding 10 years and patients given eradication therapy for H pylori less than six months before randomisation.
We excluded patients who had previous gastric surgery, previously documented ulcer disease or endoscopic oesophagitis, irritable bowel syndrome, or clinically significant laboratory abnormalities. We did not permit a course of treatment within 30 days before randomisation or during the treatment period with a non-steroidal anti-inflammatory drug, aspirin (>325 mg/day), antibiotic, H2 receptor antagonist, proton pump inhibitor, misoprostol, sucralfate, prokinetic agent, or bismuth compound. Women of childbearing potential had to have a negative pregnancy test at baseline and maintain effective contraception.
We performed the Helisal rapid blood test (Cortecs Diagnostics, Deeside, UK) at the pre-entry visit as an initial screening test to exclude patients negative for H pylori.9 Patients had to have both a positive Helisal test result and a positive 13C-urea breath test result before randomisation.10
Randomisation and interventions
A computer randomisation was generated in blocks of four consecutive patients and given to each centre in sealed, sequentially numbered envelopes. Active and placebo medications were identical in appearance and were packaged into blister packages placed in a sealed box by non-study personnel. The randomisation code was broken only at the end of the study after the database was locked.
We allocated patients randomly to either omeprazole 20 mg, metronidazole 500 mg, and clarithromycin 250 mg (“eradication arm”) or omeprazole 20 mg, placebo metronidazole, and placebo clarithromycin (“placebo arm”) twice daily for seven days. The follow up period was 12 months, with assessments at monthly intervals. During these clinic and telephone visits, the study coordinator interviewed the patients. We did not include these scheduled visits in the economic analysis. We repeated the 13C-urea breath test at three months and 12 months after the end of treatment to determine H pylori status. Investigators remained blinded to results of breath tests throughout the study.
During follow up, patients were managed by their family practitioners according to their usual clinical practice. Recurrent dyspepsia during follow up did not result in discontinuation from the study. Endoscopy or barium radiography was not performed at the beginning of the study but could be done during follow up at the family practitioners' discretion. Family practitioners could prescribe H pylori eradication treatment and other treatments such as H2 antagonists or proton pump inhibitors as clinically indicated. Information about drugs consumed, tests performed, and all adverse events was recorded.
Adherence to drugs
Patients were considered adherent by pill count if 12 of the 14 doses were taken during the treatment phase. No patient was withdrawn as a result of poor adherence.
Outcome measures
Global overall symptoms of dyspepsia
We assessed the global overall severity of dyspepsia symptoms over the preceding four weeks by using the following seven point Likert-type scale (GOS scale): (1) no problem; (2) minimal problem—can be easily ignored without effort; (3) mild problem—can be ignored with effort; (4) moderate problem—cannot be ignored but does not influence daily activities; (5) moderately severe problem—cannot be ignored and occasionally limits daily activities; (6) severe problem—cannot be ignored and often limits concentration on daily activities; (7) very severe problem—cannot be ignored, markedly limits daily activities, and often requires rest. This seven point scale was amended from previously validated five point and seven point scales.11,12
All enrolled patients had epigastric pain or discomfort and a symptom score of at least moderate severity (⩾4/7) over the previous month. For the primary outcome measure, we defined treatment success as a score of either 1 (none) or 2 (minimal) on the symptom scale at the final visit.13 As secondary outcome measures, we determined the proportion of patients becoming completely asymptomatic and treatment success according to H pylori status.
Other symptoms and subgroups of dyspepsia
At each visit, patients were asked to rate the severity of specific symptoms of dyspepsia over the previous month with the same seven point scale as for global overall symptoms. We carried out retrospective analysis of treatment success for patients with reflux predominant symptoms compared with those for whom the reflux symptoms were not predominant (non-reflux predominant).
Quality of life questionnaire
We assessed quality of life by using the validated, self administered quality of life in reflux and dyspepsia (QOLRAD) instrument.14 This disease specific instrument uses a seven point Likert-type scale in which higher scores indicate better quality of life. Results are reported as average change in each of five dimensions.
Gastrointestinal symptom rating scale questionnaire
The gastrointestinal symptom rating scale (GSRS) questionnaire is a well validated and self administered instrument. It includes 15 questions on different gastrointestinal symptoms, with a seven point Likert-type scale in five dimensions.15 The severity of symptoms reported increases with decreasing score.
Dyspepsia related health utilisation costs
Our objective was to compare the mean annual cost of H pylori eradication treatment with that of placebo. Study personnel measured dyspepsia related use of health resources prospectively at monthly intervals by telephone and clinic interviews with a health resource utilisation questionnaire. Direct costs included visits to the physician (specialist, family physician) and other healthcare professionals, drugs (prescription, over the counter), and investigations (for example, laboratory tests, radiography, endoscopy). Indirect costs of decreased productivity as a consequence of days lost through dyspepsia took into consideration whether the patient was employed, unemployed, or a senior citizen (aged over 65) and were calculated from Canadian labour force and unpaid work estimates.16,17 We calculated the cost for each health resource from the frequency of resources consumed and their unit prices. We aggregated indirect and direct costs (Province of Ontario, Canada, Ministry of Health perspective) to determine the societal perspective. Because of the duration of the study, we did not discount costs.
Eradication of H pylori
We calculated the proportion of patients in whom H pylori was eradicated on the basis of the result of the urea breath test at 12 months or, in the case of a missing 12 month value, the result at three months.
Determination of sample size
We based calculations on estimates of the difference in rates of treatment success between treatments. The assumed treatment success rate was 39% for the eradication arm and 20% for the placebo arm. In order to achieve a two tailed significance level of 0.05 and a power of 90%, we needed 120 evaluable patients in each arm. To allow for a maximum dropout rate of 25%, we needed 150 patients per arm.
Statistical evaluation
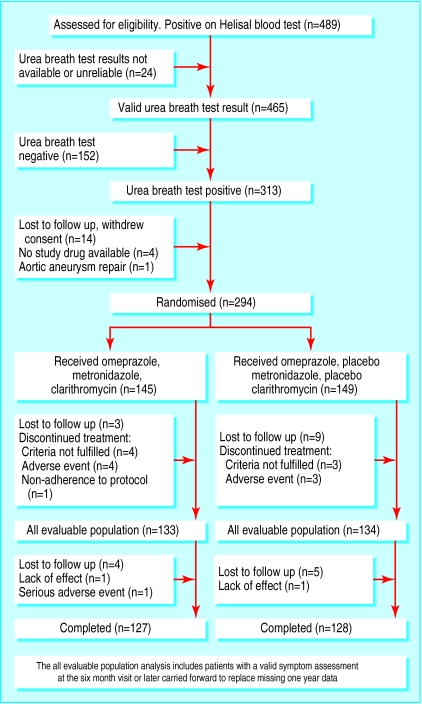
The intention to treat analysis included all randomised patients. Patients who discontinued at any time were considered treatment failures. We undertook a more clinically applicable analysis—“all evaluable patients”— in those patients who had data on symptoms at the 6-12 month assessments (figure). We carried data forward from six months and beyond to replace missing 12 month data. We used the Cochran-Mantel-Haenszel test to compare proportions of success by treatment group.
The main objective of the economic analysis was to measure and describe the costs per patient over the year of the study. As costs were not normally distributed, we used corrected α percentile bootstrap methods to measure mean costs per patient.18,19
Results
The disposition of patients enrolled and randomised into the study is shown in the figure. Of patients with positive Helisal test results, 152 (33%) had a negative 13C-urea breath test result. A total of 294 patients were randomised, and the two groups were well matched (table 1).
Table 1.
Baseline demographic characteristics of randomised patients (intention to treat). Values are numbers (percentages) unless stated otherwise
| Characteristic | Eradication group (n=145) | Placebo group (n=149) |
|---|---|---|
| Male | 69 (48) | 79 (53) |
| White | 128 (88) | 139 (93) |
| Mean age in years (range) | 50 (18-82) | 49 (19-81) |
| Current smoker | 42 (29) | 50 (34) |
| Consumer of alcohol | 83 (57) | 93 (62) |
| Previous Helicobacter pylori eradication treatment | 4 (3) | 1 (1) |
| Mean (SD) global overall symptom score (GOS) at presentation | 4.8 (0.8) | 4.9 (0.9) |
| Mean (maximum) years since first onset of dyspepsia | 10 (66) | 11 (57) |
| Adherent to drugs (⩾12 of 14 doses) | 138 (95) | 145 (97) |
The proportion of patients who were considered a treatment success was significantly greater for the eradication arm than for the placebo arm, with comparable results in the intention to treat and all evaluable patients analyses (table 2). The number needed to treat to achieve one treatment success in the eradication arm was 7 (95% confidence interval 4 to 63). A significant benefit for the eradication arm was also seen when we used the most stringent endpoint of defining only completely asymptomatic patients as responders. The treatment responses in patients with reflux predominant dyspepsia and non-reflux predominant dyspepsia were of the same order of magnitude as for the overall groups (table 2).
Table 2.
Treatment outcomes at 12 months
| Treatment | No of patients responding | Response rate (% (95% CI)) |
|---|---|---|
| Treatment success (GOS 1 or 2)—intention to treat | ||
| Eradication group (n=145) | 72 | 50 (42 to 58) |
| Placebo group (n=149) | 54 | 36 (28 to 44) |
| Difference | 14 (2 to 25), P=0.02* | |
| Treatment success (GOS 1 or 2)—all evaluable patients | ||
| Eradication group (n=133) | 72 | 54 (46 to 63) |
| Placebo group (n=134) | 54 | 40 (32 to 49) |
| Difference | 14 (1 to 26), P=0.03* | |
| Patients completely asymptomatic (GOS=1)—intention to treat | ||
| Eradication group (n=145) | 41 | 28 (21 to 36) |
| Placebo group (n=149) | 22 | 15 (9 to 20) |
| Difference | 13 (4 to 24), P=0.008* | |
| Treatment success of reflux predominant dyspepsia subgroup—intention to treat | ||
| Eradication group (n=54) | 23 | 43 (29 to 56) |
| Placebo group (n=53) | 17 | 32 (20 to 45) |
| Difference | 11 (NT) | |
| Treatment success of non-reflux predominant dyspepsia subgroup—intention to treat | ||
| Eradication group (n=91) | 49 | 54 (44 to 64) |
| Placebo group (n=96) | 37 | 39 (29 to 48) |
| Difference | 15 (NT) | |
GOS=global overall symptom score; NT=not tested.
Statistical comparison by Cochran-Mantel-Haenszel test.
The distribution of ulcer-like, dysmotility-like, and reflux-like dyspepsia subgroups was similar in both groups: 131 (90%), 76 (52%), and 122 (84%) in the eradication group (n=145) and 134 (90%), 93 (62%), and 129 (87%) in the placebo group (n=149). The subgroups showed considerable overlap, and only 29 (<10%) patients were in one category only. All dyspepsia subgroups showed a trend towards greater treatment success in the eradication arm than in the placebo arm (49% (64/131) v 36% (48/134) for ulcer-like dyspepsia, 39% (30/76) v 29% (27/93) for dysmotility-like dyspepsia, and 49% (60/122) v 36% (46/129) for reflux-like dyspepsia).
In multiple logistic regression analysis including age, sex, and treatment as predictors, only eradication treatment was significantly (P=0.009) associated with treatment success.
Results according to H pylori status
H pylori was eradicated in 75% (109/145) of the patients in the eradication arm and in 14% (21/149) of those in the placebo arm in the intention to treat population. During follow up, a second course of H pylori eradication treatment resulted in eradication in only 2 of 11 treated patients in the eradication arm compared with 15 of 23 treated patients in the placebo arm. The evaluable eradication rate in patients who received only the initial course of study treatment was 80% (107/134) in the eradication arm and 4.4% (6/136) in the placebo arm. In secondary analysis, patients who had H pylori eradicated had a treatment success rate of 54% (69/127; 95% confidence interval 45% to 63%) compared with 39% (54/137; 31% to 48%) in those who remained H pylori positive. For individual symptoms, eradication of H pylori also relieved epigastric pain or discomfort and belching symptoms but not heartburn, regurgitation, bloating, nausea, early satiety, or postprandial fullness (data not shown).
Quality of life assessments
Table 3 shows the impact of eradication treatment on disease specific measures of quality of life. The difference in the change in scores from pretreatment to study end showed significantly greater improvement in three of the five domains for the eradication arm. The gastrointestinal symptom rating scale assessment showed a significant change at 12 months in the eradication arm for the constipation dimension only (data not shown).
Table 3.
Change in quality of life measured with quality of life in reflux and dyspepsia instrument (QOLRAD)
| Domain | Mean difference in change in quality of life (eradication arm−placebo arm)* | Range | P value |
|---|---|---|---|
| Emotional distress | 0.34 | 0.04-0.65 | 0.03 |
| Sleep disturbance | 0.18 | −0.10-0.46 | 0.21 |
| Problems with eating or drinking | 0.20 | −0.10-0.50 | 0.20 |
| Physical and social functioning | 0.25 | 0.01-0.48 | 0.04 |
| Vitality | 0.39 | 0.08-0.70 | 0.02 |
A positive value indicates greater symptom improvement in the eradication arm.
Health resource utilisation
Table 4 shows selected values for direct and indirect costs. The mean total annual costs from the perspectives of society and the Ontario Ministry of Health were lower for the eradication arm than the placebo arm, although the differences were not significant (table 5). Few patients had endoscopy or upper gastrointestinal barium examination in the follow up year (table 6). The increased costs for patients randomised to placebo were primarily incurred through increased visits to the physician and drugs for dyspepsia (table 6). The proportion of patients needing additional prescriptions was 50% (73/145) in the eradication arm and 58% (87/149) in the placebo arm. The total number of prescriptions for dyspepsia was also higher in the placebo arm than in the eradication arm (75 v 67 for proton pump inhibitors, 117 v 56 for H2 antagonists, 19 v 12 for prokinetic agents).
Table 4.
Selected values for direct and indirect costs
| Item | Costs ($C)* |
|---|---|
| Drugs†: | |
| Omeprazole 20 mg | 2.20 per tablet |
| Clarithromycin 250 mg | 1.48 per tablet |
| Metronidazole 500 mg | 0.056 per tablet |
| Hospital cost‡ | 432.05 per day |
| Visits to doctor§: | |
| Gastroenterologist | First visit 106.95, subsequent 23.45 |
| Surgeon | First visit 55.90, subsequent 19.20 |
| Visit to nurse¶ | 37.27 per visit |
| Endoscopy§ (physician charge) | 94.60 |
| Upper gastrointestinal barium meal§ (physician charge) | 84.85 |
| 13C-urea breath test** | 80.00 |
| Laboratory tests (selected)‡‡: | |
| Full blood count | 8.77 per test |
| Creatinine | 2.74 per test |
| Blood sugar | 1.88 per test |
| Helisal rapid whole blood test | 22.00 per test |
| Lost productivity17: | |
| Men aged 20-65 | 79.39 per day |
| Men aged >65 | 19.27 per day |
| Women aged 20-65 | 73.84 per day |
| Women aged >65 | 21.61 per day |
1 $C≈0.60 US$≈£0.43
Ontario Drug Benefit Formulary/Comparative Drug Index. Ontario Ministry of Health 35, Toronto, Canada, 1999. (Non-prescription drug costs were determined from the Medis Health and Pharmaceutical Services Inc Distributing Catalogue, Montreal, Canada, 1999.)
Canadian Coordinating Office for Health Technology Assessment (CCOHTA). A Manual of Standard Costs for Pharmacoeconomic Studies in Canada: Feasibility Study. Ottawa, Canada, 1995. (www.ccohta.ca)
OHIP Schedule of Benefits: Physician Services Under the Health Insurance Act, 1999. Toronto, Canada.
Ontario Ministry of Health. System-Linked Research Unit. Approach to the Measurement of Costs (Expenditures) when Evaluating Health and Social Programmes. 1995. McMaster University, Hamilton, ON, Canada.
MDS Laboratories charge, Ontario, Canada.
Ontario Ministry of Health. OHIP Schedule of Laboratory Services. 1999. Ontario, Canada.
Table 5.
Mean (range) total costs to society and the Ministry of Health in $C by treatment arm (intention to treat population)
| Treatment arm | No of patients | Societal cost* | Ministry of Health cost† |
|---|---|---|---|
| Eradication | 142 | 477 (27-3069) | 136 (0-1066) |
| Placebo | 146 | 530 (31-3315) | 181 (0-1860) |
1 $C≈0.60 US$≈£0.43.
Difference in cost $C53 (95% CI −$C86 to $C180).
Difference in cost $45 (−$20 to $114).
Table 6.
Main events counted to estimate use of resources over the one year follow up
| Eradication group (No of events) | Eradication costs ($C) | Placebo group (No of events) | Placebo costs ($C) | |
|---|---|---|---|---|
| Admissions to hospital for stomach problems | 1 | 432 | 6 | 2 592 |
| Visits to family practitioner | 120 | 2 186 | 150 | 2 787 |
| Visits to specialist (surgeon or gastroenterologist) | 24 | 1 631 | 32 | 2 033 |
| Upper gastrointestinal barium study | 13 | 1 103 | 14 | 1 188 |
| Upper gastrointestinal endoscopy | 11 | 1 041 | 16 | 1 514 |
| Cost of prescription drugs for dyspepsia* | 179 prescriptions (73 patients) | 25 816 | 299 prescriptions (87 patients) | 38 974 |
| Cost of non-prescription drugs for dyspepsia† | – | 3 527 | – | 4 486 |
| Laboratory tests | 24 | 714 | 36 | 1 254 |
| Days of work missed | 263 (30 patients) | 16 910 | 226 (24 patients) | 13 200 |
| Other‡ | 53 | 2 138 | 61 | 2 663 |
1 $C≈0.60 US$≈£0.43.
Costs include drug treatment at start of study; costs taken from a log of gastrointestinal medications; includes antibiotics given for repeat Helicobacter pylori eradication treatment during the study.
Cost of non-prescription drugs paid by the patient as reported in the questionnaire; the number and types of drugs taken were not captured.
Includes visits to a nurse, imaging studies (abdominal and chest radiography, ultrasonography of abdomen and pelvis, computed tomography of abdomen, barium enema), sigmoidoscopy, one colonoscopy, and transportation costs.
Adverse events
The population consisted of all 294 randomised patients. Sixty one (42%) patients in the eradication arm and 62 (42%) patients in the placebo arm reported at least one adverse event. Diarrhoea, headache, increased abdominal pain, nausea, flatulence, and taste perversion were the most common events reported. One patient in the eradication arm stopped treatment owing to a skin rash. In the placebo arm, two patients stopped their pills because of adverse events: one had crampy abdominal pain and loose bowel movements, and the other had epigastric pain. Minor elevations of liver enzymes (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) occurred more often in the eradication group than in the placebo group, and all resolved within two to four weeks after the end of treatment.
Two deaths occurred during the study, both in the eradication arm. The first patient was diagnosed with metastatic brain cancer (primary tumour unknown) 10 months into the follow up phase and died before the 12 month visit. The second patient was a 69 year old man who was admitted to hospital with worsening dysphagia three months into follow up. He had no alarm symptoms at entry to the study. Investigations revealed inoperable oesophageal cancer, and the patient died one month later.
Discussion
H pylori is known to cause duodenal ulcers and gastric ulcers and is linked to gastric cancer20 and MALToma (mucosal associated lymphoid tumour),21 but its association with dyspepsia remains unclear. Most studies of H pylori and dyspepsia have been done in patients with functional (that is, investigated) dyspepsia. Meta-analyses of these trials have shown either no benefit from eradication of H pylori 5 or at best a small benefit with a number needed to treat of 15.4
Patients do not present to the family physician with an identified cause for their dyspepsia, as they are uninvestigated at first presentation. They may have functional dyspepsia or diseases such as peptic ulcer or gastro-oesophageal reflux disease. Unfortunately, symptoms do not reliably predict endoscopic findings or allow reliable diagnosis.3 The Rome definition of dyspepsia considers the symptoms of heartburn and acid regurgitation to be synonymous with gastro-oesophageal reflux disease and not part of the symptom complex of dyspepsia,22 but it is well known that most patients have multiple, overlapping symptoms,1,23 as we confirmed in this study. Even among patients with proved peptic ulcers, 28% can have heartburn or acid reflux as the predominant presenting symptom.24 Therefore, a definition of dyspepsia that excludes reflux symptoms does not fit the conceptual framework of family physicians, and we believe that these symptoms form part of the symptom complex of dyspepsia.2,8
Effect of H pylori eradication on symptoms of dyspepsia
Our study showed consistent results in favour of eradication of H pylori for most outcome measures, including global improvement (to mild or no symptoms) and complete resolution of dyspepsia, improvement in several specific symptoms (epigastric pain or discomfort, belching), and improvement in some aspects of quality of life. The number needed to treat to achieve one treatment success was 7 (4 to 63). The 14% clinical gain observed in this study may be attributable to the expected proportion of 5-15% of H pylori positive patients with a true ulcer diathesis.25 This is speculative, as we did not perform endoscopy at the beginning of the study. Patients in whom H pylori was eradicated had better relief of symptoms than those in whom infection persisted, which is consistent with the hypothesis that H pylori is responsible for dyspepsia in some patients.
Although extensive overlap of symptoms makes it impossible to completely exclude patients with gastro-oesophageal reflux disease, we excluded patients with reflux disease previously diagnosed by endoscopy or 24 hour oesophageal pH study and patients with symptoms of only heartburn or acid regurgitation without epigastric pain or discomfort. Studies in patients with reflux disease who test positive for H pylori show that eradication of H pylori either does not affect the subsequent clinical course of gastro-oesophageal reflux disease26 or may worsen it. Inclusion of such patients in our study would have biased the results towards no effect. In this study, we saw a trend towards improvement and not worsening of dyspepsia in patients with predominant reflux symptoms (not statistically powered for these comparisons). These results are in keeping with a study in patients with peptic ulcers and concomitant reflux oesophagitis, in which symptoms improved after eradication of H pylori.24 Our data thus suggest that a proportion of patients with uninvestigated dyspepsia with predominant reflux symptoms and epigastric pain or discomfort benefit from treatment to eradicate H pylori, and our results are robust and generalisable to primary care.
Diagnosis and eradication of H pylori
Thirty three per cent of patients who were positive for H pylori by whole blood screening had a negative 13C- urea breath test. Thus whole blood testing is unreliable for use in a “test and treat” strategy, and we recommend the more accurate 13C-urea breath test as the diagnostic method of choice.27
The 80% H pylori eradication rate in this study is consistent with eradication rates achieved with omeprazole-metronidazole-clarithromycin in the community.28 The treatment was well tolerated, and adherence was high. The frequency of adverse events was similar in both arms of the study, and most were minor. In this study, one patient (age 69) was diagnosed with oesophageal cancer three months after inclusion. At the time of randomisation, alarm symptoms (particularly dysphagia) were absent. We believe it is unlikely that earlier endoscopy could have prevented this patient's death.
Treatment guidelines
Most dyspepsia guidelines recommend investigations in patients over 50.6,8,29 We agree that endoscopy should be considered in patients at an earlier age in areas with high prevalence of gastric cancer.30 However, in Canada, gastric cancer has steadily declined over the past 40 years. Our study and the recently reported Canadian adult dyspepsia empiric treatment—prompt endoscopy (CADET-PE) study were not restricted in age. No cases of gastric cancer occurred in 1040 patients with uninvestigated dyspepsia in the prompt endoscopy study.31 Although these findings are suggestive, adequately powered studies are needed to determine whether an age limit of over 50 is safe in patients with uninvestigated dyspepsia.
Economic analysis
The cost analysis shows benefits in favour of eradication of H pylori, although the differences were not statistically significant. The study was not powered to detect economic differences. The cost data do, however, provide another justification to advocate the “test for H pylori and treat” strategy. As the time horizon for this study was only one year, economic benefits would be expected to increase over time for patients cured of their dyspepsia. Nevertheless, it is important to keep in mind that at least half of patients will need further prescriptions for dyspepsia after anti-H pylori treatment. We have done further economic modelling and analyses, which support the view that treatment to eradicate H pylori is cost effective.32
Conclusion
This primary care study has shown that the “test with 13C-urea breath test and treat to eradicate H pylori” strategy in patients with uninvestigated dyspepsia provides long term relief from symptoms and may reduce healthcare costs.
Figure.
Flow of participants through the study
Acknowledgments
We thank Joanna Lee, AstraZeneca Canada, for statistical work. We also acknowledge the assistance of the other members of the CADET Summary Group: Alan Thomson, Alan Barkun, and David Armstrong. The CADET-Hp Study Group of principal investigators are G Achyuthan, Regina; D Barr, London; K Bayly, Saskatoon; W Booth, Antigonish; M Cameron, Regina; S Cameron, Halifax; H S Conter, Halifax; S J Coyle, Winnipeg; B N Craig, Saint John; R K Dunkerley, London; J Hii, Vancouver; W P House, Vancouver; E Howlett, Saskatoon; F F Jardine, Manuels; D Johnson, Winnipeg; K Kausky, Whistler; H Langley, Kingston; K R Loader, Brandon; P V Mayer, Kingston; D M McCarty, Edmonton; S Moulavi, Montreal; M Murty, Orleans; W O'Mahony, Corunna; P O'Shea, St John's; G Pannozzo, Waterloo; J Price, Portage La Prairie; P Sackman, Calgary; C L Sanderson-Guy, Nepean; K Saunders, Winnipeg; D Shu, Coquitlam; RJ Smith, Mount Pearl; T Tobin, Guelph; G R Webb, Grand Bay; P Whitsitt, Oshawa; W Winzer, Orleans; and P Wozniak, Cambridge.
Footnotes
Funding: The study was financially supported by AstraZeneca Canada Inc.
Competing interests: NC and SJOVvanZ have acted as consultants and have received research support and honorariums for giving talks on this subject by the sponsor, AstraZeneca Canada, who manufacture omeprazole. PS and RAF are former employees of AstraZeneca Canada, and SE and EG are current employees of AstraZeneca Canada (sponsors of the study).
References
- 1.Tougas G, Chen Y, Hwang P, Liu MM, Eggleston A. Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the DIGEST study. Am J Gastroenterol. 1999;94:2845–2854. doi: 10.1111/j.1572-0241.1999.01427.x. [DOI] [PubMed] [Google Scholar]
- 2.Chiba N, Bernard L, O'Brien BJ, Goeree R, Hunt RH. A Canadian physician survey of dyspepsia management. Can J Gastroenterol. 1998;12:83–90. doi: 10.1155/1998/175926. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Silverstein MD, Agreus L, Nyren O, Sonnenberg A, Holtmann G. AGA technical review: evaluation of dyspepsia. Gastroenterology. 1998;114:582–595. doi: 10.1016/s0016-5085(98)70542-6. [DOI] [PubMed] [Google Scholar]
- 4.Moayyedi P, Soo S, Deeks J, Forman D, Mason J, Innes M, et al. Systematic review and economic evaluation of Helicobacter pylori eradication treatment for non-ulcer dyspepsia. BMJ. 2000;321:659–664. doi: 10.1136/bmj.321.7262.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with nonulcer dyspepsia. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;134:361–369. doi: 10.7326/0003-4819-134-5-200103060-00008. [DOI] [PubMed] [Google Scholar]
- 6.Hunt RH, Fallone CA, Thomson AB. Canadian Helicobacter pylori consensus conference update: infections in adults. Can J Gastroenterol. 1999;13:213–217. doi: 10.1155/1999/180751. [DOI] [PubMed] [Google Scholar]
- 7.Chiba N. Definitions of dyspepsia: time for a reappraisal. Eur J Surg. 1998;164(suppl 583):14–23. doi: 10.1080/11024159850191184. [DOI] [PubMed] [Google Scholar]
- 8.Veldhuyzen van Zanten SJ, Flook N, Chiba N, Armstrong D, Barkun A, Bradette M, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. CMAJ. 2000;162(suppl 12):S3–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Moayyedi P, Carter AM, Catto A, Heppell RM, Grant PJ, Axon AT. Validation of a rapid whole blood test for diagnosing Helicobacter pylori infection. BMJ. 1997;314:119. doi: 10.1136/bmj.314.7074.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mock T, Yatscoff R, Foster R, Hyun JH, Chung IS, Shim CS, et al. Clinical validation of the Helikit: a 13C urea breath test used for the diagnosis of Helicobacter pylori infection. Clin Biochem. 1999;32:59–63. doi: 10.1016/s0009-9120(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 11.Veldhuyzen van Zanten SJO, Tytgat KMAJ, Pollak PT, Goldie J, Goodacre RL, Riddell RH, et al. Can severity of symptoms be used as an outcome measure in trials of non-ulcer dyspepsia and Helicobacter pylori associated gastritis? J Clin Epidemiol. 1993;46:273–279. doi: 10.1016/0895-4356(93)90075-c. [DOI] [PubMed] [Google Scholar]
- 12.Junghard O, Lauritsen K, Talley NJ, Wiklund IK. Validation of 7-graded diary cards for severity of dyspeptic symptoms in patients with non-ulcer dyspepsia. Eur J Surg. 1998;164(suppl 583):106–111. doi: 10.1080/11024159850191355. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clin Trials. 1989;10:407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 14.Wiklund IK, Junghard O, Grace E, Talley NJ, Kamm M, Veldhuyzen van Zanten SJO, et al. Quality of life in reflux and dyspepsia patients. Psychometric documentation of a new disease-specific questionnaire (QOLRAD) Eur J Surg. 1998;164(suppl 583):41–49. [PubMed] [Google Scholar]
- 15.Svedlund J, Sjodin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 16.Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. New York: Oxford Medical Publications, Oxford University Press; 1997. Cost benefit analysis; pp. 205–231. [Google Scholar]
- 17.Labour force and unpaid work of Canadians: selected labour force, demographic, cultural, educational and income characteristics by sex (based on the 1991 standard occupational classification) for Canadian provinces, territories and CMAs, 1996 census (20% sample data). Ottawa, Canada: Statistics Canada; 1996. [Google Scholar]
- 18.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320:1197–1200. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinod HD. Bootstrap methods: applications in econometrics. In: Maddala GS, Rao CR, Vinod HD, editors. Handbook of statistics 11: econometrics. Elsevier Science, Chapman and Hall, CRC, 1993. pp. 629–661. [Google Scholar]
- 20.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 21.Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, et al. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection [see comments] Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 22.Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, Tytgat GN. Functional gastroduodenal disorders. Gut. 1999;45(suppl 2):II37–42. doi: 10.1136/gut.45.2008.ii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tytgat GN. GERD remains an intriguing enigma. Gastroenterology. 2001;120:787. doi: 10.1053/gast.2001.23052. [DOI] [PubMed] [Google Scholar]
- 24.McColl KEL, Dickson A, El-Nujumi A, el-Omar E, Kelman A. Symptomatic benefit 1-3 years after H. pylori eradication in ulcer patients: impact of gastroesophageal reflux disease. Am J Gastroenterol. 2000;95:101–105. doi: 10.1111/j.1572-0241.2000.01706.x. [DOI] [PubMed] [Google Scholar]
- 25.Laine L. Helicobacter pylori and complicated ulcer disease. Am J Med. 1996;100:52–9S. doi: 10.1016/s0002-9343(96)80229-4. [DOI] [PubMed] [Google Scholar]
- 26.Moayyedi P, Bardhan C, Young L, Dixon MF, Brown L, Axon AT. Helicobacter pylori eradication does not exacerbate reflux symptoms in gastroesophageal reflux disease. Gastroenterology. 2001;121:1120–1126. doi: 10.1053/gast.2001.29332. [DOI] [PubMed] [Google Scholar]
- 27.Chiba N, Veldhuyzen van Zanten SJ. 13C-Urea breath tests are the noninvasive method of choice for Helicobacter pylori detection. Can J Gastroenterol. 1999;13:681–683. [PubMed] [Google Scholar]
- 28.Chiba N, Marshall CP. Omeprazole once or twice daily with clarithromycin and metronidazole for Helicobacter pylori. Can J Gastroenterol. 2000;14:27–31. doi: 10.1155/2000/916417. [DOI] [PubMed] [Google Scholar]
- 29.Malfertheiner P.on behalf of the European Helicobacter Pylori Study Group (EHPSG). Current European concepts in the management of Helicobacter pylori infection. The Mäastricht consensus report Gut 1997418–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam SK, Talley NJ. Report of the 1997 Asia Pacific consensus conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:1–12. doi: 10.1111/j.1440-1746.1998.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomson ABR, Armstrong D, Barkun AN, Chiba N, Veldhuyzen van Zanten SJO, Daniels S, et al. Is prompt endoscopy necessary in uninvestigated dyspeptics? Prevalence of upper gastrointestinal abnormalities—the CADET-PE study. Gastroenterology. 2001;120;(suppl 1):A50–A51. [Google Scholar]
- 32.Chiba N, Veldhuyzen van Zanten SJO, Grace EM, Sinclair P, Simons WR, Lee JSM. The cost-effectiveness of a test and treat approach in primary care patients. Gut. 2000;47(suppl 1):A113–A114. [Google Scholar]