Summary
Skeletal muscle function and regenerative capacity decline during aging, yet factors driving these changes are incompletely understood. Muscle regeneration requires temporally coordinated transcriptional programs to drive myogenic stem cells to activate, proliferate, fuse to form myofibers, and to mature as myonuclei, restoring muscle function after injury. We assessed global changes in myogenic transcription programs distinguishing muscle regeneration in aged mice from young mice by comparing pseudotime trajectories from single-nucleus RNA sequencing of myogenic nuclei. Aging-specific differences in coordinating myogenic transcription programs necessary for restoring muscle function occur following muscle injury, likely contributing to compromised regeneration in aged mice. Differences in pseudotime alignment of myogenic nuclei when comparing aged with young mice via dynamic time warping revealed pseudotemporal differences becoming progressively more severe as regeneration proceeds. Disruptions in timing of myogenic gene expression programs may contribute to incomplete skeletal muscle regeneration and declines in muscle function as organisms age.
Keywords: myonuclei, pseudotime, MuSC, differentiation, skeletal muscle regeneration, aging, single-nucleus RNA sequencing, regeneration, cell fate
Highlights
-
•
Comparison of pre- and post-fusion nuclei during regeneration in young and aged mice
-
•
Quantified altered gene expression timing in aged mice during muscle regeneration
-
•
Timing of myogenic gene networks are disrupted in aged mice compared with young mice
-
•
In aging, disrupted gene expression timing is exacerbated as regeneration proceeds
In this article, Olwin, Dowell, and colleagues identify pervasive changes in gene expression timing during skeletal muscle regeneration occurring in aged mice compared with young mice. These changes in timing of gene expression networks, as identified via single-nucleus RNA sequencing and quantified with in-depth bioinformatic analyses, likely contribute to aging muscle phenotypes.
Introduction
Skeletal muscle is critical for overall health. Deteriorating skeletal muscle function and reduced regeneration occurring during aging negatively impact mobility and muscle force, contributing to physical and mental decline in elderly individuals (Akima et al., 2001; Gariballa and Alessa, 2018; López-Otín et al., 2013; McCormick and Vasilaki, 2018). Skeletal muscle is comprised of long syncytial cells (myofibers) formed by fusion of skeletal muscle progenitors during development and during muscle repair and whose nuclei share a common cytoplasm yet are transcriptionally heterogeneous (Dos Santos et al., 2020; Kim et al., 2020; Petrany et al., 2020; Wen et al., 2021).
Myofibers are maintained in adult skeletal muscle by a population of quiescent muscle stem cells (MuSCs) that, in response to injury, activate, proliferate as mononuclear myogenic progenitors, and then fuse, generating sufficient myonuclear numbers and diversity to repopulate regenerated myofibers (Lepper et al., 2011; Murphy et al., 2011). While most progenitors differentiate and fuse producing myonuclei, a subset undergoes self-renewal, reacquiring quiescence and replenishing the MuSC pool (Collins and Partridge, 2005; Cutler et al., 2022; Kuang et al., 2007; Olguin and Olwin, 2004). Though the transcriptional changes driving MuSC activation and proliferation are well studied, the mechanisms responsible for maturing and diversifying myonuclei once progenitors have fused are largely unexplored.
Failure to maintain and repair skeletal muscle in aged organisms is attributed in part to deficits in MuSC function that include delays in exiting quiescence, premature differentiation, failure to transplant, and cumulative transcriptional changes (Bernet et al., 2014; Blau et al., 2015; Chakkalakal et al., 2012; Chen et al., 2020; Day et al., 2010; Dos Santos et al., 2020; Kim et al., 2020; Kimmel et al., 2020, 2021). Temporal expression of myogenic transcription factors (TFs) is disrupted in progenitors isolated from aged mice (Bernet et al., 2014; Kimmel et al., 2020, 2021), eliciting potentially widespread and severe downstream affects in their gene regulatory networks. Aging-associated defects in MuSC transcriptional dynamics are exacerbated upon differentiation (Kimmel et al., 2020, 2021) supporting the idea that disruptions in gene expression timing during regeneration worsen as regeneration proceeds. Yet to what extent these temporal differences drive deficiencies in skeletal muscle repair and function in aged organisms is largely unexplored and cannot be sufficiently recapitulated in culture, as myonuclei fail to organize into the specialized structures found in vivo and never express mature isoforms of skeletal muscle proteins (Afshar Bakooshli et al., 2019; Dessauge et al., 2021; Yoshimoto et al., 2020).
Evaluating transcriptional changes driving myogenesis in vivo is complicated by the multinucleated and heterogeneous nature of skeletal muscle cells. Single-cell RNA sequencing provides sufficient resolution of mononuclear progenitors but fails to capture substantial myonuclear numbers (McKellar et al., 2021; Williams et al., 2022). RNA fluorescence in situ hybridization (FISH) provides spatial and transcriptional information but is low throughput, while spatial transcriptomics lacks the resolution to examine individual myonuclei (Kim et al., 2020; Moffitt et al., 2022). Numerous studies have identified individual genes and pathways through direct experimentation that are disrupted in aged mouse skeletal muscle, but aging impacts universal properties of transcription (Stoeger et al., 2022), thus large-scale transcriptomic interrogations in vivo followed by in-depth computational analyses are uniquely situated to further improve our understanding of skeletal muscle aging.
To better understand how the transcriptional changes occurring during muscle regeneration in young and aged mice differ, we constructed a pseudotime trajectory of myogenic differentiation from single-nucleus RNA sequencing (snRNA-seq) of myogenic nuclei isolated from both mononuclear and multinucleated cells prior to and during regeneration in young and aged mice. Ordering nuclei along pseudotime enabled parsing heterogeneous differentiation states present among myogenic nuclei taken at discrete times during regeneration such that nuclei from young and aged mice could be aligned and compared based on their relative cell-fate status. Comparing how the pseudotime ordering of nuclei differs between young or aged mice revealed that genes with aging-associated alterations in pseudotemporal expression are part of myogenic transcriptional programs that are essential for the reacquisition of muscle function after an injury. Further analysis of the differences between myogenic nuclei from aged compared with young mice using dynamic time warping revealed that global transcriptomic differences are larger in post-fusion myogenic nuclei compared with mononuclear progenitors. Collectively, alterations in gene expression timing may amplify as progenitors differentiate into myonuclei and may thus contribute to reduced muscle regeneration and declines in muscle function as mice age.
Results
MuSC expansion dynamics are disrupted during regeneration of aged mouse TA muscle
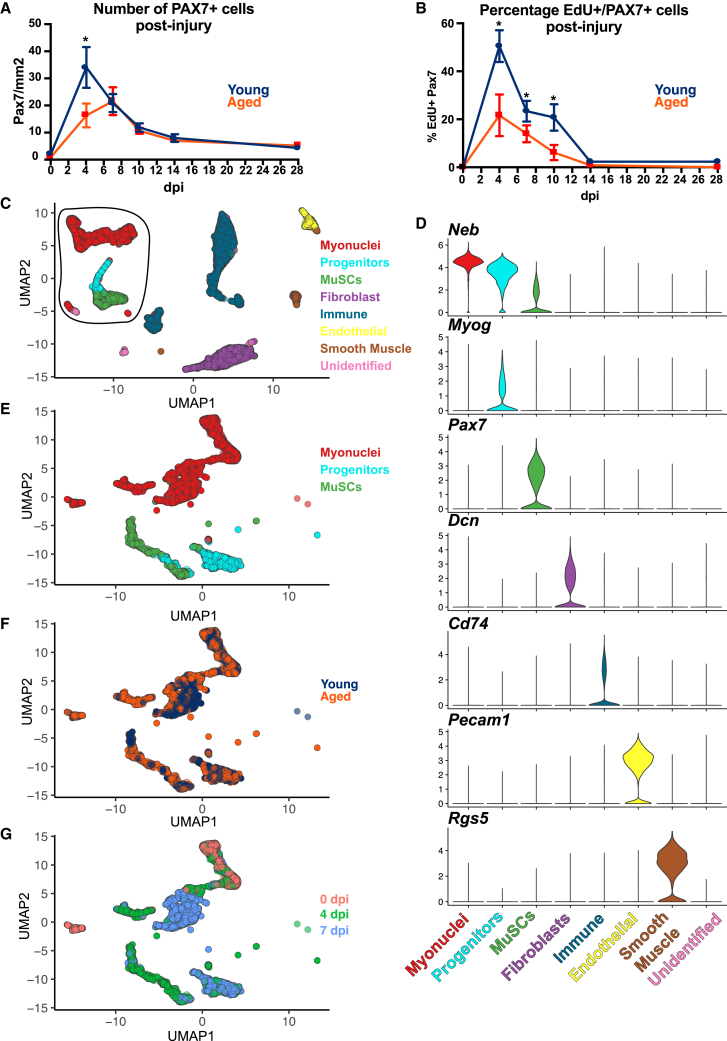
MuSCs from aged mice are less proliferative and differentiate prematurely when cultured compared with MuSCs from young mice (Bernet et al., 2014; Chakkalakal et al., 2012; Shefer et al., 2006). To identify differences in MuSC behavior in aged mice in vivo, we quantified MuSC number, identified by PAX7 immunoreactivity, in the tibialis anterior (TA) muscle for up to 28 days post-injury (dpi) in aged and young mice. MuSCs exit quiescence, and their myogenic progenitor progeny expand rapidly, reaching a peak by 4 dpi in young mouse muscle, then slowly decline to within 2-fold of initial MuSC numbers by 28 dpi (Figure 1A). In aged mice, PAX7+ cell expansion is delayed, with myoblast numbers peaking at 7 dpi with less than half the peak number in young mice (Figure 1A). Nevertheless, at 7 dpi, numbers of MuSCs and myoblasts in young and aged mice begin to decline synchronously such that by completion of regeneration, similar numbers of MuSCs are present in both age groups (Figure 1A).
Figure 1.
MuSC kinetics and sequencing in regenerating muscle of young and aged mice
(A) PAX7+ cell quantification from regenerating TA muscles in young and aged mice (n = 3).
(B) Percentage of PAX7+ and EdU+ cells in regenerating young and aged mice. EdU was administered 6 h prior to tissue harvest. ∗p < 0.05, one-way ANOVA (n = 3).
(C) UMAP clustering of nuclei from sequencing single cells and single nuclei of skeletal muscle from young and aged mouse TA muscles at 0 (n = 4; 2 young, 2 aged), 4 (n = 5; 2 young, 3 aged), and 7 dpi (n = 4; 2 young, 2 aged). Black circle demarks myogenic nuclei used for further analysis.
(D) Violin plots of selected genes used to identify cell types from snRNA-seq.
(E–G) Subset of myogenic nuclei used for downstream analyses (E) identified (F) by age and (G) by dpi.
See also Figures S1 and S2 and Tables S1 and S2.
Reduced MuSC numbers in aged mice may result from reduced MuSC proliferation, a slower cell cycle, cell death, or from some other cause. To evaluate these possibilities, we used the nucleotide analog ethynul-2′-deoxyuridine (EdU), which is incorporated into DNA during replication, to identify recently divided PAX7+ cells. Young and aged mice were injured and treated with EdU for 6 h prior to tissue harvest, and the percentage of EdU+/PAX7+ cells was quantified at different time points (Figure 1B). The percentage of EdU+/PAX7+ cells was reduced in aged mice compared with young mice at all time points up to 14 dpi, indicating fewer dividing MuSCs and myogenic progenitors (Figure 1B). Although the proportion of dividing PAX7+ cells was diminished in aged mice, the overall proliferative trend is similar in both aged and young mice, as the percentage of EdU+/PAX7+ cells peaks at 4 dpi and then decreases (Figure 1B).
snRNA-seq of post-injury skeletal muscle in young and aged mice
Temporal differences in the expansion of MuSCs and their progeny between young and aged mice at 4 or 7 dpi suggests that temporal coordination of myogenic transcriptional networks driving regeneration may be altered. Probing gene expression changes prior to, during, and following cell fusion is challenging, requiring assessment of transcripts from mononuclear cells as well as myonuclei in syncytial myofibers, rendering use of standard single-cell sequencing ineffective. To evaluate aging-associated transcriptomic differences in myogenic nuclei during muscle regeneration in aged mice, we conducted snRNA-seq on the TA muscles of young and aged mice at 0 dpi (uninjured); at 4 dpi, when myonuclear production is largely complete (Cutler et al., 2022); and at 7 dpi, when myonuclei are likely maturing (Figures 1C, S1A, and S1B). Dimensional reduction was conducted on single-nuclear transcriptomes aggregated across all time points and combined from both young and aged mice (Figures S1A and S1B). Nuclear clustering revealed the expected cell types including skeletal muscle-associated mononuclear cells (immune cells, fibroadipogenic progenitors [FAPs], endothelial cells, etc.) and myogenic mononuclear cells (MuSCs and myogenic progenitors), as well as myonuclei within multinucleated myofibers (Figures 1C, 1D, and S1C).
We subclustered all MuSCs, myogenic progenitors, and myonuclei based on 4 criteria: (1) inclusion in Ttn+/Neb+ clusters, (2) expression of the myogenic genes Pax7, Myod1, Myog, Ckm, or Mylk2, (3) lack of expression of Cd74 or Pecam1, and (4) exclusion from the fibroblast, Schwann cell, smooth muscle cell, and immune cell clusters (Figure S1C) (Petrany et al., 2020; Schmidt et al., 2019; Trinick, 1992; Woodfin et al., 2007; Zernecke et al., 2008). MuSCs, myogenic progenitors, and myonuclei (Figure 1E) arrange in unique as well as overlapping clusters when plotted by age (Figure 1F) or injury time point (Figure 1G). This comprehensive myogenic subset, comprised of myogenic nuclei from all injury time points and young as well as aged mouse skeletal muscle, was used for further analyses. Comparing GO processes for myogenic nuclei between young and aged mice at 4 and 7 dpi identified filament assembly and myoblast proliferation as significant at 4 dpi (Figure S2A; Table S1) and MuSC differentiation and innervation as significant at 7 dpi (Figure S2B; Table S1) (Gene Ontology Consortium et al., 2021). Among the most significantly affected genes within these GO processes are genes encoding receptors Fgfr1, Fgfr4, and Notch3 (Figure S2C), genes encoding sarcomeric proteins Ttn and Neb (Figure S2D), and TFs involved in myoblast proliferation, Six1 and Runx1 (Figure S2E; Table S2) (Gioftsidi et al., 2022; Le Grand et al., 2012; Olwin et al., 1994; Trinick, 1992; Umansky et al., 2015). Although the majority of myonuclei are generated by 4 dpi and the majority of MuSCs self-renew between 5 and 7 dpi (Cutler et al., 2022), we observed expression of genes characterizing myogenic progenitors including Pax7, Myod1, and Myog in nuclei from 7 dpi mice (Figure S2F). Thus, among nuclei obtained at 4 and 7 dpi, there is a diversity of myogenic states present, suggesting heterogeneity in the timing of MuSC activation, proliferation, and differentiation following an induced injury (Figure S2G).
Pseudotime trajectory of myogenic nuclei during muscle regeneration in young and aged mice
MuSC activation, proliferation, and differentiation appear asynchronous, and therefore correlating nuclear transcriptomes with progression through these cell states using a given injury time point is impossible (Figure S2G). Thus, we applied Moncole3, a tool for inferring pseudotime trajectories from single-cell sequencing experiments, to better understand how aging-specific transcriptional alterations in MuSCs and their progeny affect myonuclear transcription (Qiu et al., 2017; Trapnell et al., 2014). The Monocle3-inferred uniform manifold approximation and projection (UMAP) contains two major lobes separated by a hinge region and a continuous branched trajectory representing an average path of pseudotime (Figure 2A). Nuclei comprising the initiation of pseudotime are situated in the bottom of the left lobe and express high levels of Pax7 (Figure 2B), among other genes (Figures S3A–S3D), identifying quiescent and activated MuSCs (Zammit et al., 2006). Nuclei in the hinge region bridging the left and right lobes are enriched for Mymk expression, a gene required for fusion of mononuclear progenitors (Figure 2C) (Millay et al., 2013). Mature myosin isoforms encoded by Myh4, and other genes expressed in mature muscle, are present in the larger right lobe occupying the end of the pseudotime trajectory (Figures 2D and S3E–S3G) (Petrany et al., 2020; Trinick, 1992; Yoshimoto et al., 2020). Thus, the pseudotime trajectory comprises a comprehensive span of myogenic differentiation beginning with quiescent MuSCs, continuing through progenitor fusion, and ending with myonuclear maturation.
Figure 2.
Pseudotime trajectory of myogenic differentiation in young and aged mouse skeletal muscle
(A) A Monocle-inferred pseudotime trajectory of myogenic nuclei colored by assigned pseudotime value. Arrow represents path of increasing pseudotime.
(B–E) Pseudotime trajectory with nuclei colored (B–D) by gene expression or (E) by age.
(F and G) Pseudotime trajectory plots comprised of nuclei derived from either (F) young or (G) aged mice.
(H) Pseudotime trajectory with nuclei colored by dpi. Two dotted arrows depict an alternative orientation of the trajectory initiating from the hinge region. (I) Pseudotime trajectory plot with nuclei from 0 dpi, (J) 4 dpi, or (K) 7 dpi experiments. See Figure 1C legend for mouse sample sizes.
Nuclei from young and aged mice were both included when generating the pseudotime trajectory (Figure 2E). Qualitatively, distributions of nuclei from young and aged mice are similar throughout the trajectory, implying that the broad transcriptional processes driving myogenic differentiation during skeletal muscle regeneration are largely conserved in aged mice (Figure 2E). Nuclei from young mice (Figure 2F) and from aged mice (Figure 2G) comprising the pseudotime trajectory are derived from uninjured (0 dpi), 4 dpi, and 7 dpi TA muscle (Figure 2H). Nuclei from 0 dpi TA muscles occupy the beginning and end of pseudotime, thus relating the transcriptional changes between the initial and final transcriptional states of MuSCs and mature myonuclei, respectively (Figure 2I). Indeed, nuclei from 4 dpi mice (Figure 2J) and 7 dpi mice (Figure 2K) distribute throughout the pseudotime trajectory, bridging populations of quiescent MuSCs to mature myonuclei along a continuous path of myogenic differentiation (Figure 2A).
Heterogeneity of 4 and 7 dpi samples is evident from the dispersion of these nuclei across the trajectory, further reinforcing a diversity of myogenic states among nuclei at these time points (Figure S2G). While the majority of myonuclei are generated within the first 5 dpi, after 5 dpi, myonuclear production is reduced (Cutler et al., 2022), and myonuclei are likely undergoing maturation. We identified two distinct groups of 7 dpi myogenic nuclei clustered on either side of the trajectory’s hinge region that represents progenitor fusion (Figures 2H and 2K). Expression of Egfr, Mest, Itm2a, Mgp, and Ncoa7, genes associated with MuSC self-renewal, are enriched in transcriptomes of prefusion 7 dpi nuclei from the left lobe when compared with post-fusion 7 dpi nuclei in the right lobe (Figures S3H–S3K; Table S3) (Cutler et al., 2022; Wang et al., 2019), whereas in the right lobe, myonuclei are enriched for Myh3 and Myh8 expression, two myosin isoforms expressed in immature myonuclei during regeneration (Table S3) (Schiaffino et al., 2015). Thus, nuclei in the right lobe collected from 7 dpi TA muscles are most likely maturing nascent myonuclei, and the hinge region in the pseudotime trajectory represents the commitment of MuSCs to either self-renew or terminally differentiate (Figure 2H).
MuSCs and progenitors have altered gene expression dynamics in aged mice
Dramatic differences in transcriptional states between MuSCs and mature myonuclei may obscure subtle aging-associated differences existing within each population independently. Thus, we partitioned the trajectory into two major branches: branch 1 (B1), beginning with MuSC nuclei and ending on the distal tip of the hinge region, and B2, initiating from the hinge region and extending to the end of the myogenic trajectory (Figures S4A–S4E). Nuclei in the hinge region were initially included in both subsets to better anchor each such that when conducting dimensional reduction, a representative trajectory is inferred for both B1 and B2 (Figures S4D–S4E). An additional subset was generated from B2 nuclei to exclude nuclei in and just after the hinge region, as determined by the point of decreasing Myh3 and Myh8 expression and increasing Myh1 and Myh4 expression (Figures 2D, S3E, and S4F–S4H). Thus, B1 represents MuSC and myogenic progenitor dynamics through the point of cell fusion, and the B2 myonuclear subset represents post-fusion myonuclei maturing within a syncytial myofiber.
To identify aging-associated changes in transcriptional dynamics, we used Tradeseq, a program for comparing pseudotime trajectories across biological conditions (Figure 3A) (Van den Berge et al., 2020). Although temporal changes in TF expression between myogenic progenitors isolated from young and aged mice when differentiated in vitro are reported (Bernet et al., 2014; Kimmel et al., 2020, 2021), these changes have not been evaluated in vivo. Unlike differential gene expression tests that identify changes in transcript levels, Tradeseq analyses reveal gene expression changes occurring as a function of pseudotime, offering a more relevant strategy to interrogate temporally resolved transcriptomic data. Tradeseq was used to generate expression trajectories for individual genes expressed within B1 and the B2 myonuclear subset and to conduct statistical tests identifying genes with significantly different pseudotime trajectory patterns between nuclei from young and aged mice (Figure 3A). Out of ∼21,000 genes expressed in B1 nuclei, ∼7,000 exhibited differential pseudotime trajectories, while in the B2 subset, ∼5,000 genes were detected with differential pseudotime trajectories (Table S4). We selected the 1,000 genes with the most significant p values from B1 as well as the B2 myonuclear subset comparisons, restricting our analysis to the most significant aging-associated changes during regeneration. Among these 1,000 genes identified from each branch, a group was common to both subsets, which was surprising considering the disparate processes represented in these respective segments of the myogenic pseudotime trajectory (Figure 3B). Among B1-specific genes, GO processes involving localization of proteins to synapses and myogenic progenitor fusion were detected as exhibiting significantly altered pseudotime in nuclei from aged mice compared with young mice (Figures 3C and 3D; Table S5). Genes with altered pseudotime in nuclei from aged compared with young mice that are common to B1 and B2 are involved in T-tubule organization, myofibril assembly, and neuromuscular junction (NMJ) formation (Figures 3E and 3F; Table S5), while metabolic processes including glycolysis and the TCA cycle exhibited the most significant altered pseudotime specific to B2 (Figures 3G and 3H; Table S5). Though the majority of pseudotemporal alterations in gene expression specific to aged mice are unique to either B1 or B2 nuclei, a minority are common to both branches, raising the possibility that alterations in temporal expression of genes in B1 are directly related to the pseudotime changes occurring among nuclei from aged mice in B2.
Figure 3.
Differences in pseudotime trajectories of genes during early and late myogenesis in regenerating TA muscles
(A) Analysis pipeline schematic (see Figure S4).
(B) Venn diagram between top 1,000 genes with significantly different pseudotime trajectories from B1 and the B2 myonuclear subset.
(C and D) Averaged pseudotime expression plots for genes comprising enriched GO categories from B1-specific genes.
(E–H) Overlap of (E and F) B1 and the B2 myonuclear subset genes and (G and H) B2 myonuclear subset-specific genes. n is number of genes averaged in each plot.
Genes with altered expression dynamics in aged mice comprise distinct yet overlapping hierarchies between B1 and B2
Cellular transcriptomes organize into distinct gene networks comprised of groups of co-expressed genes involved in common biological processes (van Dam et al., 2017; Fionda, 2019; Stuart et al., 2003; Yin et al., 2021). Ensuring temporally coordinated expression of gene networks during regeneration are myogenic TFs, which, in response to specific signals, activate or repress gene expression, driving myogenesis. Because of the large number of pathways regulated by myogenic TFs, perturbed gene expression dynamics in MuSCs and progenitors may propagate (by direct and indirect associations) through regulatory hierarchies into later stages of regeneration, affecting transcriptional networks in post-fusion myonuclei. Direct identification of gene networks involved in myogenesis is difficult but can be inferred by genes exhibiting similar temporal or co-expression patterns with each other and with TFs (van Dam et al., 2017; Yin et al., 2021). If in aged mice, temporal regulation of gene networks involved in cell-fate transitions and terminal differentiation of progenitors is altered, then myonuclear gene expression and mature muscle function may be impacted.
To identify TFs putatively regulating genes whose expression timing differs in myogenic nuclei between young and aged mice in B1, we mined single-cell RNA-seq datasets using the Archs4_TFs_Coexp database with the 1,000 genes exhibiting the most significant aging-associated changes in pseudotime expression dynamics. Querying this database enables the identification of TFs enriched for their co-expression with the provided genes (Lachmann et al., 2018). Among the 1,000 genes with the most significant aging-associated changes in pseudotime in B1, 334 TFs were enriched, each associated with expression of a gene cohort representing a putative gene network. Predicted co-expressed TFs include critical myogenic regulators Myf6, Myod1, Myog, Mef2a, and Mef2c, as well as TFs with less well-established roles in myogenesis, Twist1, Smad3, Snai2, Tbx15, and Nfat5 (Table S6). The 100 most enriched TF-associated gene groups, comprised of ∼800 unique genes from the initial 1,000 genes used for this analysis, were plotted in a correlation matrix, visualizing the degree of overlap across these TF-associated gene groups (Figure 4A). Hierarchical clustering of TF-associated gene groups identified 4 distinct clusters (Figure 4A; Table S6). Averaged pseudotime expression patterns of genes comprising the clusters of TF-associated groups were plotted, revealing distinct pseudotime expression patterns associating with each cluster (Figures S5A–S5D). Qualitatively, a total of just 6 trajectory patterns were detected across the 100 TF-associated gene groups comprised of ∼800 unique genes represented in the heatmap, suggesting that genes with differential pseudotime trajectories between young and aged mouse nuclei in B1 comprise hierarchical groups associated with myogenesis, each with characteristic pseudotemporal expression patterns (Figures S5A–S5D; Table S6).
Figure 4.
Hierarchical clustering of genes with differential pseudotime between B1 and B2
(A) Heatmap of 100 most enriched TF-associated groups from B1 genes with differential trajectories in nuclei from aged mice. Rows and columns are TF-associated gene groups, and the heatmap is colored by the percentage of genes comprising both groups that are shared between them.
(B) Analogous heatmap to (A) but for TF-associated gene groups identified from the B2 myonuclear subset.
(C) Venn diagram depicting overlap of TFs predicted as co-expressed among genes with differential trajectories from either B1 or the B2 myonuclear subset. Overlap was only considered for the 100 groups with the most significant enrichment from each branch.
(D) Heatmap of only TF-associated gene groups where the TF was predicted as co-expressed among genes with differential trajectories from both branches. Heatmap is colored by the percentage of genes overlapping between TF-associated groups from B1 and B2 myonuclear subset predictions.
See also Figures S5 and S6 and Table S6.
Performing TF co-expression analysis on nuclei from B2, we identified 252 TFs enriched as co-expressed (Figure 3A; Table S6). The 100 most enriched TF-associated gene groups predicted from the B2 myonuclear subset were plotted as a heatmap, identifying only a single defined cluster with minimal overlap among most TF-associated gene groups (Figure 4B; Table S6). Additionally, a greater diversity of pseudotime expression patterns were observed among the B2 TF-associated gene groups compared with groups identified from B1 (Figures S6A and S6B), suggesting that genes with altered pseudotime in B2 myonuclei comprise less-defined hierarchies compared with genes expressed in B1. Nevertheless, 63 of the 100 most significant TF predictions from B1 are also present among the 100 most enriched TFs from B2, though the genes comprising these TF-associated gene groups are largely distinct between B1 and B2 (Figures 4C and 4D; Table S6). Common TFs enriched among genes with altered pseudotemporal expression dynamics in both B1 and B2 are thus potential regulators of disrupted gene networks across pre- and post-fusion myogenic nuclei in aged mice. These mutually co-expressed TFs include those critical for myogenesis (Myf6, Myod1, Myog, Mef2c, and Mef2a), suggesting that although the genes comprising B1 and B2 myogenic gene networks are minimally overlapping, genes with disrupted pseudotemporal expression in aged mice are putatively linked through hierarchical regulatory networks (Figure 4D). Thus, during muscle regeneration in aged mice, temporal alterations may propagate from prefusion progenitors to post-fusion myonuclei, affecting temporal regulation of interconnected gene networks encoding skeletal muscle structural and metabolic proteins.
Dynamic time-warping analysis of myogenic differentiation in young and aged mice
Whether temporal changes in gene expression occurring in aged mice compared with young mice are exacerbated as MuSCs commit to terminal differentiation, fuse, and mature as myonuclei cannot be inferred from gene network analyses conducted on B1 and B2. To address this question, an integrated analysis that examines aggregate differences between the transcriptomes of nuclei from young and aged mice at different regenerative stages is required. We iteratively subsampled nuclei from the complete pseudotime trajectory (Figure 2A), equalizing numbers of nuclei across injury time points, and subjected them to dynamic time-warping (DTW) alignment. DTW is a method that aligns points of two independent time series by locally compressing or expanding time scales for each based on a specified similarity metric, preserving the overall order of each series but not necessarily a one-to-one correspondence of each point along the trajectory (Cacchiarelli et al., 2018; Kimmel et al., 2021; Sakoe and Chiba, 1978). Euclidean distances based on global nuclear gene expression profiles, rather than on changes to individual gene trajectories, were calculated between nuclei from young and aged mice at different points in pseudotime.
We plotted the averaged differences of pseudotime values obtained from subsamples of young and aged mouse nuclei along each point of the DTW-transformed timescale, revealing predominantly positive deviations of the aged pseudotime trajectory (Figure 5A). The magnitude of deviation is smallest in B1 nuclei, larger in the hinge region, and peaks in the middle of B2 (Figure 5A). These deviations are specific to comparisons of nuclei from young compared with aged mice, as isochronic (i.e., same-age) comparisons reveal minimal deviations in the pseudotime ordering of nuclei (Figures 5B and 5C).
Figure 5.
DTW analysis of the myogenic trajectory
(A–C) Averaged difference of pseudotime values between aligned myogenic nuclei from young and aged mice (A) and isochronic comparisons between young vs. young (B) and aged vs. aged (C) along the pseudotime trajectory (see Figure 1C legend for mouse sample sizes). The x axis is the DTW-transformed timescale. Each gray line is a DTW alignment on a single bootstrap (n = 30). Black line is the average of bootstraps. Parallel green lines demarcate the hinge region. Dotted red line depicts theoretical perfect alignment.
(D) Pseudotime values for nuclei from young and aged mice plotted by DTW-transformed indices used to generate (A)–(C). Each gray line is a DTW alignment on a single bootstrap. The black line is the average of bootstraps. Red line indicates path of theoretical perfect alignment of the two trajectories. Parallel green lines demarcate the hinge region separating B1 and B2.
(E and F) Similar plots to (D) but for the B1 and B2 subsets independently. Gray boxes indicate regions with most significant distortions of the aged trajectory used for area under the curve analysis.
To quantify the relative magnitudes of aging-associated deviations in pseudotime trajectories associated with B1 and B2, the aligned pseudotime values for young and aged mouse nuclei were plotted against each other and compared with a theoretically perfect alignment of the myogenic trajectories inferred from young and aged mice (Figure 5D). Regions of maximal distortion of the trajectory from aged mice within B1 and B2 were identified, and a larger area under the curve value was calculated for the B2 segment compared with the B1 segment, demonstrating a distinct increase in magnitude of distortion associated with B2 (Figures 5E and 5F). Thus, independent of sample size differences across injury time points, B2, comprised of primarily maturing myonuclei, exhibits more significant aging-associated alterations in the myogenic trajectory compared with the changes occurring in B1 comprised of MuSCs and prefusion progenitors.
If temporal processes regulating expression are altered for a given gene, then complex downstream effects may ensue that are independent of persistent changes in gene levels. Because differences in both gene expression levels and pseudotime expression dynamics were detected during regeneration when comparing transcriptomes from young and aged mouse nuclei, we asked whether changes in pseudotime were more or less severe compared with changes in gene levels. Plotting the average gene expression levels for all genes expressed in nuclei from young mice against those from aged mice revealed a high correlation (R2 = 0.91) between the two age groups, revealing that the changes occurring in gene expression levels are discriminatory rather than global in aged mice (Figure 6A). In contrast, there was poor correlation (R2 = 0.329) when plotting the standard error of gene expression across pseudotime for nuclei from young mice against those from aged mice (Figure 6B).
Figure 6.
Changes in gene expression pseudotime dynamics are more severe than changes in gene expression levels when comparing myogenic nuclei from aged and young mice
(A and B) Scatterplot of average expression values of individual genes with black lines representing perfect correlations (A) and scatterplot of standard error of gene expression across pseudotime of individual genes (B) expressed in myogenic nuclei from either young or aged mice. See Figure 1C legend for mouse sample sizes.
(C) A schematic depicting how altered gene expression dynamics propagate through interconnected transcriptional networks during muscle regeneration.
Discussion
Gene networks involved in related biological processes require coordinated temporal control of gene expression (van Dam et al., 2017; Fionda, 2019; Stuart et al., 2003; Yin et al., 2021). Altering gene expression dynamics regulating these networks may interfere with complex cell-fate transitions required for progenitor cells to differentiate and acquire transcriptional states necessary for organ- and tissue-specific functions. During skeletal muscle regeneration, aging-associated changes in gene expression dynamics are exacerbated during MuSC differentiation in vitro (Kimmel et al., 2020, 2021). However, differentiation in culture is not representative of regeneration in vivo where transcriptional states associated with mature myonuclei are never attained (Afshar Bakooshli et al., 2019; Bernet et al., 2014; Kimmel et al., 2020, 2021; Yoshimoto et al., 2020).
We compared changes in timing of gene expression in vivo through pseudotime analysis of myogenic nuclei sequenced from regenerating skeletal muscle between aged and young mice. This analysis revealed that the genes exhibiting the largest changes in pseudotemporal expression trajectories appear to comprise distinct transcriptional networks involved in progenitor proliferation, innervation, and metabolic pathways conferring myofiber contractile speeds (Chakkalakal et al., 2012; Jang and Van Remmen, 2011; Miljkovic et al., 2015). However, aging affects transcriptomes globally (Stoeger et al., 2022), and thus we employed DTW analysis, which enabled us to quantify the aggregate differences in global gene expression dynamics rather than focus on specific pathways. We identified that specific segments of the pseudotime trajectory exhibit larger aging-associated differences than others, where peak differences appear in three discrete waves, each associated with a distinct stage of myogenesis. The first wave occurs in the middle of B1, where myogenic progenitors are balancing opposing cell-fate choices to terminally differentiate or self-renew. The second wave of distorted pseudotime, which is larger than the first, occurs in the hinge region, where myogenic cells fuse. The final and largest wave of age-distorted pseudotime is observed in myonuclei following fusion, where the transcriptional changes driving myonuclear maturation occur.
During regeneration of skeletal muscle, temporal regulation of gene networks is necessary to repair muscle and reacquire muscle function. In aged mice, the timing for gene networks driving myogenesis and muscle maturation diverges from that in young mice and worsens as regeneration proceeds, potentially propagating through interconnected gene networks associated with expression of mature muscle genes (Figure 6C). Although we have not identified mechanistic details, our data support that alterations in temporal coordination of gene expression in myogenic networks contributes to defects in muscle regeneration and to declining muscle function in aged organisms. Comprehensive analyses of the mechanisms underlying mistimed gene expression during regeneration in aged mouse skeletal muscle will require future follow-up studies that are currently impractical, requiring temporally altering the expression of large numbers of genes in combination with each other to test the effects on muscle regeneration in young mice. The temporal alterations we observed in gene networks involved in myogenesis provide a novel insight for aging research to base future mechanistic studies where altered temporal coordination of myogenic gene expression as well as changes in the expression levels of specific transcripts contribute to impaired muscle regeneration and declining muscle function in aging organisms.
Experimental procedures
Resource availability
Corresponding authors
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, Bradley Olwin (olwin@colorado.edu).
Materials availability
No unique reagents were generated for this study.
Mice
Young mice were between 4 and 8 months old and were a mix of male and female. The aged mice used were collected between 24 and 28 months old and were a mix of male and female. For injuries, mice were injected with BaCl2 into the TA and extensor digitorum longus (EDL) muscle. See the supplemental experimental procedures for more detail.
PAX7 and EdU quantification
See the supplemental experimental procedures for details on PAX7 and EdU quantification. Briefly, for injuries, mice were anesthetized and injected with BaCl2 into the TA and EDL muscle. To deliver EdU, mice were given intraperitoneal (i.p.) injections.
Statistical tests were performed on PAX7 quantified cells per mm2 for young and aged mice (n = 3). Significance was assessed using two-tailed unpaired Student’s t test with the two-stage step-up method of Benjamini, Krieger and Yekutieli multiple comparison adjustment with a q <0.05 considered significant.
Nuclear isolation, 10× Genomics, and sequencing
The protocol for nuclear isolation was similar to that described previously (Cutler et al., 2017). See the supplemental experimental procedures for specific modifications.
Data processing
See the supplemental experimental procedures for more detail on data processing and analysis.
Briefly, Cellranger was used to process Fastq files and aggregate technical replicates, creating gene-count matrices for each sequencing experiment. Feature matrices were then loaded into R and assessed for quality. Seurat was used for data normalization, dimensional reduction, and clustering. Differential expression tests were done using Seurat, and genes with p value <0.05 using the Wilcoxon rank-sum test were deemed significant.
For trajectory inference, a tutorial to generate pseudotime trajectories from Seurat objects was used as a guide (https://cole-trapnell-lab.github.io/monocle3/docs/trajectories/). Tradeseq was used as recommended, and a tutorial (https://statomics.github.io/tradeSeq/articles/tradeSeq) was used as a guide.
See the supplemental experimental procedures for more details on GO process identification and TF-associated gene clustering.
DTW analysis
We used the fastdtw (v.0.3.4) Python package implementation of the DTW algorithm to perform our analysis (see the supplemental experimental procedures for more detail).
Author contributions
B.O., B.P., N.D.B., T.A., and M.H. conceived the experiments. A.A.C., A.V.D., B.P., N.D.B., and T.A. performed the experiments. J.V.K., B.O., A.A.C., and R.D. prepared the manuscript. J.V.K., B.O., A.A.C., J.T.S., M.A.A., R.D., A.R., and M.H. provided intellectual and computational support. J.V.K. and J.T.S. performed bioinformatic analyses. J.V.K. constructed figures and wrote the manuscript. B.O., R.D., and M.A.A. supervised research. All authors read and approved the manuscript.
Acknowledgments
This work was funded by grants from the ALSAM Foundation (B.O.), the Glenn Foundation for Medical Research (B.O.), and NIAMS AR070630 (B.O.) and AR049446 (B.O.).
Conflict of interests
B.O. discloses a potential conflict of interest as a scientific advisory board member for Satellos Biosciences. A.R. is an employee and shareholder of Edge-wise Therapeutics.
Published: June 13, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.05.005.
Contributor Information
Robin Dowell, Email: robin.dowell@colorado.edu.
Bradley Olwin, Email: olwin@colorado.edu.
Supplemental information
Data and code availability
Code is available on the GitHub repository jeku7901-CU/Kurland_et_al_SCR_2023, and sequencing data have been deposited on GEO under accession number GEO: GSE180225.
References
- Afshar Bakooshli M., Lippmann E.S., Mulcahy B., Iyer N., Nguyen C.T., Tung K., Stewart B.A., van den Dorpel H., Fuehrmann T., Shoichet M., et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife. 2019;8:e44530. doi: 10.7554/eLife.44530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akima H., Kano Y., Enomoto Y., Ishizu M., Okada M., Oishi Y., Katsuta S., Kuno S. Muscle function in 164 men and women aged 20???84 yr. Med. Sci. Sports Exerc. 2001;33:220–226. doi: 10.1097/00005768-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Bernet J.D., Doles J.D., Hall J.K., Kelly Tanaka K., Carter T.A., Olwin B.B. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014;20:265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H.M., Cosgrove B.D., Ho A.T.V. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015;21:854–862. doi: 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacchiarelli D., Qiu X., Srivatsan S., Manfredi A., Ziller M., Overbey E., Grimaldi A., Grimsby J., Pokharel P., Livak K.J., et al. Aligning single-cell developmental and reprogramming trajectories identifies molecular determinants of myogenic reprogramming outcome. Cell Syst. 2018;7:258–268.e3. doi: 10.1016/j.cels.2018.07.006. [DOI] [PubMed] [Google Scholar]
- Chakkalakal J.V., Jones K.M., Basson M.A., Brack A.S. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Datzkiw D., Rudnicki M.A. Satellite cells in ageing: use it or lose it. Open Biol. 2020;10:200048. doi: 10.1098/rsob.200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.A., Partridge T.A. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4:1338–1341. doi: 10.4161/cc.4.10.2114. [DOI] [PubMed] [Google Scholar]
- Cutler A.A., Dammer E.B., Doung D.M., Seyfried N.T., Corbett A.H., Pavlath G.K. Biochemical isolation of myonuclei employed to define changes to the myonuclear proteome that occur with aging. Aging Cell. 2017;16:738–749. doi: 10.1111/acel.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A.A., Pawlikowski B., Wheeler J.R., Dalla Betta N., Elston T., O’Rourke R., Jones K., Olwin B.B. The regenerating skeletal muscle niche drives satellite cell return to quiescence. iScience. 2022;25:104444. doi: 10.1016/j.isci.2022.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K., Shefer G., Shearer A., Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev. Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessauge F., Schleder C., Perruchot M.-H., Rouger K. 3D in vitro models of skeletal muscle: myopshere, myobundle and bioprinted muscle construct. Vet. Res. 2021;52:72. doi: 10.1186/s13567-021-00942-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos M., Backer S., Saintpierre B., Izac B., Andrieu M., Letourneur F., Relaix F., Sotiropoulos A., Maire P. Single-nucleus RNA-seq and FISH identify coordinated transcriptional activity in mammalian myofibers. Nat. Commun. 2020;11:5102. doi: 10.1038/s41467-020-18789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fionda V. Encyclopedia of Bioinformatics and Computational Biology. Elsevier; 2019. Networks in biology; pp. 915–921. [Google Scholar]
- Gariballa S., Alessa A. Association between muscle function, cognitive state, depression symptoms and quality of life of older people: evidence from clinical practice. Aging Clin. Exp. Res. 2018;30:351–357. doi: 10.1007/s40520-017-0775-y. [DOI] [PubMed] [Google Scholar]
- Gene Ontology Consortium, Carbon S., Douglass E., Good B.M., Unni D.R., Harris N.L., Mungall C.J., Basu S., Chisholm R.L., Dodson R.J., et al. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–D334. doi: 10.1093/nar/gkaa1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioftsidi S., Relaix F., Mourikis P. The Notch signaling network in muscle stem cells during development, homeostasis, and disease. Skeletal Muscle. 2022;12:9. doi: 10.1186/s13395-022-00293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y.C., Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011;46:193–198. doi: 10.1016/j.exger.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Franke V., Brandt B., Lowenstein E.D., Schöwel V., Spuler S., Akalin A., Birchmeier C. Single-nucleus transcriptomics reveals functional compartmentalization in syncytial skeletal muscle cells. Nat. Commun. 2020;11:6375. doi: 10.1038/s41467-020-20064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel J.C., Hwang A.B., Scaramozza A., Marshall W.F., Brack A.S. Aging induces aberrant state transition kinetics in murine muscle stem cells. Development. 2020;147:dev183855. doi: 10.1242/dev.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel J.C., Yi N., Roy M., Hendrickson D.G., Kelley D.R. Differentiation reveals latent features of aging and an energy barrier in murine myogenesis. Cell Rep. 2021;35:109046. doi: 10.1016/j.celrep.2021.109046. [DOI] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann A., Torre D., Keenan A.B., Jagodnik K.M., Lee H.J., Wang L., Silverstein M.C., Ma’ayan A. Massive mining of publicly available RNA-seq data from human and mouse. Nat. Commun. 2018;9:1366. doi: 10.1038/s41467-018-03751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F., Grifone R., Mourikis P., Houbron C., Gigaud C., Pujol J., Maillet M., Pagès G., Rudnicki M., Tajbakhsh S., Maire P. Six1 regulates stem cell repair potential and self-renewal during skeletal muscle regeneration. J. Cell Biol. 2012;198:815–832. doi: 10.1083/jcb.201201050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C., Partridge T.A., Fan C.-M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick R., Vasilaki A. Age-related changes in skeletal muscle: changes to life-style as a therapy. Biogerontology. 2018;19:519–536. doi: 10.1007/s10522-018-9775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar D.W., Walter L.D., Song L.T., Mantri M., Wang M.F.Z., De Vlaminck I., Cosgrove B.D. Large-scale integration of single-cell transcriptomic data captures transitional progenitor states in mouse skeletal muscle regeneration. Commun. Biol. 2021;4:1280. doi: 10.1038/s42003-021-02810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic N., Lim J.-Y., Miljkovic I., Frontera W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015;39:155–162. doi: 10.5535/arm.2015.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay D.P., O’Rourke J.R., Sutherland L.B., Bezprozvannaya S., Shelton J.M., Bassel-Duby R., Olson E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature. 2013;499:301–305. doi: 10.1038/nature12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt J.R., Lundberg E., Heyn H. The emerging landscape of spatial profiling technologies. Nat. Rev. Genet. 2022;23:741–759. doi: 10.1038/s41576-022-00515-3. [DOI] [PubMed] [Google Scholar]
- Murphy M.M., Lawson J.A., Mathew S.J., Hutcheson D.A., Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin H.C., Olwin B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev. Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin B.B., Arthur K., Hannon K., Hein P., McFall A., Riley B., Szebenyi G., Zhou Z., Zuber M.E., Rapraeger A.C., et al. Role of FGFs in skeletal muscle and limb development. Mol. Reprod. Dev. 1994;39:90–100. doi: 10.1002/mrd.1080390114. [DOI] [PubMed] [Google Scholar]
- Petrany M.J., Swoboda C.O., Sun C., Chetal K., Chen X., Weirauch M.T., Salomonis N., Millay D.P. Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers. Nat. Commun. 2020;11:6374. doi: 10.1038/s41467-020-20063-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H., Trapnell C. Reversed graph embedding resolves complex single-cell developmental trajectories (Genomics) Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoe H., Chiba S. Dynamic programming algorithm optimization for spoken word recognition. IEEE Trans. Acoust. Speech Signal Process. 1978;26:43–49. doi: 10.1109/TASSP.1978.1163055. [DOI] [Google Scholar]
- Schiaffino S., Rossi A.C., Smerdu V., Leinwand L.A., Reggiani C. Developmental myosins: expression patterns and functional significance. Skeletal Muscle. 2015;5:22. doi: 10.1186/s13395-015-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Schüler S.C., Hüttner S.S., von Eyss B., von Maltzahn J. Adult stem cells at work: regenerating skeletal muscle. Cell. Mol. Life Sci. 2019;76:2559–2570. doi: 10.1007/s00018-019-03093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G., Van de Mark D.P., Richardson J.B., Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeger T., Grant R.A., McQuattie-Pimentel A.C., Anekalla K.R., Liu S.S., Tejedor-Navarro H., Singer B.D., Abdala-Valencia H., Schwake M., Tetreault M.-P., et al. Aging is associated with a systemic length-associated transcriptome imbalance. Nat. Aging. 2022;2:1191–1206. doi: 10.1038/s43587-022-00317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J.M., Segal E., Koller D., Kim S.K. A gene-coexpression network for global discovery of conserved genetic modules. Science. 2003;302:249–255. doi: 10.1126/science.1087447. [DOI] [PubMed] [Google Scholar]
- Trapnell C., Cacchiarelli D., Grimsby J., Pokharel P., Li S., Morse M., Lennon N.J., Livak K.J., Mikkelsen T.S., Rinn J.L. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinick J. Understanding the functions of titin and nebulin. FEBS Lett. 1992;307:44–48. doi: 10.1016/0014-5793(92)80899-R. [DOI] [PubMed] [Google Scholar]
- Umansky K.B., Gruenbaum-Cohen Y., Tsoory M., Feldmesser E., Goldenberg D., Brenner O., Groner Y. Runx1 transcription factor is required for myoblasts proliferation during muscle regeneration. PLoS Genet. 2015;11:e1005457. doi: 10.1371/journal.pgen.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam S., Võsa U., van der Graaf A., Franke L., de Magalhães J.P. Gene co-expression analysis for functional classification and gene–disease predictions. Briefings Bioinf. 2017;19:575–592. doi: 10.1093/bib/bbw139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berge K., Roux de Bézieux H., Street K., Saelens W., Cannoodt R., Saeys Y., Dudoit S., Clement L. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 2020;11:1201. doi: 10.1038/s41467-020-14766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Feige P., Brun C.E., Hekmatnejad B., Dumont N.A., Renaud J.-M., Faulkes S., Guindon D.E., Rudnicki M.A. EGFR-aurka signaling rescues polarity and regeneration defects in dystrophin-deficient muscle stem cells by increasing asymmetric divisions. Cell Stem Cell. 2019;24:419–432.e6. doi: 10.1016/j.stem.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Englund D.A., Peck B.D., Murach K.A., McCarthy J.J., Peterson C.A. Myonuclear transcriptional dynamics in response to exercise following satellite cell depletion. iScience. 2021;24:102838. doi: 10.1016/j.isci.2021.102838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Yokomori K., Mortazavi A. Heterogeneous skeletal muscle cell and nucleus populations identified by single-cell and single-nucleus resolution transcriptome assays. Front. Genet. 2022;13:835099. doi: 10.3389/fgene.2022.835099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A., Voisin M.-B., Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2007;27:2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- Yin W., Mendoza L., Monzon-Sandoval J., Urrutia A.O., Gutierrez H. Emergence of co-expression in gene regulatory networks. PLoS One. 2021;16:e0247671. doi: 10.1371/journal.pone.0247671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y., Ikemoto-Uezumi M., Hitachi K., Fukada S.I., Uezumi A. Methods for accurate assessment of myofiber maturity during skeletal muscle regeneration. Front. Cell Dev. Biol. 2020;8:267. doi: 10.3389/fcell.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit P.S., Relaix F., Nagata Y., Ruiz A.P., Collins C.A., Partridge T.A., Beauchamp J.R. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- Zernecke A., Bernhagen J., Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117:1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code is available on the GitHub repository jeku7901-CU/Kurland_et_al_SCR_2023, and sequencing data have been deposited on GEO under accession number GEO: GSE180225.