Abstract
Traditional protein-based vaccine approaches to COVID-19 were overshadowed by the new mRNA and adenoviral vector vaccine approaches which were first to receive marketing authorization. The current study tested for the first time in repurposed aged (median 15.4 years) cynomolgus macaques, a novel Advax-CpG55.2™ adjuvanted recombinant extracellular domain spike protein trimer antigen for immunogenicity, protection and safety. Nine animals received two intramuscular injections 10 days apart of recombinant spike protein (25 μg) with Advax-CpG55.2™ (10 mg/200 μg) and 5 controls received saline injections. Serum antibody levels were followed for 3 months and then the animals were challenged with SARS-CoV-2 virus. Clinical signs, local reactions, body weight, food consumption and antibody levels were monitored till termination on either day 3 or 7 post-infection. Two weeks after the second dose, 8/9 immunized macaques had high serum spike and receptor binding domain binding antibodies that were able to cross-neutralize Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and, to a lesser extent, Omicron variants (B.1.1.529 ). Antibody levels decayed over the subsequent 3 months, and minimal neutralizing antibody was detectable immediately prior to the challenge which used a vaccine-homologous Wuhan-like ancestral virus. Of the nine vaccinated animals, only one 18-year-old female sacrificed at d3 had low levels of lung virus, versus 100 % of the control animals. Four of 5 (80 %) control animals had positive lung staining for SARS-CoV-2 virus versus just 1 of 9 (11 %) in the immunized group. The immunized animals exhibited better maintenance of appetite post-challenge. Neutralizing antibody levels rebounded rapidly in immunized animals, post-challenge. This data supports the benefits of Advax-CpG adjuvanted recombinant spike protein vaccine in protecting against a homologous SARS-CoV-2 infection.
Keywords: COVID-19, SARS-Cov-2, Vaccine, Adjuvant, Advax, Pandemic, Coronavirus
1. Introduction
Over 3 years from the initial outbreak, the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 virus (SARS‑CoV‑2) remains a global public health issue. It is likely that 80–90 % of the entire global population has by now being infected by this extremely transmissible virus [1]. Vaccines remain a key tool to control the spread and impact of SARS-CoV-2, with multiple candidates in use globally through various emergency use authorization (EUA) and provisional approval mechanisms [2]. Issues remain, however, with rapidly waning immunity, a need for regular boosters to maintain immunity, and immune escape by new virus variants, amongst others. New vaccine technologies, such as mRNA-based platforms are temperature sensitive with strict cold chain requirements [3], [4] presenting challenges for use in developing countries that lack required cold storage infrastructure [5], [6]. They can also cause rare but serious side effects including anaphylaxis [7], central venous thrombosis [8] and myocarditis [9], [10].
Several recombinant subunit spike protein vaccines have received marketing authorization (MA), including Vaxine/Cinnagen’s SpikoGen® vaccine, that received an emergency use authorization on October 6th, 2021 in Iran and Novavax’s Nuvaxoid® vaccine that received its first authorizations in Indonesia and the Philippines in November 2021. SpikoGen® and Nuvaxoid® are both produced in insect cell cultures using the baculovirus transient transfection system but have notable differences. SpikoGen® is based on a soluble spike trimer that lacks the transmembrane domain that normally anchors the spike protein to cell membranes [11]. SpikoGen® vaccine comprises the soluble spike protein extracellular domain (ECD) that assembles into a native trimer structure. Nuvaxoid® comprises full-length spike protein that forms nanoparticles that need to be maintained in solution using lipids and surfactants [12]. Another notable difference is these vaccines contain different adjuvants; SpikoGen® uses a polysaccharide-based adjuvant system known as Advax-CpG55.2™, whereas Nuvaxoid® uses a saponin-based adjuvant system known as Matrix-M™ [12]. These adjuvants are very important as highly pure recombinant proteins have low immunogenicity [13]. Advax-CpG adjuvant combines plant-derived delta inulin with CpG55.2, a synthetic human toll-like receptor 9 (TLR9) agonist. CpG55.2 was the first human vaccine adjuvant to be designed by artificial intelligence [14], [15]. Advax-CpG adjuvant has been shown in preclinical studies to significantly boost vaccine immunogenicity with a positive safety profile [16], [17], [18], [19]. Advax-CpG adjuvant was a key part of previous coronavirus vaccines shown to be protective against severe acute respiratory syndrome coronavirus (SARS-CoV) [20] and Middle East respiratory syndrome coronavirus (MERS-CoV) [21]. Advax adjuvant has previously been tested in human clinical trials of influenza [NCT03945825; NCT03038776] and hepatitis B [NCT01951677] vaccines which have supported its efficacy and safety.
We previously reported the immunogenicity and protective efficacy of an Advax-CpG55.2™ adjuvanted recombinant spike protein ECD vaccine based on the original Wuhan virus strain in ferret [11] and hamster [22] challenge models. This vaccine platform formed the basis for the now licensed, SpikoGen® vaccine [23]. In the current study, we assessed the vaccine’s ability to induce neutralizing antibodies, including against heterologous variants, in repurposed aged cynomolgus macaques. The animals were then challenged with a homologous Wuhan-like SARS-CoV-2 virus strain, USA-WA1/2020, with the results confirming vaccine protection of immunized animals and with no features to suggest antibody-disease enhancement (ADE), a problem previously encountered with some SARS-CoV vaccines [20].
2. Methods
2.1. Vaccines and adjuvants
The study vaccine was based on the ECD construct (CoV-2-S-ΔTM) corresponding to amino acids 14—1213 of the spike protein of the Wuhan-Hu-1 strain (accession number: NC 045512) (Wuhan) with an added polyhistidine tail to assist in purification. It was produced using a baculovirus expression system, as previously described [11]. The purified CoV-2-S-ΔTM protein was formulated with Advax-CpG55.2™ adjuvant. It formed the prototype for the now licensed human SpikoGen® vaccine [23] which shares the same key features [11].
2.2. Ethics statement
All NHP immunizations were performed at AlphaGenesis primate facility in South Carolina and then the animals transferred to the University of Georgia for the challenge studies. The AlphaGenesis Institutional Animal Care and Use Committee approved the immunization studies, and the University of Georgia Institutional Animal Care and Use Committee approved the SARS-CoV-2 challenges, all of which were conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals, The Animal Welfare Act, and the CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories guide.
2.3. NHP immunization and challenge
All NHP were repurposed experimental animals held at the AlphaGenesis NHP facilities in South Carolina. Fourteen aged cynomolgus monkeys were used, 9 in the active vaccine group and 5 controls. Apart from one younger animal aged 4.6 years, the rest of the animals were middle aged to elderly with a median age of 15.4 years and the oldest being just over 20 years (Table 1 ). Most were female (11/14, 79 %) ex-breeders. The 9 animals in the vaccine group had previously received two intramuscular injections of a DNA plasmid encoding spike protein that had failed to induce detectable serum spike-binding IgG. The active immunization group received two intramuscular injections 10 days apart of recombinant CoV-2-S-ΔTM protein (25 μg) adjuvanted with Advax/CpG55.2 (10 mg/200 μg) adjuvant. The 5 control animals received a saline placebo injection. Blood was obtained at baseline (day −4) and at regular intervals thereafter as depicted in Fig. 1 A. The animals were transferred to the animal facility at University of Georgia where they had an additional blood sample taken on d103 post-vaccination prior to being challenged with SARS-CoV-2 virus on d103, which was 93 days after their most recent immunization. SARS-CoV-2 passage (P) 3 isolate USA-WA1/2020 (BEI resources, NR-52281, GenBank accession number MN985325.1) was used to generate the animal exposure stock (P4). The stock was generated by infecting VERO-E6 cells in DMEM containing 2 % FBS and viral supernatant was harvested at four days post infection. Animals were infected with a total of 3 ml of 1 × 106 plaque-forming units (PFU)/ml of the SARS-CoV-2 viral strain, USA-WA1/2020 (NR-52281; BEI Resources, Manassas, VA, USA) under isofluorane anesthesia; i.e., 1 ml drop-wise with a 3 ml syringe via the intranasal route (0.5 ml into each nostril), 1 ml drop-wise onto the surface of the eye and 1 ml drop-wise on to the back palate and throat. Mortality, clinical signs, local reactions, body weight, food consumption and immunogenicity were monitored throughout the challenge period. Animals from each group were euthanized at either d3 or d7 post-infection with a post-mortem performed on each animal by a trained pathologist. At necropsy, the dorsal and ventral surfaces of the lungs were photographed and samples including lower lung kept fresh for virus evaluation and lung tissue from each of the 6 lobes, trachea, nasal turbinates, and tracheobronchial (hilar) lymph nodes was placed in 10 % buffered formalin for histopathology. A histologic description, morphologic diagnoses, and comment were given in the reports of the individual animals, with the full pathology report on each animal included in the Supplementary material.
Table 1.
Pre and post-challenge data for individual monkeys.
| Control | Control | Control | Control | Control | Vaccine | Vaccine | Vaccine | Vaccine | Vaccine | Vaccine | Vaccine | Vaccine | Vaccine | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal ID | 2020–3 | 2020–4 | 2020–5 | 2020–1 | 2020–2 | 2020–6 | 2020–7 | 2020–8 | 2020–9 | 2020–10 | 2020–11 | 2020–12 | 2020–13 | 2020–14 |
| Day of sacrifice | D3 | D3 | D3 | D7 | D7 | D3 | D3 | D3 | D7 | D7 | D7 | D7 | D7 | D7 |
| Age | 14.1 | 17.1 | 19.8 | 4.6 | 9.75 | 11.25 | 12.5 | 18.4 | 19.6 | 19.5 | 20.25 | 15.25 | 16.15 | 16.5 |
| Sex (F/M) | F | F | F | F | M | F | F | F | F | M | M | F | F | F |
| Weight (kg) | 4.11 | 4.81 | 3.67 | 3.62 | 7.71 | 3.08 | 9.48 | 4.42 | 5.27 | 4.66 | 7.81 | 5.94 | 7.45 | 4.97 |
| Wuhan cVNT2 wks post 2nd dose | 0 | 0 | 0 | 0 | 0 | 640 | 640 | 160 | 640 | 320 | 640 | 0 | 0 | 640 |
| Wuhan cVNTpre-challenge | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total Lung Score 0–84 | 39 | 21 | 27 | 25 | 51 | 0 | 5 | 27 | 4 | 0 | 27 | 6 | 19 | 22 |
| Type II hyperplasia 0–18 | 11 | 5 | 3 | 5 | 12 | 0 | 0 | 5 | 0 | 0 | 6 | 1 | 5 | 6 |
| Syncytia present | Y | N | Y | N | Y | N | Y | Y | N | N | Y | N | Y | N |
| Perivascular score 0–18 | 0 | 2 | 2 | 1 | 0 | 4 | 3 | 3 | 5 | 3 | 4 | 3 | 3 | 3 |
| Total BALT 0–18 | 6 | 5 | 3 | 8 | 3 | 4 | 1 | 0 | 6 | 5 | 7 | 10 | 5 | 10 |
| Bronchitis score 0–18 | 9 | 1 | 4 | 3 | 1 | 5 | 7 | 4 | 5 | 0 | 0 | 1 | 2 | 11 |
| Trachea score 0–3 | 1 | 1 | 2 | 1 | 0 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 2 |
| Nasal turbinate score 0–3 | 3 | 2 | 3 | 2 | 1 | 1 | 3 | 3 | 2 | 0 | 2 | 2 | 2 | 2 |
| SARS-CoV-2 IHC | Y | N | Y | Y | Y | N | N | Y | N | N | N | N | N | N |
| Lung virus load | 2.0 × 105 | 1.2 × 106 | 1.2 × 104 | 5.5 × 105 | 2.0 × 103 | N.D. | N.D. | 4.2. × 102 | N.D | N.D | N.D | N.D | N.D | N.D |
Fig. 1.
Kinetics of spike antibody responses in immunized macaques. Schematic of study (A). Nine cynomolgus macaques up to 20 years of age received 2 intramuscular doses of Advax-CpG55.2 adjuvanted recombinant spike protein vaccine 25 µg, 10 days apart. The remaining 5 animals received a saline placebo. Serum spike protein (Sp)-binding IgG (B) and RBD-binding IgG (C) by ELISA and neutralization activity by lentivirus pseudotype (pVNT) assay (D) pre- and post-immunization and on days 3 and 7 post SARS-CoV-2 virus challenge.
2.4. Haematoxylin & eosin (H&E) and immunohistochemistry staining of lungs
To assess the viral replication and pathological effect of infection, animals were euthanized d3 or d7 post-infection. The right lung lobes were taken for viral plaques, the incision was tied with surgical suture, and the lung was inflated with 10 ml formalin. Lungs were removed and placed into formalin for 1 week prior to paraffin embedding. Lungs were embedded into paraffin and were cut using a Leica microtome. Transverse 4 µm sections were placed onto Apex superior adhesive glass slides (Leica biosystem Inc, Buffalo Grove, IL, USA), which were coated for a positive charge, and were processed for H&E staining. Briefly, sections were deparaffinized in xylene and hydrated using different concentrations of ethanol (100 %, 95 %, 80 % and 75 %) for 2 min each. Deparaffinized and hydrated lung sections were stained with hematoxylin (MilliporeSigma, MA, USA) for 8 min at RT, differentiated in 1 % acid alcohol for 10 sec, and then counterstained with eosin (MilliporeSigma, MA, USA) for 30 sec. Slides were then dehydrated with 95 % and 100 % ethanol, cleared by xylene, and mounted using Permount® mounting media (ThermoFisher scientific, MA, USA). Lung lesions were scored by a board-certified veterinary pathologist blinded to the study groups as follows: Alveolar (ALV) score: 1 = focal, 2 = multifocal, 3 = multifocal to coalescing, 4 = majority of section infiltrated by leukocytes; Perivascular cuffing (PVC) score: 1 = 1 layer of leukocytes surrounding blood vessel, 2 = 2–5 layers, 3 = 6 – 10 layers, 4 = greater than 10 cells thick; Interstitial Pneumonia (IP) score: 1 = alveolar septa thickened by 1 leukocyte, 2 = 2 leukocytes thick, 3 = 3 leukocytes, 4 = 4 leukocytes. Additional lung sections were stained for SARS-CoV-2 or cytokeratin (clone AE1/AE3, Leica Biosystems, Deer Park, IL, USA) by immunohistochemistry. For lung immunohistochemistry, the deparaffinized and hydrated lung tissue sections were subjected to antigen retrieval by sub-boiling in 10 nM sodium citrate buffer at pH6 for 10 min and then incubated in 3 % fresh-made hydrogen peroxide for 10 min to inactivate endogenous peroxidase at RT. The lung sections were blocked with 5 % horse serum in PBS for 1 h at RT, incubated with SARS-CoV-2 nucleoprotein polyclonal antibody at 1:500 dilution (Invitrogen, Carlsbad, CA, USA) overnight at 4 °C, and then incubated with biotinylated goat-antibody Rabbit IgG H&L (Abcam, Waltham, MA, USA) at 1:1000 dilution for 1 h at RT. The avidin–biotin-peroxidase complex (VectStain Standard ABC kit, Vector Laboratories, Burlingham, CA, USA) was used to localize the biotinylated antibody, and DAB (Vector Laboratories, CA, USA) was utilized for color development. Sections were then counterstained with hematoxylin, and then mounted using Permount® mounting media (ThermoFisher Scientific, Waltham, MA, USA). Images were obtained by Aperio digital slide scanner AT2 (Leica biosystem, Buffalo Grove, IL, USA).
2.5. Lung tissue virus titration
Plaque assays were used to quantify infectious virus in tissue homogenates. Briefly, 10-fold serial dilutions were prepared in BA-1 media supplemented with antibiotics. Confluent Vero E6 cell monolayers were grown in 6-well tissue culture plates. The growth media was removed from the cell monolayers and each well was inoculated with 0.1 ml of the appropriate diluted sample. The plates were rocked every 10–15 min for 45 min and then overlaid with 0.5 % agarose in in MEM without phenol red and incubated for 1 day at 37 °C, 5 % CO2. A second overlay with neutral red dye was added at 24–30 h and plaques were counted at 48–72 h post-plating. Viral titers are reported as the log10 plaque forming units (PFU) per 100 mg of tissue. Samples were considered negative for infectious virus if viral titers were below the limit of detection (LOD). The theoretical limit of detection was calculated using the equation: LOD = log [1/(N × V)], where N is the number of replicates per sample at the lowest dilution tested; V is the volume used for viral enumeration (volume inoculated/well in ml). For lung tissues the LOD was 10 PFU/100 mg.
2.6. Spike and RBD-binding IgG ELISA
Recombinant Spike (Sp) and receptor binding domain (RBD)-specific antibodies were determined by ELISA, as previously described (11) with minor modification. Briefly, 1 µg/ml Sp or 0.5 µg/ml RBD antigen (both prepared in-house) were used to coat 96-well ELISA plates (100 µl/well). After blocking plates, 100 µl/well of 1:1000-diluted serum samples were added to wells. After 2 h incubated at RT, plates were washed and wells received 100 µl/well HRP-conjugated anti-human IgG antibody (Sigma-Aldrich) then incubated 1 h at RT. After washing, TMB substrate (KPL, SeraCare, Gaithersburg, MD, USA) was added and incubated for 10 min at RT before the reaction was stopped with 100 µl 1 M Phosphoric Acid. The optical density was measured at 450 nm (OD450nm) using a VersaMax plate reader and analyzed using SoftMax Pro Software. Average OD450nm values obtained from negative control wells were subtracted.
2.7. SARS-CoV-2 spike pseudotype lentivirus neutralization assay
A non-replicative SARS-CoV-2 spike protein lentivirus pseudotype virus neutralization (pVNT) assay was developed to evaluate neutralization activity in sera, as previously described [11]. In brief, lentiviral particles pseudotyped with SARS-CoV-2 spike envelope were produced by co-transfecting HEK 293 T cells with a GFP encoding 3rd generation lentiviral plasmid pHRSIN-CSGW, psPAX2 and plasmid-expressing codon-optimized but C-terminal truncated SARS-CoV-2 S protein (pCG1-SARS-2-S Delta18). Neutralization activity of donor sera was measured using a single round infection of ACE2-HEK 293 T with spike pseudotype lentiviral particles. Virus particles were pre-incubated with serially diluted donor sera for 1 h at 37 °C. Virus-serum was then added onto ACE2-HEK 293 T cells seeded at 2,500 cells per well in a 384-well tissue culture plate (Corning #CLS3985) a day before. Following spinoculation at 1200xg for 1 h at 18 °C, the cells were moved to 37 °C for a further 72 h. Entry of pseudotyped particles was assessed by imaging GFP-positive cells and total cell numbers imaged through live nuclei counter staining using NucBlue (Invitrogen, #R37605). Total cell counts and % GFP-positive cells were acquired using the InCell imaging platform (IN Cell Analyzer 2500HS, Cytiva) followed by enumeration with InCarta high content image analysis software (Cytiva). Neutralization was measured by reduction in % GFP expression relative to control group infected with the virus particles without any serum treatment.
2.8. SARS-CoV-2 live virus neutralization assay
A high-content microscopy approach was used to assess the ability of NHP sera to inhibit SARS-CoV-2 viral infection and the resulting cytopathic effect in live permissive cells. Briefly, serum samples were diluted in cell culture medium (MEM-2 % FCS) to create a 2-fold dilution series (e.g. 1:20 to 1:160). Each dilution was then mixed (in duplicate) with an equal volume of virus solution at about 8x103 TCID50/ml (so that dilution series becomes 1:40–1:320), followed by 1 h incubation at 37 °C. Meanwhile, freshly trypsinized Vero E6 cells were and plated in 384-well plates (Corning) at 5x103 cells per well in 40 μl. After 1 h of virus-serum coincubation, 40 μl were added to the cell-plate for a final well volume of 80 μl. Plates were incubated for 72 h until readout (37 °C, 5 % CO2, >90 % relative humidity), which occurred by staining cellular nuclei with NucBlue dye (Invitrogen) and imaging the entire well’s area with a high-content fluorescence microscopy system (Cytiva). The number of cells per well was determined using InCarta image analysis software (Cytiva). The percentage of viral neutralization for each well was calculated with the formula N = (D-(1-Q))x100/D, where “Q” is the well’s nuclear count divided by the average nuclear count of the untreated control wells (i.e. without virus or serum), and “D” equals 1 minus the average Q-value for the positive infection control wells (i.e. cells + virus, without serum). Therefore, the average nuclear counts for the infected and uninfected cell controls are defined as 0 % and 100 % neutralization levels, respectively. The threshold for determining the neutralization endpoint titer of diluted serum samples mixed with virus was set to N ≥ 50 %.
2.9. Statistical analysis
GraphPad Prism 8.3.1 for Windows was used for drawing graphs and statistical analysis (GraphPad Software, San Diego, CA, USA). Limit of detection for viral plaque titers was 10 PFU/100 mg, so titers below this were allocated a value of half the limit of detection to allow for statistical analysis. PRNT90 are presented as geometric mean titers, the minimum dilution tested for neutralizing antibody was 1:20, and a titer of 10 was used for a negative result to assist statistical analysis. For statistical comparisons of lung histology scores, we combined data from animals sacrificed at the D3 and D7 time points to increase the sample size per group and increase statistical power. For all comparisons, p < 0.05 was considered to represent a significant difference. In figures * = p < 0.05; ** = p < 0.01; *** = p < 0.001 and ****, p < 0.0001.
3. Results
3.1. Vaccine immunogenicity
The overall study schema is depicted in Fig. 1A. Despite being repurposed animals that had previously received two intramuscular injections of a plasmid intended to express spike protein, the animals did not have measurable serum Sp- or RBD-binding IgG or SARS-CoV-2 neutralizing antibodies at baseline. The plasmid used was later found to be defective as it did not induce spike protein expression when used to transfect HEK cells, in vitro (data not shown). D7 sera after the first protein vaccine dose, showed detectable levels of Sp-binding IgG by ELISA in the vaccine, but not the control, group (Fig. 1B). After the second vaccine dose, Sp-binding IgG levels increased further, peaking around 2 weeks after the 2nd dose. Sp-binding IgG levels then decayed almost back to baseline over the ensuring 3 months. In response to the virus challenge, Sp-binding IgG showed a rapid jump in immunized but not in the control animals, consistent with an amnestic memory response. Notably, the control animals showed low or no Sp-binding IgG at d7 post challenge. Only a subfraction of Sp-binding IgG is neutralizing, and hence RBD-binding IgG may serve as a better guide to neutralizing antibody levels [24]. The pattern of serum RBD-binding IgG in immunized animals directly mirrored Sp-binding IgG levels, with a strong rise after the first vaccine dose, a peak shortly after the second dose and a subsequent decline back towards baseline over the ensuring 3 months. Starting d3 post challenge and more evident by d7 post-challenge, there was a sharp rebound in RBD-binding IgG restricted to immunized animals, which reached similar levels to peak levels after the 2nd dose (Fig. 1C). The control animals had no detectable serum RBD-binding IgG except for a small amount detected in just some of the control animals at d7 post challenge.
The kinetics of the neutralizing antibody was assessed by a Wuhan pVNT assay (Fig. 1D). The pVNT results mirrored the Sp- and RBD-binding IgG levels, with a peak soon after the second dose, then reducing back to baseline over 3 months, then a sharp increase post SARS-CoV-2 challenge, reaching a similar level as the post 2nd dose peak. Notably, no control animals had a detectable serum pVNT response even at d7 post-challenge.
Next, to assess the ability of the vaccine to induce cross neutralizing antibodies against variants of concern, serum pVNT responses in immunized animals 2 weeks after the 2nd dose against Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2) and Omicron BA.1, BA.2 and BA.4/5 (B.1.1.529 and sub-variants) were compared to Wuhan. Two weeks after the 2nd dose, immunized animals had a pVNT GMT of 367 against Wuhan, with just 1 out of 9 animals showing a low but still detectable pVNT response after 3 months (Fig. 2 ). Two weeks after the second dose high levels of cross-neutralization were seen against the Alpha (B.1.1.7) (GMT 526) and Beta (B.1.351) (GMT 546) variants. While some animals exhibited high neutralization of Gamma (P.1) and Delta (B.1.617.2) variants, at levels similar to the ancestral strains, other animals exhibited much lower neutralization titers against these newer variants. There was much lower cross-neutralization of the Omicron (B.1.1.529) subvariants; BA.1 (GMT 35), BA.2 (GMT 19) and BA.4/5 (GMT 11).
Fig. 2.
Serum neutralizing antibody responses in cynomolgus macaques against SARS-CoV-2 variants by pVNT assay 2 weeks post the 2nd dose of Advax-CpG55.2 adjuvanted recombinant spike protein (column and error bars represent GMT and 95 % confidence intervals). Numbers at top of figure depict the GMT against each variant.
Conventional virus neutralizing assays (cVNT) that use wild type SARS-CoV-2 virus have to be performed under BSL3 conditions, restricting their use. cVNT assays were performed against Wuhan, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2) variants using sera obtained 2 weeks after the 2nd dose, immediately prior to challenge, and d7 post-challenge (Supplementary Fig. S1A). The cVNT assays showed largely the same pattern as the pVNT assay, with highest cVNT levels against Wuhan and Alpha (B.1.1.7), but with a ∼ 4-fold drop in titer against Beta (B.1.351), Gamma (P.1) and Delta (B.1.617.2). As seen for pVNT, cVNT levels in immunized animals decayed over time and became largely undetectable against all the tested variants after ∼ 3 months. cVNT levels in immunized animals rebounded strongly by d7 post-challenge including against heterologous variants whereas the control animals did not show cVNT activity against any of the variants even at d7 post-challenge. When the pattern of cVNT against the variant strains were examined across individual immunized animals, a wide variation was seen; some immunized animals neutralized all the variants tested, whereas others only neutralized the ancestral strains (Supplementary Fig. S1B).
3.2. Vaccine effects on clinical symptoms
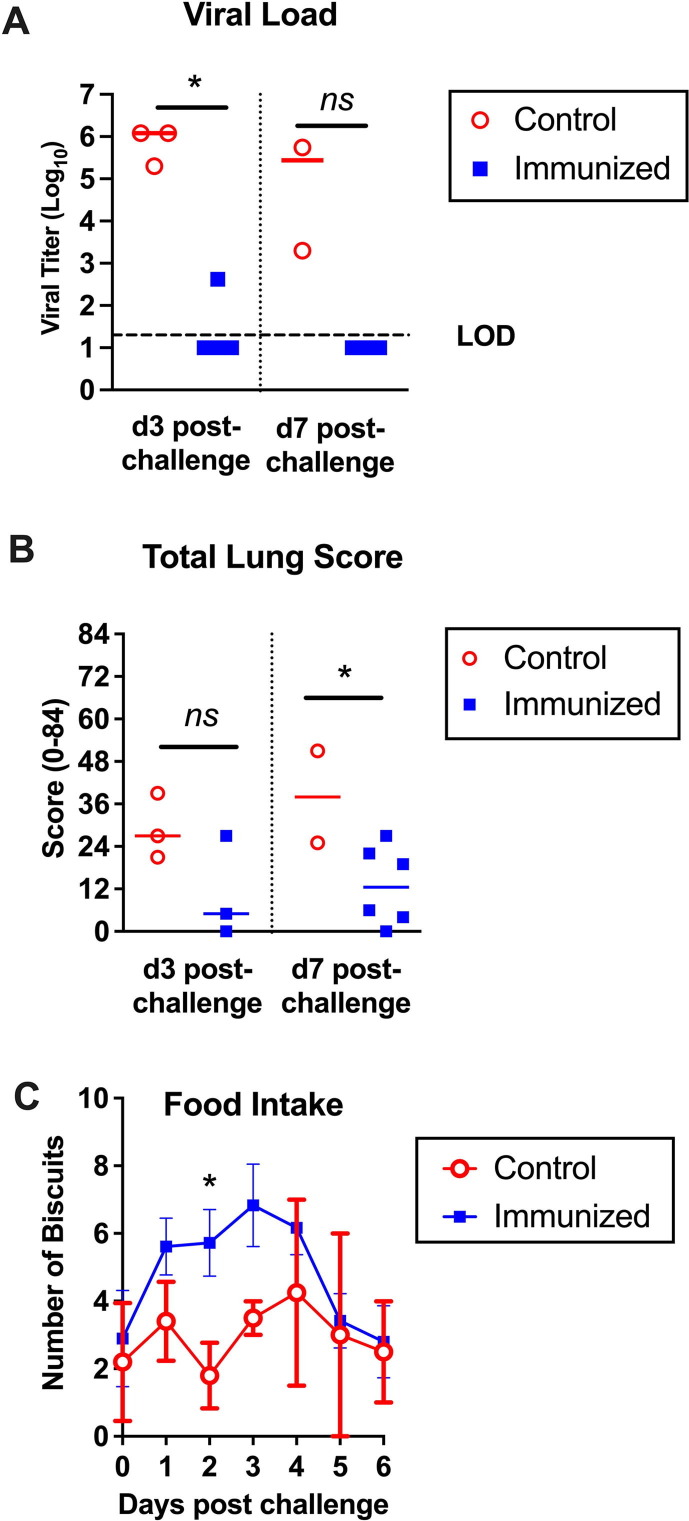
COVID-19 generally causes a mild self-limiting disease in NHP with few clinical signs of infection apart from decreased appetite and changes in bowel movement [25]. No significant differences were detected in body score, oxygen saturation, respiratory rate or rectal temperature between the immunized and control animals (Supplementary Fig. S2). However, the animal handlers reported that the control animals were less active than the immunized animals, with some controls having less interest in eating and exhibiting diarrhea. The daily biscuit count through the challenge period showed a significant increase in the number of daily biscuits consumed by the immunized when compared to control animals, with the biggest differences at days 2–3 post challenge (Fig. 3 C). This supported the impression of the handlers that the control animals were more unwell than the vaccinated animals during the challenge period.
Fig. 3.
Advax-CpG55.2 adjuvanted recombinant spike protein protection against virus replication, lung pathology and clinical illness in cynomolgus macaques. Animals were sacrificed either day 3 or day 7 post-challenge and assessed for SARS-CoV-2 viral load in the lower lung lobe (A), total lung score by histology (B) and daily ingested biscuit count (C).
3.3. Vaccine effects on lung virus replication
To assess vaccine effects on viral spread to the lungs, virus titers were measured in tissue obtained from the lower lung lobe of each animal, post-mortem (Fig. 3A). All control animals had recoverable live virus by plaque assays when sacrificed at d3 or d7 post-challenge. By contrast, there was no recoverable live virus in the lungs of 8/9 (89 %) of the immunized animals, with the remaining animal being a 18 year old female who despite no symptoms had a low level of lung virus detected when sacrificed at d3 (Table 1).
3.4. Vaccine effects on lung pathology
To evaluate whether the vaccine could protect against lung pathology, portions of the lung collected from all animals sacrificed at d3 and d7 were subjected to H&E staining and immunohistochemistry for SARS-CoV-2 virus. Total lung scores were for terminal bronchiole and interstitial changes and were a sum of 4 parameters (amount of involvement, septal thickening, type II cell hyperplasia, and alveolar cells) derived from all 6 lobes, resulting in a total potential score of 84. Mean total lung scores at post-mortem were lower across the immunized animals when compared to the controls (12.2 vs 32.6, p = 0.01, two-tailed t-test) with two immunized animals having a total lung score of 0 (Fig. 3B and Table 1). All animals in the control group developed a mild to moderate pneumonia (representative histology of 2 infected control animals is shown in Supplementary Fig. S3); most had tracheitis, bronchitis and lymphoplasmacytic rhinitis, often with eosinophils (Details on individual animals shown in Supplementary material). By contrast, when immunized monkeys exhibited pneumonia it was minimal to mild with some tracheitis, bronchitis and lymphoplasmacytic rhinitis. Pneumonia was characterized as broncho-interstitial/interstitial with type II cell hyperplasia, syncytia and variable alveolar exudate with sloughed epithelial cells, as previously reported for cynomolgus macaques [26]. Both immunized and control animals developed some degree of lymphoid hyperplasia of the tracheobronchial lymph nodes. Pneumonia was usually most severe in middle and lower lung lobes. Changes in the parenchyma of animals with pneumonia were characterized by multiple foci of alveolar septal thickening with variable amounts of type II hyperplasia which was more severe on d7. In less severe foci, the alveoli were either empty or contained histiocytic cells. As severity increased alveoli contained large mononuclear cells (suspected to be sloughed type II cells), syncytia, and macrophages, and in the most severe cases, the exudate included lymphocytes and neutrophils, edema, and less frequently bits of fibrin. In many lungs, these changes could be observed extending from terminal bronchioles. In addition, some lungs showed significant perivascular infiltrations by lymphocytes often admixed with eosinophils.
Type II cell hyperplasia was used as part of the total lung score, but was also separated out as it might be an indicator of alveolar damage or viral replication as the virus is suspected to replicate in type II cells [27] with positive staining for SARS-CoV-2 in these cells in this study supporting this. For each animal, each lobe was individually scored (0–3) and summed (resulting in a score of 0–18). All control animals had type II cell hyperplasia with a mean score of 7.2 ± 3.6 versus a mean score of 2.6 ± 2.6 for the immunized group, p = 0.026, two-tailed t-test (Supplementary Fig. S4).
SARS-CoV-2 causes the development of syncytial cells making these a marker of virus infection [28]. Syncytial cells were reported by the study pathologist in the lungs of 3/5 (60 %) of the unvaccinated animals and 4/9 (44.4 %) of the immunized animals although the levels of syncytial cells did not correlate with the degree of pneumonia. BALT was scored separately for each lobe (0–3) and summed resulting in a total score of 0–18. The mean BALT scores increased from d3 to d7 in both groups (Supplementary Fig. S4) and was highest in the immunized animals. Perivascular inflammation in the lung was scored separately from the total lung score. For each animal, each lobe was scored 0–3 and then summed resulting in a total score of 0–18 and again was higher in the immunized animals. The perivascular infiltration scores varied from 0 to 5 (out of 18) with the immunized animals having higher scores than the controls (3–5 vs 0–2, respectively).
Nasal turbinate scores (rhinitis) decreased from d3 to d7 in both immunized and control animals. All animals had rhinitis that varied from mild to severe was lymphoplasmacytic in all groups. The two animals in the immunized group with lung scores of 0 also had little or no rhinitis. Tracheal scores for tracheitis were scored separately and decreased from d3 to d7. All but one animal had tracheitis, which was lymphoplasmacytic often with varying numbers of eosinophils, and varied from mild to moderate. Bronchitis was scored separate from the lung parenchyma. Like the rest of the upper airway, the scores decreased from d3 to d7 in both immunized and control groups. It was characterized by lymphocytic infiltration, often with eosinophils.
3.5. Immunohistochemistry for SARS-CoV-2 and cytokeratin
Immunohistochemistry for SARS-CoV-2 nuclear protein was performed on lung, targeting histologic sections with lesions (example shown in (Supplementary Fig. S3). Positivity for SARS-CoV-2 staining was scant to mild and multifocal and not in all areas of pneumonia. When present it was seen as linear staining, suspected to be type I cells and in hypertrophied type II cells and rarely syncytia. Four of 5 (80 %) control animals had positive staining for SARS-CoV-2 nuclear protein, consistent with all this group having recoverable lung virus by plaque assay whereas only 1 out of 9 (11 %) animals in the immunized group had positive staining, a 18 year old female (Animal ID: 2020–8) which was also the only one of the immunized animals to have a low level of recoverable lung virus when sacrificed at d3 (Table 1) (p = 0.023, Fishers exact test).
4. Discussion
Recombinant protein-based COVID-19 vaccines make a useful addition to the other vaccine technologies on offer to prevent SARS-CoV-2 infection. In addition to efficacy, protein-based vaccines have a robust safety record. The results showed that two intramuscular doses of Advax-CpG55.2™ adjuvanted CoV-2-S-ΔTM vaccine protected cynomolgus macaques, many of which were elderly, against a homologous virus challenge. Although not designed as a formal GLP safety and toxicology study, it was reassuring that no adverse safety issues were identified. In particular, 8 of the 9 immunized monkeys had no live virus recovered from the lungs at d3 or d7 post-challenge together with negative lung staining for SARS-CoV-2, an important finding as major lung involvement is common in severe and lethal COVID-19 disease [29]. While SARS-CoV-2 does not typically cause mortality in the macaque model [25], control animals still had high levels of infectious virus in the lungs at d7 and the lung viral load correlated with total lung pathology scores, consistent with the cynomolgus macaque being a stringent challenge model reflective of human disease [26].
Advax-CpG55.2™ is a new polysaccharide adjuvant formulation where the CpG55.2 component was developed through artificial intelligence, with machine learning being used to screen large numbers of oligonucleotides for human TLR9 agonist activity [30]. This NHP protection data further supports the efficacy and safety of this Advax-CpG55.2™ adjuvanted recombinant spike protein ECD vaccine as also demonstrated in ferret [11] and hamster [22] models. Advax-CpG adjuvant has been previously demonstrated to broaden the antibody repertoire against viral antigens, inducing broad protection against a range of JEV-serotype flaviviruses when combined with an inactivated JEV antigen [31], and expanding the B cell receptor repertoire against influenza in a human trial where it was administered with a seasonal influenza vaccine [32]. Although based on the original Wuhan spike protein sequence, the Advax-CpG55.2™ adjuvanted vaccine induced broadly neutralizing antibody in the NHP against a wide range of variants of concern including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). The drop in the neutralization titers against these variants was relatively modest, with good neutralization of Beta (B.1.351), despite Beta being a known early immune escape variant [33].
A particular strength of our study was that we were able to include older macaques with a median age of 15 years and extending up to over 20 years of age. This is equivalent to a middle aged to elderly human population, based on an ageing factor of 3 to 1 [25], [34]. Notably, elderly humans are most susceptible to severe COVID-19 infection [35]. The older age of our animals might explain why the unimmunized control animals did not spontaneously clear the virus infection from their lungs, even at day 7 post-infection. A further strength of our study was that animals were not challenged immediately after vaccination when serum antibodies were at their peak, as has been the trend for other reported challenge studies where, for example, challenges were performed just 2-4 weeks after the final immunization [36], [37], [38], [39]. Instead, our animals were rested for 3 months after the second dose which meant their serum antibodies had declined significantly from their peak at the time of the challenge. Indeed, neutralizing antibody levels had largely decayed back to baseline levels in many of the immunized animals by the time of challenge, but they nevertheless demonstrated robust lung protection. This finding also was important in excluding the possibility of vaccine-induced antibody-dependent disease enhancement (ADE). A study of SARS-CoV-2 reinfection in elderly macaques showed reinfection caused severe pneumonia with increased levels of various serum cytokines and chemokines compared with that seen during the primary infection, a potential signal that ADE might occur in some COVID-19 situations [25]. The lack of evidence of ADE in our vaccinated macaques was reassuring, given the absence of serum neutralizing antibody at the time of challenge, a context in which ADE caused by low residual levels of non-neutralizing antibodies might occur [40].
COVID-19 vaccine studies in animals and humans have generally shown a good correlation between serum spike antibody levels and protection against systemic disease [41], [42], [43]. What is interesting in our study is that we saw good lung protection in the immunized animals despite the absence of measurable serum neutralizing antibody immediately prior to challenge. This means that pre-exposure serum neutralizing antibody levels might not be the only potential mediator of SARS-CoV-2 protection. Instead, the level of memory B cells may provide a better correlate of SARS-CoV-2 protection, reflecting their ability to rapidly produce high affinity antibody in response to infection. The early amnestic rebound in serum antibody levels in the immunized animals suggested that the vaccine had induced a strong underlying memory B cell response able to respond rapidly to infection. T cell responses were not able to be measured in this study. However, in murine studies the vaccine induced memory CD4 and CD8 T cells plus cytotoxic T lymphocytes that were able to efficiently kill spike-labelled target cells [11]. Hence, T cell immunity may be equally important to COVID-19 protection as humoral immunity [44].
The antigen in the study vaccine had the transmembrane domain of the spike protein removed to enable its production as a secreted soluble form, contrasting with the membrane-bound spike protein in the Nuvaxoid® vaccine [12]. Maintaining the correct structure of the spike protein is critical as it readily transitions from a pre-fusion to a post-fusion conformation [45] and holding the spike protein antigen in the pre-fusion state has been suggested to lead to production of greater quantities of neutralizing antibodies [46], [47]. Our spike protein ECD antigen induced high serum titers of neutralizing antibody indicating it was in the correct prefusion trimeric form despite absence of the transmembrane domain. As seen with other vaccines, neutralization of the Omicron variants (B.1.1.529) BA.1, BA.2 and particularly BA.4/5, was lower than for other strains, consistent with Omicron’s known immune-escape properties. This suggests that two doses of Wuhan-based vaccine may be inadequate to provide robust protection against Omicron (B.1.1.529) with additional vaccine doses being required.
Potential limitations of this study were the relatively small sample size, although this size was typical for NHP studies given their complex logistics and cost. There was a high consistency of responses within each group and significant differences were apparent in all major parameters including neutralizing antibody levels, lung virus load, and total lung scores between the vaccine and the saline control groups. Hence, the group size was adequate to show vaccine safety and efficacy. The obtained results closely reflected those obtained in immunized hamsters [22] and ferrets [11]. Despite the repurposed animals having had previously received two intramuscular injections of a plasmid intended to express spike protein, this did not induce measurable serum antibodies and the plasmid used was later found to be defective as it did not induce spike protein expression when used in vitro to transfect HEK cells. While we cannot exclude the possibility that this plasmid injection might have contributed in some way to the protection observed via, for example, priming of T cell responses, this was considered unlikely given the complete inability of the plasmid to induce spike protein expression in vitro. Another limitation was that challenges were only performed with a vaccine-homologous virus strain. However, it was reassuring that most of the immunized animals exhibited broadly neutralizing antibodies against Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) suggesting that the study vaccine should afford some protection against these variants. Notably, SpikoGen® vaccine which is based on the same platform received an emergency use authorization on October 6th, 2021 in Iran after demonstrating efficacy against COVID-19 infection in its pivotal Phase 3 trial. In conclusion, this study demonstrated that a vaccine platform based on Advax-CpG55.2™ adjuvanted recombinant spike protein ECD was safe and well tolerated, providing protection of cynomolgus macaques against SARS-CoV-2 lung infection. Reassuringly, there was no evidence of ADE even although serum neutralizing antibody levels were low or undetectable at the time of challenge. This supports the ongoing clinical development of this protein-based COVID-19 vaccine platform.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: YHO, GA and NP are affiliated with Vaxine Pty Ltd which holds the rights to COVAX-19/Spikogen vaccine and Advax™ and CpG55.2™ adjuvants.
Acknowledgements
We wish to thank Alberto Stella and Stuart Turville at the Kirby Institute for assisting with cVNT assays and Melissa Ferguson at AlphaGenesis for assistance with coordinating the NHP studies. This work was supported by funding from Vaxine, Macrovax Trust, Bridgenorth Pastoral Co, National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Contracts HHS-N272201400053C, HHSN272201800044C, and HHSN272201800024C, MTPConnect Biomedical Translation Bridge Program, and a Fast Grant administered by George Mason University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.063.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.World Health Organisation. Weekly operational update on COVID-19 -20 September 2021; 2021. p. 1–16.
- 2.Ndwandwe D., Wiysonge C.S. COVID-19 vaccines. Curr Opin Immunol. 2021;71:111–116. doi: 10.1016/j.coi.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaiser J. Temperature concerns could slow the rollout of new coronavirus vaccines. Science. 2020;16 [Google Scholar]
- 4.Sheikh A.B., Pal S., Javed N., Shekhar R. COVID-19 vaccination in developing nations: challenges and opportunities for innovation. Infect Disease Reports. 2021;13:429–436. doi: 10.3390/idr13020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu E., Ritchie H., Ortiz-Ospina E., Roser M., Hasell J., Appel C., et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 6.Loembé M.M., Nkengasong J.N. COVID-19 vaccine access in Africa: Global distribution, vaccine platforms, and challenges ahead. Immunity. 2021;54:1353–1362. doi: 10.1016/j.immuni.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel G., Kupferschmidt K. Side effect worry grows for AstraZeneca vaccine. Science. 2021;372:14. doi: 10.1126/science.372.6537.14. [DOI] [PubMed] [Google Scholar]
- 9.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Honda-Okubo Y., Huang Y., Jang H., Carlock M.A., Baldwin J., et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021;39:5940-5953 doi: 10.1016/j.vaccine.2021.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark T., Cassidy-Hanley D. Recombinant subunit vaccines: potentials and constraints. Dev Biol. 2005;121:153–163. [PubMed] [Google Scholar]
- 14.Petrovsky N., Cooper P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–5926. doi: 10.1016/j.vaccine.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas S., Abraham A., Baldwin J., Piplani S., Petrovsky N. Vaccine design. Springer; 2022. Artificial intelligence in vaccine and drug design; pp. 131–146. [DOI] [PubMed] [Google Scholar]
- 16.Görander S., Honda-Okubo Y., Bäckström M., Baldwin J., Bergström T., Petrovsky N., et al. A truncated glycoprotein G vaccine formulated with Advax-CpG adjuvant provides protection of mice against genital herpes simplex virus 2 infection. Vaccine. 2021;39:5866–5875. doi: 10.1016/j.vaccine.2021.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Sakala I.G., Honda-Okubo Y., Li L., Baldwin J., Petrovsky N. A M2 protein-based universal influenza vaccine containing Advax-SM adjuvant provides newborn protection via maternal or neonatal immunization. Vaccine. 2021;39:5162–5172. doi: 10.1016/j.vaccine.2021.07.037. [DOI] [PubMed] [Google Scholar]
- 18.Honda-Okubo Y., Baldwin J., Petrovsky N. Advax-CpG adjuvant provides antigen dose-sparing and enhanced immunogenicity for inactivated poliomyelitis virus vaccines. Pathogens. 2021;10:500. doi: 10.3390/pathogens10050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichinger K.M., Kosanovich J.L., Gidwani S.V., Zomback A., Lipp M.A., Perkins T.N., et al. Prefusion RSV F immunization elicits Th2-mediated lung pathology in mice when formulated with a Th2 (but not a Th1/Th2-balanced) adjuvant despite complete viral protection. Front Immunol. 2020;11:1673. doi: 10.3389/fimmu.2020.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.-H., Tseng C.-T.-K., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adney D.R., Wang L., Van Doremalen N., Shi W., Zhang Y., Kong W.-P., et al. Efficacy of an adjuvanted Middle East respiratory syndrome coronavirus spike protein vaccine in dromedary camels and alpacas. Viruses. 2019;11:212. doi: 10.3390/v11030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L., Honda-Okubo Y., Baldwin J., Bowen R., Bielefeldt-Ohmann H., Petrovsky N. Covax-19/Spikogen® vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters. Vaccine. 2022;40:3182–3192. doi: 10.1016/j.vaccine.2022.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabarsi P., Anjidani N., Shahpari R., Mardani M., Sabzvari A., Yazdani B., et al. Evaluating the efficacy and safety of SpikoGen(R), an Advax-CpG55.2-adjuvanted severe acute respiratory syndrome coronavirus 2 spike protein vaccine: a phase 3 randomized placebo-controlled trial. Clin Microbiol Infect. 2022;29:215–220. doi: 10.1016/j.cmi.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urano E., Okamura T., Ono C., Ueno S., Nagata S., Kamada H., et al. COVID-19 cynomolgus macaque model reflecting human COVID-19 pathological conditions. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2104847118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salguero F.J., White A.D., Slack G.S., Fotheringham S.A., Bewley K.R., Gooch K.E., et al. Comparison of rhesus and cynomolgus macaques as an infection model for COVID-19. Nat Commun. 2021;12:1260. doi: 10.1038/s41467-021-21389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges J.P., Vladar E.K., Huang H., Mason R.J. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax. 2022;77:203–209. doi: 10.1136/thoraxjnl-2021-217561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchrieser J., Dufloo J., Hubert M., Monel B., Planas D., Rajah M.M., et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020;39:e106267. doi: 10.15252/embj.2020106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosmuller H., Matter M., Fend F., Tzankov A. The pulmonary pathology of COVID-19. Virchows Arch. 2021;478:137–150. doi: 10.1007/s00428-021-03053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna V., Li L., Fung J., Ranganathan S., Petrovsky N. Prediction of novel mouse TLR9 agonists using a random forest approach. BMC Mol Cell Biol. 2019;20:56. doi: 10.1186/s12860-019-0241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komiya T., Honda-Okubo Y., Baldwin J., Petrovsky N. An advax-adjuvanted inactivated cell-culture derived Japanese encephalitis vaccine induces broadly neutralising anti-flavivirus antibodies, robust cellular immunity and provides single dose protection. Vaccines (Basel) 2021;9:1235. doi: 10.3390/vaccines9111235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L., Honda-Okubo Y., Li C., Sajkov D., Petrovsky N. Delta inulin adjuvant enhances plasmablast generation, expression of activation-induced cytidine deaminase and B-cell affinity maturation in human subjects receiving seasonal influenza vaccine. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabidi N.Z., Liew H.L., Farouk I.A., Puniyamurti A., Yip A.J.W., Wijesinghe V.N., et al. Evolution of SARS-CoV-2 variants: implications on immune escape, vaccination, therapeutic and diagnostic strategies. Viruses. 2023;15:944. doi: 10.3390/v15040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattison J.A., Vaughan K.L. An overview of nonhuman primates in aging research. Exp Gerontol. 2017;94:41–45. doi: 10.1016/j.exger.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 36.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.-T., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488. doi: 10.1016/j.cell.2020.12.015. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry E.J., Barouch D.H. T cell immunity to COVID-19 vaccines. Science. 2022;377:821–822. doi: 10.1126/science.add2897. [DOI] [PubMed] [Google Scholar]
- 45.Mercurio I., Tragni V., Busto F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. CellMol Life Sci. 2021;78:1501–1522. doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalnin K.V., Plitnik T., Kishko M., Zhang J., Zhang D., Beauvais A., et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines. 2021;6:61. doi: 10.1038/s41541-021-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juraszek J., Rutten L., Blokland S., Bouchier P., Voorzaat R., Ritschel T., et al. Stabilizing the closed SARS-CoV-2 spike trimer. Nat Commun. 2021;12:244. doi: 10.1038/s41467-020-20321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.