Abstract
Background
Post intensive care syndrome is defined as the presence of any impairment affecting the physical, psychiatric, or cognitive domains as a result of critical illnesses.
Objectives
To explore functional, cognitive and psychological outcomes at 30 days post hospital discharge among survivors of COVID-19–associated acute respiratory distress syndrome, who required mechanical ventilation.
Methods
Prospective cohort study. We included adult patients with COVID-19–associated acute respiratory distress syndrome, invasively ventilated in two ICUs in Buenos Aires. We measured functional, cognitive and psychological impairments with Barthel index, Montreal Cognitive Assessment test, Patient Health Questionnaire-9 and General Anxiety Disorder-7. Primary outcome was post-intensive care syndrome. Secondary outcome was mortality at 60 days.
Results
We admitted 40 patients, median age was 69 (60–75) and mostly male (75%). Mortality at 60 days was 37%. Cox regression analysis identified diabetes and Apache II as independent predictors of mortality. Out of 22 patients studied, 14 (64%) developed PICS after discharge. With a physical, cognitive and psychological impairment in 64%, 41% and 32% of patients, respectively. Obesity, days of mechanical ventilation, Apache II, vasopressors use, delirium duration and cumulative midazolam dose were associated with functional dependence.
Conclusions
We identified a high prevalence of functional, cognitive and mental impairment at 30 days after hospital discharge in COVID-19–associated acute respiratory distress syndrome survivors, invasively ventilated. The physical domain was the most frequently affected. These findings suggest the need for long-term follow-up of this population.
Keywords: Post-intensive care syndrome, COVID-19, Acute respiratory distress syndrome, Depression, Cognitive impairments, Functional dependency
Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-Cov-2 (Severe Acute Respiratory Syndrome Coronavirus-2) spread rapidly globally, the World Health Organization declared the pandemic on March 11, 2020.1 Between 5 and 10% of patients may present with pneumonia along with Acute Respiratory Distress Syndrome (ARDS) and multiple organ failure requiring admission to the Intensive Care Unit (ICU) .2
Survivors of prolonged stay in the ICU may experience mid and long term impairment that impact their quality of life.3 Post intensive care syndrome (PICS) is defined as the presence of any impairment affecting the physical, psychiatric, or cognitive domains as a result of critical illnesses.4 Some studies have shown that this syndrome affects between 20 and 80% of ICU survivors. A prospective study that included 821 patients with ARDS or shock found that 40% of survivors had cognitive outcomes comparable to moderate head trauma and 26% had a pattern similar to Alzheimer's disease.5 Cognitive impairment is present in more than a third of patients receiving mechanical ventilation. In this study, delirium duration was independently associated with occurrence of PICS and with worse global cognitive results at 3 and 12 months. This cognitive, functional and psychological impairment that affects patients who survive in ICU, and particularly those who require mechanical ventilation, is accompanied by a very large medical, social and economic impact.6
Recently, studies conducted in high-income countries have shown a high burden of physical, cognitive, and mental impairment in ICU survivors after COVID-19.7 , 8 However, there is little information on PICS in middle-income countries such as Argentina. Our main objectives were: 1) To explore functional, cognitive, and psychological outcomes at 30 days post hospital discharge of survivors of COVID-19–associated ARDS requiring invasive mechanical ventilation during the third wave of COVID-19 pandemic. 2) To investigate mortality at 60 days in patients who required invasive mechanical ventilation for ARDS caused by COVID-19.
Methods
Design and participants
We conducted our prospective cohort study in the ICUs of Juan A. Fernández Hospital and Trinidad Ramos Mejía Medical Center, in Buenos Aires Metropolitan Area (AMBA) in Argentina, during the third wave of COVID-19 pandemic: between December 2021 and September 2022.
The inclusion criteria were: age over 18, diagnosis of ARDS secondary to COVID-19 infection confirmed by reverse transcription polymerase reaction (RT-PCR) and requirement of invasively mechanical ventilation for more than 72 h. Patients with an order to limit therapeutic effort stated by themselves in advance or by the treating medical team with the consent of the patient's family were excluded from the study. This information was collected from medical records.
Survivors with cognitive or functional impairment before admission were excluded from the PICS evaluation. A member of the research team through direct interview whit a family member or the patients’ GPs assessed the cognitive and functional state prior to hospitalization. The Barthel index was the instrument used to assess the pre-existing functional dependence (Defined with Barthel< 100) of the participants.
The patients were followed up from the day of intubation and beginning of mechanical ventilation until day 30 after discharge.
The ethics Committee of Hospital Fernández and the Bioethics Commission of La Matanza (COMUBI) approved the Study. All participants or their legal representatives signed the informed consent.
Data collection
We collected the data from the participants according to the study protocol and good research practice guidelines, between December 2021 and September 2022, using standardized forms for initial evaluation and during ICU stay, and for follow-up at 30 days. A member of the research team collected the data prospectively from medical records and personal interviews with a family member or the participant using previously validated instruments.
Upon admission, the following data were drawn from the patients’ medical records: clinical and demographic variables; comorbidities, Charlson comorbidity score and doses of vaccines received; laboratory parameters, disease severity using the Acute Physiology And Chronic Health Evaluation (Apache II) score, organ failure using the Sepsis related Organ Failure Assessment (SOFA) score, and ARDS classification according to the definition of Berlin.9
During the ICU stay the following data were recorded: daily doses of sedatives, analgesics and neuromuscular blockers; vasopressors use, systemic corticosteroids, prone position, hemodialysis and tracheostomy requirements.
A researcher (HE, CA, DM, EH) assessed the occurrence of delirium daily using the Confusion Assessment Method on the Intensive Care (CAM-ICU) scale; a positive CAM-ICU during hospitalization was considered delirium. Sedation levels were also recorded daily and we defined deep sedation or coma on a Richmond Agitation-Sedation Scale (RASS) of −4 or −5. Cognitive function was assessed with the Montreal Cognitive Assessment (MoCA) Test at the time of discharge from ICU.10
In addition, the following events were recorded in the ICU: nosocomial infections, thromboembolic disease, and neurological complications including stroke, seizures, and hypoxic encephalopathy. The number of days on mechanical ventilation, ICU and hospital length of stay were also recorded.
At 30 days post hospital discharge, a neurologist (CZ, CC, LT, or JP) in person assessed the patients’ cognitive, physical, and mental functions through 4 questionnaires. Instruments previously validated for use in Argentina and recommended by the Monitoring and Rehabilitation Committee of the Argentine Society of Intensive Care (SATI) were used.11
Outcome measurements and definitions
The primary outcome was the appearance of PICS 30 days after hospital discharge, defined as deterioration in at least one domain: physical, cognitive, or psychological. And the co-occurrence of PICS problems, which was defined as a simultaneous impairment in two or more domains.
A neurologist personally interviewed the participants using 4 valid instruments for questionnaires. Physical function was assessed using the Barthel index, used to measure the person's ability to perform ten basic activities of daily living (ADL), obtaining a quantitative estimate of the degree of dependency of the subject with a score from 0 to 100; a score of 100 points indicates total independence in ADL.12 The Patient Health Questionnaire-9 (PHQ-9) was used to assess depression,13 characterized by a score equal to or greater than 10. Anxiety was assessed using the General Anxiety Disorder-7 (GAD-7) whose diagnosis was defined by scores equal to or greater than 10.14 The alteration of the psychic domain was defined according to the presence of depression or anxiety. The MoCA test was used to assess cognitive function, where a score < 26 was considered cognitive impairment. All MoCA results were adjusted according to the educational level of the participants. Following the version validated in Argentina,15 one point is added if the subjects meet the following criteria: having completed 12 years or less of formal education in case the MoCA score was less than 30 (Supplementary Material).
Mortality was analyzed at 60 days after starting mechanical ventilation. The data were collected by a member of the research team (CA, DM, VS, EH) by telephone call to the patients or their relatives.
Statistical analyses
Continuous variables were presented as median and interquartile range (IQR), categorical variables as absolute and relative frequencies. Wilcoxon Rank Sum test was used to compare continuous variables and the Fisher test for categorical variables. The linear correlation between continuous variables was evaluated with Spearman's non-parametric test. We used the Wilcoxon Signed-Rank sum Test to compare non-independent continuous variables.
Mortality at 60 days was assessed using survival analysis. Kaplan-Meier curves were plotted to compare the probability of survival. The differences between groups were analyzed with log-rank test.
Cox regression was used to analyze independent predictors of mortality. Variables associated with mortality in the bivariate analysis with a p value <0.20, were evaluated in a multivariable model. For the final selection of variables, we considered clinical and statistical relevance, and at least 10 events per predictor variable in the model. Harrell's C index was calculated to determine the discrimination capacity of the model. The assumption of proportional risks was tested by visual inspection of smoothed Hazard and with Schoenfeld statistical test.
All statistical tests were two-tailed with a level of statistical significance of p<0.05.
For the analysis we used the statistical package Stata Version 14.0.
Results
Between 1/12/2021 and 30/09/2022, 52 patients with COVID-19 who required invasive mechanical ventilation were admitted to the ICU; Seven patients who did not present with ARDS or pneumonia associated with COVID-19, 4 patients who died within the first 48 h after admission to the ICU and had orders for limitation of therapeutic efforts, and 1 patient who did not give his consent to participate in the study were excluded (Fig.1 ).
Fig. 1.
Flowchart diagram of patients’ selection and exclusion.
Finally, 40 patients were included; the median age was 69 years (IQR: 60–75) 30 (75%) of them were men. The most frequent comorbidities were obesity 25 (62.5%), diabetes 14 (35%), and arterial hypertension 21 (52.5%); and 30 patients (75%) had received at least one dose of the COVID-19 vaccine.
The median Apache II score was 19 (IQR: 16–27) and the baseline SOFA score was 8 (IQR: 6–9).
During the ICU stay, 38 patients (95%) were on vasopressors and 24 (60%) were treated in prone position. Table 1 describes the clinical and demographic characteristics of the patients and the differences between survivors and non-survivors.
Table 1.
Clinical and demographic characteristics of the patients.
|
All patients (n = 40) |
Survivors (n = 25) |
Non-Survivors (n = 15) |
p-value | |
|---|---|---|---|---|
| Age (years) | 69 (60–75) | 68 (59–74) | 70 (67–75) | 0.25 |
| > 65 years, n (%) | 27 (67.5) | 15 (60) | 12 (80) | 0.31 |
| Male, n (%) | 30 (75) | 17 (68) | 13 (86.6) | 0.27 |
| BMI (kg/m2) | 31 (27–36) | 32.6 (27–38) | 30.7 (25–34) | 0.12 |
| Obesity (BMI > 30 kg/m2) | 25 (62.5) | 17 (68) | 8 (53) | 0.50 |
| Comorbidities, n (%) | ||||
| Smoking | 20 (50) | 12 (61.5) | 6 (28.5) | 0.096 |
| Diabetes mellitus | 14 (35) | 5 (20) | 9 (60) | 0.017 |
| Arterial hypertension | 21 (52.5) | 11 (44) | 9 (66.6) | 0.20 |
| Respiratory disease | 5 (12.5) | 5 (20) | 0 (0) | 0.14 |
| Heart disease | 9 (22.5) | 4 (16) | 5 (33.3) | 0.24 |
| Inmunosuppressed | 7 (17.5) | 4 (16) | 3 (20) | 1 |
| Chronic Renal disease | 1 (2.5) | 0 (0) | 1 (6.6) | 0.37 |
| Charlson Comorbidity Score | 3.5 (2–5) | 3 (2–5) | 5 (3–6) | 0.019 |
| Vaccinated, n (%) | 30 (75) | 20 (80) | 10 (66.6) | 0.45 |
| Ferritin (ng/mL) | 1202 (652–1944) | 1202 (601–2039) | 1238 (698–1743) | 0.93 |
| D-dimer (mg/L) | 2,13 (0.86–4.49) | 1.91 (0.88–4.4) | 3.3 (0.5–4.17) | 0.75 |
| pH on day 1 | 7.34 (7.22–7.41) | 7.35 (7.23–7.41) | 7.34 (7.20–7.40) | 0.67 |
| Apache II | 19 (16–27) | 18 (16–21) | 29 (18–32) | 0.0049 |
| SOFA at admision | 8 (6–9) | 7 (6–8) | 8 (7–10) | 0.096 |
| VAP, n (%) | 22 (55) | 15 (60) | 7 (46.6) | 0.51 |
| Vasopressors use, n (%) | 38 (95) | 23 (92) | 15 (100) | 0.38 |
| Prone position, n (%) | 24 (60) | 12 (48) | 12 (80) | 0.056 |
| Tracheostomy, n (%) | 22 (55) | 17 (68) | 5 (33) | 0.05 |
Data are shown as numbers (%), median [IQR]. Abbreviations: BMI: body mass index, VAP: ventilator-associated pneumonia, SOFA: sequential organ failure assessment.
In-hospital and 60-days mortality was 37% (15/40 patients). Cox regression identified diabetes (adjusted hazard ratio (HR): 3.31 (95% CI: 1.16–9.32)) and Apache II (adjusted HR: 1.13 (95% CI: 1.04–1.22)) as independent predictors of mortality (Table S1 in supplementary material). The model presented good discrimination with a Harrell's C index: 0.83. Fig.2 shows survival curve for patients with and without diabetes.
Fig. 2.
Kaplan-Meier survival curves for diabetic and non-diabetic patients.
Twenty-five patients survived, three were excluded due to prior cognitive or functional impairments. A total of 22 patients completed surveys to identify PICS. Survivors’ median age was 68 years (IQR: 58–71); were predominantly male (68%) and obese (59%). ICU and hospital lengths of stay (LOS) were 43 days (IQR: 21–56) and 60 days (IQR: 43–63), respectively. Median duration of invasive mechanical ventilation was 37 days (IQR: 12–46), delirium occurred in 17 patients (77%), 18 (82%) received neuromuscular blockers, 15 (68%) required tracheostomy, and 10 patients (45%) were referred to a rehabilitation center.
At the time of discharge from the ICU, 13 patients (59%) had cognitive impairment. The median MoCA was 23.5 (IQR: 14–27).
There were no losses to follow up. The time between ICU discharge and PICS assessment was 59 days (IQR: 50–66).
Functional outcome: 14 patients (64%) had functional dependence on ADL (Barthel index <100 points), the median Barthel index was 60 (IQR: 20–100). 2 patients presented with moderate dependency (Barthel between 90 and 61), 5 severe dependence (Barthel between 60 and 21) and 7 presented with total dependence (Barthel ≤ 20).
In the bivariate analysis, patients with functional dependence were more obese (85% vs 25%, p: 0.009) had more days on mechanical ventilation (41 days (IQR: 35–54) vs 18 days (IQR: 7–34), p: 0.0045), a higher Apache II score (19 (IQR: 17–24) vs 14 (IQR: 13–17), p: 0.03), a higher cumulative dose of midazolam (89 mg/kg (IQR: 51–129) vs 31 mg/kg (IQR: 18–53), p: 0.020), more days on vasopressor (12 days (IQR: 9–15) vs 1.5 days (IQR: 0–5), p: 0.0018), a longer delirium duration (11 days (IQR: 4–19) vs 2 days (IQR: 0–4.5), p: 0.016) and ICU length of stay (LOS) (52 days (IQR: 40–63) vs 29 days (IQR: 12–43), p: 0.037) (Table 2 ).
Table 2.
Clinical and demographic characteristics of patients with and without functional dependence.
|
All patients (n = 22) |
Funcional dependence (n = 14) |
Funcional independence (n = 8) |
p-value | |
|---|---|---|---|---|
| Age (years) | 68 (58–71) | 68 (63–70) | 54.5 (44–69) | 0.16 |
| > 65 years, n (%) | 12 (54) | 9 (64.3) | 3 (37.5) | 0.37 |
| Male, n (%) | 15 (68) | 9 (64.3) | 6 (75) | 0.49 |
| BMI (kg/m2) | 32 (27–38) | 33 (30–39) | 27 (23–31) | 0.051 |
| Comorbidities, n (%) | ||||
| Obesity | 14 (59) | 12 (85.7) | 2 (25) | 0.008 |
| Smoking | 13 (68) | 10 (71) | 3 (37.5) | 0.11 |
| Diabetes | 4 (18) | 1 (7.1) | 3 (37.5) | 0.22 |
| Hypertensión | 9 (41) | 5 (35) | 4 (50) | 0.66 |
| Charlson score | 3 (1–4) | 3 (2–4) | 1.5 (0.5–4) | 0.36 |
| Vaccinated n (%) | 18 (81) | 11 (78.5) | 7 (87.5) | 0.53 |
| Ferritin (ng/L) | 1406 (633–1988) | 1217 (596–1812) | 1406 (1000–2090) | 0.85 |
| Apache II | 18.5 (14–21) | 19.5 (17–24) | 14.5 (13–17) | 0.03 |
| SOFA on admission | 8 (6–8) | 8 (7–8) | 7 (5–8) | 0.47 |
| Severe ARDS, n (%) | 6 (27) | 6 (42.8) | 0 (0) | 0.051 |
| VAP, n (%) | 14 (63.6) | 10 (71.4) | 4 (50) | 0.38 |
| Duration of MV (days) | 37 (12–46) | 41 (35–54) | 18 (7–34) | 0.0045 |
| Prone position, n (%) | 9 (41) | 8 (57) | 1 (12.5) | 0.074 |
| Vasopressors use, n (%) | 20 (91) | 14 (100) | 6 (75) | 0.12 |
| Tracheostomy, n (%) | 15 (68) | 11 (78.5) | 4 (50) | 0.34 |
| Days on NMB | 4.5 (3–14) | 10 (3–20) | 4 (1.5–7) | 0.17 |
| Days on midazolam | 12 (6–21) | 19 (6–26) | 5.5 (3.5–12) | 0.031 |
| Midazolam (mg/kg) | 61 (23–101) | 89.68 (51.75- 129.61) | 31.41 (18.36- 53.51) | 0.020 |
| MP pulses, n (%) | 8 (36.3) | 4 (28) | 4 (50) | 0.38 |
| Delirium, n (%) | 17 (77.2) | 12 (85.7) | 5 (62.5) | 0.30 |
| DFD in 28 days | 22 (11–26) | 18 (6–24) | 26 (21–28) | 0.063 |
| Delirium duration (days) | 5 (1–14) | 11 (4–19) | 2 (0–4.5) | 0.016 |
| ICU LOS (days) | 43 (21–56) | 52 (40–63) | 29 (12–43) | 0.037 |
| Hospital LOS (days) | 60 (43–63) | 61 (56–63) | 36 (14–61) | 0.61 |
| Days of vasopressors | 9 (4–13) | 12 (9–15) | 1.5 (0–5) | 0.0018 |
| Referral on hospital discharge, n (%) | ||||
| Home | 12 (54.5) | 4 (28.57) | 8 (100) | |
| Rehabilitation center | 10 (45.4) | 10 (71.43) |
Data are shown as numbers (%), median [IQR]. Abbreviations: BMI: body mass index, SOFA sequential organ failure assessment, ARDS: acute respiratory distress syndrome, smokers: includes current and former smokers, VAP: ventilator-associated pneumonia, NMB: neuromuscular blockers, MP: methylprednisolone, DFD: Delirium free days LOS: length of stay.
Compared with non-obese patients, the obese had a longer hospital LOS (61 days (IQR: 56–64) vs (46 (IQR: 14–60), p: 0.05), more days on mechanical ventilation (39 days (IQR: 17–61) vs 30 days (IQR: 7–38), p. 0.10) and UCI-LOS (52 days (IQR: 25–58) vs 41 days (IQR: 12–43), p. 0.080), they were on benzodiazepines (19 days (IQR: 6–26) vs 6.5 days (IQR: 3–12), p. 0.036) and on NMB (11.5 days (IQR: 4–20) vs 2.5 days (IQR: 0–4), p: 0.006); and a higher cumulative doses of NMB (51 mg/kg (IQR: 18–87) vs 14.2 mg/kg (IQR: 0–34), p: 0.031). There were no differences in age, sex, Apache II, and SOFA scores.
In a secondary analysis, the Barthel index had a negative and significant correlation with Apache II (rho: −0.54, p: 0.0081), days of corticosteroid use (rho: −0.62, p: 0.0018), cumulative dose of midazolam (rho: −0.56, p: 0.0057), cumulative dose of NMB (rho: −0.45, p: 0.032), days of vasopressor support (rho: −0.76, p: 0.0000), days of invasive mechanical ventilation (rho: −0.63, p: 0.0014), UCI LOS (rho: −0.48, p: 0.02) and coma duration (rho: −0.55, p: 0.0075); and a positive correlation with coma and delirium free days at 28 days (rho: 0.65, p: 0.0009).
Cognitive outcome: 9 patients (41%) had cognitive function impairment, the median MoCA was 26 (IQR: 20–28). The most frequently affected cognitive ability was delayed recall with a score of 3.5 (2–4.5) in a scale of 0–5. Patients with cognitive impairment had a higher Apache II (21 (IQR: 19–25) vs 16 (IQR:13–19), p: 0.0032), baseline SOFA (8 (IQR: 8–9) vs 6 (IQR : 4–8), p: 0.026) and cumulative doses of midazolam (93 mg/kg (IQR: 87–129) vs 39 mg/kg (IQR: 21–62), p: 0.02); they were also on invasive mechanical ventilation much longer (46 days (IQR: 40–54) vs 30 days (IQR: 7–37), p: 0.0082) and ICU LOS (56 days (IQR: 48–63) vs 41 days (IQR: 16–44, p: 0.03) (Table 3 ).
Table 3.
Clinical and demographic characteristics of patients with and without cognitive impairment.
|
All patients (n = 22) |
MoCA <26 (n = 9) |
MoCA ≥ 26 (n = 13) |
p-value | |
|---|---|---|---|---|
| Age (years) | 68 (58–71) | 69 (63–71) | 63 (44–70) | 0.22 |
| > 65 years, n (%) | 12 (54) | 6 (66.6) | 6 (46) | 0.41 |
| Male, n (%) | 15 (68) | 5 (55.5) | 10 (76.9) | 0.48 |
| BMI (kg/m2) | 32 (27–38) | 33 (30–38) | 32 (27–33) | 0.48 |
| Comorbidities, n (%) | ||||
| Obesity | 14 (59) | 7 (77.7) | 7 (53.8) | 0.38 |
| Smoking | 13 (68) | 6 (66.7) | 7 (53.8) | 0.67 |
| Diabetes | 4 (18) | 0 (0) | 4 (30) | 0.98 |
| Hypertensión | 9 (41) | 3 (33) | 6 (46) | 0.67 |
| Charlson score | 3 (1–4) | 3 (2–4) | 2 (1–3) | 0.19 |
| Vaccinated, n (%) | 18 (81) | 7 (77.7) | 11 (84.6) | 0.6 |
| Ferritin (ng/L) | 1406 (633–1988) | 1066 (517–1582) | 1406 (934–2090) | 0.42 |
| Apache II | 18.5 (14–21) | 21 (19–25) | 16 (13–19) | 0.0032 |
| SOFA on admission | 8 (6–8) | 8 (8–9) | 6 (4–8) | 0.026 |
| Severe ARDS, n (%) | 6 (27) | 4 (44.4) | 2 (15.3) | 0.18 |
| VAP, n (%) | 14 (63.6) | 7 (77.7) | 7 (53.8) | 0.38 |
| Duration of MV (days) | 37 (12–46) | 46 (40–54) | 30 (7–37) | 0.0082 |
| Prone position, n (%) | 9 (41) | 6 (66.7) | 3 (23) | 0.079 |
| Vasopressors use n (%) | 20 (91) | 9 (100) | 11 (84.6) | 0.49 |
| Tracheostomy, n (%) | 15 (68) | 8 (88.9) | 7 (53.8) | 0.16 |
| Days on NMB | 4.5 (3–14) | 9 (3–22) | 7 (4–14) | 0.32 |
| Days on midazolam | 12 (6–21) | 20 (6–26) | 10 (4–13) | 0.10 |
| Midazolam (mg/Kg) | 61 (23–101) | 93.57 (87–129.6) | 39.2 (21.4–62.7) | 0.02 |
| MP pulses, n (%) | 8 (36.3) | 2 (22) | 6 (46) | 0.38 |
| Delirium, n (%) | 17 (77.2) | 7 (77.7) | 10 (76.9) | 1 |
| DFD in 28 days | 22 (11–26) | 16 (0–23) | 24 (20–27) | 0.08 |
| Delirium duration (days) | 5 (1–14) | 13 (9–15) | 1 (4–6) | 0.14 |
| ICU LOS (days) | 43 (21–56) | 56 (48–63) | 41 (16–44) | 0.03 |
| Hospital LOS (days) | 60 (43–63) | 60 (22–61) | 58 (55–64.5) | 0.34 |
| Days on vasopressors | 9 (4–13) | 12 (9–19) | 6 (1–13) | 0.08 |
| Referral on hospital discharge, n (%) | ||||
| Home | 12 (54.5) | 1 (11) | 9 (82) | |
| Rehabilitation center | 10 (45.4) | 8 (88.9) | 2 (15.3) |
Data are shown as numbers (%), median [IQR]. Abbreviations: BMI: body mass index, SOFA sequential organ failure assessment, ARDS: acute respiratory distress syndrome, smokers: includes current and former smokers, VAP: ventilator-associated pneumonia, NMB: neuromuscular blockers, MP: methylprednisolone, DFD: Delirium free days LOS: length of stay.
MoCA score negatively correlated with Apache II (rho: −0.59, p: 0.0037), and baseline SOFA (rho: −0.49, p: 0.019) and positively correlated with delirium free days at 28 days (rho: −0.46, p: 0.03).
There was a significant improvement in the MoCA score at 30 days after hospital discharge compared to the MoCA score at ICU discharge, 23.5 points (IQR: 16–27) vs 26 points (IQR: 20–28, p: 0.01).
Psychological outcome: 7 patients (32%) had impairment in the psychic domain with anxiety or depression. 4 patients had mild depression (PHQ-9 > 4) and 7 moderate to severe depression (PHQ-9 ≥ 10 points). The median of PHQ-9 was 5 (IQR: 3–12). The median of GAD-7 was 3 (IQR: 2–7). 2 patients had mild anxiety (GAD-7 > 4) and 3 had moderate anxiety (GAD-7 ≥ 10).
Occurrence of PICS
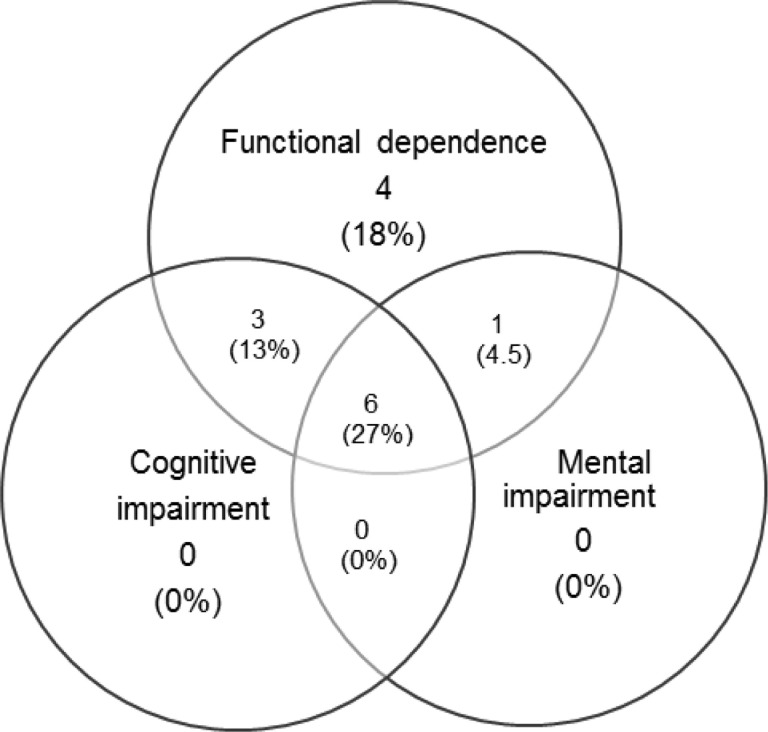
At 30 days after hospital discharge, 14 patients (64%) had PICS. Of these patients, 4 had a single domain affected, 5 with two domains, and 5 all three domains compromised. The co-occurrence of PICS (compromising 2 or more domains) was 45% (Fig.3 ).
Fig. 3.
Overlapping of Post-Intensive Care Syndrome Domains at 30 days Follow-Up.
This diagram illustrates the overlapping of PICS domains at 30 days. The proportion of patients with PICS and overlapping of the domains is shown in the circles.
All patients with PICS had functional dependence on ADLs due to compromise of the physical domain. At 30 days after hospital discharge, 10 patients continued their care at a rehabilitation center; of these, only 4 continued with the tracheostomy cannula, no patient required mechanical ventilation, and only one presented with oxygen therapy requirements. Survivors admitted to rehabilitation center had a higher Apache II score (20 (IQR: 18–24) vs 16 (IQR: 13–19), p: 0.028) and were longer on mechanical ventilation (41 days (IQR: 38–54) vs 30.5 days (IQR. 7–37), p: 0.031) compared to patients who were discharged from hospital at home. And at 30 days after discharge, they had a higher prevalence of PICS (100% vs 33%, p: 0.002) (See Table 4 ).
Table 4.
Clinical characteristics and outcome of patients according to referral on hospital discharge.
|
Rehabilitation (n = 10) |
Home (n = 12) |
p-value | |
|---|---|---|---|
| Age (years) | 68 (63–72) | 61 (43–70) | 0.14 |
| Male, n (%) | 6 (60) | 9 (75) | 0.38 |
| Charlson score | 3 (2–4) | 2 (0.5–3.5) | 0.26 |
| Apache II | 20 (18–24) | 16 (13–19) | 0.0028 |
| SOFA on admission | 8 (8–9) | 6 (5–8.5) | 0.14 |
| Duration of MV (days) | 41 (38–54) | 30 (7–37) | 0.0031 |
| Prone position, n (%) | 6 (66.7) | 3 (33) | 0.11 |
| Tracheostomy, n (%) | 8 (53.3) | 7 (46.6) | 0.38 |
| Days on NMB | 10 (4–22) | 4 (1.5–12) | 0.16 |
| Days on midazolam | 20 (6–26) | 8.5 (4–15.5) | 0.09 |
| Midazolam (mg/Kg) | 92.9 (62.6–129.6) | 43.4 (22.5–68.9) | 0.07 |
| NMB (mg/kg) | 48.8 (13.8–87.5) | 24.8 (3.9–87.5) | 0.27 |
| Delirium, n (%) | 8 (47) | 9 (53) | 1 |
| DFD in 28 days | 13.5 (0–25) | 23.5 (2 (5–19.5–27.5) | 0.11 |
| Delirium duration (days) | 11 (3–19) | 4 (5–7.5) | 0.19 |
| ICU LOS (days) | 52 (40–58) | 42 (15–50) | 0.03 |
| Hospital LOS (days) | 60 (56–61) | 55 (18–63) | 0.52 |
| Days on vasopressors | 12.5 (11–15) | 4.5 (0.5–7) | 0.014 |
| PICS n (%) | 10 (100) | 4 (33) | 0.02 |
| Functional dependence n (%) | 10 (100 | 4 (33) | 0.02 |
| Cognitive impairment n (%) | 8 (89%) | 1 (11%) | 0.002 |
| Mental impairment n (%) | 6 (85.7) | 1 (14.2) | 0.02 |
| Barthel Index | 20 (15–30) | 100 (90–100) | 0.0000 |
| MoCA score | 20 (15–24) | 27.5 (26.5–29.5) | 0.0002 |
Data are shown as numbers (%), median [IQR]. Abbreviations: BMI: body mass index, SOFA sequential organ failure assessment, ARDS: acute respiratory distress syndrome, NMB: neuromuscular blockers, MP: methylprednisolone, DFD: Delirium free days, LOS: length of stay.
The results of the questionnaires used to assess PICS domains are described in Table 5 .
Table 5.
Results of questionnaires that evaluated PICS after hospital discharge.
| Median (IQR) |
Test altered |
||
|---|---|---|---|
| n = 19 | n | % | |
| MoCA | 26 (IQR: 20–28) | 9 | 40.9% |
| Barthel Index | 60 (IQR: 20–100) | 14 | 63.6% |
| GAD-7 | 3 (IQR: 2–5) | 3 | 13.6% |
| PHQ-9 | 5 (IQR: 3–12) | 7 | 31.8% |
Data are expressed as numbers (%), median and interquartile range (IQR). MoCA: Montreal Cognitive Assessment, GAD-7: General Anxiety Disorder −7, PHQ-9: Patient Health Questionnaire-9. Altered test definition: MoCA 〈 26, Barthel < 100. GAD-7 〉 10 and PHQ-9 > 10.
Discussion
In this prospective study, we investigated functional, cognitive, and psychological outcomes in 22 survivors of COVID-19–associated ARDS requiring mechanical ventilation. We found that 64% had some degree of functional dependence in basic activities of daily life, 41% had a new cognitive deterioration and 32% psychological alterations demonstrated by anxiety or depression. PICS prevalence was 64%. Most patients had co-occurrence of PICS problems with impairment in two or more domains simultaneously.
An important finding of this study was that more than half of ARDS survivors due to COVID-19 had severe or total functional dependence 30 days after hospital discharge. We observed that the ability to move, walk and climb stairs was the most frequently impairment.
Our findings are consistent with previously published studies in that the physical domain was the most frequently affected.
Recently, a prospective study conducted in two ICUs in France evaluated 41 survivors of COVID-19–associated ARDS requiring intubation, found that between 3 and 6 months 61% had poor functional outcomes, 52% cognitive impairment and 26% anxiety or depression.7 Previously, several prospective studies found a high prevalence of functional impairment in survivors of critical COVID-19 survivors.8 , 16 , 17 Instead, Rosseau et al. in a prospective study in 33 patients with COVID-19 in Belgium, observed that the cognitive domain was the most frequently affected (47%) 3 months after ICU discharge; and only 31% presented with functional dependence.18 Patients in this cohort were younger, had less severity of illness on admission, and fewer days on mechanical ventilation and ICU stay than our patients.
In our study, we found that severity of the disease on admission, duration of mechanical ventilation and ICU LOS were the main variables associated with functional dependence after hospital discharge. These findings are in line with other studies conducted in patients with ARDS of different etiologies, demonstrating that the risk factors that may contribute to PICS development after critical illness due to COVID-19 are likely to be not different from PICS development from other etiologies.7 , 16 , 19 , 20 However, the obesity impact on the functional dependence at medium or long term has not been sufficiently studied. Numerous studies indicate that obesity is associated with increased disease severity and mortality in patients with COVID-19 while others conducted in patients with non-COVID-19 associated ARDS showed a "paradoxical or protective effect" of obesity on mortality.21 , 22 In our study, obese patients did not have higher mortality than the non-obese. However, obese survivors developed physical impairment more frequently. This finding can be accounted for by the fact that unlike non-obese patients, the obese that survived had a longer hospital stay, mechanical ventilator assistance and consequently higher cumulative doses of benzodiazepines and neuromuscular blocking drugs.
Several studies, prior to the COVID-19 pandemic, identified delirium and its duration as the main independent risk factor for long-term cognitive impairment in critically ill patients.23 , 24 Recently, a multicenter study in COVID-19 patients reported a high prevalence (55%) of delirium in the ICU.25 In our study, delirium occurrence was 77%, and patients with cognitive impairment on discharge went through a longer period of delirium in the ICU compared with patients without cognitive impairment. However, this difference was not statistically significant probably due to the study lack of power. We found that cognitive impairment was associated with severity of illness on admission (Apache II and SOFA baseline), cumulative dose of midazolam, and duration of mechanical ventilation and ICU stay. Furthermore, we observed that the MoCA score strongly negatively correlated with the Apache II score. Recently, in a prospective study of 22 patients conducted in Ireland, Apache II strongly correlated with the MoCA at 6 months.26 In a linear regression analysis, the authors observed that for each point that Apache II increases, the MoCA score decreases by 1.6 points (Coefficient β: −1.6, p: 0.04).
In line with previous studies in patients with COVID-19, we found that delayed recall was the most frequently affected cognitive ability.7
In our cohort, PICS occurrence was similar to those reported by studies prior to the COVID-19 pandemic. Marra et al. observed a 64% prevalence of PICS at 3 months after ICU discharge in non-COVID-19 patients.19 In contrast, the prevalence reported in studies with COVID-19 patients was highly variable, for example, 90% at one month after hospital discharge,8 80% at 6 months18 and 66% at one year.16 These differences could be due to heterogeneity among the studies at the moments of assessment, instruments used to evaluate PICS domains and baseline characteristics of the population. Physiologically it is expected that a trend towards an improvement in some of the evaluated domains can be observed the later the patients are assessed. In fact, we observed an improvement in the cognitive domain thirty days after discharge compared to that made at the time of discharge.
We observed that 45% of the patients had co-occurrence of PICS problems. Likewise, Maley et al. reported a co-occurrence of 47% at 6 months after discharge.17 Prior to the pandemic, a multicenter study in patients with ARDS or shock not caused by COVID-19, showed a 25% of PICS co-occurrence at 3 months.20 These data would suggest a greater PICS co-occurrence in COVID-19 patients that could be due to a set of factors that includes: severity of the disease, heavy sedation, neuromuscular blockers use, isolation from members of the family, high Delirium prevalence, prolonged mechanical ventilation, and long ICU stays that were characteristic of COVID-19 patients admitted to the ICU during the pandemic.
Our study was carried out during the third wave of the pandemic, with a population vaccination rate of 75% and a decrease in the number of hospitalizations due to COVID-19. We found an in hospital mortality of 37%. Previously, a large prospective and multicenter cohort study conducted in our country,27 which included patients with COVID-19 requiring mechanical ventilation during the first wave of the pandemic had reported a hospital mortality of 57.7%. We think that this higher mortality was probably associated with the high health personnel's workload and a very low population vaccination rate in the first wave of the pandemic.
Our results suggest that patients with COVID-19 who survive ICU admission have a high burden of functional, cognitive, and psychological impairment. In addition, many of them require specialized care with a multidisciplinary team of intensive care providers, psychologists, physical therapists, neurologists and, in many cases, admission to rehabilitation centers. In our series, ten of the 22 survivors (45%) continued their care in a rehabilitation center at 30 days after hospital discharge.
Our data are consistent with work carried out in high income countries.7 , 8 , 16., 17., 18. We believe that our research provides quantitative information, which together with previously published studies, generates valuable evidence on the burden of disease and sequelae in COVID-19 survivors.
This study has several strengths. There were no losses to follow-up, thus minimizing selection bias. The prospective design of the study allowed the recording of information on the exposure variables, outcomes, and potential confounders directly from the patient's family and medical records during follow-up. We evaluated primary outcome with personal interviews in hospitals, clinics, or through researchers’ visits to the rehabilitation center, unlike other studies that evaluated PICS via the telephone or email surveys.8 , 17
To our knowledge, this is one of the first studies to explore PICS in survivors of COVID-19 who required mechanical ventilation in Argentina and Latin America.
However, our study has several limitations. First, the sample size was small, and therefore it prevents the identification of factors independently associated with PICS occurrence. Second, this study was conducted in two Buenos Aires centers; our findings cannot be extrapolated to other centers or geographic regions. Third, we did not assess post-traumatic stress, which might have led to an underestimation of psychic domain impairment. Fourth, given the urgency of the patients’ critical illness, we were unable to personally assess participants’ cognitive function and functional dependence prior to the illness. Fifth, the absence of a control group was another limitation; we compared our data with data published in non-COVID-19 survivors before the pandemic. And finally, PICS was evaluated at 30 days after hospital discharge. We do not have long-term follow-up data that could inform us about the trajectory of post-ICU sequelae.
Implications for future practice and research
Our findings in the context of current evidence highlight the need to optimize the use of resources for adequate identification and long term follow-up of ICU COVID-19 survivors. A large multicenter study is warranted to identify potentially modifiable factors associated with PICS to improve long-term outcome. Furthermore, studies comparing PICS characteristics in ICU COVID-19 survivors with non-COVID-19 ICU survivors are an important field for future research.
Conclusions
In this prospective study, we identified a high prevalence of functional, cognitive, and psychological impairment at 30 days after hospital discharge in survivors of COVID-19-associated ARDS requiring mechanical ventilation. The physical domain was the most frequently altered. In most cases, multiple PICS domains were affected. These findings suggest the need for long-term follow-up of this population.
Authors' contributions
EM and DM conceived and designed the study. EM and FDM analyzed the data. EM and DM were in charge of the project administration. EE, DM and FDM verified the data. EM, CA, DM, VS, HE, EH, LT, GZ, JP, CCi, CCo, SPA and CP contributed to the acquisition and interpretation of the data. EM Writing-original draft, DM and FDM Writing-review and editing.
Funding
This research work was carried out thanks to the support of the SALUD INVESTIGA Scholarships for Research projects 2021–2022, granted by the Argentine Ministry of Health, through the Directorate of Health Research.
Ethical approval and consent to participate
The study was approved by the ethics committee of Juan A. Fernández Hospital and the Bioethics Commission of La Matanza (COMUBI). All participants or their legal representatives signed the informed consent.
Declaration of Competing Interest
All authors have disclosed that they do not have any conflicts of interest related to this study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2023.06.021.
Appendix. Supplementary materials
References
- 1.WHO. Coronavirus disease (COVID-2019) situation report 2020 [Available from:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 2.Osuchowski M.F., Winkler M.S., Skirecki T., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;9:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge M., Cheung A., Tansey C., et al. One-Year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 4.Needham D.M., Davidson J., Cohen H., et al. Improving long-term outcomes after discharge from intensive care unit: report from a stake-holders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 5.Pandharipande P.P., Girard T.D., Jackson J.C., et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lone N.I., Gillies M.A., Haddow C., et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med. 2016 Jul 15;194(2):198–208. doi: 10.1164/rccm.201511-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaquet P., Legouy C., Le Fevre L., et al. Neurologic outcomes of survivors of COVID-19-associated acute respiratory distress syndrome requiring intubation. Crit Care Med. 2022 Aug 1;50(8):e674–e682. doi: 10.1097/CCM.0000000000005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martillo M.A., Dangayach N.S., Tabacof L., et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: cohort study from a New York City critical care recovery clinic. Crit Care Med. 2021;49:1427–1438. doi: 10.1097/CCM.0000000000005014. [DOI] [PubMed] [Google Scholar]
- 9.Definition Task Force A.R.D.S., Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012 Jun 20;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine Z.S., Phillips N.A., Bédirian V., et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 11.Bursico M., Das Neves A., Carini F., et al. Programa de seguimiento al alta de Cuidados intensivos. Medicina Intensiva. 2019;43(4):243–254. doi: 10.1016/j.medin.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Shah S., Vanclay F., Cooper B., et al. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 13.Urtasun M., Daray F.M., Teti G.L., et al. Validation and calibration of the patient health questionnaire (PHQ-9. Argentina.BMC Psychiatry. 2019;19:29. doi: 10.1186/s12888-019-2262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer R.L., Kroenke K., Williams J.B.W., et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 15.González Palau F., Berrios W., García Basalo M.M., et al. Validation of the montreal cognitive assessment (MoCA) as a screening tool for mild cognitive impairment in the population of Buenos Aires, Argentina. Vertex. 2018 Jul;XXIX(140):261–269. Spanish. [PubMed] [Google Scholar]
- 16.Banno A., Hifumi T., Takahashi Y., et al. One-year outcomes of post intensive care syndrome in critically ill coronavirus disease 2019 patients: a single institutional study. Crit Care Explor. 2021 Dec 2;3(12):e0595. doi: 10.1097/CCE.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maley J.H., Sandsmark D.K., Trainor A., et al. Six-month impairment in cognition, mental health, and physical function following COVID-19-associated respiratory failure. Crit Care Explor. 2022 Mar 28;4(4):e0673. doi: 10.1097/CCE.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousseau A.F., Minguet P., Colson C., et al. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann Intensive Care. 2021 Jul 29;11(1):118. doi: 10.1186/s13613-021-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marra A., Pandharipande P.P., Girard T.D., et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M., Kang J., Jeong Y.J. Risk factors for post-intensive care syndrome: a systematic review and meta-analysis. Aust Crit Care. 2020;33:287–294. doi: 10.1016/j.aucc.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Sawadogo W., Tsegaye M., Gizaw A., et al. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: systematic review and meta-analysis. BMJ Nutr Prev Health. 2022 Jan 19;5(1):10–18. doi: 10.1136/bmjnph-2021-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W., Wang Y., Li W., Wang J. Association between obesity and short-and long-term mortality in patients with acute respiratory distress syndrome based on the berlin definition. Front Endocrinol (Lausanne) 2021 Feb 12;11 doi: 10.3389/fendo.2020.611435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandharipande P.P., Girard T.D., Jackson J.C., et al. BRAIN-ICU study investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013 Oct 3;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girard T.D., Jackson J.C., Pandharipande P.P., et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pun B.T., Badenes R., Heras La Calle G., et al. COVID-19 intensive care international study group: prevalence and risk factors for delirium in critically ill patients with COVID- 19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmartin M., Collins J., Mason S., et al. Post-intensive care COVID survivorship clinic: a single-center experience. Crit Care Explor. 2022 May 11;4(5):e0700. doi: 10.1097/CCE.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estenssoro E., Loudet C.I., Ríos F.G., et al. COVID-19 Study Group. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021 Sep; 9;(9):989–998. doi: 10.1016/S2213-2600(21)00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.