Abstract
MicroRNA-22 (miR-22) can be induced by beneficial metabolites that have metabolic and immune effects, including retinoic acids, bile acids, vitamin D3, and short-chain fatty acids. The tumor suppressor effects of miR-22 have been suggested, but whether miR-22 treats orthotopic hepatocellular carcinoma (HCC) is not established. The role of miR-22 in regulating tumor immunity is also poorly understood. Our data showed that miR-22 delivered by adeno-associated virus serotype 8 effectively treated HCC. Compared with FDA-approved lenvatinib, miR-22 produced better survival outcomes without noticeable toxicity. miR-22 silenced hypoxia-inducible factor 1 (HIF1α) and enhanced retinoic acid signaling in both hepatocytes and T cells. Moreover, miR-22 treatment improved metabolism and reduced inflammation. In the liver, miR-22 reduced the abundance of IL17-producing T cells and inhibited IL17 signaling by reducing the occupancy of HIF1α in the Rorc and Il17a genes. Conversely, increasing IL17 signaling ameliorated the anti-HCC effect of miR-22. Additionally, miR-22 expanded cytotoxic T cells and reduced regulatory T cells (Treg). Moreover, depleting cytotoxic T cells also abolished the anti-HCC effects of miR-22. In patients, miR-22 high HCC had upregulated metabolic pathways and reduced IL17 pro-inflammatory signaling compared with miR-22 low HCC. Together, miR-22 gene therapy can be a novel option for HCC treatment.
Keywords: liver cancer, hepatocellular carcinoma, retinoic acid, HIF1α, RORγ, immunotherapy, tumor microenvironment
Graphical abstract
Wan and colleagues discovered that miR-22 gene therapy offers a promising treatment option for HCC. This novel drug inhibits tumor growth, prolongs survival without toxic side effects, and restores liver function by affecting both hepatocytes and hepatic T cells.
Introduction
Hepatocellular carcinoma (HCC) is an emerging health burden caused by the increasing levels of obesity.1,2 However, there is a lack of optimal therapies for HCC.3 The tyrosine kinase inhibitors including sorafenib, lenvatinib, regorafenib, and cabozantinib have been used as first- or second-line drugs for patients with unresectable HCC.4,5 However, these agents are associated with considerable toxicities and poor quality of life outcomes, and the survival benefit is limited to a few months.6 Although immunotherapy has been revolutionary in the management of different types of cancer, the outcomes of HCC treatment are poor.7,8 Therefore, there is an urgent need to identify novel treatment options for HCC.
MicroRNA-22 (miR-22) is implicated in the development of different cancers, including liver, colorectal, cholangiocarcinoma, prostate, breast, and stomach.9 miR-22 is generally considered a tumor suppressor but may have oncogenic effects depending on the experimental models.9,10,11,12,13,14,15,16,17 For the liver, surprisingly, it has been shown that miR-22 promotes HBV-related HCC development.18 However, we have uncovered that the expression of miR-22 is induced by beneficial metabolites generated by the microbiota and the host within the gut-liver axis.19,20 These metabolites include retinoic acid (RA), short-chain fatty acids (SCFAs), bile acids (BAs), and vitamin D3, which have metabolic and anti-cancer benefits.19,20,21 Signaling regulated by these chemicals and their receptors is usually compromised during dysbiosis-associated inflammation or liver tumorigenesis.22,23,24,25,26 In the past, we have shown that miR-22 has anti-cancer effects in the colon by exporting the nuclear receptors RARβ and NUR77 to the cytosol and targeting mitochondria to induce apoptosis.20 Other mechanisms include silencing of cyclin A2 (CCNA2) and inhibition of cancer cell migration.19,27 miR-22 also silences fibroblast growth factor 21 and its receptor FGFR1 to inhibit metabolism-driven growth and proliferation controlled by ERK1/2 signaling.28 Further, miR-22 levels are inversely correlated with serum α-fetoprotein (AFP) levels, suggesting its tumor-suppressive role in HCC.29 However, whether miR-22 treats HCC has not been studied in orthotopic preclinical models.
The role of miR-22 in the modulation of the tumor immune microenvironment is less understood. Recent studies showed that miR-22 downregulates checkpoint molecule programmed death-ligand 1, which inhibits T cell-mediated immune responses in colon cancer.30 miR-22 is highly expressed in regulatory T cells (Treg) from multiple sclerosis patients.31 Additionally, miR-22 indirectly regulates the T helper 17 (Th17) responses by controlling the activation of myeloid dendritic cells in an emphysema mouse model.32 However, it is still unclear how miR-22 regulated the functions of T cells. Thus, the current study examined the anti-HCC effects of miR-22 particularly focusing on its immunomodulatory functions in T cells using orthotopic mouse HCC models.
Our data revealed that miR-22 treated HCC and prolonged survival time. Compared with lenvatinib treatment, miR-22 therapy provided a longer survival time without detectable toxicity. Moreover, miR-22 treatment effectively silenced hypoxia-inducible factor 1 (HIF1α) and enhanced RA signaling in both hepatocytes and T cells, which improved metabolism and anti-tumor immunity. The miR-22-targeted metabolism and inflammation signaling observed were human relevant, as indicated by comparing miR-22 high vs. low human HCC. miR-22 gene therapy, targeting both hepatocytes and T cells, may become a novel option for HCC treatment.
Results
miR-22 treats HCC
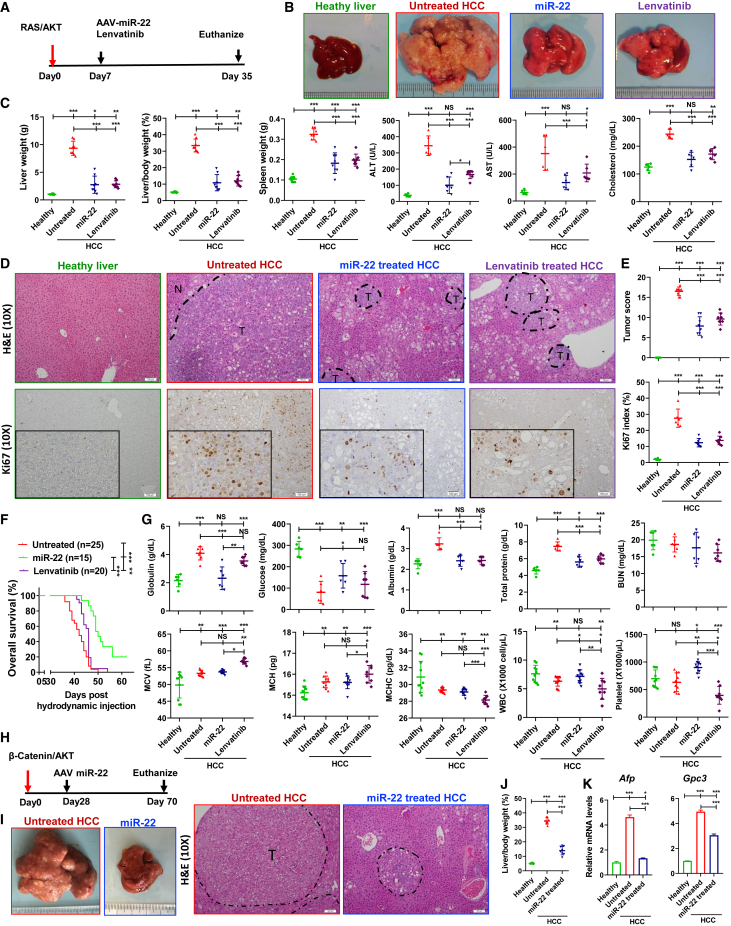
Activation of AKT and the neuroblastoma RAS viral oncogene homolog (RAS) is frequently observed in patients with HCC.6,33 To examine the anti-HCC effect of miR-22, RAS/AKT-induced HCC female mice were treated with either AAV8-miR-22 (miR-22 treated) or AAV8 control (untreated). Lenvatinib, an FDA-approved HCC drug, was used for comparison (Figure 1A). In untreated control groups, 5 weeks post oncogene injection, the liver-to-body weight (L/B) ratio reached 33.5%, 8-fold higher than that in healthy mice. Tumors accounted for more than 90% of liver sections. Treatment with miR-22 and lenvatinib reduced the L/B ratio to 10.9% and 12.0%, respectively (Figures 1B and 1C). The effectiveness of both treatments was confirmed by histological evaluation (Figures 1D and 1E). miR-22 and lenvatinib reduced the serum alanine transaminase (ALT), aspartate aminotransferase (AST), and cholesterol levels, indicating improved liver function (Figures 1B and 1C). Additionally, miR-22 and lenvatinib treatment markedly reduced the number of Ki67-positive cells (Figures 1D and 1E). Splenomegaly is typically found in HCC patients and mice, likely due to increased infiltration of foreign cells in the spleen34; both miR-22 and lenvatinib reduced spleen size significantly (Figure 1C).
Figure 1.
miR-22 treats HCC and prolongs survival in female HCC mice
(A) Study design for miR-22 and lenvatinib treatment in RAS/AKT-induced HCC model, (B) representative liver morphology, (C) liver weight, L/B ratio, spleen weight, serum ALT, AST, and cholesterol levels, and (D and E) H&E-stained liver sections and Ki67 IHC staining. The cellularity of the proliferating cells is seen at high magnification (insets). The tumor score was quantitively evaluated, which is detailed in Table S3. The percentage of Ki67-positive cells was determined in five random x10 microscopic fields for each section. Scale bar, 100 μm. (F) Kaplan-Meier survival curves of overall survival of three groups (n = 15–25/group). (G) Toxicology for the studied groups. (H) Study design of miR-22 treatment in β-catenin/AKT-induced HCC model. (I) Representative liver morphology and H&E-stained liver sections (scale bar, 100 μm), (J) L/B ratio, and (K) hepatic mRNA levels of HCC markers Afp and Gpc3 for studied group. Data represent mean ± SD (n = 6–8/group for B, C, E, G, I, J, and K). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA (C, E, F, G, J, and K).
The anti-HCC effect of miR-22 was also confirmed in male mice. miR-22 treatment significantly reduced tumor burden as indicated by significant reductions in the liver weight and L/B ratio in male HCC mice (Figures S1A–S1C). In addition, terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) analysis that showed apoptosis of HCC cells was demonstrated in miR-22-treated HCC (Figure S1D).
miR-22-treated mice have longer survival time without toxicity compared with lenvatinib-treated mice
In the RAS/AKT-HCC female mice, the median survival time of the HCC mice was 42 days. Two mice treated with miR-22 remained alive 60 days post oncogene injection. Excluding these two, miR-22 treatment was found to extend the survival time to 50 days. In contrast, the median survival time of the lenvatinib-treated group was 46 days (Figure 1F).
Patients with HCC commonly have elevated serum globulin levels, which was also observed in our mouse models.35 miR-22 treatment reduced serum globulin levels; however lenvatinib did not. Moreover, HCC mice had reduced serum glucose levels, which are also observed in patients with HCC as indicators of hypoglycemia and poor prognosis.36 Serum glucose levels were also reduced in response to miR-22 treatment but not lenvatinib. Both miR-22 and lenvatinib reversed the elevation in total protein and albumin levels. Blood urea nitrogen (BUN) levels were not affected by HCC or either treatment, suggesting no renal toxicity (Figure 1G).
Complete blood counts indicated that HCC mice had macrocytic anemia, as evidenced by increased mean corpuscular volume and mean corpuscular hemoglobin, as well as reduced mean corpuscular hemoglobin concentration (Figure 1G). Macrocytic anemia is commonly observed in HCC.37 Lenvatinib exacerbated this condition, whereas miR-22 did not. Furthermore, lenvatinib-treated mice had the lowest white blood cell and platelet counts. In contrast, miR-22 treatment increased the levels of both (Figure 1G).
miR-22 treats β-catenin-positive HCC.
β-Catenin activation promotes immune escape.38 β-Catenin-positive HCC, accounting for 50% of human HCC cases, is resistant to anti-PD-1 treatment.38 The anti-cancer effect of miR-22 was studied in β-catenin/AKT-induced female mouse HCC. miR-22 treatment was initiated 4 weeks after oncogene delivery (Figure 1H) and significantly reduced the L/B ratio compared with that in untreated mice (Figure 1J). The gross morphology and histology are shown in Figure 1I. Furthermore, miR-22 reduced the expression of the HCC markers Afp and Gpc3 (Figure 1K).
miR-22 treatment restores hepatic metabolism and reduces inflammatory signaling
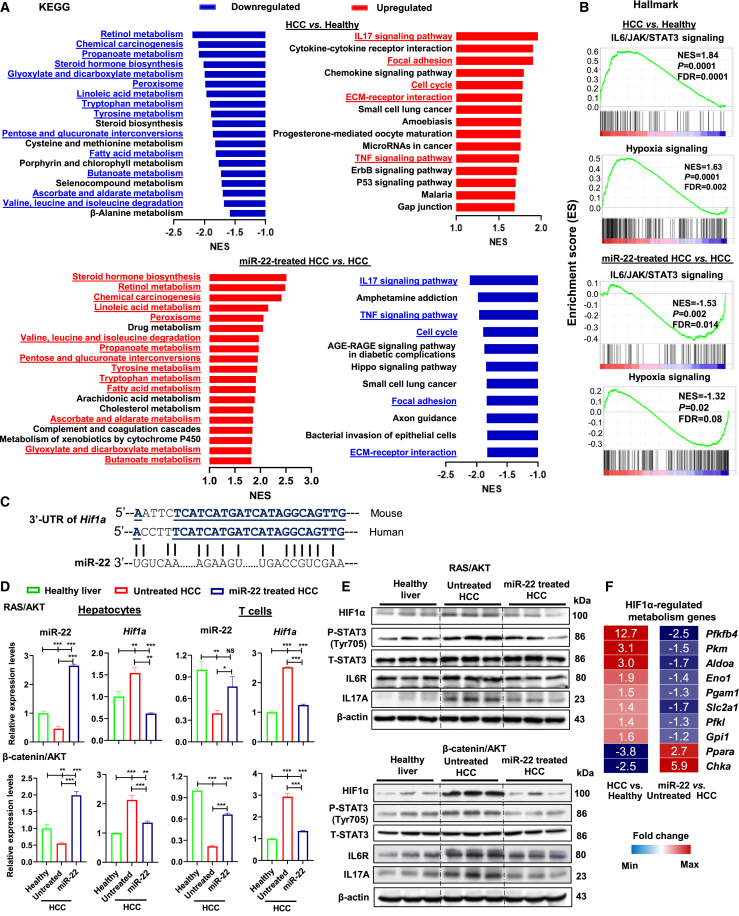
To uncover the pathways altered due to HCC development and the response to miR-22 treatment, RNA sequencing was performed using the livers derived from female HCC mice, followed by gene set enrichment analysis (GSEA) using the Kyoto Encyclopedia of Genes and Genomes (KEGG) gene set. Compared with healthy livers, downregulated pathways found in HCC were related to the metabolism of retinol (RA), propanoate and butanoate (SCFAs), fatty acids, steroid hormones (BAs), peroxisomes (functions of BA), tryptophan, sugar, and amino acids. Among these, many are miR-22 inducers (pathways in parentheses). Importantly, miR-22 treatment induced 14 of the 19 downregulated pathways (Figure 2A).
Figure 2.
miR-22 treatment restores metabolic programs and reduces inflammatory signaling accompanied by reduced HIF1α expression in the liver, hepatocytes, and T cells
(A) Pathways enriched due to HCC formation or miR-22 treatment revealed by GSEA based on KEGG gene sets. miR-22-reversed pathways are underlined and highlighted in red (upregulated) or blue (downregulated). NES, normalized enrichment score. (B) Enriched IL6/JAK/STAT3 and hypoxia signaling by comparing HCC vs. healthy livers or miR-22 treated vs. untreated HCC as demonstrated by GSEA based on hallmark gene sets. (C) Human and mouse miR-22 have conserved sequences, which partially pair with the 3′ UTR of the human and mouse Hif1a gene. (D) The level of miR-22 and HIF1α in hepatocytes and T cells isolated from livers of healthy, HCC, and miR-22-treated HCC mice (n = 3). (E) The levels of indicated proteins in the HIF1α/IL6/STAT3/IL17 axis were determined by western blot (n = 3). (F) The fold changes of HIF1α-regulated metabolism-related genes are shown in the heatmap based on RNA-seq data. Data represent mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA (D).
The upregulated pathways in HCC were related to inflammation, including IL17 signaling, cytokine-cytokine receptor interaction, extracellular matrix (ECM)-receptor interaction, and chemokine signaling. Additionally, cell cycle, p53 signaling, and focal adhesion pathways were upregulated in HCC. In contrast, miR-22 treatment reduced IL17 signaling, TNF signaling, cell cycle, and ECM-receptor interaction (blue, Figure 2A, lower panel). Increased IL17 signaling in HCC was accompanied by enriched IL6/JAK/STAT3, crucial for IL17 transcription and Th17 differentiation (Figure 2B). Increased hypoxia signaling observed in HCC was also inhibited by miR-22 treatment (Figure 2B). Overall, miR-22 treatment improved metabolism, inhibited inflammation, and reduced hypoxia signaling.
Hepatocytes and T cells isolated from HCC have reduced miR-22 and increased HIF1α, which are reversed by miR-22 treatment
Hypoxia induces HIF1α, a master transcription factor that regulates metabolism and inflammation.39,40 HIF1α is an miR-22 target41; an miR-22 homolog motif is shown in the 3′ untranslated region (3′ UTR) of the Hif1a gene (Figure 2C). Thus, the expression levels of miR-22 and HIF1α were quantified in the liver, as well as in isolated hepatocytes and T cells, using western blotting or RT-PCR, respectively. The purity of isolated cells was validated by the expression of cell-type-specific markers (Figure S2). In both HCC hepatocytes and T cells, reduced miR-22 and increased Hif1a expression were observed. miR-22 treatment reversed the expression pattern found in both the RAS/AKT- and β-catenin/AKT models (Figure 2D). Taken together, miR-22 treatment impacted both hepatocytes and T cells.
Western blotting validated these findings at the protein level. In both RAS/AKT- and β-catenin/AKT HCC models, untreated HCC had increased HIF1α, activated STAT3, and increased inflammatory signaling of IL6R and IL17A, which were all reduced by miR-22 (Figure 2E).
HIF1α has known metabolic roles, and the expression of HIF1α-regulated metabolic genes was altered due to HCC development and the response to miR-22 treatment. HCC increased the expression of glycolysis-related genes including Pfkfb4, Pkm, Aldoa, Eno1, Pgam1, Slc2a1, Pfkl, and Gpi1.42 Whereas, two known HIF1α-suppressed lipid metabolism-related genes, Ppara (fatty acid oxidation) and Chka (phospholipid synthesis), were reduced in HCC livers.43,44 Upon miR-22 treatment, these changes were reversed (Figure 2F).
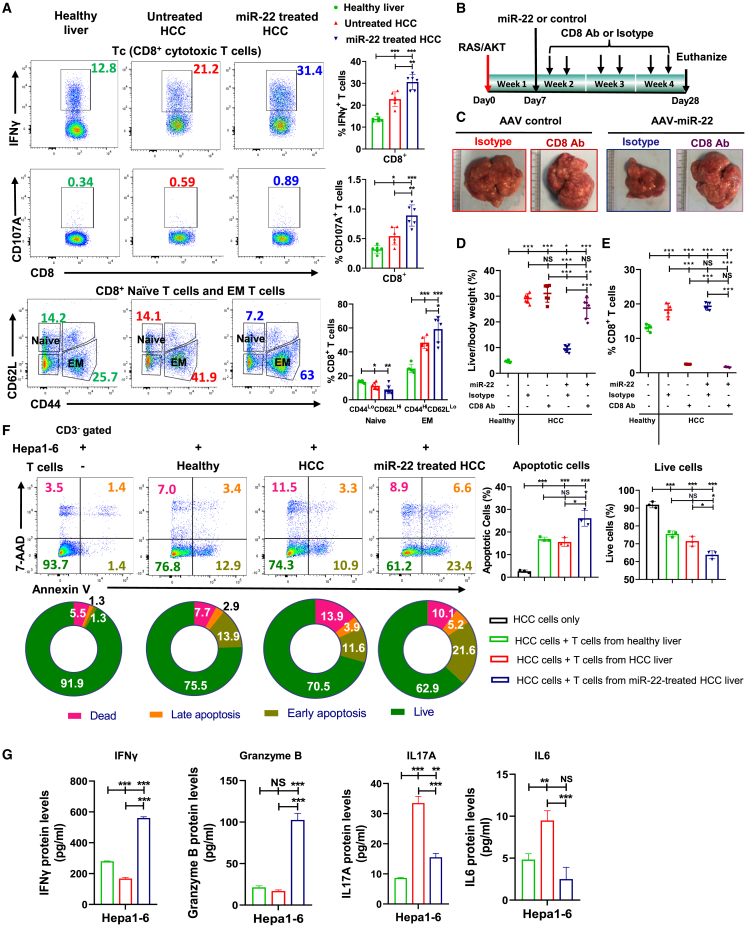
The anti-HCC effect of miR-22 is cytotoxic T cell dependent
Hypoxia and HIF1α can induce tumor cells to become resistant to cytotoxic T cells.45 Flow cytometry was performed to investigate the contribution of cytotoxic T cells to the anti-HCC effect of miR-22. Treatment with miR-22 increased CD8+IFNγ+/CD8+CD107A+ T cells, indicating activation of cytotoxic CD8+ T cells (Figure 3A). In parallel, miR-22 increased CD8+ effector memory T cells, which are known to enhance the therapeutic effects of anti-PD-1 in head and neck cancers (Figure 3A).46 CD8 blockade was then performed (Figure 3B). While anti-CD8 antibody did not affect the L/B ratio and tumor development, CD8 blockade inhibited the anti-HCC effects of miR-22 (Figures 3C and 3D). Flow cytometric analysis confirmed that the anti-CD8 antibody significantly reduced the number of CD8+ T cells in the liver (Figures 3E and S3).
Figure 3.
The anti-HCC effect of miR-22 is cytotoxic T cell dependent, and miR-22 activates cytotoxic T cells to induce apoptosis of HCC cells
(A) Representative flow cytometry plots and percentage of CD8+IFNγ+, CD8+CD107A+, and CD8+ naive and EM T cells. Hepatic lymphocytes were isolated from livers of healthy, HCC, and miR-22-treated HCC mice followed by flow cytometry. (n = 6). (B) Study design of anti-CD8 antibody blockade. (C) Representative liver morphology, (D) L/B ratio (n = 6), and (E) percentage of CD8+ T cells measured by flow cytometry in studied groups (n = 4). (F) Representative flow cytometry plots of Annexin V/7-AAD staining and apoptosis rates of mouse HCC Hepa1-6 cells co-cultured with hepatic T cells. Hepatic-isolated T cells from healthy livers, HCC, and miR-22-treated HCC were co-cultured with Hepa1-6 at a 1:1 ratio for 36 h. (G) The concentrations of IFNγ, granzyme B, IL17A, and IL6, and in the supernatant were quantified by ELISA. Data are representative of two independent experiments (F and G). Data represent mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA (A, D, E, F, and G).
miR-22 activates cytotoxic T cells and induces apoptosis of HCC cells
The essential role of cytotoxic CD8+ T cells in miR-22 treatment was further examined. T cells isolated from livers of healthy, HCC, and miR-22-treated HCC mice were co-cultured with mouse Hepa1-6 HCC cells for 36 h, followed by an apoptosis assay. The data showed that T cells from miR-22-treated mice induced the highest apoptotic rate (Figure 3F), and the culture supernatant also had the highest levels of IFNγ and granzyme B. Compared with healthy controls, HCC T cells produced elevated levels of IL17A and IL6, which were reduced by miR-22 treatment (Figure 3G).
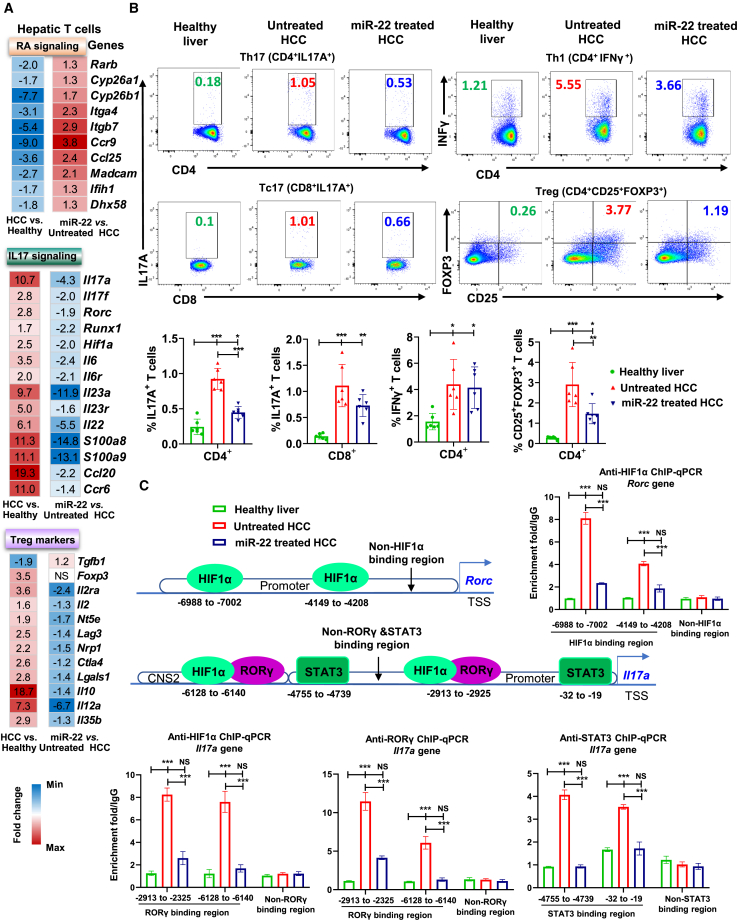
miR-22 increases RA and reduces Th17/Treg signaling in hepatic T cells
We have previously shown that RA via RARβ induces miR-22, which in turn induces RARβ by silencing HDCAs. Thus, RARβ is involved in both upstream and downstream signaling of miR-22.20 This positive regulatory loop ensures sustained RARβ expression. RA, via RARβ, has profound effects on the inhibition of Th17 cell differentiation.47 Hepatic transcriptomics revealed an improved retinol metabolism in response to miR-22 treatment. Therefore, we investigated RA signaling in hepatic T cells.
Consistent with the bulk liver transcriptomic data, RA signaling was reduced in HCC T cells, which was reversed by miR-22 treatment. This was evident by the induction of genes encoding RARβ, as well as RA oxidation enzymes CYP26A1 and CYP26B1. Moreover, miR-22-treated T cells showed increased expression of RA-regulated genes that control immunity,48 including the gut-homing genes Itga4, Itgb7, Ccr9, Ccl25, and Madcam1. Other miR-22-induced RA signaling genes, Ifih1 and Dhx58, can recruit T cells to inflammation sites and enhance CD8+ T cell survival (Figure 4A).49
Figure 4.
miR-22 suppresses IL17 signaling in the T cells by reducing the recruitment of HIF1α/RORγT/STAT3 in the Il17a promoter
(A) The fold changes of RA signaling and Th17/Treg-related genes in hepatic T cells were quantified by RT-PCR and are shown in the heatmap. Hepatic T cells were isolated from livers of healthy, HCC, and miR-22-treated HCC mice followed by flow cytometry (n = 3). (B) Representative flow cytometry plots and percentage of Th17 (CD4+IL17A+), Tc17 (CD8+IL17A+), Th1 (CD4+IFNγ+), and Treg (CD4+CD25+FOXP3+) T cells in studied groups (n = 6). (C) ChIP-qPCR using anti-HIF1α, anti-RORγt, and anti-STAT3 antibodies in hepatic T cells. Hepatic T cells that were isolated from three mice for each studied group were subjected to ChIP assay. The primers for amplifying non-binding regions were used as a negative control. The regulatory regions of the Il17a and Rorc genes are shown with the binding locations of the indicated proteins. The numbers are relative to the transcription start site. Binding enrichment was expressed relative to the IgG-negative control. CNS, conserved non-coding sequence. Data represent mean ± SD, ∗∗p < 0.01; ∗∗∗p < 0.001 by one-way ANOVA (B and C).
IL17 signaling genes significantly upregulated in HCC included Il17a/f, Il17a transcription regulators Rorc, Runx1, and Hif1a, upstream regulatory cytokines Il6, Il23a, and their receptors, as well as Th17 cell downstream signaling Il22, Ccl20, Ccr6, S100a8, and S100a9. miR-22 treatment suppressed all these IL17 signaling genes (Figure 4A). Tregs have immunosuppressive functions in cancer. Many Treg genes were elevated in HCC T cells but repressed by miR-22 treatment, including Il2, surface markers Il2ra, Nt5e, Lag3, Nrp1, Ctla4, and downstream factors Lgals1, Il10, Il12a, and Il35b (Figure 4A).
Similarly, in β-catenin-positive HCC, qRT-PCR data showed that many genes involved in RA signaling were downregulated in HCC T cells but induced by miR-22. In contrast, IL17 signaling-associated genes were induced in HCC and reduced in miR-22-treated T cells (Figure S4).
miR-22 treatment reduces IL17-producing T cells as well as Treg
Flow cytometry was performed to validate these findings. Compared with healthy livers, HCC had increased CD3+IL17A+ T cells, which miR-22 treatment then reduced (Figure S5). The CD4+ subsets (Th17, Treg, Th1) and Tc17 (CD8+IL17A+) T cells were increased in HCC. While miR-22 treatment reduced Th17 and Treg cells, it did not affect Th1 and Tc17 cells (Figure 4B). Taken together, miR-22 suppressed the expansion of IL17-producing cells and Tregs, which likely favored cytotoxic CD8+ T cell activation.50
HCC mice spleens had increased Treg cells, whereas Th17, Tc17, and Th1 cells were similar to healthy mice. In contrast, miR-22 treatment reduced Treg and Th1 cells but had no effect on Th17 and Tc17 cells (Figure S6). Thus, Th17 expansion in HCC and its reduction by miR-22 were tumor specific.
miR-22 silences HIF1α and inhibits Il17a expression by reducing the recruitment of HIF1α/RORγT/STAT3
There are two RORγT and two STAT3 binding sites in the regulatory region of the Il17a gene (Figure 4C).51,52 Moreover, the Rorc promoter contains two hypoxia response elements (HREs).51 The occupancy of the transcription factors HIF1α, RORγT, and STAT3 was examined by chromatin immunoprecipitation-qPCR (ChIP-qPCR) in HCC T cells. In untreated HCC T cells, HIF1α bound to the two HREs located in the Rorc promoter, while miR-22-treated T cells showed reduced binding. Similarly, miR-22-treated HCC T cells showed reduced recruitment of HIF1α, RORγT, and STAT3 to the Il17a promoter (Figure 4C). These results showed that miR-22 silenced the Il17a gene, leading to Th17 cell reduction by decreasing the recruitment of RORγT/HIF1α/STAT3 to Il17a promoter in T cells.
Overexpression of IL23/IL17 attenuates the anti-HCC effect of miR-22
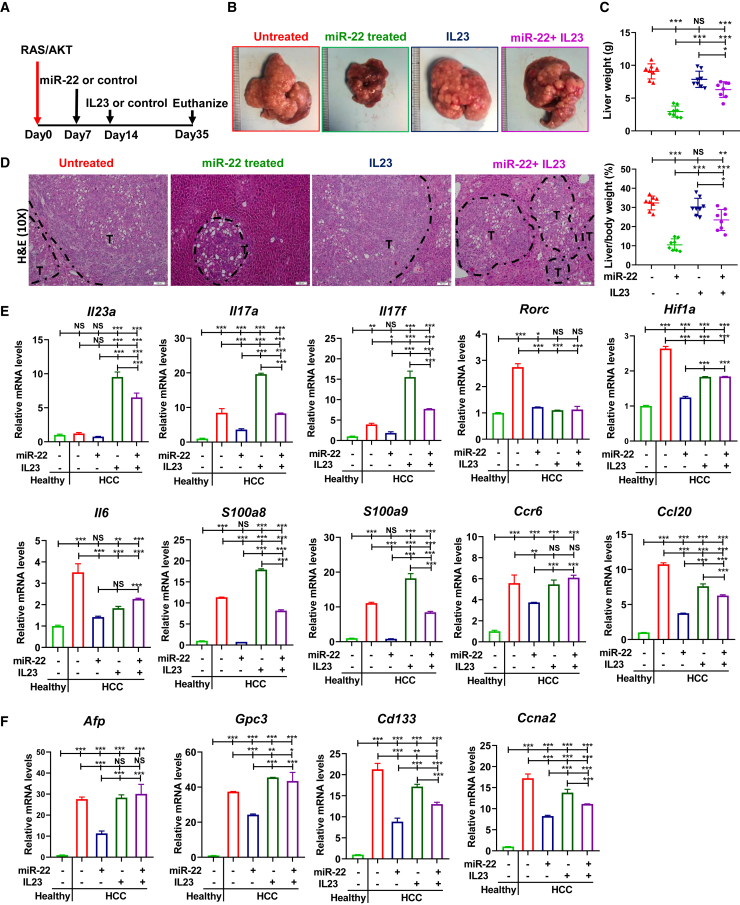
To further establish that IL17A signaling inhibition contributes to the anti-HCC effect of miR-22, a low dose of IL23 minicircle DNA was introduced to boost IL17 (Figure 5A).53 The results showed inducing IL17 attenuated the anti-HCC effect of miR-22, as evidenced by tumor load and histology (Figures 5B–5D). Furthermore, in HCC T cells, IL23 increased Il23, Il17a, Il17f, S100a8, S100a9, Ccr6, and Ccl20 levels (Figure 5E). IL23 overexpression prevented miR-22 reduction of HCC markers Afp, Gpc3, Cd133, and Ccna2 (Figure 5F). Together, reduced IL17 signaling contributed to the anti-HCC effects of miR-22.
Figure 5.
Overexpression of IL23/IL17 attenuates the anti-HCC effect of miR-22
(A) Study design for miR-22 and IL23 overexpression in male HCC mice. (B) Representative liver morphology, (C) liver weight and L/B ratio, and (D) H&E-stained liver sections in each group (n = 8); scale bar, 100 μm. (E) mRNA levels of IL17A signaling-related genes in hepatic T cells isolated from indicated groups (n = 3). (F) Hepatic mRNA levels of HCC markers in each group (n = 3). Data represent mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by one-way ANOVA (C, E, and F).
The similarity between human and mouse HCC based on miR-22 expression
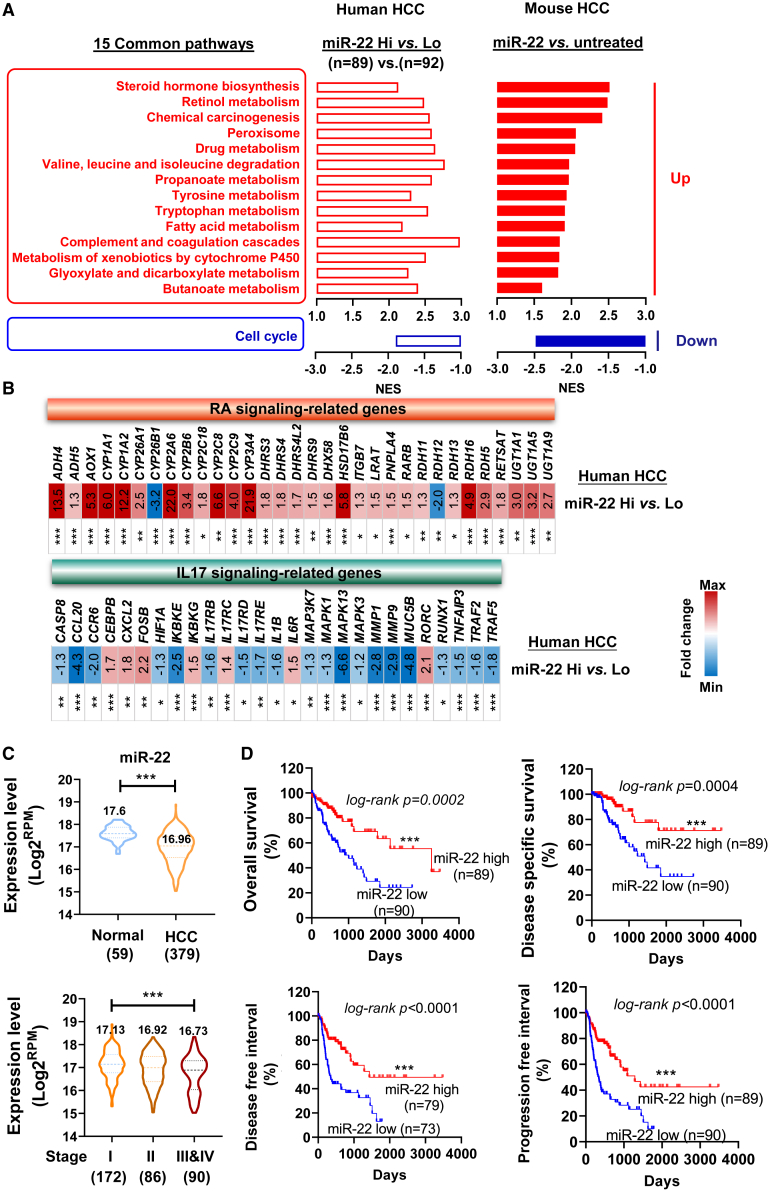
Based on miR-22 expression, the TCGA LIHC dataset was grouped into miR-22 high (miR-22 Hi, n = 89) and miR-22 low (miR-22 Lo, n = 92) HCC. Comparing the two groups, 30 pathways (27 upregulated and three downregulated pathways) were significantly changed (Figure S7). In mouse HCC, miR-22 treatment enriched 30 pathways (19 upregulated and 11 downregulated; Figure 2A); 15 pathways overlapped with human pathways by comparing miR-22 high vs. low human HCC (Figure 6A). Human miR-22 Hi HCC or miR-22-treated mouse HCC consistently showed increased retinol, propanoate, butanoate, and tryptophan metabolism, improved steroid biosynthesis, as well as glycolysis and detoxification, but reduced cell cycle signaling. This congruence suggests human relevance for the mice data.
Figure 6.
Mouse and human HCC have similar gene expression profiles based on miR-22 levels revealing human relevance of the findings
(A) Fifteen common pathways were identified by comparing miR-22 Hi (high, n = 89) vs. miR-22 Lo (low, n = 92) human HCC and miR-22 treated vs. untreated mouse HCC. (B) The fold changes of RA and IL17 signaling-related genes in miR-22 Hi vs. miR-22 Lo human HCC. (C) The levels of miR-22 in human HCC vs. normal livers and different stages of HCC. The numbers in parentheses are case numbers. Data were shown with medium ±5 to 95 percentiles. (D) Kaplan-Meier survival curves of HCC patients with high and low miR-22 levels based on TCGA LIHC. p values were calculated by the log rank test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by unpaired two-tailed Student’s t test (B and C).
Based on the KEGG pathways, the expression of genes involved in retinol metabolism and IL17 signaling was analyzed in miR-22 Hi vs. Lo HCC (Figure 6B). For retinol metabolism, 30/32 related transcripts showed higher levels in miR-22 Hi HCC than in miR-22 Lo HCC. In addition, miR-22 Hi HCC had reduced IL17 signaling in 20/27 transcripts. Fold changes with significance levels are indicated in Figure 6B.
Reduced miR-22 in human HCC predicts poor survival outcomes
miR-22 expression in human HCC was analyzed using the TCGA LIHC dataset. miR-22 levels were significantly lower in 379 HCC specimens compared with the 59 healthy livers. Additionally, miR-22 levels were much lower in stage III and IV HCC (90 cases) than in stage I (172 cases) (Figure 6C). Furthermore, survival analyses revealed that miR-22 levels positively correlated with overall survival, disease-specific survival, disease-free interval, and progression-free interval (Figure 6D). In summary, reduced miR-22 expression predicted poor survival outcomes in patients with HCC.
Discussion
The current study revealed that miR-22 could be a new therapeutic target for HCC treatment. miR-22 gene therapy was effective and prolonged survival without causing detectable toxicity. The current study is also the first to reveal the metabolic and anti-tumor immunity roles of miR-22 in the liver; miR-22 targeted both hepatocytes and T cells by silencing HIF1α and increasing RA signaling, both of which have metabolic and anti-inflammatory effects.
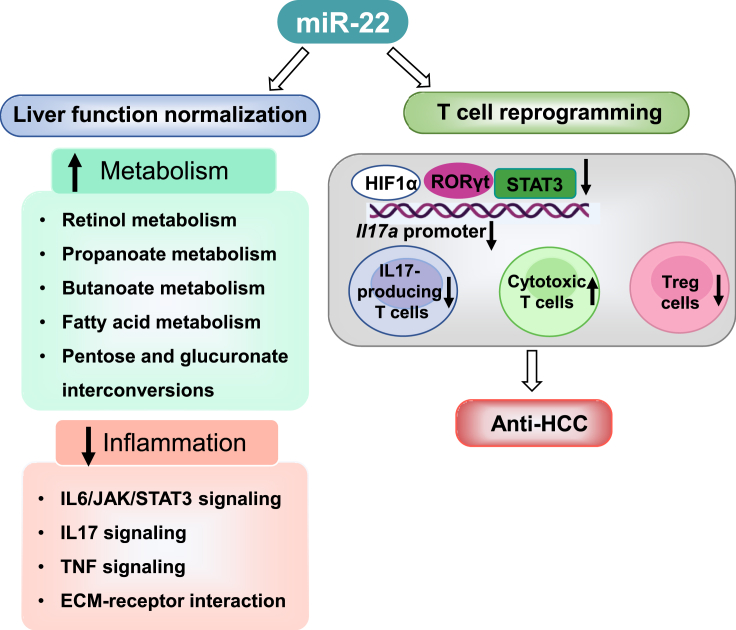
Our data revealed that miR-22 had profound effects on reducing inflammation. In T cells, miR-22 affected Il17a expression at multiple levels: (1) miR-22 reduced HIF1α and its occupancy of the Rorc gene, leading to reduced expression. (2) miR-22 diminished the recruitment of HIF1α and RORγT to Il17a and reduced IL17 expression. (3) miR-22 inhibited IL6/IL23 signaling, deactivated STAT3, and reduced its recruitment to Il17a, suppressing IL17 levels. Additionally, miR-22 activated cytotoxic T cells, induced apoptosis in tumor cells, and suppressed Treg cells, which can inhibit the activation of anti-tumor effector cells, leading to tumor immune escape.54 These results are summarized in Figure 7.
Figure 7.
The schematic diagram summarizes miR-22 treats HCC by inducing metabolism and modulating T cell reprogramming
miR-22 treatment induces the metabolism of retinol, propanoate, butanoate, fatty acid, and sugar. Therefore, the miR-22 inducer signaling is compromised in HCC and restored due to positive treatment outcomes. Meanwhile, miR-22 inhibits inflammation pathways including IL17 signaling, cytokine-receptor interaction, and ECM-receptor interaction, which are all upregulated due to HCC development. In the T cells, miR-22 inhibits IL17 signaling at multiple levels: (1) miR-22 silences HIF1α and reduces its occupancy in the Rorc promoter. (2) miR-22 reduces Rorc expressions and the recruitment of RORγT/HIF1α to the Il17a gene leading to reduced expression. (3) miR-22 deactivates STAT3 and decreases its occupancy in the Il17a promoter, which consequentially reduces Il17a gene expression. Additionally, miR-22 reduces Treg cells. The reduced inflammatory signaling as well as immunosuppressive effects permit activation of cytotoxic T cells leading to cancer cell death.
RA self-regulates via RARβ binding to induce Rarb expression.55 RA also increases RARβ via the induction of miR-22.20 Thus, multiple pathways induce and sustain RA signaling, signifying its importance. Increasing RA signaling has several benefits. RA and its receptors, including RXRα, facilitate lipid metabolism by dimerizing with FXR and PPARα. RA also inhibits the production of Th17 cells by blocking IL23/IL6 signaling and reducing Rorc expression.47,56 In line with this, our data revealed that restoration of RA signaling by miR-22 was accompanied by reduced Rorc, Runx1, Il6, Il6r, Il23, and Il23r mRNA in T cells. Furthermore, IL23 overexpression boosted IL17 signaling and attenuated the anti-HCC effects of miR-22, indicating that miR-22 treatment was partly mediated by the inhibition of inflammatory IL17A signaling. Thus, miR-22 and RA mutually regulate one another. Whether miR-22-suppressed IL17 signaling is RA dependent would be of interest for future studies.
Patients with HCC have a high frequency of Tregs, which is correlated with poor prognosis and reduced survival.57 Depleting these tumor-infiltrating Treg cells improves immunotherapy of HCC.58 Our data revealed that miR-22 reduced Tregs and the expression of Treg marker genes in HCC cells. However, RA plays a role in Treg expansion, and this mechanism is partially mediated by RARα-mediated TGFβ and FOXP3 induction.47,51 Whether miR-22-regulated RA signaling affects Treg regulation in the HCC environment remains to be addressed.
Interestingly, the synthesis of RA in intestinal dendritic cells is controlled by aldehyde dehydrogenase ALDH1A, whose expression is induced by HDAC inhibitors, such as butyric acid and propionic acid, which are miR-22 inducers.20,47 Thus, there are interactive effects between SCFAs and RA signaling. Our transcriptomic data revealed that the metabolic pathways for SCFAs and retinol were both reduced in HCC but induced in response to miR-22 treatment. The effect of miR-22 on the regulation of the gut microbiome remains to be elucidated. These data suggest that the intertwined signaling pathways found in the gut affect liver health. Targeting these pathways in the gut-liver axis may provide novel therapeutic options for treating HCC.
Materials and methods
Mice and tumor models
Male and female 6-week-old FVB/N mice were obtained from Jackson Laboratory (Sacramento, CA, USA). Liver tumors were produced via sleeping beauty transposon (SB)-mediated hydrodynamic injection using the plasmids pT3-EF1α-HA-myr-AKT, pT3-EF1α-N90-β-catenin, pT/Caggs-Nras-v12, and pCMV-SB11 as previously described.59 Briefly, oncogene plasmids and SB were diluted in 2 mL saline and injected into the mouse tail vein within 5–7 s. Mice were housed, fed, and monitored according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis (Sacramento, CA, USA).
Drug administration
miR-22 was delivered by adeno-associated virus, serotype 8 (AAV8, Applied Biological Materials, Richmond, BC, Canada). One dose (5 x 1012 GC/kg) of AAV8-miR-22 or AAV8 blank control was injected intravenously. Lenvatinib (10 mg/kg/day, MedChemExpress, Monmouth Junction, NJ, USA) or saline was administered via oral gavage. The treatment timeline for each experiment is presented in the figures.
Hepatic lymphocyte isolation and T cell enrichment
Hepatic lymphocytes were isolated from livers of healthy, HCC, and miR-22-treated HCC mice using previously published methods.60 In detail, the livers were mechanically dissociated followed by digestion with Liberase (0.05 mg/mL, Roche Diagnostics, Basel, Switzerland) at 37°C for 30 min. The digested specimens were filtered through a 70-μm cell strainer. Hepatocytes were removed by sequential centrifugation at 50 x g (5 min, 4°C). The supernatants containing nonparenchymal cells (NPCs) were collected by centrifugation at 500 x g for 5 min at 4°C. The NPCs were further fractionated by Percoll (GE Healthcare, Little Chalfont, United Kingdom) density gradient (70%/30%) centrifugation at 690 x g for 12 min at room temperature. Red blood cells were removed by incubating in RBC lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) for 5 min at room temperature. After washing with PBS, NPCs were pelleted by centrifugation at 300 x g for 5 min. CD3+ T cells were further purified using a MojoSort mouse CD3+ T cell Isolation Kit (Biolegend, San Diego, California, USA) followed by RNA extraction, ChIP, or co-culture. The purity of CD3+ T cells was >90%.
Cloning and packaging of miR-22 overexpressed adeno-associated virus
Mature mmu-miR-22-3p (ID: MIMAT0000531, https://www.mirbase.org/cgi-bin/mature.pl?mature_acc=MIMAT0000531) was cloned into AAV8 plasmid pAAV-miro-GFP-hGH-amp vector with CMV promoter (Applied Biological Materials, Richmond, BC, USA). The AAV8 viruses were packaged and tittered by Applied Biological Materials.
RNA isolation and gene expression quantification
Total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific), and cDNA was generated using a High-Capacity RNA-to-cDNA Kit (Applied Biosystems, Carlsbad, CA, USA).19,20 qRT-PCR was performed on a QuantStudio 6 Fast real-time PCR system using Power SYBR Green PCR master mix (Applied Biosystems). Primers were designed using Primer3 Input software version 0.4.0. Primer sequences are listed in Table S1.
RNA sequencing and bioinformatics data analysis
RNA samples used for RNA sequencing were isolated from RAS/AKT-induced HCCs treated with AAV control or miR-22 as well as normal healthy livers from the same genetic background, FVB/N mice (n = 3). RNA was quantified with Nanodrop, and the quality was determined using a Qubit and Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA). Library preparation and sequencing were performed by Novogene (Sacramento, CA, USA). Libraries were prepared using a NEBNext Ultra II non-directional RNA Library Prep kit (New England Biolabs, Ipswich, MA, USA). Library quality and concentration were assessed with LabChip GX Touch nucleic acid analyzer (PerkinElmer, Waltham, MA, USA) and qPCR. Libraries were sequenced on Novaseq6000 using PE150 sequencing. Reads quality was checked using the fastqc (v0.11.7, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/).61 RNA sequencing data was analyzed using the Salmon-tximport-DESeq2 pipeline. The pair-ended reads (FASTQ format) were mapped to the reference mouse genome assembly (GRCm39, GENCODE release 25) and quantified with Salmon.62 Gene-level counts were imported with tximport,63 and differential expression analysis was performed with DESeq2 (version 1.18) with the corrected p value <0.05 and fold change >1.5.64 Pathway analysis was performed with iDEP (http://bioinformatics.sdstate.edu/idep93/) using GSEA method,65,66 which is conducted in the pre-ranked mode using a recent faster algorithm based on the fgsea package (bioRxiv, http://biorxiv.org/content/early/2016/06/20/060012).67 Functional pathways or processes with FDR <0.1 and Bonferroni value < 0.1 were accepted. KEGG gene set was used in the GSEA analysis.
TCGA analysis
miR-22 levels and survival analysis were performed using UCSC Xena (http://xena.ucsc.edu/) based on the TCGA LIHC dataset.68 GSEA analysis based on the KEGG and hallmark gene set was performed by UCSC Xena differential gene expression analysis (http://analysis.xenahubs.net/).68 The TCGA LIHC dataset includes 379 HCC and 59 adjacent normal liver specimens. For pathway analysis based on differentially expressed miR-22 levels, 181 HCC specimens were classified into two groups based on the upper and lower quartiles of miR-22 levels: (1) high miR-22 expression (miR-22 Hi) with Log2(RPM) > 17.48 (n = 89) and (2) low miR-22 expression (miR-22 Lo) with Log2(RPM) < 16.5 (n = 92).
Co-culture of hepatic T cells and HCC cells
Murine Hepa1-6 cells (ATCC CRL-1830) were seeded into 6-well plates at a density of 1 x 105/well in RPMI 1640 culture medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (R&D Systems, Minneapolis, MN, USA). At day 2, T cells isolated from livers of healthy, HCC, and miR-22-treated HCC mice were mixed with Hepa1-6 cells at a 1:1 ratio. Cells and supernatants were collected after 36 h of incubation for further flow cytometry or enzyme-linked immunosorbent assay (ELISA). For flow cytometry, 2 x 106 cells were stained with anti-CD3e antibody (BD Bioscience San Jose, California USA) to gate the CD3+ T cell population. Annexin V/7-AAD staining (Thermo Fisher Scientific) was applied to determine the apoptosis of Hepa1-6 cells (CD3– cells) in round-bottom 96-well plates.
ELISA
The levels of IL17A, IL6, IFNγ, and granzyme B in the supernatant of the co-culture system were quantified by ELISA kits according to the manufacturer’s protocol (Thermo Fisher Scientific).
Flow cytometry
Antibodies used for flow cytometric analysis are listed in Table S1. They were tested for optimal dilution in-house based on lot, clone, and vendor. 2 x 106 lymphocytes isolated from the livers and spleens were stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin (50 ng/mL and 500 ng/mL, respectively) in the presence of Golgi Stop (Thermo Fisher Scientific) for 6 h. Thereafter, the cells were washed and stained for surface antigens. Zombie Aqua fixable viability (1:500; BioLegend) was added to exclude dead cells. For surface antigen staining only, cells were fixed in 4% paraformaldehyde. For intracellular staining, cells were fixed and permeabilized using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) followed by staining of intracellular proteins. Data were acquired by a BD LSRFortessa instrument running FACS DIVA software and analyzed with FlowJo v10.7.1 software (Tree Star, OR).
Chromatin immunoprecipitation-qPCR
ChIP-qPCR was performed based on a previous publication.69 Briefly, chromatin lysate was precleared before incubation with anti-HIF1α (Novus Biologicals, Centennial, CO, USA), anti-RORγT (eBioscience), or anti-phosphor (P)-STAT3 (Cell Signaling Technology, Beverly, MA, USA). Rabbit IgG and H3Ac antibodies (MilliporeSigma, Burlington, MA, USA) were used as negative and positive controls, respectively. Samples were incubated with Dynase beads (Thermo Fisher Scientific) at 4°C overnight followed by de-crosslinking and purification. ChIP analysis was carried out according to the manufacturer’s protocol (Upstate/Millipore, Massachusetts, USA). The immunoprecipitated DNA was quantified via RT-PCR with a QuantStudio 6 Fast real-time PCR system (Applied Biosystems) using SYBR Green. Primers and antibodies used for ChIP assays are listed in Tables S1 and S2.
Histology, tumor grade, Ki67 immunohistochemistry, and TUNEL assay
Tumor score was quantitively evaluated by pathologists based on H&E staining using five criteria including the level of centrilobular vacuolar degeneration, the number of proliferation foci, mitotic index, scirrhous type foci of proliferation, and inflammatory cells.70,71 The tumor scoring criteria are described in Table S3.
Immunohistochemistry was performed as described previously.28,72 To monitor hepatocyte proliferation, immunostaining was performed with anti-Ki67 antibody (NeoMarkers, Fremont, CA, USA) in healthy livers, HCC, miR-22-treated HCC, and lenvatinib-treated HCC. The number of proliferating hepatocytes was determined by counting positive-staining cells in at least five random microscopic fields (x10) for each specimen. Positive cells were determined using QuPath software.73 To monitor apoptosis in response to miR-22, TUNEL assay was performed with TUNEL assay kit (Abcam, Cambridge, MA), according to the manufacturer’s instructions.
Serum biochemistry analysis
Blood samples were collected at the endpoint of the experiments, and serum was separated within 2 h of the collection after centrifugation at 3,000 x g for 10 min. Serum ALT, AST, cholesterol, glucose, globulin, albumin, total protein, and BUN levels were quantified using FUJI DRI-CHEM 4000 veterinary chemistry analyzer (Heska Corporation, Loveland, CO) according to manufacturer’s instruction.
Blood hematology analysis
Blood samples were collected at the endpoint of the experiments. 100 μL aliquots were analyzed within 10 min of collection using a veterinary Hematrue hematology analyzer (Heska Corporation) based on the manufacturer’s instruction.74
Western blotting
Western blots were performed as described previously.19,20,28 Proteins were extracted from the livers using a lysis buffer with cocktail protease inhibitors and phosphatase inhibitors (Thermo Fisher Scientific). Protein concentration was measured using the Pierce BCA protein assay kit, and 20–40 μg of total lysate was loaded and immunoblotted. Antibodies used were anti-IL6R (Santa Cruz Biotechnology, Santa Cruz, CA, USA), IL17A (eBioscience), phosphor (P)-STAT3, total (T)-STAT3 (Cell Signaling Technology), β-ACTIN (MilliporeSigma), and HIF1α (Novus Biologicals), which are listed in Table S1.
Cytotoxic CD8+ T cell depletion assay
At 1 week post oncogene injection, HCC-bearing mice were randomly assigned into AAV-miR-22 or AAV blank treated groups. On the same day, HCC mice from each group were randomly divided into two groups to receive 200 μg of InVivoMAb anti-mouse CD8α (BE004-1, Bio X cell, Lebanon, NH, USA) or InVivoMAb rat IgG2a isotype control (BE0089, Bio X cell) via intraperitoneal injection twice per week for six times as indicated.75
IL-23 minicircle DNA production and hydrodynamic injection in HCC mice
Minicircle (MC)-RSV.Flag.mIL23.elasti.bpA or RSV.eGFP.bpA was produced to induce IL17 signaling in HCC mice as described.76 Hydrodynamic delivery of 3 μg IL-23 or GFP MC DNA via tail vein injection was performed as previously described.77 At 1 week post oncogene injection, HCC mice were randomly assigned into AAV-miR-22 or AAV blank treated groups. At 2 weeks post oncogene injection, mice from each subgroup were further divided into two groups to receive one dose (3 μg) of either IL-23 MC or MC control.
Statistical analysis
Statistical analysis was performed using Prism software v8.2.1 (Graph Software). Data were expressed as means ± standard deviation (SD). Statistical significance between two groups was evaluated using a two-tailed Student’s t test. One-way ANOVA followed by Tukey’s t test was used to compare the statistical difference among multiple groups. Associations were analyzed by linear regression. A value of p < 0.05 was considered statistically significant.
Data availability
The source data that support the findings of this study are available. The RNA sequencing data have been deposited at the Gene Expression Omnibus under the accession number GSE215753. All the other data are available in the main text or supplemental information.
Acknowledgments
We thank Drs. Snigdha Guha, Chengfei Liu, and Ms. Jennifer Clark from UC Davis for editing and commenting on the manuscript. We also thank Dr. Daisuke Yamada for preparing IL23 minicircle plasmids. We would like to thank the following funding sources for their support: National Institutes of Health, United States (R01CA222490, R50CA243787, and T32 CA108459-15).
Author contributions
Conceptualization, methodology, and study design: Y.-J.Y.W. and Y.H. Data acquisition, data analysis, and interpretation: Y.H., T.S., F.V., and Y.-J.Y.W. Manuscript writing: Y.H. and Y.-J.Y.W. Manuscript editing and commenting: T.S., X.C., X.S.W., and S.T.H. Obtaining research funding: Y.-J.Y.W. and Y.H.
Declaration of interests
The authors declare no potential conflict of interest.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.04.019.
Supplemental information
References
- 1.Chen B., Garmire L., Calvisi D.F., Chua M.S., Kelley R.K., Chen X. Harnessing big 'omics' data and AI for drug discovery in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020;17:238–251. doi: 10.1038/s41575-019-0240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J. Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Colquhoun S.D., Wan Y.J.Y. Hepatocellular carcinoma diagnosis and treatment: an overview. Liver Res. 2020;4:159–160. doi: 10.1016/j.livres.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faivre S., Rimassa L., Finn R.S. Molecular therapies for HCC: looking outside the box. J. Hepatol. 2020;72:342–352. doi: 10.1016/j.jhep.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 6.Xu C., Xu Z., Zhang Y., Evert M., Calvisi D.F., Chen X. beta-Catenin signaling in hepatocellular carcinoma. J. Clin. Invest. 2022;132 doi: 10.1172/JCI154515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishida N., Kudo M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol. Res. 2018;48:622–634. doi: 10.1111/hepr.13191. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri F., Colquhoun S., Wan Y.J.Y. Hepatocellular carcinoma immunotherapy: the impact of epigenetic drugs and the gut microbiome. Liver Res. 2020;4:191–198. doi: 10.1016/j.livres.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J., Li Y., Ding M., Zhang H., Xu X., Tang J. Molecular mechanisms and clinical applications of miR-22 in regulating malignant progression in human cancer (Review) Int. J. Oncol. 2017;50:345–355. doi: 10.3892/ijo.2016.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansini A.P., Lorenzo Pisarello M.J., Thelen K.M., Cruz-Reyes M., Peixoto E., Jin S., Howard B.N., Trussoni C.E., Gajdos G.B., LaRusso N.F., et al. MicroRNA (miR)-433 and miR-22 dysregulations induce histone-deacetylase-6 overexpression and ciliary loss in cholangiocarcinoma. Hepatology. 2018;68:561–573. doi: 10.1002/hep.29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y., Chen X., Cheng R., Yang F., Yu M., Wang C., Cui S., Hong Y., Liang H., Liu M., et al. The Jun/miR-22/HuR regulatory axis contributes to tumourigenesis in colorectal cancer. Mol. Cancer. 2018;17:11. doi: 10.1186/s12943-017-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S., Wang X., Wu Y., Zhou T., Tian H., Liu Z., Li L., Yan Z., Zhang G. miR-22 suppresses EMT by mediating metabolic reprogramming in colorectal cancer through targeting MYC-associated factor X. Dis. Markers. 2022;2022 doi: 10.1155/2022/7843565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X., Su W., Li Y., Zhou Z., Zhou Y., Shan H., Han X., Zhang M., Zhang Q., Bai Y., et al. MiR-22-3p suppresses cell growth via MET/STAT3 signaling in lung cancer. Am. J. Transl. Res. 2021;13:1221–1232. [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi H., Hayashi M., Taniguchi K., Nakamura M., Kuranaga Y., Ito Y., Kondo Y., Sasaki H., Terai Y., Akao Y., Ohmichi M. The therapeutic potential of exosomal miR-22 for cervical cancer radiotherapy. Cancer Biol. Ther. 2020;21:1128–1135. doi: 10.1080/15384047.2020.1838031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W., Zhan F., Li D., Wang T., Huang H. RGMB-AS1/miR-22-3p/NFIB axis contributes to the progression of gastric cancer. Neoplasma. 2020;67:484–491. doi: 10.4149/neo_2020_190418N350. [DOI] [PubMed] [Google Scholar]
- 16.Vesuna F., Lisok A., van Diest P., Raman V. Twist activates miR-22 to suppress estrogen receptor alpha in breast cancer. Mol. Cell. Biochem. 2021;476:2295–2306. doi: 10.1007/s11010-021-04065-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y., Li X., Zeng C., Liu C., Hao Q., Li W., Zhang K., Zhang W., Wang S., Zhao H., et al. CD63(+) cancer-associated fibroblasts confer tamoxifen resistance to breast cancer cells through exosomal miR-22. Adv. Sci. 2020;7 doi: 10.1002/advs.202002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang R., Deng L., Zhao L., Li X., Zhang F., Xia Y., Gao Y., Wang X., Sun B. miR-22 promotes HBV-related hepatocellular carcinoma development in males. Clin. Cancer Res. 2011;17:5593–5603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 19.Yang F., Hu Y., Liu H.X., Wan Y.J.Y. MiR-22-silenced cyclin A expression in colon and liver cancer cells is regulated by bile acid receptor. J. Biol. Chem. 2015;290:6507–6515. doi: 10.1074/jbc.M114.620369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y., French S.W., Chau T., Liu H.X., Sheng L., Wei F., Stondell J., Garcia J.C., Du Y., Bowlus C.L., Wan Y.J.Y. RARbeta acts as both an upstream regulator and downstream effector of miR-22, which epigenetically regulates NUR77 to induce apoptosis of colon cancer cells. FASEB J. 2019;33:2314–2326. doi: 10.1096/fj.201801390R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Díaz S., Valle N., Ferrer-Mayorga G., Lombardía L., Herrera M., Domínguez O., Segura M.F., Bonilla F., Hernando E., Muñoz A. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 2012;21:2157–2165. doi: 10.1093/hmg/dds031. [DOI] [PubMed] [Google Scholar]
- 22.Sheng L., Jena P.K., Hu Y., Liu H.X., Nagar N., Kalanetra K.M., French S.W., French S.W., Mills D.A., Wan Y.J.Y. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J. Pathol. 2017;243:431–441. doi: 10.1002/path.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H.X., Keane R., Sheng L., Wan Y.J.Y. Implications of microbiota and bile acid in liver injury and regeneration. J. Hepatol. 2015;63:1502–1510. doi: 10.1016/j.jhep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan Y.J.Y., Jena P.K. Precision dietary supplementation based on personal gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:204–206. doi: 10.1038/s41575-019-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan L., Liu H.X., Fang Y., Kong B., He Y., Zhong X.B., Fang J., Wan Y.J.Y., Guo G.L. Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gyamfi M.A., He L., French S.W., Damjanov I., Wan Y.J.Y. Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. J. Pharmacol. Exp. Ther. 2008;324:443–453. doi: 10.1124/jpet.107.132258. [DOI] [PubMed] [Google Scholar]
- 27.Luo L.J., Zhang L.P., Duan C.Y., Wang B., He N.N., Abulimiti P., Lin Y. The inhibition role of miR-22 in hepatocellular carcinoma cell migration and invasion via targeting CD147. Cancer Cel Int. 2017;17:17. doi: 10.1186/s12935-016-0380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Y., Liu H.X., Jena P.K., Sheng L., Ali M.R., Wan Y.J.Y. miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Wang Y.S., Mugiyanto E., Chang W.C., Yvonne Wan Y.J. MiR-22 as a metabolic silencer and liver tumor suppressor. Liver Res. 2020;4:74–80. doi: 10.1016/j.livres.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian J., Wang W., Zhu J., Zhuang Y., Qi C., Cai Z., Yan W., Lu W., Shang A. Histone methyltransferase SETDB1 promotes immune evasion in colorectal cancer via FOSB-mediated downregulation of MicroRNA-22 through BATF3/PD-L1 pathway. J. Immunol. Res. 2022;2022 doi: 10.1155/2022/4012920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X., Zhou J., Zhong Y., Jiang L., Mu P., Li Y., Singh N., Nagarkatti M., Nagarkatti P. Expression, regulation and function of microRNAs in multiple sclerosis. Int. J. Med. Sci. 2014;11:810–818. doi: 10.7150/ijms.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu W., You R., Yuan X., Yang T., Samuel E.L.G., Marcano D.C., Sikkema W.K.A., Tour J.M., Rodriguez A., Kheradmand F., Corry D.B. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote T(H)17 cell-dependent emphysema. Nat. Immunol. 2015;16:1185–1194. doi: 10.1038/ni.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stauffer J.K., Scarzello A.J., Andersen J.B., De Kluyver R.L., Back T.C., Weiss J.M., Thorgeirsson S.S., Wiltrout R.H. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai Z.T., Zhang X.P., Shao M., Ao J.Y., Chen Z.H., Zhang F., Hu Y.R., Zhong C.Q., Lin J.H., Fang K.P., et al. Impact of splenomegaly and splenectomy on prognosis in hepatocellular carcinoma with portal vein tumor thrombus treated with hepatectomy. Ann. Transl. Med. 2021;9:247. doi: 10.21037/atm-20-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu L., Yang W., Shu Y.F., Xu X.F. Hepatocellular carcinoma and multiple myeloma with elevated globulin: a case report and literature review. J. Int. Med. Res. 2020;48 doi: 10.1177/0300060520920395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regino C.A., López-Montoya V., López-Urbano F., Alvarez J.C., Roman-Gonzalez A. Paraneoplastic hypoglycemia in hepatocarcinoma: case report and literature review. Cureus. 2020;12 doi: 10.7759/cureus.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J., Yan B., Yang L., Li H., Fan Y., Zhu F., Zheng J., Ma X. Macrocytic anemia is associated with the severity of liver impairment in patients with hepatitis B virus-related decompensated cirrhosis: a retrospective cross-sectional study. BMC Gastroenterol. 2018;18:161. doi: 10.1186/s12876-018-0893-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz de Galarreta M., Bresnahan E., Molina-Sánchez P., Lindblad K.E., Maier B., Sia D., Puigvehi M., Miguela V., Casanova-Acebes M., Dhainaut M., et al. Beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson G.K., Tennant D.A., McKeating J.A. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: current understanding and future directions. J. Hepatol. 2014;61:1397–1406. doi: 10.1016/j.jhep.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Chen C., Lou T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget. 2017;8:46691–46703. doi: 10.18632/oncotarget.17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamakuchi M., Yagi S., Ito T., Lowenstein C.J. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao M.H., Wong C.C. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. 2021;10:e71715. doi: 10.3390/cells10071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narravula S., Colgan S.P. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J. Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 44.Bansal A., Harris R.A., DeGrado T.R. Choline phosphorylation and regulation of transcription of choline kinase alpha in hypoxia. J. Lipid Res. 2012;53:149–157. doi: 10.1194/jlr.M021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lequeux A., Noman M.Z., Xiao M., Van Moer K., Hasmim M., Benoit A., Bosseler M., Viry E., Arakelian T., Berchem G., et al. Targeting HIF-1 alpha transcriptional activity drives cytotoxic immune effector cells into melanoma and improves combination immunotherapy. Oncogene. 2021;40:4725–4735. doi: 10.1038/s41388-021-01846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kansy B.A., Concha-Benavente F., Srivastava R.M., Jie H.B., Shayan G., Lei Y., Moskovitz J., Moy J., Li J., Brandau S., et al. PD-1 status in CD8(+) T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 2017;77:6353–6364. doi: 10.1158/0008-5472.CAN-16-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao S., Jin H., Korn T., Liu S.M., Oukka M., Lim B., Kuchroo V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammerschmidt S.I., Friedrichsen M., Boelter J., Lyszkiewicz M., Kremmer E., Pabst O., Förster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suthar M.S., Ramos H.J., Brassil M.M., Netland J., Chappell C.P., Blahnik G., McMillan A., Diamond M.S., Clark E.A., Bevan M.J., Gale M., Jr. The RIG-I-like receptor LGP2 controls CD8(+) T cell survival and fitness. Immunity. 2012;37:235–248. doi: 10.1016/j.immuni.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D., Yu W., Lian J., Wu Q., Liu S., Yang L., Li F., Huang L., Chen X., Zhang Z., et al. Th17 cells inhibit CD8(+) T cell migration by systematically downregulating CXCR3 expression via IL-17A/STAT3 in advanced-stage colorectal cancer patients. J. Hematol. Oncol. 2020;13:68. doi: 10.1186/s13045-020-00897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.R., et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R., Hirahara K., Sun H.W., Wei L., Vahedi G., et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Z., Wu X., Wu C.Y., Singh S.P., Law T., Yamada D., Huynh M., Liakos W., Yang G., Farber J.M., et al. Bile acids improve psoriasiform dermatitis through inhibition of IL-17a expression and CCL20-CCR6-mediated trafficking of T cells. J. Invest. Dermatol. 2022;142:1381–1390.e11. doi: 10.1016/j.jid.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Togashi Y., Shitara K., Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019;16:356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 55.Bushue N., Wan Y.J.Y. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev. 2010;62:1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown C.C., Esterhazy D., Sarde A., London M., Pullabhatla V., Osma-Garcia I., Al-Bader R., Ortiz C., Elgueta R., Arno M., et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity. 2015;42:499–511. doi: 10.1016/j.immuni.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Liu T., Tang W., Deng B., Chen Y., Zhu J., Shen X. Hepatocellular carcinoma cells induce regulatory T cells and lead to poor prognosis via production of transforming growth factor-beta1. Cell. Physiol. Biochem. 2016;38:306–318. doi: 10.1159/000438631. [DOI] [PubMed] [Google Scholar]
- 58.Unitt E., Rushbrook S.M., Marshall A., Davies S., Gibbs P., Morris L.S., Coleman N., Alexander G.J.M. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 59.Chen X., Calvisi D.F. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am. J. Pathol. 2014;184:912–923. doi: 10.1016/j.ajpath.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiede F., Tiganis T. Isolation and characterization of mouse intrahepatic lymphocytes by flow cytometry. Methods Mol. Biol. 2018;1725:301–311. doi: 10.1007/978-1-4939-7568-6_23. [DOI] [PubMed] [Google Scholar]
- 61.Aronesty E. Comparison of sequencing utility programs. Open Bioinforma. J. 2013;7:1–8. [Google Scholar]
- 62.Patro R., Duggal G., Love M.I., Irizarry R.A., Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soneson C., Love M.I., Robinson M.D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ge S.X., Son E.W., Yao R. iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics. 2018;19:534. doi: 10.1186/s12859-018-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sergushichev A.A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv. 2016 doi: 10.1101/060012. Preprint at. [DOI] [Google Scholar]
- 68.Goldman M.J., Craft B., Hastie M., Repečka K., McDade F., Kamath A., Banerjee A., Luo Y., Rogers D., Brooks A.N., et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020;38:675–678. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Y., Liu H.X., He Y., Fang Y., Fang J., Wan Y.J.Y. Transcriptome profiling and genome-wide DNA binding define the differential role of fenretinide and all-trans RA in regulating the death and survival of human hepatocellular carcinoma Huh7 cells. Biochem. Pharmacol. 2013;85:1007–1017. doi: 10.1016/j.bcp.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thoolen B., Maronpot R.R., Harada T., Nyska A., Rousseaux C., Nolte T., Malarkey D.E., Kaufmann W., Küttler K., Deschl U., et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010;38:5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- 71.Ha S.Y., Choi M., Lee T., Park C.K. The prognostic role of mitotic index in hepatocellular carcinoma patients after curative hepatectomy. Cancer Res. Treat. 2016;48:180–189. doi: 10.4143/crt.2014.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Y., Zhan Q., Liu H.X., Chau T., Li Y., Wan Y.J. Accelerated partial hepatectomy-induced liver cell proliferation is associated with liver injury in Nur77 knockout mice. Am. J. Pathol. 2014;184:3272–3283. doi: 10.1016/j.ajpath.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bankhead P., Loughrey M.B., Fernández J.A., Dombrowski Y., McArt D.G., Dunne P.D., McQuaid S., Gray R.T., Murray L.J., Coleman H.G., et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohr A.M., ElHassan I.O., Hannoush E.J., Sifri Z.C., Offin M.D., Alzate W.D., Rameshwar P., Livingston D.H. Does beta blockade postinjury prevent bone marrow suppression? J. Trauma. 2011;70:1043–1049. doi: 10.1097/TA.0b013e3182169326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen L., Xin B., Wu P., Lin C.H., Peng C., Wang G., Lee J., Lu L.F., Feng G.S. An efficient combination immunotherapy for primary liver cancer by harmonized activation of innate and adaptive immunity in mice. Hepatology. 2019;69:2518–2532. doi: 10.1002/hep.30528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Z.Y., He C.Y., Kay M.A. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum. Gene Ther. 2005;16:126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- 77.Adamopoulos I.E., Tessmer M., Chao C.C., Adda S., Gorman D., Petro M., Chou C.C., Pierce R.H., Yao W., Lane N.E., et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 2011;187:951–959. doi: 10.4049/jimmunol.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source data that support the findings of this study are available. The RNA sequencing data have been deposited at the Gene Expression Omnibus under the accession number GSE215753. All the other data are available in the main text or supplemental information.