Abstract
Post-coronavirus disease 2019 (COVID-19) cholangiopathy (PCC) is a rare but life-threatening complication of COVID-19 infection. PCC typically presents when patients recovering from the contagion and manifests as cholestasis in patients with no history of pre-existing liver disease. The pathogenesis of PCC is little understood. Hepatic injury in PCC could be mediated by the predilection of severe acute respiratory syndrome coronavirus 2 for cholangiocytes. Though PCC shows some resemblance to secondary sclerosing cholangitis in critically ill patients, it is considered as a separate and unique entity in the literature. Various treatment options like ursodeoxycholic acid, steroids, plasmapheresis, and endoscopic retrograde cholangiopancreatography guided interventions have been tried but with limited success. We have noticed significant improvement in liver function with antiplatelet therapy in a couple of patients. PCC can progress to end-stage liver disease necessitating liver transplantation. In this article, we discuss the current knowledge of PCC focusing on its pathophysiology, clinical manifestations, and management strategies.
Keywords: COVID-19, Liver, Post-COVID-19 syndrome, Long haulers, Cholangiopathy, Cholestasis
Core Tip: Post-coronavirus disease 2019 (COVID-19) cholangiopathy (PCC) is a rare complication of COVID-19 infection with gruesome prognosis. There is no proven treatment for this entity and patients often end up in liver transplantation. This review focusses on pathophysiology, clinical manifestations and management strategies of PCC along with our experience with antiplatelets in managing patients with PCC.
INTRODUCTION
World health organization declared coronavirus disease 2019 (COVID-19) as a global pandemic in March 2020[1]. Though severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) mainly affects the respiratory system, studies have demonstrated that organotropism of the virus can cause multisystem inflammation[2,3]. Hypercoagulability associated fatal cardiovascular, cerebrovascular, and gastrointestinal complications have been reported. Derangements in liver function tests (LFT) are the most frequent hepatic manifestation of COVID-19, and its incidence among hospitalized COVID-19 patients varies between 14% to 83%[4-10]. However, the spectrum of liver injury due to COVID-19 extends beyond just abnormalities in LFT. Other attributed hepatic effects of this contagion include vascular thromboses, cholangiopathy, and COVID-19 vaccine-related auto-immune hepatitis[11-13]. Remarkably, ‘Long haul COVID’ or ‘post-COVID syndrome’ (a collective term used to denote the persistence of symptoms or development of delayed complications beyond four weeks after the initial presentation of COVID-19) has also been known to adversely affect the liver[14,15].
Although COVID-19 results most commonly in a hepatocellular pattern of liver injury, severe cholestasis has also been occasionally noted[16-20]. Roth et al[13] labeled this unique entity of severe cholestasis as post-COVID-19 cholangiopathy (PCC)[21]. PCC typically presents when patients recover from COVID-19 and manifests as cholestasis in patients with no history of pre-existing liver disease. PCC is diagnosed in less than 1% of patients hospitalized for COVID-19[22]. Literature regarding this newly described entity is sparse, and the natural course of the disease remains unknown. We searched PubMed, Reference Citation Analysis (RCA), and Web of Science using Mesh words such as “post-Covid-19 cholangiopathy”, “COVID-19 sclerosing cholangiopathy”, “Covid-19 and liver”, and “COVID-19 and liver transplantation”. The data on pathogenesis, histology, imaging findings, clinical features, management, and outcomes were collected. This review summarizes the current knowledge of PCC, focusing on its pathophysiology, clinical manifestations, and management strategies.
PATHOGENESIS
SARS-CoV-2 utilizes angiotensin converting enzyme 2 (ACE2) receptor to enter the host cell and the internalisation process is aided by the host cell transmembrane serine protease 2. ACE2 receptors are expressed in various human organs including lung, liver, intestine, kidney, and heart[23,24]. The binding of SARS-CoV-2 to ACE2 receptor impairs ACE2 activity leading to the enhanced effect of angiotensin-2 resulting in an inflammatory and hypercoagulable state. In the liver, ACE2 receptors are more intensely expressed on cholangiocytes (59.7%) than on hepatocytes[24]. Cholangiocytes modify hepatocyte-derived bile acids, and the tight junction between these cells is essential for bile acid accumulation and excretion. Experimental studies using liver ductal organoid culture showed that SARS-CoV-2 can cause dysregulation of genes engaged in tight junction formation and bile acid transportation, thereby resulting in an impaired barrier and defective bile acid transportation. This mechanism of injury has been purported as the cause for direct cholangiocytic injury and consequent bile acid accumulation, resulting in severe and prolonged hepatic damage caused by COVID-19[25].
Ischemia injury, especially to the cholangiocytes has also been implicated in the causality of PCC. ACE2 receptors are expressed on vascular endothelial cells and SARS-CoV-2 can lead to uncontrolled inflammation through the interleukin (IL)-6 signaling pathway. Endothelitis results in hypercoagulability and thrombosis of the peribiliary vascular plexus aggravating biliary ischemia. Shreds of evidence in favor of this hypothesis include endothelial swelling with luminal narrowing of the hepatic arterioles and portal venous endophlebitis reported on histological examination of PCC specimens. Furthermore, improvement in liver function on treatment with antiplatelets in two of our patients (detailed below) also implicates the role of microvascular events in the pathogenesis of PCC[26]. On the contrary, a few studies noted no significant microvascular thrombi in their patients’ livers with PCC[22,27,28].
Due to their similar clinicopathological features, many researchers believe that PCC is a variant of secondary sclerosing cholangitis in critically ill patients (SSC-CIP). SSC-CIP is a rare form of secondary sclerosing cholangitis which occurs in patients with no history of hepatobiliary disease after a long intensive care unit stay for various conditions requiring prolonged mechanical ventilation and high-dose vasopressors. SSC-CIP was first described by Scheppach et al[29]. The pathogenesis of SSC-CIP is not fully elucidated, but the main mechanism appears to be bile duct ischemia. Other proposed causes include changes in the composition of the bile and biliary infection[30]. Ischemia leads to necrosis and sloughing of biliary epithelium resulting in biliary cast formation. Ischemia can also damage hepatobiliary transporters involved in the protective barrier mechanism of cholangiocytes from toxic bile salts. Progression to cirrhosis may occur over several months[31,32]. SSC-CIP has a mortality of over 50% in severe cases and up to a fifth of patients require a liver transplant (LT)[33,34]. Damages to extra and intrahepatic biliary ducts, cholangiocyte necrosis and biliary epithelial destruction, ductular reaction, and progressive fibrosis portal tracts are features common to PCC and SSC-CIP. Though PCC is typically described in patients with severe COVID-19 who required prolonged mechanical ventilation, few authors have reported cases of severe cholestasis in patients with mild to moderate COVID-19[17,18]. In contrast to acquired immunodeficiency syndrome cholangiopathy, the opportunistic infection has not been implicated as an etiological factor of PCC.
Some authors attributed PCC to ketamine related hepatobiliary damage, a condition called Ketamine induced cholangiopathy. Ketamine is metabolised in the liver and is used for sedation of patients with respiratory distress. Two recent articles reported patients with severe COVID-19 developed cholestatic liver disease with features of sclerosing cholangitis after exposure to ketamine. Nonetheless, a majority of other reports of PCC do not mention the use of ketamine. Antiviral drugs, particularly remdesivir and immunomodulatory agents like tocilizumab (IL-6 receptor antagonist) used in the management of COVID-19 are known to cause hepatic injury. The use of these agents has not been uniformly reported in any of the published cases of PCC. Moreover, there is insufficient evidence to prove that these medications may cause cholangiopathy.
CLINICAL, BIOCHEMICAL & IMAGING FEATURES
Patients with PCC are predominantly males (80%) and their median age at presentation is over 50 years[13,23,35-37] (Table 1). Patients typically present with jaundice with or without pruritus several weeks or months after the initial admission in intensive care units for severe COVID-19[13,37]. Significantly, these patients have no prior history of liver disease. Diabetes mellitus is the most common comorbid condition reported[37]. LFTs at the time of admission following COVID-19 diagnosis are almost always near-normal. In a cohort of 24 patients with PCC from various German centers, the median serum total bilirubin level at admission was 0.6 mg/dL (N: 0.6-1.2 mg/dL), while at the time of diagnosis of PCC it was 11.9 mg/dL[37]. The highest serum total bilirubin level reported in a patient with PCC was 42.4 mg/dL[16]. Gross elevation of serum alkaline phosphatase (ALP) levels, with peak levels above 1000 U/L (N: 20-140 U/L) have been commonly reported[13,22,27]. Remarkably, these biochemical changes in PCC are similar to that observed in patients with SSC-CIP[37].
Table 1.
A summary of reported cases of post-coronavirus disease 2019 cholangiopathy in the literature
|
Ref.
|
Number of patients
|
Age, median (range)
|
Sex
|
Time interval between COVID-19 diagnosis and presentation with features of PCC
|
Peak bilirubin (mg/dL)
|
Peak ALP (U/L)
|
MRCP
|
Biopsy
|
Treatment
|
Outcome reported
|
| Edwards et al[21], 2020 | 1 | 59 | Male | Time to elevation of bilirubin: 15 d. Peak at 79 d | 14.6 | 4000 | Beading of intrahepatic ducts | Not reported | ERCP for sludge clearance | Alive (planning biopsy to decide on liver transplantation) |
| Roth et al[13], 2021 | 3 | 34 (25-40) | 2 males, 1 female | Around 6 mo | Patient 1-7 gm/dL, patient 2-24 and patient 3-15 | 16 × ULN | Two of three: Hepatomegaly. One of three: Extrahepatic bile ducts dilatation and one of three: Intrahepatic bile ducts strictures and dilatations with beaded aspect or solely dilatation | Two of three: Mild and moderate bile ducts paucity. Three of three moderate ductular reaction, cholangiocytes swelling and regenerative changes with portal tract inflammation, hepatic artery endothelial swelling, hepatic veins endophlebitis and periportal fibrosis | Conservative | Two patients discharged home, one still hospitalized |
| Faruqui et al[22], 2021 | 12 | 58 (38-73) | Male: 92%; female: 8% | Mean interval-118 d | Range: 2-35 | Range: 965-2544 | Eleven of twelve patents showed beaded images of intrahepatic bile ducts, seven of twelve patients showed bile ducts thickening and hyperenhancement, ten of twelve patients showed peribiliary diffusion high signal | Performed in four of twelve pts. Acute or chronic large bile ducts obstruction, mild fibrosis of some portal tracts, Keratin 7 immunostain positivity | UDCA. UDCA slightly improved some lab tests (AST and ALT) but GGT and ALP remained elevated | Four of twelve died for complications consequent to sclerosing cholangiopathy, 2/12 listed for transplantation, 5/12 continuing conservative management |
| Durazo et al[27], 2021 | 1 | 47 | Male | Around 2 mo | 19 | 1644 | Mild intrahepatic bile ducts dilatation with focal strictures and beaded aspect, no dilatation of CBD | Inflammatory mononuclear infiltrates of bile ducts walls with increased collagen deposition, liver abscesses and bile lakes associated with bile duct injury with vacuolization and neutrophilia. Endothelial cell swelling, lumen obliteration of arterial vessels and obliterative portal venopathy | Liver transplantation | Alive with normal LFT at 7 mo after liver transplantation |
| Tafreshi et al[42], 2021 | 1 | 38 | Male | Cholestasis at a few months after initial hospital admission | 9.8 | 3665 | Mild dilatation of intrahepatic bile ducts with beaded aspect, dilatation of CBD and periportal oedema | Cholangiocytes injury, ductular proliferation, canalicular cholestasis, a bile lake and focal bridging fibrosis | Under evaluation for liver transplantation | Under evaluation for liver transplantation |
| Lee et al[38], 2021 | 1 | 64 | Male | 51 d | 7.8 | 1600 | Mild intrahepatic biliary ductal dilatation and mild patchy T2 hyperintensity within the right hemiliver, concerning for cholangitis | Explant pathology: Bridging fibrosis, severe bile duct injury, ductular reaction and leucocytes and plasma cells infiltrate | Liver transplantation | Alive at 8 mo and returned to work |
| Klindt et al[43], 2021 | 1 | 47 | Male | Around 50 d | 18 | 1700 | Alterations of medium and small intrahepatic bile ducts | Enlarged portal tracts with phlogistic infiltrate, ductular reaction with degenerative alterations of bile duct epithelium; focal biliary metaplasia of periportal hepatocytes. A few bile infarcts and perivenular canalicular cholestasis | Liver transplantation | Alive |
| Rojas et al[44], 2021 | 1 | 29 | Female | Around 2 mo | 19 | 6000 | Only a cystic lesion in liver segment V | Low periportal phlogistic infiltrate without necrosis but with a severe obstructive cholestatic pattern | UDCA and cholestyramine | Slight improvement at the time of reporting |
| Bütikofer et al[28], 2021 | 4 | 59 (54-67) | Male: 3, female: 1 | 70-153 d | 3.81-26.05 | (12.85-21.26) × ULN | Diffuse irregularities of the bile ducts with dilatations and strictures | Portal inflammation with pericellular fibrosis | UDCA | 2 patients: Deceased. 1 patient: Listed for liver transplantation (MELD-17). 1 patient: Persistently marked increased ALP and GGT at 9 mo of follow up |
| Rela et al[16], 2022 | 1 | 50 | Male | 4 wk | 42.4 | 248 | Mild prominence of central intrahepatic, common hepatic, and common bile ducts with minimal beading of the right posterior sectoral and segment 2 ducts | Mild portal tract inflammation with lymphocytes, histiocytes and few eosinophils, with loss of interlobular bile ducts | Auxiliary partial orthotopic liver transplantation | Asymptomatic at 6 mo follow-up with good graft function and recovering function in native liver remnant |
| Kulkarni et al[17], 2022 | 15 | Unvaccinated: 59 (24-67). Vaccinated: 52 (29-67) | Unvaccinated: Male (8/8, 100%). Vaccinated: Male (5/7, 71.4%) | The median time to the development of cholestasis was 35 (19-44) d and in vaccinated group and 39.5 (27-57) in the unvaccinated group | Unvaccinated group: 22.95 (4.2-48.5), vaccinated group 17 (8.3-32.4) | 312 (239-517) U/L in the vaccinated group and 571.5 (368-1058) U/L in the unvaccinated group | Normal in all patients | Architectural distortion, fibrosis, cholestasis, and ductular reaction with duct openia in unvaccinated group. Cholestasis and inflammation and no fibrosis in vaccinated group | UDCA. Plasma exchange: 5. Oral steroids: 4 | 2-died. 2-liver transplantation. 2-listed for liver transplantation. 1-declined liver transplantation. 2-recovered. All 7 in vaccinated group recovered |
| Mayorquín-Aguilar et al[45], 2022 | 3 | 46 (45-52) | Male | 12-14 wk in two patients and 20 d in another patient | 17.32 (5.8-22.7) | 1328 (705-1695) | Irregular morphology of intrahepatic and extrahepatic bile ducts. Multiple areas of stenosis in the distal intrahepatic bile ducts | Intracanalicular cholestasis, portal inflammation, ductular reaction, and moderate portal fibrosis | UDCA, cholestyramine, and sertraline | Persisitent cholestasis in one patient and disease progressed to cirrhosis in another patient. Third patient expired due to unrelated cause |
| Hunyady et al[37], 2023 | 24 | 57 (19-73) | Out of 24 patients, 20 were male, 4 females | 91 d (IQR: 64-154 d) | Peak bilirubin 24.3 mg/dL. Median bilirubin 11.9 mg/dL (6.0-24.3) | Peak ALP 1100 U/L. Median ALP 925 U/L. (555-1100) | Strictures or dilatation of biliary system, rarefication of biliary tree including contrast filling defects or detection of biliary casts | - | UDCA in 16 (66.7%) patients, ERCP with sphincterotomy done in 20 (83.3%) patients. Cast extraction done in 11 (45.8% patients). 3 patients underwent liver transplantation | 3 patients underwent liver transplantation. 2 patients had transplant free survival |
ULN: Upper limit of normal; UDCA: Ursodeoxycholic acid; COVID-19: Coronavirus disease 2019; ALP: Alkaline phosphatase; PCC: Post-coronavirus disease 2019 cholangiopathy; IQR: Interquartile range; ERCP: Endoscopic retrograde cholangiopancreatography; GGT: Gamma glutamyl transpeptidase; MELD: Model for end-stage liver disease; CBD: Cannabidiol; LFT: Liver function test; MRCP: Magnetic resonance cholangiopancreatography; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
In a series of 12 cases reported by Faruqui et al[22] mean interval between the initial diagnosis of COVID-19 and the diagnosis of PCC by magnetic resonance cholangiopancreatography (MRCP) was 118 d. All 12 patients in their series, showed some structural changes in the biliary system on MRCP[35]. Intrahepatic bile duct strictures and the beaded appearance of intrahepatic bile ducts were the most commonly noted findings[13,22]. Ghafoor et al[35] studied magnetic resonance imaging/MRCP of 17 patients with PCC, and noted that none of the patients had cirrhosis or vascular thrombosis. Strictures in the form of beading of intrahepatic bile ducts were seen in 14 (82.3%) patients and biliary casts were seen in 2 (11.8%) patients. In addition to biliary abnormalities, liver contour irregularities and signal intensity changes in PCC livers can also be ascertained on MRCP.
HISTOPATHOLOGY
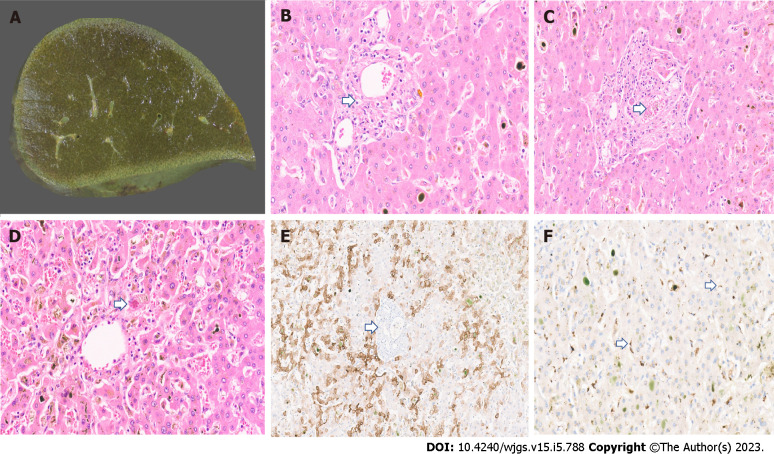
On macroscopic examination, the PCC livers are described to have a greenish discoloration[13,38,39] (Figure 1A). Brightfield microscopy may show portal/periportal fibrous expansion with bile ductular proliferation and degenerative cholangiocyte injury accompanied by leucocytes[13,39]. Loss of interlobular bile ducts has been described in PCC (Figure 1B). Ductular bile plugs and bile lakes are reported. Lobular disarray, hepatocanalicular bilirubinostasis with rosetting, and patchy lobular inflammation may be seen. Hilar bile ducts with inflammation and fibrosis have been described. Portal veins may show fibrin thrombi (Figure 1C). A case from the authors’ series also showed Mallory Denk bodies along with patchy sinusoidal dilatation and congestion with hepatocyte atrophy (Figure 1D). In late cases, bridging fibrosis and cirrhosis have been reported[13,38]. Immunostaining with CK7 may show biliary metaplasia of hepatocytes (Figure 1E). Pathognomonic findings of PCC on immunohistochemistry include a granular cytoplasmic positivity for SARS-CoV-2 within hepatocytes and sinusoidal macrophages (Figure 1F).
Figure 1.
Histopathology imaging. A: Explant liver cut surface with greenish discolouration; B: Explant liver displaying duct loss (arrow) and bilirubinostasis [× 20, hematoxylin and eosin (H&E)]; C: Fibrin thrombi in portal vein (arrow, × 18, H&E); D: Mallory Denk body in a hepatocyte (arrow) and mild lobular inflammation (× 18, H&E); E: CK7 immunostaining with duct loss (arrow) and biliary metaplasia of hepatocytes (× 15); F: Severe acute respiratory syndrome coronavirus 2 immunostaining with granular brown positivity in hepatocytes and macrophages (arrow, × 15).
TREATMENT
Given that PCC is a recently described disease arising out of the COVID-19 pandemic, little is known about its natural history. Anecdotal evidence of various treatment modalities is present in the literature, and currently, there is no well-defined treatment algorithm. Though universally used for PCC, medical treatment with ursodeoxycholic acid and cholestyramine does not seem to offer much clinical or biochemical improvement. Antiplatelet medications have serendipitously shown benefits (two patients from the authors’ series). However, the exact indication, timing, dose, and duration of this regimen remain unknown. Franzini et al[40] recently published a video demonstration of their experience with cholangioscopy (SpyGlass@) to assess bile duct changes and removal of biliary casts. In patients with biliary casts or cholangitis, interventions using endoscopic retrograde cholangiopancreatography may offer transient improvement of the clinical condition, but abnormal liver functions are likely to persist even after an anatomical clearance of the extrahepatic ducts[13,36].
LIVER TRANSPLANTATION FOR PCC
In most patients, the disease causes progressive biliary injury with worsening cholestasis and recurrent infections[16,22]. The first successful LT for PCC was reported by Durazo et al[27]. A 47-year-old man with PCC and worsening liver and renal function underwent deceased donor liver transplantation on day 108 from the initial presentation with COVID-19. Histopathology of the explanted liver showed features of severe sclerosing cholangitis with hepatic abscesses. The patient improved well and graft function was normal at 7 mo after LT. Subsequently, various authors reported their experience with LT for patients with PCC[16,22,38]. Our team reported a living donor auxiliary right lobe LT (APOLT) in a 50-year-old patient at 12 wk after the initial diagnosis of COVID-19. The patient underwent a right trisectionectomy with caudate lobectomy. The right lobe was retrieved robotically from a related donor and implanted orthotopically. Interestingly, hepatobiliary scintigraphy at six months follow-up showed 90% and 10% in the graft and native livers respectively, reflecting some native liver recovery[16]. The authors’ premise was that since the natural course of PCC is unknown, there remains the possibility of spontaneous liver recovery. Thus, the allograft in APOLT potentially acts as a bridge till native regeneration occurs, providing the patient with a realistic possibility of becoming immunosuppression-free. While LT is an effective curative option for patients with PCC, it is naïve to offer it to every patient with PCC. It is also sobering to realise that several variables of this management continue to be undefined. These include vital data to define which cohort of patients are likely to recover without an LT. It is likely that these questions will have answers as experience grows with this disease entity.
COVID-19 VACCINATION AND PCC
In the current era of near-universal COVID-19 vaccination, it is important to re-evaluate the natural course of PCC. Vaccination has been shown to reduce the severity of COVID-19 and improve outcomes[41]. Kulkarni et al[17] compared 8 unvaccinated patients with 7 vaccinated patients with post-COVID-19 cholestasis and showed that serum ALP and gamma glutamyl transpeptidase (GGT) were significantly lower in the vaccinated group. Furthermore, all patients in the vaccinated group improved with conservative management while a majority in the unvaccinated group required LT. Again, literature in this regard is scarce, but intuitively, COVID-19 vaccination is likely to play a positive role in preventing/attenuating the course of PCC.
OUR EXPERIENCE
Our experience with PCC is limited to four patients. All of them presented with severe cholestatic jaundice following initial recovery from COVID-19 illness[26] (Table 2). All were men in their 5th or 6th decade of life. Unlike other reported series, only half of our patients had a history of mechanical ventilation for their COVID-19 related respiratory illness. Clinical recovery from COVID-19 was complete and all of them had been discharged between 7 and 21 d. None of them had a history of any underlying liver disease. LFTs were uniformly unremarkable at the time of COVID-19. All these patients were readmitted with fatigue and jaundice four to six weeks following their COVID-19 and a couple of them developed pruritus. Peak enzymes were aspartate aminotransferase 4-8 times upper limit normal (ULN), alanine aminotransferase 3-10 ULN, ALP 4-6 ULN, and GGT 5-15 ULN. Peak bilirubin varied between 15 to 42 mg/dL (N: 0.6-1.2 mg/dL). Interestingly, none of them developed coagulopathy, ascites, or hepatic encephalopathy. Abdominal imaging in 3 patients was unremarkable. MRCP of one patient demonstrated mild prominence of central intrahepatic, common hepatic, and common bile ducts with minimal beading of the right posterior sectoral and segment 2 ducts. Liver biopsies showed loss of interlobular bile ducts, degenerative features in residual ducts, hepatocanalicular bilirubinostasis, and fibrin thrombi in some vessels.
Table 2.
A summary of authors’ experience with post coronavirus disease 2019 cholangiopathy
|
Case
|
Age/sex
|
ICU admission for COVID
|
Mechanical ventilation for COVID
|
Medications received for COVID
|
LFT at initial admission for COVID
|
LFT (peak values)
|
Time interval1
|
MRCP
|
Biopsy
|
Treatment
|
Outcome
|
| 1 | 67/male | Yes (hypoxia-high flow oxygen) | No | Remdesivir, methylprednisolone | Bilirubin 0.84 mg/dL, AST 54 U/L, ALT 32 U/L, ALP 127 U/L, GGT 78 U/L and albumin 4.1 mg/dL | Bilirubin 12.7 mg/dL, AST 322 U/L, ALT 527 U/L, ALP 474 U/L and GGT 1318 U/L, albumin 3.2 g/dL, INR 0.98 | 4 wk | Normal except for cholelithiasis | Mild portal fibrous expansion with diffuse loss of interlobular bile ducts | Prednisolone, ursodeoxycholic acid | Hyperbilirubinemia continued to worsen. Expired of pneumonia |
| 2 | 50/male | Yes | Yes | Methylprednisolone, Remdesivir, antibiotics and thromboprophylaxis | Bilirubin 0.5 mg/dL, AST 43 U/L, ALT 57 U/L, ALP 60 U/L, GGT 64 U/L, albumin 2.8 mg/dL | Bilirubin 31.3 mg/dL, ALP 248 U/L, GGT 355 U/L, AST 176 U/L and ALT 200 U/L | 6 wk | Mild prominence of central intrahepatic, common hepatic, and common bile ducts with minimal beading of the right posterior sectoral and segment 2 ducts | Mild portal tract inflammation with lymphocytes, histiocytes and few eosinophils, with loss of interlobular bile ducts | Auxiliary partial orthotopic liver transplantation | Asymptomatic at 6 mo follow-up with good graft function and recovering function in native liver remnant |
| 3 | 58/male | No | No | Doxycycline, ivermectin, methylprednisolone. Remdesivir, paracetamol along with zinc and other vitamin supplements | Bilirubin 1.99 mg/dL, AST 145 U/L, ALT 140 U/L, ALP 70 U/L, GGT 65 U/L and albumin 4.1 g/dL | Bilirubin 10 mg/dL, AST 167 U/L, ALT 181 U/L, ALP 532 U/L and GGT 728 U/L | 6 wk | Normal | Bile duct degenerative changes in majority of portal tracts along with prominent centrilobular hepatocanalicular bilirubinostasis | Aspirin, clopidogrel, prednisolone, ursodeoxycholic acid | Improved, LFT at 18 mo of follow up. Bilirubin 1.3 mg/dL, AST 101 U/L, ALT 101 U/L, ALP 309 U/L |
| 4 | 52/male | Yes | Yes | Amoxycillin, remedesivir, IV methyl prednisolone | Bilirubin 1 mg/dL, albumin 4.2 g/dL, ALT 25 U/L, AST 49 U/L, ALP 110 U/L, GGT 62 U/L | Bilirubin 33.9 mg/dL ALP 390 U/L, ALT 41 U/L, GGT 94 U/L, AST 58 U/L | 6 wk | Normal | Bile ducts showed mild injury, lobular bilrubinostasis | Therapeutic plasma exchange, aspirin, clopidogrel, prednisolone, ursodeoxycholic acid | Improved, LFT at 6 mo of follow up. Bilirubin 1.1 mg/dL, ALP 101 U/L, AST 23 U/L, ALT 32 U/L |
Time interval between coronavirus disease 2019 (COVID-19) diagnosis and presentation with features of post-COVID-19 cholangiopathy.
COVID: Coronavirus disease; ALP: Alkaline phosphatase; GGT: Gamma glutamyl transpeptidase; MELD: Model for end-stage liver disease; LFT: Liver function test; MRCP: Magnetic resonance cholangiopancreatography; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ICU: Intensive care unit; INR: International normalized ratio.
Of the 4 patients, one died of worsening symptoms and sepsis. The second patient developed progressive jaundice and underwent APOLT (described above)[16]. He remains well on 9 mo follow-up and is due to have a hepatobiliary scintigraphy at 12 mo to reassess native liver regeneration. The third patient remained symptomatic with worsening hyperbilirubinemia and was listed for LT. On evaluation, he was noted to have double-vessel coronary artery disease which required stenting. Following stent placement, he was commenced on aspirin and clopidogrel. Interestingly, there was a significant improvement in LFT within six weeks of initiating antiplatelet therapy. He was discharged home without the need for a LT and his clinical improvement was attributed to anti-platelet drugs. With this experience, the fourth patient was commenced early on antiplatelet therapy and he too had a remarkable improvement in his clinical and biochemical status. Both these patients remain well on 6- and 7-mo’ follow-up respectively. This finding underpins the theory of microvascular events in the pathogenesis of PCC.
CONCLUSION
PCC is a recently described entity of severe cholestasis that has been recognized in patients recovering from severe COVID-19 infection. While the exact etiopathogenesis remains unknown, purported theories include SSC-CIP, microthromboses, direct liver injury, and autoimmune etiology among others. Although relatively uncommon at present, it is likely that this disease will be more commonly encountered in the near future. Its natural course remains undefined, but with accumulating evidence several successful management strategies have been proposed. There remains a need for concentrated, multicenter studies to further elucidate this disease which can potentially have high morbidity if not identified and managed appropriately.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 23, 2022
First decision: January 2, 2023
Article in press: April 7, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fouad MM, Egypt; Freund O, Israel S-Editor: Wang JJ L-Editor: A P-Editor:Wu RR
Contributor Information
Fadl H Veerankutty, Institute of Liver Disease and Transplantation, Dr. Rela Institute and Research Centre, Chennai 600044, India. fadl_05@yahoo.com.
Kushan Sengupta, Institute of Liver Disease and Transplantation, Dr. Rela Institute and Medical Centre, Chennai 600044, India.
Mukul Vij, Department of Pathology, Institute of Liver Disease and Transplantation, Chennai 600044, India.
Ashwin Rammohan, Institute of Liver Disease and Transplantation, Dr. Rela Institute and Medical Centre, Chennai 600044, India.
Dinesh Jothimani, Institute of Liver Disease and Transplantation, Dr. Rela Institute and Medical Centre, Chennai 600044, India.
Ananthavadivelu Murali, Gastroenterology and Hepatology, Clinician’s Point, Chennai 600014, Tamil Nadu, India.
Mohamed Rela, Institute of Liver Disease and Transplantation, Dr. Rela Institute and Medical Centre, Chennai 600044, India.
References
- 1.Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang PP, Cai J, Zhou XD, Jiang DP, Fei XC, Huang XQ, Zhao L, Zhang H, Wu HB, Ren Y, Liu ZH, Zhang HR, Chen C, Fu WJ, Li H, Xia XY, Chen R, Wang Y, Liu XD, Yin CL, Yan ZX, Wang J, Jing R, Li TS, Li WQ, Wang CF, Ding YQ, Mao Q, Zhang DY, Zhang SY, Ping YF, Bian XW. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31:836–846. doi: 10.1038/s41422-021-00523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freund O, Eviatar T, Bornstein G. Concurrent myopathy and inflammatory cardiac disease in COVID-19 patients: a case series and literature review. Rheumatol Int. 2022;42:905–912. doi: 10.1007/s00296-022-05106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807–817. doi: 10.1002/hep.31404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. doi: 10.1016/j.aohep.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123:102688. doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 12.McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol Commun. 2022;6:255–269. doi: 10.1002/hep4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt C. COVID-19 long haulers. Nat Biotechnol. 2021;39:908–913. doi: 10.1038/s41587-021-00984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23:194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rela M, Rajakannu M, Veerankutty FH, Vij M, Rammohan A. First report of auxiliary liver transplantation for severe cholangiopathy after SARS-CoV-2 respiratory infection. Am J Transplant. 2022;22:3143–3145. doi: 10.1111/ajt.17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni AV, Khelgi A, Sekaran A, Reddy R, Sharma M, Tirumalle S, Gora BA, Somireddy A, Reddy J, Menon B, Reddy DN, Rao NP. Post-COVID-19 Cholestasis: A Case Series and Review of Literature. J Clin Exp Hepatol. 2022;12:1580–1590. doi: 10.1016/j.jceh.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jothimani D, Vij M, Sanglodkar U, Patil V, Sachan D, Narasimhan G, Kaliamoorthy I, Rela M. Severe Jaundice in a COVID-19 Patient-Virus or Drug? J Clin Exp Hepatol. 2021;11:407–408. doi: 10.1016/j.jceh.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindholm CR, Zhang X, Spengler EK, Daniel KE. Severe Cholestatic Hepatitis Secondary to SARS-CoV-2. ACG Case Rep J. 2022;9:e00753. doi: 10.14309/crj.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faruqui S, Shanbhogue K, Jacobson IM. Biliary Tract Injury in Patients With COVID-19: A Review of the Current Literature. Gastroenterol Hepatol (N Y) 2022;18:380–387. [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 23.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ILTS 2022 Joint International Congress of ILTS, ELITA & LICAGE, May 4-7, 2022. Transplantation. 2022;106:1–214. [Google Scholar]
- 27.Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, Loy V, Kim J, Zimmerman MA, Hong JC. Post-Covid-19 Cholangiopathy-A New Indication for Liver Transplantation: A Case Report. Transplant Proc. 2021;53:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bütikofer S, Lenggenhager D, Wendel Garcia PD, Maggio EM, Haberecker M, Reiner CS, Brüllmann G, Buehler PK, Gubler C, Müllhaupt B, Jüngst C, Morell B. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41:2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheppach W, Druge G, Wittenberg G, Mueller JG, Gassel AM, Gassel HJ, Richter F. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001;29:438–441. doi: 10.1097/00003246-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 30.Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Hetzer R, Schaffartzik W, Tryba M, Neuhaus P, Seehofer D. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015;19:131. doi: 10.1186/s13054-015-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirchner GI, Scherer MN, Obed A, Ruemmele P, Wiest R, Froh M, Loss M, Schlitt HJ, Schölmerich J, Gelbmann CM. Outcome of patients with ischemic-like cholangiopathy with secondary sclerosing cholangitis after liver transplantation. Scand J Gastroenterol. 2011;46:471–478. doi: 10.3109/00365521.2010.537683. [DOI] [PubMed] [Google Scholar]
- 32.Leonhardt S, Veltzke-Schlieker W, Adler A, Schott E, Eurich D, Faber W, Neuhaus P, Seehofer D. Secondary Sclerosing Cholangitis in Critically Ill Patients: Clinical Presentation, Cholangiographic Features, Natural History, and Outcome: A Series of 16 Cases. Medicine (Baltimore) 2015;94:e2188. doi: 10.1097/MD.0000000000002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voigtländer T, Negm AA, Schneider AS, Strassburg CP, Manns MP, Wedemeyer J, Lankisch TO. Secondary sclerosing cholangitis in critically ill patients: model of end-stage liver disease score and renal function predict outcome. Endoscopy. 2012;44:1055–1058. doi: 10.1055/s-0032-1325733. [DOI] [PubMed] [Google Scholar]
- 34.Lin T, Qu K, Xu X, Tian M, Gao J, Zhang C, Di Y, Zhang Y, Liu C. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014;8:118–126. doi: 10.1007/s11684-014-0306-6. [DOI] [PubMed] [Google Scholar]
- 35.Ghafoor S, Germann M, Jüngst C, Müllhaupt B, Reiner CS, Stocker D. Imaging features of COVID-19-associated secondary sclerosing cholangitis on magnetic resonance cholangiopancreatography: a retrospective analysis. Insights Imaging. 2022;13:128. doi: 10.1186/s13244-022-01266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleem N, Li BH, Vuppalanchi R, Gawrieh S, Gromski MA. Critical Illness Cholangiopathy in COVID-19 Long-haulers. Tech Innov Gastrointest Endosc. 2022;24:351–353. doi: 10.1016/j.tige.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunyady P, Streller L, Rüther DF, Groba SR, Bettinger D, Fitting D, Hamesch K, Marquardt JU, Mücke VT, Finkelmeier F, Sekandarzad A, Wengenmayer T, Bounidane A, Weiss F, Peiffer KH, Schlevogt B, Zeuzem S, Waidmann O, Hollenbach M, Kirstein MM, Kluwe J, Kütting F, Mücke MM. Secondary Sclerosing Cholangitis Following Coronavirus Disease 2019 (COVID-19): A Multicenter Retrospective Study. Clin Infect Dis. 2023;76:e179–e187. doi: 10.1093/cid/ciac565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee A, Wein AN, Doyle MBM, Chapman WC. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-244168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cesar Machado MC, Filho RK, El Bacha IAH, de Oliveira IS, Ribeiro CMF, de Souza HP, Parise ER. Post-COVID-19 Secondary Sclerosing Cholangitis: A Rare but Severe Condition with no Treatment Besides Liver Transplantation. Am J Case Rep. 2022;23:e936250. doi: 10.12659/AJCR.936250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franzini TAP, Guedes MMF, Rocha HLOG, Fleury CA, Bestetti AM, Moura EGH. CHOLANGIOSCOPY IN A POST-COVID-19 CHOLANGIOPATHY PATIENT. Arq Gastroenterol. 2022;59:321–323. doi: 10.1590/S0004-2803.202202000-58. [DOI] [PubMed] [Google Scholar]
- 41.Freund O, Tau L, Weiss TE, Zornitzki L, Frydman S, Jacob G, Bornstein G. Associations of vaccine status with characteristics and outcomes of hospitalized severe COVID-19 patients in the booster era. PLoS One. 2022;17:e0268050. doi: 10.1371/journal.pone.0268050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tafreshi S, Whiteside I, Levine I, D'Agostino C. A case of secondary sclerosing cholangitis due to COVID-19. Clin Imaging. 2021;80:239–242. doi: 10.1016/j.clinimag.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klindt C, Jensen BE, Brandenburger T, Feldt T, Killer A, Schimmöller L, Antoch G, Senff T, Hauka S, Timm J, Bahners BH, Seidl M, Esposito I, Luedde T, Bode JG, Keitel V. Secondary sclerosing cholangitis as a complication of severe COVID-19: A case report and review of the literature. Clin Case Rep. 2021;9:e04068. doi: 10.1002/ccr3.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rojas M, Rodríguez Y, Zapata E, Hernández JC, Anaya JM. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. doi: 10.1016/j.jtauto.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayorquín-Aguilar JM, Lara-Reyes A, Revuelta-Rodríguez LA, Flores-García NC, Ruiz-Margáin A, Jiménez-Ferreira MA, Macías-Rodríguez RU. Secondary sclerosing cholangitis after critical COVID-19: Three case reports. World J Hepatol. 2022;14:1678–1686. doi: 10.4254/wjh.v14.i8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]