Abstract
Mesenchymal stromal/stem cells (MSCs) have shown significant therapeutic potential, and have therefore been extensively investigated in preclinical studies of regenerative medicine. However, while MSCs have been shown to be safe as a cellular treatment, they have usually been therapeutically ineffective in human diseases. In fact, in many clinical trials it has been shown that MSCs have moderate or poor efficacy. This inefficacy appears to be ascribable primarily to the heterogeneity of MSCs. Recently, specific priming strategies have been used to improve the therapeutic properties of MSCs. In this review, we explore the literature on the principal priming approaches used to enhance the preclinical inefficacy of MSCs. We found that different priming strategies have been used to direct the therapeutic effects of MSCs toward specific pathological processes. Particularly, while hypoxic priming can be used primarily for the treatment of acute diseases, inflammatory cytokines can be used mainly to prime MSCs in order to treat chronic immune-related disorders. The shift in approach from regeneration to inflammation implies, in MSCs, a shift in the production of functional factors that stimulate regenerative or anti-inflammatory pathways. The opportunity to fine-tune the therapeutic properties of MSCs through different priming strategies could conceivably pave the way for optimizing their therapeutic potential.
Keywords: Mesenchymal stromal/stem cells; Mesenchymal stromal/stem cell therapeutic properties; Mesenchymal stromal/stem cell paracrine effects; Mesenchymal stromal/stem cell priming; Pro-inflammatory priming; Hypoxic priming, 3D culture priming
Core Tip: Mesenchymal stromal/stem cells (MSCs) have demonstrated promising therapeutic results in the field of regenerative medicine. However, due to their heterogeneity, the application of MSCs in clinical trials has shown moderate or poor efficacy. Here, we review data on the principal priming approaches for enhancing the therapeutic potential of MSCs. We found that different priming strategies can modify MSC properties and, in this case some therapeutic effects on different disease models can be obtained in relation to dose and/or combination of the priming factors used. The production of priming type-specific functional factors in MSCs could pave the way toward implementing new MSC-based therapies.
INTRODUCTION
Mesenchymal stromal/stem cells (MSCs) are multipotent adult stem cells involved in the homeostasis of tissue regeneration and, because of their therapeutic potential, have been extensively investigated in various clinical conditions[1-6]. Though MSC treatment was initially thought to promote tissue regeneration thanks to MSC multipotency of differentiation[7-9], recent evidence has revealed that the efficacy of MSC-based therapies is, at least in part, linked to the production of functional paracrine factors. These cells are able to secrete numerous products, e.g., growth factors, cytokines, chemokines, and extracellular vesicles (EVs), which can regulate many pathophysiological processes, such as fibrosis, immune dysregulation, angiogenesis, and stimulation of tissue resident stem cells, in order to coordinate both tissue regeneration and functional recovery[10-12]. In injured tissue, MSC engraftment is limited because they undergo cell death, and their beneficial effects are exerted through secretion of various functional factors that not only enhance the function of resident cells, but also attract immune and progenitor cells, contributing to the coordination of tissue repair[13,14]. Therefore, considering the importance of the paracrine component in mediating MSC functions, there is growing interest in the molecular basis of MSC secretion involved in the therapeutic function of these cells.
Over the years, a large number of tissues, including placenta, adipose, umbilical cord, dental pulp, bone marrow, synovial membrane, liver and others, have been used as a source of MSCs[15-20]. It is quite clear that MSCs derived from all these sources possess a wide variety of functional effects, which they apply physiologically to their own original tissue, regulating homeostasis and regeneration. Interestingly, these effects may be useful for therapeutic applications of MSCs[3,21]. Currently, there are 1487 clinical trials registered at clinicaltrials.gov aimed at studying MSC therapeutic efficacy in the treatment of several clinical disorders, including lung, liver, kidney, orthopedic, cardiovascular, neurodegenerative, and immune diseases. In different clinical settings, MSC-therapies have been tested, showing tolerable safety, and demonstrating therapeutic benefits, and this has led to regulatory approvals of some MSC-based therapeutic products in several countries. In 2012, Cartistem, a MSC product based on the use of umbilical cord-derived MSCs for the treatment of traumatic or degenerative osteoarthritis, was approved by Korea’s Ministry of Food and Drug Safety[22]. Moreover, Remestemcel-L, based on the use of bone marrow-derived MSCs (BM-MSCs), has been investigated in a phase 3 clinical trial in patients with steroid-refractory acute graft-versus-host disease (GVHD)[23]. Recently, due to the immunomodulatory properties of Remestemcel-L, which are able to work against cytokine storm linked to several inflammatory conditions, this therapy has also been tested for the treatment of coronavirus disease 2019-associated multisystem inflammatory syndrome[24]. The increasing interest in the clinical applications of MSCs as a cellular therapy has also been evidenced by the burgeoning of several companies that sell MSC therapies to United States clinics[25]. However, this has highlighted that in some cases the propensity for economic gain has outweighed the clinical advantages, despite the lack of solid scientific evidence that supports the broad use of MSCs in treating various human disorders. Indeed, in many clinical trials it has been shown that MSCs have moderate or poor efficacy, and the results from some studies are controversial[26-31]. In particular, due to both the inconsistent criteria used for the MSC identity across studies, and MSC heterogeneity, which depends on the different MSC origin[32] and the diverse harvesting and culture strategies[33], the clinical results obtained after MSC therapy are frequently variable. This makes it very difficult to obtain reliable conclusions regarding MSC therapeutic efficacy. Thus, while MSCs demonstrate a good margin of safety as cellular treatment, they have usually been therapeutically ineffective in humans[21].
These issues have underscored the urgent need to optimize the clinical use of MSCs or enhance MSC therapeutic effects. After determining the most appropriate cell source to use both in terms of invasiveness for cell isolation and cell yield, specific standardized production methods are needed to ensure MSC therapeutic abilities and, therefore, their clinical efficacy. MSCs can be considered a key regulatory component in the tissue stem cell niche and, starting with the physiological role that these cells play in regulating tissue regeneration following injury[3,4,6,34-39], specific priming strategies can be understood and adapted for MSC clinical application. In this regard, much attention has been paid to the opportunity of MSC pre-conditioning to prime the cells before their clinical use. In this case, the therapeutic properties of MSCs can be modulated by pre-treatment of cells with hypoxia, cytokines, as well as growing MSCs under three-dimensional (3D) culture. In those instances, in response to MSC priming, the phenotype of MSCs was switched toward an anti-inflammatory, pro-trophic and more regenerative potential, which results in an enhanced therapeutic function of the cells[3,40-45].
In this review, we summarize the principal priming methods aimed at improving MSC efficacy as a therapeutic product. We would also like to highlight the fact that specific priming strategies can be considered more suitable for some types of diseases, leading to new therapeutic approaches that could be used to develop more powerful and predictable MSC therapies.
THE SECRETION OF PARACRINE FACTORS MEDIATE THE THERAPEUTIC FUNCTION OF MSCs
The secretion of functional products is central to MSC-based therapy, as demonstrated in numerous studies. Indeed, individual components of MSC secretome, such as functional proteins and EVs, are involved in the regulation of various biological processes, including angiogenesis, immunoregulation, wound healing, and tissue repair/protection[14,46-49]. Among the MSC-derived functional products, exosomes (EXOs), belonging to EVs, are anuclear particles ranging from 50 to 200 nm in size that are constitutively released from the endosomal compartment of MSCs. They contain a plethora of functional protein and other molecules, including microRNAs (miRNAs), which mediate several MSC properties[15,50,51]. EXOs are key components of intercellular communication, because they are released into the intercellular space where they exert local paracrine or distal systemic effects[52]. In fact, EXOs are able to regulate numerous biological processes, including angiogenesis[53], cell proliferation[54], and the activation/inhibition of immune cells[55]. Interestingly, EXO content can be changed by various priming stimuli[3,40,55]. Recently, it has been revealed that EXO-derived miRNAs play a critical role in mediating EXO effects[56]. MiRNAs are 19-22-nucleotide-long non-coding RNAs that regulate mRNA translation, and are involved in many cellular processes[56,57]. Therefore, even if some therapeutic functions of the MSCs are mediated by cell-to-cell contact, the secretion of paracrine factors can be considered the main mechanism by which MSCs elicit functional responses in target cells[3,40,58,59]. In many in vitro and in vivo disease models, MSC-derived products have been identified as responsible for therapeutic effects[60-63]. For example, promising preclinical therapeutic effects have been obtained using MSC-derived EVs. In particular, regarding BM-MSC-derived EVs, Haga et al[64] found that these functional factors were able to reduce hepatic injury by modulating cytokine expression in a mouse model of fulminant hepatic failure. Reis et al[65] demonstrated that the administration of EXOs in a rat model of gentamycin-induced kidney injury, was able to improve the kidney injury score. Moreover, it has been shown that EXOs derived from umbilical cord-derived MSCs were able to accelerate wound healing in a rat skin burn model[66], and EXOs derived from BM-MSCs overexpressing hypoxia-inducible factor (HIF)-1α accelerated bone regeneration and angiogenesis in a rabbit model of steroid-induced avascular bone necrosis[67].
MSCs can also secrete a number of cytokines/chemokines that control both the innate and adaptive immune responses, resulting in immunoregulation and the induction of tolerance[68]. Indeed, it has been shown that MSCs can produce both anti- and pro-inflammatory factors which, depending on their ratio, regulate the pro- or anti-inflammatory activity of MSCs[69]. In this case, final immunoregulatory properties may be affected by cell culture conditions that can prime/enhance MSC properties[3,70,71]. MSCs also have the ability to roll and adhere to post-capillary venules, and migrate to injured tissues, contributing to tissue repair/regeneration[72]. In this case, once MSCs reach the site of the injury, these cells put in place an active regulation by producing paracrine factors that impact tissue survival/repair, and activate tissue resident stem cells[3,73,74]. The secretion of various soluble factors has also been found to be responsible for the pro-angiogenic and anti-apoptotic effects of MSCs[75]. Though not well understood, the beneficial effects of conditioned media (CM) derived from MSCs have been clearly demonstrated by various experimental findings, supporting the concept of paracrine effects[76]. Several preclinical studies have tested the efficacy of CM in different diseases models. MSC-derived CM has been shown capable of improving cell viability and reducing inflammation in both in vitro and in vivo models of lung ischemia/reperfusion injury (IRI)[59,77]. Moreover, it has been demonstrated that BM-MSC-derived CM was able to reduce lung inflammation and edema in a mouse model of lipopolysaccharide-induced lung injury[78], and to improve renal tissue pathology in a mouse model of cisplatin-induced kidney injury[79]. Youdim et al[80], in a rat model of fulminant hepatic failure, found that the CM derived from BM-MSCs reduced leukocytic infiltrates and hepatocellular death. The CM derived from the same cells, in a mouse model of antigen-induced arthritis, was also able to reduce joint swelling, cartilage loss, and tumor necrosis factor (TNF)-α secretion[81]. In a rat model of lung fibrosis and hypertension, using CM derived from adipose MSCs (AdMSCs), demonstrated the ability of secretome to reduce collagen deposition and improve lung blood flow[82]. In a rabbit model of surgical bone lesion, Linero and Chaparro[83] found that the CM produced from AdMSC cultures induced bone regeneration.
THE SECRETION OF MSC PARACRINE FACTORS CAN BE MODULATED BY VARIOUS PRIMING STRATEGIES
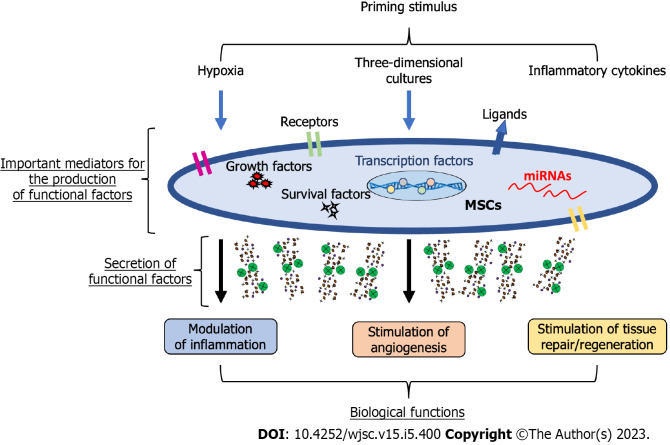
Given the heterogeneity of results supporting the efficacy of MSCs in the treatment of different human disorders, there is a need to improve the therapeutic properties of MSCs, and the best way might be that of preconditioning/priming. Though this approach has been widely used in the field of immunology, has also been effectively applied to MSCs[3,84,85]. Among commonly used priming strategies, leading approaches can be attributed to three main categories: (1) MSC priming with inflammatory molecules; (2) MSC priming with hypoxia; and (3) MSC priming with 3D cultures. These priming signals activate potential MSC mediators, including surface receptors and ligands, signalling molecules that induce survival/growth, regulatory molecules such as miRNAs, and transcription factors, which can modify the MSC phenotype[86-89], with a consequent boosting of MSC therapeutic functions (Figure 1).
Figure 1.
Potential mechanisms mediating mesenchymal stromal/stem cell-primed therapeutic properties. Mesenchymal stromal/stem cells (MSCs) can be primed through different signals, including hypoxia, three-dimensional cultures, and inflammatory cytokines to obtain a therapeutic phenotype. The potential mediators of this new phenotype comprise a plethora of regulatory molecules within MSCs, including surface receptors and ligands, signalling molecules inducing survival/growth, regulatory molecules such as microRNAs, and transcription factors regulating several pathways. Thus, primed MSCs can modulate inflammation, stimulate angiogenesis, and promote tissue repair/regeneration. MSCs: Mesenchymal stromal/stem cells; miRNAs: MicroRNAs.
Priming with inflammatory molecules
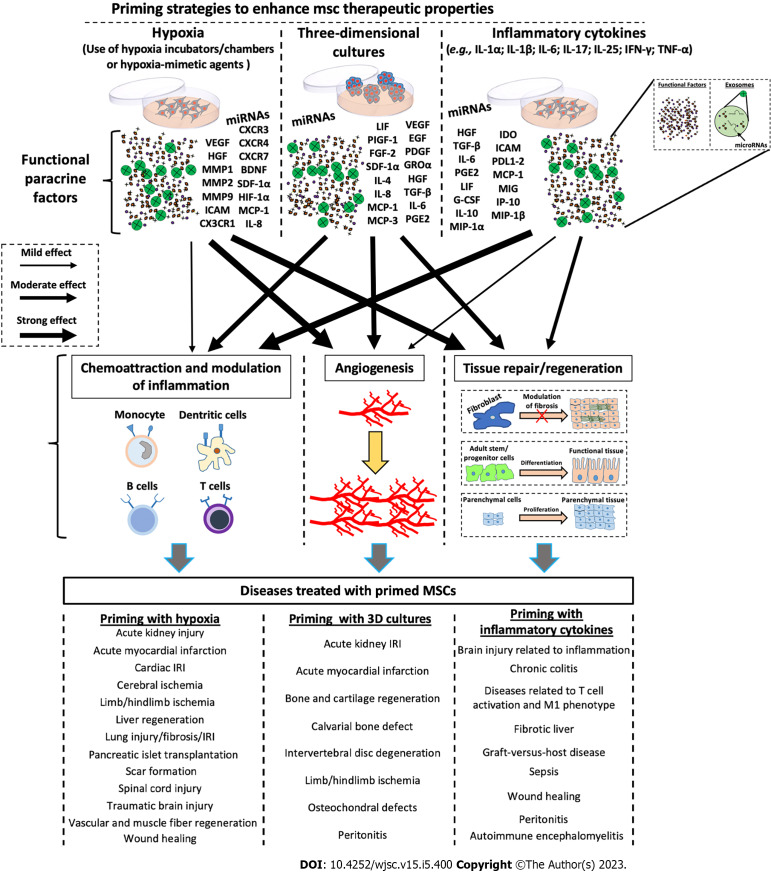
Numerous studies have revealed that the immunosuppressive properties of MSCs are not intrinsically possessed, but require priming of MSCs by inflammatory factors[90-92]. Depending on the inflammatory conditions, it has been demonstrated that MSC phenotypes can be polarized into MSC type 1 (with pro-inflammatory properties) and MSC type 2 (with immunosuppressive properties)[93,94]. Several strategies have been implemented to modulate/enhance the secretion of functional molecules in MSCs. As shown in Figure 2, the treatment of MSCs with inflammatory cytokines, including interferon-gamma (IFN-γ), interleukin (IL)-1α/β, IL-25, IL-6, TNF-α, and IL-17 enhanced the immunomodulatory properties of MSCs[40,95-112]. These treatments increase the production/secretion of functional factors, including hepatocyte growth factor (HGF), transforming growth factor-β, IL-6, prostaglandin E2 (PGE2), leukemia inhibitory factor, granulocyte colony-stimulating factor, IL-10, macrophage inflammatory protein-1α, indoleamine 2,3-dioxygenase (IDO), intercellular adhesion molecule, programmed death ligand 1-2, monocyte chemoattractant protein (MCP)-1, monokine induced by IFN-γ, induced protein 10, and macrophage inflammatory protein-1β, which in turn confer more paracrine immunomodulatory properties to MSCs (Figure 2). It has been demonstrated that CM enriched with the above-described factors was able to inhibit T cell proliferation/activation, reduce the secretion of inflammatory mediators, and induce monocyte polarization towards anti-inflammatory the M2 phenotype[40,102,105-112]. It has been shown that the treatment with inflammatory cytokines was also able to improve the immunomodulatory capabilities of EXOs, and these effects appear to be mediated by specific miRNAs, such as miR-21, miR-23a, miR-26b, miR-125b, miR-130b, miR-140, miR-146a, miR-203a, miR-223, miR-224, and miR-320a[40,109,111,113].
Figure 2.
Schematic representation of the molecular effects after priming of mesenchymal stromal/stem cells. Mesenchymal stromal/stem cells (MSCs) can be primed through various stimuli, including hypoxia, three-dimensional cultures, and pro-inflammatory cytokines to enhance their therapeutic potential. Each priming method induces the production of specific factors (e.g., trophic factors, angiogenetic factors, chemokines, cytokines, and exosomes containing both proteins and microRNAs), which induce the activation of biological processes such as angiogenesis, tissue repair/regeneration, chemoattraction, and modulation of inflammation. Each priming strategy seems to stimulate the production of functional factors in a different way, thus eliciting different responses. miRNA: MicroRNA; VEGF: Vascular endothelial-derived growth factor; CXCR: Chemokine receptor; HGF: Hepatocyte growth factor; MMP: Matrix metallopeptidase; BDNF: Brain-derived neurotrophic factor; SDF: Stromal cell-derived factor; HIF: Hypoxia-inducible factor; ICAM: Intercellular adhesion molecules; MCP: Monocyte chemoattractant protein; IL: Interleukin; LIF: Leukemia inhibitory factor; PIGF: Placental growth factor; EGF: Epidermal growth factor; FGF: Basic fibroblast growth factor; PDGF: Platelet-derived growth factor; GRO: Growth-related oncogene; TGF: Transforming growth factor; PGE2: Prostaglandin E2; IDO: Indoleamine 2,3-dioxygenase; PDL1-2: Programmed death ligand 1-2; MIG: Monokine induced by interferon-gamma; G-CSF: Granulocyte colony-stimulating factor; IP-10: Induced protein 10; MIP: Macrophage inflammatory protein; IRI: Ischemia/reperfusion injury; MSCs: Mesenchymal stromal/stem cells; 3D: Three-dimensional.
Priming with hypoxia
Differently from inflammatory cytokines, hypoxic treatment of MSCs seems to stimulate primarily the secretion of functional factors involved in the processes of angiogenesis and tissue proliferation/regeneration (Figure 2). Hypoxic preconditioning was able to promote angiogenic potential of MSCs via the activation of the HIF-1α-GRP78-Akt axis, and the overproduction of vascular endothelial-derived growth factor (VEGF) and HGF[114]. Lee and Joe[115] demonstrated that hypoxia priming induces an increase in HIF-1α expression and consequent VEGF production, improving the ability of MSCs to stimulate migration and tube formation of human umbilical vein endothelial cells (HUVECs). Moreover, Bader et al[116] found that hypoxic preconditioning induces the anti-apoptotic and pro-angiogenic effects of MSCs compared with untreated cells. In particular, Bcl-xL, BAG1, and VEGF were overexpressed after hypoxic priming, enhancing HUVEC proliferation and migration. Hypoxic MSCs are also able to produce numerous factors related to tissue remodelling, including matrix metallopeptidase 1 (MMP1), MMP2, and MMP9[117-119], as well as crucial factors such as IL-8 and MCP-1, involved in the chemotaxis and activation of innate immune responses[120,121]. Also with regard to EVs, hypoxic priming has been shown to have important effects. Xue et al[122] discovered that EXOs derived from hypoxia-treated MSCs were able to increase migration and tube formation of HUVECs through the PKA signalling pathway. Moreover, Ge et al[123] demonstrated the efficacy of hypoxic MSC-derived EXOs in enhancing angiogenesis. In particular, they showed that hypoxic EXOs containing miR612 promoted, through HIF-1α activation, the production of VEGF in human brain microvascular endothelial cells, inducing proliferation, migration, and angiogenic activities of these cells.
Priming with 3D culture of MSCs
Various in vitro strategies have been applied for the production of MSCs, with improved therapeutic properties, and priming with inflammatory factors may impact the expression of HLA-DR, thus altering allogeneic therapeutic possibilities[124-126]. MSC priming through 3D culture techniques, which allows for the generation of MSC spheroids, strictly recapitulates the in vivo MSC niche and enhances the phenotypic profile of MSCs, increasing both trophic and immunomodulatory functionalities. MSC spheroid action is exerted by the paracrine secretion of functional factors that possess anti-inflammatory, angiogenic, anti-fibrotic, anti-apoptotic, and mitogenic properties (Figure 2)[127]. Recently, through omics approaches, such as RNA sequencing and analysis of DNA methylation, it has been demonstrated that, compared with conventional 2D culture, MSC spheroids were able to modify their transcriptome profile by overexpressing genes that can regulate proliferation/differentiation, as well as immunomodulatory and angiogenic processes[128]. Concerning immunomodulatory and regenerative effects, 3D culture of MSCs seems to have more intermediary functions than the above-mentioned priming strategies (priming with inflammatory molecules or hypoxia) (Figure 2). 3D MSC spheroids have been shown to be capable of secreting multiple functional factors. For example, it has been found that various regenerative and immunomodulatory factors, such as stromal cell-derived factor-1α, growth-related oncogene α, MCP-1/3; IL-4, IL-10; epidermal growth factor (EGF), leukemia inhibitory factor, placental growth factor-1, VEGF-A/D, HGF, insulin like growth factor 1, TNFAIP6, STC1, platelet-derived growth factor B, transforming growth factor-β, PGE2, and IDO were up-regulated in 3D MSC spheroids compared with those of the MSCs cultivated under conventional 2D conditions[43,44,59,73,128-131] (Figure 2). The paracrine effects of 3D MSC appear to be also mediated by EVs. In particular, EXOs derived from MSC 3D cultures have been shown to have higher yields and enhanced activity. Indeed, compared with 2D cultures, EXOs isolated from CM of MSC spheroids were able to inhibit T cell proliferation and stimulate angiogenesis in vitro[44], as well as attenuate inflammation and periodontitis in vivo[132], and stimulate tissue regeneration in both in vitro and in vivo models[133].
THERAPEUTIC PROPERTIES OF PRIMED MSCs IN PRECLINICAL MODELS
Principal priming strategies to treat chronic immune-related disorders
By virtue of their immunomodulatory properties, MSCs are being studied to treat numerous chronic conditions, including GVHD and inflammatory bowel disorders, in order to attenuate inflammation and induce tissue recovery (Table 1). As already mentioned, treating MSCs with inflammatory factors enhances their immunomodulatory properties, and renders these cells able to inhibit T cell proliferation/activation and induce monocytes toward an anti-inflammatory phenotype. This quality makes these cells more clinically effective when applied to chronic inflammatory-related diseases (Figure 2). Indeed, several experimental studies have demonstrated that the treatment of MSCs with inflammatory factors, such as IFN-γ, IL-1β, and IL-25, enhanced MSC therapeutic effects in in vivo models of chronic colitis[95,96,98]. Rafei et al[134], in a mouse in vivo model of autoimmune encephalomyelitis, found that treatment with allogeneic MSCs primed with IFN-γ reduced clinical signs in a dose-dependent manner. In this study the authors showed that, though the priming treatment induced the increase of CCL2 and MHCI/II expression in IFN-γ-primed MSCs, it inhibited manifestations of autoimmune encephalomyelitis while keeping their immunogenicity low. The use of IFN-γ- or TNF-α-primed MSCs has also been shown to attenuate symptoms of GVHD[101,103]. In these cases, in the first study it was shown that therapeutic effects of MSCs were mediated by overproduction of IDO induced through the IFN-γ-JAK-STAT1 pathway[101]. In the second study, the therapeutic function of MSCs was activated by TNF-α, which induced overexpression of Chi3 L1 and consequent suppression of T-helper 17 cells[103]. Recently, it has been revealed that the priming of MSCs with IL-1β relieved the side effects of sepsis[109,111]. In particular, Song et al[109] demonstrated that IL-1β makes MSCs more effective in inducing macrophage polarization toward an anti-inflammatory M2 phenotype, and this effect was mediated, at least in part, through overproduction of EXOs containing miR146a. Similar results on M2 macrophage polarization were also obtained by Yao et al[111], who revealed the ability of IL-1β to stimulate the production of MSC-derived EXO containing miR21. The therapeutic efficacy of MSCs primed with IFN-γ was also found in an in vivo model of colonic wounds. Particularly, García et al[135] showed that these cells were able to enhance healing of colonic mucosal wounds in both immunocompromised and immunocompetent mice. Similar results were also obtained using MSCs primed with TNF-α, which were able to accelerate wound closure and angiogenesis in an in vivo model of wound healing[99]. The priming with inflammatory cytokines seems to also be effective for the treatment of chronic liver diseases. Indeed, treatment with IL-6 improved the ability of MSCs to reduce liver injury[104]. The study reported that in a mouse in vivo model of liver fibrosis, treatment with IL-6-primed MSCs reduced both fibrosis and apoptosis, and improved liver functions[104]. Moreover, TNF-α-primed MSCs were also able to attenuate inflammation in an in vivo model of peritonitis[136]. In this study, the authors demonstrated that TNF-α induced the overproduction of the anti-inflammatory factor TSG-6, generating a mechanism that reduces inflammation in an in vivo model of zymosan-induced peritonitis[136]. Interestingly, in a similar experimental model, Bazhanov et al[137] found that after intraperitoneal injection MSCs formed 3D aggregates, and stimulated the production of anti-inflammatory cytokines, such as IL-10 and PGE2. In this regard, Bartosh et al[138] showed that the priming of MSCs with 3D culture decreased inflammation in an in vivo model of peritonitis[138]. In particular, the authors suggest that MSC spheroids overexpressed TSG-6, and these cells were more effective than conventional MSCs as therapy for diseases characterized by unresolved inflammation.
Table 1.
Representative priming strategies of mesenchymal stromal/stem cells and their application in preclinical studies
|
MSCs
|
Dose
|
Priming treatments
|
Study model
|
Observed therapeutic effects
|
Ref.
|
| AMSCs | 1 × 105 MSCs/5 × 105 PBMCs | IFN-γ | In vitro model of T cell activation and monocyte M1/M2 polarization | Regulation of T cell activation/anergy and induction of M2-like polarized phenotype in monocytes | [40] |
| BM-MSCs | 0.5 × 106 MSCs/mouse | IFN-γ | In vivo model of chronic colitis | Attenuation of inflammation and colitis | [96] |
| BM-MSCs | NA | IFN-γ; TNF-α | In vitro model of MLR | Inhibition of allogeneic MLR | [97] |
| CB-MSC-derived EVs | NA | IFN-γ | In vivo model of acute kidney injury and in vitro model of T cell activation | Regulation of T cell activation and amelioration of kidney injury with unprimed MSCs only | [100] |
| BM-MSCs and CB-MSCs | 1 × 106 MSCs/mouse | IFN-γ | In vivo model of GVHD | Reduction of the symptoms of GVHD | [101] |
| BM-MSCs | 1 × 104 MSCs/2 × 103 macrophages | IFN-γ; LPS; TNF-α | In vitro model of monocyte M1/M2 polarization | Induction of monocyte polarization toward an anti-inflammatory M2 phenotype | [102] |
| UC-MSCs | 1 × 106 MSCs/mouse | IFN-γ; TNF-α | In vivo model of GVHD | Reduction of the symptoms of GVHD | [103] |
| BM-MSCs | 2.5 × 105 MSCs/5 × 105 macrophages | IFN-γ; IL-1β | In vitro model of monocyte M1/M2 polarization | Induction of monocyte polarization toward an anti-inflammatory M2 phenotype | [105] |
| BM-MSC-derived CM | NA | IFN-γ; IL-1α/β; TNF-α | In vitro model of LPS-injured microglial cells | Reduction in the secretion of inflammatory factors | [106] |
| AdMSCs; BM-MSCs; CB-MSCs. | NA | IFN-γ | In vitro model of T cell activation | Suppression of T cell proliferation | [110] |
| BM-MSCs | NA | IFN-γ; spheroids | In vitro model of T cell activation | Suppression of T cell activation and proliferation | [112] |
| BM-MSCs | 2 × 106 MSCs/mouse | IFN-γ | Autoimmune encephalomyelitis | Attenuation of pathologic manifestations | [134] |
| BM-MSCs | 1 × 106 MSCs/mL | IFN-γ | In vitro model of T cell activation and in vivo model of colonic wounds | Regulation of T cell activation and acceleration of healing of colonic mucosal wounds | [135] |
| UC-MSCs | 2 × 106 MSCs/mouse | IL-1β | In vivo model of chronic colitis | Attenuation of inflammation and colitis | [98] |
| UC-MSCs | 1 × 106 MSCs/mouse | IL-1β | In vivo model of sepsis | Increase in survival rate | [109] |
| MSC-derived EVs | 40 μg/mouse | IL-1β | In vitro model of monocyte M1/M2 polarization and in vivo model of sepsis | Induction of monocyte M2 polarization and amelioration of sepsis | [111] |
| AdMSC-derived CM | 20 μL/rat | TNF-α | In vivo model of wound healing | Acceleration of wound closure and angiogenesis | [99] |
| BM-MSCs | 1.6 × 106 MSCs/mouse | TNF-α | In vivo model of peritonitis | Attenuation of inflammatory responses | [136] |
| BM-MSCs | 5 × 106 MSCs/rat | IL-25 | In vivo model of chronic colitis | Attenuation of inflammation and colitis | [95] |
| BM-MSCs | 1 × 106 MSCs/mL | IL-6 | In vivo model of liver fibrosis | Reduction of liver injury and fibrosis | [104] |
| BM-MSCs | 3.91 × 104 MSCs/3.91 × 106 T cells | IL-17 | In vitro model of T cell activation | Suppression of T cell proliferation/activation and Th1 cytokines | [108] |
| AdMSCs | 5 × 105 MSCs/mouse | Hypoxia | In vivo model of hindlimb ischemia | Improvement of angiogenesis | [114] |
| BM-MSC-derived CM | 100 μL/mouse | Hypoxia | In vivo model of wound healing | Acceleration of skin wound healing | [120] |
| BM-MSCs | 2.5 × 105 MSCs/mouse | Hypoxia | In vivo model of pancreatic islet transplantation | Reversion of impaired glucose tolerance | [121] |
| BM-MSCs | 5 × 105 MSCs/mouse | Hypoxia | In vivo model of hindlimb ischemia | Improvement of angiogenesis | [139] |
| AdMSCs | 5 × 105 MSCs/mouse | Hypoxia | In vivo model of hindlimb ischemia | Improvement of functional recovery and neovascularization | [140] |
| AdMSC-derived CM | NA | Hypoxia | In vivo model of partial hepatectomy | Enhanced liver regeneration | [142] |
| AdMSCs | 2 × 106 MSCs/rat | Hypoxia | In vivo model of acute kidney injury | Improvement of angiogenesis and inhibition of ROS generation | [145] |
| AdMSC-derived CM | 100 μL/mouse | Hypoxia | In vivo model of acute kidney injury | Improvement of renal function and reduction of inflammation | [146] |
| BM-MSCs | 1 × 106 MSCs/rat | Hypoxia | In vivo model of lung IRI | Attenuation of pathologic lung injury score by inhibiting inflammation and generation of ROS and anti-apoptotic effects | [147] |
| BM-MSCs | NA | Hypoxia | In vivo model of radiation-induced lung injury | Improvement of antioxidant ability | [148] |
| BM-MSCs | 1 × 106 MSCs/rat | Hypoxia | In vivo model of myocardial infarction | Improvement of angiogenesis and function | [150] |
| BM-MSCs | 1 × 106 MSCs/mouse | Hypoxia | In vivo model of myocardial infarction | Prevention of apoptosis in cardiomyocytes | [151] |
| BM-MSC-derived EVs | 1 μg of EVs/mouse | Hypoxia | In vivo model of myocardial infarction | Reduction of cardiac fibrosis | [152] |
| BM-MSC-derived EVs | 50 μg of EVs/rat | Hypoxia | In vivo model of cardiac IRI | Reduction of IRI and improvement of cardiomyocyte survival | [153] |
| BM-MSC-derived EVs | 200 μg of EVs/20 g | Hypoxia | In vivo model of myocardial infarction | Improved cardiac repair by amelioration of cardiomyocyte apoptosis | [154] |
| BM-MSCs | 1 × 106 MSCs/rat | Hypoxia | In vivo model of cerebral ischemia | Enhanced angiogenesis and neurogenesis | [157] |
| BM-MSC-derived CM | 100 μg of CM/kg | Hypoxia | In vivo model of traumatic brain injury | Improved neurogenesis, motor and cognitive function | [158] |
| UC-MSCs | 1 × 105 MSCs/rat | Hypoxia | In vivo model of spinal cord injury | Increase in axonal preservation and decrease of apoptosis | [159] |
| PMSC-derived CM | 100 μL/mouse | Hypoxia | In vivo model of scar formation | Reduction of scar formation | [162] |
| BM-MSCs | 5 × 106 MSCs/rat | Hypoxia | In vivo model of partial hepatectomy | Enhanced liver regeneration | [164] |
| DP-MSCs | N.A. | Hypoxia | In vivo model of dental pulp injury | Regeneration of dental pulp with a rich vasculature | [167] |
| AF-MSC-derived CM | N.A. | Hypoxia | In vivo model of wound healing | Acceleration of skin wound healing | [168] |
| AMSC-derived CM and EVs | 200 μL CM and 5 μg EVs/1 × 105 PBMCs, and 100 μL CM and 5 μg EVs/1 × 104 HUVECs | 3D cultures/spheroids | In vitro model of T cell activation and HUVEC cells | Induction of angiogenesis and inhibition of T cell proliferation | [44] |
| AMSCs | 250 μL CM/ 1.5 × 105 alveolar epithelial cells | 3D cultures/spheroids | In vitro model of lung IRI | Attenuation of IRI side effects by improving the efficacy of in vitro EVLP | [59] |
| AMSC-derived CM | 50 μL CM/ 1 × 104 liver cells | 3D cultures/spheroids | In vitro model of liver IRI | Attenuation of IRI side effects by inhibiting inflammation and apoptosis | [131] |
| BM-MSCs | 3 × 106 MSCs/mouse | 3D cultures/spheroids | In vivo model of peritonitis | Production of anti-inflammatory cytokines | [137] |
| BM-MSCs | 1.5 × 106 MSCs/mouse | 3D cultures/spheroids | In vivo model of peritonitis | Attenuation of inflammatory responses | [138] |
| CB-MSCs | 1 × 107 MSCs/mouse | 3D cultures/spheroids | In vivo model of hindlimb ischemia | Improvement of survival and angiogenesis | [141] |
| AdMSCs | 2 × 106 MSCs/rat | 3D cultures/spheroids | In vivo model of acute kidney injury | Reduction of apoptosis and tissue damage, promotion of vascularization, and amelioration of renal function | [143] |
| UC-MSC-derived EVs | 200 μg of EVs/mouse | 3D cultures/spheroids | In vivo model of acute kidney injury | Attenuationof pathological changes and improvement of renal function | [144] |
| BM-MSCs | 2 × 106 MSCs/rat | 3D cultures/spheroids | In vivo model of myocardial infarction | Promotion of cardiac repair | [155] |
| BM-MSCs | 5 × 105 MSCs/rat | 3D cultures/spheroids | In vivo model of myocardial infarction | Stimulation of a vascular density and improvement of cardiac function | [156] |
| AdMSCs | 1 × 107 MSCs/mouse | 3D cultures/spheroids | In vivo model of hindlimb ischemia | Improvement of angiogenesis | [163] |
| AdMSCs | 2 × 106 MSCs/rabbit | 3D cultures/spheroids | In vivo model of disc degeneration | Induction of disc repair | [169] |
| BM-MSCs | NA | 3D cultures/spheroid | In vivo model of bilateral calvarial defects | Induction of bone regeneration | [170] |
| SMSCs | NA | 3D cultures/spheroid | In vivo model of osteochondral defects | Induction of cartilage regeneration | [171] |
MSCs: Mesenchymal stem cells; BM-MSCs: Bone marrow-derived mesenchymal stem cells; AMSCs: Amnion-derived mesenchymal stem cells; UC-MSCs: Umbilical cord-derived mesenchymal stem cells; AdMSCs: Adipose-derived mesenchymal stem cells; CB-MSCs: Cord blood-derived mesenchymal stem cells; WJ-MSCs: Wharton’s Jelly-derived mesenchymal stem cells; PMSCs: Placenta-derived mesenchymal stem cells; AF-MSCs: Amniotic fluid derived mesenchymal stem cells; SMSCs: Synovial derived mesenchymal stem cells; EVs: Extracellular vesicles; CM: Conditioned medium; NA: Not available; GVHD: Graft-versus-host disease; IRI: Ischemia-reperfusion injury; 3D: Three-dimensional; IFN: Interferon; TNF: Tumor necrosis factor; IL: Interleukin; MLR: Mixed lymphocyte reactions; LPS: Lipopolysaccharide; HUVEC: Human umbilical vein endothelial cell.
Overall, the above-mentioned studies suggest that treatment with pro-inflammatory cytokines or the 3D culture of MSCs represents promising priming strategies for enhancing the MSC immunoregulatory phenotype, making these cells more suitable for clinical disorders related to exacerbated immune responses (Figure 2).
Main priming strategies for treating acute injury
Priming strategies for MSCs have been considered a crucial tool for enhancing their therapeutic effects, making these cells more suitable for application in the field of regenerative medicine[3,85]. However, while the priming of MSCs with pro-inflammatory cytokines potentially represents the principal strategy modulating inflammation in chronic immune-related disorders (or, in any case, conditions in which the inflammation is exacerbated), the priming of MSCs with hypoxia is thought to represent the more appropriate priming strategy for boosting MSC effects for the stimulation of tissue function recovery after acute injury (Figure 2). This has been demonstrated in numerous study models, and on different organs (Table 1). For example, hypoxia pre-conditioning significantly improved blood flow recovery in mouse models of hindlimb ischemia. Rosová et al[139] demonstrated that hypoxic MSCs better migrate to the injured site compared with non-hypoxic MSCs, thus speeding up the restoration of blood flow. The authors demonstrated that the observed effects were likely mediated by the HGF-cMET axis. It has been shown that hypoxia helps MSCs to better integrate in the damaged tissue. Han et al[140] revealed that hypoxic priming enhanced survival and proliferation of transplanted MSCs, thus improving the regeneration of hindlimb ischemic tissues. After MSC treatment, the authors observed inhibition of apoptosis and promotion of neovascularization and, as they showed the increased expression of the normal cellular prion protein upon hypoxia pre-conditioning, they identified this prion as a potential target for MSC therapy. In a similar manner, Lee et al[115] recently identified GRP78 as new potential target for the development of functional MSCs. GRP78 has been shown to be induced by hypoxia, thus increasing transplanted-MSC survival and proliferation in a mouse model of hindlimb ischemia. Moreover, the authors found that the HIF-1α-GRP78-Akt axis regulates the suppression of cell death signals, and increases angiogenic cytokine secretion, thus strongly improving tissue recovery from the damage[114]. Recently, it has been found that mild hypoxia can be induced in MSCs when they are cultured as spheroids. Various studies have clearly demonstrated that 3D culture conditions induce hypoxia in the core of the spheroid, thus stimulating the production of both growth and pro-angiogenic factors, which in turn stimulate the fast recovery of damaged tissues in mouse models of hindlimb ischemia[141,142]. Interestingly, it has also been shown that the CM derived from MSCs primed by 3D culture attenuated injury and inflammation in two IRI in vitro models of both lung and liver[59,131]. 3D pre-conditioning has been shown to also be effective for other type of diseases, such as acute kidney injury (AKI). Xu et al[143] found that 3D pre-conditioned MSCs, when transplanted in mice with AKI, are more viable than the 2D cultured cells, and exhibit higher paracrine secretions, as evidenced by the increased levels of VEGF and TSG-6. Furthermore, the authors show that the paracrine secretion, which also includes basic fibroblast growth factor, insulin like growth factor, and EGF, significantly improved renal function and reduced tissue apoptosis, thus speeding up the regeneration of renal tissues upon injury[143]. Recently, the secretome of 3D MSCs transplanted for the treatment of AKI was furtherly investigated. For example, Cao et al[144] found that the paracrine effect on AKI was mediated not only by soluble factors, such as anti-inflammatory cytokines, but also by EXOs, whose production is increased after 3D pre-conditioning. Furthermore, by using a cisplatin-inducing AKI model in mice, the authors showed that the increased number of EXOs upon 3D culture enhanced the renoprotective and anti-inflammatory efficacy of MSCs[144]. Treatment of AKI with MSC therapy has been implemented in recent years by defining new protocols of MSC pre-conditioning. Along with 3D culturing, hypoxia priming has been used for the treatment of IRI-inducing AKI in animal models, and Zhang et al[145] demonstrated that hypoxia priming enhanced angiogenic and antioxidative MSCs properties in a rat model of renal IRI. In addition, in the same model, the authors found that transplanted MSCs attenuated renal apoptosis by reducing cleaved caspase3 activation. Notably, hypoxia also enhanced MSC therapeutic potential in a cisplatin-induced mouse model of AKI. Overath et al[146] found that hypoxic conditions increased the efficacy of transplanted MSCs in attenuating renal damage upon injury both by reducing creatinine and N-GAL serum levels, and decreasing pro-inflammatory cytokine release. MSC hypoxia pre-conditioning has also been found to be strongly effective for the treatment of IRI in the lung. For example, MSC infusion in lung perfusates demonstrated that hypoxic MSCs quickly migrate from the pulmonary artery to the lung tissue, where they attenuate parenchymal damage by reducing oxidative stress, inflammation, and apoptosis, and by stimulating cell proliferation and survival[147]. In a similar manner, MSC hypoxia has been found to have important effects also for radiation-induced lung injury (RILI). A mouse model of RILI was recently established by exposing the lungs of mice to irradiation, thus generating tissue damage. Upon irradiation, the authors demonstrated that hypoxic MSCs reside for longer in the injured tissue compared with normoxic MSCs. In addition, Li et al[148] showed that hypoxia-primed MSCs enhanced cell viability and proliferation, as well as anti-oxidative and anti-apoptotic capabilities in lung parenchymal cells. Finally, the authors highlighted the role of HIF-1 in modulating resistance to lung hypoxic stress induced by RILI, thus promoting tissue repair and regeneration upon injury.
The use of MSCs as cellular therapy has also been shown to be effective for the treatment of acute myocardial injury in several preclinical models (Table 1). Also in this case, to ameliorate the therapeutic effects of MSCs various priming strategies have been evaluated. In particular in myocardial infarction (MI), it has been widely believed that tissue injury is related to ischemia and the hypoxic environment. Therefore, the in vitro hypoxic condition was tested to improve MSC therapeutic effects in MI animal models[149]. In a mouse model of MI, it was found that intramyocardial injection of hypoxia-preconditioned MSCs reduces infarct size, influences heart remodelling by modulating vasculogenesis, and improves heart functions, promoting cell survival[150,151]. Of note, expression analysis in hypoxic MSCs has revealed an increase in expression of pro-survival and pro-angiogenic factors, including HIF-1α, ANGPT1, VEGF, Flk-1, Bcl-2, Bcl-xL, and these proteins can act in a paracrine manner on MI, inducing functional recovery[150]. It has also been observed that hypoxic MSCs influence the expression of specific miRNAs that can be secreted through EVs. In particular, Feng et al[152] demonstrated that after hypoxic treatment of MSCs an increase of miR22 was observed in EXOs, and this miRNA was considered responsible for targeting Mecp2, with beneficial effects on survival of cardiomyocytes exposed to ischemia. Similarly, EVs derived from hypoxic MSCs overexpressing miR26 were able to reduce the damage from ischemia/reperfusion in a rat model[153]. In the same way, in an MI mouse model the intracardial injection of hypoxic-preconditioned MSC-derived EXOs was able to positively regulate cardiomyocyte proliferation and survival, and this effect was ascribable to the overexpression of miR125b[154]. In addition to the use of hypoxia priming, the use of 3D culture has also been shown to be effective in the improvement of MSC therapeutic effects on the treatment of acute myocardial injury. You et al[155], in an acute MI rat model, found that treatment with 3D-primed MSCs resulted in a retention of MSCs at the epicardium, where MSCs exerted cardiac protection/repair, and functional recovery. Moreover, in the same animal model, Wang et al[156] revealed that 3D MSCs were able to stimulate vascular density and improve cardiac function after MI.
Over the last decade, MSCs have also been intensively studied for their potential use in the treatment of neurological acute injury, including cerebral ischemia, traumatic brain injury, and spinal cord damage. For example, in an in vivo model of cerebral ischemia, it has been shown that hypoxic-preconditioned MSCs enhanced angiogenesis and neurogenesis after ischemia[157]. In an in vivo model of traumatic brain injury, Chang et al[158] demonstrated that the priming of MSCs with hypoxia improved their therapeutic function, and resulted in an amelioration of neurogenesis, and motor and cognitive functions. Moreover, in a rat model of spinal cord injury, hypoxic MSCs were also able to increase axonal preservation and decrease apoptosis[159].
Principal priming strategies for stimulating tissue regeneration
MSCs are involved in tissue homeostasis, which is necessary for physiologically coordinating regeneration/repair of tissue, also after injury[3,6,36]. Thus, the use of MSCs in regenerative therapies is garnering great interest due to their potentially numerous clinical applications.
In the complex process of cutaneous wound healing, a central role is played by fibroblasts, which contribute, through the interaction with surrounding cells, to the production of ECM, glycoproteins, adhesive molecules, and various growth factors[160]. Recent evidence suggests that CM produced by primed MSCs from different sources, such as bone marrow[120], adipose tissue[160], amnion fluid[161], and placenta[162] enhanced the migration and proliferation of fibroblasts in vitro, and accelerated wound healing in in vivo models (Table 1). In all these cases, hypoxia treatment represented the chosen priming strategy for driving MSCs in increasing secretion of various angiogenic factors, cytokines, and chemokines. Therefore, the priming of MSCs with hypoxia might well represent the main approach to improving the therapeutic effects of MSCs to be applied in the stimulation of tissue regeneration (Figure 2). This idea has also been supported by other studies (Table 1). Indeed, in both hepatectomized mouse and rat models, it has been demonstrated that hypoxic MSCs produce crucial functional molecules, including HGF and VEGF, which were considered responsible for the induction of liver regeneration[163,164]. Kuo et al[165] showed that systemic infusion of MSCs restored liver function and promoted liver regeneration in rodents. In this regard, in a rat massive hepatectomy model, Yu et al[164] found that hypoxia-conditioned MSCs secreted significantly more VEGF than normoxia-conditioned cells, and the infusion of primed MSCs promoted proliferation of hepatocytes and liver regeneration. Several studies have focused on the signalling pathways up-regulated by MSC during liver regeneration. Lee et al[163] using a partially hepatectomized mouse model, found that treatment with hypoxic MSC-derived CM increased the viability of hepatotoxic hepatocytes, and enhanced liver regeneration through JAK/STAT3 signalling. These data were also confirmed by Lee et al[166], who confirmed the activation of JAK/STAT3 signalling induced by MSC CM during mouse liver generation. Hypoxic MSCs that secrete high level of VEGF were also able to regenerate pulp-like tissues and vasculature similar to the native pulp in a rat model of pulp repair[167]. HGF and VEGF produced by hypoxic MSCs were considered by Chang et al[158] to be responsible for improvement of neuronal proliferation. Moreover, Zhilai et al[159] demonstrated that both HGF and VEGF produced by hypoxia-primed MSCs facilitated axonal survival in a rat model of spinal cord injury. Han et al[140], in a murine hindlimb ischemia model, found that the expression levels of EGF, VEGF, fibroblast growth factor, and HGF were significantly higher in ischemic tissue treated with hypoxic MSCs, where an improvement of neovascularization was observed. The efficacy of hypoxic MSCs was also tested in reducing scar formation and inducing wound healing in various in vivo models[120,162,168].
Despite the fact that the principal MSC priming strategy used for both in vitro and in vivo regeneration experiments was hypoxia treatment, 3D culture of MSCs as priming strategy has also been investigated in tissue regeneration (Figure 2). In fact, MSC spheroids have also shown therapeutic abilities with regard to both bone and cartilage regeneration. In particular, it has been found that treatment with MSC spheroids was effective in inducing disc repair in an in vivo model of disc degeneration, bone regeneration in an in vivo model of bilateral calvarial defects, and cartilage regeneration in an in vivo model of osteochondral defects[169-171].
CONCLUSION
The therapeutic effects of MSCs have been demonstrated in both in vitro and in vivo studies. Nevertheless, due to their heterogeneity related mainly to tissue source, which can impact MSC functional properties[85,172], the application of MSCs in clinical trials has shown moderate or poor efficacy. MSCs are considered key regulators of tissue repair and, in this case, different stimuli are crucial in modulating the functional properties of these cells. In fact, it is believed that inflammation and low oxygen levels are essential signals for triggering MSC activity in a suitable manner. Moreover, it has recently been shown that different priming approaches can eliminate the functional heterogeneity of MSCs[173]. Therefore, specific priming strategies have been implemented to improve the regenerative and immunomodulatory properties of MSCs. In this review, we have explored data regarding the principal priming approaches used to enhance the therapeutic potential of MSCs. The above-mentioned data underscore that several factors play a role in the ability to modify MSC properties. Moreover, some therapeutic effects, on different disease models, can be obtained in relation to dose and/or combination of the priming factors used.
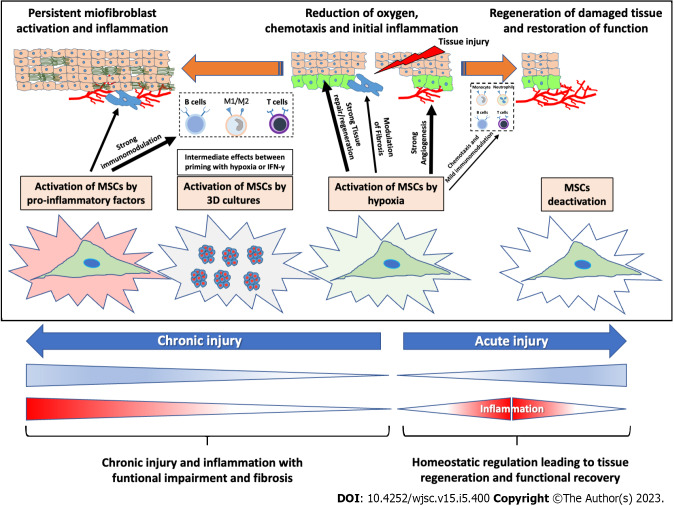
Several diseases have in common tissue injury and repair processes, in which inflammation plays a central role in coordinating different pathways that regulate tissue regeneration and functional recovery. Indeed, after acute injury, a low level inflammation (acute inflammation) occurring after specific triggers, is crucial in stimulating wound healing and tissue repair, facilitating the resolution of inflammation and restoring tissue structure/function (inflammation drives regeneration). On the other hand, in the case of abnormal damage repair, chronic unregulated inflammation can lead to pathological processes, including hormonal metabolic changes, which culminate in the onset of specific diseases, including cancer and fibrosis[174,175]. Therefore, the regulation of both acute and chronic inflammation is essential for a proper restorative response and, in this scenario, MSCs can have a crucial physiopathological role. In fact, it has been shown that when MSCs coordinate damaged tissue for repair, they undergo local stimuli such as inflammatory cytokines, and hypoxia, which in turn boost and direct the reaction of MSCs to orchestrate tissue regeneration[85,176]. In Figure 3, we depict a hypothetical model that occurs during physiopathologic tissue injury and repair. In this model, MSCs are activated differently by various microenvironment stimuli to manage tissue functional recovery. One of the first factors that arises after tissue injury is the establishment of a hypoxic and weakly inflammatory microenvironment, which in turn activates local cells to protect/regenerate tissues[3,177]. Hypoxia rapidly up-regulates the level of intercellular adhesion molecule-1 in local-inflamed endothelium, promoting MSC migration to injured tissues[178,179]. Moreover, a mild inflammation may stimulate MSCs to release chemokines for attracting immune cells and amplifying immune responses[180]. Once MSCs reach the site of injury, the paracrine properties of MSCs to release chemotactic and angiogenic factors is significantly amplified under hypoxic conditions[181]. In this case, naïve MSC are activated to recruit neutrophils and stimulate the formation of new blood vessels. Neutrophil action is followed by monocyte/macrophage activity that ensures sustained release of pro-inflammatory cytokines and potentiation of the fibroproliferative response[182,183]. If these processes are not adequately regulated, a state of chronic inflammation occurs. Thus, cytokines such as IFN-γ, TNF-α, and IL-1 accumulate in the injured tissues, and the inflammatory environment becomes central in affecting the regulatory role of MSCs that exhibit immunosuppressive capacities[184]. The MSC phenotype is switched into a lower regenerative potential and a higher anti-inflammatory phenotype (Figure 3). Thus, high amounts of pro-inflammatory cytokine confer a dramatic immunomodulatory ability to MSCs[40,91,124,125,185,186] which, in turn, act as a homeostatic regulator to control the inflammatory response. Overall, this scenario describes what occurs when MSCs are exposed to low levels of both oxygen and inflammation, and their phenotype is potentially inclined to low immunomodulation and high stimulation of tissue regeneration. Otherwise, high levels of inflammation can imprint a MSC phenotype inclined toward high immunomodulation and weak stimulation of tissue regeneration (Figure 3). In this regard, Vigo et al[87] found that IFN-γ can orchestrate MSCs functions in a dose-manner, and this is reflected in the opportunity to modulate MSC properties before their use in clinical practice. In addition, considering the heterogeneous immune regulatory functions of MSCs due to intrinsic characteristics of individual clones, the priming of MSCs with pro-inflammatory factors can equally amplify immune therapeutic properties of MSCs, and eliminate the variances among different MSC clones[173].
Figure 3.
Schematic illustration of the physiological role and biological action of mesenchymal stromal/stem cells primed in vivo in a model of tissue injury and repair. During tissue injury and repair, mesenchymal stromal/stem cells (MSCs) are differently activated by various microenvironment stimuli to orchestrate tissue repair and functional recovery. First, naïve MSC activation (hypoxic activation) leads to the release of both angiogenic factors and chemokines, which stimulate the formation of new blood vessels, the recruitment of neutrophils, and the expression of adhesion molecules. Neutrophil action is followed by macrophage activity, which ensures sustained release of pro-inflammatory cytokines, and potentiation of the fibroproliferative response. If this process is not adequately regulated, a state of chronic inflammation occurs; the MSC phenotype is switched into an anti-inflammatory phenotype. MSCs: Mesenchymal stromal/stem cells; 3D: Three-dimensional.
Priming with inflammatory signals polarizes MSCs toward an anti-inflammatory and pro-trophic phenotype allowing, on the one hand, the regulation of inflammatory responses, and on the other the final remodelling and recovery of damaged tissue. Likewise, different priming strategies can be used to direct the therapeutic effects of naïve MSCs toward specific pathological processes. As also highlighted by the studies we have noted in this review, while hypoxic priming of MSCs could be used mainly to treat acute disease, to principally stimulate angiogenesis and tissue regeneration, inflammatory cytokines could be used mainly to prime MSCs for treating chronic immune-related disorders. The change of perspective from regeneration to inflammation implies in the MSCs the shift in the production of functional factors that stimulate regenerative or anti-inflammatory pathways (Figure 2). Interestingly, the 3D culture of MSCs as priming strategy appears to be an intermediate functional priming between the two mentioned above. The production of priming type-specific functional factors in MSCs could well pave the way for optimizing their therapeutic potential, aimed at a greater effectiveness as an advanced therapy medicinal product.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 27, 2023
First decision: February 28, 2023
Article in press: April 17, 2023
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Cao HC, China; Cheng YH, United States; Qin Y, China; Shen Y, China; Yang T, China; Li SC, United States S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
Contributor Information
Vitale Miceli, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy. vmiceli@ismett.edu.
Giovanni Zito, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy.
Matteo Bulati, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy.
Alessia Gallo, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy.
Rosalia Busà, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy.
Gioacchin Iannolo, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy.
Pier Giulio Conaldi, Department of Research, IRCCS ISMETT (Istituto Mediterraneo per i Trapianti e Terapie ad alta Specializzazione), Palermo 90127, Italy.
References
- 1.Chinnici CM, Russelli G, Bulati M, Miceli V, Gallo A, Busà R, Tinnirello R, Conaldi PG, Iannolo G. Mesenchymal stromal cell secretome in liver failure: Perspectives on COVID-19 infection treatment. World J Gastroenterol. 2021;27:1905–1919. doi: 10.3748/wjg.v27.i17.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cittadini E, Brucculeri AM, Quartararo F, Vaglica R, Miceli V, Conaldi PG. Stem cell therapy in the treatment of organic and dysfunctional endometrial pathology. Minerva Obstet Gynecol. 2022;74:504–515. doi: 10.23736/S2724-606X.21.04919-8. [DOI] [PubMed] [Google Scholar]
- 3.Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22020763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmelzer E, Miceli V, Chinnici CM, Bertani A, Gerlach JC. Effects of mesenchymal stem cell coculture on human lung small airway epithelial cells. BioMed research international 2020; 2020: 9847579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019;27:2029–2035.e5. doi: 10.1016/j.celrep.2019.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum AJ, Grande DA, Dines JS. The use of mesenchymal stem cells in tissue engineering: A global assessment. Organogenesis. 2008;4:23–27. doi: 10.4161/org.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8 doi: 10.3390/cells8080784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. doi: 10.3389/fimmu.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 11.Fayyad-Kazan M, Fayyad-Kazan H, Lagneaux L, Najar M. The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy. 2016;8:839–842. doi: 10.2217/imt-2016-0037. [DOI] [PubMed] [Google Scholar]
- 12.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7:92. doi: 10.1038/s41392-022-00932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti G, Russo E, Corrao S, Anzalone R, Kruzliak P, Miceli V, Conaldi PG, Di Gaudio F, La Rocca G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines. 2022;10 doi: 10.3390/biomedicines10112822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burja B, Barlič A, Erman A, Mrak-Poljšak K, Tomšič M, Sodin-Semrl S, Lakota K. Human mesenchymal stromal cells from different tissues exhibit unique responses to different inflammatory stimuli. Curr Res Transl Med. 2020;68:217–224. doi: 10.1016/j.retram.2020.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parolini O, Alviano F, Bagnara GP, Bilic G, Bühring HJ, Evangelista M, Hennerbichler S, Liu B, Magatti M, Mao N, Miki T, Marongiu F, Nakajima H, Nikaido T, Portmann-Lanz CB, Sankar V, Soncini M, Stadler G, Surbek D, Takahashi TA, Redl H, Sakuragawa N, Wolbank S, Zeisberger S, Zisch A, Strom SC. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 19.Tesarova L, Jaresova K, Simara P, Koutna I. Umbilical Cord-Derived Mesenchymal Stem Cells Are Able to Use bFGF Treatment and Represent a Superb Tool for Immunosuppressive Clinical Applications. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21155366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walter SG, Randau TM, Hilgers C, Haddouti EM, Masson W, Gravius S, Burger C, Wirtz DC, Schildberg FA. Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21124382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, De Biasio M, Heinelt M, Reeve B, Abdi R, Alturki M, Fallatah M, Almalik A, Alhasan AH, Shah K, Karp JM. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6:eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med. 2017;6:613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, Waller EK, Burke E, Skerrett D, Shpall E, Martin PJ. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020;26:835–844. doi: 10.1016/j.bbmt.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckard AR, Borow KM, Mack EH, Burke E, Atz AM. Remestemcel-L Therapy for COVID-19-Associated Multisystem Inflammatory Syndrome in Children. Pediatrics. 2021;147 doi: 10.1542/peds.2020-046573. [DOI] [PubMed] [Google Scholar]
- 25.Rubin R. Unproven but Profitable: The Boom in US Stem Cell Clinics. JAMA. 2018;320:1421–1423. doi: 10.1001/jama.2018.13861. [DOI] [PubMed] [Google Scholar]
- 26.Fričová D, Korchak JA, Zubair AC. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regen Med. 2020;5:20. doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019;2019:9628536. doi: 10.1155/2019/9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malliaras K, Kreke M, Marbán E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90:532–541. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 29.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 30.Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280–284. doi: 10.1182/asheducation-2011.1.280. [DOI] [PubMed] [Google Scholar]
- 31.Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. doi: 10.1186/s13045-021-01037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattar P, Bieback K. Comparing the Immunomodulatory Properties of Bone Marrow, Adipose Tissue, and Birth-Associated Tissue Mesenchymal Stromal Cells. Front Immunol. 2015;6:560. doi: 10.3389/fimmu.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YH, Tao YC, Wu DB, Wang ML, Tang H, Chen EQ. Cell heterogeneity, rather than the cell storage solution, affects the behavior of mesenchymal stem cells in vitro and in vivo. Stem Cell Res Ther. 2021;12:391. doi: 10.1186/s13287-021-02450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 35.Brack AS, Rando TA. Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell. 2012;10:504–514. doi: 10.1016/j.stem.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558:449–453. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 37.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20:847–856. doi: 10.1038/nm.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, Conaldi PG. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-γ Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front Immunol. 2020;11:54. doi: 10.3389/fimmu.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1276–1292. doi: 10.1177/0271678X18776802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol. 2018;9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miceli V, Chinnici CM, Bulati M, Pampalone M, Amico G, Schmelzer E, Gerlach JC, Conaldi PG. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. Biochem Biophys Res Commun. 2020;522:171–176. doi: 10.1016/j.bbrc.2019.11.069. [DOI] [PubMed] [Google Scholar]
- 44.Miceli V, Pampalone M, Vella S, Carreca AP, Amico G, Conaldi PG. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019;2019:7486279. doi: 10.1155/2019/7486279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 46.Chang C, Yan J, Yao Z, Zhang C, Li X, Mao HQ. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv Healthc Mater. 2021;10:e2001689. doi: 10.1002/adhm.202001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavoie JR, Rosu-Myles M. Uncovering the secretes of mesenchymal stem cells. Biochimie. 2013;95:2212–2221. doi: 10.1016/j.biochi.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Park WS, Ahn SY, Sung SI, Ahn JY, Chang YS. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr Res. 2018;83:214–222. doi: 10.1038/pr.2017.249. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Lee CW, Wang YF, Huang S, Shin LY, Wang YH, Wan Z, Zhu X, Yung PSH, Lee OK. The Role of Paracrine Regulation of Mesenchymal Stem Cells in the Crosstalk With Macrophages in Musculoskeletal Diseases: A Systematic Review. Front Bioeng Biotechnol. 2020;8:587052. doi: 10.3389/fbioe.2020.587052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 52.Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33:1744–1762. doi: 10.1016/j.cmet.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Looze C, Yui D, Leung L, Ingham M, Kaler M, Yao X, Wu WW, Shen RF, Daniels MP, Levine SJ. Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochem Biophys Res Commun. 2009;378:433–438. doi: 10.1016/j.bbrc.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasirishargh A, Kumar P, Ramasubramanian L, Clark K, Hao D, Lazar SV, Wang A. Exosomal microRNAs from mesenchymal stem/stromal cells: Biology and applications in neuroprotection. World J Stem Cells. 2021;13:776–794. doi: 10.4252/wjsc.v13.i7.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iannolo G, Sciuto MR, Cuscino N, Carcione C, Coronnello C, Chinnici CM, Raffa GM, Pilato M, Conaldi PG. miRNA expression analysis in the human heart: Undifferentiated progenitors vs. bioptic tissues-Implications for proliferation and ageing. J Cell Mol Med. 2021;25:8687–8700. doi: 10.1111/jcmm.16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galderisi U, Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med Res Rev. 2014;34:1100–1126. doi: 10.1002/med.21322. [DOI] [PubMed] [Google Scholar]
- 59.Miceli V, Bertani A, Chinnici CM, Bulati M, Pampalone M, Amico G, Carcione C, Schmelzer E, Gerlach JC, Conaldi PG. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22020510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammadipoor A, Antebi B, Batchinsky AI, Cancio LC. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir Res. 2018;19:218. doi: 10.1186/s12931-018-0921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Műzes G, Sipos F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells. 2022;11 doi: 10.3390/cells11152300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pileggi A, Xu X, Tan J, Ricordi C. Mesenchymal stromal (stem) cells to improve solid organ transplant outcome: lessons from the initial clinical trials. Curr Opin Organ Transplant. 2013;18:672–681. doi: 10.1097/MOT.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ragni E, Parolini O, Silini AR. Editorial: MSC-Derived Extracellular Vesicles and Secreted Factors as "Cell-Free" Therapeutic Alternatives in Regenerative Medicine. Front Bioeng Biotechnol. 2022;10:842128. doi: 10.3389/fbioe.2022.842128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haga H, Yan IK, Takahashi K, Matsuda A, Patel T. Extracellular Vesicles from Bone Marrow-Derived Mesenchymal Stem Cells Improve Survival from Lethal Hepatic Failure in Mice. Stem Cells Transl Med. 2017;6:1262–1272. doi: 10.1002/sctm.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y, Shi H, Wu L, Zhu W, Qian H, Xu W. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells. 2015;33:2158–2168. doi: 10.1002/stem.1771. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Liu D, Li C, Zhou S, Tian D, Xiao D, Zhang H, Gao F, Huang J. Exosomes secreted from mutant-HIF-1α-modified bone-marrow-derived mesenchymal stem cells attenuate early steroid-induced avascular necrosis of femoral head in rabbit. Cell Biol Int. 2017;41:1379–1390. doi: 10.1002/cbin.10869. [DOI] [PubMed] [Google Scholar]
- 68.Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol. 2008;20:55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Suzdaltseva Y, Goryunov K, Silina E, Manturova N, Stupin V, Kiselev SL. Equilibrium among Inflammatory Factors Determines Human MSC-Mediated Immunosuppressive Effect. Cells. 2022;11 doi: 10.3390/cells11071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller L, Tunger A, Wobus M, von Bonin M, Towers R, Bornhäuser M, Dazzi F, Wehner R, Schmitz M. Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front Cell Dev Biol. 2021;9:637725. doi: 10.3389/fcell.2021.637725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci. 2020;41:653–664. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 73.Lo Nigro A, Gallo A, Bulati M, Vitale G, Paini DS, Pampalone M, Galvagno D, Conaldi PG, Miceli V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front Med (Lausanne) 2021;8:746298. doi: 10.3389/fmed.2021.746298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 75.Zimmerlin L, Park TS, Zambidis ET, Donnenberg VS, Donnenberg AD. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie. 2013;95:2235–2245. doi: 10.1016/j.biochi.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sagaradze G, Grigorieva O, Nimiritsky P, Basalova N, Kalinina N, Akopyan Z, Efimenko A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miceli V, Bertani A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells. 2022;11 doi: 10.3390/cells11050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 80.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kay AG, Long G, Tyler G, Stefan A, Broadfoot SJ, Piccinini AM, Middleton J, Kehoe O. Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Sci Rep. 2017;7:18019. doi: 10.1038/s41598-017-18144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rathinasabapathy A, Bruce E, Espejo A, Horowitz A, Sudhan DR, Nair A, Guzzo D, Francis J, Raizada MK, Shenoy V, Katovich MJ. Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis. Br J Pharmacol. 2016;173:2859–2879. doi: 10.1111/bph.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One. 2014;9:e107001. doi: 10.1371/journal.pone.0107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82–93. doi: 10.4252/wjsc.v6.i2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131. doi: 10.1186/s13287-019-1224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta S, Rawat S, Krishnakumar V, Rao EP, Mohanty S. Hypoxia preconditioning elicit differential response in tissue-specific MSCs via immunomodulation and exosomal secretion. Cell Tissue Res. 2022;388:535–548. doi: 10.1007/s00441-022-03615-y. [DOI] [PubMed] [Google Scholar]
- 87.Vigo T, Procaccini C, Ferrara G, Baranzini S, Oksenberg JR, Matarese G, Diaspro A, Kerlero de Rosbo N, Uccelli A. IFN-γ orchestrates mesenchymal stem cell plasticity through the signal transducer and activator of transcription 1 and 3 and mammalian target of rapamycin pathways. J Allergy Clin Immunol. 2017;139:1667–1676. doi: 10.1016/j.jaci.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 88.Wobma HM, Tamargo MA, Goeta S, Brown LM, Duran-Struuck R, Vunjak-Novakovic G. The influence of hypoxia and IFN-γ on the proteome and metabolome of therapeutic mesenchymal stem cells. Biomaterials. 2018;167:226–234. doi: 10.1016/j.biomaterials.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3:90–104. doi: 10.1038/s41551-018-0325-8. [DOI] [PubMed] [Google Scholar]
- 90.Jauković A, Kukolj T, Obradović H, Okić-Đorđević I, Mojsilović S, Bugarski D. Inflammatory niche: Mesenchymal stromal cell priming by soluble mediators. World J Stem Cells. 2020;12:922–937. doi: 10.4252/wjsc.v12.i9.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 92.Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- 93.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng W, Su J, Hu Y, Huang Q, Shi H, Wang L, Ren J. Interleukin-25 primed mesenchymal stem cells achieve better therapeutic effects on dextran sulfate sodium-induced colitis via inhibiting Th17 immune response and inducing T regulatory cell phenotype. Am J Transl Res. 2017;9:4149–4160. [PMC free article] [PubMed] [Google Scholar]
- 96.Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 97.English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473–481. doi: 10.1038/cmi.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 100.Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, Kankuri E, Mervaala E, Laitinen S. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, Sung KW, Koo HH, Yoo KH. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine. 2018;28:261–273. doi: 10.1016/j.ebiom.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin T, Pajarinen J, Nabeshima A, Lu L, Nathan K, Jämsen E, Yao Z, Goodman SB. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res Ther. 2017;8:277. doi: 10.1186/s13287-017-0730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu W, Yuan F, Bai H, Liu Y, Li X, Wang Y, Zhang Y. hUC-MSCs Attenuate Acute Graft-Versus-Host Disease through Chi3l1 Repression of Th17 Differentiation. Stem Cells Int. 2022;2022:1052166. doi: 10.1155/2022/1052166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nasir GA, Mohsin S, Khan M, Shams S, Ali G, Khan SN, Riazuddin S. Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med. 2013;11:78. doi: 10.1186/1479-5876-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. doi: 10.1186/s13287-018-1039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79. doi: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sivanathan KN, Rojas-Canales D, Grey ST, Gronthos S, Coates PT. Transcriptome Profiling of IL-17A Preactivated Mesenchymal Stem Cells: A Comparative Study to Unmodified and IFN-γ Modified Mesenchymal Stem Cells. Stem Cells Int. 2017;2017:1025820. doi: 10.1155/2017/1025820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sivanathan KN, Rojas-Canales DM, Hope CM, Krishnan R, Carroll RP, Gronthos S, Grey ST, Coates PT. Interleukin-17A-Induced Human Mesenchymal Stem Cells Are Superior Modulators of Immunological Function. Stem Cells. 2015;33:2850–2863. doi: 10.1002/stem.2075. [DOI] [PubMed] [Google Scholar]
- 109.Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 110.Torres Crigna A, Uhlig S, Elvers-Hornung S, Klüter H, Bieback K. Human Adipose Tissue-Derived Stromal Cells Suppress Human, but Not Murine Lymphocyte Proliferation, via Indoleamine 2,3-Dioxygenase Activity. Cells. 2020;9 doi: 10.3390/cells9112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021;264:118658. doi: 10.1016/j.lfs.2020.118658. [DOI] [PubMed] [Google Scholar]
- 112.Zimmermann JA, Hettiaratchi MH, McDevitt TC. Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs. Stem Cells Transl Med. 2017;6:223–237. doi: 10.5966/sctm.2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng Part A. 2017;23:1212–1220. doi: 10.1089/ten.tea.2016.0548. [DOI] [PubMed] [Google Scholar]
- 114.Lee JH, Yoon YM, Lee SH. Hypoxic Preconditioning Promotes the Bioactivities of Mesenchymal Stem Cells via the HIF-1α-GRP78-Akt Axis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SG, Joe YA. Autophagy mediates enhancement of proangiogenic activity by hypoxia in mesenchymal stromal/stem cells. Biochem Biophys Res Commun. 2018;501:941–947. doi: 10.1016/j.bbrc.2018.05.086. [DOI] [PubMed] [Google Scholar]
- 116.Bader AM, Klose K, Bieback K, Korinth D, Schneider M, Seifert M, Choi YH, Kurtz A, Falk V, Stamm C. Hypoxic Preconditioning Increases Survival and Pro-Angiogenic Capacity of Human Cord Blood Mesenchymal Stromal Cells In Vitro. PLoS One. 2015;10:e0138477. doi: 10.1371/journal.pone.0138477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu C, Zhao L, Duan J, Li L. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med. 2019;23:1657–1670. doi: 10.1111/jcmm.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee SM, Jun DW, Kang HT, Oh JH, Saeed WK, Ahn SB. Optimal Hypoxic Preconditioning of Human Embryonic Stem Cell-Derived Mesenchymal Stem Cells (hES-MSCs) and Their Characteristics. Int J Stem Cells. 2021;14:221–228. doi: 10.15283/ijsc20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Page P, DeJong J, Bandstra A, Boomsma RA. Effect of serum and oxygen concentration on gene expression and secretion of paracrine factors by mesenchymal stem cells. Int J Cell Biol. 2014;2014:601063. doi: 10.1155/2014/601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, Liu X, Qi S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9:e96161. doi: 10.1371/journal.pone.0096161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiang C, Xie QP. Protection of mouse pancreatic islet function by coculture with hypoxia pretreated mesenchymal stromal cells. Mol Med Rep. 2018;18:2589–2598. doi: 10.3892/mmr.2018.9235. [DOI] [PubMed] [Google Scholar]
- 122.Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, Chen Y, Ma B, Wei J, Han Q, Zhao RC. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018;27:456–465. doi: 10.1089/scd.2017.0296. [DOI] [PubMed] [Google Scholar]
- 123.Ge L, Xun C, Li W, Jin S, Liu Z, Zhuo Y, Duan D, Hu Z, Chen P, Lu M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J Nanobiotechnology. 2021;19:380. doi: 10.1186/s12951-021-01126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.François M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- 126.van Megen KM, van 't Wout ET, Lages Motta J, Dekker B, Nikolic T, Roep BO. Activated Mesenchymal Stromal Cells Process and Present Antigens Regulating Adaptive Immunity. Front Immunol. 2019;10:694. doi: 10.3389/fimmu.2019.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kouroupis D, Correa D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front Bioeng Biotechnol. 2021;9:621748. doi: 10.3389/fbioe.2021.621748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gallo A, Cuscino N, Contino F, Bulati M, Pampalone M, Amico G, Zito G, Carcione C, Centi C, Bertani A, Conaldi PG, Miceli V. Changes in the Transcriptome Profiles of Human Amnion-Derived Mesenchymal Stromal/Stem Cells Induced by Three-Dimensional Culture: A Potential Priming Strategy to Improve Their Properties. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23020863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen LC, Wang HW, Huang CC. Modulation of Inherent Niches in 3D Multicellular MSC Spheroids Reconfigures Metabolism and Enhances Therapeutic Potential. Cells. 2021;10 doi: 10.3390/cells10102747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Follin B, Juhl M, Cohen S, Pedersen AE, Kastrup J, Ekblond A. Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture. Tissue Eng Part B Rev. 2016;22:322–329. doi: 10.1089/ten.teb.2015.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zito G, Miceli V, Carcione C, Busà R, Bulati M, Gallo A, Iannolo G, Pagano D, Conaldi PG. Human Amnion-Derived Mesenchymal Stromal/Stem Cells Pre-Conditioning Inhibits Inflammation and Apoptosis of Immune and Parenchymal Cells in an In Vitro Model of Liver Ischemia/Reperfusion. Cells. 2022;11 doi: 10.3390/cells11040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, Shen Z, Qin W, Lin Z, Huang S. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:43. doi: 10.1038/s41368-021-00150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee SY, Lee JW. 3D Spheroid Cultures of Stem Cells and Exosome Applications for Cartilage Repair. Life (Basel) 2022;12 doi: 10.3390/life12070939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009;17:1799–1803. doi: 10.1038/mt.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.García JR, Quirós M, Han WM, O'Leary MN, Cox GN, Nusrat A, García AJ. IFN-γ-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials. 2019;220:119403. doi: 10.1016/j.biomaterials.2019.119403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bazhanov N, Ylostalo JH, Bartosh TJ, Tiblow A, Mohammadipoor A, Foskett A, Prockop DJ. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Res Ther. 2016;7:27. doi: 10.1186/s13287-016-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bartosh TJ, Ylöstalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2016;7:e2395. doi: 10.1038/cddis.2016.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bhang SH, Lee S, Shin JY, Lee TJ, Kim BS. Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization. Tissue Eng Part A. 2012;18:2138–2147. doi: 10.1089/ten.tea.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee JH, Han YS, Lee SH. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic Activities of Adipose-Derived Mesenchymal Stem Cells. Biomol Ther (Seoul) 2016;24:260–267. doi: 10.4062/biomolther.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu Y, Shi T, Xu A, Zhang L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J Cell Mol Med. 2016;20:1203–1213. doi: 10.1111/jcmm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, Hu R, Wei Q, Shen A, Fu Y, Liu B. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11:206. doi: 10.1186/s13287-020-01719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang W, Liu L, Huo Y, Yang Y, Wang Y. Hypoxia-pretreated human MSCs attenuate acute kidney injury through enhanced angiogenic and antioxidative capacities. Biomed Res Int. 2014;2014:462472. doi: 10.1155/2014/462472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Overath JM, Gauer S, Obermüller N, Schubert R, Schäfer R, Geiger H, Baer PC. Short-term preconditioning enhances the therapeutic potential of adipose-derived stromal/stem cell-conditioned medium in cisplatin-induced acute kidney injury. Exp Cell Res. 2016;342:175–183. doi: 10.1016/j.yexcr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 147.Liu YY, Chiang CH, Hung SC, Chian CF, Tsai CL, Chen WC, Zhang H. Hypoxia-preconditioned mesenchymal stem cells ameliorate ischemia/reperfusion-induced lung injury. PLoS One. 2017;12:e0187637. doi: 10.1371/journal.pone.0187637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li B, Li C, Zhu M, Zhang Y, Du J, Xu Y, Liu B, Gao F, Liu H, Cai J, Yang Y. Hypoxia-Induced Mesenchymal Stromal Cells Exhibit an Enhanced Therapeutic Effect on Radiation-Induced Lung Injury in Mice due to an Increased Proliferation Potential and Enhanced Antioxidant Ability. Cell Physiol Biochem. 2017;44:1295–1310. doi: 10.1159/000485490. [DOI] [PubMed] [Google Scholar]
- 149.Pulido-Escribano V, Torrecillas-Baena B, Camacho-Cardenosa M, Dorado G, Gálvez-Moreno MÁ, Casado-Díaz A. Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J Stem Cells. 2022;14:453–472. doi: 10.4252/wjsc.v14.i7.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 151.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 152.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park H, Park H, Mun D, Kang J, Kim H, Kim M, Cui S, Lee SH, Joung B. Extracellular Vesicles Derived from Hypoxic Human Mesenchymal Stem Cells Attenuate GSK3β Expression via miRNA-26a in an Ischemia-Reperfusion Injury Model. Yonsei Med J. 2018;59:736–745. doi: 10.3349/ymj.2018.59.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, Shen H, Zhang GG, Bai YP. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.You Y, Kobayashi K, Colak B, Luo P, Cozens E, Fields L, Suzuki K, Gautrot J. Engineered cell-degradable poly(2-alkyl-2-oxazoline) hydrogel for epicardial placement of mesenchymal stem cells for myocardial repair. Biomaterials. 2021;269:120356. doi: 10.1016/j.biomaterials.2020.120356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang CC, Chen CH, Hwang SM, Lin WW, Huang CH, Lee WY, Chang Y, Sung HW. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- 157.Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 2013;124:165–176. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- 159.Zhilai Z, Biling M, Sujun Q, Chao D, Benchao S, Shuai H, Shun Y, Hui Z. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–435. doi: 10.1016/j.brainres.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 160.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 161.Yoon BS, Moon JH, Jun EK, Kim J, Maeng I, Kim JS, Lee JH, Baik CS, Kim A, Cho KS, Lee HH, Whang KY, You S. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 162.Du L, Lv R, Yang X, Cheng S, Ma T, Xu J. Hypoxic conditioned medium of placenta-derived mesenchymal stem cells protects against scar formation. Life Sci. 2016;149:51–57. doi: 10.1016/j.lfs.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 163.Lee SC, Jeong HJ, Lee SK, Kim SJ. Hypoxic Conditioned Medium From Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration Through JAK/STAT3 Signaling. Stem Cells Transl Med. 2016;5:816–825. doi: 10.5966/sctm.2015-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, Zhang M, He J, Zheng S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013;4:83. doi: 10.1186/scrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121, 2121.e1. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee SK, Lee SC, Kim SJ. A novel cell-free strategy for promoting mouse liver regeneration: utilization of a conditioned medium from adipose-derived stem cells. Hepatol Int. 2015;9:310–320. doi: 10.1007/s12072-014-9599-4. [DOI] [PubMed] [Google Scholar]
- 167.Kuang R, Zhang Z, Jin X, Hu J, Shi S, Ni L, Ma PX. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016;33:225–234. doi: 10.1016/j.actbio.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jun EK, Zhang Q, Yoon BS, Moon JH, Lee G, Park G, Kang PJ, Lee JH, Kim A, You S. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci. 2014;15:605–628. doi: 10.3390/ijms15010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Muttigi MS, Kim BJ, Kumar H, Park S, Choi UY, Han I, Park H, Lee SH. Efficacy of matrilin-3-primed adipose-derived mesenchymal stem cell spheroids in a rabbit model of disc degeneration. Stem Cell Res Ther. 2020;11:363. doi: 10.1186/s13287-020-01862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Suenaga H, Furukawa KS, Suzuki Y, Takato T, Ushida T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J Mater Sci Mater Med. 2015;26:254. doi: 10.1007/s10856-015-5591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Suzuki S, Muneta T, Tsuji K, Ichinose S, Makino H, Umezawa A, Sekiya I. Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration. Arthritis Res Ther. 2012;14:R136. doi: 10.1186/ar3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cai S, Fan C, Xie L, Zhong H, Li A, Lv S, Liao M, Yang X, Su X, Wang Y, Wang H, Wang M, Huang P, Liu Y, Wang T, Zhong Y, Ma L. Single-cell RNA sequencing reveals the potential mechanism of heterogeneity of immunomodulatory properties of foreskin and umbilical cord mesenchymal stromal cells. Cell Biosci. 2022;12:115. doi: 10.1186/s13578-022-00848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Szabó E, Fajka-Boja R, Kriston-Pál É, Hornung Á, Makra I, Kudlik G, Uher F, Katona RL, Monostori É, Czibula Á. Licensing by Inflammatory Cytokines Abolishes Heterogeneity of Immunosuppressive Function of Mesenchymal Stem Cell Population. Stem Cells Dev. 2015;24:2171–2180. doi: 10.1089/scd.2014.0581. [DOI] [PubMed] [Google Scholar]
- 174.Granata OM, Cocciadiferro L, Miceli V, Polito LM, Campisi I, Carruba G. Metabolic profiles of androgens in malignant human liver cell lines. Ann N Y Acad Sci. 2006;1089:262–267. doi: 10.1196/annals.1386.028. [DOI] [PubMed] [Google Scholar]
- 175.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529:307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–C682. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]
- 177.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- 178.Li X, Wang Q, Ding L, Wang YX, Zhao ZD, Mao N, Wu CT, Wang H, Zhu H, Ning SB. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res Ther. 2019;10:267. doi: 10.1186/s13287-019-1384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liang X, Arullampalam P, Yang Z, Ming XF. Hypoxia Enhances Endothelial Intercellular Adhesion Molecule 1 Protein Level Through Upregulation of Arginase Type II and Mitochondrial Oxidative Stress. Front Physiol. 2019;10:1003. doi: 10.3389/fphys.2019.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc. 2009;41:2607–2611. doi: 10.1016/j.transproceed.2009.06.119. [DOI] [PubMed] [Google Scholar]
- 181.Paquet J, Deschepper M, Moya A, Logeart-Avramoglou D, Boisson-Vidal C, Petite H. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl Med. 2015;4:809–821. doi: 10.5966/sctm.2014-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol. 2010;7:182–189. doi: 10.1038/cmi.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yao Y, Xu XH, Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cassano JM, Schnabel LV, Goodale MB, Fortier LA. Inflammatory licensed equine MSCs are chondroprotective and exhibit enhanced immunomodulation in an inflammatory environment. Stem Cell Res Ther. 2018;9:82. doi: 10.1186/s13287-018-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]