Abstract
In 2011, Ehrlichia muris eauclairensis (EME) was described as a human pathogen spread by the blacklegged tick, Ixodes scapularis. Until very recently, its reported distribution was limited to the upper midwestern United States, mainly in Minnesota and Wisconsin. In this study, we report the detection of EME DNA in 4 of 16,146 human biting I. scapularis ticks submitted from Massachusetts to a passive tick surveillance program. Active tick surveillance yielded evidence of EME local transmission in the northeastern United States through detection of EME DNA in 2 of 461 host-seeking I. scapularis nymphs, and in 2 white-footed mice (Peromyscus leucopus) of 491 rodent samples collected in the National Ecological Observatory Network (NEON) Harvard Forest site in Massachusetts.
Keywords: Ehrlichia muris eauclairensis, Ixodes scapularis, Peromyscus leucopus, Massachusetts
Introduction
Ehrlichia spp. are the causative agents of ehrlichiosis. They are obligatory intracellular bacteria that are transmitted to vertebrates by ticks (Dumler et al., 2001). To date, six Ehrlichia species (E. canis, E. chaffeensis, E. ewingii, E. minasensis, E. muris, and E. ruminantium), and several Candidatus Ehrlichia species have been discovered (Aguiar et al., 2019; Pritt et al., 2017). Among them, E. canis, E. chaffeensis, E. ewingii, and E. muris are known to infect humans. In the United States, E. chaffeensis and E. ewingii are human pathogens transmitted by Amblyomma americanum ticks; E. muris is a human pathogen transmitted by Ixodes scapularis ticks in the upper midwestern United States (Pritt et al., 2017). An isolation of the “Panola Mountain Ehrlichia” has been associated with a human case of illness after the bite of a nymphal A. americanum from the southern United States (Loftis et al., 2008).
The type strain AS145 of E. muris was originally isolated from a mouse in Japan (Wen et al., 1995). In 2009, an E. muris-like agent (EMLA) was identified as a causative agent of human ehrlichiosis in Wisconsin and Minnesota (Pritt et al., 2011). In 2017, the Japanese isolate AS145 was proposed as E. muris subsp. muris, which is transmitted by Haemaphysalis flava ticks in Japan and Ixodes persulcatus ticks in eastern Europe. The EMLA isolates from the upper midwestern United States were proposed as a taxonomically distinct subspecies, E. muris eauclairensis (EME), which is associated with I. scapularis ticks in Minnesota and Wisconsin (Pritt et al., 2017). Previously, we reported the detection of a novel clade of E. muris from Ixodes cookei in the northeastern United States (Xu et al., 2018).
Despite the broad distribution of I. scapularis ticks in the United States, EME has historically been limited to the upper midwestern region of the United States. Heretofore, EME has been reported in white-footed mice (Peromyscus leucopus) (Castillo et al., 2015) and I. scapularis ticks in only three states: Wisconsin, Minnesota, and Michigan (Fleshman et al., 2022). In humans, 3 Wisconsin residents and 1 Minnesota resident out of 4247 people in 45 states tested positive for EME (Pritt et al., 2011). This study identified EME in I. scapularis ticks and white-footed mice (P. leucopus) in Massachusetts, indicating the presence of an enzootic transmission cycle of EME local to New England.
Materials and Methods
Collection and identification of ticks and rodents
From June 2014 to November 2020, 16,146 I. scapularis nymphal and adult ticks were submitted to the TickReport public testing program (https://www.tickreport.com) from Massachusetts residents and tested for E. muris (Table 1 and Map in Supplementary Data). I. scapularis ticks were first identified at the genus-level using morphologic keys, and then were differentiated from other species by a species-specific TaqMan PCR assay or by amplifying and sequencing a fragment of the tick 16S rRNA gene (Xu et al., 2019).
Table 1.
Ehrlichia muris eauclairensis Samples Collected in Massachusetts
| Positive sample sites in Massachusetts | Organism (tick/mouse) | Life stage | Sample size | EME positives | DNA sequences |
|---|---|---|---|---|---|
| Hardwick, Palmer, Oakham, and Petersham | Ixodes scapularis | Adult female | 16,146 | 4 (0.025%) | gltA and groEL genes |
| NEON Harvard Forest | I. scapularis | Nymph | 461 | 2 (0.434%) | groEL and rrs-IGS genes |
| NEON Harvard Forest | Peromyscus leucopus | — | 491 | 2 (0.407%) | gltA and groEL genes |
EME, Ehrlichia muris eauclairensis; NEON, National Ecological Observatory Network.
From 2015 to 2021, 461 nymphal I. scapularis ticks were tested for pathogens of the 649 total that were collected from the National Ecological Observatory Network (NEON) Harvard Forest site in Massachusetts (NEON, 2021b; NEON, 2021c), by a tick-dragging method (Table 1). A 1 square meter white cloth was dragged along the ground at a slow pace around the 160 meters perimeter of each of six sampling plots (40 × 40 meter) each year. The tick species of NEON samples were identified by a morphological characterization method at the U.S. National Tick Collection, which also archives the ticks not sent for pathogen testing.
From 2020 to 2021, 491 blood and ear samples from various rodent species were also tested for tick-borne pathogens (NEON, 2021a). These samples were collected as part of the mark-recapture effort for small mammals from the Harvard Forest & Quabbin Watershed NEON site, using Sherman live traps (Table 1). The 11,900-acre Harvard Forest site is located ∼105 km west of Boston, Massachusetts in the county of Worcester. The site is dominated by northern hardwood and coniferous forest, with some areas used for agriculture and represents a typical rural northeastern wildland, linking suburban areas outside Boston with the wildlands throughout New England (https://www.neonscience.org/field-sites/harv).
DNA extraction and E. muris molecular identification
Tick and rodent samples were first sorted into individual tubes. The total nucleic acids were extracted from each sample using the Lucigen Masterpure Complete DNA and RNA Purification kit (Lucigen Corporation, Middleton, WI) following the manufacturer's protocols. A Taqman real-time PCR targeting P13 gene was used for E. muris screening (Xu et al., 2018). The assay was performed in 16-μL reaction volumes using the Brilliant III qPCR Master Mix (Agilent, La Jolla, CA) in a CFX96 Touch Real-Time PCR Detection System. The reaction contained 8-μL Master Mix, 200 nM forward primer (TACCTAATTCTTCTCAAGAGATTCAGTTG), 200 nM reverse primer (ATGATGATACTGCGAACAACTATAAGAG), 200 nM dual-labeled probe (Cy5-ATATTGATAAAAGAGTCAGTGTTGATCCGTATGAGTTAGGGTT-BHQ), 1-μL template DNA, and water up to 16 μL.
An internal control was used for checking tick DNA quality. Cycling conditions included an initial activation of the Taq DNA polymerase at 95°C for 10 min, followed by 40 cycles: 95°C for 15 s and 60°C for 1 min. E. muris positivity of the samples was confirmed by amplifying and sequencing the citrate synthase (gltA) and heat shock protein (groEL) genes (Telford et al., 2011). DNA from EME positive tick samples (N = 2, I. scapularis nymphs) collected from the NEON Harvard Forest site through drag sampling were sent to CDC, Fort Collins, for verification and additional testing. A previously described multiplex PCR amplicon assay was utilized for testing and verification, consisting of two primer sets 859–860 (groEL), and 2149–2150 (rrs-IGS) (Hojgaard et al., 2022).
Ethical statement
All animal protocols have been approved by Battelle's Institutional Animal Care and Use Committee (IACUC).
Results
Four E. muris positive adults were identified from 16,146 I. scapularis ticks (0.025%) submitted to TickReport from Massachusetts from June 2014 to November 2020. The first E. muris positive tick was submitted from Hardwick, Massachusetts in 2017. Three more E. muris positive ticks were later submitted from Palmer, Oakham, and Petersham, Massachusetts in 2019 and 2020. Surprisingly, the gltA and groEL gene sequences (Supplementary Data) of these samples were identical to EME and all four ticks were adult female I. scapularis collected from people residing in Massachusetts without a travel history outside of Massachusetts.
From 2015 to 2021, 461 nymphal I. scapularis ticks collected from the NEON Harvard Forest site in Massachusetts were tested for E. muris. Two nymphs (0.434%, 1 in 2017 and 1 in 2019) were positive for E. muris (NEON, 2021b) using both the Taqman PCR and NGS sequencing assays described earlier. The E. muris groEL and rrs-IGS sequences from these two samples were identical to each other, as well as to the EME control sample (Supplementary Data). In addition to tick samples, 2 P. leucopus blood samples (0.407%) were positive for E. muris of the 491 rodent blood or ear samples collected from the same site in 2020 and 2021 (NEON, 2021a). EME may have different abundances in blood and tissues. All obtained E. muris sequences of the gltA and groEL genes from ticks and rodents were identical to each other, as well as to EME.
Discussion
Since its discovery in 2009 (Pritt et al., 2011), EME has been thought to be localized in the upper midwestern United States. It has been detected in I. scapularis ticks, strictly in Minnesota (Johnson et al., 2018), Wisconsin (Pritt et al., 2011; Telford et al., 2011), and Michigan (Fleshman et al., 2022). EME positive samples were also detected in P. leucopus mice from Minnesota and Wisconsin (Castillo et al., 2015) and human blood from five states: Indiana, Michigan, Minnesota, North Dakota, and Wisconsin (Dahlgren et al., 2016).
This study identified EME in two nymphal I. scapularis ticks collected from the NEON Harvard Forest site in Massachusetts and four adult I. scapularis ticks from people residing in Massachusetts without a travel history. More importantly, two P. leucopus mice collected from the NEON Harvard Forest site were also positive for EME. These data greatly expand the known geographic distribution of the pathogen, indicating that the number of states reporting EME prevalence might be underestimated. Similar to previous studies in the Upper Midwest (Castillo et al., 2015; Johnson et al., 2018; Pritt et al., 2011), I. scapularis ticks and P. leucopus mice also appear to be important in maintaining the enzootic cycle of EME in Massachusetts.
In general, the prevalence of EME in ticks appears to be low. Only two (0.026%) EME positives were found in 7800 human-biting I. scapularis ticks from 33 states in the northeastern, midwestern, and southeastern regions (Xu et al., 2018). However, the reported infection rate in the upper midwestern United States was higher: EME was detected in 17 of 697 (2.4%) I. scapularis ticks collected in Wisconsin and Minnesota (Pritt et al., 2011), and 2 of 36 (5.6%) adult ticks in Wisconsin (Stauffer et al., 2020). The prevalence of EMLA infection was 0.94% from 760 I. scapularis adult ticks collected in Wisconsin between 1992 and 1997 (Telford et al., 2011), and 4.6% of 196 I. scapularis ticks removed from soldiers in Wisconsin and 0.5% of 365 ticks in Minnesota for a 15-year period (Stromdahl et al., 2014).
Johnson et al. (2018) reported EME in 13 of 64 sampling sites throughout Minnesota with an estimated nymphal infection prevalence rate of 1.29% (95% CI: 0.77–2.04). Blood analysis of 75,007 patients collected from 2004 to 2013 from 50 states found that 69 patients (0.1%) were positive for EME (Dahlgren et al., 2016). Unlike Lyme disease agents in I. scapularis ticks, the prevalence of EME is also low in Massachusetts. Only four EME (0.025%) positives were found from 16,146 I. scapularis ticks submitted from Massachusetts. In the NEON Harvard forest site, the prevalence of EME was 0.434% in nymphal I. scapularis ticks and 0.407% in rodents.
Although a mouse model for ehrlichiosis caused by EME has been developed (Saito et al., 2015), the natural animal reservoir of this pathogen is largely unknown and probably involves small rodents, such as Microtus agrestis and Myodes glareolus in Western Siberia and Eothenomys kageus in Japan. The white-footed mouse, P. leucopus, is considered an important host for nymphal I. scapularis and a reservoir of EME (Pritt et al., 2011). Interestingly, the woodland deer mouse, Peromyscus maniculatus, was likely responsible for feeding and infecting more ticks with pathogens (including EME) than P. leucopus in the upper midwestern United States (Larson et al., 2021).
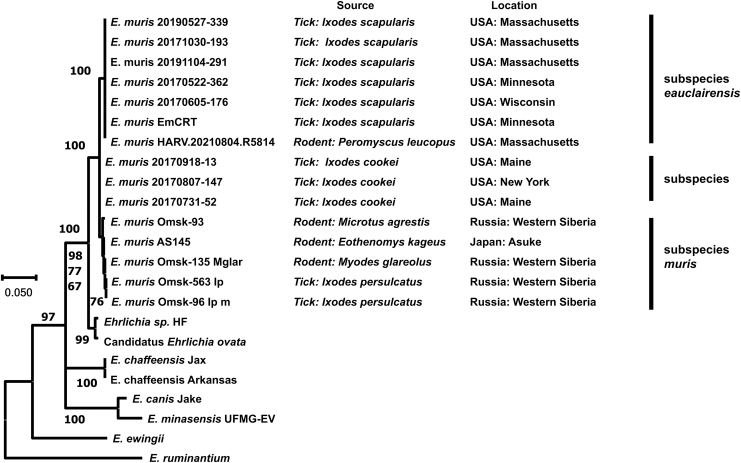
Although P. maniculatus has been found in Berkshire, Franklin, and Hampshire counties in Massachusetts, the reservoir competence of P. maniculatus to EME is unknown in this region. Moreover, there is a distinct E. muris clade in I. cookei ticks in the northeastern United States (Xu et al., 2018). Because I. scapularis and I. cookei may feed on the same host species, the transmission cycle of two different E. muris subspecies can be complicated and overlapping. Further studies are needed to understand the importance of Ixodes species ticks as a vector and Peromyscus species as a reservoir of E. muris maintenance and transmission in the northeastern United States (Fig. 1).
FIG. 1.
Phylogenetic tree of Ehrlichia citrate synthase (gltA) and heat shock protein (groEL) genes constructed by the maximum-likelihood method of MEGA11 software. The total length of two concatenated genes is 1045 bp. Hasegawa–Kishino–Yano with invariable sites was selected as the best model based on Bayesian information criterion scores. Numbers on the branches represent bootstrap support with 500 bootstrap replicates. Scale bar indicates nucleotide substitutions per site.
It has been suggested that EME possibly evolved in midwestern I. scapularis populations that were geographically isolated until very recently from populations in the northeastern and southeastern United States during the Pleistocene glacial period (Fleshman et al., 2022). The finding of EME in the northeastern I. scapularis populations indicates three possibilities: (1) EME first evolved in the midwestern region, then was very recently transmitted from the midwestern to the northeastern United States; (2) EME was also present in the northeastern I. scapularis populations and was not detected until this study due to the low pathogen prevalence and lack of large-scale surveillance; or (3) EME was present at low prevalence at the time the midwestern and northeastern populations were geographically isolated and it has evolved in these populations, but went largely undetected due to limited testing with specific molecular detection assays.
A retrospective analysis on Ixodes ticks and human samples from Massachusetts may help us to better understand the transmission history and rate of spread of EME in New England. Although >115 ehrlichiosis cases caused by EME have been identified in patients in the Upper Midwest since 2009 (https://www.cdc.gov/ehrlichiosis/stats/index.html), no EME-associated cases of human ehrlichiosis have been reported in Massachusetts. The results of this study indicate that the known presence and prevalence of EME might be underestimated. In 1994, an ehrlichiosis case and a substantial frequency of seropositivity for Ehrlichia were reported among residents on Cape Cod (Rynkiewicz and Liu, 1994), where A. americanum populations were not established until the 2010s (Telford et al., 2019). Alternatively, this ehrlichiosis case may relate to Anaplasma phagocytophilum due to cross-reactivity among antigens of Ehrlichia and Anaplasma.
We found identical DNA sequences of EME samples in Massachusetts and the upper midwestern. However, little is known about the possible linkage between the low genetic diversity and EME's pathogenicity and host specificity. Further study is warranted to better understand the vector competence, the natural enzootic cycle, and the ecological niche of EME. The potential range in New England should also be monitored. Most importantly, human ehrlichiosis should be considered as a possible diagnosis for tick bite victims in New England.
Supplementary Material
Acknowledgments
The authors thank Dr. Allison Snow and Eric Siegel for helpful comments on earlier versions of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the CDC. The National Ecological Observatory Network is a program sponsored by the National Science Foundation and operated under cooperative agreement by Battelle. This material is based in part on study supported by the National Science Foundation through the NEON Program.
Author Disclosure Statement
Data presented in this study were collected while TickReport was offered as a service of the University of Massachusetts Amherst. TickReport service is now offered by MedZu, Inc. (Amherst, MA) under a licensing agreement with the University of Massachusetts, Amherst. S.M.R. and G.X. are the principal owners of MedZu, Inc., as recognized by their respective conflicts management plans through the University of Massachusetts Amherst. No conflicting financial interests exist for the other authors.
Funding Information
This study was partially supported by the Centers for Disease Control (CDC) New England Center of Excellence in Vector-borne Disease (CDC award U01CK000661).
Supplementary Material
References
- Aguiar DM, Araujo JP Jr., Nakazato L, et al. Complete genome sequence of an Ehrlichia minasensis strain isolated from cattle. Microbiol Resour Announc 2019;8(15):e00161-19; doi: 10.1128/MRA.00161-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo CG, Eremeeva ME, Paskewitz SM, et al. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus. Ticks Tick Borne Dis 2015;6(2):155–157; doi: 10.1016/j.ttbdis.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Dahlgren FS, Heitman KN, Behravesh CB. Undetermined human Ehrlichiosis and Anaplasmosis in the United States, 2008–2012: A catch-all for passive surveillance. Am J Trop Med Hyg 2016;94(2):299–301; doi: 10.4269/ajtmh.15-0691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 2001;51(Pt 6):2145–2165; doi: 10.1099/00207713-51-6-2145 [DOI] [PubMed] [Google Scholar]
- Fleshman AC, Foster E, Maes SE, et al. Reported county-level distribution of seven human pathogens detected in host-seeking Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J Med Entomol 2022;59(4):1328–1335; doi: 10.1093/jme/tjac049 [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Osikowicz LM, Rizzo MF, et al. Using next generation sequencing for molecular detection and differentiation of Anaplasma phagocytophilum variants from host seeking Ixodes scapularis ticks in the United States. Ticks Tick Borne Dis 2022;13(6):102041; doi: 10.1016/j.ttbdis.2022.102041 [DOI] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis 2018;9(6):1499–1507; doi: 10.1016/j.ttbdis.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RT, Bron GM, Lee X, et al. Peromyscus maniculatus (Rodentia: Cricetidae): An overlooked reservoir of tick-borne pathogens in the Midwest, USA? Ecosphere 2021;12(11):e03831; doi: 10.1002/ecs2.3831 [DOI] [Google Scholar]
- Loftis AD, Mixson TR, Stromdahl EY, et al. Geographic distribution and genetic diversity of the Ehrlichia sp. from Panola Mountain in Amblyomma americanum. BMC Infect Dis 2008;8:54; doi: 10.1186/1471-2334-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Ecological Observatory Network (NEON). Rodent pathogen status, tick-borne (DP1.10064.002), RELEASE-2022. 2021a; doi: 10.48443/6pfn-t955. Available from: https://data.neonscience.org [Last accessed: November 16, 2022]. [DOI]
- National Ecological Observatory Network (NEON). Tick pathogen status (DP1.10092.001), RELEASE-2022. 2021b; doi: 10.48443/nygx-dm71. Available from: https://data.neonscience.org [Last accessed: November 16, 2022]. [DOI]
- National Ecological Observatory Network (NEON). Ticks sampled using drag cloths (DP1.10093.001), RELEASE-2022. 2021c; doi: 10.48443/7jh5-8s51. Available from: https://data.neonscience.org [November 16, 2022]. [DOI]
- Pritt BS, Allerdice MEJ, Sloan LM, et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol 2017;67(7):2121–2126; doi: 10.1099/ijsem.0.001896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med 2011;365(5):422–429; doi: 10.1056/NEJMoa1010493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz DL, Liu LX. Human ehrlichiosis in New England. N Engl J Med 1994;330(4):292–293; doi: 10.1056/NEJM199401273300418 [DOI] [PubMed] [Google Scholar]
- Saito TB, Thirumalapura NR, Shelite TR, et al. An animal model of a newly emerging human ehrlichiosis. J Infect Dis 2015;211(3):452–461; doi: 10.1093/infdis/jiu372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer MT, Mandli J, Pritt BS, et al. Detection of zoonotic human pathogens from Ixodes scapularis in Wisconsin. J Vector Ecol 2020;45(1):147–149; doi: 10.1111/jvec.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromdahl E, Hamer S, Jenkins S, et al. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and the northeastern United States over a 15 year period (1997–2012). Parasit Vectors 2014;7:553; doi: 10.1186/s13071-014-0553-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, 3rd, Buchthal J, Elias P. Early questing by lone star tick larvae, New York and Massachusetts, USA, 2018. Emerg Infect Dis 2019;25(8):1592–1593; doi: 10.3201/eid2508.181293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, 3rd, Goethert HK, Cunningham JA. Prevalence of Ehrlichia muris in Wisconsin Deer ticks collected during the mid 1990s. Open Microbiol J 2011;5:18–20; doi: 10.2174/1874285801105010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen B, Rikihisa Y, Mott J, et al. Ehrlichia muris sp. nov., identified on the basis of 16S rRNA base sequences and serological, morphological, and biological characteristics. Int J Syst Bacteriol 1995;45(2):250–254; doi: 10.1099/00207713-45-2-250 [DOI] [PubMed] [Google Scholar]
- Xu G, Pearson P, Dykstra E, et al. Human-biting Ixodes ticks and pathogen prevalence from California, Oregon, and Washington. Vector Borne Zoonotic Dis 2019;19(2):106–114; doi: 10.1089/vbz.2018-2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Pearson P, Rich SM. Ehrlichia muris in Ixodes cookei ticks, Northeastern United States, 2016–2017. Emerg Infect Dis 2018;24(6):1143–1144; doi: 10.3201/eid2406.171755 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.