Abstract
Background:
Bartonella ancashensis is a recently described Bartonella species endemic to Peru, where it causes verruga peruana in humans. While the arthropod vector of B. ancashensis transmission is unknown, human coinfections with Bartonella bacilliformis suggest that phlebotomine sand flies are a vector.
Materials and Methods:
To address the hypothesis that sand flies are involved in the bacterium's transmission, Lutzomyia longipalpis sand flies were used as an infection model, together with green fluorescent protein-expressing B. ancashensis.
Results:
Results showed that bacterial infections were clearly established, limited to the anterior midgut of the female fly, and maintained for roughly 7 days. At 3–7 days postinfection, a prominent microcolony of aggregated bacteria was observed in the anterior midgut, immediately distal to the stomodeal valve of the esophagus. In contrast, eggs, diuretic fluid, feces, and other tissues were not infected.
Conclusion:
These results suggest that certain sand fly species within the endemic zone for B. ancashensis may play a role in the bacterium's maintenance and possibly in its transmission to humans.
Keywords: Bartonella ancashensis, sand fly, infection model, arthropod vector, Lutzomyia longipalpis
Introduction
Bartonella ancashensis is a gram-negative alphaproteobacterium, first isolated from two children in 2003 during a drug trial screening of 127 verruga peruana patients in Caraz, Ancash, Peru (Blazes et al., 2013; Mullins et al., 2015; Mullins et al., 2013). Genomic sequencing, multilocus sequence typing, and intergenic spacer typing indicated that the species was novel, with its closest relative being the gram-negative alphaproteobacterium, Bartonella bacilliformis (Mullins et al., 2015). Before this discovery, verruga peruana was presumed to be a chronic manifestation of Carrión's disease; a sand fly-vectored human illness caused by a B. bacilliformis infection, that is endemic to South America.
Carrión's disease manifests as Oroya fever; a potentially life-threatening hematic syndrome involving acute hemolytic anemia, and/or verruga peruana; a chronic infection of vascular endothelial cells characterized by nonlife-threatening hemangiomas of the skin and bacteremia (Minnick et al., 2014). While the geographic distribution of B. ancashensis overlaps that of B. bacilliformis, the bacterium has not been isolated from patients with Oroya fever, to date. As such, B. ancashensis is thought to be less virulent (Blazes et al., 2013; Mullins et al., 2017; Mullins et al., 2015; Mullins et al., 2013). The apparent overlap in geographic ranges between these two pathogens, similarities in their chronic pathological manifestations during infections, and possible coinfections involving the two bacteria (Mullins et al., 2017) obfuscates our understanding of the epidemiology of Carrión's disease.
This situation also complicates the proper diagnosis and treatment of Carrión's disease, which is currently based on the patient's symptoms, blood smears, and blood cultures (Ellis et al., 1999). We hypothesize that, such as B. bacilliformis, B. ancashensis transmission involves a sand fly vector. To investigate this, we used a Lutzomyia longipalpis sand fly infection model to follow tissue colonization by green fluorescent protein (GFP)-expressing B. ancashensis in vitro over time.
Materials and Methods
Ethics statement
The Institutional Biosafety Committee and Institutional Review Board at the University of Montana granted approval for the experimental use of B. ancashensis, L. longipalpis, and human blood (IBC 2022-002; IRB 120-20). Formal consent was obtained from the blood donor (coauthor MFM).
Bacterial culture
B. ancashensis type strain 20.00 (Mullins et al., 2015) was cultivated on HIBB medium (Bacto heart infusion agar [Becton Dickinson; Franklin Lakes, NJ] supplemented with 4% defibrinated sheep blood and 2% sheep serum [Quad-Five, Ryegate, MT] by volume). Cultures were routinely grown for 4–5 days at 30°C with 5% CO2. HIBB plates were supplemented with 25 μg/mL kanamycin (HIBB-kan25) to cultivate B. ancashensis transformed with pJMB-GFP. When needed, B. ancashensis was freshly harvested from HIBB plates using a sterile razor blade, as previously described (Battisti and Minnick, 2008), and suspended in 300 μL ice-cold, sterile physiological saline (0.9% NaCl; w/v) per plate.
Generation of GFP-expressing B. ancashensis
B. ancashensis (type strain 20.00) was transformed with pJMB-GFP by electroporation, as previously described for B. bacilliformis (Battisti and Minnick, 1999). Positive clones were identified by kanamycin resistance on HIBB-kan25, pJMB-GFP plasmid content, and an intense GFP signal when samples of bacterial colonies were observed by ultraviolet (UV) fluorescence microscopy.
Sand fly handling and maintenance
L. longipalpis (LLJB, Brazil; L3/pupae, NR-44001) was obtained from BEI Resources (Manassas, VA). Adults were released daily from commercial larval “pots” into 30.5 cm3 plexiglass holding cages (21st Century Plastics, Missoula, MT) until the pots were depleted of adults. Holding cages were maintained in a growth chamber (Percival Model 136NL) at 25°C, 100% relative humidity, in total darkness. All work was done in a dedicated and secure insectary with air filters and negative ventilation. Flies were fed ad libitum on sterile, 30% sucrose-water in moist cotton balls placed on the tops of the cages and were replaced every 48 h. Sand flies were allowed to mature for ≥10 days before artificial bloodmeal feedings.
Artificial bloodmeal feedings
Fresh human blood (6 mL) was collected by venipuncture into anticoagulant acid-citrate dextrose solution B tubes (BD Vacutainer 364816; Becton Dickinson). After gentle mixing, blood cells were centrifuged (1000 × g for 5 min at 4°C), and the plasma supernatant aseptically drawn off and discarded. Blood cells were subsequently washed three times by gently mixing the pellet in 4 mL ice-cold, sterile physiological saline, followed by centrifugation (1000 × g for 5 min at 4°C). The final cell pellet was brought to 6 mL with physiological saline and used immediately or was refrigerated overnight at 10°C before use.
Artificial bloodmeals were prepared by combining freshly-harvested B. ancashensis cells and washed erythrocytes (∼1 × 109 cells of each type) to yield a multiplicity of infection (MOI) of 1.0. Cell counts were performed using a hemocytometer for erythrocytes, and by counting viable (GFP+) bacteria in 10, 0.22-mm fields on three slides using UV fluorescence microscopy and a fluorescein isothiocyanate (FITC) filter (1000 × magnification). The number of bacteria per mL was calculated using the average number of bacteria per field times the dilution factor times a conversion factor of 1.273 × 106. The bloodmeal mix was incubated for 4 h at 37°C, before sand fly feeding, to allow for equilibration and infection of erythrocytes by bacteria.
Artificial bloodmeal feedings were performed using a 14 mm glass mosquito feeder (Chemglass Life Sciences, Vineland, NJ) overlaid with the defeathered skin of a 1-day-old frozen chick (Layne Laboratories, Arroyo Grande, CA), as previously described (Battisti et al., 2015). The feeder was connected to a constantly circulating water bath to maintain the bloodmeal at 39°C. The center vestibule of the feeder was filled with the bloodmeal mixture, while the outer vestibule contained circulating warm water. The glass feeder (chick skin facing downwards) was placed on top of a fly feeding cup that was covered with tulle cloth, containing 50–100 sand flies transferred from the holding cage. A damp sponge and aluminum foil were placed around the entire feeder and cup, and feeding occurred for 60 min in the dark at 25°C.
Afterward, two cotton balls soaked with 10% sucrose water plus kanamycin (40 μg/mL) were provided for feeding the flies ad libitum. Infected insects were maintained in feeding cups in the growth chamber for up to 9 days with cotton balls replaced at 48 h intervals.
Quantification of B. ancashensis over the course of infection
Alimentary tracts of infected sand flies were aseptically isolated daily for 7 days postfeeding using a dissecting scope and sterile microtools (i.e., insect needles embedded in wooden applicator sticks). Individual abdominal midguts were suspended in 50 μL sterile physiological saline and macerated in 1.5 mL microcentrifuge tubes using a pestle and vortexer. Resulting cell suspensions were 10-fold serially diluted with heart infusion broth, vortexed, then spread-plated onto HIBB-kan25 plates. Colony counts were performed visually after incubating for 10 days at 30°C with 5% CO2. A GFP+ phenotype, by fluorescence microscopy, was used to verify that the colonies were B. ancashensis.
Feces and diuretic fluid sampling from infected sand flies
Three randomly selected dried fecal piles and diuretic fluids were collected from feeder cups at 6 days postinfection using 10 μL sterile physiological saline and a micropipettor, then cultured for 7 days on HIBB-kan25 plates, as previously described (Battisti et al., 2015). Three additional samples of each type were collected and observed by UV fluorescence microscopy.
Imaging
Microscopic imaging was done using a BX31 UV fluorescence microscope (Olympus; Waltham, MA) and a FITC filter. Images in results are representative of at least three different insects per time point postinfection, from five separate bloodmeal feedings, and were captured using a DP74 microscope digital camera (Olympus) and cellSens imaging software version 3.1 (Olympus).
Limitations of the study
A colony of a bona fide sand fly vector for B. bacilliformis (e.g., Lutzomyia verrucarum) was not available at the time of the study, thus we used a model organism (Lutzomyia longipalpis). Uninfected sand flies were not examined as a control in this study.
Results
Microscopic examination of L. longipalpis colonization by B. ancashensis
To examine the possibility that sand flies could maintain B. ancashensis, we artificially infected L. longipalpis using fresh, washed human blood spiked with a GFP+ strain of the bacterium at an MOI of 1.0. Bacterial colonization of sand flies was followed over time by observing various tissues by UV fluorescence microscopy until no apparent infection was observed in any of the sampled insects. Overall, the results of these experiments showed that L. longipalpis remained infected with viable (GFP+) bacteria for up to 7 days, and the infections were restricted to the abdominal midgut.
At 1 day postinfection, female sand flies that had taken a bloodmeal were easily identified, as they appeared larger, darker, and had a prominent reddish-colored abdomen (Supplementary Fig. S1). Dissections and UV microscopy were not conducted at this time point to allow the peritrophic membrane (PM) to fully develop around the bloodmeal.
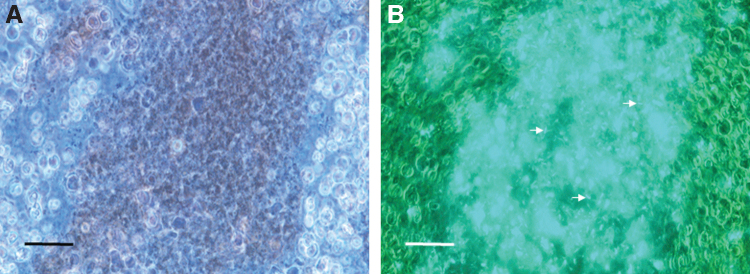
At 2 days postinfection, the PM was well formed and provided for easy dissection and removal of the entire alimentary tract from the sand flies. When observed microscopically, the entire anterior midgut was filled with intact human erythrocytes and GFP+ B. ancashensis (Fig. 1). However, no other tissues were found to contain bacteria (not shown).
FIG. 1.
Micrographs of sand fly anterior midgut contents at 2 days postfeeding. (A) Bloodmeal showing human erythrocytes by phase-contrast microscopy. (B) Corresponding fluorescence micrograph revealing GFP+ Bartonella ancashensis cells (examples are arrowed) in the dark-colored, central area of erythrocytes (1000 × magnification; scale bars = 35 μm). GFP, green fluorescent protein.
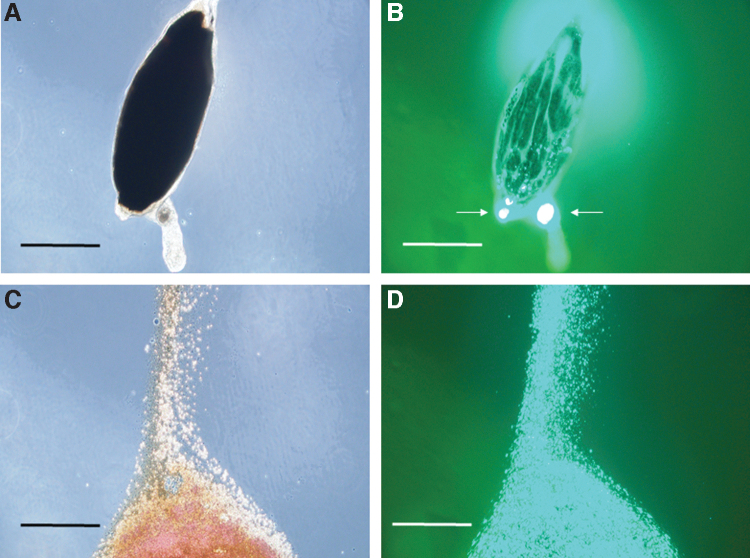
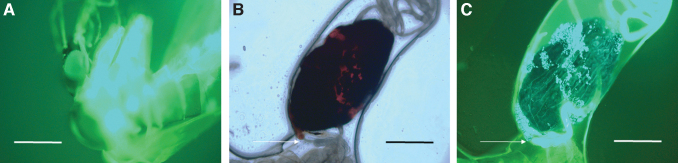
By 3 days postinfection, a prominent bacterial aggregation (microcolony) appeared at the anterior end of the abdominal midgut, immediately distal to the stomodeal valve (SV) of the esophagus, in all insects observed (Fig. 2). In a few instances, a small number of GFP+ bacteria had apparently escaped the PM and adhered to its exterior (Fig. 2B). The bloodmeal content of the anterior midgut from these insects was indistinguishable from flies observed at 2 days and consisted of a high concentration of erythrocytes and GFP+ B. ancashensis (Fig. 2C, D). As on day 2, GFP+ bacteria were confined to the anterior midgut of the insect and were not detectable in the head, thorax, or thoracic midgut (Supplementary Fig. S2).
FIG. 2.
Micrographs of an isolated sand fly anterior midgut at 3 days postfeeding. (A) The bloodmeal is surrounded by a well-formed PM and is a dark, rusty color by phase-contrast microscopy. (B) Corresponding fluorescence micrograph revealing GFP+ B. ancashensis cells, forming a microcolony at the anterior end of the anterior midgut below the SV (arrowed). Some bacteria appear to be external to the PM but still within the anterior midgut. (C) Posterior end of anterior midgut leaking erythrocytes to the external milieu after applying a coverslip. (D) Corresponding fluorescence micrograph (C), revealing GFP+ B. ancashensis cells mixed in with the bloodmeal (400 × magnification; scale bars = 160 μm in (A, B), and 53 μm in (C, D). PM, peritrophic membrane; SV, stomodeal valve.
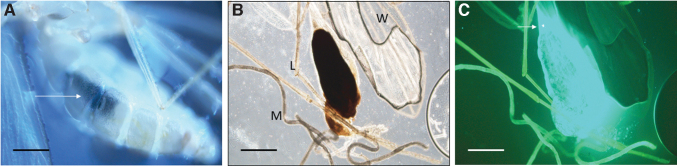
At 4–6 days postinfection, the bloodmeal in infected sand flies was partially digested and rusty-brown in color. Despite autofluorescence of various insect body parts under UV fluorescence microscopy, GFP+ bacteria were readily discernible throughout the anterior midgut. Over the 4–6 days time period, the microcolony at the anterior of the anterior midgut became more prominent, in contrast to a waning bacterial population located elsewhere in the anterior midgut (Figs. 3–5). In a few instances, GFP+ bacteria that had apparently escaped the PM and adhered to its exterior were also observed (Fig. 5C).
FIG. 3.
Micrographs of infected sand flies at 4 days postfeeding. (A) Intact sand fly with visible bloodmeal in the anterior midgut (arrowed). At this time the bloodmeal was dark brown when observed by light microscopy (10 × magnification; scale bar = 200 μm). (B) Isolated anterior midgut containing the bloodmeal. Other structures shown include a wing (W), malpighian tubules (M), and legs (L). (C) Corresponding fluorescence micrograph revealing GFP+ B. ancashensis throughout the anterior midgut with an apparent microcolony at the anterior end (arrowed) (400 × magnification; scale bars = 160 μm).
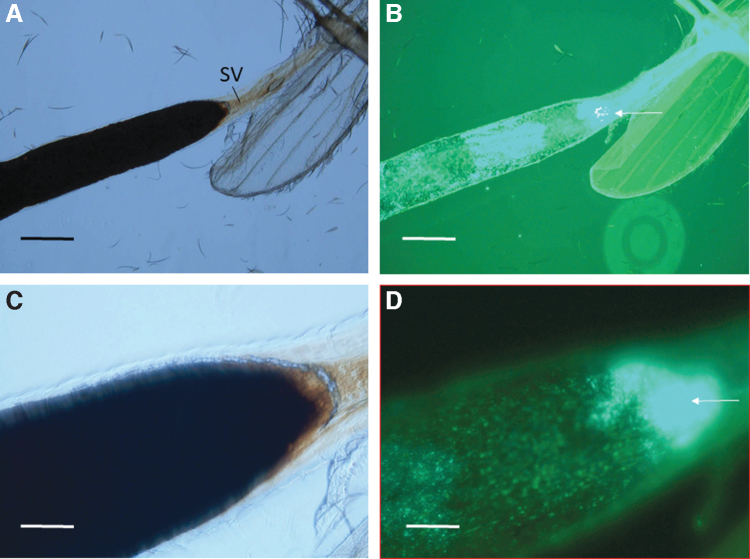
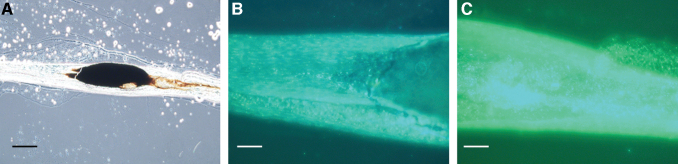
FIG. 4.
Micrographs of an isolated sand fly anterior midgut at 5 days postfeeding. The anterior portion of the anterior midgut is above the wing. (A) The blood meal was a dark, rusty color by phase-contrast microscopy (100 × magnification; scale bars = 160 μm). The SV is indicated. (B) Corresponding fluorescence micrograph revealing GFP+ B. ancashensis cells in the lumen of the anterior midgut with a microcolony at the anterior end of the anterior midgut just below the SV (arrowed). (C) Closeup image of (A) phase-contrast microscopy (400 × magnification; scale bars = 50 μm). (D) Corresponding fluorescence micrograph to (C), revealing GFP+ B. ancashensis cells just below the SV.
FIG. 5.
Micrographs of an infected sand fly at 6 days postfeeding. (A) UV fluorescence micrograph showing absence of apparent infection in head, thorax, and thoracic midgut (100 × magnification; scale bar = 3000 μm). (B) Anterior midgut showing rusty-brown bloodmeal by phase-contrast microscopy. The SV at the anterior is arrowed. (C) Corresponding fluorescence micrograph showing heavy colonization by GFP+ B. ancashensis below the SV and outside the PM but still within the confines of the anterior midgut (400 × magnification; scale bars = 100 μm in B, C). UV, ultraviolet.
Sand flies observed at the 7-day time point exhibited considerable variation regarding their infection status. While roughly half of the flies showed reduced bloodmeal sizes (Fig. 6), the other half had no detectable bloodmeal remaining when observed by dissection or light microscopy. These qualitative differences possibly resulted from the variable bloodmeal volumes initially taken by the sand flies. In flies that still possessed a visible bloodmeal, the number of GFP+ B. ancashensis cells was markedly reduced compared to insects observed at earlier time points.
FIG. 6.
Micrographs of an infected sand fly anterior midgut at 7 days postfeeding. (A) Intact anterior midgut showing a brownish-black bloodmeal by phase-contrast microscopy (100 × magnification; scale bar = 140 μm). In certain flies, the bloodmeal was completely absent at this time point. Fluorescence microscopy revealed relatively low densities of GFP+ B. ancashensis cells at 7 days, especially in the (B) posterior portion of the anterior midgut. (C) A microcolony was still apparent at the anterior end of the anterior midgut (400 × magnification; scale bar = 35 μm in B, C).
However, bacteria remained at low density throughout the abdominal midgut, and remained concentrated as a microcolony in the anterior end of the anterior midgut (Fig. 6). When anterior midguts from insects at the 7 days postinfection time were aseptically isolated and cultured on HIBB-kan25, GFP+ bacteria were rescued, suggesting that the B. ancashensis present remained viable for at least a week in the sand fly anterior midgut (not shown).
At 8–9 days postinfection, GFP+ B. ancashensis was not detected in female sand flies by dissection, light, or fluorescence microscopy, and the bloodmeals were completely digested. However, the presence of eggs in these insects indicated that they had previously fed on blood, since oviposition requires a bloodmeal by L. longipalpis (Milleron et al., 2008). However, none of the eggs observed in flies after 8 days postinfection contained GFP+ B. ancashensis by UV microscopy (Supplementary Fig. S3).
Diuretic fluid and feces from infected sand flies did not possess GFP+ B. ancashensis cells when observed by fluorescence microscopy at 1000 × magnification. Moreover, B. ancashensis was not recovered from these samples when cultured on HIBB-kan25 plates.
Viability of B. ancashensis in L. longipalpis
Quantification of bacteria by plate counts using isolated abdominal midguts from different insects gave inconsistent results (not shown). Although disappointing, this was not unexpected, especially considering the wide range of bloodmeal volumes and bacterial loads taken up during feeding by individual sand flies. As a corollary, we also observed considerable variation in the sizes of bloodmeals on any given day during the dissections. To demonstrate bacterial load variability, plate counts on one sand fly at 3 days postinfection yielded 4.47 × 107 B. ancashensis CFU's/mL, while in another sand fly at 1 day postinfection, the midgut yielded 5 × 105 B. ancashensis CFU's/mL. Despite variability in the bacterial load quantification results, we were able to culture viable B. ancashensis from isolated abdominal midguts/bloodmeals each day for up to 7 days postinfection.
Discussion
In this study, we addressed the hypothesis that B. ancashensis colonizes and persists in a sand fly vector, by using a L. longipalpis sand fly model of infection and GFP fluorescently tagged bacteria. Results of the study showed that L. longipalpis can be infected and maintain viable, GFP+ B. ancashensis in the anterior midgut for 7 days. These results are similar to those we reported previously for GFP+ B. bacilliformis in the L. longipalpis infection model (Battisti et al., 2015). Interestingly, both B. bacilliformis and B. ancashensis infections of L. longipalpis were restricted to the bloodmeal of the abdominal midgut. In contrast, B. bacilliformis infection of L. verrucarum (a bona fide sand fly vector for B. bacilliformis [Noguchi et al., 1929]) showed a much longer infection (>14 days) and involved the anterior midgut lumen outside the blood meal (i.e., in the intraperitrophic space).
Once the bona fide sand fly vector(s) for B. ancashensis is identified, it would be prudent to determine if it also provides for longer term maintenance of the bacterium versus L. longipalpis. One noticeable difference between B. bacilliformis and B. ancashesis, in the L. longipalpis model, was the appearance of a small number of B. ancashensis cells that had apparently escaped from the blood meal and adhered to the exterior of the PM in a subset of sand flies (Figs. 2B and 5C). The mechanism of bacterial escape to this location is unknown. Taken as a whole, the results suggest that one or more sand fly species inhabiting the endemic zone of B. ancashensis could conceivably maintain the pathogen for at least a short term and possibly serve as a vector of transmission during this time.
Although bona fide sand fly vectors of B. ancashensis have not yet been identified, those phlebotomine sand fly species that have been shown to harbor B. bacilliformis or its DNA are logical starting points for screening. These include Lutzomyia maranonensis (Ulloa et al., 2018), Lutzomyia noguchii (Noguchi et al., 1929), Lutzomyia peruensis (Villaseca et al., 1999), Lutzomyia robusta (Carrazco-Montalvo, 2017), and L. verrucarum (Noguchi et al., 1929; Romero, 2004). A closer examination of the various sand fly species inhabiting the endemic zone for B. ancashensis and B. bacilliformis is needed to better understand the epidemiology of this understudied pathogen. It may also be prudent to screen other insects living in the endemic zone, especially considering the increasing number of vectors involved in the transmission of other Bartonella species.
An additional novel discovery was the consistent formation of a bacterial aggregate or “microcolony” distal to the SV of the esophagus at the anterior portion of the abdominal midgut. These microcolonies were observed as early as 3 days postinfection (Fig. 2) and maintained for up to 7 days in infected sand flies (Fig. 6). It is possible that the microcolonies resulted from biofilm formation, as previously reported for Bartonella henselae (Okaro et al., 2021). However, the actual composition of the B. ancashensis microcolony remains to be determined. Assuming that the microcolony also forms in the anterior portion of the anterior midgut of a bona fide sand fly vector(s), it could conceivably enhance transmission by allowing bacteria to be regurgitated into the thoracic midgut, mouth parts, and finally into a human host's circulatory system during hematophagy.
A similar transmission scenario is seen in Yersinia pestis-infected fleas, where biofilm formation in the proventricular valve of the foregut eventually blocks its closure and allows the bacterium to be regurgitated during hematophagy (Hinnebusch et al., 2017).
Conclusions and Future Directions
In conclusion, this study has shown that B. ancashensis can establish an infection of the anterior midgut of female L. longipalpis sand flies, and the infection is maintained for ∼7 days. At 3–7 days postinfection, a prominent microcolony of aggregated bacteria forms in the anterior midgut of the fly, immediately distal to the SV of the esophagus. Eggs, diuretic fluid, feces, and other tissues are not infected. While our results suggest that certain sand fly species within the endemic zone for B. ancashensis may play a role in the bacterium's maintenance and possibly in its transmission to humans, numerous questions remain unanswered.
For example, how many of the reported cases of verruga peruana in historically nonendemic areas of South America were actually caused by B. ancashensis but attributed to B. bacilliformis (Alexander, 1995; Amano et al., 1997; Cooper et al., 1997; Ellis et al., 1999; Kosek et al., 2000; Maco et al., 2004; Maguiña and Gotuzzo, 2000; Maguiña-Vargas, 1998; Pachas-Cavez, 2001)? Is B. ancashensis actually vectored by sand flies? Do coinfections involving B. ancashensis and B. bacilliformis in sand flies result in cotransmission through a single vector (Mullins et al., 2017)? We believe that the study has paved the way to begin to address these questions.
Supplementary Material
Acknowledgments
We sincerely thank Dr. Al Richards for the generous gift of B. ancashensis strain 20.00 and Dr. James M. Battisti for technical advice on sand fly handling. We are grateful to the Walter Reed Army Institute of Research for Lutzomyia longipalpis (LLJB, Brazil; L3/pupae, NR-44001) obtained through BEI Resources, National Institute of Allergy and Infectious Diseases (NIAID), and National Institute of Health (NIH). Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Author Disclosure Statement
The authors declare that they have no known competing financial interests or personal relationships that could constitute a conflict of interest or to have influenced the work reported in this paper.
Funding Information
This work was supported by NIH grant R03AI146927 (to M.F.M.).
Supplementary Material
References
- Alexander B. A review of bartonellosis in Ecuador and Colombia. Am J Trop Med Hyg 1995;52(4):354–359; doi: 10.4269/ajtmh.1995.52.354 [DOI] [PubMed] [Google Scholar]
- Amano Y, Rumbea J, Knobloch J, et al. Bartonellosis in Ecuador: Serosurvey and current status of cutaneous verrucous disease. Am J Trop Med Hyg 1997;57(2):174–179; doi: 10.4269/ajtmh.1997.57.174 [DOI] [PubMed] [Google Scholar]
- Battisti JM, Lawyer PG, Minnick MF. Colonization of Lutzomyia verrucarum and Lutzomyia longipalpis sand flies (Diptera: Psychodidae) by Bartonella bacilliformis, the etiologic agent of Carrión's disease. PLoS Negl Trop Dis 2015;9(10):e0004128; doi: 10.1371/journal.pntd.0004128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti JM, Minnick MF. Development of a system for genetic manipulation of Bartonella bacilliformis. Appl Environ Microbiol 1999;65(8):3441–3448; doi: 10.1128/AEM.65.8.3441-3448.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti JM, Minnick MF. Laboratory maintenance of Bartonella quintana. Curr Protoc Microbiol 2008; Chapter 3:Unit 3C.1.1–3C.1.13; doi: 10.1002/9780471729259.mc03c01s10 [DOI] [PubMed] [Google Scholar]
- Blazes DL, Mullins K, Smoak BL, et al. Novel Bartonella agent as cause of verruga peruana. Emerg Infect Dis 2013;19(7):1111–1114; doi: 10.3201/eid1907.121718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrazco-Montalvo AR. Detección molecular de Bartonella bacilliformis en flebótomos (Diptera: Psychodidae) en la zona fronteriza Ecuatoriana-Peruana. Thesis. Colegio de Ciencia Biológicas y Ambientales, University of San Francisco de Quito USFQ. Ecuador, 2017. Available from: https://repositorio.usfq.edu.ec/bitstream/23000/6480/1/131141.pdf [Last accessed: March 7, 2023].
- Cooper P, Guderian R, Orellana P, et al. An outbreak of bartonellosis in Zamora Chinchipe Province in Ecuador. Trans R Soc Trop Med Hyg 1997;91(5):544–546; doi: 10.1016/s0035-9203(97)90019-5 [DOI] [PubMed] [Google Scholar]
- Ellis BA, Rotz LD, Leake JA, et al. An outbreak of acute bartonellosis (Oroya fever) in the Urubamba region of Peru, 1998. Am J Trop Med Hyg 1999;61(2):344–349; doi: 10.4269/ajtmh.1999.61.344 [DOI] [PubMed] [Google Scholar]
- Hinnebusch BJ, Jarrett CO, Bland DM. “Fleaing” the plague: Adaptations of Yersinia pestis to its insect vector that lead to transmission. Annu Rev Microbiol 2017;71:215–232; doi: 10.1146/annurev-micro-090816-093521 [DOI] [PubMed] [Google Scholar]
- Kosek M, Lavarello R, Gilman RH, et al. Natural history of infection with Bartonella bacilliformis in a nonendemic population. J Infect Dis 2000;182(3):865–872; doi: 10.1086/315797 [DOI] [PubMed] [Google Scholar]
- Maco V, Maguiña C, Tirado A, et al. Carrion's disease (Bartonellosis bacilliformis) confirmed by histopathology in the High Forest of Peru. Rev Inst Med Trop Sao Paulo 2004;46(3):171–174; doi: 10.1590/s0036-46652004000300010 [DOI] [PubMed] [Google Scholar]
- Maguiña C, Gotuzzo E. Bartonellosis. New and old. Infect Dis Clin North Am 2000;14(1):1–22, vii; doi: 10.1016/s0891-5520(05)70215-4 [DOI] [PubMed] [Google Scholar]
- Maguiña-Vargas C. Bartonellosis o Enfermedad de Carrión. Nuevos Aspectos de Una Vieja Enfermedad. A.F.A. Editores Importadores S.A.: Lima, Peru; 1998. [Google Scholar]
- Milleron RS, Meneses CR, Elnaiem DA, et al. Effects of varying moisture on egg production and longevity of Lutzomyia longipalpis (Diptera: Psychodidae). J Med Entomol 2008;45(1):160–165; doi: 10.1603/0022-2585(2008)45[160:eovmoe]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Minnick MF, Anderson BE, Lima A, et al. Oroya fever and verruga peruana: Bartonelloses unique to South America. PLoS Negl Trop Dis 2014;8(7):e2919; doi: 10.1371/journal.pntd.0002919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins KE, Hang J, Clifford RJ, et al. Whole-genome analysis of Bartonella ancashensis, a novel pathogen causing verruga peruana, rural Ancash Region, Peru. Emerg Infect Dis 2017;23(3):430–438; doi: 10.3201/eid2303.161476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins KE, Hang J, Jiang J, et al. Molecular typing of “Candidatus Bartonella ancashi,” a new human pathogen causing verruga peruana. J Clin Microbiol 2013;51(11):3865–3868; doi: 10.1128/JCM.01226-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins KE, Hang J, Jiang J, et al. Description of Bartonella ancashensis sp. nov., isolated from the blood of two patients with verruga peruana. Int J Syst Evol Microbiol 2015;65(10):3339–3343; doi: 10.1099/ijsem.0.000416 [DOI] [PubMed] [Google Scholar]
- Noguchi H, Shannon RC, Tilden EB, et al. Etiology of Oroya fever: XIV. The insect vectors of Carrion's disease. J Exp Med 1929;49(6):993–1008; doi: 10.1084/jem.49.6.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaro U, George S, Anderson B. What Is in a Cat Scratch? Growth of Bartonella henselae in a biofilm. Microorganisms 2021;9(4):835; doi: 10.3390/microorganisms9040835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachas Chavez PE. Enfermedad de Carrión (Bartonelosis) en el Peru. Ministerio de Salud. Oficina General de Epidemiologia: Lima, Peru; 2001. Available from: https://cdn.www.gob.pe/uploads/document/file/391445/Enfermedad_de_Carri%C3%B3n__Bartonelosis__en_el_Per%C3%BA20191017-26355-grx1zo.pdf?v=1571312750 [Last accessed: March 7, 2023].
- Peterkova-Koci K, Robles-Murguia M, Ramalho-Ortigao M, et al. Significance of bacteria in oviposition and larval development of the sand fly Lutzomyia longipalpis. Parasit Vect 2012;5:145; doi: 10.1186/1756-3305-5-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S. Detection of Bartonella bacilliformis by real-time PCR in naturally infected sand flies. Thesis. Department of Preventive Medicine and Biometrics, University of the Health Sciences. Bethesda, MD, 2004. Available from: https://www.semanticscholar.org/paper/Detection-of-Bartonella-bacilliformis-by-Real-Time-Romero/424840897baf345fdd29b9c5035036d93bea02c2 [Last accessed: March 7, 2023].
- Ulloa GM, Vásquez-Achaya F, Gomes C, et al. Molecular detection of Bartonella bacilliformis in Lutzomyia maranonensis in Cajamarca, Peru: A new potential vector of Carrion's disease in Peru? Am J Trop Med Hyg 2018;99(5):1229–1233; doi: 10.4269/ajtmh.18-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseca P, Padilla C, Ventura G, et al. Importancia de la Lutzomyia peruensis en la transmisión de la enfermedad de Carrión en el valle Sagrado de los Incas. Urubamba-Cusco, Peru. Rev Med Exp 1999;15:28–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.