Abstract
Purpose:
Histrelin implant (HI) is a gonadotropin-releasing hormone agonist (GnRHa) used in pediatrics to treat central precocious puberty (CPP) and for pubertal suppression in transgender/non-binary (TG/NB) youth with gender dysphoria. HI is designed for annual removal/replacement; however, effectiveness has been reported beyond 1 year. No previous study has assessed prolonged HI use in TG/NB youth. We hypothesize that HI is effective >12 months in TG/NB youth as described in children with CPP.
Methods:
This retrospective, two-center study included 49 subjects with 50 HI retained ≥17 months, in TG/NB (42) and CPP (7). Pubertal suppression was assessed biochemically and/or clinically (testicular/breast exams). Escape from pubertal suppression and HI removal is also characterized.
Results:
Most implants (42/50) maintained clinical/biochemical suppression for the duration of the study. The average use of a single HI was 37.5±13.6 months. Pubertal suppression escape occurred in eight subjects at average 30.4 months from placement: five had only biochemical; two clinical; and one both clinical and biochemical escape. After an average of 32.9 months, only 3/23 HI removed had adverse effects (HI broken, difficult removal).
Conclusion:
Extended use of HI in our TG/NB and CPP subjects was efficacious, resulting in sustained biochemical and clinical pubertal suppression in most. Suppression escape occurred at 15–65 months. Complications at HI removal were infrequent. Keeping HI for extended time would improve cost and morbidity, while maintaining efficacy and safety for most patients.
Keywords: gender dysphoria, histrelin implant, puberty suppression, transgender youth
Introduction
Histrelin (acetate) is a gonadotropin-releasing hormone agonist (GnRHa), which suppresses pulsatile secretion of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and gonadal hormone production, designed for annual removal/replacement.1 It has been utilized in both pediatric and adult populations when complete suppression of sex hormones is required as in central precocious puberty (CPP) in children, or adjuvant therapy for prostate and breast cancers in adults. Histrelin implant (HI) has been utilized to treat CPP since 2005.2 More recently, HI has been used for pubertal suppression in transgender/non-binary (TG/NB) youth with gender dysphoria (GD).
Although use of GnRHa for this indication is off-label, pubertal suppression is recommended as per current guidelines from the Endocrine Society and WPATH.3,4 Oftentimes, providers and families face barriers to utilizing this implantable GnRHa, particularly for off-label use, due to high cost of therapy5 paired with restricted insurance coverage. In addition, some children require sedation with general anesthesia for placement/removal of the HI. Leaving implants in place for longer than 12 months has been shown to be effective in several small pediatric and adult case series.6–9
The objective of this study is to present retrospective data on clinical and biochemical outcomes of extended HI use in a pediatric cohort of 49 subjects from two pediatric centers.
Methods
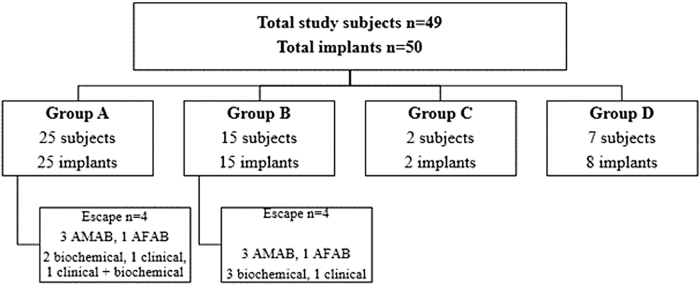
A retrospective chart review was performed in two U.S. pediatric centers using electronic medical records (Slicer/dicer, i2i) to retrieve patients with GD or CPP, seen between January 2010 and December 2020, with HI in place for ≥17 months. This study was approved by individual IRB committees in both institutions. Although we typically asked for follow-up assessment to be done at 18-month visit following HI insertion, several subjects had pre-visit laboratory assessment at 17 months, and therefore that cutoff was used in the study. Exclusion criteria were as follows: HI removed <17 months and subjects with HI in place >17 months, but lacking follow-up data. Forty-nine subjects met the inclusion criteria and long-term outcomes of 50 implants were described (Fig. 1).
FIG. 1.
Group A: TG/NB youth with histrelin implant alone. Group B: TG/NB youth on GAHT and HI. Group C: TG/NB youth on HI and GAHT with irregular follow-ups. Group D: subjects with central precocious puberty. AFAB, assigned female at birth; AMAB, assigned male at birth; GAHT, gender-affirming hormone therapy; HI, histrelin implant; TG/NB, transgender/nonbinary.
All HI placements/removals were done by surgical teams, with local or general anesthesia depending on age, ability to cooperate during the procedure, anxiety level, and/or preference.
The choice of retaining versus replacing HI after 12 months was based on providers' decision to offer it and patient/family preference. Risks and benefits of the extended HI use were discussed with each subject's family. Safety was assessed as presence of complications related to HI removal or replacement after the extended use: infections, bleeding, difficulties with removal, and HI breakage. Subjects were advised to have follow-up care every 3–6 months. At each visit >12 months of placement, the option of HI removal/replacement was discussed.
Our study cohort was subdivided into the following: group A -TG/NB youth treated with HI alone (n=25), group B -TG/NB youth receiving gender-affirming hormone therapy (GAHT) and HI (n=15), group C -TG/NB youth (n=2) who did not adhere to recommended testing and follow-ups (included to highlight the importance of follow-up care), and group D -subjects with CPP (n=7). Four subjects (two in group A and two in group B) had Vantas™ (histrelin acetate 50 mcg/day); the others had SupprelinLA™ (histrelin acetate 65 mcg/day).
All relevant study data were retrieved retrospectively from medical records. Efficacy of HI was monitored clinically and by measurements of the random, unstimulated concentrations of the sex hormones and LH. The majority of laboratory tests were done with pediatric assays pre-HI and post-HI insertion: LH (immunoassay), ultrasensitive estradiol (Liquid chromatography/mass spectrometry), and testosterone (Liquid chromatography with tandem mass spectrometry). Appropriate pubertal suppression was defined as estradiol <20 pg/mL; testosterone (T) <30 ng/dL; and/or lack of Tanner stage progression on the physical examination. For subjects >13 years of age, we used higher T concentration cutoff (T<40 ng/dL) due to adrenarche. LH concentrations alone were not used to guide treatment as leuprolide stimulation was not performed.
Clinical examinations were conducted by pediatric endocrinologists and included breast Tanner staging (by palpation) and testicular size measurements with Prader beads. We also assessed the presence of menstrual cycles in subjects assigned female at birth (AFAB).
Pubertal progression/escape was defined by clinical examination: increase in Tanner stage and/or biochemically: hormonal concentrations over above-mentioned ranges. Wilcoxon paired signed rank tests were performed on the non-normally distributed hormone variables of LH, T, and estradiol (at the baseline and while on HI treatment). These hormonal data are presented as median, 25th (Q1), and 75th%iles (Q3). As age was normally distributed for all groups, paired t-tests were implemented to compare the distributions between groups. Version 4.0.4 of the software Tool R was used for statistical analysis. Statistical significance was considered p<0.05. Analysis was performed for the group as a whole, and for subgroups, divided by sex assigned at birth.
Results
Of 49 subjects in the study, 42 (85.7%) were TG/NB youth and 7 were with CPP (14.3%); 27 were AFAB and 22 were assigned male at birth (AMAB). The details of each group, total implants, and those who escaped suppression are depicted in Figure 1.
Baseline characteristics and demographics of this cohort are depicted in Table 1. There were no significant differences in Tanner staging between groups A, B, and D. The mean ages of groups A and B were not significantly different, and both A and B were older than patients with CPP, as expected. Before HI, 11/49 subjects had received injectable GnRHa: group A (4), group B (3), and group D (4).
Table 1.
Baseline Characteristics of the Cohort (n=49 Subjects)
| Age at presentation for pubertal suppression, years, mean±SD | |
| Total cohort | 11.6±2.4 |
| Group A (n=25) | 11.7±1.6 |
| Group B (n=15) | 12.9±1.7 |
| Group C (n=2) | 14.7±2.2 |
| Group D (n=7) | 7.9±2.4 |
| Tanner stage 2–5, mean, (range) | 2.9 (2–5) |
| Group A (n=25) | 2.5 (2–5) |
| Group B (n=15) | 3.1 (2–5) |
| Group C (n=2) | 5 |
| Group D (n=7) | 3.0 (2–5) |
| Race, n (%) | |
| White | 29 (59.2) |
| Black/AA | 3 (6.1) |
| Asian | 7 (14.3) |
| Multiracial | 2 (4.1) |
| Other/unspecified | 8 (16.3) |
| Ethnicity, n (%) | |
| Non-Hispanic/Latino | 34 (69.4) |
| Hispanic/Latino | 10 (20.4) |
| Declined to answer | 5 (10.2) |
| Indication for treatment | |
| GD, n (%) | 42 (85.7) |
| AFAB | 22 (52.4) |
| AMAB | 20 (47.6) |
| Central precocious puberty, n (%) | 7 (14.2) |
| AFAB | 5 (71.4) |
| AMAB | 2 (28.6) |
AFAB, assigned female at birth; AMAB, assigned male at birth; GD, gender dysphoria; SD, standard deviation.
Treatment outcomes of 50 HI are presented in Table 2. We demonstrated pubertal suppression for a minimum of 15 months after the insertion, as confirmed by laboratory testing and/or clinical examination in all 50 implants. The average use of a single HI was 37.5±13.7 months. The longest biochemically confirmed HI effectiveness was 48 months in AFAB with CPP. In group A, the longest clinical effectiveness was in a transmale: LH and estradiol were suppressed at 43 months, and he was amenorrheic until HI removal at 45 months.
Table 2.
Treatment Outcomes of the Extended Use of HI
| Months from HI placement to the implant removal or end of study period; mean±SD, (range) | |
| Total number of implants (N=50) | 37.9±13.6 (18–71) |
| Group A (n=25)a | 41.9±12.8 (22–71) |
| Group B (n=15) | 35.9±14.5 (21–71) |
| Group C (n=2) | 25 (21–29) |
| Group D (n=8) | 28.6±11.1 (17–50) |
| Months from HI placement to last confirmed pubertal suppression (labs/examination), mean±SD, (range) | |
| N=48 implants (group C excluded) | 26.3±9.1 (17–65) |
| Group A (n=25) | 25.5±13.4 (17–43) |
| Group B (n=15) | 26±9.8 (19–46) |
| Group D (n=8) | 26±11.7 (17–48) |
| Implant removal: time from placement, months, mean±SD, (range) | |
| Total cohort (23/50 implants) | 32.9±11.5 (17–62) |
| Group A (9/25) | 40.3±12.2 (24–62) |
| Group B (5/15) | 27.6±6.1 (24–36) |
| Group C (1/2) | 29 |
| Group D (8/8) | 27.3±12.4 (17–50) |
Eight subjects later on started GAHT.
GAHT, gender-affirming hormone therapy; HI, histrelin implant.
Subjects were advised to have follow-up care every 3–6 months; however, some had less frequent or irregular visits (range 4–17 months). Sex hormone concentrations at baseline and follow-up were available on 38/49 subjects (Table 3).
Table 3.
Hormone Values at Baseline and the Latest on Treatment (n=38 Subjects)
| Baseline (before GnRHa) Median (Q1, Q3) |
Histrelin implant (17–65 months post-insertion) Median (Q1, Q3) |
|
|---|---|---|
| Total cohort | ||
| *LH (mlU/mL) | 0.83 (0.44, 2.35) | 0.14 (0.06, 0.31) |
| *T (ng/dL) | 51 (24.7, 301) | 9 (6, 17) |
| *Estradiol (pg/mL) | 24 (9, 34) | 3.8 (0.0, 5.5) |
| Group A (n=21) | ||
| *LH (mlU/mL) | 0.63 (0.4, 1.4) | 0.17 (0.1, 0.3) |
| AFAB (n=12) | ||
| *LH (mlU/mL) | 0.44 (0.19, 0.72) | 0.12 (0.04, 0.28) |
| *Estradiol (pg/mL) | 17.6 (9.3, 28.9) | 3.5 (1.5, 5) |
| AMAB (n=9) | ||
| *LH (mlU/mL) | 1.1 (0.63, 1.36) | 0.19 (0.11, 0.31) |
| *T (ng/dL) | 36 (10, 78) | 8 (5, 10) |
| Group B (n=12) | ||
| *LH (mlU/mL) | 1.65 (0.67, 2.19) | 0.15 (0.06, 0.30) |
| AFAB (n=3) | ||
| LH (mlU/mL) | 0.3 (0.22, 1.32) | 0.14 (0.11, 0.15) |
| Estradiol (pg/mL) | 5 (2.5, 81.5) | 9 (4.5, 11.5) |
| AMAB (n=9) | ||
| LH (mlU/mL) | 1.7 (1.22, 2.14) | 0.3 (0.04, 0.31) |
| *T (ng/dL) | 73.5 (44.4, 460.8) | 16.5 (8.3, 18.5) |
| Group D (n=5) | ||
| LH (mlU/mL) | 2.5 (2.5, 3.9) | 0.08 (0.05, 0.14) |
| Estradiol (pg/mL) | 45 (31.5, 82.3) | 2 (0, 4) |
Excluded are four subjects from group A; three from group B; two from group C; AMAB subjects from group D (n=2) due to insufficient laboratory data.
Q1–25th percentile, Q3–75th percentile.
p-Value <0.05.
GnRHa, gonadotropin-releasing hormone agonist; T, testosterone.
Pubertal suppression per patient group
Group A included 25 TG/NB subjects (15 AFAB and 10 AMAB). HI was placed on average at 11.7±1.6 years (Table 1). Group A had biochemically effective HI in place, on average 25.5±13.4 months (range 17–43). Most subjects (21/25) had hormonal values at baseline and during the follow-up, showing statistically significant decreases in LH, T (in AMAB), and LH, estradiol (in AFAB), while HI was in place (Table 3). Implant outcomes: 9/25 implants were removed during the study period, on average at 40.3±12.1 (range 24–62) months post-placement (Table 2).
Group B included 15 TG/NB adolescents (5 AFAB and 10 AMAB), who started pubertal suppression at an average age of 12.8±1.6 years. They were initially on HI alone, and 5–18 months (average 12.3±4.0 months) after their HI placement, they were started on GAHT. While on regimen of gradually increasing GAHT, the endogenous hormone concentrations remained low, as confirmed by laboratory data at an average of 26±9.8 months post-HI (range 19–46) (Table 2). Once on GAHT, it was difficult to confirm with certainty that pubertal suppression was due to HI and not a result of GAHT. The transfemales were on 3.9±1.9 mg/day (range 2–8 mg) of oral/sublingual estradiol, and two received transdermal estradiol 75 mcg weekly.
Their average estradiol concentration was 99.4±66 pg/mL (range 4–232), which was within the guidelines (2) and peer-concordant. Follow-up laboratory testing was available on 8/10, and all achieved desired T suppression: average T 17.5±11.7 ng/dL (range 6–38). All transfemales had reached Tanner 3–5 breast development. Transmales were on 12.5–40 mg subcutaneous T (SC-T) weekly (exception was a single subject receiving transdermal T patches 2 mg/day) and had average serum T concentration of 259±176 ng/dL. Notably, not all study subjects reached a full T replacement dose during the study period.
Implant outcomes: 5/15 removed without complications and one was replaced.
Group C consisted of 2 TG/NB adolescents (both AFAB) who had HI placed at an average 14.7±2.2 years at Tanner stage 5. These subjects had incomplete data due to having irregular follow-ups. One subject was initially lost for 1 year, but returned for several visits up to 18 months, and had sustained amenorrhea, but had no laboratory studies. Subsequently, he transferred care and had uneventful HI removal at 29 months post-insertion. The second subject reported amenorrhea during the only (telemedicine) visit at 21 months post-HI placement.
Group D included seven subjects with CPP (five AFAB and two AMAB), who had a total of eight implants. One AFAB subject had two implants placed over the course of study follow-up, both in place ≥17 months. As expected, this group started treatment at a significantly younger age (mean 7.9±2.3 years than groups A and B, p<0.05). An AMAB subject had 2 mL testicles, but pubertal LH=1.39 mIU/mL, T=20 ng/dL consistent with CPP. Implant effectiveness was confirmed by the laboratory/clinical examination at 24.9±10.9 months (range 17–48 months) for all eight implants (Tables 2 and 3). All eight implants have been removed, at an average 27.5±10.8 months (range 17–50 months).
Hormonal data
In most subjects, HI efficacy was confirmed by both clinical and laboratory assessments. However, due to coronavirus disease 2019 (COVID-19)-related quarantine, care transfers, and retrospective study design, follow-up laboratory testing was not available in 11 subjects. Table 3 represents median hormone values of 38/49 subjects (77.5%) who had pre- and post-HI placement data. During the extended HI use, median hormone concentrations of LH, T, and estradiol, of the total cohort compared to pre-treatment, showed statistically significant decreases.
In Group A, (including subgroups) median LH and sex hormone values also decreased significantly, as did median LH in Group B, as a whole, and median T value in AMAB (Table 3). Importantly, the median hormone values for LH, T, and estradiol at 12 months compared to the end of the study (17–65 months) were not significantly different.
Escape from pubertal suppression
Clinical and/or laboratory evidence of pubertal progression was defined as escape and occurred in eight TG/NB subjects (16%) out of 50 HI, group A: n=4 and group B: n=4; six had SupprelinLA and two Vantas implant, five of these were replaced. Two AFAB subjects from group C had irregular follow-ups, but reported persistent amenorrhea. Four subjects were on GAHT at the time of pubertal escape. Five subjects had only biochemical, two clinical and one both clinical/biochemical pubertal escape. Escape occurred at an average 30.4±16.5 months (range 15–65) post-insertion (Table 4).
Table 4.
Laboratory Assessment of Study Subjects with Pubertal Escape (n=8)
| Time of escape from HI placement (months, average±SD) | LH (mIU/mL) | T (ng/dL) | Estradiol (pg/mL) | GAHT | HI outcome | Escape biochemical/clinical | |
|---|---|---|---|---|---|---|---|
| Group A (n=4) | 33.3±21.6 | ||||||
| A1 | 27 | n/a | n/a | — | Replaced | Clinical (breast enlargement) | |
| A2 | 24 | n/a | 104 | — | Replaced | Biochemical | |
| A3 | 65 | 2.2 | 390 | 138 | 2 mg estradiol oral | In place, added Lupron | Biochemical and clinical (testicular enlargement, voice change) |
| A4 | 7 | 0.93 | 20 | — | Replaced (29 months) | Biochemical | |
| 17 | 1.15 | 52 | — | ||||
| 21 | 0.9 | 34 | — | ||||
| 25 | 1.05 | 16 | 17 | 1.5 mg estradiol oral | |||
| Group B (n=4) | 27.5±12.2 | ||||||
| B1 | 28 | n/a | 51 | 75 mcg weekly transdermal estradiol | Replaced | Biochemical | |
| B2 | 44 | 64 | 106 | 4 mg estradiol oral | In place, Increased estradiol | Biochemical | |
| B3 | 23 | 0.09 | 182 | 3 | 12.5 mg SC-T bi-weekly | Replaced | Clinical (spotting) |
| B4 | 15 | 0.97 | 80 | — | In place, started estradiol | Biochemical | |
| All subjects (n=8) Average, months±SD |
30.4±16.5 |
n/a, not available; SC-T, subcutaneous testosterone.
Subject B4 (AMAB) had biochemical escape at 15 months, with T=80 ng/dL and LH=0.97 mIU/mL. Interestingly, this subject did not achieve full biochemical pubertal suppression on depot-Lupron before switching to HI. Family opted to keep HI, start estradiol 2 mg/day, and at 25 months follow-up, T=16 ng/dL and LH=0.653 mIU/mL. Subject A4 experienced a transient increase in LH and T at 17 months. At 21 months (on the same treatment), hormone concentrations spontaneously decreased. HI was replaced at 29 months post-insertion as per family's choice. Subject B3 (AFAB) receiving SC-T, reported spotting at 23 months, despite having well suppressed LH=0.09 mIU/mL; estradiol=3 pg/mL; and T=182 ng/dL, and had HI replaced.
He reported irregular T dosing due to discomfort with injections and it is possible that spotting was related to precipitous decrease in T concentrations. Subject A3, concomitantly on estradiol 2 mg/day, with limited follow-up, experienced increase in T from 6 ng/dL at 53 months to 390 ng/dL at 65 months and testicular enlargement and voice changes, consistent with clinical and biochemical pubertal escape. Two transfemales (B2 and B1) had mild increase of T noted biochemically at 44 and 28 months, respectively, B1 had HI replacement, and B2 had estradiol dose adjustment to suppress T.
Experience with implant removal
A total of 23 implants were removed during the study follow-up at an average 32.9±11.5 months (range 17–62), as depicted in Table 2.
To assess safety of extended HI use, we reviewed surgical reports. Those were available from 22 cases and 19/22 (86.4%) had uneventful procedures. Complications were as follows: one implant was removed in 2 pieces at 45 months, and one implant broke into several pieces at 62 months, with the potential of a small, retained piece (group A). The third, minor complication was in a child with CPP whose implant removal at 18 months post-insertion was described as “difficult due to encapsulation.” Subsequent implant removal in the same child at 17 months post-insertion was uneventful. None of the subjects had excessive bleeding or infection related to HI replacement/removal.
Discussion
This is the largest and the longest retrospective study of extended histrelin implant use in the pediatric population and the first study describing use of HI beyond 12 months in TG/NB youth. A previous pediatric study confirming the efficacy of HI at 24 months included 33 subjects with CPP.6
Our findings are of interest to physicians and families for a number of reasons. Most importantly, this review showed that extended use of HI beyond a year in our two centers has been safe and efficacious, both for children with CPP and for TG/NB youth. Safety was assessed as follows: lack of infections, bleeding, and only rare complications with removal. As noted in our cohort, it is reasonable to expect the HI to be effective for 2 years or longer in most patients, as previously shown in CPP.6
In patients who opt for extended HI use, we advise monitoring for clinical and biochemical evidence of escape as early as 15 months post-insertion, and every 3–4 months thereafter to identify any escape as early as possible.
Except for one patient who did not respond well to GnRHas in general, and escaped suppression at 15 months, all other patients maintained suppression for 17 months or longer. Four TG/NB youth opted for Vantas (HI approved for adult use) due to the significantly lower cost of this preparation compared to SupprelinLA pediatric product. In our cohort, 2/8 of the pubertal escapes were with Vantas implants. Due to small numbers, it is difficult to conclude if efficacy of the two histrelin products is comparable for extended use, although efficacy seemed comparable when used for 12 months.10
We discussed risks and benefits of extended HI use with each subject's family (Table 5). By extending HI use, patients could benefit from fewer procedures and reduced need for general/local anesthesia, decreasing the risk of scarring, infection, pain, and nausea. Fewer surgical interventions can reduce overall treatment cost (especially important for those who self-pay or have high co-pays). In our experience, families and patients have been very interested in extended HI use.
Table 5.
Pros/Cons for Extended Use of Histrelin Implant
| Annual implant replacement | Extended implant use (>12 months) | |
|---|---|---|
| Effective suppression | Very good | May wane after 18–24 months |
| Ease of extraction | Typically, easy to remove | May be more difficult the longer HI is kept |
| Cost overall | Higher | Lower |
| Implant | Higher | Lower |
| Procedure/s | Higher | Lower |
| Laboratory monitoring | Lower | Higher the longer HI is kept |
| Out of pocket | Higher | Lower |
| Frequency of laboratory testing | Lower, predictable | Higher, variable |
| Risk for worsening GD | Lower | Possibly higher, due to concern about suppression escape or more frequent physical examinations |
| Time requirement (authorization, surgical time, outpatient vs. operating room) | Higher | Lower |
| Frequency of general anesthesia | Higher | Lower |
Potential risks related to extended implant use (Table 5) can be difficulties removing/replacing the implant and/or possibility of the implant breaking. Unrecognized pubertal escape can result in pubertal progression and can lead to bone age advancement with negative height outcomes in patients with CPP, and for youth with GD development of possibly irreversible body changes resulting in increased dysphoria. We advised families that more frequent clinic and laboratory testing were needed to monitor pubertal resumption if HI was kept in-place for extended time. At each visit, the option of HI removal/replacement was discussed and families could opt for removal/replacement.
Despite advances in insurance approvals for GnRHa for TG/NG patients as reported by Stevens et al.,11 restricted access to GnRHs is an ongoing issue. As per a 2017 study,12 only 29.6% of prescribed GnRHa received insurance coverage.
The time-consuming process of obtaining HI authorization/appeals for an off-label use frequently places a large burden on physicians, staff, health systems, and families. Importantly, even with an approval granted, it must be renewed annually and out-of-pocket portions for the medication and/or procedures can be very costly for families with high deductibles. Furthermore, at the times of travel and in-person visit restrictions due to COVID-19 pandemic, and periodic shortages of some injectable GnRHa, it is advantageous to provide seamless pubertal suppression with HI for extended periods of time.
While extended use of an implant may increase the risk of difficulty with removal, the majority of our subjects had minimal or no complications with the removal, similar to the findings of Swendiman et al. who noted no correlation between difficulty of removal and length of use, even with extended use, longer than 4 years.13
In our experience, removal of the implant was related to breakage in only 2/23 cases (8.7%), which kept their HI for >3 years, significantly less than 29% reported by Eugster.14 The main concern of implant breaking is the risk of retaining a piece leading to additional surgical procedures to locate it and the risk of protracted pubertal suppression. Another potential risk of extended HI use is patients not returning for regular follow-ups as it occurred in subjects in group C. This reinforces the importance of discussing follow-ups with families.
Seven subjects had unstimulated baseline LH concentrations below the typical pubertal threshold; however, clinical examinations demonstrated pubertal initiation. Therefore, we used clinical judgment when deciding to initiate treatment, keep or replace the implant. The low unstimulated baseline LH concentrations and small subgroups might have prevented the test from detecting a difference between pre-treatment and on-treatment hormone values, leading to some results not reaching statistical significance (AFAB in group B and CPP group). Larger cohort is necessary to reveal whether there is a difference in hormone concentrations before and while on treatment in these subgroups.
For TG/NB patients starting GAHT, continuation of pubertal suppression with HI while on initial, lower GAHT doses is advised. We discuss with patients that taking GAHT can make assessment of HI efficacy more difficult, since GAHT contributes to suppression of endogenous hormones. A study of adult transgender women taking oral estradiol found that 27.9% of subjects did not reach goal T suppression, despite estradiol doses of 6–8 mg/day.15
Per another study,16 while on average 2.9 mg/day of estradiol (range 0.5–10 mg/day) plus spironolactone average dose of 145 mg/day (range 25–400 mg), the majority of adult transgender women were not able to achieve T<50 ng/dL.
Among our transfemales, in 8/10 who had follow-up laboratory testing, 100% achieved desired T suppression with HI in place, while on sublingual/oral estradiol 3.8±1.9 mg/day, range 2–8 mg/day (one subject was on 8 mg/day) or 75 mcg weekly transdermal estradiol (2 subjects). With T concentrations within goal range, the estradiol dose adjustments were made to ensure adequate growth, breast development, and bone maturation. In transmales, suppression with HI allowed for a gradual increase in GAHT dose without concerns of menstruation starting/recurring. Although not U.S. Food and Drug Administration (FDA) approved, SC-T administration was shown to be safe and efficacious in TG/NB youth17 and was the primary method of T treatment in both centers.
Although we report eight cases of pubertal escape, 5/7 subjects had only biochemical increase in hormone concentrations without detectable clinical progression (Table 4). The earliest biochemical escapes at 15–17 months (subjects B4 and A4) were transient and did not result in immediate HI replacement. In subject A4, T concentration was similar to cisgender female adolescents and suggested goal for transfemales on GAHT of <50 ng/dL.2 Furthermore, such a T concentrations was not expected to cause virilization, and there was no worsening dysphoria or anxiety. For subject B4, who had biochemical escape, both T and LH decreased after estradiol start.
Strict biochemical suppression criteria that we used in this study, based on children with CPP,18 may not be feasible or required in clinical practice for TG/NB youth, due to presence of adrenarche, as well as different age and stage of pubertal suppression at start. Although there is no consensus on cutoffs for random LH, T, or estradiol during HI use, Neely et al. showed good correlation of random and stimulated LH concentrations.18
Furthermore, pediatric CPP study19 showed mild increases in random LH concentrations even when pubertal suppression was confirmed by stimulation testing. None of the suppressed subjects in our cohort had random LH concentrations >1 mIU/mL (while in the study by Neely et al.,18 highest random LH measured, while suppressed, was 1.5 mIU/mL), nor received implant replacement based on increased LH alone.
The study subjects who required implant replacement opted for extended use of subsequent implant. This implied satisfaction with extended HI use, although formal survey was not performed.
Limitations of the study include retrospective design, nonstandardization of assays used, timing of laboratory and clinical examinations, and lack of GnRH stimulation testing. Due to COVID-19, several families declined clinic visits or laboratory testing, limiting our ability to obtain additional hormone values and clinical assessments on some of the subjects. Although there may be a potential for selection bias, our chart review did not reveal any subject who received elective HI replacement at 12–15 months due to pubertal escape.
Conclusion
Extended use of HI (≥17 months) in TG/NB and CPP youth was efficacious and resulted in sustained biochemical and clinical pubertal suppression in majority of our study subjects. Our retrospective chart review revealed pubertal escape in 16% of implants at 15–65 months, with only 3/50 (6%) having clinical evidence of escape. Complications at HI removal were infrequent. Keeping HI beyond 12 months would be advantageous for cost and morbidity, while maintaining efficacy and safety for most patients. Our study indicates that the decision to replace HI should be based on both clinical and biochemical assessment.
We suggest the use of clinical examinations in combination with laboratory studies to determine optimal time for placement and removal of the HI. Random serum hormone concentrations in our group were not sensitive measurements of pubertal start and pubertal suppression. Additional prospective studies would be helpful to assess efficacy of extended HI in a larger number of study subjects.
Acknowledgments
The authors express gratitude to statistician Zaineb Boulil (Department of Pediatric Research, Rady Children's Hospital, San Diego) for assistance in statistical analysis.
Abbreviations Used
- AFAB
assigned female at birth
- AMAB
assigned male at birth
- COVID-19
coronavirus disease 2019
- CPP
central precocious puberty
- FDA
U.S. Food and Drug Administration
- FSH
follicle-stimulating hormone
- GAHT
gender-affirming hormone therapy
- GD
gender dysphoria
- GnRHa
gonadotropin-releasing hormone agonist
- HI
histrelin implant
- LH
luteinizing hormone
- SC-T
subcutaneous testosterone
- SD
standard deviation
- TG/NB
transgender/nonbinary
Authors' Contributions
E.P.-T. collected, reviewed, and analyzed data, and co-authored the article. R.S.N. reviewed, assisted in data analysis, and co-authored the article. M.M. collected, reviewed data, assisted in data analysis, and co-authored the article.
Author Disclosure Statement
E.P.-T. and R.S.N. have no conflicts to disclose. M.M. served on the Advisory Board for Endo Pharmaceuticals in 2018 and 2020.
Funding Information
No funding was received for this article.
Cite this article as: Pine-Twaddell E, Newfield RS, Marinkovic M (2023) Extended use of histrelin implant in pediatric patients, Transgender Health 8:3, 264–272, DOI: 10.1089/trgh.2021.0130.
References
- 1. SUPPRELIN® LA [package insert]. Malvern, PA: Endo Pharmaceuticals, Inc. Available at: https://www.supprelinla.com/hcp Accessed February 21, 2019.2021.
- 2. Hirsch H, Gillis D, Strich D, et al. The histrelin implant: a novel treatment for central precocious puberty. Pediatrics. 2005;116:e798–e802. [DOI] [PubMed] [Google Scholar]
- 3. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869–3903. [DOI] [PubMed] [Google Scholar]
- 4. Coleman E, Bockting W, Botzer M, et al. WPATH- “Standards of Care” (SOC) version 7. Int J Transgend. 2011;13:165–232. [Google Scholar]
- 5. Gonadotropin Releasing Hormone Agonists. Available at: https://www.goodrx.com/gonadotropin-releasing-hormone-agonists Accessed June 20, 2021.
- 6. Lewis KA, Goldyn AK, West KW, et al. A single histrelin implant is effective for 2 years for treatment of central precocious puberty. J Pediatr. 2013;163:1214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spitz IM, Chertin B, Lindenberg T, et al. Long-acting gonadotropin-releasing hormone implant to maintain medical castration for two years in men with prostate cancer. N Engl J Med. 1999;340:1439. [DOI] [PubMed] [Google Scholar]
- 8. Chertin B, Spitz IM, Lindenberg T, et al. An implant releasing the gonadotropin hormone-releasing hormone agonist histrelin maintains medical castration for up to 30 months in metastatic prostate cancer. J Urol. 2000;163:838–844. [PubMed] [Google Scholar]
- 9. Schlegel PN, Kuzma P, Frick J, et al. Effective long-term androgen suppression in men with prostate cancer using a hydrogel implant with the GnRH agonist histrelin. Urology. 2001;58:578–582. [DOI] [PubMed] [Google Scholar]
- 10. Olson-Kennedy J, Streeter LH, Garofalo R, et al. Histrelin implants for suppression of puberty in youth with gender dysphoria: a comparison of 50 mcg/day (Vantas) and 65 mcg/day (SupprelinLA). Transgend Health. 2021;6:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stevens J, Gomez-Lobo V, Pine-Twaddell E. Insurance coverage of puberty blocker therapies for transgender youth. Pediatrics. 2015;136:1029–1031. [DOI] [PubMed] [Google Scholar]
- 12. Nahata L, Quinn GP, Caltabellotta NM, et al. Mental health consequences and insurance denials among transgender adolescents, LGBT Health. 2017;4:188–193. [DOI] [PubMed] [Google Scholar]
- 13. Swendiman RA, Vogiatzi MG, Alter CA, et al. Histrelin implantation in the pediatric population: a 10-year institutional experience. J Pediatr Surg. 2019;54:1457–1461. [DOI] [PubMed] [Google Scholar]
- 14. Eugster EA. Experience with the histrelin implant in pediatric patients. Endocr Dev. 2016;30:54–59. [DOI] [PubMed] [Google Scholar]
- 15. Leinung MC, Feustel PJ, Joseph J. Hormonal treatment of transgender women with oral estradiol. Transgend Health. 2018;3:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang JJ, Jolly D, Chan KJ, et al. Testosterone levels achieved by medically treated transgender women in a United States Endocrinology Clinic Cohort. Endocr Pract. 2018;24:135–142. [DOI] [PubMed] [Google Scholar]
- 17. Laurenzano SE, Newfield RS, Lee E, Marinkovic M. Subcutaneous testosterone is effective and safe as gender affirming hormone therapy in transmasculine and gender-diverse adolescents and young adults: a single center's 8-year experience. Transgend Health. 2021;6:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neely EK, Silverman LA, Geffner ME, et al. Random unstimulated pediatric luteinizing hormone levels are not reliable in the assessment of pubertal suppression during histrelin implant therapy. Int J Pediatr Endocrinol. 2013;2013:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis KA, Eugster EA. Random luteinizing hormone often remains pubertal in children treated with the histrelin implant for central precocious puberty. J Pediatr. 2013;162:562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]