Abstract
Nuclear reprogramming of somatic cells into a pluripotent status has the potential to create patient-specific induced pluripotent stem cells for regenerative medicine. Currently, however, the epigenetic mechanisms underlying this pluripotent reprogramming are poorly understood. To delineate this epigenetic regulatory network, we utilized a chromatin RNA in situ reverse transcription sequencing (CRIST-seq) approach to identify long noncoding RNAs (lncRNAs) embedded in the 3-dimensional intrachromosomal architecture of stem cell core factor genes. By combining CRIST-seq and RNA sequencing, we identified Oct4-Sox2 interacting lncRNA 9 (Osilr9) as a pluripotency-associated lncRNA. Osilr9 expression was associated with the status of stem cell pluripotency in reprogramming. Using short hairpin RNA (shRNA) knockdown, we showed that this lncRNA was required for the optimal maintenance of stem cell pluripotency. Overexpression of Osilr9 induced robust activation of endogenous stem cell core factor genes in fibroblasts. Osilr9 participated in the formation of the intrachromosomal looping required for the maintenance of pluripotency. After binding to the Oct4 promoter, Osilr9 recruited the DNA demethylase ten-eleven translocation 1, leading to promoter demethylation. These data demonstrate that Osilr9 is a critical chromatin epigenetic modulator that coordinates the promoter activity of core stem cell factor genes, highlighting the critical role of pluripotency-associated lncRNAs in stem cell pluripotency and reprogramming.
Keywords: long noncoding RNA, reprogramming, stem cell, pluripotency, Oct4, Sox2, DNA demethylation
Graphical abstract

By combining CRIST-seq and RAT-seq, Zhu et al. characterize lncRNA Osilr9 as a novel pluripotency-associated chromatin epigenetic modulator. Mechanistically, they reveal that Osilr9 maintains stem cell pluripotency by coordinating the intrachromosomal promoter-enhancer loop and TET1-induced promoter demethylation in the Oct4, a master pluripotency self-renewal factor gene.
Introduction
Induced expression of the four transcription factors, Oct4, Sox2, Klf4, and c-Myc (OSKM), can epigenetically reprogram somatic cells to a pluripotent state, resulting in the generation of induced pluripotent stem cells (iPSCs).1,2 This nuclear reprogramming approach has the potential to create patient-specific iPSCs for regenerative medicine. However, the generation of iPSCs from somatic cells using these defined factors is typically an extremely inefficient process,3,4 with only a small minority of the cells in the culture dish completing the nuclear remodeling needed to yield a substantial iPSC population. This is particularly true for reprogramming somatic cells derived from the elderly, probably because of accrued epigenetic changes.5 The identification of cellular factors that determine the efficiency of reprogramming may lead to the successful therapeutic application of these pluripotent cells.
Successful reprogramming starts with a series of epigenetic events, including the downregulation of somatic cell-specific gene expression, followed by the activation of the pluripotency gene regulatory network.6 Chromatin regulators, including epigenetic writers, readers, and erasers, have emerged as determining factors of this nuclear remodeling, acting as facilitators or barriers to successful reprogramming. It is, thus, critical to identify those epigenetic factors that determine cell fate in iPSC induction. Previously, we examined the promoter DNA binding and chromatin architecture in iPSCs that have completed the reprogramming process, comparing these cells with fibroblasts that express the lentiviral OSKM factors yet fail to become iPSCs (non-iPSCs). In non-iPSCs, the endogenous stemness genes were not activated, as there was an absence of intrachromosomal looping between the promoters and enhancers of the stemness genes.7,8,9
Long noncoding RNAs (lncRNAs) have recently been shown to take part in epigenetic, transcriptional, and post-transcriptional control of gene expression.10,11,12 A large number of lncRNAs are differentially expressed in the pluripotent state.13 Many of these lncRNAs are associated with pluripotency and stemness in embryonic stem cells and iPSCs; other lncRNAs promote differentiation of pluripotent cells.14,15,16 We have devised a chromatin RNA in situ reverse transcription sequencing (CRIST-seq) approach to profile lncRNAs that interact with the promoters of the stem cell core factor genes, such as Oct4 and Sox2.13,17 These lncRNAs regulate the pluripotency network through various epigenetic mechanisms.18,19,20,21,22 In this study, we used CRIST-seq in combination with RNA sequencing (RNA-seq), identifying Oct4-Sox2 interacting lncRNA 9 (Osilr9) as a critical pluripotency-associated lncRNA that is specifically activated in somatic cell reprogramming. Notably, Osilr9 epigenetically induced the activation of endogenous stem cell core factors by coordinating intrachromosomal looping and by the recruitment of DNA demethylases ten-eleven translocation (TET) 1. This study highlights the critical role of pluripotency-associated lncRNAs in stem cell pluripotency and reprogramming.
Results
CRIST-seq identifies Osilr9 as a pluripotency-associated lncRNA
To explore epigenetic mechanisms underlying chromatin remodeling, we focused on the lncRNAs that are embedded in or interact with loci that may be involved in the regulation of pluripotency. We used a CRIST-seq approach17 to profile lncRNAs that interact with the promoter of Oct4 and Sox2 (Figure 1A), two key transcription factors required for the transformation of somatic cells into iPSCs. To profile the Oct4/Sox2-associated lncRNAs, Cas9 guide RNAs (gRNAs) and control gRNA (gCT) were transfected into iPSCs and chromatin-associated RNAs were in situ reverse transcribed using biotin-deoxycytidine triphosphate (dCTP). After Cas9-FLAG immunoprecipitation and streptavidin bead purification, an Illumina cDNA library was constructed and sequenced to identify Oct4/Sox2-interacting RNAs.17 The resulting CRIST-seq data were then combined with RNA-seq data13 to identify pluripotency-associated RNAs. Using this strategy, we were able to profile lncRNAs that not only interacted with the Oct4 and Sox2 promoters but also were differentially activated in reprogramming (Figure 1B).
Figure 1.
Mapping pluripotency-associated lncRNAs by RNA-seq and CRIST-seq
(A) CRIST-Seq assay. dCas9, Catalytically inactive CRISPR Cas9; FLAG, a tag octa-peptide at the N-terminal of Cas9; gRNA, Cas9 gRNAs that target the Oct4/Sox2 promoter. After fixation, the Oct4/Sox2 promoter-interacting RNAs were reverse transcribed into cDNAs in the isolated nuclei with biotin-dCTP. The Cas9 Oct4/Sox2 promoter biotin-cDNA complex was immunoprecipitated by a Cas9-FLAG antibody, and biotin-cDNAs were further purified from genomic DNAs by biotin-streptavidin beads. The CRIST-captured cDNAs were Illumina sequenced to identify the RNA components in the Oct4 and Sox2 promoters. (B) Profiling pluripotency-associated lncRNAs by CRIST-seq and RNA-Seq. The Oct4/Sox2-interacting lncRNAs identified by CRIST-seq were integrated with RNA-seq data. The combination of these two datasets helps identify lncRNAs that interact with the Oct4 and Sox2 promoters and are also expressed differentially in reprogramming. (C) Pluripotency-associated RNA candidates identified by RNA-Seq and CRIST-Seq. The RNA candidates are ranked on the basis of the RNA expression-fold between iPSCs and fibroblasts (FIB) from the high (red) to the low (green). Gm28168 (Osilr9) was chosen for further study as it lacks protein-coding potential and has not been characterized previously.
Among the identified candidate RNAs (Figure 1C), some were predicted to have the potential to code known and unknown proteins. They were, therefore, not included in the list of lncRNAs. Next, we quantitated the dynamic expression of these lncRNAs during reprogramming and focused on noncoding RNAs that were closely associated with known stem cell marker genes, such as Oct4 and Sox2. We recently described the role of one of these lncRNAs, Platr10.18 Among the other candidates, lncRNA ENSMUSG00000101776 (Gm28268) was upregulated when fibroblasts completed reprogramming into iPSCs. CRIST-seq showed that Osilr9 specifically interacted with the Oct4 and Sox2 loci. No interaction signals were detected in the Cas9 random (Cas9-CT) CRIST-seq control group (Figure S1). RNA-seq showed that Osilr9, located on the mouse X chromosome, was highly expressed in E14 embryonic pluripotent stem cells as compared with fibroblasts (Figure S2). We thus chose to study this lncRNA, and we have referred to it as Oct4-Sox2 interacting lncRNA 9 (Osilr9). We characterized its gene structure using 5′RACE and 3′RACE and obtained its full-length cDNA (Figure S3).
LncRNA Osilr9 is activated during pluripotent stem cell induction
To determine the role of Osilr9 in pluripotency, we first examined whether its expression was associated with pluripotent reprogramming. We collected cells at different stages of reprogramming, including fibroblasts, iPSCs, and non-iPSCs that expressed the lentiviral OSKM factors but failed to complete reprogramming. An embryonic pluripotent stem cell line E14 was used as the positive control. We found that Osilr9 was highly expressed in fully reprogrammed iPSCs and E14-positive control cells (Figures 2A and S2B). In contrast, Osilr9 was essentially undetectable in fibroblasts and non-iPSCs that fail to complete the reprogramming process.
Figure 2.
Osilr9 is a pluripotency-associated lncRNA
(A) Differential expression of Osilr9 in reprogramming. FIB, fibroblast; non-iPSC, cells that express four OSKM cocktail factors, but failed to complete reprogramming; E14, mouse pluripotent stem cell line used as a positive control. Gene expression was measured by qPCR and was normalized to β-actin. For comparison, the value of positive control E14 was set as 1. (B) The expression of Osilr9 and stem cell core factors in embryoid body (EB) differentiation. E14 cells were collected at different stages of EB differentiation for qPCR. (C) Subcellular localization of Osilr9 lncRNA. RNAs extracted from each subcellular fraction were analyzed by RT-PCR and qPCR. β-Actin was used as the cytoplasmic control and U6 was used as the nuclear control. (D) RNA FISH of Osilr9. LncRNA probes were synthesized using DIG-11-dUTP and detected by anti-digoxigenin-fluorescein (green). DAPI was used to stain the nucleus of E14 cells (blue). Data shown are mean ± standard error of technical replicates from three independent experiments. ∗p < 0.05 as compared with E14 and iPSC (Kruskal-Wallis H test).
We also collected cells on different days after embryoid body differentiation. Osilr9 abundance decreased when E14 cells were induced to differentiate into embryoid bodies (Figure 2B). This downregulation of Osilr9 paralleled the decrease in abundance of three stemness genes Oct4, Sox2, and Nanog.
Using small nuclear RNA U6 as a positive control in a cellular fractionation assay, we found that Osilr9 was localized to the nucleus (Figure 2C). An RNA fluorescence in situ hybridization (RNA FISH) assay confirmed its nuclear localization (Figure 2D).
LncRNA Osilr9 is required for the maintenance of stem cell pluripotency
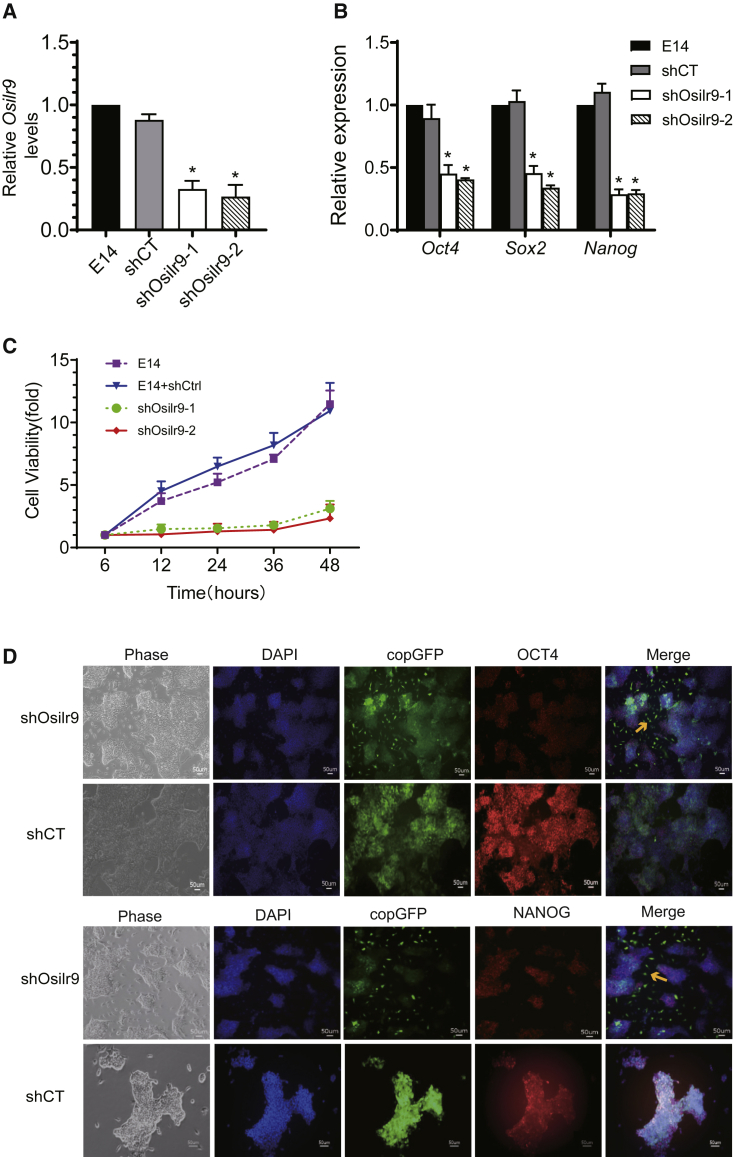
To further explore the role of Osilr9, we silenced Osilr9 in E14 cells using two short hairpin RNA (shRNA) lentiviruses (shOsilr9-1 and shOsilr9-2). Each lentivirus carried two shRNAs (Figure S4A). Osilr9 knockdown was tracked using a copGFP marker in the lentiviral vector (Figure S4B). After puromycin selection, stably-transduced bulk cells emitting the copGFP green signal were collected for qPCR quantitation. Osilr9 was significantly knocked down by the two shRNAs in E14 cells (Figure 3A). Depletion of Osilr9 significantly reduced the expression of Oct4, Sox2, and Nanog (Figure 3B). In addition, we used an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay to examine the cell viability of these three groups of cells. Osilr9 knockdown decreased the cell viability of E14 cells (Figure 3C).
Figure 3.
Osilr9 is required for the maintenance of pluripotency
(A) Osilr9 knockdown in E14 cells. After lentiviral shRNA transduction and puromycin selection, stably transduced bulk E14 cells were collected for qPCR analyses. shCT, random shCT; shOsilr9-1 and −2, Lenti Osilr9 shRNA vector 1 and Osilr9 shRNA vector 2, with each vector containing two separate shRNAs. For comparison, the abundance of Osilr9 in E14 cells was set as 1. (B) Expression of stem cell core factors in Osilr9-knockdown E14 cells. Stably transduced bulk E14 cells were collected for PCR quantitation of stem cell core factors. (C) Effects of Osilr9 on cell viability. An MTT assay was performed to determine the cell viability of E14 cells transduced with two Osilr9 shRNAs and E14 cell controls. (D) Pluripotent marker staining in Osilr9 knockdown E14 cells. Osilr9 shRNA and random shRNA lentiviral vectors carry the copGFP tracker gene (green). After lentiviral transduction and puromycin selection, cells were fixed and immunostained by antibodies against stem cell pluripotent markers OCT4 (top) and NANOG (bottom) (red), respectively. Yellow arrow, shOsilr9 transduced E14 cells were copGFP-positive and were negative for OCT4 and NANOG immunostaining with differentiated morphology. shCT, lentiviruses carrying the random shCT. Data shown are mean ± standard error of technical replicates from three independent experiments. ∗p < 0.05 as compared with E14 and random shCT controls (Kruskal-Wallis H test).
We also examined whether Osilr9 knockdown would affect the fate of treated E14 cells (Figure 3D). In the random shRNA control (shCT) group, the copGFP-positive cells maintained the same cellular morphology as pluripotent stem cells. However, shRNA knockdown of Osilr9 dramatically altered cell morphology (top panel 1, shOsilr9), with the Osilr9-deficient cells becoming enlarged and flat. Pluripotency was examined in Osilr9 knockdown E14 cells by immunostaining of the two stem cell markers, OCT4 and NANOG. Osilr9 shRNA knockdown induced E14 cells to differentiate and lose these pluripotency-associated markers (top panel 4, unmarked regions). Similar results were also obtained by using alkaline phosphatase staining (Figure S5). These data suggest that lncRNA Osilr9 silencing abrogates the pluripotency of E14 cells.
Loss of Osilr9 induced cells to differentiate preferentially toward the mesoderm lineage, as shown by the high expression of mesoderm markers Hand 1 and Brachyury (Figure S6). These data suggest that Osilr9 may be a critical inhibitory factor supporting early mesoderm development.
LncRNA Osilr9 activates stem cell core factors in fibroblasts
We then initiated a series of studies to delineate the molecular mechanisms by which Osilr9 regulates reprogramming. To determine if Osilr9 could activate the expression of stem cell core factor genes in fibroblasts, we synthesized a lenti-pCMV-DsRed/Puro-Osilr9 plasmid and packaged the Osilr9 lentivirus in 293T cells (Figure S7A). Fibroblasts were transduced with Osilr9 lentiviruses. After puromycin selection, more than 90% of cells expressed Osilr9 as determined by using DsRed as a tracking marker (Figures S7B and S7C). Using qPCR, we confirmed a more than 8-fold overexpression of Osilr9 as compared with the vector control (Figure 4A). Oct4, Sox2, and Nanog were upregulated in parallel with Osilr9 overexpression in these cells (Figure 4B).
Figure 4.
Osilr9 activates stem cell core factor genes
(A) Lentiviral Osilr9 overexpression in fibroblasts. Osilr9, lentiviruses carrying the full-length Osilr9 cDNA; Vector, empty lentiviral vector; lncCT, lncRNA random control. After lentiviral infection, cells were selected by puromycin and showed a greater than 92% transduction of DsRed fluorescence. The bulk population of stably transduced cells was collected for qPCR. (B) Upregulation of endogenous stem cell core factor genes in Osilr9-overexpressing fibroblasts. The bulk population of stably transduced cells was collected for qPCR. U6 was used as the negative control. (C) Cell proliferation analyses of Osilr9 transduced fibroblasts. Random lncRNA (lncCT) was used as the control. (D) Osilr9 enhances promoter activities of core pluripotent factors. The 293T cells were co-transfected with Osilr9 plasmid, reporter plasmid, Renilla control, and reporter plasmid DNAs. Forty-eight hours after transfection, cells were collected for luciferase activity measurement using the Firefly-Renilla Dual luciferase kit. Reporter plasmid, empty vector and random lncRNA (lncCT) vectors were used as the controls. The luciferase activity was calculated by adjusting over the Renilla reading. For comparison, the untreated fibroblasts were set as 1. Snhg14 was used as a positive control in the reporter assay. Data shown are mean ± standard error of technical replicates from three independent experiments. ∗p < 0.05. ns, not statistically significant, as compared with the Vector and lncCT controls (Kruskal-Wallis H test or Mann-Whitney U test).
In addition, we assessed the effect of Osilr9 on cell proliferation. Fibroblasts transduced with Osilr9 or lncCT lentiviruses were incubated with carboxyfluoresceinsuccinimidyl ester (CFSE) for 5 days. Fluorescein cell sorting (FACS) analysis showed that, compared with the lncCT group, the Osilr9 overexpression group had an increased cell proliferation rate, suggesting that Osilr9 promotes cell growth (Figure 4C).
Next, we explored whether Osilr9 could activate the promoter activities of these stemness genes. We constructed luciferase plasmids containing the promoters of these three pluripotency transcript factors, and then co-transfected them with the Osilr9 overexpression plasmid in 293T cells. Forty-eight hours after transfection, cells were harvested and luciferase activity was measured using the dual-luciferase reporter assay system. Like Snhg14, a known lncRNA that activates stemness genes,23 Osilr9 also increased the luciferase activity of the promoters of Oct4, Sox2, and Nanog in 293T by 15-, 2-, and 3-fold, respectively, as compared with the vector control and random controls (Figure 4D). These data indicate that lncRNA Osilr9 activates these stemness genes.
Osilr9 activates Oct4 promoter expression by inducing DNA demethylation
During reprograming, the Oct4 gene is epigenetically regulated by promoter DNA methylation. There are two CpG islands in the Oct4 promoter, and the status of DNA methylation in these two CpG islands correlates with the promoter activity. Induced DNA demethylation using CRISPR dCas9-TET1 epigenetic targeting can activate the endogenous Oct4 gene.24 Using both in situ reverse transcription-associated trap (RAT) and chromatin isolation by RNA purification (ChIRP) assays, we found that Osilr9 directly interacted with the Oct4 promoter (Figure S8). We, thus, hypothesized that Osilr9 might regulate Oct4 by altering the promoter epigenotype.
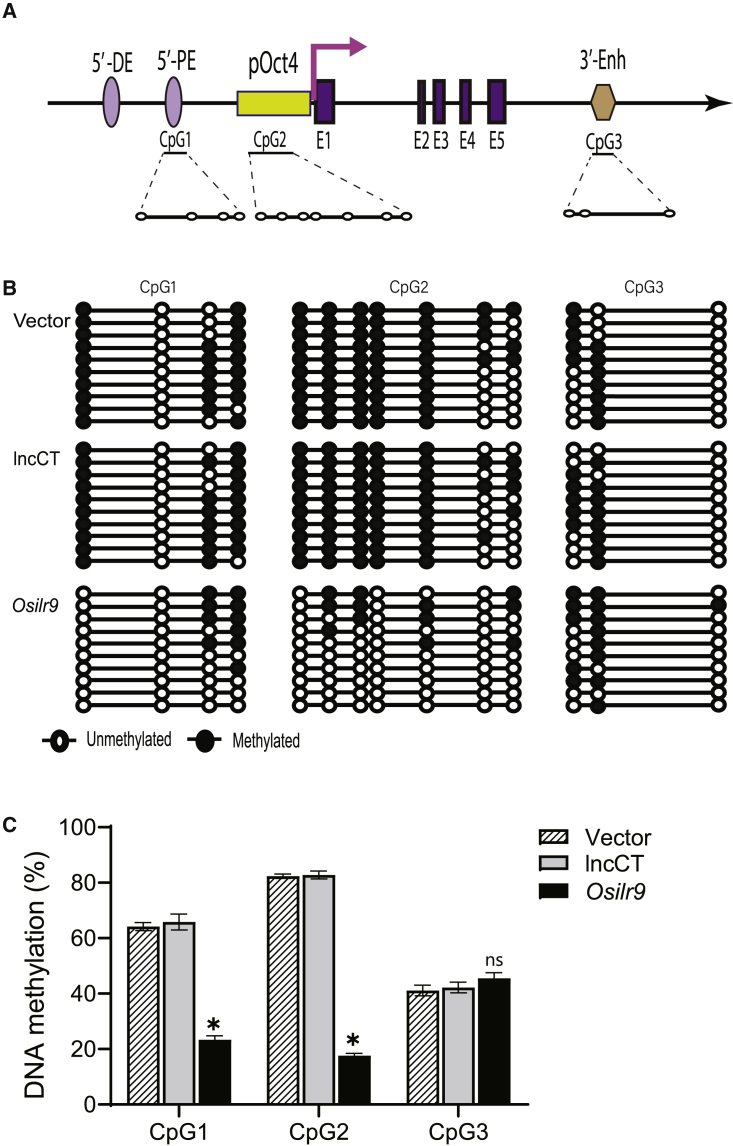
We isolated genomic DNAs from Osilr9-overexpressing fibroblasts and used sodium bisulfite sequencing to examine the status of DNA methylation in the Oct4 locus (Figure 5A). As expected, the empty vector control and the lncCT group did not affect DNA methylation in this locus (Figure 5B, panels 1 and 2). However, after Osilr9 overexpression, we observed an extensive decrease in DNA methylation in the Oct4 promoter, particularly at CpG1 and CpG2 islands (Figure 5B, panel 3). Quantitation of DNA methylation in three independent assays also showed CpG demethylation in the Osilr9-overexpressing fibroblasts (Figure 5C). These data suggest that Osilr9 may trigger the activation of the Oct4 and its promoter through DNA demethylation.
Figure 5.
Osilr9 induces DNA demethylation at the Oct4 promoter locus
(A) CpG islands at the Oct4 locus. CpG1: 5ʹ-distal enhancer (5ʹ-DE); CpG2: CpG2: promoter region (P); and CpG3: 3ʹ-enhancer (3ʹ-Enh). (B) Induced Oct4 CpG demethylation in Osilr9-overexpressing fibroblasts. DNAs were extracted from Osilr9 stably transduced bulk fibroblasts, treated by sodium bisulfite, amplified with the Oct4-specific primers, cloned into the pJET vector, and sequenced. Solid circle, methylated CpG; open circle, un-methylated CpG. Each line represents sequencing from one clone. A total of 10 clones were sequenced for each group. (C) Quantitation of DNA methylation in the Oct4 promoter. The status of DNA methylation was calculated as the percentage of methylated CpGs in each island. Data shown are mean ± standard error from three independent assays in fibroblasts. ∗p < 0.05. ns, not statistically significant, as compared with the Vector and lncCT controls (Kruskal-Wallis H test).
LncRNA Osilr9 recruits TET family enzymes and induces DNA methylation
DNA methylation is regulated by TET family enzymes that convert 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC).25 To determine if Osilr9 activates its target genes by coordinating with TET family enzymes, we compared the expression of the TET family enzymes in E14 cells after Osilr9 knockdown. We found that both the Tet1 and Tet2 expression levels were downregulated in parallel with Osilr9 downregulation, while TET3 was not affected (Figure S9).
We then determined if Osilr9 recruits TET1 and TET2 demethylases to the Oct4 locus. Osilr9-overexpressing and vector control fibroblasts were collected, and chromatin immunoprecipitation (ChIP) was performed to assess the binding of TET demethylases to the Oct4 locus. The TET1/2-RNA chromatin complex was immunoprecipitated by the anti-TET1/2 antibody. An anti-IgG antibody was used as the ChIP control. The anti-TET1/2 pulled-down chromatin complex was quantitated by qPCR for its enrichment at Oct4 regulatory elements. As compared with the vector control, Osilr9 overexpression significantly enhanced the binding of TET1 demethylase to Oct4 regulatory elements, particularly the gene promoter (Figure 6A). However, Osilr9 did not significantly affect the recruitment of TET2 demethylase to the Oct4 locus (Figure 6B).
Figure 6.
Osilr9 recruits TET1 DNA demethylase
(A) Osilr9 recruits TET1 enzyme to the Oct4 locus. Stably transduced bulk Osilr9-overexpressing fibroblasts were collected, and ChIP was performed to determine the binding of TET1 demethylase to the Oct4 locus. The TET-RNA chromatin complex was immunoprecipitated with an antibody against TET1. IgG was used as a ChIP control. The enrichment of the Oct4 regulatory elements in the anti-TET1 pulldown chromatin complex was measured by qPCR. The values were normalized to that of the IgG control. 5ʹ-PE, 5ʹ-proximal enhancer; pOc4-1, distal Oct4 promoter; pOct4-2, proximal Oct4 promoter; 3ʹ-Enh, 3ʹ-enhancer. Data shown are mean ± standard error of technical replicates from three independent experiments. ∗p < 0.05. ns, not statistically significant, as compared with the vector control (Mann-Whitney U test). (B) The TET2-Oct4 interaction in Osilr9-overexpressing fibroblasts. Fold enrichments of TET2 to the Oct4 locus were measured in TET2 ChIP samples and were calculated by normalizing over that of IgG controls. ∗p < 0.05. ns, not statistically significant, as compared with the vector control (Mann-Whitney U test). (C) RIP mapping of the Osilr9 lncRNA fragment that interacts with TET1 demethylase. Bulk Osilr9-overexpressing fibroblasts were collected for the RIP mapping. After sonication and immunoprecipitation, the TET1-interacting Osilr9 lncRNA fragments were mapped by qPCR using overlapping primers (A–F). Mouse IgG was used as the negative control and anti-EZH2 as the positive control. RIP data are represented by fold enrichment relative to the input. Data shown are mean ± standard error of technical replicates from three independent experiments. ∗p < 0.05 and ∗∗∗p < 0.001, as compared with the IgG control (Student's t-test). (D) The Osilr9 IGV binding signal of TET1 RIP-seq data. The TET1 binding fragment in Osilr9 was mapped by RIP sequencing. The Osilr9 binding signal was analyzed using the IGV program. Anti-IgG was used as the RIP-seq control.
We then examined which part of the Osilr9 lncRNA directly interacts with the TET1 enzyme. Osilr9-overexpressing fibroblasts were collected for the RNA-binding protein immunoprecipitation (RIP) assay. After sonication, the TET1-RNA chromatin complex was immunoprecipitated by an anti-TET1 antibody. An anti-EZH2 antibody was used as the positive control and an anti-IgG antibody was used as the RIP negative control. The pulled-down RNA fragments were reverse transcribed and quantitated by quantitative PCR (qPCR) using primers covering different Osilr9 fragments. As compared with the IgG control, there was significant enrichment in the 5′ fragment of Osilr9 in the TET1 antibody-precipitated complex (Figure 6C).
The TET1 antibody-precipitated cDNAs were then used for Illumina library sequencing. A detailed IGV analysis of the TET1 RIP-seq indicated that Osilr9 binding to TET1 was increased only in the TET1 antibody immunoprecipitated chromatin complex, but not in the IgG immunoprecipitation control (Figure 6D). Of note, the TET1 binding site was primarily located in the exon 1 region of Osilr9.
Taken together, these data suggest that Osilr9 may control DNA methylation by multiple mechanisms. In addition to its ability to maintain optimal transcription of DNA demethylases TET1 and TET2, Osilr9 also recruits TET1 DNA demethylase to the Oct4 promoter, where the enzyme induces DNA demethylation to promote the expression of Oct4.
Osilr9 coordinates pluripotency-specific intrachromosomal looping
Epigenetic remodeling plays a key role in cell-fate conversion from somatic cells into pluripotent status. During this chromatin remodeling, the genome undergoes major epigenetic alterations to reacquire the euchromatin characteristic of pluripotent cells.26,27,28 For example, the Oct4 locus forms a pluripotency-associated intrachromosomal loop in iPSCs that juxtaposes a downstream enhancer to the gene’s promoter, enabling its activation to initiate reprogramming.8 Since Osilr9 binds to these regulatory elements, we asked whether this lncRNA participates in the orchestration of intrachromosomal looping for reprogramming.
We compared intrachromosomal looping between E14 cells, shCT, and Osilr9 knockdown E14 cells using a chromatin conformation capture (3C) technique29 using specific 3C primers (Figure 7A). As previously reported,8 we detected reprogramming-associated intrachromosomal interaction products in E14 cells, including the JH4780/JH4783 and JH4780/JH4785 loops between the 5′ upstream enhance and the promoter, as well as the JH4792/JH4783 loops between the 3′ enhancer and the promoter (Figure 7B). Treatment of E14 cells with the shCT did not significantly affect this intrachromosomal looping. However, shRNA knockdown of Osilr9 abolished these intrachromosomal loops and caused E14 cells to exit from pluripotency.
Figure 7.
Osilr9 orchestrates pluripotency-specific intrachromosomal looping
(A) Location of 3C primers used to detect the interaction between the Oct4 promoter and enhancer. Enh, enhancers; pOct4, Oct4 promoter; E1-E5, exons; Arrows, intrachromosomal interactions. (B) Knockdown of Osilr9 abolishes the intrachromosomal interaction loop in E14 cells. shCT, negative control shRNA; shOsilr9-1, shOsilr9-1, two shRNA lentiviruses that target Osilr9 lncRNA. After puromycin selection, bulk E14 cells were collected for the 3C assay. The 3C interaction was quantitated by qPCR and was standardized over the 3C input control. For comparison, the relative 3C interaction was calculated by setting the 5ʹ- control site as 1. (C) Osilr9 induces de novo formation of intrachromosomal looping in fibroblasts. FIB, fibroblasts; Fib + Vector, fibroblasts transduced with vector control; Fib + Osilr9, fibroblasts overexpressed Osilr9. Stably transduced Osilr9 bulk fibroblasts were collected for the 3C assay. (D) Sequencing of the Oct4 intrachromosomal loop products. Blue line on the top of the sequence, the 3C ligation product between the BamH1 and Bgl2 sites. Data shown are mean ± standard error from three independent experiments. ∗p < 0.05, as compared with the controls (Kruskal-Wallis H test).
We then asked whether overexpression of lncRNA Osilr9 was able to trigger the formation of these intrachromosomal loops. Osilr9-overexpressing fibroblasts were collected for the 3C assay. As expected, no three-dimensional (3D) interactions at these sites were detected in fibroblasts or in vector-transduced control fibroblasts, but we observed de novo formation of intrachromosomal interactions in fibroblasts when Osilr9 was overexpressed in these cells (Figure 7C).
We sequenced the 3C products and confirmed the presence of intrachromosomal loops. As predicted, the sequences covered the ligated BamHI/BglII sites, which were flanked by sequences from the promoter and enhancers of Oct4, respectively (Figure 7D). Overall, our data suggest that Osilr9 is critical for the maintenance of the intrachromosomal interactions that are known to be associated with reprogramming and the maintenance of pluripotency.8
Discussion
The epigenetic mechanisms underlying the reprogramming of somatic cells into iPSCs remain largely unknown. In particular, we do not understand why the majority of the defined factor-expressing cells are unable to complete the reprogramming process. As an initial step to delineate the reprogramming-associated regulatory network, we focused on the promoter chromatin component factors, particularly the lncRNAs that regulate the stem cell core factor genes through epigenetic mechanisms.30,31,32 In this study, by using combined RNA-seq and CRIST-seq, we identified Osilr9 as a crucial regulator that helps to maintain the pluripotency of iPSCs. Osilr9 is differentially expressed during the reprogramming progress, as it is nearly silenced in fully differentiated fibroblasts, but is highly expressed in iPSCs. Knockdown of Osilr9 induces the loss of pluripotency in E14 cells, while lentivirus-induced expression Osilr9 increases the abundance of endogenous stem cell core factors in fibroblasts. Osilr9 induces Oct4 promoter demethylation by recruiting TET1/2, two DNA demethylases that are required for the initiation of reprogramming. TET1/2 genes are also downregulated in the Osilr9-knocked-down E14 cells. Finally, Osilr9 plays a vital role in the formation of the intrachromosomal looping structure that is required for the activation of stemness gene expression. Knockdown of Osilr9 in E14 cells abolishes this intrachromosomal long-range interaction in parallel with the exit of E14 cells from pluripotency, while there is de novo formation of this looping structure in fibroblasts in cells that overexpress Osilr9. Thus, Osilr9 plays a crucial role in coordinating a topological network and a pluripotency-associated epigenotype that is necessary to initiate reprogramming.
Subcellular localization may help to predict the function of lncRNAs.33 Nuclear lncRNAs usually act as guides and tethers to enhance or activate the expression of specific genes. They can help to guide chromatin factors, such as epigenetic writers, readers, and modifiers, bind to specific genomic loci, or function as scaffolds to tether-related functional complexes.34 LncRNAs can interact with proteins and with RNA by base-pairing with other nucleic acids,35,36 thereby activating or repressing gene transcription. In contrast, the lncRNAs localized to the cytoplasm often regulate gene expression by base-pairing complementary regions on target RNAs or sequestering microRNAs.37 In this study, we demonstrate that Osilr9 is highly enriched in the nucleus, and that it exerts its activity by binding to the Oct4 promoter and recruiting TET proteins to induce DNA demethylation and, thus, enhance the expression of stemness genes. Osilr9 is also involved in the formation of pluripotency-associated intrachromosomal loops, which bring the distant enhancer in close proximity to the Oct4 promoter as an essential step in iPSC induction. Thus, Osilr9 controls the pluripotency state in reprogramming through this multi-faceted network.
DNA methylation is an epigenetic modification that is involved in the repression of gene transcription, the maintenance of genomic integrity, the establishment of parent-specific imprinting patterns, and the repression of transposable elements.38 DNA methylation of promoter CpGs is associated with transcriptional silencing and is essential for mouse embryonic development.39 In contrast, DNA methylation-deficient embryonic stem cells (ESCs) are unable to differentiate, indicating that DNA methylation is required for the cell to exit from pluripotency.40 An important epigenetic modification that takes place during reprogramming is the removal of DNA methylation on the promoters of pluripotency-regulated genes.41,42 TET family proteins can catalyze DNA demethylation by converting 5mC to 5hmC, which is further oxidized to 5-formylcytosine and 5-carboxylcytosine.43,44 Accumulating evidence shows that the TET family enzymes play an important role in the development and stem cell pluripotency maintenance.45,46,47 Recently, several lncRNAs have been demonstrated to interact with the Oct4/Sox2 loci.11,14,17,48 Notably, these lncRNAs regulate the pluripotency and differentiation network using different epigenetic mechanisms. After binding to the regulatory elements of pluripotency-associated genes, like promoters and intragenic enhancers, these RNA molecules coordinate the construction of pluripotency-specific chromatin loops, with the recruitment of TET demethylases, promoter epigenotypes alterations, and the enhancer RNA pathway.18,19,21,22 In this study, using a RIP-PCR assay, we demonstrate that Osilr9 can recruit TET1 to the Oct4 promoter, where the induced demethylation promotes the expression of the Oct4 gene for reprogramming.
Sox2 is another core regulatory gene required for the establishment and maintenance of pluripotency. Like Oct4, it is also activated during reprogramming. However, unlike Oct4, the molecular mechanisms underlying the activation of Sox2 in reprogramming is not well defined. Quantitation of DNA methylation at the Sox2 promoter did not show noticeable differences between pluripotent and somatic cells.41 In this study, we found that Sox2 is slightly upregulated in Osilr9-overexpressing fibroblasts. Similarly, DNA methylation was not significantly different in the treated group. Thus, it seems that Sox2 may be controlled by epigenetic modifications outside the promoter area, possibly at its 5′- and -3′′-enhancer loci.41,49 Future studies are needed to address if Osilr9 regulates the Sox2 gene at these super-enhancer areas during changes in stem cell fate.
In this study, we also noted that both Tet1 and Tet2, but not Tet3, were downregulated in Osilr9-knockdown ESCs (Figure S7), suggesting that Osilr9 may be a critical factor in maintaining the optimal expression of the Tet family genes. It is expected that the downregulation of these two critical DNA demethylases in Osilr9 knockdown cells may induce genome-wide DNA hypermethylation, resulting in the downregulation of downstream target genes. It may partially account for the downregulation of stemness genes, like Oct4, in Osilr9-KD cells (Figure 3B). However, we do not currently have direct evidence to support this hypothesis. Future studies are needed to delineate the epigenetic control of Tet1 and Tet2 by Osilr9. Furthermore, we show that TET2 does not bind to the Oct4 regulator loci in Osilr9-overexpressing fibroblasts (Figure 6B). Thus, TET2 demethylase may not be involved in the process of CpG DNA demethylation in the Oct4 promoter. It will be interesting to explore if Osilr9 and TET2 can form a partnership to regulate the Oct4 gene using other potential epigenetic mechanisms, including modifications in the super enhancer regulatory region.
Proper remodeling of 3D chromatin architecture is very important for early mammalian development,50,51 as remodeling plays an important role in controlling the cell fate and identity.52 The reprogramming of somatic cells to a pluripotent status also requires chromatin remodeling to switch off the somatic program and establish the pluripotent state. For example, pluripotency-specific intrachromosomal looping is observed between the gene promoter and enhancer regulatory regions in the Oct4 locus.8 This intrachromosomal looping juxtaposes distal enhancers into physical proximity with the Oct4 promoter, thereby activating it to initiate reprogramming. In this study, we showed that Osilr9 is essential for the maintenance of this pluripotency-specific intrachromosomal loop structure. Notably, Osilr9 knockdown abolishes this 3D chromatin loop structure and causes E14 cells to exit from pluripotency. Our data, thus, suggest that, during reprogramming, Osilr9 is actively transcribed and that its transcripts bind to regulatory elements, like the promoter and enhancers of the Oct4. By coordinating with other chromatin factors and lncRNAs, Osilr9 organizes a topological cross-talk network that is necessary for the establishment and maintenance of pluripotency.
As a major critical epigenetic modification, DNA methylation is closely associated with chromatin structure. By comparing iPSCs and unreprogrammed cells that express the viral OSKM insert, Zhang et al demonstrated that the promoter-enhancer intrachromosomal loop was a critical epigenetic barrier to induction of pluripotency.7 To overcome this reprogramming barrier, Morgan et al53 used an inducible CRISPR-dCas9 approach to induce chromatin loop reorganization. They found that this enforced functional intrachromosomal looping was able to activate the Oct4 gene in fibroblasts. Using the same approach, Wang et al.54 showed that this artificial chromatin loop enhanced reprogramming efficiency. It is assumed that the induced Oct4 activation is accompanied by CpG demethylation in the promoter. However, it is not clear how this induced chromatin loop directly alters the status of DNA methylation in the Oct4 CpG islands, or vice versa.
In summary, this study identifies Osilr9 as a critical pluripotency-associated lncRNA. Osilr9 is not expressed or is expressed at a very low level in somatic cells, such as fibroblasts, as well as in non-iPSCs that fail to complete pluripotent reprogramming. However, after the completion of reprogramming, Osilr9 became activated in iPSCs. Osilr9 depletion caused E14 cells to differentiate and lose pluripotency. Osilr9 maintains stem cell pluripotency through multiple epigenetic mechanisms, including the activation of endogenous stem cell core factor genes, coordination of intrachromosomal looping, and regulation of TET1 to induce DNA demethylation. Thus, this lncRNA functions as a chromatin epigenetic modulator in the regulatory network of stem cell pluripotency and reprogramming.
Materials and methods
CRIST-Seq to map the Oct4/Sox2-interacting lncRNAs
We used CRIST-Seq to identify lncRNAs that bind to the Oct4 and Sox2 promoters. As previously described,17 two gRNAs were designed for each promoter and were ligated to a lentiviral dCas9 vector under the control of U6 and H1 promoters, respectively. We also constructed a random gRNA (gCT) in the same vector as the control. The Cas9-gRNA lentiviruses were produced in 293T cells, as previously described,55,56,57 and were used to infect E14 cells. Transduced cells were selected by puromycin.58,59 Cells were cross-linked with 2% formaldehyde and lysed with cell lysis buffer. The nuclei were isolated and nuclear RNAs were reverse transcribed into cDNAs using dCTP-biotin labeling. After nuclear lysis, the biotin-labeled cDNA/Cas9 complex was immunoprecipitated with anti-FLAG antibodies (#F7425, Sigma). An anti-IgG antibody was used as the background control.13 The biotin-labeled cDNAs were further purified from genomic DNAs with M-280 streptavidin beads (Invitrogen). The Oct4-Sox2 promoter-interacting cDNAs were used for Illumina cDNA sequencing (Shanghai Biotechnology).17
After CRIST-seq, we removed the called peaks that overlapped with that of the IgG control, and the CRIST-Seq signal intensities were further normalized by comparison with the non-targeting Cas9 gCT control using parameters of fold change difference of 2 or more and a p value of less than 0.05, with a false discovery rate of less than 0.1. The adjusted CRIST-seq data were then used for mapping the Oct4 and Sox2 RNA interactions.13,17
Identification of lncRNAs by RNA-seq in reprogramming
As previously reported,13 RNA-seq was performed to identify lncRNAs that are differentially expressed in reprogramming. We used TRIzol (Invitrogen) to isolate RNAs from fibroblasts and iPSCs, and prepared indexed libraries for paired-end sequencing using a HiSeq4000 (Shanghai Biotechnology). Gene counts were normalized as the reads per kilobase of transcript per million mapped reads. Cuff diff was used to identify lncRNAs that are differentially expressed in reprogramming, with a fold change of more than 2 and a p value of less than 0.05 with an unpaired two-sided t test.
Cell reprogramming
Reprogramming was performed using mouse fibroblasts OSKM lentiviruses as previously reported.7,60 Briefly, fibroblasts were infected with OSKM lentiviruses in the presence of polybrene (8 μg/mL). Three days after infection, cells were transferred and cultured on mitomycin C-inactivated MEF feeder cells in the ES medium (DMEM/F12 supplemented with 20% KSR, 10 ng/mL leukemia inhibitory factor [LIF, Sigma], 10 ng/mL β-fibroblast growth factor (PeproTech), 0.1 mM β-mercaptoethanol, L-glutamine, and 1 × 10−4 M non-essential amino acids.61 The iPSC colonies were isolated and characterized by immunostaining stem cell markers, alkaline phosphatase staining, karyotype analysis, and teratoma formation as previously described.7,60,62 Fibroblast-like cells that expressed OSKM transgenes but failed to complete reprogramming were used as non-iPSCs for the assays.7,13
RNA extraction and cDNA synthesis
As previously described,19,22 RNAs were extracted using Trizol reagent (Invitrogen) and stored at −80°C. For cDNA synthesis, 400–800 ng RNAs were digested with DNase I (Millipore Sigma) to remove genomic DNA contamination. cDNAs were synthesized with M-MLV reverse transcriptase at 37°C for 1 h and 95°C for 10 min; and were diluted 10-fold for PCR and RT-qPCR assays.
Quantitation of gene expression by qPCR
Reverse transcriptase (RT)-PCR was carried out with a Bio-Rad Thermol Cycler: 1 cycle at 98°C for 5 min, 33 cycles at 95°C for 20 s, 62°C for 15 s, 72°C for 15 s, and 1 cycle at 72°C for 10 min. qRT-PCR was performed in triplicate using SYBR GREEN PCR Master (Applied Biosystems) with a StepOnePlus real-time PCR system (ABI Prism 7900HT; Applied Biosystems). The threshold cycle (Ct) values of target genes were normalized over that of the β-actin control.22
Cytoplasmic and nuclear fractionation assay
Cells were treated with Trypsin-EDTA and gently resuspended in DMEM. Then cells were spun down in Falcon tubes for 5 min at 3,000 rpm in a Beckman tabletop centrifuge and washed with PBS. After completely aspirating the PBS, 800 μL hypotonic buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl) was added to samples and placed on ice for 2 min. We added 10% Nonidet P-40 to a final concentration of 0.4% (35 μL). Samples were inverted a few times and spun at 3,000 rpm for 7 min. Supernatants (450 μL, cytoplasmic fractions) were collected for processing, and the pellet (nuclear fraction) was gently resuspended in 500 μL hypotonic buffer and spun at 3,000 rpm for 2 min. This washing step was repeated three to four times. After removing the buffer following the last spin, the samples were briefly spun again to remove the remaining supernatant from cells. Both the cytoplasmic and nuclear fractions were processed for RNA extraction, cDNA synthesis, and real-time PCR as recommended by manufacturer.
RNA FISH
RNA FISH was carried out by modifying the method published previously.63,64,65 The RNA FISH probe was prepared as an antisense single-stranded DNA (ssDNA) by asymmetric PCR.66 Briefly, the probe was synthesized by 3 X Klen-Taq I DNA polymerase mix, iPSC cDNA template, diluted forward primer JH5194 and undiluted reverse primer JH5195 listed in Table S1. For hybridization, 0.1 μg ssDNA probe and 10 μg salmon sperm DNA (Boehringer) were precipitated with ethanol and resuspended in 10 μL RNA hybridization buffer (2× SSC, 10% dextran sulfate, 0.2 mg/mL BSA [Invitrogen], 2 mM VCR, 10% formamide). After sequential RNA FISH, slides were counterstained with DAPI, and FISH signals were detected using the Keyence BZ-X710 fluorescence microscope with a green fluorescent protein (GFP) filter (EX 470/40 nm, DM 495nm, BA 525/50 nm), Cy5 filter (EX 620/60 nm, DM 660 nm, BA 700/75 nm), and DAPI filter (EX 360/40 nm, DM 400 nm, BA 460/50 nm), respectively. Images were captured and merged to confirm the subcellular localization.
Characterization of full-length Osilr9
The full-length lncRNA Osilr9 sequence was determined using 5′RACE and 3′RACE system to characterize the both cDNA ends following the manufacturer’s instructions (Invitrogen). For 5′-racing, total RNAs were reverse transcribed using Maxima Reverse Transcriptase with random hexamer oligonucleotides for 30 min. The first racing PCR was performed by adding PolyG primer (SJ774) to the mixture for 30 min. DNAs were diluted (1:9) and were used for the second nested PCR using 5′-adaptor RACE primer (SJ775) and 5′-GSP (JH5658). PCR bands were cut out and cloned in a pJET vector for sequencing. For the 3′-end, we used PolyT primer (SJ773) to synthesize the first strand of cDNA, followed by first racing PCR using 3′-RACE primer (SJ771) and gene-specific primer 1 (GSP, JH6548). After diluting the first PCR product (1:500), the second PCR was performed using 3′ RACE primer (SJ772) and GSP2 (JH6549). Primers are listed in Table S1.
Embryoid body differentiation
E14 cells were digested by collagenase IV (Invitrogen) and cultured using a floating method in a 6-cm culture dish without LIF. After 3 days, embryoid bodies (EBs) were transferred to 0.1% gelatin-coated 10-cm plates for suspension culture with slow shaking for up to 12 days. The media were replenished by sedimentation every other day. EBs were collected at different time points and used for gene expression analysis using RT-qPCR.
Knockdown of Osilr9 lncRNA in E14 cells
LncRNA Osilr9 was knocked down with two separate shRNA lentiviral vectors (shOsilr9-1 and shOsilr9-2). Each shRNA vector contained two shRNAs. shRNAs were designed online (http://katahdin.cshl.edu/homepage/siRNA/RNAi). For cloning, two pairs of shRNAs (5′- GACAGATTCTCTCCTCTGAGTTTCA-3′; 5′-ATGATGGTAGAGGACATCAAGAAGG-3′ and 5′- TATTCTGTTGCTGATGCTCAAATCT-3′; 5′-CTGATAGACGGTAACAGAATCACCA-3′) were linked to the H1 and U6 promoter using PCR and were ligated into the EcoR1/BamH1 site in a pGreenPuro vector (#SI505A-1, SBI). The copGFP reporter in the vector was used to track lentivirus transduction. A random shRNA (GCAGCAACTGGACACGTGATCTTAA) was cloned in the same vector as the assay control (shCT). After lentiviral transduction, E14 cells were selected by puromycin. The bulk population of stably transduced cells was collected for quantitation of Osilr9 lncRNA and related genes using RT-qPCR. Primers are listed in Table S1.
Histoimmunochemical staining of stem cell markers
Stem cell markers in iPSC colonies were examined using immunofluorescent staining.67 Briefly, cells were fixed by 4% paraformaldehyde for 10 min at room temperature, washed three times in PBS for 5 min each, permeabilized with freshly made 0.5% v/v Triton X-100/PBS on ice for 5 min, and then blocked in 1% w/v BSA for 30 min at room temperature. After incubation with primary antibodies (2 μg/mL 1:500) for 1–3 h at room temperature or overnight at 4°C, the samples were washed three times in PBS for 5 min each, and then incubated with secondary antibodies for 1 h at room temperature. The following antibodies were used for immunostaining: rabbit anti-OCT4 antibody (AB-3029, 1:100 dilution; Millipore) and rabbit anti-NANOG antibody (sc-33759, 1:100 dilution; Santa Cruz). Cell samples were subsequently incubated with Cy3 or Alexa Fluor 647 labeled secondary antibodies for 1 h, and were counterstained with Hoechst 33258 (Invitrogen). Alternatively, the pluripotency of stem cells was examined using a Fluorescent Mouse ES/iPS Cell Characterization kit (Cat.#SCR077, Millipore) following the protocol provided by the manufacturer. Fluorescence images were acquired with a Zeiss AxioCam Camera.
MTT assay
Cell viability was examined using the Cell Growth determination KIT MTT Bases (Sigma, Stock No. CGD-1) following the manufacturer’s instructions. Cells were cultured in 96-well plates. Medium was removed when cells were adherent to the plate and MTT SOLUTION was added in an amount equal to 10% of the culture volume. Cells were incubated in a 37°C incubator with 5% CO2 for 4 h. After removing the MTT SOLUTION, MTT SOLVENT was added in an amount equal to the original culture volume. After gentle shaking, the absorbance was spectrophotometrically measured at a wavelength of 570 nm. Cell numbers were quantitated at 6, 12, 24, 36, and 48 h. Every group had three wells and the study was repeated for three replicates.
Lentiviral overexpression of Osilr9 lncRNA in fibroblasts
The full-length Osilr9 lncRNA was amplified with PCR primers containing the EcoRI and Xho1 restriction sites. The PCR products were gel purified, cut by restriction enzymes, and ligated into the EcoRI /Xho1 site in pCMV-DsRed/Puro vector The Osilr9 lncRNA clone was confirmed by sequencing and then packaged in 293T packing cells.19,22 After transducing fibroblasts, cells were selected using 5 μg/μL puromycin. The DsRed reporter in the vector was used to track the lentivirus transduction efficiency. After 14 days of selection, more than 92% of cells were positive for DsRed as quantitated by FACS. The bulk population of cells was collected for RT-qPCR quantitation of Osilr9 and target gene expression as well as the DNA methylation assay.
CFSE cell proliferation assay
Cell proliferation was assessed using CellTace Cell Proliferation Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Fibroblasts transduced with Osilr9 or lncCT lentiviruses were stained with 5 μM CFSE dye for 20 min at 37°C. Stained cells were cultured at 37°C and 5% CO2 for 5 days. Cells were analyzed by flow cytometry.
Luciferase reporter assay
A 2-kb genomic DNA fragment covering the Oct4 promoter sequence was amplified and cloned into a pGL3 vector to create a pOct4-luciferase reporter structure.19,22 For the luciferase assay, cells were seeded at a density of 5 × 104 cells/well in 24-well plates. Cells were co-transfected with pOct4-luciferase reporter vector, Osilr9 overexpression vector, and Renilla luciferase control vector (Promega) DNAs using Lipofectamine 3000 (Invitrogen, CA). The empty lentiviral vector and random lncRNA vector were used as the controls. Forty-eight hours after transfection, cells were collected to measure firefly and Renilla luciferase activities using the dual-luciferase reporter system (Promega) using a luminometer (Turner BioSytems). The relative activity of the pOct4-luciferase was adjusted over that of the Renilla control and was normalized by setting the untreated control cells as 1. All luciferase assays were repeated three times with three culture replicates in each assay.
RAT assay
As previously described,18,19 a RAT assay was used to identify the genome-wide regulatory elements that interact with Osilr9 (Figure S4A). Briefly, 1.0 × 107 E14 cells were cross-linked with 2% formaldehyde and lysed with lysis buffer (10 mM Tris [pH 8.0], 10 mM NaCl, 0.2% NP-40, 1× protease inhibitors). Nuclei were isolated and reverse-transcribed in reverse transcription buffer containing Osilr9-specific primers, biotin-14-dCTP, RNase inhibitor, and Maxima Reverse Transcriptase (Thermo Fisher Scientific) for 30 min at 65°C. The biotin-cDNA/chromatin DNA complex was sonicated for 3 min (10s on and 10 s off) at 40% amplification and subsequently purified using M280 Streptavidin beads (Invitrogen). After de-cross-linking with proteinase K at 65°C overnight, genomic DNAs that interacted with Osilr9 were isolated and digested by MboI (Invitrogen). A DNA library was constructed using NEBNext ChIP-Seq Library Prep Master Mix Set for Illumina sequencing. Enriched target genes were analyzed by qPCR. The primers are listed in Table S1.
ChIRP assays
To confirm the interaction of Osilr9 with regulatory elements of the Oct4 gene, a ChIRP assay was performed following the protocol as previously described.68 Briefly, anti-sense DNA probes were constructed with BiotinTEG at the 3′- end. We cross-linked 1.0 × 107 E14 cells with 1% glutaraldehyde and lysed with lysis buffer. Cell lysates were sonicated for 5 min (15 s on and 20 s off) at 40% amplification. DNA was extracted with a Qiagen PCR purification kit (Qiagen). We removed a 10-μL ChIRP sample as the RNA INPUT and DNA INPUT. Chromatin (1 mL), hybridization buffer (2 mL), and probes (1 μL of 100 pmol/μL probe per 1 mL chromatin) were added to a 15-mL Falcon tube. Samples were mixed well and incubated at 37°C for 4 h with shaking. C-1 magnetic beads were used to pull down the chromatin complex. The DNA fraction in the ChIRP samples was extracted for quantitation by qPCR. The primers are listed in Table S1.
Quantitation of CpG DNA methylation by sodium bisulfite sequencing
After puromycin selection, the bulk population of lentivirally-transduced cells was collected for assay. Genomic DNA was extracted from those fibroblasts that carried the empty control vector, the lncCT control and Osilr9 with the EZ DNA Methylation-Gold kit (Cat. no. D5005). PCR was performed using BS-treated DNA samples as templates. PCR products were run on 2% agarose gels to verify product size and extracted using a QIAquick gel extraction kit (Qiagen). Purified PCR products were cloned into the pJET vector (Thermo Fisher Scientific) for sequencing. Specific primers were shown in Table S1.
3C assays
A 3C assay was used to determine intrachromosomal interactions in the Oct4 locus as previously reported.18,19 Briefly, fibroblasts and E14 cells were cross-linked with 2% formaldehyde and lysed with Hi-C lysis buffer (10 mM Tris-HCl, PH 8.0, 10 mM NaCl, 0.2% NP-40, 1× protease inhibitors). An aliquot of nuclei (2 × 106) was digested with 100 U BamHI/BglII at 37°C overnight. Chromatin DNA was diluted with NEB ligation buffer and ligated with 2,000 U of T4 DNA ligase (Thermo Fisher Scientific). After reversing the cross-links, DNA samples were purified for PCR amplification using primers that are derived from different regions of the Oct4 locus (Table S1). The 3C PCR products were cloned and sequenced to validate the intrachromosomal interaction by checking for the presence of the BamHI/BglII ligation site.
RIP assay
A RIP assay was performed to examine the interaction of Osilr9 lncRNA with TET1/2 demethylases. The RIP assay was performed using the MagnaRIPTM RIP Kit (Millipore) according to the manufacturer’s instructions. Fibroblasts carrying Osilr9 were collected and lysed using RIP lysis buffer The lysates were incubated in RIP buffer containing magnetic beads conjugated with anti-TET1/2 antibody (Abcam). After digestion with proteinase K, the immunoprecipitated RNAs were isolated and reverse transcribed for Illumina library sequencing and qPCR quantitation using target gene primers (Table S1). RIP-qPCR was performed in three replicates. The Ct values were normalized over the input and compared with the IgG control.
Statistical analyses
All experiments were performed in triplicate. The data were expressed as the mean ± standard error and were analyzed using SPSS software (IBM SPSS Statistics 26). Data were shown as mean ± standard error. A normal quantile-quantile plot was used to check the distribution of the assay data. For non-parametric data, the Mann-Whitney U test was used to compare statistical differences between two groups, and the Kruskal-Wallis H test was used to compare statistical differences between multiple groups. For parametric data, the Student's t-test and one-way ANOVA (Bonferroni test) were used to compare statistical differences for variables among treatment groups. Results were considered statistically significant when the p value was less than 0.05.
Acknowledgments
Supported by the National Key R&D Program of China (2018YFA0106902, 2020YFA0707704), the Innovative Program of National Natural Science Foundation of China (82050003), National Natural Science Foundation of China (31871297, 81874052, 81900701, 81900327, 82001670, 82101675, 32000431, 32200657, 82002429, 82270859), Fund of Jilin Provincial Science and Technology Department (20210101311JC, 20190303146SF, 20200602032ZP, 20200201390JC, 20190901006JC, 20200801046GH), Provincial Science Fund of Jilin Province Development and Reform Commission (2021C10 and 2020C038-4), Natural Science Fund of Jilin Provincial Finance Department (JLSWSRCZX2020-023, JLSWSRCZX2020-100), the Youth Fund of Jilin Provincial Health Commission (2016Q035), Fund of Changchun City Science and Technology Bureau (21ZGY28), China Guanghua Fund, and the Youth Fund of First Hospital of Jilin University (JDYY11202102, 2020-CXM-01, JDYYGH2019004, JDYY 102019002, JDYY 102019034, 2020B33), California Institute of Regenerative Medicine (CIRM) grant (RT2-01942), and the Department of Veterans Affairs (BX002905).
Author contributions
Y.Z. and J.F.H. conceived and designed the study; Y.Z. and Z.Y. performed most of the experiments and organized the data; C.F., X.W., L.J., L.Z., Z.D., C.W., Y.W., J.C., Y.N., and W.W. conducted cell assays, cell culture, and transduction; J.F.H., L.W., J.C., G.W., and A.R.H. supervised the project and acquired funding; J.F.H. wrote and revised the manuscript; A.R.H. edited the manuscript. All authors have reviewed and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.12.010.
Contributor Information
Jiuwei Cui, Email: cuijw@jlu.edu.cn.
Guixia Wang, Email: gwang168@jlu.edu.cn.
Andrew R. Hoffman, Email: arhoffman@stanford.edu.
Ji-Fan Hu, Email: jifan@stanford.edu, jifanhu@jlu.edu.cn.
Wei Li, Email: liwei66@jlu.edu.cn.
Supplemental information
Data availability statement
Sequencing data used in this study have been deposited in NIH GEO databases with accession numbers GSE116605 and GSE107945.13,17
References
- 1.Takahashi K., Okita K., Nakagawa M., Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 2.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Park I.H., Zhao R., West J.A., Yabuuchi A., Huo H., Ince T.A., Lerou P.H., Lensch M.W., Daley G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Rohani L., Johnson A.A., Arnold A., Stolzing A. The aging signature: a hallmark of induced pluripotent stem cells? Aging Cell. 2014;13:2–7. doi: 10.1111/acel.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelmohsen K., Panda A., Kang M.J., Xu J., Selimyan R., Yoon J.H., Martindale J.L., De S., Wood W.H., 3rd, Becker K.G., Gorospe M. Senescence-associated lncRNAs: senescence-associated long noncoding RNAs. Aging Cell. 2013;12:890–900. doi: 10.1111/acel.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Jiao W., Sun L., Fan J., Chen M., Wang H., Xu X., Shen A., Li T., Niu B., et al. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell. 2013;13:30–35. doi: 10.1016/j.stem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Bikard D., Jiang W., Samai P., Hochschild A., Zhang F., Marraffini L.A. Programmable repression and activation of bacterial gene expression using an engineered CRISPR-Cas system. Nucleic Acids Res. 2013;41:7429–7437. doi: 10.1093/nar/gkt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S.L., Wu Y., Yu H., Zhang P., Zhang X.Q., Ying L., Zhao H.F. Inhibition of Bcl-2 expression by a novel tumor-specific RNA interference system increases chemosensitivity to 5-fluorouracil in Hela cells. Acta Pharmacol. Sin. 2006;27:242–248. doi: 10.1111/j.1745-7254.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Fang F. Long noncoding RNA mediated regulation in human embryogenesis, pluripotency, and reproduction. Stem Cell. Int. 2022;2022:8051717. doi: 10.1155/2022/8051717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J., Wang Y., Wang C., Hu J.F., Li W. LncRNA functions as a new emerging epigenetic factor in determining the fate of stem cells. Front. Genet. 2020;11:277. doi: 10.3389/fgene.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo F., Li L., Yang W., Hu J.F., Cui J. Long noncoding RNA: a resident staff of genomic instability regulation in tumorigenesis. Cancer Lett. 2021;503:103–109. doi: 10.1016/j.canlet.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Du Z., Jia L., Wang Y., Wang C., Wen X., Chen J., Zhu Y., Yu D., Zhou L., Chen N., et al. Combined RNA-seq and RAT-seq mapping of long noncoding RNAs in pluripotent reprogramming. Sci. Data. 2018;5:180255. doi: 10.1038/sdata.2018.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y., Yan Z., Tang Z., Li W. Novel approaches to profile functional long noncoding RNAs associated with stem cell pluripotency. Curr. Genomics. 2020;21:37–45. doi: 10.2174/1389202921666200210142840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherstyuk V.V., Medvedev S.P., Zakian S.M. Noncoding RNAs in the regulation of pluripotency and reprogramming. Stem Cell Rev. Rep. 2018;14:58–70. doi: 10.1007/s12015-017-9782-9. [DOI] [PubMed] [Google Scholar]
- 16.Smith K.N., Miller S.C., Varani G., Calabrese J.M., Magnuson T. Multimodal long noncoding RNA interaction networks: control panels for cell fate specification. Genetics. 2019;213:1093–1110. doi: 10.1534/genetics.119.302661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S., Wang Y., Jia L., Wen X., Du Z., Wang C., Hao Y., Yu D., Zhou L., Chen N., et al. Profiling the long noncoding RNA interaction network in the regulatory elements of target genes by chromatin in situ reverse transcription sequencing. Genome Res. 2019;29:1521–1532. doi: 10.1101/gr.244996.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z., Wen X., Wang Y., Jia L., Zhang S., Liu Y., Zhou L., Li H., Yang W., Wang C., et al. Chromatin lncRNA Platr10 controls stem cell pluripotency by coordinating an intrachromosomal regulatory network. Genome Biol. 2021;22:272. doi: 10.1186/s13059-021-02444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Jia L., Wang C., Du Z., Zhang S., Zhou L., Wen X., Li H., Chen H., Nie Y., et al. Pluripotency exit is guided by the Peln1-mediated disruption of intrachromosomal architecture. J. Cell Biol. 2022;221:e202009134. doi: 10.1083/jcb.202009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia L., Wang Y., Wang C., Du Z., Zhang S., Wen X., Zhou L., Li H., Chen H., Li D., et al. Oplr16 serves as a novel chromatin factor to control stem cell fate by modulating pluripotency-specific chromosomal looping and TET2-mediated DNA demethylation. Nucleic Acids Res. 2020;48:3935–3948. doi: 10.1093/nar/gkaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Jia L., Wang Y., Du Z., Zhou L., Wen X., Li H., Zhang S., Chen H., Chen N., et al. Genome-wide interaction target profiling reveals a novel Peblr20-eRNA activation pathway to control stem cell pluripotency. Theranostics. 2020;10:353–370. doi: 10.7150/thno.39093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y., Yan Z., Du Z., Zhang S., Fu C., Meng Y., Wen X., Wang Y., Hoffman A.R., Hu J.F., et al. Osblr8 orchestrates intrachromosomal loop structure required for maintaining stem cell pluripotency. Int. J. Biol. Sci. 2020;16:1861–1875. doi: 10.7150/ijbs.45112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S., Du Z., Zhu Y., Chen J., Jia L., Wang Y., Wang C., Hoffman A.R., Cui J., Hu J.F. Profiling lncRNAs in the regulatory elements of target genes by chromatin in situ reverse transcription trap sequencing. Genome Res. 2019;29:1521–1532. doi: 10.1101/gr.244996.118. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107945 Datasets Gene Expression Omnibus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J.G., Park J.S., Ko J.H., Kim Y.S. Regulation of gene expression by altered promoter methylation using a CRISPR/Cas9-mediated epigenetic editing system. Sci. Rep. 2019;9:11960. doi: 10.1038/s41598-019-48130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Best J., Schotten C., Theysohn J.M., Wetter A., Müller S., Radünz S., Schulze M., Canbay A., Dechêne A., Gerken G. Novel implications in the treatment of hepatocellular carcinoma. Ann. Gastroenterol. 2017;30:23–32. doi: 10.20524/aog.2016.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergmann J.H., Li J., Eckersley-Maslin M.A., Rigo F., Freier S.M., Spector D.L. Regulation of the ESC transcriptome by nuclear long noncoding RNAs. Genome Res. 2015;25:1336–1346. doi: 10.1101/gr.189027.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brambrink T., Foreman R., Welstead G.G., Lengner C.J., Wernig M., Suh H., Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chédin F. Nascent connections: R-loops and chromatin patterning. Trends Genet. 2016;32:828–838. doi: 10.1016/j.tig.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekker J., Rippe K., Dekker M., Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 30.Rad S.M., Mohammadi-Sangcheshmeh A., Bamdad T., Langroudi L., Atashi A., Lotfinia M., Arefian E., Gastal E.L., Soleimani M. Pluripotency crossroads: junction of transcription factors, epigenetic mechanisms, MicroRNAs, and long non-coding RNAs. Curr. Stem Cell Res. Ther. 2017;12:300–311. doi: 10.2174/1574888X12666170216155850. [DOI] [PubMed] [Google Scholar]
- 31.Panda A., Zylicz J.J., Pasque V. New insights into X-chromosome reactivation during reprogramming to pluripotency. Cells. 2020;9:2706. doi: 10.3390/cells9122706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilieva M., Uchida S. Long noncoding RNAs in induced pluripotent stem cells and their differentiation. Am. J. Physiol. Cell Physiol. 2022;322:C769–C774. doi: 10.1152/ajpcell.00059.2022. [DOI] [PubMed] [Google Scholar]
- 33.Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai M.C., Manor O., Wan Y., Mosammaparast N., Wang J.K., Lan F., Shi Y., Segal E., Chang H.Y. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E., Chang H.Y. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K.C., Yang Y.W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B.R., Protacio A., Flynn R.A., Gupta R.A., et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Facciorusso A., Villani R., Bellanti F., Mitarotonda D., Vendemiale G., Serviddio G. Mitochondrial signaling and hepatocellular carcinoma: molecular mechanisms and therapeutic implications. Curr. Pharm. Des. 2016;22:2689–2696. doi: 10.2174/1381612822666160209153624. [DOI] [PubMed] [Google Scholar]
- 38.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 39.Okano M., Bell D.W., Haber D.A., Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 40.Jackson M., Krassowska A., Gilbert N., Chevassut T., Forrester L., Ansell J., Ramsahoye B. Severe global DNA hypomethylation blocks differentiation and induces histone hyperacetylation in embryonic stem cells. Mol. Cell. Biol. 2004;24:8862–8871. doi: 10.1128/MCB.24.20.8862-8871.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imamura M., Miura K., Iwabuchi K., Ichisaka T., Nakagawa M., Lee J., Kanatsu-Shinohara M., Shinohara T., Yamanaka S. Transcriptional repression and DNA hypermethylation of a small set of ES cell marker genes in male germline stem cells. BMC Dev. Biol. 2006;6:34. doi: 10.1186/1471-213X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okita K., Ichisaka T., Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 43.Wu X., Zhang Y. TET-mediated active DNA demethylation: mechanism, function and beyond. Nat. Rev. Genet. 2017;18:517–534. doi: 10.1038/nrg.2017.33. [DOI] [PubMed] [Google Scholar]
- 44.Joshi K., Liu S., Breslin S J P., Zhang J. Mechanisms that regulate the activities of TET proteins. Cell. Mol. Life Sci. 2022;79:363. doi: 10.1007/s00018-022-04396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross S.E., Bogdanovic O. TET enzymes, DNA demethylation and pluripotency. Biochem. Soc. Trans. 2019;47:875–885. doi: 10.1042/BST20180606. [DOI] [PubMed] [Google Scholar]
- 46.Yang J., Bashkenova N., Zang R., Huang X., Wang J. The roles of TET family proteins in development and stem cells. Development. 2020;147:dev183129. doi: 10.1242/dev.183129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran K.A., Dillingham C.M., Sridharan R. The role of alpha-ketoglutarate-dependent proteins in pluripotency acquisition and maintenance. J. Biol. Chem. 2019;294:5408–5419. doi: 10.1074/jbc.TM118.000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao H., Xu D., Cai Y., Han X., Tang L., Gao F., Qi Y., Cai D., Wang H., Ri M., et al. Very long intergenic non-coding (vlinc) RNAs directly regulate multiple genes in cis and trans. BMC Biol. 2021;19:108. doi: 10.1186/s12915-021-01044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stelzer Y., Shivalila C.S., Soldner F., Markoulaki S., Jaenisch R. Tracing dynamic changes of DNA methylation at single-cell resolution. Cell. 2015;163:218–229. doi: 10.1016/j.cell.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borsos M., Torres-Padilla M.E. Building up the nucleus: nuclear organization in the establishment of totipotency and pluripotency during mammalian development. Genes Dev. 2016;30:611–621. doi: 10.1101/gad.273805.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du Z., Zheng H., Huang B., Ma R., Wu J., Zhang X., He J., Xiang Y., Wang Q., Li Y., et al. Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature. 2017;547:232–235. doi: 10.1038/nature23263. [DOI] [PubMed] [Google Scholar]
- 52.Hörnblad A., Remeseiro S. Epigenetics, enhancer function and 3D chromatin organization in reprogramming to pluripotency. Cells. 2022;11:1404. doi: 10.3390/cells11091404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan S.L., Mariano N.C., Bermudez A., Arruda N.L., Wu F., Luo Y., Shankar G., Jia L., Chen H., Hu J.F., et al. Manipulation of nuclear architecture through CRISPR-mediated chromosomal looping. Nat. Commun. 2017;8:15993. doi: 10.1038/ncomms15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J., Yu H., Ma Q., Zeng P., Wu D., Hou Y., Liu X., Jia L., Sun J., Chen Y., et al. Phase separation of OCT4 controls TAD reorganization to promote cell fate transitions. Cell Stem Cell. 2021;28:1868–1883.e11. doi: 10.1016/j.stem.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 55.Sun J., Li W., Sun Y., Yu D., Wen X., Wang H., Cui J., Wang G., Hoffman A.R., Hu J.F. A novel antisense long noncoding RNA within the IGF1R gene locus is imprinted in hematopoietic malignancies. Nucleic Acids Res. 2014;42:9588–9601. doi: 10.1093/nar/gku549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Li W., Guo R., Sun J., Cui J., Wang G., Hoffman A.R., Hu J.F. An intragenic long noncoding RNA interacts epigenetically with the RUNX1 promoter and enhancer chromatin DNA in hematopoietic malignancies. Int. J. Cancer. 2014;135:2783–2794. doi: 10.1002/ijc.28922. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z.H., Niu B., Zeitz M.J., Ge S., Qian G., Higgins M.J., Hoffman A.R., Hu J.F. Long non-coding RNA Kcnq1ot1 regulates Kcnq1 imprinting by building a long-range intra-chromosomal loop. J. Cell Biol. 2014;204:61–75. doi: 10.1083/jcb.201304152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li T., Hu J.F., Qiu X., Ling J., Chen H., Wang S., Hou A., Vu T.H., Hoffman A.R. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex-2 intrachromosomal loop. Mol. Cell. Biol. 2008;28:6473–6482. doi: 10.1128/MCB.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S., Zhong B., Chen M., Yang L., Yang G., Li Y., Wang H., Wang G., Li W., Cui J., et al. Epigenetic reprogramming reverses the malignant epigenotype of the MMP/TIMP axis genes in tumor cells. Int. J. Cancer. 2014;134:1583–1594. doi: 10.1002/ijc.28487. [DOI] [PubMed] [Google Scholar]
- 60.Chen M., Zhang H., Wu J., Xu L., Xu D., Sun J., He Y., Zhou X., Wang Z., Wu L., et al. Promotion of the induction of cell pluripotency through metabolic remodeling by thyroid hormone triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials. 2012;33:5514–5523. doi: 10.1016/j.biomaterials.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X.Q., Pan X.H., Wang W., Chen Q., Pang R.Q., Cai X.M., Hoffman A.R., Hu J.F. Transient in vitro epigenetic reprogramming of skin fibroblasts into multipotent cells. Biomaterials. 2010;31:2779–2787. doi: 10.1016/j.biomaterials.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai Y., Chen X., Yu D., Li T., Cui J., Wang G., Hu J.F., Li W. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming-induced senescence stress. Exp. Cell Res. 2015;337:61–67. doi: 10.1016/j.yexcr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Barakat T.S., Gribnau J. Combined DNA-RNA fluorescent in situ hybridization (FISH) to study X chromosome inactivation in differentiated female mouse embryonic stem cells. J. Vis. Exp. 2014;88:e51628. doi: 10.3791/51628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonev B., Cavalli G. Organization and function of the 3D genome. Nat. Rev. Genet. 2016;17:661–678. doi: 10.1038/nrg.2016.112. [DOI] [PubMed] [Google Scholar]
- 65.Andriole G.L., Crawford E.D., Grubb R.L., 3rd, Buys S.S., Chia D., Church T.R., Fouad M.N., Isaacs C., Kvale P.A., Reding D.J., et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J. Natl. Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y., Liu S., Zhou L., Li X., Meng Y., Li Y., Li L., Jiao B., Bai L., Yu Y., et al. Aberrant shuttling of long noncoding RNAs during the mitochondria-nuclear crosstalk in hepatocellular carcinoma cells. Am. J. Cancer Res. 2019;9:999–1008. [PMC free article] [PubMed] [Google Scholar]
- 67.Banerji S., Cibulskis K., Rangel-Escareno C., Brown K.K., Carter S.L., Frederick A.M., Lawrence M.S., Sivachenko A.Y., Sougnez C., Zou L., et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abi-Ghanem J., Chusainow J., Karimova M., Spiegel C., Hofmann-Sieber H., Hauber J., Buchholz F., Pisabarro M.T. Engineering of a target site-specific recombinase by a combined evolution- and structure-guided approach. Nucleic Acids Res. 2013;41:2394–2403. doi: 10.1093/nar/gks1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data used in this study have been deposited in NIH GEO databases with accession numbers GSE116605 and GSE107945.13,17