Summary
Radiofrequency heat ablation is an ideal radical treatment for hepatocellular carcinoma (HCC). However, insufficient radiofrequency ablation (IRFA) could lead to high recurrence of HCC. N7-methylguanosine (m7G) on tRNAs, an evolutionally conservative modification in mammals and yeast, modulates heat stress responses and tumor progression, while its function in HCC recurrence after IRFA remains unknown. Here, we found that IRFA significantly upregulates the level of m7G tRNA modification and its methyltransferase complex components METTL1/WDR4 in multiple systems including HCC patient-derived xenograft (PDX) mouse, patients’ HCC tissues, sublethal-heat-treated models of HCC cell lines, and organoids. Functionally, gain-/loss-of-function assays showed that METTL1-mediated m7G tRNA modification promotes HCC metastasis under sublethal heat exposure both in vitro and in vivo. Mechanistically, we found that METTL1 and m7G tRNA modification enhance the translation of SLUG/SNAIL in a codon frequency-dependent manner under sublethal heat stress. Overexpression of SLUG/SNAIL rescued the malignant potency of METTL1 knockdown HCC cells after sublethal heat exposure. Our study uncovers the key functions of m7G tRNA modification in heat stress responses and HCC recurrence after IRFA, providing molecular basis for targeting METTL1-m7G-SLUG/SNAIL axis to prevent HCC metastasis after radiofrequency heat ablation treatment.
Keywords: hepatocellular carcinoma, insufficient radiofrequency ablation, m7G, METTL1, recurrence, tRNA modifications
Graphical abstract

Radiofrequency ablation (RFA) is an ideal radical treatment for hepatocellular carcinoma (HCC) but leads to a high recurrence rate. Kuang and colleagues revealed that METTL1-mediated m7G tRNA modification regulates SLUG/SNAIL axis to promote HCC metastasis after insufficient radiofrequency ablation, providing a molecular basis for preventing HCC metastasis after RFA.
Introduction
Hepatocellular carcinoma (HCC) is the most common and refractory primary liver cancer, which ranks sixth among diagnosed cancer and the third of tumor mortality worldwide.1 Radiofrequency ablation (RFA) is one of the HCC guideline-recommended radical cures for tumors ≤3 cm because of its advantages including minor invasiveness, low complications rate, and good repeatability as well as comparable efficacy to liver resection in overall survival.2,3,4 However, the 5-year recurrence rate of RFA remains approximately 60%–70%,5,6 and the mechanism of metastasis after RFA is still undetermined.
Temperature in an ablation area is over 50°C and kills tumor cells by inducing coagulative degeneration or necrosis in RFA therapy. However, tumor cells in a marginal zone are exposed to sublethal heat stress with temperatures ranging between 41°C and 50°C and therefore suffer from reversible heat damage.7 In response to sublethal heat exposure, the tumor cells induce dynamic epigenetic changes to induce stress responsive gene expression and maintain cell homeostasis and survival,8,9 which eventually leads to HCC recurrence and metastasis after RFA, while the underlying mechanisms are still poorly understood.
Recent studies uncovered that epigenetic modifications on various RNA species play vital roles in cell’s quick response to heat stress in various bio-systems.10,11,12 Upon heat treatment, the dynamic epigenetic modifications on RNAs facilitate the immediate stress responses mainly by regulation of stress-related gene expression.11,12,13 The transfer RNAs (tRNAs) are extensively modified, and the modifications on tRNAs are essential for tRNA stability and mRNA translation. Initially, the tRNA modifications are divided into two groups based on their roles in yeast: the essential modifications that are required for yeast survival and the non-essential modifications that function in specific conditions such as stress responses.14,15 N7-methylguanosine (m7G) on tRNAs is a non-essential, highly conserved modification presented in eukaryotes, prokaryotes, and even some archaea. Depletion of m7G tRNA modification has no effect on yeast growth in normal cultured condition, while yeast without m7G tRNA modification cannot survive from heat stress, supporting the critical function of m7G tRNA modification in heat response.16,17 The m7G tRNA modification is catalyzed by Trm8/Trm82 complex in yeast, while the mammalian m7G modification is catalyzed by the homologous METTL1/WDR4 protein complex, in which METTL1 is the only known m7G catalytic enzyme, and WDR4 acts in stabilizing the methyltransferase complex.18 Previous studies have uncovered that METTL1/WDR4-mediated m7G modification plays a key role in mammals’ physiological and pathological conditions.19,20,21 Recently, several studies reported that METTL1 is highly expressed in human cancers and closely associated with malignancy development, chemotherapy resistance, and poor prognosis.22,23,24,25,26,27,28 However, whether METTL1-mediated m7G tRNA modification plays a pivotal role in promoting HCC recurrence after RFA remains unknown.
In this study, we demonstrated that sublethal heat stress significantly elevates the levels of m7G tRNA modification and METTL1/WDR4 both in vivo and in vitro. We further revealed that METTL1 promotes HCC metastasis under sublethal heat exposure both in vitro and in vivo. Mechanistically, METTL1-mediated m7G tRNA modification selectively regulates translation of key genes of epithelial-mesenchymal transition (EMT) procedure in a codon frequency-dependent manner, which shed lights on clinical treatment to prevent HCC metastasis after RFA.
Results
Sublethal heat stress upregulates METTL1 expression and m7G modification in HCC
To explore the potential function of N7-methylguanosine (m7G) tRNA modification in thermo resistance of HCC during RFA, we firstly established an insufficient radiofrequency ablation (IRFA) orthotopic HCC patient-derived xenograft (PDX) mouse model to simulate clinical HCC ablation with high fidelity. Using an infrared imager, we monitored the temperature changes in tumor during ablation, and recorded the temperature in transitional zone (TZ) suffering sublethal heat stress in a range of 41°C–50°C (Figure 1A). We found that IRFA significantly upregulates m7G tRNA modification level and expression of both METTL1 and WDR4 in TZ (Figures 1B–1D and S1A). Most importantly, our data revealed that levels of both METTL1 and WDR4 are low in the non-tumor tissues but significantly increase in the recurrent HCC tissues after RFA treatment. Peri-tumor tissues (Peri) were used as control because of the adjacent normal characteristic and the unavailability of healthy liver tissue (Figures 1E and 1F). We further demonstrated that sublethal heat treatment significantly upregulates METTL1 and WDR4 expression in patient HCC-derived organoids (Figures 1G and 1H). Accordingly, we verified in a sublethal heat stress HCC cell model that sublethal heat treatment increases m7G tRNA modification and the level of its methyltransferase complex components METTL1/WDR4 in HCC cells (Figures 1I, 1J, S1B, and S1C). When considering the critical function of m7G tRNA modification on yeast survival upon heat stress, our data may suggest that the upregulated m7G tRNA modification induced by IRFA could be associated with thermo resistance of HCC during RFA.
Figure 1.
Elevated level of m7G tRNA modification and methyltransferase complex components METTL1/WDR4 in HCC exposed to sublethal heat stress
(A) During eccentric RFA process in vivo, the dynamic thermal changes (upper panel) were recorded by infrared imaging system. The distance distribution curve of temperature (lower panel) shows central zone (>50°C area), transitional zone (41°C–50°C area), and normal reference zone (<40°C area). (B–D) Comparison of METTL1 and WDR4 expression between unablated control and reference zone and transitional zone tissues from patient-derived xenograft mouse model after insufficient RFA through IHC (B) and WB (C). m7G tRNA modification was higher expressed in transitional zone than reference zone as detected by anti-m7G northwestern blot (C, the last two lanes). IHC staining analysis (B) showed left panels for representative images and right panels for quantification data (N = 8). Quantification data (D) of the bands are shown (N = 6). (E and F) Representative image (E) and quantitative analysis (F) of IHC staining against METTL1 (upper panel) and WDR4 (lower panel) in matched pre-/post-RFA tissues of HCC patients (N = 14). (G–J) Organoid sublethal heat stress model (G) and HCC cells sublethal heat stress model (I) showed extra evidence of increased expression of METTL1/WDR4 or m7G tRNA modification under heat stress in vitro. Quantification data of the bands are provided in (H) and (J) respectively (three technical replicates). U6 snRNA was used as a loading control for northwestern blot. β-Actin was used as a loading control for WB. Scale bars: 200 μm, 100 μm, and 20 μm. Data presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student’s t test). Ctl, control; CZ, central zone; TZ, transitional zone; RZ, reference zone; Peri, peri-tumor tissues; PDX, patient-derived xenograft; PDOs, patient-derived organoids; Pts, patients; IHC, immunohistochemistry; WB, western blot.
METTL1 knockdown inhibits malignant behavior of HCC cells under sublethal heat stress
Given that sublethal heat stress increases m7G tRNA modification and METTL1/WDR4 expression, we hypothesized that METTL1 may play essential oncogenic function under sublethal heat stress. Therefore, we constructed three stable METTL1 knockdown HCC cell lines with two independent short hairpin RNAs. Our data confirmed that METTL1 knockdown reduces the expression of m7G modification in tRNAs by northwestern blot (Figures 2A, S2A, S2D, and S2E). Consistent with previous research, we found that sublethal heat stress significantly inhibits HCC cell growth (Figures 2B and S2F), clone formation ability (Figures 2C, 2D, S2G, and S2H) and cell cycle progression (Figures 2E and S2B), and loss of METTL1 further suppresses HCC cell growth and colony formation under sublethal heat treatment. On the other hand, sublethal heat stress induces HCC cell apoptosis, and this trend is even more pronounced when METTL1 is silenced (Figures 2F and S2C). Moreover, sublethal heat stress treatment significantly promotes the migration and invasion ability of three HCC cells, but METTL1 silencing significantly impairs this trend (Figures 2G–2J, S2I, and S2J). Therefore, our data suggested that METTL1 plays an important function in the malignant characteristics of HCC under sublethal heat stress.
Figure 2.
Knockdown of METTL1 impairs malignant behavior of HCC cells under sublethal heat stress
(A) WB verified METTL1 suppression using METTL1 shRNA lentivirus in both SNU449 and MHCC97H cells. m7G tRNA modification in METTL1 knockdown HCC cells was further confirmed by anti-m7G northwestern blot. (B) Cells viability of METTL1 knockdown SNU449 (left panel) and MHCC97H (right panel) cells under sublethal heat stress were detected by CCK-8 assay (six technical replicates). (C and D) Clone formation assay of METTL1 knockdown SNU449 (upper panel) and MHCC97H (lower panel) cells under sublethal heat stress. Representative images (C) and quantification data (D) are shown (three technical replicates). (E) Quantification data of cell cycle assay in METTL1 knockdown SNU449 (left panel) and MHCC97H (right panel) cells under sublethal heat stress (three technical replicates). (F) Quantification data of cell apoptosis assay in METTL1 knockdown SNU449 (left panel) and MHCC97H (right panel) cells under sublethal heat stress (three technical replicates). (G and H) Migration assay of METTL1 knockdown SNU449 (upper panel) and MHCC97H (lower panel) cells shows METTL1 silencing significantly suppressed metastasis increased by sublethal heat stress in vitro. Representative images (G) and quantification data (H) are shown (three technical replicates). (I and J) Invasion assay of METTL1 knockdown SNU449 (upper panel) and MHCC97H (lower panel) cells after sublethal heat stress. Loss of METTL1 significantly suppressed invasive ability forced by sublethal heat stress in vitro. Representative images (I) and quantification data (J) are shown (three technical replicates). U6 snRNA was used as a loading control for northwestern blot. β-Actin was used as a loading control for WB. Scale bar: 20 μm. Data presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student’s t test, one-way ANOVA). D, day; H, heat; shNC, negative control; sh1, shMETTL1-1; sh2, shMETTL1-2; WB, western blot.
Overexpression of METTL1 promotes malignant capability of HCC cells after sublethal heat stress in m7G tRNA modification dependent manner
To further determine whether m7G tRNA modification is involved in METTL1’s regulation upon HCC malignant ability under sublethal heat stress, we then overexpressed the wild-type (oeWT) and its catalytic inactive mutant (oeMUT) METTL1 in HCC cells. The increased m7G modification level in tRNAs is observed in HCC cells with oeWT METTL1 but not oeMUT METTL1, when compared with oeNC (Figures 3A and S3A–S3C). We found, under normal culture conditions, oeWT METTL1 significantly promotes HCC cell growth (Figures 3B and S3D), clone formation (Figures 3C, 3D, S3E, and S3F), migration (Figures 3E, 3F, S3G, and S3H), and invasion (Figures 3G, 3H, S3G, and S3H). Yet, overexpression of the catalytic inactive mutant METTL1 influences little on HCC progression (Figures 3B–3H and S3D–S3H). Consistently, overexpression of WT METTL1 but not the mutant METTL1 dramatically promotes the malignancy of HCC cells under sublethal heat treatment (Figures 3B–3H and S3D–S3H). Therefore, our data demonstrated that METTL1 is critical for HCC cell malignancy in an m7G tRNA methyltransferase activity-dependent manner under sublethal heat stress.
Figure 3.
Overexpression of METTL1 promotes malignant capability of HCC cells after sublethal heat stress in m7G tRNA modification-dependent manner
(A) WB confirmed METTL1 overexpression in both SNU449 and MHCC97H cells. m7G tRNA modification in HCC cells with overexpression of wild-type and catalytic inactive METTL1 was further confirmed by anti-m7G northwestern blot. (B) Overexpression of wild-type rather than catalytic inactive METTL1 promotes viability of both SNU449 (left panel) and MHCC97H (right panel) cells under normal condition and alleviated the growth suppressive effect induced by heat stress (six technical replicates). (C and D) Representative images (C) and quantification data (D) of clone formation assay in HCC cells overexpressed wild-type and catalytic inactive METTL1 after sublethal heat stress (three technical replicates). (E and F) Migration ability of HCC cells that overexpressed wild-type rather than catalytic inactive METTL1 was significantly enhanced under normal condition, which was more striking under heat stress. Representative images (E) and quantification data (F) are shown (three technical replicates). (G and H) Invasion ability of HCC cells that overexpressed wild-type rather than catalytic inactive METTL1 was obviously boosted under normal condition and was more striking under heat stress. Representative images (G) and quantification data (H) are shown (three technical replicates). U6 snRNA was used as a loading control for northwestern blot. β-Actin was used as a loading control for WB. Scale bar: 20 μm. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student’s t test, one-way ANOVA). D, day; H, heat; oe, overexpression; NC, negative control; WT, wild-type; MUT, catalytic inactive mutant; WB, western blot.
Sublethal heat stress promotes the malignant potency of HCC cells through METTL1-m7G-mediated translation control mechanism
Given that tRNAs are essential factors in mRNA translation, we further profiled tRNA expression and mRNA translation in HCC cells exposed to sublethal heat treatment. Our m7G tRNA reduction and cleavage sequencing (TRAC-seq) revealed that tRNA m7G modification is significantly upregulated in HCC cells after sublethal heat treatment (Figures 4A, 4B, S4A, and S4B). In addition, the expression levels of the majority of m7G modified tRNAs are increased after sublethal heat exposure, especially ThrTGT and LysCTT tRNAs (Figure 4C). We next performed polyribosome-bound mRNAs sequencing (polysome-mRNA-seq) to analyze the changes of mRNA translation efficiency after heat treatment. We found that the genes with altered translation efficiency are significantly enriched in EMT process (Figure S4C). These data revealed that sublethal heat treatment leads to dynamic changes of tRNA modification/expression and mRNA translation in HCC cells.
Figure 4.
Sublethal heat stress increases translation efficiency of SLUG/SNAIL in m7G tRNA decoded codon frequency-dependent manner
(A) Quantitative comparison of cleavage scores in MHCC97H cells with or without heat stress by analyzing m7G tRNA reduction and cleavage sequencing (TRAC-seq) data. (B) Quantitative comparison of expression fold change between m7G and N-m7G tRNAs. Fold change was calculated using ratio of tRNA expression level of the heat-treated group to the control group. (C) Expression profile of the 21 m7G-modified tRNAs identified by TRAC-seq. After calculating the combined cleavage score of all tRNA genes belonging to each tRNA type, we quantified expression of every tRNA and then normalized the expression of indicated tRNA type by its overall average level in both groups and transformed by log2. (D and E) Increased translational efficiencies (TEs) of SLUG and SNAIL increased after sublethal heat treatment were impaired in METTL1 knockdown SNU449 cells, as analyzed by Venn diagram overlapping METTL1 knockdown SNU449 cells with EMT gene set. TEs were calculated through dividing the ribosome binding transcripts’ signals by the input RNA-seq signals. Fold changes were calculated using ratio of TEs in the heat-treated group to the control group and TEs in the METTL1 knockdown heat-treated group to the control heat-treated group. (F) qRT-PCR analysis of SLUG and SNAIL with METTL1 knockdown SNU449 and MHCC97H cells after sublethal heat stress (three technical replicates). (G) WB analysis of SLUG and SNAIL in METTL1 knockdown SNU449 and MHCC97H cells after sublethal heat stress. (H) Validation that LysCTT overexpression in METTL1-depleted cells rescues expression of both SLUG and SNAIL after sublethal heat stress by WB. U6 snRNA was used as a loading control for northwestern blot. β-Actin was used as a loading control for WB. Data are presented as mean ± SD. ∗∗p < 0.01, ∗∗∗p < 0.001 (Mann-Whitney test; Student’s t test, one-way ANOVA). oe, overexpression; CTT, LysCTT; shNC, negative control; sh1, shMETTL1-1; sh2, shMETTL1-2; shM1, shMETTL1; TE, translational efficiency; WB, western blot.
Our translation profiling further revealed that the translation efficiencies of key EMT genes, SLUG and SNAIL, increase significantly after sublethal heat stress, and knockdown of METTL1 decreases their mRNA translation in heat-treated HCC cells (Figures 4D and 4E). We further verified that the protein levels and mRNA translation efficiencies of SLUG and SNAIL increased upon heat treatment in HCC cells and PDX tissues (Figures S4D–S4H). Moreover, depletion of METTL1 significantly decreases the protein levels and mRNA translation efficiencies of SLUG and SNAIL, suggesting that METTL1 promotes the translation of SLUG and SNAIL mRNAs in the heat-treated HCC cells (Figures 4F, 4G, and S5A–S5D). Further analysis of codon composition revealed that the frequency of codon AAG (Lys) is the highest among the m7G tRNA decoded codons in both SLUG and SNAIL (Figure S5E). Moreover, its corresponding tRNA LysCTT is dramatically increased upon heat treatment (Figure 4C). These data indicate that m7G tRNAs, tRNA LysCTT for special, modulate SLUG and SNAIL translation in a condon-dependent frequency. We therefore performed LysCTT rescue assays and found overexpression of LysCTT in METTL1-depleted cells rescue protein levels of SLUG and SNAIL after heat sublethal stress (Figure 4H and S5F), strengthening the functional link between METTL1-mediated tRNA m7G modification and SLUG and SNAIL mRNA translation.
Ectopic expression of SLUG/SNAIL rescues the malignant potency of METTL1 knockdown HCC cells after sublethal heat stress
To further verify whether SLUG/SNAIL serve as the downstream effectors of METTL1 in regulation of HCC progression under sublethal heat stress, we performed rescue assay by SLUG/SNAIL overexpression in stable METTL1 knockdown HCC cells (Figures 5A, S6A, S6B, S7A, and S7B). Our data revealed that overexpression of SLUG or SNAIL significantly promotes the cell growth and colony formation in METTL1-depleted HCC cells under sublethal heat stress (Figures 5B–5D and S7C–S7E). Moreover, the migration and invasion capability are restored after overexpression of SLUG/SNAIL in METTL1-depleted HCC cells (Figures 5E–5H and S7F-S7I). Therefore, SLUG/SNAIL are essential downstream targets of METTL1 for their function in promoting the malignant potency of HCC cells under sublethal heat stress.
Figure 5.
Ectopic expression of SLUG rescues the malignant potency of METTL1 knockdown HCC cells after sublethal heat stress
(A) Validation of SLUG overexpression and METTL1 suppression by WB. (B) Cells viability of METTL1 knockdown SNU449 (left panel) and MHCC97H (right panel) cells with or without overexpression of SLUG under sublethal heat stress (six technical replicates). (C and D) Clone formation assay of METTL1 knockdown SNU449 (upper panel) and MHCC97H (lower panel) cells with or without overexpression of SLUG under sublethal heat stress. Representative images (C) and quantification data (D) are shown (three technical replicates). (E and F) Migration assay showed that overexpression of SLUG rescued metastatic capability impaired by METTL1 silencing under sublethal heat stress in both SNU449 (upper panel) and MHCC97H (lower panel) cells. Representative images (E) and quantification data (F) are shown (three technical replicates). (G and H) Invasion assay showed that overexpression of SLUG salvaged metastatic capability jeopardized by METTL1 silencing under sublethal heat stress in both SNU449 (upper panel) and MHCC97H (lower panel) cells. Representative images (G) and quantification data (H) are shown (three technical replicates). β-Actin was used as a loading control for WB. Scale bar: 20 μm. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student’s t test, one-way ANOVA). D, day; H, heat; oe, overexpression; shNC, negative control; shM1, shMETTL1; WB, western blot.
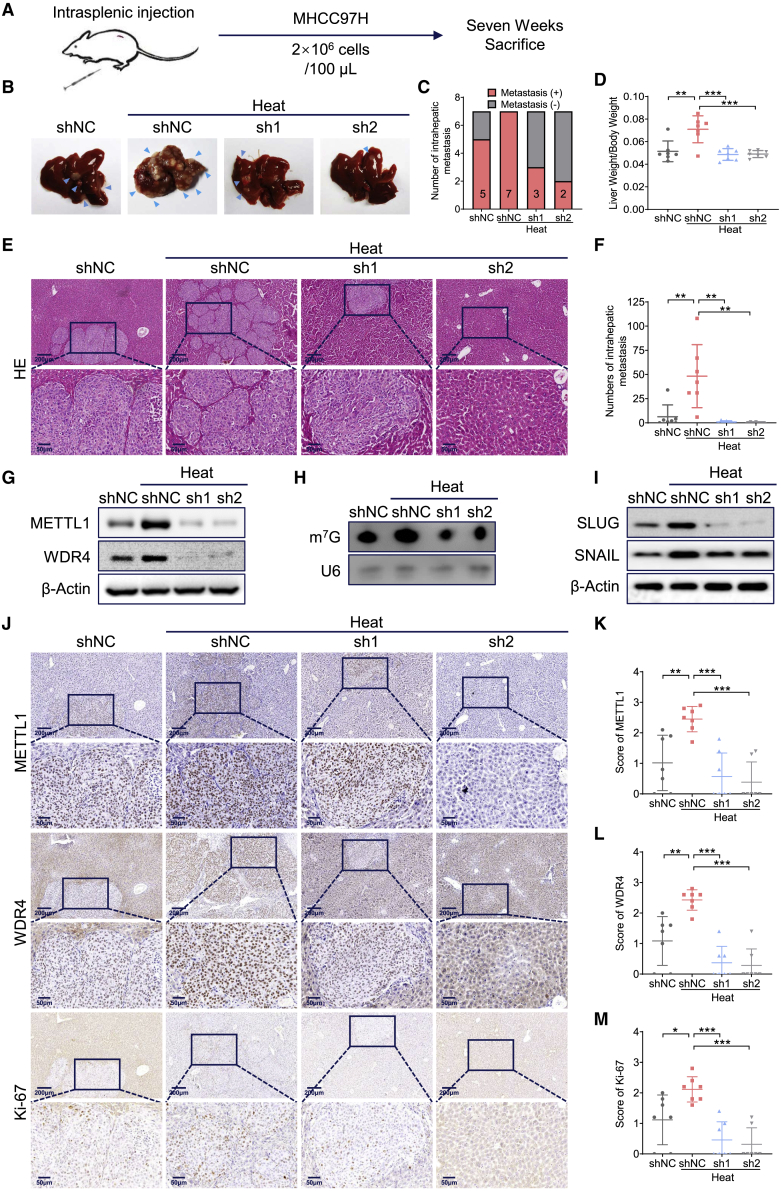
METTL1 accelerates HCC metastasis after heat treatment in vivo
We further studied METTL1’s oncogenic function in HCC recurrence after IRFA using in vivo HCC metastasis models. In the intrasplenic injection model, the heat-treated METTL1 knockdown MHCC97H cells and control cells were injected into the spleen of NCG mice to evaluate liver metastases (Figure 6A). Our data revealed that more liver metastatic tumors form in a group of controlled cells with heat treatment (7/7), while METTL1 depletion significantly decreases HCC tumors (3/7 and 2/7 respectively) (Figures 6B–6F). Moreover, expression level of METTL1, WDR4, Ki-67, SLUG, and SNAIL as well as the level of m7G tRNA modification of tumor tissues all rise in controlled group with heat treatment but decrease in METTL1 knockdown group (Figures 6G–6M and S8A–S8C).
Figure 6.
Loss of METTL1 significantly suppresses intrahepatic metastasis of HCC cells after sublethal heat stress in vivo
(A) Flow chart of the construction of intrasplenic injection model. (B and C) Representative images (B) of liver metastasis in control and METTL1 knockdown HCC cells with or without heat treatment (N = 7). Blue arrow: liver metastasis. Quantitative comparison of mice with liver metastasis after intrasplenic injection of heat-treated or control HCC cells (C). (D) Quantitative comparison of tumor burden by calculating liver weight to body weight ratio of each mouse. (E and F) Representative images (E) and quantification data (F) of liver metastasis in each group with or without heat treatment by H&E staining. (G–I) Analysis of expression levels of METTL1/WDR4 (G) and SLUG/SNAIL (I) by WB, and m7G tRNA modification level (H) by northwestern blot in HCC metastatic tissue with or without heat treatment. (J–M) Representative images (J) and quantification data of METTL1 (K), WDR4 (L), and Ki-67 (M), all of which were detected higher in group with sublethal heat stress by IHC staining. U6 snRNA was used as a loading control for northwestern blot. β-Actin was used as a loading control for WB. Scale bars: 200 μm and 50 μm. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student’s t test, one-way ANOVA). shNC, negative control; sh1, shMETTL1-1; sh2, shMETTL1-2; HE, hematoxylin and eosin staining; IHC, immunohistochemistry staining; WB, western blot.
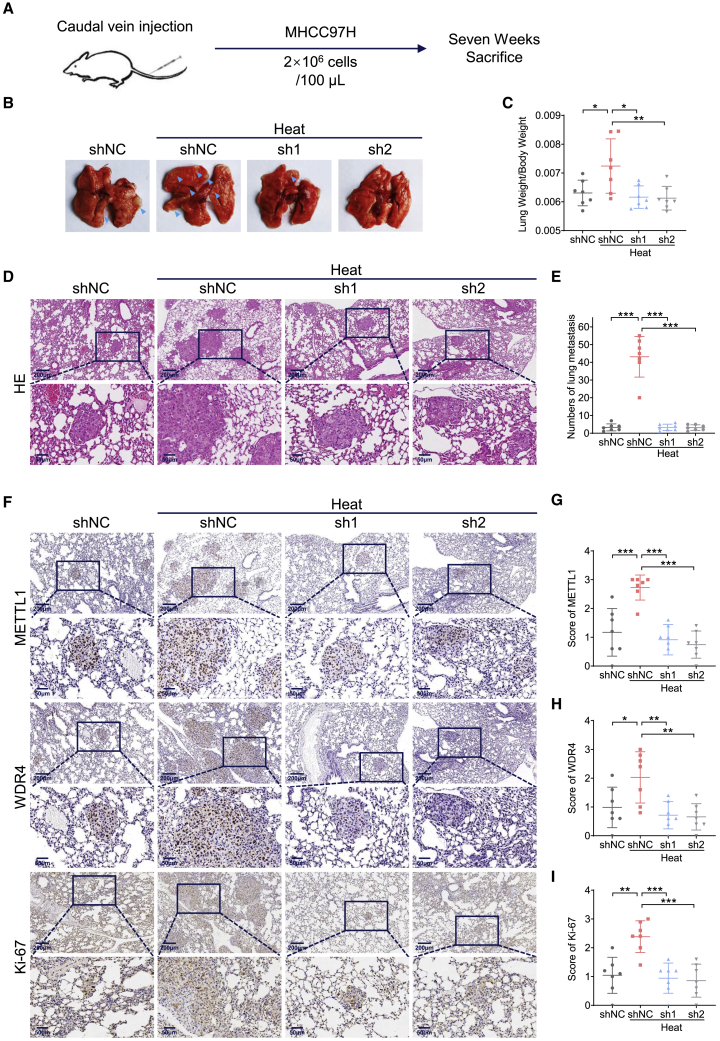
We further established the caudal vein injection model (Figure 7A) with METTL1 knockdown MHCC97H cells to assess the METTL1’s function in regulation of metastasis in lung after heat treatment. We found, as expected, that more lung metastases occur in controlled cells group with heat treatment (Figures 7B, 7D, and 7E). Lung weight to body weight ratio also showed a higher burden of lung metastases induced by sublethal heat stress (Figure 7C). Importantly, depletion of METTL1 significantly suppresses the lung metastases and proliferation activity of HCC cells, as revealed by the decreased tumor burden and reduced Ki-67 level in tumors from the caudal vein injection model (Figures 7F–7I). Therefore, our data uncovered the essential role of METTL1 in promoting in vivo metastasis after heat treatment.
Figure 7.
Loss of METTL1 significantly inhibits lung metastasis of HCC cells after sublethal heat stress in vivo
(A) Flow chart of the establishment of caudal vein injection mouse model. (B) Representative images of lung metastasis in control and METTL1 knockdown HCC cells with or without heat treatment (N = 7). Blue arrow: lung metastasis. (C) Quantitative comparison of tumor burden by calculating lung weight to body weight ratio of each mouse. Sublethal heat stress induced more lung metastasis in vivo. (D and E) Representative images (D) and quantification data (E) of lung metastasis in each group with or without heat treatment by H&E staining. (F–I) Representative images (F) and quantification data of METTL1 (G), WDR4 (H), and Ki-67 (I), all of which were detected higher in group with sublethal heat stress by IHC staining. Scale bars: 200 μm and 50 μm. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Student’s t test, one-way ANOVA). shNC, negative control; sh1, shMETTL1-1; sh2, shMETTL1-2; HE, hematoxylin and eosin staining; IHC, immunohistochemistry staining.
METTL1 promotes HCC metastasis after IRFA in vivo
To further demonstrate the functional link between METTL1 and HCC recurrence after IRFA, we performed IRFA in an orthotopic xenograft implantation HCC model. Mice were sacrificed after 8 weeks to evaluate metastasis in different organs including liver and lung (Figure 8A). More intrahepatic metastases were detected in shNC + IRFA group (7/7) after quantification of HE staining of all tissues (Figures 8B, 8C, 8E, and 8F). Of note, we found extrahepatic metastasis in shNC + IRFA group, including lung, abdominal wall, spleen, kidney, diaphragm, intestine, and mesentery (Figures 8D, 8G, 8H, and S9A–S9J). As expected, METTL1 knockdown could significantly inhibit the trend induced by IRFA, both in metastasis of liver and other organs, especially lung metastasis. These data demonstrated the critical physiological function of METTL1 in promoting HCC metastasis after IRFA.
Figure 8.
METTL1 promotes HCC metastasis after IRFA in vivo
(A) Flow chart of the construction of IRFA orthotopic xenograft implantation HCC model. Subcutaneous tumor, orthotopic xenograft model, and IRFA model were subsequently established within 9 weeks. (B) Representative images of intrahepatic metastases after IRFA in orthotopic xenograft HCC mouse model are shown (N = 7). Blue arrow: intrahepatic metastases. (C) Quantitative comparison of mice with intrahepatic metastases in each group after IRFA. Red number means the transferred mice. (D) Quantitative comparison of mice with lung metastasis in each group after IRFA. Red number means the transferred mice. (E and F) Representative images (E) and quantification data (F) of intrahepatic metastasis in each group after IRFA by H&E staining. (G and H) Representative images (G) and quantification data (H) of lung metastasis in each group after IRFA by H&E staining. Scale bars: 200 μm and 50 μm. Data are presented as mean ± SD. ∗p < 0.05, ∗∗p < 0.01. (Student’s t test, one-way ANOVA). IRFA, insufficient radiofrequency ablation; shNC, negative control; sh1, shMETTL1-1; sh2, shMETTL1-2; HE, hematoxylin and eosin staining; W, week.
Overall, our results demonstrated that METTL1 promotes HCC metastasis under sublethal heat stress through promoting SLUG/SNAIL mRNA translation in an m7G tRNA modification-dependent manner.
Discussion
Recurrence rate after RFA remains high in HCC patients, and the underlying mechanism is elusive. Epigenetic modifications, like m6A, have been confirmed to play a critical role in maintaining cell homeostasis under stress conditions by rapidly upregulating the expression of key genes.10,29,30 m7G tRNA modification was found to be not essential for yeast growth under normal culture condition but indispensable for yeast growth under heat stress condition.16,17 These studies indicated that m7G tRNA modification may be involved in heat stress responses and HCC recurrence after RFA. In this study, we delineated that METTL1/WDR4-mediated m7G tRNA modification elevates dramatically and then quickly promotes the expression of pivotal EMT genes to promote HCC cells survival and metastasis after RFA in vitro and in vivo. Previous studies reported that METTL1 depends on m7G to modulate the processing of let-7e miRNA, which could target HMGA2 to regulate tumor progression.31,32,33,34 Then, we detected the levels of let-7e-5p miRNA in sublethal heat treatment model of HCC cell lines and found that sublethal heat stress did not affect expressions of let-7e-5p miRNA (data not shown), indicating that the let-7e-5p-HMGA2 system is not involved in HCC progression after IRFA. Therefore, our research provides evidence that HCC cells rapidly promote translation under stress conditions in a tRNA epigenetic modification-dependent manner.
Tumor cells selectively upregulate specific gene expression to achieve efficient stress responses. Previous studies have revealed that epigenetic modification plays a critical role in selective translation control.35,36,37 For instance, the post-translational modifications of GAPDH induce metabolic diseases such as obesity and type 2 diabetes,38 and the N1-methyladenosine (m1A) modification in the mitochondrial tRNALys impacts translation elongation and the stability of selected nascent chains in protein synthesis.39 Moreover, m6A modification promotes heat shock protein mRNA translation under heat stress.10 In this study, we found that tRNA m7G modification plays a critical role in regulating downstream gene expression after sublethal heat stress. Polysome-mRNA-seq data revealed that sublethal heat stress selectively promotes the translational efficiencies of EMT genes SLUG and SNAIL. In addition, we further showed that sublethal heat stress increases expression of specific tRNAs through m7G modification, such as ThrTGT tRNA and LysCTT tRNA, and then urges the translation of SLUG and SNAIL in a codon frequency-dependent manner. The elevated specific tRNAs are reported to promote metastasis by regulating translation of transcripts with more cognate codons in cancers.40 Therefore, our data provides evidence for how HCC cells conduct translation control under stress condition through an epigenetic modification perspective.
Previous studies show that epigenetic modifications on RNAs modulate the level of their targets by increasing stability or reducing degradation.17,41,42,43,44 As to tRNAs, the cellular location is dynamic under stress conditions such as heat treatment, oxidative stress, nutritive deficiency, and so on.45,46 Of note, cells transport less-modified tRNAs into the nucleus for complete degradation and selectively regulate appropriate tRNAs for rapid translation in responding to stress.47 In this study, we detected an absolute increase of m7G-modified tRNA copies from whole-cell lysate, indicating m7G modification promotes the stability of tRNAs under sublethal heat stress. Our study uncovers the key functions of m7G tRNA modification in heat stress responses and HCC recurrence after IRFA, providing a molecular basis for targeting METTL1-m7G-SLUG/SNAIL axis to prevent HCC metastasis after radiofrequency heat ablation treatment. Follow-up studies including screening the small molecule inhibitors against METTL1 would potentially help developing new therapeutic strategies to IRFA to reduce stress-induced changes.
In conclusion, our study found that the expression of METTL1, tRNA m7G modification, and LysCTT tRNA are positively correlated with the malignant behavior of HCC after IRFA in vivo and in vitro, which may serve as biomarkers for screening or molecular stratification of patients at high risk of recurrence. Moreover, we determined that targeting METTL1 or LysCTT tRNA suppresses HCC recurrence after RFA and provides a theoretical foundation for clinical drug development and intervention strategies.
Materials and methods
HCC cell lines and animals
HCC cell lines SNU449, MHCC97H, and Huh7 were purchased from American Type Culture Collection (ATCC, USA), and all were confirmed through short tandem repeat assay. Cell culture was performed according to the ATCC guidelines, including 37°C under 5% CO2 and 95% humidity, Roswell Park Memorial Institute-1640 (RPMI-1640; SNU449) or Dulbecco’s modified Eagle’s medium (DMEM; MHCC97H and Huh7) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco, USA).
NCG mice (6 weeks old, male) were purchased from GemPharmatech (Jiangsu, China). According to the animal administration protocol approved by the Institutional Ethics Committee for Clinical Research and Animal Trials of The First Affiliated Hospital, Sun Yat-sen University, mice were housed in specific pathogen-free condition, with free water and 12 h light/12 h dark cycle.
HCC patient samples
HCC specimens involved in this study are in accordance with ethical guidelines of the 1975 Declaration of Helsinki and requirements of Institutional Review Board of The First Affiliated Hospital of Sun Yat-sen University. 14 pre-RFA or post-RFA HCC patients’ paraffin tissue specimens were used in this study, collected in the First Affiliated Hospital of Sun Yat-sen University from 2003–19. With the patients’ informed consents, fresh surgical specimens of HCC patients were used to construct the PDX animal model.
Cell viability and clone formation assays
Trypsinized HCC cells were diluted with complete medium to a concentration of 2000/100 μL. For cell counting kit-8 (CCK-8) assay, 2000 cells/well were seeded into 96-well plates, then cell viability was evaluated at 6, 24, 48, 72, and 96 h thereafter by CCK-8 reagent (Dojindo, Japan). According to the manufacturer’s instructions, samples were incubated for 2 h and detected under 450 nm OD Value of a microplate reader (Thermo, USA). For clone formation assays, 2000 cells/well were seeded in six-well plates and grown for 2–3 weeks until visible; then clone numbers (≥50 cells/colony) were calculated after fixing with 4% paraformaldehyde and 0.5% crystal violet.
Cell cycle and apoptosis assays
Cell cycle and apoptosis assays were performed at 24 h after sublethal heat treatment in stable METTL1 knockdown HCC cells. According to the manufacturer’s instruction, cell cycle assay was detected with PI/RNase Staining Kit (Dojindo, Japan), and cell apoptosis was carried out with Annexin V-FITC Apoptosis Detection Kit (Absin, China), followed with flow cytometry analysis with CytoFLEX (Beckman Coulter, USA) and Flowjo software version 8.1.
Migration and invasion assays
For migration assay, 1 × 105 cells (SNU449 and MHCC97H) or 0.5 × 105 cells (Huh7) in 500 μL serum-free medium were seeded into the upper chamber of a transwell insert (pore size, 8 μm; Corning Falcon) and then placed into a well of 24-well plates containing 750 μL cell culture medium supplemented with 20% FBS in the lower chamber. For invasion assay, chambers were pre-coated with 100 μL Matrigel (Corning, USA) for 1 h in 37°C condition, and then cells were set as the same condition described in the migration assay. After 24 h or 48 h, cells that had penetrated to the lower surface were fixed in 4% formaldehyde solution and stained by 0.5% crystal violet. Finally, cells were quantified based on five random fields under the microscope.
RNA extraction and reverse transcription-qPCR
Total RNA was extracted by Trizol reagent (Invitrogen, USA) as the manufacturer’s instruction described. 2 μg total RNA in a 20-μL reaction system was then used to obtain cDNA by reverse transcription. Real-time polymerase chain reactions (PCR) assay was performed to detect gene expression changes of SLUG and SNAIL by TB GreenTM Premix Ex TaqTM II (Takara, Japan) in LightCycler 480 real-time PCR system (Thermo, USA), with the diluted cDNA samples in 1:10. After three repetitions for each sample, the results were calculated by the 2-ΔΔCt method, with β-actin as an internal control. Primer sequences were as follows:
SLUG forward: 5′-TGTGACAAGGAATATGTGAGCC-3′;
SLUG reverse: 5′-TGAGCCCTCAGATTTGACCTG-3′;
SNAIL forward: 5′-TCGGAAGCCTAACTACAGCGA-3′;
SNAIL reverse: 5′-AGATGAGCATTGGCAGCGAG-3′;
β-actin forward: 5′-TTGCTGACAGGATGCAGAAG-3′;
β-actin reverse: 5′-ACTCCTGCTTGCTGATCCACAT-3′.
Northern blot, northwestern blot, and western blot
Detailed assays that included northern blot, northwestern blot, and western blot (WB) were performed as previous research described.20,48 For northern blot, 2 μg total RNA was separated in 10% TBE-UREA gel for 2 h electrophoresis and then were transferred onto a positively charged nylon membrane (Thermo, USA). The RNA on the membrane were crosslinked for three minutes with ultraviolet light and then blotted with digoxigenin-labeled probes against tRNA LysCTT or the loading control of U6 snRNA. For northwestern blot, membranes containing separated RNA were blotted with anti-m7G antibody (MBL, Japan) overnight at 4°C. Finally, the digoxigenin and anti-m7G signals were detected according to the WB protocol previously recorded.20 All probe sequences and primary antibodies are listed in Table S1.
Hematoxylin and eosin staining and immunohistochemistry analysis
Hematoxylin and eosin staining (H&E) and immunohistochemistry (IHC) staining were performed as previously reported.25 All tissue specimens were dehydrated with 4% paraformaldehyde, embedded in paraffin, and then were cut into 5-μm-thick sections. For H&E staining, tissue sections were deparaffinized with xylene, rehydrated with a descending gradient ethanol, stained with H&E in order, then dehydrated with elevated gradient ethanol and transparent with xylene. Finally, sections were scanned under microscope to observe tumor morphology and count tumor numbers. For IHC staining, tissue sections were deparaffinized with xylene and rehydrated with ethanol, after which antigen retrieval was performed with heat treatment for 3 min. Sections were blocked with 20% goat serum for 30 min. Primary antibodies (METTL1, WDR4, and Ki-67) were added and incubated overnight at 4°C. Secondary antibodies were incubated for 1 h at room temperature, followed by staining with DAB reagent (DAKO) and counterstaining with hematoxylin. Staining densities of METTL1, WDR4, and Ki-67 were evaluated by calculating intensity of positive staining from five random fields.
Polyribosome-bound mRNAs sequencing
To explore translation efficiency changes of specific genes under heat treatment, ribosome nascent-chain complex-associated mRNA-sequencing and qRT-PCR were performed as previous research recorded.49 Multiple reagents were used, including cycloheximide solution (100 μg/mL), ribosome buffer (RB buffer) (20 mM HEPES-KOH, pH 7.4, 15 mM MgCl2, 200 mM KCl, 100 μg/mL cycloheximide, and 2 mM dithiothreitol), lysis buffer (1% Triton X-100 in RB buffer), and sucrose buffer (30% sucrose in RB buffer). Cells were incubated with cycloheximide solution medium (100 μg/mL) at 37°C for 15 min. Immediately, pre-treated cells were washed with pre-cold PBS, collected with 1 mL lysis buffer and incubated on ice for 30 min, after which cell lysates were centrifuged at 16,200 g for 10 min at 4°C. Then, 90% of the supernatant was transferred on the surface of 11 mL sucrose buffer (13.2 mL ultracentrifuge tube, 331372) and centrifuged under 185,000 g for 5 h at 4°C in a SW41Ti rotor (Beckman Coulter, USA), after which polysome fractions were collected. Finally, RNA samples isolated from the remaining supernatants (Input) and polysome-mRNA (Treated) were detected by sequencing and qRT-PCR.
Polysome-mRNA-seq data and gene enrichment analysis
As previously described,50 cDNA library construction and sequencing of RNA samples were performed by the Beijing Genomics Institute using BGISEQ-500 platform (BGI-shenzhen, China). With the alignment tool Bowtie2,51 reads were highly mapped to human reference genome (GRCh38). FPKM (fragments per kilobase of transcript per million mapped reads) were used for normalizing the gene expression level. Translational efficiency was calculated as follows: (FPKM of polysome-mRNA-seq)/(FPKM of input). The differential translated genes were distinguished by setting value, including at least greater than 1 for FPKM, 1.5-fold change for translation efficiency. Gene enrichment analysis of elevated genes identified in polysome-mRNA-seq data was conducted by gene set enrichment analysis (gsea-msigdb.org). Gene sets were arranged according to the enrichment p value, and p value < 0.05 was considered significant.
m7G tRNA reduction and cleavage sequencing
To analyze the dynamics of specific tRNAs after heat treatment, TRAC-seq was performed as previous research described.25,52,53,54 After extracting 100 μg total RNA of heat-treated and controlled cells by Trizol, the small RNA extraction kit (Invitrogen, USA) was used to isolate small RNA according to the instructions. Then the small RNA was subjected to demethylation, NaBH4 reduction, and aniline cleavage. For demethylation, recombinant wild-type and D135S AlkB protein was added, and half of the demethylated RNA were used as input to analyze the expression level of tRNAs after sequencing. For reduction and cleavage, the remaining half samples were treated with 0.1 M NaBH4 for 30 min on ice at dark, followed by aniline-acetate solution (H2O:glacial acetic acid:aniline, 7:3:1) at room temperature for 2 h at dark to perform specific cleavage of the m7G site. Finally, samples were subjected to oligo purification (ZYMO Research, USA) and cDNA library construction, using the Multiplex Small RNA Library Prep set for Illumina kit (New England Biolabs), and then cDNA was sequenced using Illumina Nextseq 500.
TRAC-seq data and analysis
Analysis of m7G modification and tRNA expression was performed as previously described.25,52 The raw sequencing data was performed with adaptor trimming and quality filtering using Trim Galore! (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore), after which the cleaned sequences were aligned using Bowtie2 tool (http://bowtie-bio.sourceforge.net) to the reference genome (RNA sequences of human downloaded from GtRNAdb database). For mature tRNA sequences, a “CCA” sequence was added to the 3′ end. The human tRNA sequences and Bowtie indexes were used (https://github.com/rnabioinfor/TRAC-Seq/tree/master/bowtie_index.) in the mapping process, and reads below 50 hits were considered the best alignment as reported. For analysis of m7G tRNA modification, clean reads from sequencing data of chemically treated samples and controlled input samples were mapped to the human mature tRNA sequences. Bedtools (http://bedtools.readthedocs.io/en/latest/) was then used for processing the alignments to record the read depth of each site and the number of reads starting at that position. The cleavage score was calculated to detect m7G tRNA modification level, which related to ratios between the number of reads starting at site i (cleaved reads in chemical treated samples) and the read depth of site i (tRNA abundance in controlled input samples).55 Finally, the cleavage score of i sites was calculated as follows:
In addition, positions (between 46 and 48) with cleavage score >3 and cleavage ratio >0.1 in both chemically treated and controlled input samples were considered as the candidate m7G sites.
For analysis of tRNA expression, read count for each tRNA sequenced in the input samples (AlkB demethylated) was calculated according to previous method.54 After normalized as reads per million by the total number of small RNA reads that are matched to tRNAs in each sample, tRNAs with expression greater than 500 were used for subsequent analysis.
Plasmids construction and lentiviral transduction
METTL1 wild-type and catalytic inactive mutant (aa160-163, LFPD to AFPA) plasmid as well as LysCTT tRNA plasmid were generated as previously described.20,25 SLUG and SNAIL expression plasmids were all generated by cloning the full-length ORF of SLUG gene (NM_003068.5) and SNAIL gene (NM_005985.4) into pCDH-CMV vector (GeneCopoeia, China). For overexpression of LysCTT tRNA, SLUG, and SNAIL, the corresponding plasmid (including control vector) was transfected into cells using Lipofectamine 3000 reagent (Invitrogen, USA) following the manufacturer’s instructions.
Lentiviral vectors expressing pLKO.1 shRNA constructs targeting METTL1 (shMETTL1, shM1; shMETTL1-1, sh1; shMETTL1-2, sh2) and negative control (shNC) were purchased from Horizon Discovery. Viruses were collected by co-transfecting shRNA constructs with packaging vector pCMVΔR8.9 and enveloped vector pCMV-VSVG into 293T cells with Lipofectamine 3000 reagent. After 48 h, collected viruses were added to infect HCC cells with 8 μg/mL Polybrene (Solarbio, China). The stable METTL1 knockdown cells were established by validating with puromycin (Solarbio, China) (5.0 μg/mL for SNU449 and MHCC97H, 2.5 μg/mL for Huh7) for 48 h.
Sublethal heat treatment model in vitro
The sublethal heat treatment model was established following the previous study.56 HCC cells with 70% density were exposed to pre-heated medium at 46°C, then immediately placed onto 46°C constant temperature bathwater for 15 min. In addition, fresh medium was replaced after 6 h. With informed consent from HCC patients and approval from the Institutional Review Board of FAH-SYSU, HCC organoids were also used in vitro. After treated with the same conditions of the sublethal heat treatment cell model, organoids were collected for WB analysis.
Insufficient radiofrequency ablation in vivo
To perform IRFA in vivo, the electrode (STARmed, South Korea) was inserted into the eccentric tumor position, and the tumor was ablated with 5 Watts generator output for 30 s. TZ and reference zone (RZ) could be distinguished with temperature range recorded by the infrared camera (MAG62-25-150, Magnity Electronics China). Tumor tissues from TZ and RZ after IRFA were used for detecting expression changes of tRNA m7G modification and other markers including METTL1, WDR4, SLUG, and SNAIL.
IRFA animal model
For the orthotopic HCC PDX mouse model, postoperative tumor tissues of HCC patients were collected after removal of necrotic tissue, cut into 1 mm3 pieces, and transplanted into the right flanks of NCG mice (N = 3). 3–4 months later, the subcutaneous xenograft was isolated, cut into 1 mm3 pieces, and then embedded into the left liver lobe of other NCG mice (N = 16) using tunnel planting method. Finally, tumor tissues treated with or without IRFA were collected for further analysis.
For the cell line-derived orthotopic xenograft mouse model, control and METTL1 knockdown MHCC97H (1 × 107 cells/100 μL) cells were first injected into the right flanks of NCG mice to establish subcutaneous tumor. Each visible subcutaneous tumor was then cut into 1 mm3 pieces and implanted into the left liver lobe of NCG mice (N = 7, each group). After 2–3 weeks, intrahepatic tumors were treated with IRFA. To detect the role of METTL1 in accelerating HCC metastasis after IRFA, mice were sacrificed after recording weight of liver and lung to collect tumor, liver, and lung tissues after 3 weeks.
Metastatic animal model
METTL1 knockdown MHCC97H cells were treated with or without heat treatment at 46°C for 15 min. All mediums were replaced after 6 h. Until 24 h later, cells were digested and resuspended using PBS solution with 2 × 106/100 μL cells. Immediately, prepared cells were separately injected into the spleen of NCG mice (N = 7, each group) as intrasplenic injection model or caudal vein of other NCG mice (N = 7, each group) as caudal vein injection model. All mice were sacrificed after 7 weeks. The livers (intrasplenic injection model) or lungs (caudal vein injection model) were collected and subsequently fixed with formalin, embedded in paraffin, and cut into 5-μm-thick sections, after which H&E staining was performed for calculating numbers of tumor metastasis, and IHC was performed for detecting the expression changes of METTL1, WDR4, and Ki-67 in vivo.
Statistical analysis
All data analysis was performed using Graphpad prism 8.0 and demonstrated as the mean ± standard deviation (SD). Unpaired, Student’s t test or Mann-Whitney test or one-way ANOVA statistical analyses were applied and p value considered significant, including p < 0.001 (∗∗∗), p < 0.01 (∗∗), p < 0.05 (∗). Significant changes were observed after three consistent repetitions. For quantification of IHC, scores related to staining intensities were evaluated by pathologists with double blind method, in which 0 is for no positive cell, 1 for weak intensity, 2 for mediate intensity, and 3 for strong intensity. For quantifying number of migrated or invasive tumor cells and colony formation, five representative regions were selected randomly and then were counted by the software ImageJ.
Data availability
Data of polysome-mRNA-seq and TRAC-seq are deposited in public repository NCBI/GEO (GSE:199966).
Acknowledgments
We are grateful to the patients for their agreement with HCC samples usage. We sincerely thank Dr. Shuibin Lin for data analysis and manuscript revision. We thank Dr. Huanjing Hu and Dr. Minghui He for their kind assistances in analyzing data of polysome-mRNA-seq and TRAC-seq. This work was supported by Key Project of National Natural Science Foundation of China (grant no.: 82130083); National Key Research and Development Program of China (grant no.: 2020AAA0109504); National Natural Science Foundation of China (grant nos.: 81825013, 82173191, 82172047); Guangdong Basic and Applied Basic Research Foundation (grant nos.: 2019B151502009, 2020A1515110114, 2021A1515010450) and National high level talents special support plan—“Ten thousand plan”—Young top-notch talent support program (grant no.: 80000-41180003). It was also supported by Natural Science Foundation of Guangdong Province (grant no.: 18zxxt12) and General Project of China Postdoctoral Science Foundation (2021M703732).
Author contributions
Project design: M.K. and S.P. Experimental advancement: S.P.; S.Z., X.Z., and Y.W. performed the in vitro assays; S.Z., Y.W., and X.Z. contributed to the in vivo studies. S.Z. and T.S. analyzed the TRAC and polysome-mRNA-seq sequencing data; S.Z., T.S., H.X., S.C., and L.X. performed other data analysis; S.Z. and T.S. wrote the draft, and all authors revised and approved the final manuscript.
Declaration of interests
The authors have no conflict of interest.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2022.08.004.
Contributor Information
Lixia Xu, Email: xulixia@mail.sysu.edu.cn.
Tianhong Su, Email: suth3@mail.sysu.edu.cn.
Ming Kuang, Email: kuangm@mail.sysu.edu.cn.
Supplemental information
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Omata M., Cheng A.L., Kokudo N., Kudo M., Lee J.M., Jia J., Tateishi R., Han K.H., Chawla Y.K., Shiina S., et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European association for the study of the liver. Electronic address: easloffice@easloffice.eu EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R., Zhu A.X., Murad M.H., Marrero J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 5.Kang T.W., Kim J.M., Rhim H., Lee M.W., Kim Y.S., Lim H.K., Choi D., Song K.D., Kwon C.H.D., Joh J.W., et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology. 2015;275:908–919. doi: 10.1148/radiol.15141483. [DOI] [PubMed] [Google Scholar]
- 6.Lee T.Y., Lin J.T., Ho H.J., Wu M.S., Wu C.Y. Evaluation of the effect of cumulative operator experience on hepatocellular carcinoma recurrence after primary treatment with radiofrequency ablation. Radiology. 2015;276:294–301. doi: 10.1148/radiol.15141864. [DOI] [PubMed] [Google Scholar]
- 7.Chu K.F., Dupuy D.E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 8.Dedon P.C., Begley T.J. A system of RNA modifications and biased codon use controls cellular stress response at the level of translation. Chem. Res. Toxicol. 2014;27:330–337. doi: 10.1021/tx400438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Home T., Jensen R.A., Rao R. Heat shock factor 1 in protein homeostasis and oncogenic signal integration. Cancer Res. 2015;75:907–912. doi: 10.1158/0008-5472.CAN-14-2905. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J., Wan J., Gao X., Zhang X., Jaffrey S.R., Qian S.B. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su T., Huang M., Liao J., Lin S., Yu P., Yang J., Cai Y., Zhu S., Xu L., Peng Z., et al. Insufficient radiofrequency ablation promotes hepatocellular carcinoma metastasis through N6-methyladenosine mRNA methylation-dependent mechanism. Hepatology. 2021;74:1339–1356. doi: 10.1002/hep.31766. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson E., Cui Y.H., He Y.Y. Context-dependent roles of RNA modifications in stress responses and diseases. Int. J. Mol. Sci. 2021;22:1949. doi: 10.3390/ijms22041949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Licht K., Jantsch M.F. Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J. Cell Biol. 2016;213:15–22. doi: 10.1083/jcb.201511041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopper A.K., Phizicky E.M. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 15.Grosjean H. In: Fine-tuning of RNA Functions by Modification and Editing. Grosjean H., editor. Springer Berlin Heidelberg; 2005. Modification and editing of RNA: historical overview and important facts to remember; pp. 1–22. [DOI] [Google Scholar]
- 16.Alexandrov A., Grayhack E.J., Phizicky E.M. tRNA m7G methyltransferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA. 2005;11:821–830. doi: 10.1261/rna.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrov A., Chernyakov I., Gu W., Hiley S.L., Hughes T.R., Grayhack E.J., Phizicky E.M. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov A., Martzen M.R., Phizicky E.M. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA. 2002;8:1253–1266. doi: 10.1017/s1355838202024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaheen R., Abdel-Salam G.M.H., Guy M.P., Alomar R., Abdel-Hamid M.S., Afifi H.H., Ismail S.I., Emam B.A., Phizicky E.M., Alkuraya F.S. Mutation in WDR4 impairs tRNA m(7)G46 methylation and causes a distinct form of microcephalic primordial dwarfism. Genome Biol. 2015;16:210. doi: 10.1186/s13059-015-0779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin S., Liu Q., Lelyveld V.S., Choe J., Szostak J.W., Gregory R.I. Mettl1/Wdr4-Mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell. 2018;71:244–255.e5. doi: 10.1016/j.molcel.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun D.A., Shril S., Sinha A., Schneider R., Tan W., Ashraf S., Hermle T., Jobst-Schwan T., Widmeier E., Majmundar A.J., et al. Mutations in WDR4 as a new cause of Galloway-Mowat syndrome. Am. J. Med. Genet. A. 2018;176:2460–2465. doi: 10.1002/ajmg.a.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian Q.H., Zhang M.F., Zeng J.S., Luo R.G., Wen Y., Chen J., Gan L.G., Xiong J.P. METTL1 overexpression is correlated with poor prognosis and promotes hepatocellular carcinoma via PTEN. J. Mol. Med. 2019;97:1535–1545. doi: 10.1007/s00109-019-01830-9. [DOI] [PubMed] [Google Scholar]
- 23.Ma J., Han H., Huang Y., Yang C., Zheng S., Cai T., Bi J., Huang X., Liu R., Huang L., et al. METTL1/WDR4-mediated m(7)G tRNA modifications and m(7)G codon usage promote mRNA translation and lung cancer progression. Mol. Ther. 2021;29:3422–3435. doi: 10.1016/j.ymthe.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orellana E.A., Liu Q., Yankova E., Pirouz M., De Braekeleer E., Zhang W., Lim J., Aspris D., Sendinc E., Garyfallos D.A., et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell. 2021;81:3323–3338.e14. doi: 10.1016/j.molcel.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Z., Liu H., Liao J., Huang C., Ren X., Zhu W., Zhu S., Peng B., Li S., Lai J., et al. N(7)-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Mol. Cell. 2021;81:3339–3355.e8. doi: 10.1016/j.molcel.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z., Zhu W., Zhu S., Sun K., Liao J., Liu H., Dai Z., Han H., Ren X., Yang Q., et al. METTL1 promotes hepatocarcinogenesis via m(7) G tRNA modification-dependent translation control. Clin. Transl. Med. 2021;11:e661. doi: 10.1002/ctm2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han H., Yang C., Ma J., Zhang S., Zheng S., Ling R., Sun K., Guo S., Huang B., Liang Y., et al. N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat. Commun. 2022;13:1478. doi: 10.1038/s41467-022-29125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto M., Fujiwara M., Hori M., Okada K., Yazama F., Konishi H., Xiao Y., Qi G., Shimamoto F., Ota T., et al. tRNA modifying enzymes, NSUN2 and METTL1, determine sensitivity to 5-fluorouracil in HeLa cells. PLoS Genet. 2014;10:e1004639. doi: 10.1371/journal.pgen.1004639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B., Qian S.B. Translational reprogramming in cellular stress response. Wiley Interdiscip Rev. RNA. 2014;5:301–315. doi: 10.1002/wrna.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engel M., Eggert C., Kaplick P.M., Eder M., Röh S., Tietze L., Namendorf C., Arloth J., Weber P., Rex-Haffner M., et al. The role of m(6)A/m-RNA methylation in stress response regulation. Neuron. 2018;99:389–403.e9. doi: 10.1016/j.neuron.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y., Zhao Z., Xu C., Zhou Z., Zhu Z., You T. HMGA2 induces transcription factor Slug expression to promote epithelial-to-mesenchymal transition and contributes to colon cancer progression. Cancer Lett. 2014;355:130–140. doi: 10.1016/j.canlet.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira-Mateos C., Sánchez-Castillo A., Soler M., Obiols-Guardia A., Piñeyro D., Boque-Sastre R., Calleja-Cervantes M.E., Castro de Moura M., Martínez-Cardús A., Rubio T., et al. The transcribed pseudogene RPSAP52 enhances the oncofetal HMGA2-IGF2BP2-RAS axis through LIN28B-dependent and independent let-7 inhibition. Nat. Commun. 2019;10:3979. doi: 10.1038/s41467-019-11910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J., Liu Z., Shao C., Gong Y., Hernando E., Lee P., Narita M., Muller W., Liu J., Wei J.J. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 2011;71:349–359. doi: 10.1158/0008-5472.CAN-10-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pandolfini L., Barbieri I., Bannister A.J., Hendrick A., Andrews B., Webster N., Murat P., Mach P., Brandi R., Robson S.C., et al. METTL1 promotes let-7 MicroRNA processing via m7G methylation. Mol. Cell. 2019;74:1278–1290.e9. doi: 10.1016/j.molcel.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Advani V.M., Ivanov P. Translational control under stress: reshaping the translatome. Bioessays. 2019;41:e1900009. doi: 10.1002/bies.201900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu C., Begley T.J., Dedon P.C. tRNA modifications regulate translation during cellular stress. FEBS Lett. 2014;588:4287–4296. doi: 10.1016/j.febslet.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bond S.T., Howlett K.F., Kowalski G.M., Mason S., Connor T., Cooper A., Streltsov V., Bruce C.R., Walder K.R., McGee S.L. Lysine post-translational modification of glyceraldehyde-3-phosphate dehydrogenase regulates hepatic and systemic metabolism. FASEB J. 2017;31:2592–2602. doi: 10.1096/fj.201601215R. [DOI] [PubMed] [Google Scholar]
- 39.Richter U., Evans M.E., Clark W.C., Marttinen P., Shoubridge E.A., Suomalainen A., Wredenberg A., Wedell A., Pan T., Battersby B.J. RNA modification landscape of the human mitochondrial tRNA(Lys) regulates protein synthesis. Nat. Commun. 2018;9:3966. doi: 10.1038/s41467-018-06471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodarzi H., Nguyen H.C.B., Zhang S., Dill B.D., Molina H., Tavazoie S.F. Modulated expression of specific tRNAs drives gene expression and cancer progression. Cell. 2016;165:1416–1427. doi: 10.1016/j.cell.2016.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng F., Xu J., Cui B., Liang Q., Zeng S., He B., Zou H., Li M., Zhao H., Meng Y., et al. Oncogenic AURKA-enhanced N(6)-methyladenosine modification increases DROSHA mRNA stability to transactivate STC1 in breast cancer stem-like cells. Cell Res. 2021;31:345–361. doi: 10.1038/s41422-020-00397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abakir A., Giles T.C., Cristini A., Foster J.M., Dai N., Starczak M., Rubio-Roldan A., Li M., Eleftheriou M., Crutchley J., et al. N(6)-methyladenosine regulates the stability of RNA:DNA hybrids in human cells. Nat. Genet. 2020;52:48–55. doi: 10.1038/s41588-019-0549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Li A., Sun B.F., Yang Y., Han Y.-N., Yuan X., Chen R.-X., Wei W.-S., Liu Y., Gao C.-C., et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 44.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chatterjee K., Nostramo R.T., Wan Y., Hopper A.K. tRNA dynamics between the nucleus, cytoplasm and mitochondrial surface: location, location, location. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861:373–386. doi: 10.1016/j.bbagrm.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwenzer H., Jühling F., Chu A., Pallett L.J., Baumert T.F., Maini M., Fassati A. Oxidative stress triggers selective tRNA retrograde transport in human cells during the integrated stress response. Cell Rep. 2019;26:3416–3428.e5. doi: 10.1016/j.celrep.2019.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirchner S., Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat. Rev. Genet. 2015;16:98–112. doi: 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.W., Li Z., Moore P.S., Monaghan A.P., Chang Y., Nichols M., John B. A sensitive non-radioactive northern blot method to detect small RNAs. Nucleic Acids Res. 2010;38:e98. doi: 10.1093/nar/gkp1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T., Cui Y., Jin J., Guo J., Wang G., Yin X., He Q.Y., Zhang G. Translating mRNAs strongly correlate to proteins in a multivariate manner and their translation ratios are phenotype specific. Nucleic Acids Res. 2013;41:4743–4754. doi: 10.1093/nar/gkt178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen K., Liu J., Liu S., Xia M., Zhang X., Han D., Jiang Y., Wang C., Cao X. Methyltransferase SETD2-mediated methylation of STAT1 is critical for interferon antiviral activity. Cell. 2017;170:492–506.e14. doi: 10.1016/j.cell.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 51.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin S., Liu Q., Jiang Y.Z., Gregory R.I. Nucleotide resolution profiling of m(7) G tRNA modification by TRAC-Seq. Nat. Protoc. 2019;14:3220–3242. doi: 10.1038/s41596-019-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng G., Qin Y., Clark W.C., Dai Q., Yi C., He C., Lambowitz A.M., Pan T. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods. 2015;12:835–837. doi: 10.1038/nmeth.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cozen A.E., Quartley E., Holmes A.D., Hrabeta-Robinson E., Phizicky E.M., Lowe T.M. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods. 2015;12:879–884. doi: 10.1038/nmeth.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz S., Bernstein D.A., Mumbach M.R., Jovanovic M., Herbst R.H., León-Ricardo B.X., Engreitz J.M., Guttman M., Satija R., Lander E.S., et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan L., Chen S., Wei G., Li Y., Liao J., Jin H., Zou Y., Huang M., Peng Z., Guo Y., et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40. doi: 10.1016/j.canlet.2019.05.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of polysome-mRNA-seq and TRAC-seq are deposited in public repository NCBI/GEO (GSE:199966).