Abstract
Preeclampsia (PE) is characterized by new onset hypertension in association with elevated soluble fms-like tyrosine kinase-1 (sFlt-1) and preproendothelin-1 (PPET-1) levels. Currently there is no effective treatment for PE except for early delivery of the fetal placental unit, making PE a leading cause for premature births worldwide. Administration of 17-hydroxyprogesterone caproate (17-OHPC) is used for prevention of recurrent preterm birth. This study was designed to test the hypothesis that 17-OHPC improves hypertension and ET-1 in response to elevated sFlt-1 in pregnant rats. sFlt-1 was infused into normal pregnant (NP) Sprague-Dawley rats (3.7 μg·kg−1·day−1 for 6 days, gestation days 13–19) in the presence or absence of 17-OHPC (3.32 mg/kg) administered via intraperitoneal injection on gestational days 15 and 18. Mean arterial blood pressure (MAP), pup and placenta weights, renal cortex PPET-1 mRNA levels and nitrate-nitrite levels were measured on GD 19. Infusion of sFlt-1 into NP rats elevated mean arterial pressure (MAP) compared with control NP rats: 115 ± 1 (n = 13) vs. 99 ± 2 mmHg (n = 12, p < 0.05). 17-OHPC attenuated this hypertension reducing MAP to 102 ± 3 mmHg in sFlt-1 treated pregnant rats (n = 8). Neither pup nor placental weight was affected by sFlt-1 or 17-OHPC. Importantly, renal cortex PPET-1 mRNA levels were elevated 3 fold in NP + sFlt-1 rats compare to NP rats, which decreased with 17-OHPC administration. Plasma nitrate-nitrite levels were 44 ± 9 μM in NP rats (n = 9), 20 ± 3 μM in NP + sFlt-1 (n = 7), which increased to 42 ± 11 μM NP + sFlt-1 + 17OHPC (n = 6). Administration of 17-OHPC improves clinical characteristics of preeclampsia in response to elevated sFlt-1 during pregnancy.
Keywords: Preproendothelin-1, sFlt-1, 17-OHPC, Preeclampsia
1. Introduction
Preeclampsia (PE) is a multi-system hypertensive disorder characterized by fetal growth restriction, preterm birth, placental abruption, HELLP syndrome, eclampsia, cardiovascular disease, and end-organ damage [1]. PE complicates approximately 10% of all pregnancies, is a significant contributor to maternal and perinatal morbidity, and remains a leading cause of iatrogenic preterm birth [1,2]. Delivery of the fetal-maternal unit remains the only definitive cure of PE despite many years of research, and further treatment strategies are needed [3]. Interventions that could safely and effectively prolong pregnancy and reduce the risk of iatrogenic preterm birth has the potential to significantly improve maternal and fetal antenatal care.
The diagnosis of PE is made by new onset hypertension with additional evidence of multi-system vascular dysfunction [4]. This vascular dysfunction manifests clinically as hypertension, proteinuria, worsening kidney function, neurological symptoms, hemolysis, blood clotting disorders, and increased vascular resistance. On a cellular and molecular level, the disorder is marked by deficiency of trophoblast invasion and placental ischemia, ultimately resulting in decreased vasodilators, increased inflammatory cytokines, chronic immune activation, and imbalance in circulating angiogenic factors [3,5–13].
In PE the imbalance and dysregulation between proangiogenic and antiangiogenic factors is thought to be a main contributing factor to the laboratory signs and clinical manifestations associated with the disorder [14]. Placental growth factor (PIGF) and soluble fms-like tyrosine-1 (sFlt-1) are two important biomarkers of placental function and PE [13]. sFlt-1 is an antiangiogenic factor notoriously associated with intrauterine growth restriction (IUGR) and PE [15]. In vivo studies have shown that women with PE have lower concentrations of PIGF and higher concentrations of sFlt-1 compared to control subjects [13,16–18]. Utilizing a ratio between antiangiogenic sFlt-1 and proangiogenic placental growth factor (PIGF), there are ongoing investigations into utilization of these placental biomarkers to predict PE [19–22]. Disordered angiogenesis is a hallmark of PE and restoration of the balance between proangiogenic and antiangiogenic factors is hypothesized to be a possible treatment option.
Endothelin-1, also known as preproendothelin-1, is produced and secreted by endothelial cells [23]. It is a potent vasoconstrictor and acts in concert with the vasodilator nitric-oxide to maintain vascular tone [23]. Endothelin-1 is known to contribute to the pathophysiology of PE with studies demonstrating that ET-1 is increased in PE [24]. Attenuation of the endothelin axis and blockage of endothelin-1 is now being considered a treatment option for PE [25]. The effect of progesterone supplementation on modulating the relationship between endothelin-1 as a vasoconstrictor and nitric-oxide as a vasodilator is an area of great interest in furthering our understanding of PE.
Progesterone supplementation through a progesterone derivative, such as 17-OHPC, has been used for many years to lower the recurrence risk of preterm birth [26–29]. The utilization of 17-OHPC in patients at risk of PE or for those who have already developed the clinical manifestations of preterm PE is still an area needing further exploration, and the benefit of 17-OHPC to reduce placental ischemia has not yet been clearly defined [26,30]. Previous data from Kiprono et al indicate that women with PE have lower levels of progesterone when compared to gestational age- and race-matched non-PE control pregnant women [31,32]. Furthermore, studies have shown that supplementation of RUPP rat model of PE with 17-OHPC decreases blood pressure and inflammation, with attenuation of pro-inflammatory cytokines that accompany an increase of NO bioavailability [33,34]. Improvement in UARI, litter size, IUGR and hypertension in response to placental ischemia was also demonstrated. More recent studies by Amaral et al. have shown that supplementation of 17-OHPC in the RUPP model increased NO bioavailability while decreasing sFlt-1 [35].
This current study was designed to test the hypothesis that 17-OHPC improves hypertension and ET-1 in response to sFlt-1 induced hypertension in pregnant rats.
2. Materials and methods
Pregnant Sprague-Dawley rats purchased from Harlan Sprague Dawley (Indianapolis, IN) were used in this study. Animals were housed in a temperature-controlled room (23 °C) with a 12:12-h light–dark cycle with free access to standard rat chow and water. All experimental procedures executed in this study were in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
2.1. sFlt-1 induced hypertension
Surgical procedures were carried out under appropriate anesthesia, 2% isoflurane, and analgesics were given post-operatively as needed. Pregnant rat dams weighting approximately 200–250 g were randomly assigned to either Normal pregnant + sFlt-1 or NP control groups. sFlt-1 (recombinant mouse VEGF R1/Flt-1 Fc Chimera, R&D Systems catalog # 471-F1–100) miniosmotic pump was infused into normal pregnant (NP) Sprague-Dawley rats (3.7 μg·kg−1·day−1 for 6 days, gestation days 13–19) as described previously [36] in the presence or absence of 17-OHPC. Also analgesics were used to provide comfort for the surgical rats include 0.25% sensor care administered topically or 5 mg/kg Carprofen administered via subcutaneous injection, once daily for 2–3 days following surgical procedure.
2.2. Administration of 17-OHPC
A subset of sFlt-1 pregnant rats were injected with 17-hydroxyprogesterone caproate (17-OHPC) at days 15 (first injection) and 18 of gestation (second injection). The 17-OHPC (Makena, AMAG Pharmaceuticals) was administered intraperitoneal as 0.5 cm3 solution of 3.32 mg/kg 17-OHPC to pregnant rats as described previously [32]. This dose is equivalent to a typical human dose for the prevention of preterm labor and has been demonstrated to be effective in rat models of PE [32,34,35]. We have shown that 17-OHPC had no blood pressure effects on NP rats and therefore 17-OHPC was not administered to NP rats in this study [35].
2.3. Measurement of mean arterial blood pressure
On day 18 of gestation, using the isoflurane anesthesia (Webster, Sterling, MA), carotid arterial catheters were inserted for blood pressure measurements. The catheters inserted were V3 tubing (SCI, Scientific Commodities, Inc., Lake Havasu City, AZ), which is tunneled to the back of the neck and exteriorized. On day 19 of gestation, mean arterial blood pressure was analyzed after placing the rats in individual restraining cages. Arterial pressure was monitored with a pressure transducer (Cobe III tranducer CDX Sema) and recorded continuously for 30 min after a 30-min stabilization period. Subsequently, blood samples were collected, tissues were harvested, and placenta and pup weights were recorded under anesthesia.
2.4. Determination of circulating nitrate–nitrite levels
Plasma collected from all pregnant rats was utilized to measure nitrate-nitrite levels using Nitrate/Nitrite Colorimetric Assay Kit from Cayman Chemical following instructions outlined by the manufacturer. The inter-assay coefficient of variation is 3.4% while intra-assay coefficient of variation is 2.7%.
2.5. Determination of renal cortex preproendothelin-1 mRNA levels
Real-time polymerase chain reaction (qRT-PCR) was used to determine renal cortex preproendothelin-1 (PPET-1) levels. The tissues were stored at −80 °C. Total RNA was then extracted from the homogenized tissues using the RNeasy Protect Mini Kit (Qiagen). The isolation procedure was performed according to the manufacturer’s provided instructions. After RNA was isolated, concentration and quality were verified using a spectrophotometer (Eppendorf BioPhotometer). cDNA was synthesized from 1 μg of RNA using the iScript cDNA Synthesis Kit (BioRad). Real-time polymerase chain reaction (qRT-PCR) was performed using iQ SYBR Green Supermix (BioRad) and the CFX96 Touch Real-Time PCR Detection System (BioRad). The following primer sequences provided by Life technologies were used for PPET: forward 1, CTAGGTCTAAGCGATCCTTG, and reverse 1, TCTTTGTCTGCTTGGC. Levels of mRNA were calculated using the mathematical formula 2−ΔΔCt (2avg. Ct gene of interest − avg Ct beta actin) recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997).
2.6. Statistical analysis
All of the data are expressed as mean ± SEM. Comparisons of control with experimental groups were analyzed by one-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis. A value of p < 0.05 was considered statistically significant.
3. Results
Infusion of sFlt-1 into NP rats elevated mean arterial pressure (MAP) compared with control NP rats: 117 ± 1 (n = 13) vs. 99 ± 2 mmHg (n = 12, p < 0.05). Administration of 17-OHPC blunted the hypertension lowering MAP to 102 ± 3 mmHg in sFlt-1 treated pregnant rats (n = 8, Fig. 1). 17-OHPC did not change pup, placenta weights or litter size in response to sFlt-1 during pregnancy (Fig. 2).
Fig. 1.
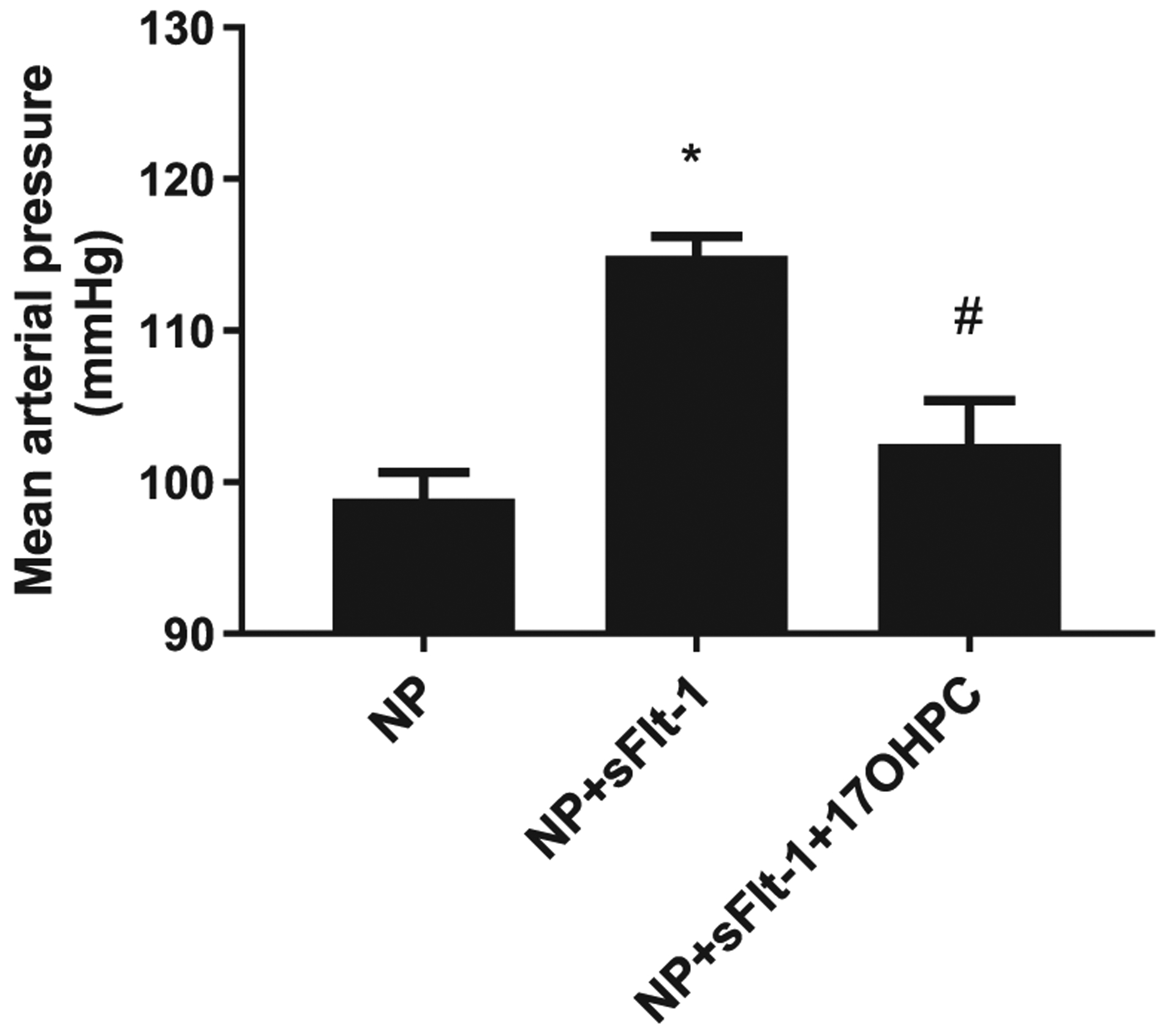
17-OHPC reduces mean arterial blood pressure (MAP) in response to sFlt-1 induced hypertension in pregnant rats (n = 11–17/group). Data are shown as means ± S.E.M. *p < 0.05 vs. NP, #p < 0.05 vs. NP + sFlt-1. One-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values.
Fig. 2.
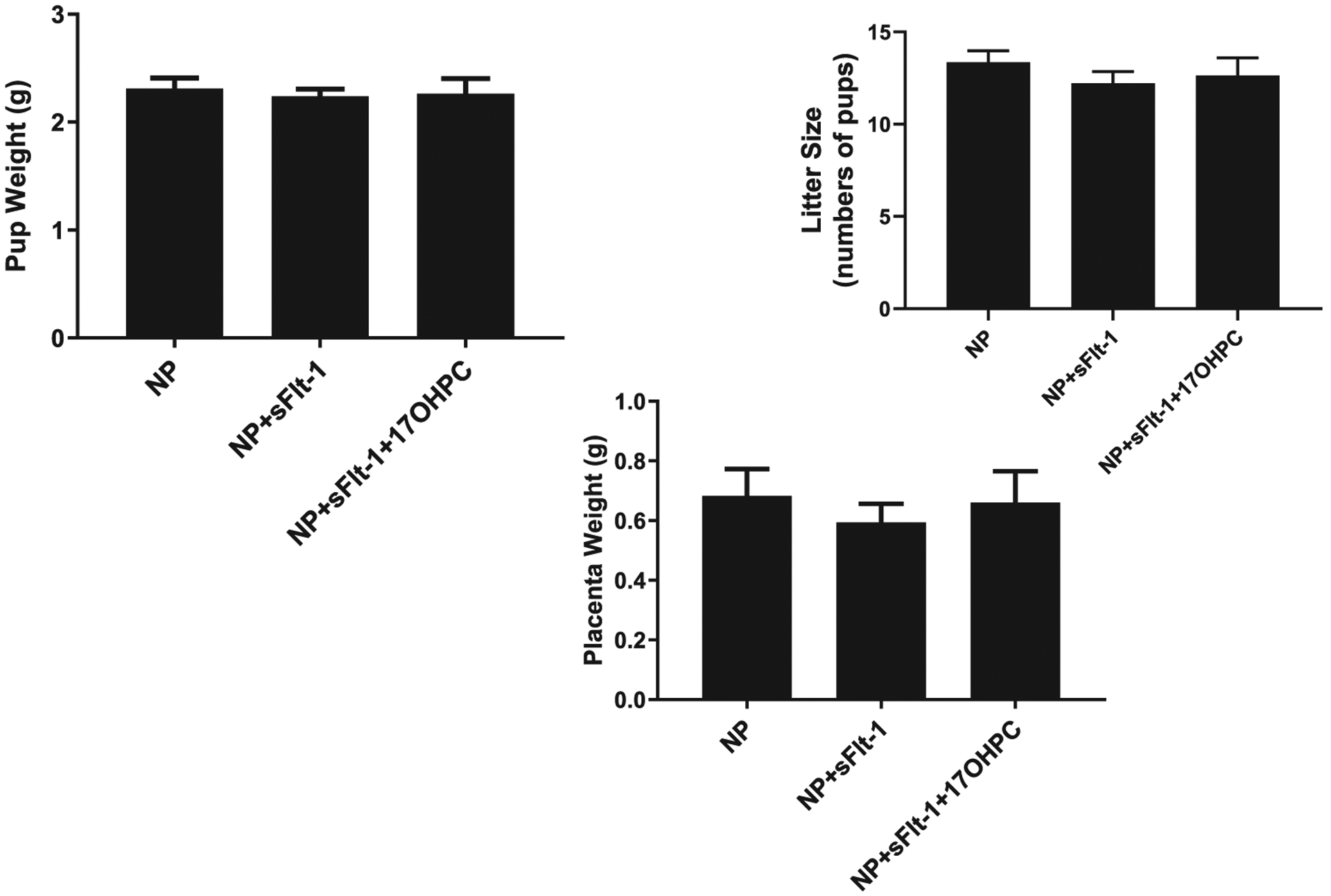
17-OHPC did not change either pup (panel A) or placenta weights (panel B) in response to sFlt-1 induced hypertension in pregnant rats (n = 11–17/group). Data are shown as means ± S.E.M. One-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values.
Importantly, renal cortex PPET-1 mRNA levels were elevated 3 fold in NP + sFlt-1 rats compare to NP rats, which was normalized with 17-OHPC administration, (n = 5–7/group, p < 0.05, Fig. 3).
Fig. 3.
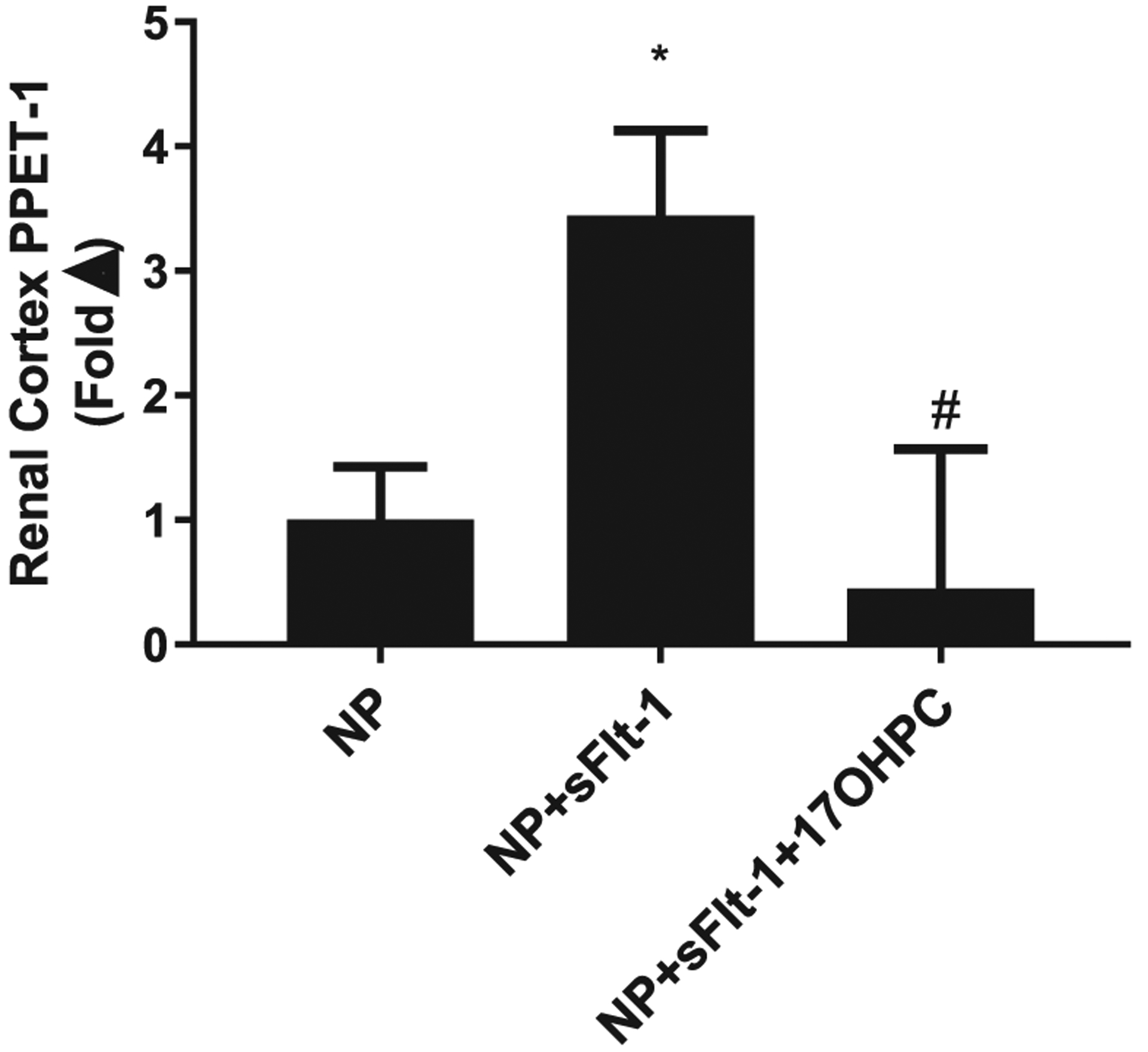
17-OHPC improves renal cortex PPET-1 (n = 5–7/group). Data are shown as means ± S.E.M. *p < 0.05 vs. NP, #p < 0.05 vs. NP + sFlt-1. One-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values.
Plasma nitrate-nitrite levels were 44 ± 9 μM in NP rats (n = 9), 20 ± 3 μM in NP + sFlt-1 (n = 7), which increased to 42 ± 11 μM NP + sFlt-1 + 17OHPC (n = 6). Neither pup nor placental weight was affected by sFlt-1 or 17-OHPC. We have recently published that 17-OHPC given to NP rats did not show any difference in pregnancy outcomes when compared with untreated NP rats [35] (Fig. 4).
Fig. 4.
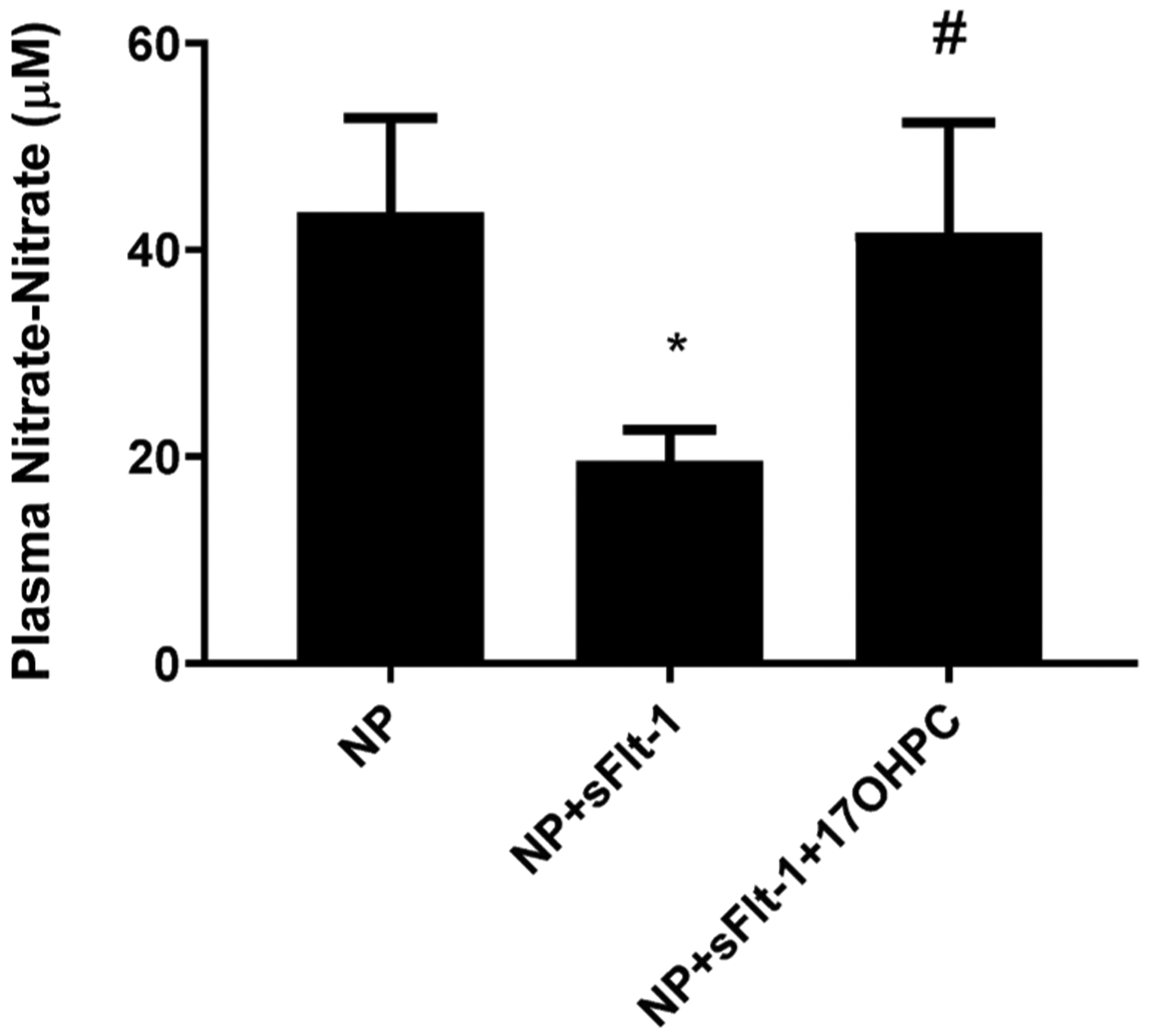
17-OHPC increases circulating nitrate-nitrite levels (n = −6–9/group) in response to sFlt-1 during pregnancy. Data are shown as means ± S.E.M. One-way ANOVA and Bonferroni as post hoc analysis were performed to generate p values.
4. Discussion
The principal findings of this study are: 17-OHPC administered at days 15 and 18 of gestation into sFlt-1 induced hypertension in pregnant rats reduces blood pressure and renal ET-1 while elevating circulating nitrate-nitrite levels.
Progesterone is an endogenous steroid and sex hormone that plays a critical role in the maintenance of normal pregnancy. In addition to its many other function, progesterone has anti-inflammatory as well as vasodilatory effects [31,37,38]. The ability of progesterone to modulate the immune system is two-fold, determined by the availability of the hormone or progesterone sensitivity of the lymphocytes [39]. It is believed the mechanism of action of 17-OHPC involves interaction with the progesterone receptors, facilitating an increase in nitric oxide production and resultant promotion of uterine relaxation.
Prior data showed that 17-OHPC administration on day 18 of gestation in the RUPP rat model improved hypertension in RUPP rats which was associated with improved inflammation by attenuating CD4+ T cells and other pro-inflammatory cytokines. Improvements were also noted in renal and placental ET-1, UARI, litter size, vascular eNOS expression and nitric oxide (NO) bioavailability [32–34].
Pregnancy is associated with immuno-modulation in order to assure a successful and healthy pregnancy. Preeclampsia is associated with physiologic alterations resulting in a state of chronic inflammation [6,8,11,40]. This state of chronic inflammation plays an important role in the pathophysiology of PE, with increased inflammatory mediators, and imbalance in circulating angiogenic factors. These factors have been shown to play an important role in stimulating vasoactive pathways such as endothelin-1 (ET-1), AT1-AA and sFlt-1 and oxidative stress, while reducing vasodilatory factors such as nitric oxide [9,31,41–43].
The exact mechanism by which sFlt-1 production leads to hypertension is an area of current investigation [14,36]. It has been demonstrated that sFlt-1 increases in response to placental ischemia via AT1 receptor activation by endogenous ANGII [44]. Furthermore, our lab has shown that infusion of agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) significantly increased endothelin-1 in renal cortices and placenta, as well as increasing sFlt-1 compared to normal pregnant controls [45].
Studies have been performed attempting to restore the balance between the antiangiogenic sFlt-1 and angiogenic placental growth factor (PIGF) [46]. Zhu et al infused PIGF into the RUPP rat model and demonstrated that restoring the angiogenic/antiangiogenic balance by infusing PIGF decreased vasoconstriction while improving blood pressure [46]. The mechanism by which PIGF infusion decreases blood pressure is through enhancement of endothelial nitric oxide cGMP pathway [46].
The endothelin type A receptor has been hypothesized as a potential target for therapeutics in treating PE [24]. Murphy et al explored the relationship between sFlt-1 and ET-1 by infusion of sFlt-1 into pregnant rats and pregnant rats treated with a selective endothelin type 1 receptor antagonist [36]. In their study, sFlt-1 infusion into pregnant rats causes hypertension and increases ET-1 mRNA expression in the renal cortices by approximately 3-fold compared to normal pregnant rats. Endothelin type A receptor blockade completely mitigated blood pressure response in sFlt-1 infused rats, and had no effect on blood pressure in normal pregnant rats [36]. In agreement with it, an important finding of this current study is that 17-OHPC improves pregnancy outcomes in response to sFlt-1 induced hypertension in pregnant rats.
5. Conclusion
This study demonstrated that 17-OHPC improves hypertension in response to elevated sFlt-1 in pregnant rats. Furthermore, 17-OHPC improved renal cortex PPET-1 and NO levels. More studies are needed to investigate the role of progesterone in regards to modulation of sFlt-1 induced hypertension and attenuation of renal preproendothelin-1 in PE.
Funding
This research was supported by grants from the National Institutes of Health RO1HD067541 and P20GM121334 (BL, LA)/Office of Research, UMMC (BL) AHA 19CDA34670055 (LA) and AMAG-LUMARA Pharmaceutical Company (BL, LA).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Creasy RK, Resnik R, Greene MF, Iams JD, Lockwood CJ, Creasy and resnik’s maternal-fetal medicine: principles and practice.1 online resource.
- [2].Duley L, Maternal mortality associated with hypertensive disorders of pregnancy in africa, asia, latin america and the caribbean, Br. J. Obstet. Gynaecol 99 (1992) 547–553. [DOI] [PubMed] [Google Scholar]
- [3].Noris M, Perico N, Remuzzi G, Mechanisms of disease: pre-eclampsia, Nat. Clin. Practice Nephrol 1 (2005) 98–114, quiz 120. [DOI] [PubMed] [Google Scholar]
- [4].American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet. Gynecol 122 (2013) 1122–1131. [DOI] [PubMed] [Google Scholar]
- [5].Schlembach D, Pre-eclampsia–still a disease of theories, Fukushima J. Med. Sci 49 (2003) 69–115. [PubMed] [Google Scholar]
- [6].Granger JP, Inflammatory cytokines, vascular function, and hypertension, Am. J. Physiol 286 (2004) R989–R990. [DOI] [PubMed] [Google Scholar]
- [7].Redman CW, Sargent IL, Latest advances in understanding preeclampsia, Science 308 (2005) 1592–1594. [DOI] [PubMed] [Google Scholar]
- [8].Conrad KP, Benyo DF, Placental cytokines and the pathogenesis of preeclampsia, Am. J. Reprod. Immunol 37 (1997) 240–249. [DOI] [PubMed] [Google Scholar]
- [9].LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP, Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia, Curr. Hypertens. Rep 9 (2007) 480–485. [DOI] [PubMed] [Google Scholar]
- [10].Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M, Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia, J. Obstet. Gynaecol. Res 36 (2010) 239–247. [DOI] [PubMed] [Google Scholar]
- [11].Lamarca B, The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia, Minerva Ginecol. 62 (2010) 105–120. [PMC free article] [PubMed] [Google Scholar]
- [12].Tam Tam KB, Cockrell K, Arany M, Speed J, Martin JN Jr, Lamarca B, Granger JP, Endothelin type a receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance, Am. J. Obstet. Gynecol 204 (4) (2011) 330.e331–330.e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Holme AM, Roland MC, Henriksen T, Michelsen TM, In vivo uteroplacental release of placental growth factor and soluble fms-like tyrosine kinase-1 in normal and preeclamptic pregnancies, Am. J. Obstet. Gynecol 215 (782) (2016) e781–782 e789. [DOI] [PubMed] [Google Scholar]
- [14].Gilbert JS, Babcock SA, Granger JP, Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression, Hypertension 50 (2007) 1142–1147. [DOI] [PubMed] [Google Scholar]
- [15].Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP, Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction, Am. J. Physiol 294 (2008) H541–H550. [DOI] [PubMed] [Google Scholar]
- [16].Kaitu’u-Lino TJ, Brownfoot FC, Beard S, Cannon P, Hastie R, Nguyen TV, Binder NK, Tong S, Hannan NJ, Combining metformin and esomeprazole is additive in reducing sflt-1 secretion and decreasing endothelial dysfunction – implications for treating preeclampsia, PLoS ONE 13 (2018), e0188845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Alasztics B, Gullai N, Molvarec A, Rigo J Jr., the role of angiogenic factors in preeclampsia, Orv. Hetil 155 (2014) 1860–1866. [DOI] [PubMed] [Google Scholar]
- [18].Unal ER, Robinson CJ, Johnson DD, Chang EY, Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia, Am. J. Obstet. Gynecol 197 (211) (2007) e211–e214. [DOI] [PubMed] [Google Scholar]
- [19].Black C, Al-Amin A, Stolarek C, Kane SC, Rolnik DL, White A, da Silva Costa F, Brennecke S, Midpregnancy prediction of pre-eclampsia using serum biomarkers sflt-1 and plgf, Pregnancy Hypertens. 16 (2019) 112–119. [DOI] [PubMed] [Google Scholar]
- [20].Kwiatkowski S, Kwiatkowska E, Torbe A, The role of disordered angiogenesis tissue markers (sflt-1, plgf) in present day diagnosis of preeclampsia, Ginekol. Pol 90 (2019) 173–176. [DOI] [PubMed] [Google Scholar]
- [21].Boulanger H, Lefevre G, Ahriz Saksi S, Achiche J, Bailleul S, Ekoukou D, Drouin D, Sault C, Stawiarski N, Dupuis E, potential value of placental angiogenic factors as biomarkers in preeclampsia for clinical physicians, Nephrol. Ther (2019). [DOI] [PubMed] [Google Scholar]
- [22].Kwiatkowski S, Dolegowska B, Kwiatkowska E, Rzepka R, Marczuk N, Loj B, Mikolajek-Bedner W, Torbe A, Do the physiological aging of the placenta and the changes in angiogenesis marker sflt-1 and plgf concentrations predispose patients to late-onset preeclampsia? J. Matern. Fetal Neonatal. Med 32 (2019) 11–20. [DOI] [PubMed] [Google Scholar]
- [23].Marasciulo FL, Montagnani M, Potenza MA, Endothelin-1: the yin and yang on vascular function, Curr. Med. Chem 13 (2006) 1655–1665. [DOI] [PubMed] [Google Scholar]
- [24].Bakrania B, Duncan J, Warrington JP, Granger JP, The endothelin type a receptor as a potential therapeutic target in preeclampsia, Int. J. Mol. Sci 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saleh L, Danser JA, van den Meiracker AH, Role of endothelin in preeclampsia and hypertension following antiangiogenesis treatment, Curr. Opin. Nephrol. Hypertens 25 (2016) 94–99. [DOI] [PubMed] [Google Scholar]
- [26].Meis PJ, The role of 17 alpha-hydroxyprogesterone caproate in the prevention of preterm birth, Women’s Health 2 (2006) 819–824. [DOI] [PubMed] [Google Scholar]
- [27].Merlob P, Stahl B, Klinger G, 17alpha hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth, Reprod. Toxicol 33 (2012) 15–19. [DOI] [PubMed] [Google Scholar]
- [28].Sfakianaki AK, Norwitz ER, Mechanisms of progesterone action in inhibiting prematurity, J. Mater.-Fetal Neonatal Med 19 (2006) 763–772. [DOI] [PubMed] [Google Scholar]
- [29].Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA, Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth, Cochrane Database Syst. Rev 7 (2013). CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Committee on Practice Bulletins-Obstetrics TACoO, Gynecologists. Practice bulletin no. 130: Prediction and prevention of preterm birth, Obstet. Gynecol 120 (2012) 964–973. [DOI] [PubMed] [Google Scholar]
- [31].Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J, Wallukat G, Martin JN Jr., Dechend R, Lamarca B, Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin ii type i receptor in response to elevated interleukin-6 during pregnancy, Am. J. Obstet. Gynecol (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kiprono LV, Wallace K, Moseley J, Martin J Jr., Lamarca B, Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia, Am. J. Obstet. Gynecol (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, Granger JP, Martin JN Jr., Lamarca B, Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy, Am. J. Hypertens 22 (2009) 1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr., LaMarca B, 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model, Hypertension 65 (2015) 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, Vaka VR, McKenzie J, LaMarca B, Continued investigation into 17-ohpc: results from the preclinical rupp rat model of preeclampsia, Hypertension (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murphy SR, LaMarca BB, Cockrell K, Granger JP, Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats, Hypertension 55 (2010) 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Selles J, Polini N, Alvarez C, Massheimer V, Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation, Life Sci. 69 (2001) 815–827. [DOI] [PubMed] [Google Scholar]
- [38].Simoncini T, Fu XD, Caruso A, Garibaldi S, Baldacci C, Giretti MS, Mannella P, Flamini MI, Sanchez AM, Genazzani AR, Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors, Hum. Reprod 22 (2007) 2325–2334. [DOI] [PubMed] [Google Scholar]
- [39].Szekeres-Bartho J, Halasz M, Palkovics T, Progesterone in pregnancy; receptor-ligand interaction and signaling pathways, J. Reprod. Immunol 83 (2009) 60–64. [DOI] [PubMed] [Google Scholar]
- [40].Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, Granger J, Hypertension in response to il-6 during pregnancy: role of at1-receptor activation, Int. J. Interferon Cytokine Mediator Res 2011 (2011) 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ, Preeclampsia is associated with lower percentages of regulatory t cells in maternal blood, Hypertens. Pregnancy 28 (2009) 300–311. [DOI] [PubMed] [Google Scholar]
- [42].Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, Treszl A, Decreased number of foxp3+ regulatory t cells in preeclampsia, Acta Obstet. Gynecol. Scand 87 (2008) 1229–1233. [DOI] [PubMed] [Google Scholar]
- [43].Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B, Rigo J Jr., Molvarec A, The frequency of peripheral blood cd4+ cd25high foxp3+ and cd4+ cd25− foxp3+ regulatory t cells in normal pregnancy and pre-eclampsia, Am. J. Reprod. Immunol 68 (2012) 175–180. [DOI] [PubMed] [Google Scholar]
- [44].Murphy SR, Cockrell K, Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: role of angiotensin ii, Physiol. Rep 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].LaMarca B, Parrish MR, Wallace K, Agonistic autoantibodies to the angiotensin ii type i receptor cause pathophysiologic characteristics of preeclampsia, Gend. Med 9 (2012) 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA, Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy, Am. J. Physiol. Regul. Integr. Comp. Physiol 311 (2016) R505–R521. [DOI] [PMC free article] [PubMed] [Google Scholar]


