Abstract
Coronary artery abnormalities are the most important complications in children with Kawasaki disease (KD). Two-dimensional transthoracic echocardiography currently is the standard of care for initial evaluation and follow-up of children with KD. However, it has inherent limitations with regard to evaluation of mid and distal coronary arteries and, left circumflex artery and the poor acoustic window in older children often makes evaluation difficult in this age group. Catheter angiography (CA) is invasive, has high radiation exposure and fails to demonstrate abnormalities beyond lumen. The limitations of echocardiography and CA necessitate the use of an imaging modality that overcomes these problems. In recent years advances in computed tomography technology have enabled explicit evaluation of coronary arteries along their entire course including major branches with optimal and acceptable radiation exposure in children. Computed tomography coronary angiography (CTCA) can be performed during acute as well as convalescent phases of KD. It is likely that CTCA may soon be considered the reference standard imaging modality for evaluation of coronary arteries in children with KD.
Keywords: Coronary artery abnormalities, Computed tomography coronary angiography, 2D-echocardiography, Kawasaki disease, Imaging modality, Acquired heart disease
Core Tip: In recent years advances in computed tomography technology have enabled explicit evaluation of coronary arteries along their entire course including major branches with optimal and acceptable radiation exposure in children. Computed tomography coronary angiography (CTCA) can be performed during acute as well as convalescent phases of Kawasaki disease (KD). It is likely that CTCA may soon be considered the gold standard imaging modality for evaluation of coronary arteries in children with KD.
INTRODUCTION
Kawasaki disease (KD) is the most common vasculitis in children with a special predilection for coronary arteries. Coronary artery abnormalities (CAAs) are usually proportional to the extent of inflammation and without appropriate treatment, up to a quarter of patients with KD can develop CAAs. With early diagnosis and prompt institution of therapy, the incidence of CAAs is less than 5%, however there may be non-responsiveness to standard intravenous immunoglobulin therapy posing risk of developing severe CAAs[1-3]. CAAs can be assessed by several imaging techniques. 2D-echocardiography is the preferred imaging modality for coronary arteries evaluation both during the acute phase as well as during convalescence[4]. However, there has been increasing interest about other evolving modalities that can address the limitations of echocardiography[4,5]. In this context, we have discussed the role of computed tomography coronary angiography (CTCA) for detection of CAAs in KD and compared its performance with other available modalities. Current guidelines on use of CTCA have also been discussed along with the proposed use of CTCA in management of children with KD.
CORONARY ARTERY ABNORMALITIES IN KD AND NEED OF IMAGING
Coronary artery abnormalities (CAA) are the dreaded complications of KD requiring prompt and accurate diagnosis. In the acute phase of illness, typical CAAs include dilatation and aneurysm formation. These may resolve, remodel or persist during convalescence or may be complicated by thrombosis and steno-occlusive lesions mandating long term surveillance[6-10]. Concerns have also been raised that KD may act as a risk factor for premature atherosclerosis[11]. Precise imaging is required for assessment of these complications.
2D-echocardiography is the standard imaging modality for coronary arteries at presentation and during follow-up[12]. However, it has several limitations and these preclude its use in certain circumstances[12,13].
Other imaging techniques that have been used for coronary artery evaluation include catheter angiography (CA), CTCA and magnetic resonance coronary angiography (MRCA).
CA is an invasive procedure with uncontrolled radiation exposure[12], whereas MR angiography is technically difficult and very few centers have requisite expertise[4]. With the availability of modern CT scanners there is increasing interest in CTCA as an imaging modality of KD. It is non-invasive, has optimized sub-millisievert radiation exposure and has the potential to address all the limitations of CA and echocardiography[14-19]. Moreover CTCA has an ability to detect the earliest changes of atherosclerosis[20,21]. In this scenario CTCA seems promising and its incorporation into the management algorithm of KD should be considered. In this review we have discussed the emerging role of CTCA in evaluation of children with KD for detection of CAAs.
CTCA IN KD: NEEDS AND CHALLENGES
Transthoracic echocardiography is the presently standard of care for KD. However, it has several limitations. CA is invasive, has significant radiation exposure and cannot be repeated frequently.
After the advent of 64-Slice CT platforms, CTCA has become feasible. Radiation associated with CTCA is the biggest challenge as such radiation exposure has been linked to malignancies in children[22,23]. The older platforms also had sub-optimal image quality due to inherent high heart rates in children[14,24]. In recent years, however, CT technology has undergone a remarkable progress enabling the radiologist to acquire high resolution coronary artery images with acceptable radiation exposure and at any heart rate. This was possible due to higher slice CT (128, 256 and 320 slice) platforms and dual source (DS) CT scanners[14-18]. DSCT has superior diagnostic performance as compared to single-source CT in the evaluation of coronary arteries and this gives an advantage of scanning in children who otherwise have inherent high heart rates and obviates the need of large doses of beta-blockers[17,18].
As a result, CTCA is now being increasingly used for evaluation of children with KD and CAAs. It provides information about coronary arteries and the major branches with exquisite details of luminal caliber and intramural changes. Moreover, it can be repeated on follow-up as it is non-invasive. CTCA is especially useful in older children and adolescents who often have a poor acoustic window for echocardiography.
CTCA: CALCIUM SCORING AND LUMINOGRAPHY
CT calcium scoring is a technique that identifies calcium deposition in coronary arteries without the need of intravenous contrast (Figure 1). Calcium deposition in coronary arteries is a strong predictor of previous coronary artery involvement in patients with KD. Kahn et al[21] reported coronary artery calcium scoring using a low radiation dose CT protocol on 70 patients with KD at median of 14.8 years’ follow-up and showed that coronary calcification was not present in patients with KD and normal coronaries during acute phase of disease. Ten out of 14 subjects with CAAs in acute phase had coronary calcification. A subsequent study from the same group of investigators affirmed that calcium deposition was not seen in patients with KD who did not develop CAAs during acute phase of disease[25]. The authors have shown that calcium scoring by CT is also a useful tool for identification of unidentified CAAs in patients with remote history of KD. It was also noted that sensitivity and specificity of calcium scoring to identify presence of CAAs is highest when scans were performed after more than 10 years of follow-up. Whether the degree of calcification has prognostic value in patients with KD is still conjectural. Zero calcium score in patients with remote history of KD is reassuring if performed at least 10 years after the initial illness.
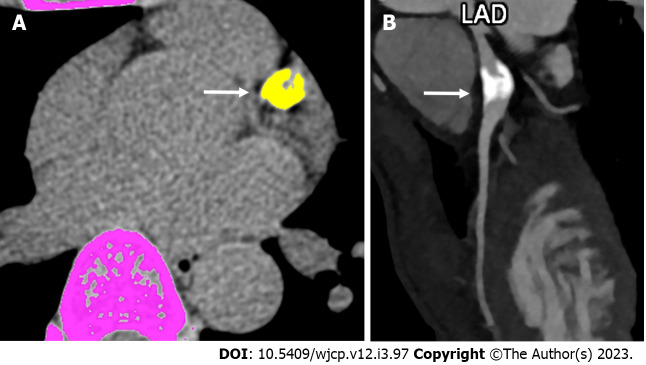
Figure 1.
Computed tomography derived calcium score and role of angiography in convalescent phase of Kawasaki disease. A: Computed tomography (CT) derived calcium scoring in a 19 years male patient on follow-up with history of Kawasaki disease in childhood shows a thickly calcified lesion in the proximal course of left anterior descending coronary artery (color marked as yellow with an arrow (calcium score was 910); B: Subsequent CT coronary angiographic curved reformatted image shows calcified aneurysm (arrow). LAD: Left anterior descending.
Angiography or luminography is acquisition of data after injection of intra-venous contract for evaluation of CAA. Acquisition of data should preferably be done with low radiation protocols and following strategies should be employed to reduce the radiation exposure:
Lower kilovoltage (kVp) for acquisition: kVp below 80 is appropriate - this reduces radiation exposure up to 70%[26-28].
Prospective ECG triggering: In this technique data acquisition is regulated by ECG signal and the X-ray tube current is either switched off or markedly lowered according to phase of R–R interval. This allows reduction of radiation exposure up to 90% as compared to retrospective ECG-gating, where X-ray exposure is given continuously[29-31]. Duan et al[17] and Kim et al[32] reported mean effective dose of 0.36 ± 0.06 mSv and 0.6 ± 0.5 mSv respectively on DSCT. Even in our experience the radiation had always been below 1 mSv[18].
(ECG)-controlled tube current modulation: With this technology up to 50% reduction in radiation exposure is achievable[32,33].
High pitch: Pitch in CT refers to area coverage with overlap and has inverse relationship with radiation exposure. With DSCT higher pitch (up to 3.4) there is further reduction in radiation exposure[34-36].
Iterative image reconstruction method: This is a newer development that allows to reconstruct data post-acquisition with high resolution images even at low radiation parameters (lower kilovoltage and tube current values)[36,37].
Using the dose saving strategies discussed above, radiation exposure can be brought down significantly below 1 mSv level[18,36].
CTCA: STRENGTH AND ADVANTAGES
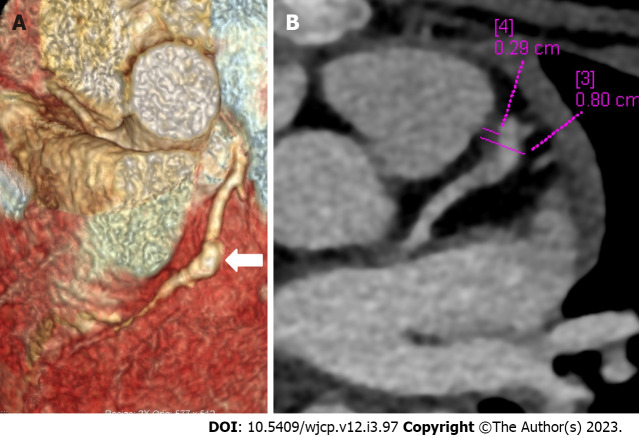
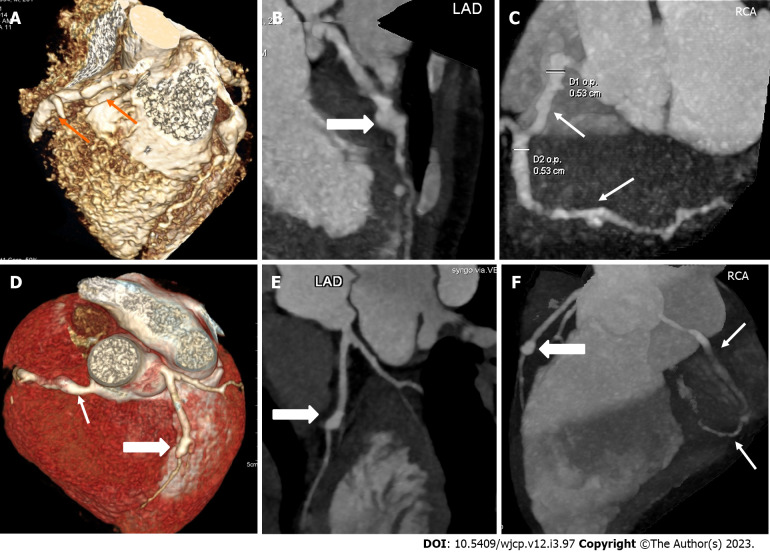
CTCA with the current technologies of radiation optimization and fast scanning has capability to image the entire course of all coronary arteries especially left circumflex coronary artery which is difficult to evaluate on 2D-echocardiography (Figure 2). CTCA also allows to detect CAAs in middle and distal segments of coronary arteries[38]. These are usually missed on echocardiography (Figure 3). It also precisely identifies location and morphology of aneurysms (Figure 4) and thrombo-occlusive lesions (Figures 5 and 6). Mural abnormalities (calcifications, plaque, and adherent thrombus to luminal wall) are also well characterized on CTCA (Figures 1, 5 and 6).
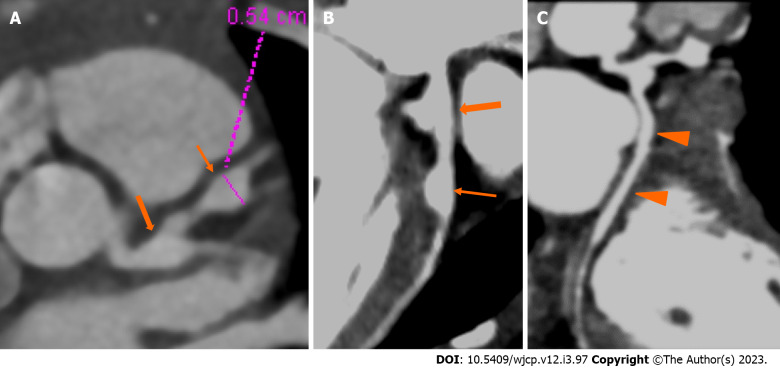
Figure 2.
Computed tomography coronary angiography images showing its strength to evaluate coronary artery abnormalities in left circumflex artery. A: 3 years male child at presentation shows fusiform aneurysm at bifurcation of left main coronary artery [left main coronary artery (LMCA)- thick arrows in A and B] with extension into osteo-proximal segment of left anterior descending (LAD). Note a skip fusiform aneurysm in proximal LAD (thin arrow in b). C: Proximal and mid segments of left circumflex (LCX) are dilated (arrow heads in C). Echo demonstrated LMCA and LAD aneurysms however cannot evaluate LCX due to its orientation and course.
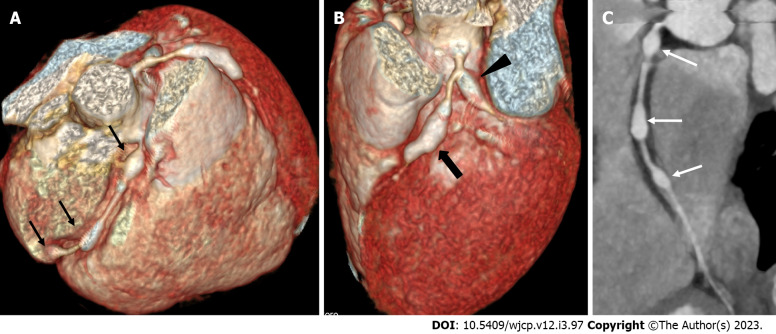
Figure 3.
Computed tomography coronary angiography images showing its ability to evaluate mid and distal segments of coronary arteries. Computed tomography coronary angiography (A and B: Volume rendered images; C: Curved reformatted image of RCA) of 4 years male at presentation demonstrate skip fusiform aneurysms in RCA (Thin arrows in A and C) and fusiform aneurysms in proximal LAD (thick arrow in B) and proximal left circumflex (arrow head in B). Fusiform aneurysm in mid and distal RCA could not be visualized on transthoracic echocardiography.
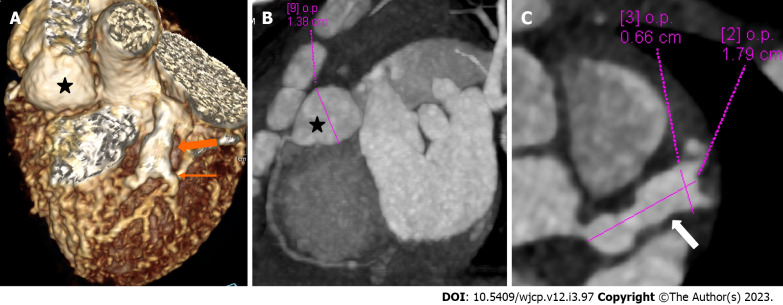
Figure 4.
Panel of computed tomography coronary angiography images showing its ability to precisely identify location and morphology of aneurysms with extension into side branches. Computed tomography coronary angiography (A: Volume rendered image; B: Curved reformatted image and C-axial image) of 4 years female child in acute phase (presentation) demonstrate giant saccular aneurysm in proximal resonance coronary angiography (asterisk in A and B). Fusiform aneurysm is seen in proximal left anterior descending (thick arrow in A) with extension into diagonal-1 branch (thin arrow in A).
Figure 5.
Computed tomography coronary angiography images depicting complications in coronary artery abnormalities (thrombus). Computed tomography coronary angiography [(A: Volume rendered image; B: Axial image in plane of left anterior descending (LAD)] during follow up at shows a giant fusiform aneurysm in mid segment of LAD (thick arrow in A) with a hypodense plaque like tissue attached to the anterior wall (B) suggestive of thrombus. Child was having chest pain and ECHO at presentation and during the current episode was reported as normal; ECHO fails to elicit coronary artery abnormalities and its complication of thrombus in the aneurysm.
Figure 6.
Computed tomography coronary angiography images showing its role in follow-up imaging and its role in assessment of compications. Computed tomography coronary angiography (CTCA) at presentation in 1 year male (top row A-C) shows a giant complex aneurysm in mid segment of left anterior descending (LAD) (thick arrow in A and B) with multiple segmental aneurysms along the entire course of resonance coronary angiography (RCA). Follow-up CTCA after 3 years of presentation shows remodelling of LAD aneurysm (now becomes fusiform with mural calcification and severe stenosis its distal aspect (thick arrows D-F), also note resolution of aneurysmal dilatations of RCA (lower row D-F).
LIMITATIONS OF CTCA
CTCA has few limitations as it may not be available at all the centers, need of sedation in infants and young children, portability and radiation exposure. However, these are minor considering the advantages derived and moreover advanced CT scanners with radiation optimization are now finding space in large centers. Sedation if so, needed in infants and young children is of short duration and general anesthesia is not required in our experience[38-40].
CTCA: COMPARISON WITH OTHER IMAGING MODALITIES
2D-echocardiography
It is known that CAAs in patients with KD can involve all segments of main coronary arteries. 2D-echocardiography remains the imaging modality of choice of evaluation of CAAs in patients with KD because of its inherent advantages – it is easily available, is inexpensive and can be repeated as often as required. However, it has only a limited role in evaluation of middle and distal coronary arteries and the left circumflex coronary artery[41]. CTCA provides an ideal method for evaluation of all segments of coronary arteries[38,42-45]. Several investigators have reported a good correlation between echocardiography and CTCA for the size of the aneurysms.
MRCA
It is still under evaluation for assessment of CAAs in children with KD. MRCA has lower spatial (related to image accuracy that measure fineness of image) and temporal (scanning speed) resolution and poorer image quality compared to CTCA[46,47]. Moreover, MRCA takes long scan time and often requires sedation in young children[32]. Steno-occlusive lesions are better delineated on CTCA compared to MRCA[32]. MRCA, however, is better for evaluation of coronary arteries with heavy intra-mural calcifications (leading to blooming artefact on CT), assessment of myocardial perfusion and viability, and serial follow-up of thrombotic aneurysms[48].
CA
It is the gold standard for evaluation of coronary artery lumen, but is of limited value for assessment of mural abnormalities (thickening, plaque and calcifications) and intramural thrombi. Moreover, it is an invasive procedure with inordinate radiation exposure. CTCA with CA has been shown to have excellent agreement for measurement of size of aneurysms[49].
CTCA: WHEN TO ADVICE?
Current guidelines and the way forward
The American Heart Association Guidelines 2017 have recommended use of CTCA in following circumstances during follow-up when 2D-echocardiography becomes limiting[50]: (1) Due to poor acoustic window in older children; (2) Poor sensitivity to detect complications like thrombosis, stenosis; (3) Inability to detect distal abnormalities; and (4) For detection of mural abnormalities or calcification; However, based on our clinical experience over 25 years we suggest that CTCA may be considered under the following circumstances: As a baseline additional investigation in acute phase in children having significant CAAs on 2D-echocardiography for confirmation of echocardiography findings, detection of distal CAAs and for follow-up[38]. When the initial 2D-echocardiography examination in acute phase is equivocal or sub-optimal, Apparently normal 2D-echocardiography examination in acute phase with a stormy clinical course, On follow-up assessment of CAAs to document resolution or complications (e.g., thrombosis, stenosis), Long term surveillance for detection of mural abnormalities and dystrophic calcifications. At the present time there is no consensus amongst experts on the timing and frequency of carrying out CTCA during follow up of children with KD.
Recent advances in CTCA in KD
Cardiac single-photon emission computed tomography (SPECT) and CT hybrid imaging has been recently described for accurate demonstration of ischemic regions of the myocardium. Abe et al[51] have performed SPECT/CT in 17 patients with KD in chronic phase of disease and showed that this fusion imaging was capable of accurately evaluating myocardial ischemia/infarction as cardiovascular sequelae of KD and delineating the affected coronary arteries.
CONCLUSION
State of the art CT platforms now allow CTCA with high resolution images at sub-millisievert radiation exposure. CTCA has the ability to detect CAAs along the entire course of coronary arteries and delineates mural abnormalities, which otherwise are missed on current standard of care 2D-echocardiography. CTCA can be performed during acute as well as convalescent phases of KD. It is likely that CTCA may soon be considered the imaging modality of choice for evaluation of coronary arteries in children with KD and multi-centric studies focused on use of CTCA is desirable to formulate guidelines.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 4, 2023
First decision: April 13, 2023
Article in press: May 22, 2023
Specialty type: Rheumatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Rigante D, Italy S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Manphool Singhal, Departments of Radiodiagnosis and Imaging, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, Chandigarh, India. drmsinghal74@gmail.com.
Rakesh Kumar Pilania, Pediatric Allergy Immunology Unit, Department of Paediatrics, Advanced Pediatrics Center, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, Chandigarh, India.
Pankaj Gupta, Departments of Radiodiagnosis and Imaging, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, Chandigarh, India.
Nameirakpam Johnson, Pediatric Allergy Immunology Unit, Department of Paediatrics, Advanced Pediatrics Center, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, Chandigarh, India.
Surjit Singh, Pediatric Allergy Immunology Unit, Department of Paediatrics, Advanced Pediatrics Center, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, Chandigarh, India.
References
- 1.Kato H. [Natural history of Kawasaki disease vasculitis] Nihon Rinsho. 2014;72:1530–1535. [PubMed] [Google Scholar]
- 2.Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–1385. doi: 10.1161/01.cir.94.6.1379. [DOI] [PubMed] [Google Scholar]
- 3.Rigante D, Andreozzi L, Fastiggi M, Bracci B, Natale MF, Esposito S. Critical Overview of the Risk Scoring Systems to Predict Non-Responsiveness to Intravenous Immunoglobulin in Kawasaki Syndrome. Int J Mol Sci. 2016;17:278. doi: 10.3390/ijms17030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease; Council on Cardiovascular Disease in the Young; American Heart Association; American Academy of Pediatrics. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 5.Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW, Wilson W. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.cir.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki A, Kamiya T, Arakaki Y, Kinoshita Y, Kimura K. Fate of coronary arterial aneurysms in Kawasaki disease. Am J Cardiol. 1994;74:822–824. doi: 10.1016/0002-9149(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A, Kamiya T, Ono Y, Kinoshita Y, Kawamura S, Kimura K. Clinical significance of morphologic classification of coronary arterial segmental stenosis due to Kawasaki disease. Am J Cardiol. 1993;71:1169–1173. doi: 10.1016/0002-9149(93)90641-o. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Kamiya T, Ono Y, Kohata T, Kimura K, Takamiya M. Follow-up study of coronary artery lesions due to Kawasaki disease by serial selective coronary arteriography in 200 patients. Heart Vessels. 1987;3:159–165. doi: 10.1007/BF02058793. [DOI] [PubMed] [Google Scholar]
- 9.Pilania RK, Jindal AK, Bhattarai D, Naganur SH, Singh S. Cardiovascular Involvement in Kawasaki Disease Is Much More Than Mere Coronary Arteritis. Front Pediatr. 2020;8:526969. doi: 10.3389/fped.2020.526969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilania RK, Bhattarai D, Singh S. Controversies in diagnosis and management of Kawasaki disease. World J Clin Pediatr. 2018;7:27–35. doi: 10.5409/wjcp.v7.i1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi K, Oharaseki T, Naoe S. Pathological study of postcoronary arteritis in adolescents and young adults: with reference to the relationship between sequelae of Kawasaki disease and atherosclerosis. Pediatr Cardiol. 2001;22:138–142. doi: 10.1007/s002460010180. [DOI] [PubMed] [Google Scholar]
- 12.Sokmen G, Tuncer C, Sokmen A, Suner A. Clinical and angiographic features of large left main coronary artery aneurysms. Int J Cardiol. 2008;123:79–83. doi: 10.1016/j.ijcard.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Kato M, Inoue F, Fukui T, Imazeki T, Mitsui M, Matsumoto N, Takahashi M, Karasawa K, Ayusawa M, Kanamaru H, Harada K, Kanmatsuse K. Detection of coronary artery aneurysms, stenoses and occlusions by multislice spiral computed tomography in adolescents with kawasaki disease. Circ J. 2003;67:427–430. doi: 10.1253/circj.67.427. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno N, Funabashi N, Imada M, Tsunoo T, Endo M, Komuro I. Utility of 256-slice cone beam tomography for real four-dimensional volumetric analysis without electrocardiogram gated acquisition. Int J Cardiol. 2007;120:262–267. doi: 10.1016/j.ijcard.2006.07.219. [DOI] [PubMed] [Google Scholar]
- 15.de Graaf FR, Schuijf JD, van Velzen JE, Kroft LJ, de Roos A, Reiber JH, Boersma E, Schalij MJ, Spanó F, Jukema JW, van der Wall EE, Bax JJ. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography in the non-invasive evaluation of significant coronary artery disease. Eur Heart J. 2010;31:1908–1915. doi: 10.1093/eurheartj/ehp571. [DOI] [PubMed] [Google Scholar]
- 16.Pasricha SS, Nandurkar D, Seneviratne SK, Cameron JD, Crossett M, Schneider-Kolsky ME, Troupis JM. Image quality of coronary 320-MDCT in patients with atrial fibrillation: initial experience. AJR Am J Roentgenol. 2009;193:1514–1521. doi: 10.2214/AJR.09.2319. [DOI] [PubMed] [Google Scholar]
- 17.Duan Y, Wang X, Cheng Z, Wu D, Wu L. Application of prospective ECG-triggered dual-source CT coronary angiography for infants and children with coronary artery aneurysms due to Kawasaki disease. Br J Radiol. 2012;85:e1190–e1197. doi: 10.1259/bjr/18174517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singhal M, Singh S, Gupta P, Sharma A, Khandelwal N, Burns JC. Computed Tomography Coronary Angiography for Evaluation of Children With Kawasaki Disease. Curr Probl Diagn Radiol. 2018;47:238–244. doi: 10.1067/j.cpradiol.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda E, Singhal M. Role of imaging studies in Kawasaki disease. Int J Rheum Dis. 2018;21:56–63. doi: 10.1111/1756-185X.13210. [DOI] [PubMed] [Google Scholar]
- 20.Thangathurai J, Kalashnikova M, Takahashi M, Shinbane JS. Coronary Artery Aneurysm in Kawasaki Disease: Coronary CT Angiography through the Lens of Pathophysiology and Differential Diagnosis. Radiol Cardiothorac Imaging. 2021;3:e200550. doi: 10.1148/ryct.2021200550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn AM, Budoff MJ, Daniels LB, Jimenez-Fernandez S, Cox AS, Gordon JB, Burns JC. Calcium scoring in patients with a history of Kawasaki disease. JACC Cardiovasc Imaging. 2012;5:264–272. doi: 10.1016/j.jcmg.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuclear and Radiation Studies Board, Division on Earth and Life Studies, National Research Council of the National Academies. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Available from: https://nap.nationalacademies.org/catalog/11340/health-risks-from-exposure-to-low-levels-of-ionizing-radiation .
- 23.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, Zeng J, Du Z, Sun G, Guo H. Usefulness of 64-slice MDCT for follow-up of young children with coronary artery aneurysm due to Kawasaki disease: initial experience. Eur J Radiol. 2009;69:500–509. doi: 10.1016/j.ejrad.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Kahn AM, Budoff MJ, Daniels LB, Oyamada J, Gordon JB, Burns JC. Usefulness of Calcium Scoring as a Screening Examination in Patients With a History of Kawasaki Disease. Am J Cardiol. 2017;119:967–971. doi: 10.1016/j.amjcard.2016.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leschka S, Stolzmann P, Schmid FT, Scheffel H, Stinn B, Marincek B, Alkadhi H, Wildermuth S. Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol. 2008;18:1809–1817. doi: 10.1007/s00330-008-0966-1. [DOI] [PubMed] [Google Scholar]
- 27.Pflederer T, Rudofsky L, Ropers D, Bachmann S, Marwan M, Daniel WG, Achenbach S. Image quality in a low radiation exposure protocol for retrospectively ECG-gated coronary CT angiography. AJR Am J Roentgenol. 2009;192:1045–1050. doi: 10.2214/AJR.08.1025. [DOI] [PubMed] [Google Scholar]
- 28.Sun Z, Choo GH, Ng KH. Coronary CT angiography: current status and continuing challenges. Br J Radiol. 2012;85:495–510. doi: 10.1259/bjr/15296170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheffel H, Alkadhi H, Leschka S, Plass A, Desbiolles L, Guber I, Krauss T, Gruenenfelder J, Genoni M, Luescher TF, Marincek B, Stolzmann P. Low-dose CT coronary angiography in the step-and-shoot mode: diagnostic performance. Heart. 2008;94:1132–1137. doi: 10.1136/hrt.2008.149971. [DOI] [PubMed] [Google Scholar]
- 30.Husmann L, Valenta I, Gaemperli O, Adda O, Treyer V, Wyss CA, Veit-Haibach P, Tatsugami F, von Schulthess GK, Kaufmann PA. Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J. 2008;29:191–197. doi: 10.1093/eurheartj/ehm613. [DOI] [PubMed] [Google Scholar]
- 31.Shuman WP, Branch KR, May JM, Mitsumori LM, Lockhart DW, Dubinsky TJ, Warren BH, Caldwell JH. Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology. 2008;248:431–437. doi: 10.1148/radiol.2482072192. [DOI] [PubMed] [Google Scholar]
- 32.Kim JW, Goo HW. Coronary artery abnormalities in Kawasaki disease: comparison between CT and MR coronary angiography. Acta Radiol. 2013;54:156–163. doi: 10.1258/ar.2012.120484. [DOI] [PubMed] [Google Scholar]
- 33.Lee TY, Chhem RK. Impact of new technologies on dose reduction in CT. Eur J Radiol. 2010;76:28–35. doi: 10.1016/j.ejrad.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 34. 34 Ertel D, Lell MM, Harig F, Flohr T, Schmidt B, Kalender WA. Cardiac spiral dual-source CT with high pitch: a feasibility study. Eur Radiol. 2009;19:2357–2362. doi: 10.1007/s00330-009-1503-6. [DOI] [PubMed] [Google Scholar]
- 35.Gosling O, Loader R, Venables P, Roobottom C, Rowles N, Bellenger N, Morgan-Hughes G. A comparison of radiation doses between state-of-the-art multislice CT coronary angiography with iterative reconstruction, multislice CT coronary angiography with standard filtered back-projection and invasive diagnostic coronary angiography. Heart. 2010;96:922–926. doi: 10.1136/hrt.2010.195909. [DOI] [PubMed] [Google Scholar]
- 36.Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S, Kastrati A, Schömig A. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113:1305–1310. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 37.Weustink AC, Neefjes LA, Kyrzopoulos S, van Straten M, Neoh Eu R, Meijboom WB, van Mieghem CA, Capuano E, Dijkshoorn ML, Cademartiri F, Boersma E, de Feyter PJ, Krestin GP, Mollet NR. Impact of heart rate frequency and variability on radiation exposure, image quality, and diagnostic performance in dual-source spiral CT coronary angiography. Radiology. 2009;253:672–680. doi: 10.1148/radiol.2533090358. [DOI] [PubMed] [Google Scholar]
- 38.Singhal M, Pilania RK, Jindal AK, Gupta A, Sharma A, Guleria S, Johnson N, Maralakunte M, Vignesh P, Suri D, Sandhu MS, Singh S. Distal coronary artery abnormalities in Kawasaki disease: experience on CT coronary angiography in 176 children. Rheumatology (Oxford) 2023;62:815–823. doi: 10.1093/rheumatology/keac217. [DOI] [PubMed] [Google Scholar]
- 39.Dusad S, Singhal M, Pilania RK, Suri D, Singh S. CT Coronary Angiography Studies After a Mean Follow-up of 3.8 Years in Children With Kawasaki Disease and Spontaneous Defervescence. Front Pediatr. 2020;8:274. doi: 10.3389/fped.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Stijn D, Planken RN, Groenink M, Streekstra GJ, Kuijpers TW, Kuipers IM. Coronary artery assessment in Kawasaki disease with dual-source CT angiography to uncover vascular pathology. Eur Radiol. 2020;30:432–441. doi: 10.1007/s00330-019-06367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Sun K, Wang R, Li Y, Xue H, Yu L, Chen S, Xi L. Comparison study of echocardiography and dual-source CT in diagnosis of coronary artery aneurysm due to Kawasaki disease: coronary artery disease. Echocardiography. 2011;28:1025–1034. doi: 10.1111/j.1540-8175.2011.01486.x. [DOI] [PubMed] [Google Scholar]
- 42.Arnold R, Ley S, Ley-Zaporozhan J, Eichhorn J, Schenk JP, Ulmer H, Kauczor HU. Visualization of coronary arteries in patients after childhood Kawasaki syndrome: value of multidetector CT and MR imaging in comparison to conventional coronary catheterization. Pediatr Radiol. 2007;37:998–1006. doi: 10.1007/s00247-007-0566-2. [DOI] [PubMed] [Google Scholar]
- 43.Chu WC, Mok GC, Lam WW, Yam MC, Sung RY. Assessment of coronary artery aneurysms in paediatric patients with Kawasaki disease by multidetector row CT angiography: feasibility and comparison with 2D echocardiography. Pediatr Radiol. 2006;36:1148–1153. doi: 10.1007/s00247-006-0281-4. [DOI] [PubMed] [Google Scholar]
- 44.Xing Y, Wang H, Yu X, Chen R, Hou Y. Assessment of coronary artery lesions in children with Kawasaki disease: evaluation of MSCT in comparison with 2-D echocardiography. Pediatr Radiol. 2009;39:1209–1215. doi: 10.1007/s00247-009-1364-9. [DOI] [PubMed] [Google Scholar]
- 45.Jrad M, Ben Salem F, Barhoumi C, Lassoued F, Frikha W, Boukriba S, Mizouni H. The Role of Computed Tomography Coronary Angiography in Kawasaki Disease: Comparison with Transthoracic Echocardiography in a 25-Case Retrospective Study. Pediatr Cardiol. 2019;40:265–275. doi: 10.1007/s00246-018-2044-z. [DOI] [PubMed] [Google Scholar]
- 46.Schuijf JD, Bax JJ, Shaw LJ, de Roos A, Lamb HJ, van der Wall EE, Wijns W. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J. 2006;151:404–411. doi: 10.1016/j.ahj.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 47.Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, Hundley WG, Manning WJ, Printz BF, Stuber M, Woodard PK. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation. 2008;118:586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Levin DC, Halpern EJ, Fischman D, Savage M, Walinsky P. Accuracy of MDCT in assessing the degree of stenosis caused by calcified coronary artery plaques. AJR Am J Roentgenol. 2008;191:1676–1683. doi: 10.2214/AJR.07.4026. [DOI] [PubMed] [Google Scholar]
- 49.Tsujii N, Tsuda E, Kanzaki S, Kurosaki K. Measurements of Coronary Artery Aneurysms Due to Kawasaki Disease by Dual-Source Computed Tomography (DSCT) Pediatr Cardiol. 2016;37:442–447. doi: 10.1007/s00246-015-1297-z. [DOI] [PubMed] [Google Scholar]
- 50.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 51.Abe M, Fukazawa R, Ogawa S, Watanabe M, Fukushima Y, Kiriyama T, Hayashi H, Itoh Y. Usefulness of Single Photon Emission Computed Tomography/Computed Tomography Fusion-Hybrid Imaging to Evaluate Coronary Artery Disorders in Patients with a History of Kawasaki Disease. J Nippon Med Sch. 2016;83:71–80. doi: 10.1272/jnms.83.71. [DOI] [PubMed] [Google Scholar]