Abstract
The hedgehog (Hh) signaling pathway plays several diverse regulatory and patterning roles during organogenesis of the intestine and in the regulation of adult intestinal homeostasis. In the embryo, fetus, and adult, intestinal Hh signaling is paracrine: Hh ligands are expressed in the endodermally derived epithelium, while signal transduction is confined to the mesenchymal compartment, where at least a dozen distinct cell types are capable of responding to Hh signals. Epithelial Hh ligands not only regulate a variety of mesenchymal cell behaviors, but they also direct these mesenchymal cells to secrete additional soluble factors (e.g., Wnts, Bmps, inflammatory mediators) that feed back to regulate the epithelial cells themselves. Evolutionary conservation of the core Hh signaling pathway, as well as conservation of epithelial/mesenchymal cross talk in the intestine, has meant that work in many diverse model systems has contributed to our current understanding of the role of this pathway in intestinal organogenesis, which is reviewed here.
Keywords: patterning, smooth muscle development, villus development, enteric neuron development, inflammation, cancer, epithelial-mesenchymal interaction, peri-cryptal fibroblasts
INTRODUCTION
Endoderm, the innermost germ layer, gives rise to the epithelium of the entire tubular gut and, in many organisms, to the epithelial compartment of several accessory organs. The Hh ligands, Sonic Hedgehog (Shh) and Indian Hedgehog (Ihh), are expressed in the endoderm, even before gut tube folding is complete (1, 2), and continued expression of this pathway is essential for many aspects of gut organogenesis. In the intestine, Hh signaling is strictly paracrine; Shh and Ihh ligands are secreted by endodermal (epithelial) cells, and these signals are received by underlying mesenchymal (stromal) cells. Throughout development, endodermal Hh signals are utilized for multiple purposes and in multiple contexts to accomplish proper intestinal growth and patterning. The diverse temporal and spatial roles for Hh signals, combined with the fact that several different mesenchymal cell types can respond to these signals, even within the same endodermal territory, can make dissection of the phenotypic readout of altered Hh signaling particularly challenging. Adding to that challenge, Hh ligands stimulate mesenchymal cells to secrete other soluble signaling molecules (e.g., Wnts, Bmps), which then feed back to affect the patterning and phenotype of nearby epithelial cells. Thus, Hh is a central player in a critical cell–cell communication network that influences both mesenchymal and epithelial cell behavior.
In the adult intestine, the level of Hh pathway signaling is often reduced, relative to fetal levels, but Hh signaling continues to participate in homeostasis and response to injury. In this review, our goal is to summarize the literature concerning the roles of the Hh pathway in growth, patterning, and homeostasis of the mammalian small intestine. Where possible, we link actions of Hh to specific downstream cellular and molecular targets and to downstream signaling pathways. We conclude by examining some of the major unanswered questions that may drive future research in intestinal Hh signal transduction.
HEDGEHOG PATHWAY EXPRESSION IN INTESTINAL CELLS
Readers are referred to several outstanding recent reviews that provide very detailed descriptions of the Hh signaling pathway and its regulators (3–5). Here, our goals are to outline the major molecular players (Figure 1) and to highlight the dynamics of signal transduction in developing and adult intestinal tissue (Figure 2). In mammals, three hedgehog ligands are expressed: Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh). The localization of major pathway components has been most extensively studied in the mouse, and those data are highlighted here (Figure 2). Shh and Ihh are highly expressed throughout the proliferative pseudostratified epithelium of the early gut tube [embryonic day (E)9.5–14] prior to villus morphogenesis (1, 2, 6). Dhh is not epithelially expressed but may be expressed in some neuronal elements. As villi begin to emerge and the thick pseudostratified epithelium is converted to a thinner columnar layer, Shh expression is quickly suppressed in cells overlying emerging villi but retained in proliferative cells of the intervillus spaces; Ihh expression is maintained in all epithelial cells during villus development, though at reduced levels (6). Germline deletion of either Shh or Ihh results in left/right (L/R) patterning defects, enteric nerve malformations, and decreased smooth muscle thickness, as discussed further below (7). However, the much more severe phenotype seen after deletion of both Shh and Ihh, with poorly expanded mesenchyme, reduced villus formation, and little to no smooth muscle differentiation, testifies to the functional redundancy of these ligands (8).
Figure 1.
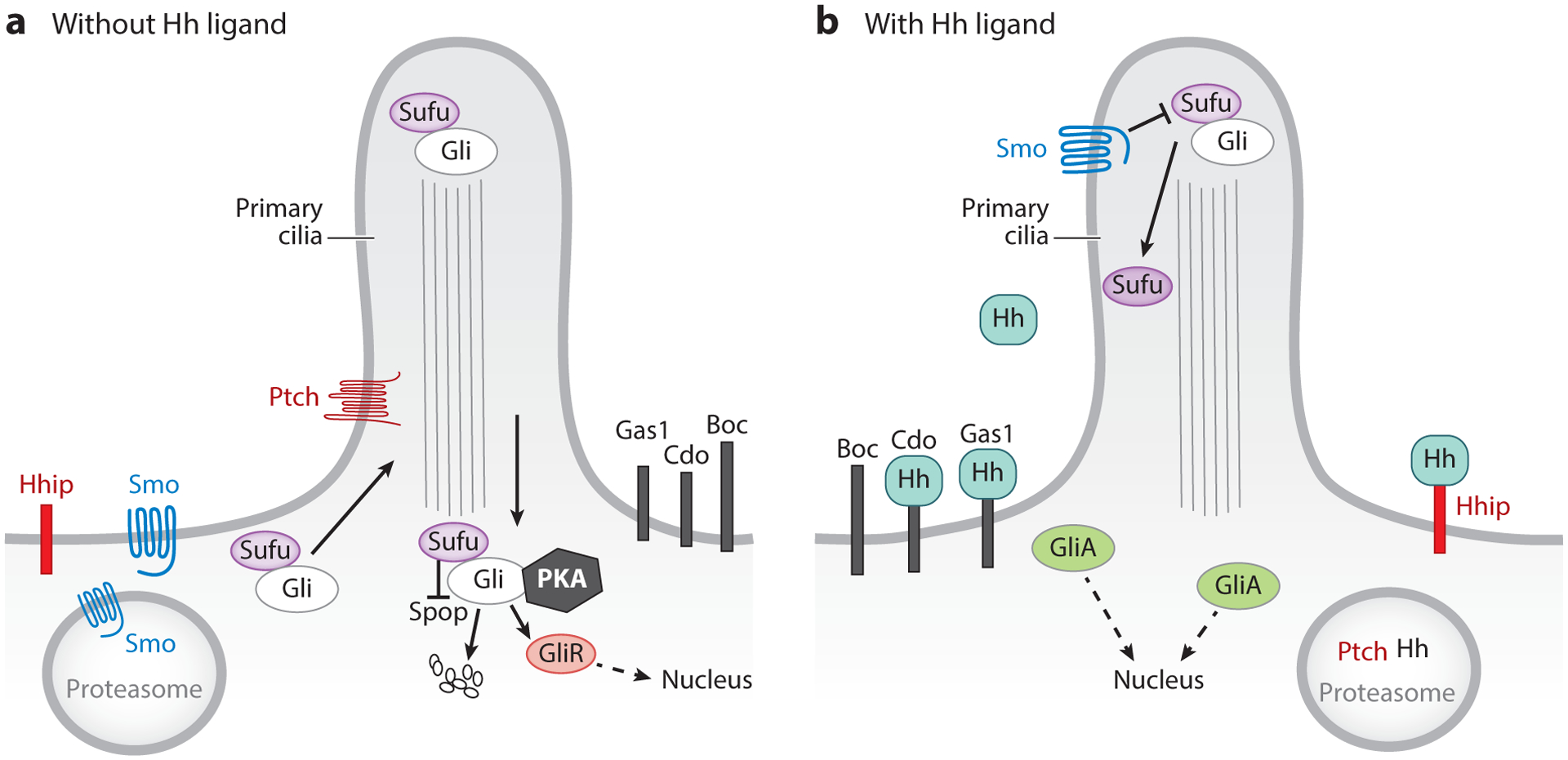
Simplified diagram of paracrine Hedgehog (Hh) signaling in the intestine. Hh ligands are secreted by epithelial cells, and signals are received in the mesenchyme by several different cell types. (a) In the absence of Hh, Ptch prevents the entry of Smoothened (Smo) into the primary cilium. Under these conditions, Sufu sequesters Gli proteins in the cytosol, where they are phosphorylated by a multikinase complex, including protein kinase A (PKA), resulting in their proteolysis to Gli repressor (GliR) forms or full degradation by complex-associated Spop ubiquitin ligase and the proteasome. (b) Binding of Hh ligands to Ptch allows entry of Smo into the cilium, where it is activated and inhibits Sufu, resulting in the stabilization of Gli activator (GliA) forms (12). Hhip is a transmembrane protein that acts as a negative regulator of Hh signaling by competing with Ptch to bind Hh. Other transmembrane proteins, such as Cdo, Boc, and Gas1, act as Ptch coreceptors to modulate Hh signaling. Endocytosis of Ptch and Hh targets them for lysosomal degradation.
Figure 2.
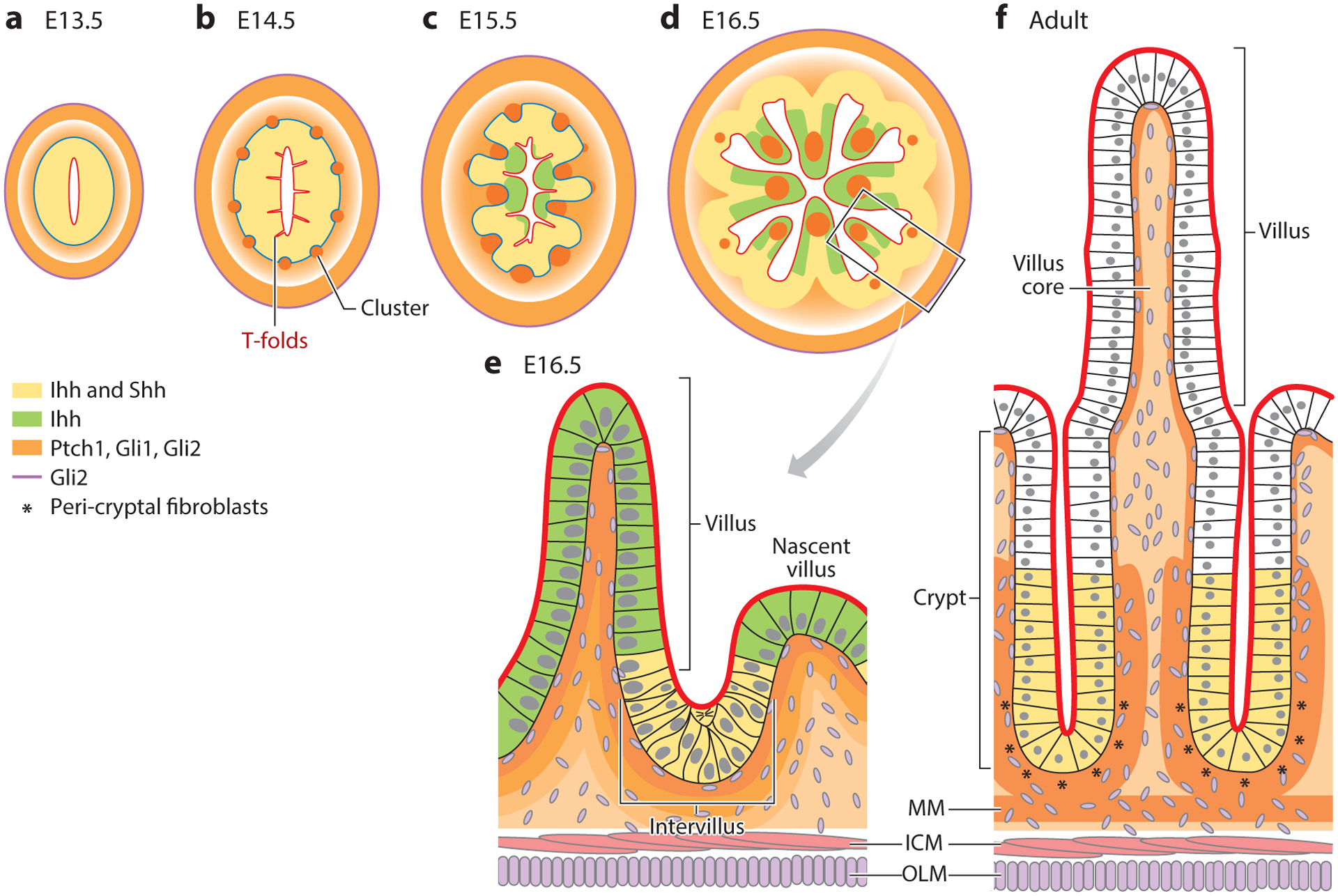
Dynamic expression of Hh pathway components during intestinal development and adult homeostasis. Expression patterns in the mouse are summarized from References 1, 2, 6, 10, and 108 and color coded as indicated. Prior to villus formation, Shh and Ihh are expressed by all epithelial cells (a,b). As villi emerge (c–e), cells above the mesenchymal clusters cease to express Shh, while Ihh expression remains in all epithelial cells, though at lower levels. In adult intestine, Shh and Ihh expression is restricted to crypts (f). Hh signals are received by multiple cell types in the mesenchyme. At E13.5, the Hh receptor, Ptch1, as well as Gli1 and Gli2 transcription factors, are expressed in a gradient with highest expression in mesenchymal cells closest to the epithelial source of Hh ligand and in the ICM. As development proceeds, all three proteins are highly expressed in mesenchymal clusters, villus cores, subepithelial fibroblasts, peri-cryptal fibroblasts (* in panel f), and the MM. Gli2, but not Ptch1 and Gli1, is expressed in the OLM and in many cells of the mesenchyme (all stages). Serosal cells (not shown) also express Gli2, and some express Gli1. Abbreviations: E, embryonic day; Hh, Hedgehog; ICM, inner circular muscle; Ihh, Indian Hedgehog; MM, muscularis mucosa; OLM, outer longitudinal muscle; Shh, Sonic Hedgehog.
The fact that Hh signaling in the intestine is paracrine is revealed by the exclusively mesenchymal nature of cells that express the primary receptor for Hh ligands, Ptch1 (6). Ptch1 is a direct target of Hh signaling, and its expression therefore provides a convenient surrogate for pathway activity. In the E14.5 intestine, a gradient of Ptch1 expression is clearly visible, with robust expression directly adjacent to the epithelium and progressively decreasing activity in mesenchymal cells located farther away (Figure 2) (6). Interestingly, smooth muscle cells of the inner circular smooth muscle [but not those of the outer longitudinal muscle (OLM)] are also Ptch1 positive, indicative of active signaling in this compartment. Another direct Hh pathway target, the pan-Hh inhibitor Hhip, is also expressed mesenchymally (9). Hhip is membrane bound but can also be secreted. Overexpression of a soluble form of Hhip in the E14.5 intestine perturbs villus formation and smooth muscle development (9), indicating that precise control of the levels of Hh signaling is required during intestinal organogenesis.
Just prior to the emergence of villi, Ptch1-positive mesenchymal cells begin to form tight clusters directly under the pseudostratified epithelium (Figure 2b). Direct modulation of Hh signaling levels using explanted intestines has revealed that cluster formation is Hh dependent (10), though the actual molecular targets that mediate clustering per se have not yet been defined.
Within hours of mesenchymal cluster formation, additional (unknown) signals from the clustered Hh responsive cells cause overlying epithelial cells to become shorter and wider, resulting in the eruption of villi. Epithelial cells on the tips of the emerging villi begin to withdraw from the cell cycle, while those in intervillus regions continue to actively proliferate. Continued expression of Ptch1 is seen in mesenchymal clusters at the tips of emerging villi (Figure 2c), in nascent clusters that form de novo to direct the next round of villus emergence (Figure 2d), as well as in mesenchymal cells that line intervillus regions (6) (Figure 2e). Later, as crypts emerge (7–10 days after birth in the mouse), the peri-cryptal fibroblasts, which tightly wrap emerging and emerged crypts, are Ptch1 positive, suggesting a continued role for active Hh signaling in homeostasis of these important stem cell niches (discussed further below). A related Hh receptor, Ptch2, is also expressed in these cells, but at much weaker levels (6).
In mesenchymal cells that transduce Hh signals, a cellular appendage called the primary cilium plays an important role in pathway regulation (Figure 1) (11). In the absence of Hh signals, Ptch is located in the ciliary membrane, where it prevents the entry of Smoothened (Smo), a GPR-family protein and key activator of Hh signal transduction. Binding of Hh ligands to Ptch relieves this suppression and allows entry of Smo into the cilium, where it is activated, resulting in the stabilization and nuclear translocation of downstream transcription factors of the Gli family (12). Thus, Smo is an essential pathway component for all canonical Hh signaling (13), though noncanonical signaling downstream of Smo has been reported (reviewed in 4, 14).
Three Gli transcription factors are expressed in the intestine. Gli1, which like Ptch1 is a direct target and readout of Hh signaling, acts primarily as a transcriptional activator. Importantly, loss of Gli1 produces no obvious phenotype in the mouse; in fact, initial activation of Hh signaling does not require Gli1, which is absent until a Hh signal has been transduced (15). Gli2 can act as activator or repressor (GliA or GliR), and this choice is dependent upon its proteolytic cleavage (Figure 1). Gli3 is primarily a repressor of Hh pathway activity. Total Gli activity within a cell is therefore dependent on the ratio of GliAs to GliRs.
In the early pseudostratified gut tube, Gli1 is expressed in mesenchymal cells surrounding the gut tube, at high levels in the inner circular (but not outer longitudinal) muscle and in scattered Gli1-positive cells of the serosa (6). Gli2 is more widely and more robustly expressed throughout the mesenchymal compartment, in both circular and longitudinal muscle layers and in most serosal cells. Gli3 expression is weak and limited primarily to the muscle layers. Upon villus morphogenesis, cells of the mesenchymal clusters are Gli1 and Gli2 positive and Gli1/Gli2-expressing cells with fibroblast morphology inhabit the cores of emerging villi (Figure 2). After crypt emergence, Ptch1-positive peri-cryptal fibroblasts also express Gli1 and Gli2 (6). It is important to note, however, that (a) at all time points, Gli2 is more widely expressed than Gli1, and (b) it is clear that multiple distinct mesenchymal cell types express these proteins, but the exact identities of each of these cell populations have not been clarified. Future single-cell RNAseq studies will begin to resolve this important source of diversity in Hh signaling.
In addition to the essential core pathway components described above, multiple other molecular players can modify Hh signaling outcomes, and many of these are not well studied with respect to expression levels, location, or even function in the context of the intestine. First, at the membrane level, multiple coreceptors, including Cdo, Boc, and Gas1, have been identified (16–20). Indeed, Gas1-null mice have shortened intestines with a poorly patterned enteric nervous system and thin muscle layers (21), similar to the phenotype of Shh-null mice (7), pinpointing Gas1 as an important coreceptor in the intestine. Second, the levels of Hh signal transduction are highly regulated via phosphorylation, proteolysis, alternative splicing, and molecular stabilization/degradation (22). A particularly important arm of this regulation is determined by molecular regulators of protein stability that control the ratio of available Gli activators/repressors. In particular, recent work has revealed the importance of Sufu and Spop as negative regulators of Gli signaling in the developing and adult intestine (23). Sufu binds Gli proteins and sequesters them in the cytosol; mutations in this protein are associated with an increased GliA/GliR ratio and activation of Hh signal transduction (23). Spop is a ubiquitin ligase that drives degradation of full-length Gli2 and Gli3 activators (24). Coquenlorge et al. (23) showed that while the deletion of Spop in the intestinal mesenchyme has no apparent phenotype in the developing intestine, deletion of Sufu results in deformed stomach, malrotation of the gut, and shortened intestines. Interestingly, loss of both Sufu and Spop results in a more dramatic phenotype, with greatly expanded mesenchyme in stomach and intestine as well as decreased organization and function of the smooth muscle layers. At the molecular level, both Gli and Wnt signaling targets are greatly increased in the double knockouts. Importantly, this phenotype can be partially reversed by deletion of one allele of Gli2, confirming both the critical role of Gli2 signaling and the need for precise control of Gli2 levels during intestinal organogenesis.
As we next introduce a robust literature on the roles of Hh signaling during intestinal development and adult homeostasis, it is important to keep in mind that many different mesenchymal/stromal cell types can transduce Hh signals. Particularly important is the fibroblast population, a highly heterogeneous group of cells. There are stromal fibroblasts, villus core fibroblasts, and subepithelial fibroblasts, and even within these spatial groups, the nomenclature is often confusing. For example, the fibroblasts that directly underlie the epithelium during villus formation and later in adult life have been referred to as subepithelial fibroblasts, subepithelial myofibroblasts, peri-cryptal fibroblasts/myofibroblasts, or just plain fibroblasts or myofibroblasts. Moreover, it is possible that cells may change back and forth from fibroblast to myofibroblast (expressing smooth muscle actin) depending upon developmental or disease state (25, 26). Recent single-cell sequencing, combined with careful immunohistochemical marker analysis, is beginning to define several distinct fibroblast populations, such as telocytes and trophocytes (25–32). For clarity in this review, we refer to (a) subepithelial fibroblasts, cells directly opposed to the epithelium in fetal stages (including those next to the epithelium inside the developing or mature villi) and (b) peri-cryptal fibroblasts, the subset of the subepithelial fibroblasts that line the crypt base and provide the stem cell niche. Within each of these populations, both myofibroblasts and fibroblasts are present; we refer primarily to fibroblasts, except where publications specifically focus on myofibroblasts.
FUNCTIONAL ROLES OF THE HEDGEHOG PATHWAY DURING INTESTINAL DEVELOPMENT
During intestinal morphogenesis, a remarkable number of different patterning events rely on proper Hh pathway activity (Figure 3), including L/R patterning, radial patterning, smooth muscle differentiation, mesenchymal expansion, and establishment of an orderly field of straight, un-branched villi. These major functions of Hh signaling are summarized below.
Figure 3.
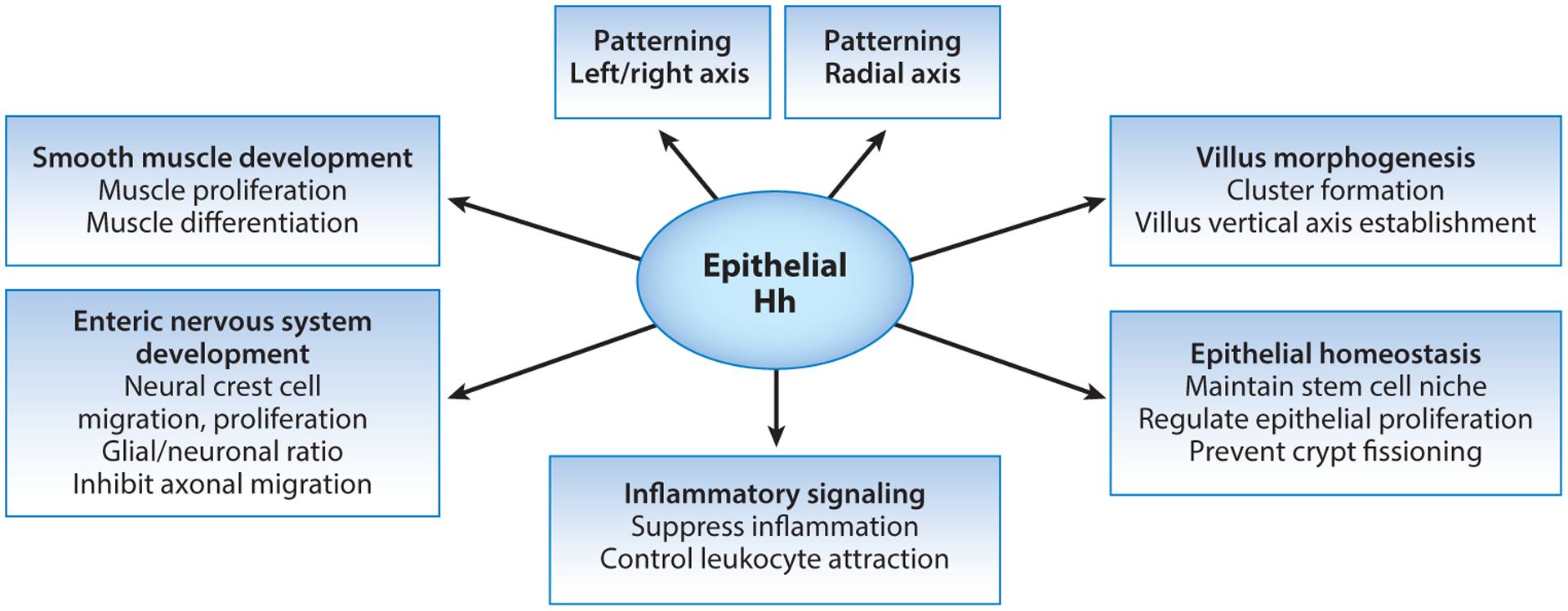
Summary of the diverse regulatory and patterning roles of Hedgehog (Hh) signaling during organogenesis of the intestine and in the regulation of adult intestinal homeostasis.
Hedgehog Participation in Left/Right Axis Patterning
Hh functions as a midline signal in many organisms. In the mouse, at the 2–3 somite stage, Shh is expressed in the node and notochord (midline structures), while Ihh is activated in the node as well as in the anterior definitive endoderm that underlies the lateral plate mesoderm (13, 33–35). This combined Hh signal participates in a major symmetry-breaking event that results in L/R patterning, thereby determining the position, shape, and/or development of multiple organs, including stomach and intestines (35). Indeed, complete loss of L/R axis specification is seen in mice null for Smo as well as in Ihh−/−;Shh−/− compound mutants (8, 13).
Murine L/R patterning is thought to result from a directional flow of signaling molecules set up by coordinated movement of cilia on the cells of the node (36). One model suggested that cells of the node release small particles carrying Hh proteins that are transported leftward by ciliary-induced flow, where they initiate the L/R patterning cascade (37). However, at the time of symmetry breaking, both Ptch1 and Gli1 (targets of Hh signaling and indicators of Hh-responsive cells) are expressed symmetrically in the lateral plate mesoderm (LPM) surrounding the node (35), arguing against the idea that polarized exposure to Hh ligands is the critical determinant of this patterning process. In the LPM, signaling downstream of Smo directly induces Foxf1 expression; Foxf1 then activates its target, Bmp4, which, in turn, facilitates the activation of Nodal signaling to establish L/R patterning through the activation of Pitx2 and Lefty2 exclusively in the left LPM (35). This process is further reinforced by a separate Hh action in cells of the prospective floorplate, where Hh-dependent activation of the Nodal antagonist, Lefty-1, provides a midline barrier to stop the spread of Nodal to the right (38, 39). Interestingly, a role for Hh signaling in L/R axis determination appears to be quite ancient, since studies in the sea urchin indicate that Hh pathway perturbation results in randomization of L/R asymmetry in these primitive deuterostomes (40).
Several L/R patterning defects in endodermal organs are observed within the spectrum of human diseases thought to relate to faulty Hh signaling. One example is annular pancreas, a condition in which the pancreas encircles the duodenum. Annular pancreas seems to be due to selective failure of the proper morphogenesis of the ventral pancreas bud (41). This condition can be genetically transmitted in humans, and an association between annular pancreas and Down’s syndrome has been noted (42, 43). Annular pancreas is seen in 42% of Ihh-null mice (7). Hebrok et al. (41) note that only 1 of 17 Shh-null mice exhibit this condition, but Ramalho-Santos et al. (7) found that 6 of 7 of their Shh-null mice had this phenotype. These differences in phenotype penetrance may be due to strain differences; the identification of the strain-specific modifiers responsible for such a powerful background effect is an important goal for the future.
Gut malrotation, another condition associated with L/R mispatterning, is observed in 100% of animals that are null for either Ihh or Shh (7). Notably, liver and lung (other endodermal organs) do not display defects in L/R patterning with the loss of a single Hh ligand (7). Thus, some aspects of the Hh-related L/R patterning cascade may be redundantly controlled by both ligands, while some are exacerbated after loss of only one ligand. Indeed, mice doubly mutant for Ihh and Shh or those lacking Smo lack L/R pattern in all visceral organs (7, 13).
Hedgehog in Enteric Nervous System Development
The enteric nervous system is derived from neural crest cells (NCCs) that delaminate from the dorsal neural tube and migrate to and surround the gut tube by following a series of attractive and repressive cues (reviewed in 44, 45). As the NCCs colonize the intestine, some become positioned to form the myenteric plexus between the inner circular muscle (ICM) and OLM, while others migrate into the subepithelial mesenchyme to form the submucosal plexus and innervate the villi. Migrating NCCs as well as proliferating NCCs in the intestine express Ptch1 and Gli1 and are responsive to Hh signaling (46, 47), but mature neurons are not (6, 48).
Functionally, perturbations in Hh signaling are associated with multiple defects in enteric nervous system development. Mice lacking Shh exhibit ectopic ganglia in the cores of the villi, while those lacking Ihh show reduced NCC proliferation and impaired migration, resulting in areas of aganglionosis, similar to human Hirschsprung’s disease (7). Interestingly, aberrant activation of the Hh pathway by overexpression of Gli1 also causes Hirschsprung’s-like phenotypes in mice, the severity of which correlates with Gli1 expression level (49). Indeed, three GLI1-activating mutations have now been identified in human Hirschsprung’s patients (50).
The Ptch1+/Gli1+ NCCs that colonize the embryonic intestine are bipotential precursors that will give rise to both neurons and glia. Transcriptomic dissection of the trajectory of differentiation of NCCs from mouse or human pluripotent stem cells revealed that Hh ligands act on the precursor cells to encourage their differentiation into neurons and glia (48). Addition of a Hh agonist (SAG) during the differentiation protocol promotes maturation, whereas treatment with the Hh inhibitor cyclopamine suppresses it. Interestingly, the ratio of neurons to glia is also sensitive to Hh levels (51, 52). Overexpression of the Gli3 repressor in NCCs leads to formation of significantly fewer glia (51). Conversely, loss of Ptch1, which results in increased Hh signaling, leads to precocious gliogenesis at the expense of neurogenesis (47, 53). Likewise, loss of Sufu in NCCs increases the ratio of GliA to GliR (increasing Hh signaling) and results in increased and precocious differentiation of neurons and glia, with skewing toward the glial fate (51). Ngan et al. (52) proposed that alterations in the neuronal:glial ratio may represent one of the molecular mechanisms underlying Hirschsprung’s disease.
Hh signaling also plays a role in enteric nerve patterning. As mentioned above, Shh mutant mice exhibit ectopic axons that project abnormally into the villus cores (7, 8, 54). Loss of Smo or the Hh coreceptor Gas1 in NCCs (decreased Hh signaling) also results in ectopic ganglia (21, 54). Jin et al. (54) explored these observations further and showed that, in vitro, enteric projections from neurospheres derived from E11.5 wild-type (WT) intestines change direction in response to localized Shh-N (a 19-kDa proteolytically processed aminoterminal portion of Shh, a potent Hh signal), while those from Gas1 mutants are not repelled. Since the myenteric plexus develops just outside of the zone of active Hh signaling (outside of the ICM), the authors proposed that axon migration in the central direction is repelled by Hh in a Gas1-dependent fashion. Interestingly, this repellant activity depends on Hh and Smo but does not appear to require transcriptional activation of Hh targets. Rather, this repulsive response is mediated by a Smo-coupled G protein (Gnaz); Smo, Gnaz, and Gas1 all localize to the termini of the neuronal axons, where repellant patterning would be expected to play out for proper myenteric plexus development.
Hedgehog as a Driver of Mesenchymal Proliferation
Hh is a well-known mitogenic signal in several settings, and abundant evidence indicates that this is also the case in gastrointestinal (GI) organs (55). In the mouse, Shh and Ihh are both expressed in the developing endoderm by E8.5 (1, 2). In compound Ihh/Shh mutants generated using Shh-Cre, the histological appearance of epithelial and mesenchymal layers of the stomach and intestine is relatively normal at E11.5. But from this point on, expansion of mesenchymal Hh-responsive cells is impaired; the gut fails to lengthen, and the stomach fails to enlarge (8). Similarly, when Smo is specifically deleted from the gut mesenchyme, both epithelium and mesenchyme show proliferation defects (56). In accord with these findings, cultured mesenchyme from stomach or intestine of E12.5 embryos exhibits a clear mitogenic response to added Hh ligand (8). Moreover, activation of the Hh pathway in gut tissues using a constitutively active form of Smo promotes robust expansion of the mesenchymal compartment and results in excessive growth of the stomach and intestine (8). Thus, Hh signaling during early intestine and stomach development is critical for the proliferation of mesenchymal progenitors that, in turn, act on nearby endodermal cells to establish correct organ size. Parenthetically, the use of Hh signals as a driver of tissue growth in the gut is ancient. In Drosophila, the anterior region of the larval hindgut is analogous to the vertebrate small intestine. Hh is expressed in epithelial cells that give rise to the small intestine, and Hh mutants exhibit a greatly shortened small intestine (57).
At least part of the mitogenic action of Hh on mesenchymal cells in several endodermally derived organs is driven by Fox family members. Foxf1, Foxf2, and Foxl1 are redundantly expressed in intestinal mesenchyme (58, 59). Functionally, conditional loss of Foxl1 or Foxf1 in intestinal tissue leads to reduced mesenchymal proliferation, and haploinsufficiency for Foxf1 results in many of the same phenotypes that are seen with loss of Shh, including proliferative defects and foregut malformations (58–60). Finally, chromatin immunoprecipitation (ChIP) assays demonstrate that Gli2 binds to consensus sites upstream from the Foxf1 and Foxl1 genes, implicating them as direct targets of Hh signaling (61). Important next questions are: Which mesenchymal cells are primarily responsible for mesenchymal expansion, and which genes downstream of the Fox transcription factors are required for this process?
Hh ligands are also important for proliferation of mesenchymal cells perinatally during the time of intestinal crypt formation. Crypts are flask-like invaginations of the epithelium that harbor the stem and progenitor cells of the intestine. The nascent intestinal crypts are surrounded by peri-cryptal fibroblasts that transduce Hh signals, as demonstrated by their positive staining in Gli1lacZ/+ and Ptch1lacZ/+ reporter mice (6). In Ihhf/f;Villin-Cre mice, which lack Ihh in intestinal epithelium, these peri-cryptal fibroblasts are dramatically reduced in number (62). Furthermore, in culture, proliferation of these cells is inhibited by GANT61, a Gli antagonist (62). These Hh-responsive peri-cryptal fibroblasts remain tightly associated with intestinal crypts during adult life and have been shown to be an important source of signaling molecules that directly regulate epithelial stem cell proliferation and differentiation (31, 63), as discussed further below.
Hedgehog Function in Radial Patterning and Smooth Muscle Development
From esophagus to colon, the developing tubular gut is characterized by a similar radial pattern: The innermost endodermal layer is encircled by loose mesenchymal cells; outside of that, 2–3 layers of oppositely oriented smooth muscle surround the gut tube. A network of enteric neurons occupies the space between these muscular layers, and together, these nerves and muscles control gut peristalsis. The notion that a gradient of Hh signals emitted from the endoderm is required for locating the muscle layer (muscularis externa) on the outermost radial position of the tubular gut was first proposed by Sukegawa et al. (64) in an analysis of chick gut development. These authors grafted Hh-expressing epithelium to the outside of the embryonic chick gizzard and observed that smooth muscle always developed in regions that were spatially separated from the epithelium, suggesting that signals from the epithelium actively inhibit smooth muscle formation in nearby cells (which are exposed to very high levels of Hh) but promote smooth muscle development in cells further away (where Hh levels are low). Indeed, the presence of the epithelium was required for muscle development, since no muscle formed in its absence. Their further analysis suggested that Hh, secreted by the epithelium, promotes the expression of high levels of the Hh target Bmp4 in the region next to the epithelium. Since Bmp4 is inhibitory to smooth muscle development, these authors proposed that muscularis externa forms only on the outside of the intestinal tube, where Hh and Bmp4 levels are low (64). Huycke et al. (65) add that Bmp2/7 expression at the outer edge of the intestine inhibits OLM development, but later when Bmp antagonists Noggin and Gremlin are expressed in ICM and nearby nerves, the OLM is permitted to form.
Several more recent studies reinforce the idea that Hh is required for smooth muscle development but suggest additional complexity in the underlying mechanism. First, in transgenic mice overexpressing Ihh in the intestinal epithelium, levels of Hh are five times higher than in those in WT littermates, but these transgenic mice do not exhibit reduced muscle as predicted if high Hh is inhibitory to smooth muscle development (66). Rather, smooth muscle is greatly increased, and robust muscle formation occurs immediately adjacent to the Hh-secreting epithelium. Similarly, Cre-mediated activation of a constitutively active form of Smo (SmoM2) in intestinal mesenchyme at E9.5 leads to robust expansion of the mesenchyme in general and smooth muscle cells in particular (8, 56). Thus, again, increasing Hh pathway activity promotes rather than inhibits smooth muscle formation. Mao et al. (8) argue that Hh actually has two actions in this regard: It expands a population of smooth muscle progenitors and promotes differentiation of the expanded precursors to a smooth muscle fate. Indeed, in addition to its role in muscularis externa development, Hh is also required for the later formation of the populations of smooth muscle that lie near the epithelium, including villus smooth muscle and muscularis mucosa; these structures are compromised in animals with reduced Hh signaling (7, 9, 66–69). Thus, there is substantial evidence for an important role for Hh in visceral smooth muscle development. Indeed, studies in the sea urchin demonstrate that reduced Hh expression inhibits gut smooth muscle development (40), indicating that this function of the Hh pathway has been highly conserved over millions of years of evolution.
It is noteworthy that the ICM, but not the OLM, expresses both Gli1 and Ptch1, indicators of active Hh signaling (6). Indeed, the intensity of signal for both of these targets is greater in the ICM than in the nearby loose mesenchyme closer to the epithelium. Thus, while a decreasing gradient of Hh signaling seems to travel across the loose mesenchyme toward the external muscle (as visualized in PtchLacZ/+ or Gli1LacZ/+ reporter animals), this declining gradient is interrupted at the ICM where a sudden spike in apparent Hh signal reception occurs (6). The basis for this is not yet fully understood, but it is interesting to note that the muscularis externa (but not the nearby loose mesenchyme) also expresses high levels of the Hh coreceptor Gas1 (Figure 3b; E13.5). Gas1 can potentiate Hh activity in some contexts by acting together with Ptch1 in a cell-autonomous manner, particularly in regions where the concentration of Hh is low (19). In Gas1-null animals, the OLM layer is disorganized, but its thickness is not significantly different from controls. The ICM, however, is greatly reduced in thickness (21). These molecular and phenotypic differences between the ICM and OLM with regard to Hh signaling are interesting in light of the fact that these two muscle layers demonstrate different pathological reactions in some forms of visceral myopathy (70, 71).
Both smooth muscle precursors and differentiated smooth muscle cells can transduce Hh signals, as both cell types are marked by the Hh signaling reporters Gli1lacZ/+ and Ptch1lacZ/+ (6). Additionally, transfection of Gli factors can promote smooth muscle differentiation in a cell-autonomous manner in the mesenchymal stem cell line NIH10T1/2 (66, 72). Zacharias et al. (66) speculated that a potential Hh target gene identified by RNA expression profiling microarray analysis, Myocardin (Myocd), may drive smooth muscle development downstream of Hh. Consistent with this finding, Kosinski et al. (67) also saw reduced Myocd expression in mice with a conditional deletion of Ihh in the intestinal epithelium. Indeed, Myocd, a master regulator of smooth muscle (73), is upregulated in response to Hh, with kinetics similar to that seen with known Hh target genes (66). Furthermore, MYOCD’s binding partner, serum response factor (Srf), contains transcription factor binding sites for the Hh target, cJun (74, 75). Together, these data suggest that Hh signaling drives an intestinal smooth muscle cell differentiation network through cJun, Srf, and Myocd.
Interestingly, Huang et al. (56) recently identified additional Hh-responsive genes that may influence smooth muscle differentiation: osteoglycin (Ogn) and lumican (Lum) (Figure 3b; E13.5). Both are small leucine-rich glycoproteins (SLRPs). Overexpression of Ogn inhibits Shh-induced Myocd expression and smooth muscle differentiation. In Smo mutant intestines, both Ogn and Lum are three- to fourfold downregulated; the authors concluded that SLRPs may negatively regulate Shh-induced smooth muscle differentiation. In this regard, the expression pattern of both Ogn and Lum, as displayed on GenePaint.org (https://www.genepaint.org), is interesting: Both are highly expressed in the loose mesenchyme underlying the epithelium and excluded from the muscle layers. Thus, SLRPs may participate in Hh-mediated radial patterning of the early gut by inhibiting smooth muscle differentiation in the peri-epithelial mesenchyme.
Together, these findings indicate a complex and multifaceted role for Hh signaling that is played out in time and space during intestinal smooth muscle development. Temporally, early Hh signaling is required to expand the pool of progenitor cells giving rise to smooth muscle cells; later Hh signaling promotes differentiation of smooth muscle cells by driving cJun (and Srf) and Myocd in the smooth muscle cell differentiation network. Spatially, patterning of smooth muscle relies on additional Hh-responsive factors, which are themselves expressed in specific spatial domains: Bmp and SLRPs can inhibit Hh near the epithelium, while coreceptors such as Gas1 can promote muscle formation in the outer mesenchyme.
Hedgehog Signaling in Intestinal Villus Development and Patterning
In transgenic mice expressing high levels of the pan-Hh inhibitor Hhip, wide areas of the epithelium exhibit complete lack of villus structures (9). This phenotype is also seen in Ihh−/−;Shh−/− mice (8) and mice lacking Smo in the mesenchymal compartment (56). Further investigation of this phenotype reveals that, at E14.5, Hh signals from the epithelium act on nearby mesenchymal cells to cause their aggregation into “villus clusters” that are associated with the emergence of the villi (Figure 2) (10). As villi emerge, the epithelial cells directly above each cluster withdraw from the cell cycle, while nearby epithelial cells located between adjacent clusters remain proliferative. Clusters are distributed in a patterned array that spreads from anterior to posterior and dorsal to ventral along the length of the intestine, between E14.5 and E16.5 in the mouse (10). Inhibiting the formation of these clusters, by treatment with the Hh inhibitors cyclopamine, 5E1, or SANT1, prevents villus development (10). Conversely, addition of the Hh agonist SAG to cultured E14 intestines results in the formation of larger clusters and wider villi. It will be important to determine both the fate of cells within the clusters and the signals emitted by these cells, which might direct cell shape change in the overlying epithelium.
The Hh-induced patterning of intestinal clusters is very similar to that seen during feather morphogenesis, a process that is also known to proceed with mesenchymal signaling centers that are reliant on Hh signals (76). Investigation of the underlying drivers of villus patterning suggests that Bmp ligands and Bmp inhibitors, both secreted by clusters, are responsible for the spot-like pattern of intestinal clusters (77). Indeed, cultured intestines, treated with the Bmp inhibitor dorsomorphin, exhibit a stripe-like pattern instead of the normal spotted one. Alan Turing’s models of two-component chemical patterning systems provide a possible framework for understanding this patterning event (78). In those models, an activator and inhibitor are secreted by the same cells; the inhibitor diffuses further from the source than the activator. Additionally, the inhibitor inhibits the activator and the activator stimulates the expression of the inhibitor. Under such conditions, increasing the concentration of the activator changes a spotted pattern into a striped one. If this model indeed applies to intestinal villus cluster patterning, then Bmp in this case should be considered the inhibitor and an unknown Bmp inhibitor acts as the patterning activator (77). The actual molecules involved in this process have yet to be identified.
Interestingly, the process of villus emergence in the chick, while also reliant on Hh signals, differs in several details from the process outlined in the mouse. For a full discussion of these differences, the reader is referred to a recent review (79). Briefly, in the chick, sequential maturation of three smooth muscle layers of the intestine (inner circular, outer longitudinal, inner longitudinal/muscularis mucosa) drives progressive folding of the epithelium into ridges and then zigzags. This largely mechanical process serves to concentrate epithelial Hh proteins at the elbows of the zigzags, accounting for the formation of villus clusters at those positions (80, 81). Once formed, the villus clusters of mouse and chick express similar factors (e.g., Gli1, Ptch1, Bmp4) and appear to drive further villus emergence.
It is interesting that in the chick, stereotypical epithelial folds determine mesenchymal cluster location/patterning, while in the mouse [and most likely, the human (79)], the formation of mesenchymal clusters precedes and determines the pattern of epithelial folds. Indeed, murine smooth muscle formation does not correlate temporally with epithelial folding as it does in the chick (77). Furthermore, in Spop/Sufu-null animals, smooth muscle development and functional muscle contraction are severely perturbed, but villus patterning and emergence are unaffected (23). It is not clear why such different modes of epithelial folding are utilized in birds and mammals, but it is noteworthy that at the time that clusters form in the mouse, the epithelium is composed of tall, thin, pseudostratified cells, forming a thick tube that would likely be resistant to folding via contraction of surrounding muscles. Nevertheless, once clusters form, folding of the epithelium is rapid, with nascent villi emerging from flat epithelium within 10 min (82). Interestingly, mechanical forces do appear to be involved in this rapid epithelial folding, but these forces are mediated by epithelial rather than mesenchymal cells. Freddo et al. (82) demonstrated that the formation of clusters beneath the thick epithelium causes overlying epithelial cells to shorten and widen, placing intraepithelial pressure on cells located between the clusters. Within these circumferentially pressurized regions (which remain pseudostratified), mitotic cell rounding and basal contraction cause rapid inward movement of the mitotic cell, generating folds (called T-folds) that establish the villus boundaries (Figure 2b). A very similar mechanism (circumferential pressure, mitotic cell rounding, and rapid basal contraction) has also been described during invagination/folding of Drosophila tracheal placodes, another pseudostratified epithelium (83).
Very recent data shed more light on this rapid folding event. Rao-Bhatia and colleagues (84) demonstrated that epithelial Hh signals mediate mesenchymal clustering by activating adhesion genes and several planar cell polarity components that insure proper cluster formation (e.g., Wnt5a, Fat4, Dchsl, Vangl1, and Vangl2). All of these components appear to be direct or indirect targets of Hh signaling. In mice mutant for Fat4, Dchsl, Vangl2 (Looptail mutation), or Wnt5a, mesenchymal clustering is perturbed, and emerging villi are misformed and occasionally fused (85). Further investigation of this phenotype in the Fat4-null background suggested that fusions arise from incomplete folding events mediated by T-folds, as the number of such folds is significantly reduced in these mice. In vitro analyses further showed that platelet-derived growth factor receptor alpha (PDGFRα)-expressing cells from E15.5 WT mice cluster spontaneously in culture, and this activity is inhibited by cyclopamine, consistent with the idea that Hh signaling controls this behavior (10). Interestingly, overexpression of Fat4 or Dchsl rescues clustering in cyclopamine-treated PDGFRα+ cells from WT mice but not those from Fat4-null mice. Additionally, WT PDGFRα+ cells migrate directionally in response to a Wnt5a gradient, but Fat4-null PDGFRα+ cells do not. Altogether, these data are consistent with the following model: (a) Prior to cluster formation, the epithelium expresses high levels of Hh ligands, while scattered mesenchymal cells coexpress Gli1/2, Wnt5a, and Pdgfrα. (b) At E14.5, in response to an as-yet-unknown trigger, epithelial Hh signals activate expression of adhesion molecules that drive the formation of small mesenchymal clusters. (c) These clusters provide a concentrated source of Wnt5a and therefore attract additional mesenchymal cells to grow the clusters. (d) Expression of Bmp activators and inhibitors by the clusters drives cluster patterning via a Turing-like field. (e) Clusters drive intraepithelial shape changes that lead to formation of patterned areas of circumferential pressure that, in turn, drive the invagination of T-folds and result in the rapid establishment of villus boundaries.
Hedgehog as a Director of Epithelial/Mesenchymal Cross Talk
In the developing intestine, inhibition of Hh signaling in a variety of mouse models leads not only to mesenchymal phenotypes as discussed above, but also to significant changes in the overlying epithelium (7, 9, 50, 56, 67–69, 86–89). Since Hh signaling is paracrine in these tissues, these effects are clearly indirect, the result of alterations in the function of mesenchymal target cells that then send aberrant signals back to the associated epithelium.
Several candidate signaling molecules could mediate these changes in epithelial state. In a model of reduced intestinal Hh signaling generated by overexpression of a soluble form of Hhip, a Hh inhibitor in the epithelium, WNT signaling was increased, while Bmp2 and Bmp4 were reduced (9, 69). Interestingly, smooth muscle antigen-positive myofibroblasts, normally located at the base of the emerging villi, were ectopically seen beneath epithelium lining the villi proper (9). Patches of ectopic proliferative epithelium that closely resembled precrypt pockets were found directly adjacent to these mislocalized myofibroblasts and were potentially responsible for the branched villus structures seen in this model (9). Thus, through cross talk with underlying fibroblast cells, epithelial Hh ligands appear to exert a polarizing influence on the emerging villi by anchoring these cells (potential sources of Wnt and/or other niche signals) to the region immediately beneath the intervillus base.
Kosinski et al. (67) crossed Villin-Cre transgenic mice onto an Ihhf/f background to delete Ihh specifically in the intestinal epithelium. Again in this model, branched villi and ectopic precrypt pockets were observed. These authors further documented an increase in the number of epithelial cells expressing the stem cell marker, Olfm4, indicating that the loss of Ihh leads to an expanded stem cell pool. This finding contrasts with the earlier study by Ramalho-Santos et al. (7), in which intestinal stem cells were reduced in Ihh-null animals. However, the earlier study did not examine specific stem cell markers, and their phenotypic analysis was restricted to one time point (E18.5). Indeed, the balance of the evidence in several reports now suggests that reduced Hh signaling results in increased epithelial proliferation and crypt fissioning that is likely due to an expanded stem cell pool (9, 67, 68, 89). Together, these data strongly bolster the conclusion that reduced Hh signaling in the intestine leads to a deranged mesenchymal compartment that is, in turn, responsible for an altered proliferation/differentiation state in the epithelium and perturbation of the vertical crypt/villus axis. Indeed, Degirmenci et al. (31) showed in adult mice that Gli-positive cells are critical suppliers of Wnts that control epithelial stem cell homeostasis in the colon; in the intestine, these Gli+ cells provide an essential reserve pool of Wnts when Wnt expression is compromised in Paneth cells.
Additional players in epithelial/mesenchymal cross talk downstream of epithelial Hh signals likely include Fox and Nkx family genes expressed in mesenchymal cells. Developmental defects seen after Foxl1 (Fkh6) gene deletion include delayed emergence of villi and formation of fewer branched and stunted villi (58). However, after birth and crypt formation, the epithelium of Foxl1 mice exhibits increased proliferation with enhanced crypt fissioning and longer villi. Downstream of Foxl1 loss, Bmp2 and Bmp4 are decreased, and Wnt signaling is increased, as discussed above (58, 90).
Loss of Nkx2–3, another mesenchymally expressed transcription factor, also produces an epithelial phenotype that is similar to that seen in mice with reduced Hh signaling (9, 67, 69): branched villi, with ectopic proliferative epithelial areas and a defect in epithelial differentiation (91, 92). Like Fox family members, several Nkx family members are known to be direct targets of Hh signaling, and binding of Gli1 to the genomic loci of these genes has been confirmed in ChIP assays (93). Thus, the changes seen when Hh ligands are lost or downregulated may be mediated, at least in part, by reduced expression of Nkx and/or Fox transcription factors in mesenchymal cells. These transcriptional targets of Hh themselves likely regulate a number of secreted signals that feed back to control epithelial proliferation/differentiation.
FUNCTIONS OF HEDGEHOG DURING ADULT INTESTINAL HOMEOSTASIS
The studies discussed above indicate that Hh signaling is critical for multiple steps of endodermal tissue organogenesis. In adult GI epithelia, expression levels of Ihh and Shh are greatly reduced. Nevertheless, the Hh pathway continues to play critical roles in homeostasis, regeneration, and cancer.
Hedgehog as a Regulator of Inflammation
Lees et al. (94) were the first to suggest that HH might have a tolerogenic influence on the adult intestine. These authors showed that in humans with inflammatory bowel disease and ulcerative colitis, the Hh pathway is downregulated. They further identified a specific genetic variant of GLI1 that is associated with these inflammatory diseases and showed that this variant is a poor transcriptional activator of the HH signaling pathway. Finally, they demonstrated that mice heterozygous for Gli1 are significantly more susceptible to inflammatory challenge with dextran sodium sulfate (DSS) (though in the absence of challenge, no increased inflammatory phenotype is seen). Together, these data indicate that the Hh pathway may be an important mediator of inflammatory response in the adult intestine.
Further supporting this hypothesis, van Dop et al. (68) conditionally deleted Ihh in adult mice and found a phenotype that had striking similarities to a wound repair response. Crypts deepened progressively as proliferation increased and fissioning of crypts became common. Crypt areas exhibited increased Wnt signaling and decreased Activin signaling, whereas Bmp signaling was decreased in villus epithelium. Also, similar to previous studies (7, 9, 66, 67), differentiated smooth muscle cells were progressively lost. In older animals, villi were stunted or lost, potentially due to reduced structural integrity in the face of declining smooth muscle (68). An inflammatory response accompanied these crypt changes, characterized by increased Tgf-β signaling, progressive fibrosis, robust myeloid cell infiltration, and increased deposition of extracellular matrix proteins such as collagen and fibronectin. The authors concluded that epithelial Hh can function as a sensor of epithelial integrity (68).
In more recent follow-up work, Westendorp et al. (95) deleted Smo from macrophages and dendritic cells and demonstrated that the lack of Hh signaling in this myeloid cell population fails to trigger the Hh-dependent inflammatory phenotype. Rather, a Hh-responsive heterogeneous population of fibroblasts (fibroblasts and myofibroblasts) sensed the reduced Hh signal and released Cxcl12, a potent immune cell chemoattractant. Parallel studies by Lee et al. (96) showed that increasing Hh signaling, either pharmacologically, by administration of the Smoothened agonist (SAG), or genetically, by deleting one allele of Ptch1, protects from DSS-induced colitis in mice. Because these investigators were unable to find stromal myeloid cells expressing Gli1, they examined gene expression in FACS-sorted Gli1-positive cells of vehicle- or SAG-treated mice and found that SAG treatment resulted in significant upregulation of interleukin (IL)-10, an anti-inflammatory cytokine that in turn recruits CD4+Foxp3+ regulatory T cells to suppress inflammation. The precise Hh-responsive stromal cell population responsible for upregulating IL-10 levels in response to Hh was not identified, and it is not clear whether these cells are the same population that, in the face of reduced Hh levels, secretes higher levels of Cxcl12. Also, while both of these studies appear to rule out a role for myeloid cells in the Hh response, in vitro analyses indicate that isolated intestinal lamina propria myeloid cells show decreased IL6 expression in response to Hh ligand (97). Together, these results bolster the notion that Hh functions as an important tolerogenic signal in the intestine and implicate several potential stromal cell populations as direct and indirect mediators of this effect.
Hedgehog as an Indirect Regulator of Epithelial Stem Cell Behavior and Tumor Control
Developmental roles for Hh in the regulation of proliferation in both mesenchymal cells (directly) and epithelial cells (indirectly) are documented above. Thus, it is perhaps not surprising that the Hh pathway has also been linked to the control of intestinal tumorigenesis. In this regard, there is ample evidence for cross talk between the Wnt and Hh pathways. Indeed, it is well known that activation of the Wnt pathway is a common cause of intestinal tumorigenesis. In the context of excess Wnt activity, loss of one allele of Smo (98) or conditional deletion of Ihh from the epithelium (99), both of which result in reduced Hh signaling, reduces the number and size of tumors seen. Peri-cryptal fibroblasts, which secrete abundant Wnt ligands and provide important niche signals to the overlying epithelium (28, 31, 100), have been shown to be Hh responsive (31, 61, 101) and represent likely gatekeepers of this Wnt/Hh signaling balance. In support of this idea, a recent analysis of the targets of Gli2 signaling in intestinal mesenchymal cells revealed a number of Wnt regulators (23). Importantly, in an Apcmin mouse model of intestinal tumorigenesis, heterozygosity for Gli2 greatly suppresses the overall Wnt response and reduces the number and size of tumors, confirming the regulatory connection between Wnt and Hh in homeostasis. Molecular regulators of the Hh pathway, such as Sufu, are also critical in this control: Tumorigenesis in the Apcmin mouse model is greatly increased in a Sufu heterozygotic background (23).
SUMMARY AND PERSPECTIVES
Through analysis of animal models of perturbed Hh signaling, we are beginning to appreciate the multiple roles played by this pathway during organogenesis and homeostasis of the gut. Indeed, Hh plays a role in L/R axis patterning, proliferation of the mesenchyme, formation of the musculature, establishment of the enteric nervous system, development of the villi, polarization of the crypt/villus axis, homeostasis of peri-cryptal myofibroblasts, control of inflammatory signaling, and probably additional processes (reviewed in 97, 102). Additionally, via epithelial-mesenchymal interactions, Hh signals indirectly control proliferation and differentiation in the epithelial compartment, even though the epithelium does not transduce Hh signals.
These findings demonstrate what Hh does in endodermally derived organs, but less information is available about how Hh carries out these functions. In the intestine, at least 10 different cell types have been identified as direct targets of Hh signals by virtue of their expression of Gli1 and/or Ptch1: smooth muscle precursors, enteric neuron precursors, differentiated smooth muscle cells, pericytes, serosal cells, myofibroblasts/fibroblasts (likely several distinct types), a subset of CD11c+ cells, two morphologically distinct types of CD11b+ cells, and a population of clustered mesenchymal cells that drive villus formation (6, 7, 10, 46–48, 89, 94). Likely, in each cell type (and at each stage of development), Hh activates a distinct set of target genes. Mapping these targets and the enhancers through which Hh signals are transduced will shed further light on how this single signaling pathway can carry out so many distinct functions in a single tissue. The identification of the basis for the context specificity of these responses is also critical to the future design of targeted therapeutics to counteract the pathological effects of deranged pathway activity.
Hh target genes can be activated or repressed by three different Gli factors. Direct Hh target cells are mapped by identification of active Gli1 expression, but Gli1-null mice have no discernable phenotype, and Gli1 is not even expressed in the absence of a Hh signal (103). Huang et al. (56) concluded that Gli2 plays the most critical role in the intestine because Gli3 deletion had little phenotypic consequence and Gli2 expression could dramatically rescue intestinal defects in a Smo-null background. Gli2 is clearly the most widely expressed Gli factor (6); it is present in both direct target cells that are Gli+ and cells that are not actively responding to Hh signals and are Gli1−. But whether expression of a Gli1 or Gli3 activator could also rescue a Smo knockout has not been tested and, to our knowledge, the intestinal phenotype of a Gli2-null animal has not been reported. Notably, when Gli1 is placed in the Gli2 locus, it can rescue Gli2 functions (103). Thus, the actual individual and collective roles of the Gli factors in intestinal organogenesis remain open for future investigation and most likely vary in individual cell types.
Another important future task will be to determine the expression patterns and functional significance of other Hh pathway molecules in the intestine. The potential role of Gas1 in patterning the muscularis externa and the participation of Spop and Sufu in muscle organization and mesenchymal expansion are discussed above. But the Hh pathway is multifactorial and its regulation is complex. Gene deletion strategies, even when targeted in time and space, are sledgehammer approaches that are helpful, but more delicate mutations done in a cell-specific manner would allow more precise interpretations. For example, abundant evidence indicates that phosphorylation, ubiquitination, and SUMOylation affect the proteolysis and stability of the Gli proteins and in some cases, the critical residues have been identified and the enzymatic effectors pinpointed (22). However, the specific roles of these different protein modifications remain unexplored. Although the generation and testing of multiple precise genetic modifications in the mouse model are painfully slow, newer strategies to generate intestinal organoids (with epithelial and mesenchymal components) from genetically modified embryonic stem cells will help speed up the massive task of probing these questions.
Clinically, the fact that Hh signaling appears to be reactivated in endodermal organs in several settings of injury suggests that harnessing this signaling pathway for selected and targeted use to improve tissue repair and regeneration could be beneficial. To do this will require either that we learn more about the factors upstream of Hh and/or that we better understand the downstream factors that Hh modulates in order to effect tissue repair. The downstream road takes us once again to target cells, target genes, and enhancers. Upstream, not much is known, but in several settings, NF-κB appears to regulate Shh (104, 105). It will be important to further explore the connections between NF-κB activation and Shh signaling in the intestine.
Hh pathway inhibitors have recently been used in clinical trials for the treatment of Gorlin’s syndrome, medulloblastoma, colon cancer, and pancreatic cancer (106). Interestingly, an analysis of Gorlin’s patients treated with the Hh inhibitor vismodegib indicates that approximately 30% of patients report GI symptoms, including diarrhea and pain, that are significant enough to cause withdrawal from the study (107). Given the findings in mice that show increased susceptibility of Gli1 heterozygotes to chemically induced colitis (94) and the demonstration that Hh downregulation is associated with inflammation in several mouse models (9, 67, 68, 89), it is possible that systemic Hh inhibition will affect immune tolerance in the human GI tract, accounting for the observed side effects to vismodegib. Two more recent studies suggest that more than one target cell population is relevant to Hh-modulated inflammation (95, 96). Precise determination of which downstream targets of Hh signaling are being altered within these cell populations might suggest improved therapies to modulate or ameliorate the inflammation that accompanies reduced Hh signaling in the intestine.
In summary, unraveling the complex phenotypes that are seen in the intestine (or any tissue) after Hh signaling is modified is complicated by (a) the existence of several distinct cellular targets of Hh signaling, (b) the lack of clarity about which gene targets are activated/repressed in each cell type, (c) redundancy of function among the three Gli proteins, (d) the complexity of the molecular alterations that regulate the pathway, and (e) downstream changes in other signaling molecules secreted by target cells when alterations in Hh signaling are sensed (indirect effects of pathway activity). It seems clear that we have plenty more to do to unravel the interesting and potentially clinically relevant secrets that this pathway has to offer. Nevertheless, as Sonic the Hedgehog once said, “An adventure is no fun if it’s too easy!”
ACKNOWLEDGMENTS
As this chapter reviews an enormous literature on Hh activity in the endoderm and intestine specifically, the authors apologize to colleagues whose work was not cited. The authors are grateful for current and past support from grants NIH/NIDDK R01-DK065850 (D.L.G.), NIH/NIDDK P01-DK062041 (D.L.G.), and NIH/NIDDK R01-DK121166 (K.D.W.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, et al. 1993. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75:1417–30 [DOI] [PubMed] [Google Scholar]
- 2.Bitgood MJ, McMahon AP. 1995. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell–cell interaction in the mouse embryo. Dev. Biol 172:126–38 [DOI] [PubMed] [Google Scholar]
- 3.Gigante ED, Caspary T. 2020. Signaling in the primary cilium through the lens of the Hedgehog pathway. Wiley Interdiscip. Rev. Dev. Biol 10.1002/wdev.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RT, Zhao Z, Ingham PW. 2016. Hedgehog signalling. Development 143:367–72 [DOI] [PubMed] [Google Scholar]
- 5.Wu F, Zhang Y, Sun B, McMahon AP, Wang Y. 2017. Hedgehog signaling: from basic biology to cancer therapy. Cell Chem. Biol 24:252–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, et al. 2009. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology 137:618–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalho-Santos M, Melton DA, McMahon AP. 2000. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127:2763–72 [DOI] [PubMed] [Google Scholar]
- 8.Mao J, Kim BM, Rajurkar M, Shivdasani RA, McMahon AP. 2010. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137:1721–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. 2005. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 132:279–89 [DOI] [PubMed] [Google Scholar]
- 10.Walton KD, Kolterud A, Czerwinski MJ, Bell MJ, Prakash A, et al. 2012. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. PNAS 109:15817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87 [DOI] [PubMed] [Google Scholar]
- 12.Rohatgi R, Milenkovic L, Scott MP. 2007. Patched1 regulates hedgehog signaling at the primary cilium. Science 317:372–76 [DOI] [PubMed] [Google Scholar]
- 13.Zhang XM, Ramalho-Santos M, McMahon AP. 2001. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell 106:781–92 [PubMed] [Google Scholar]
- 14.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. 2012. Noncanonical Hedgehog signaling. Vitam. Horm 88:55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. 2002. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129:4753–61 [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. 2006. Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell 10:657–65 [DOI] [PubMed] [Google Scholar]
- 17.Yao S, Lum L, Beachy P. 2006. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell 125:343–57 [DOI] [PubMed] [Google Scholar]
- 18.Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP. 2006. The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signaling pathway and feedback network in mice. Dev. Cell 10:647–56 [DOI] [PubMed] [Google Scholar]
- 19.Martinelli DC, Fan CM. 2007. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 21:1231–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen BL, Tenzen T, McMahon AP. 2007. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 21:1244–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biau S, Jin S, Fan CM. 2013. Gastrointestinal defects of the Gas1 mutant involve dysregulated Hedgehog and Ret signaling. Biol. Open 2:144–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu A 2019. Proteostasis in the Hedgehog signaling pathway. Semin. Cell Dev. Biol 93:153–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coquenlorge S, Yin WC, Yung T, Pan J, Zhang X, et al. 2019. GLI2 Modulated by SUFU and SPOP induces intestinal stem cell niche signals in development and tumorigenesis. Cell Rep. 27:3006–18.e4 [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Pan Y, Wang B. 2010. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development 137:2001–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roulis M, Flavell RA. 2016. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 92:116–31 [DOI] [PubMed] [Google Scholar]
- 26.Jun JI, Lau LF. 2018. Resolution of organ fibrosis. J. Clin. Investig 128:97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy N, Manieri E, Storm EE, Saadatpour A, Luoma AM, et al. 2020. Distinct mesenchymal cell populations generate the essential intestinal BMP signaling gradient. Cell Stem Cell 26:391–402.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoshkes-Carmel M, Wang YJ, Wangensteen KJ, Toth B, Kondo A, et al. 2018. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557:242–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, et al. 2018. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175:372–86.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, et al. 2020. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 580:524–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. 2018. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 558:449–53 [DOI] [PubMed] [Google Scholar]
- 32.Czerwinski M, Holloway EM, Tsai YH, Wu A, Yu Q, et al. 2020. In vitro and in vivo development of the human intestinal niche at single cell resolution. Cell Stem Cell. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazawa S, Umesono Y, Hayashi T, Tarui H, Agata K. 2009. Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. PNAS 106:22329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rink JC, Gurley KA, Elliott SA, Sánchez Alvarado A. 2009. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326:1406–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsiairis CD, McMahon AP. 2009. An Hh-dependent pathway in lateral plate mesoderm enables the generation of left/right asymmetry. Curr. Biol 19:1912–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonaka S, Shiratori H, Saijoh Y, Hamada H. 2002. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418:96–99 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Okada Y, Hirokawa N. 2005. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature 435:172–77 [DOI] [PubMed] [Google Scholar]
- 38.Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, et al. 1998. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell 94:287–97 [DOI] [PubMed] [Google Scholar]
- 39.Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, et al. 1999. Multiple left-right asymmetry defects in Shh−/− mutant mice unveil a convergence of the Shh and retinoic acid pathways in the control of Lefty-1. PNAS 96:11376–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walton KD, Warner J, Hertzler PH, McClay DR. 2009. Hedgehog signaling patterns mesoderm in the sea urchin. Dev. Biol 331:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. 2000. Regulation of pancreas development by hedgehog signaling. Development 127:4905–13 [DOI] [PubMed] [Google Scholar]
- 42.Kallen B, Mastroiacovo P, Robert E. 1996. Major congenital malformations in Down syndrome. Am. J. Med. Genet 65:160–66 [DOI] [PubMed] [Google Scholar]
- 43.Levy J 1991. The gastrointestinal tract in Down syndrome. Prog. Clin. Biol. Res 373:245–56 [PubMed] [Google Scholar]
- 44.Heanue TA, Pachnis V. 2007. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat. Rev. Neurosci 8:466–79 [DOI] [PubMed] [Google Scholar]
- 45.Uesaka T, Young HM, Pachnis V, Enomoto H. 2016. Development of the intrinsic and extrinsic inner-vation of the gut. Dev. Biol 417:158–67 [DOI] [PubMed] [Google Scholar]
- 46.Rowitch DH, St.-Jacques B, Lee SMK, Flax JD, Snyder EY, McMahon AP. 1999. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J. Neurosci 19:8954–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngan ES, Kim KH, Hui CC. 2013. Sonic hedgehog signaling and VACTERL association. Mol. Syndromol 4:32–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lau ST, Li Z, Lai FPL, Lui KNC, Li P, et al. 2019. Activation of hedgehog signaling promotes development of mouse and human enteric neural crest cells, based on single-cell transcriptome analyses. Gastroenterology 157:1556–71.e5 [DOI] [PubMed] [Google Scholar]
- 49.Yang JT, Liu CZ, Villavicencio EH, Yoon JW, Walterhouse D, Iannaccone PM. 1997. Expression of human GLI in mice results in failure to thrive, early death, and patchy Hirschsprung-like gastrointestinal dilatation. Mol. Med 3:826–35 [PMC free article] [PubMed] [Google Scholar]
- 50.Litingtung Y, Lei L, Westphal H, Chiang C. 1998. Sonic hedgehog is essential to foregut development. Nat. Genet 20:58–61 [DOI] [PubMed] [Google Scholar]
- 51.Liu JA, Lai FP, Gui HS, Sham MH, Tam PK, et al. 2015. Identification of GLI mutations in patients with Hirschsprung disease that disrupt enteric nervous system development in mice. Gastroenterology 149:1837–48.e5 [DOI] [PubMed] [Google Scholar]
- 52.Ngan ES, Garcia-Barcelo MM, Yip BH, Poon HC, Lau ST, et al. 2011. Hedgehog/Notch-induced premature gliogenesis represents a new disease mechanism for Hirschsprung disease in mice and humans. J. Clin. Investig 121:3467–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu M, Lui VCH, Sham MH, Pachnis V, Tam PKH. 2004. Sonic hedgehog regulates the proliferation, differentiation, and migration of enteric neural crest cells in gut. J. Cell Biol 166:673–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin S, Martinelli DC, Zheng X, Tessier-Lavigne M, Fan CM. 2015. Gas1 is a receptor for sonic hedgehog to repel enteric axons. PNAS 112:E73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMahon AP, Ingham PW, Tabin CJ. 2003. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol 53:1–114 [DOI] [PubMed] [Google Scholar]
- 56.Huang H, Cotton JL, Wang Y, Rajurkar M, Zhu LJ, et al. 2013. Specific requirement of Gli transcription factors in hedgehog-mediated intestinal development. J. Biol. Chem 288:17589–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoch M, Pankratz MJ. 1996. Control of gut development by fork head and cell signaling molecules in Drosophila. Mech. Dev 58:3–14 [DOI] [PubMed] [Google Scholar]
- 58.Kaestner KH, Silberg DG, Traber PG, Schutz G. 1997. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 11:1583–95 [DOI] [PubMed] [Google Scholar]
- 59.Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, et al. 2006. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development 133:833–43 [DOI] [PubMed] [Google Scholar]
- 60.Mahlapuu M, Enerback S, Carlsson P. 2001. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 128:2397–406 [DOI] [PubMed] [Google Scholar]
- 61.Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. 2009. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J. Biol. Chem 284:5936–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kosinski C, Stange D, Xu C, Chan AS, Ho C, et al. 2010. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology 139:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aoki R, Shoshkes-Carmel M, Gao N, Shin S, May CL, et al. 2016. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell. Mol. Gastroenterol. Hepatol 2:175–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, et al. 2000. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development 127:1971–80 [DOI] [PubMed] [Google Scholar]
- 65.Huycke TR, Miller BM, Gill HK, Nerurkar NL, Sprinzak D, et al. 2019. Genetic and mechanical regulation of intestinal smooth muscle development. Cell 179:90–105.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zacharias WJ, Madison BB, Kretovich KE, Walton KD, Richards N, et al. 2011. Hedgehog signaling controls homeostasis of adult intestinal smooth muscle. Dev. Biol 355:152–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosinski C, Stange DE, Xu C, Chan AS, Ho C, et al. 2010. Indian hedgehog regulates intestinal stem cell fate through epithelial-mesenchymal interactions during development. Gastroenterology 139:893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Dop WA, Heijmans J, Buller NV, Snoek SA, Rosekrans SL, et al. 2010. Loss of Indian Hedgehog activates multiple aspects of a wound healing response in the mouse intestine. Gastroenterology 139:1665–76 [DOI] [PubMed] [Google Scholar]
- 69.Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, et al. 2002. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology 122:469–82 [DOI] [PubMed] [Google Scholar]
- 70.Kansu A, Ensari A, Kalayci AG, Girgin N. 2000. A very rare cause of intestinal pseudoobstruction: familial visceral myopathy type IV. Acta Paediatr. 89:733–36 [DOI] [PubMed] [Google Scholar]
- 71.Jacobs E, Ardichvili D, Perissino A, Gottignies P, Hanssens JF. 1979. A case of familial visceral myopathy with atrophy and fibrosis of the longitudinal muscle layer of the entire small bowel. Gastroenterology 77:745–50 [PubMed] [Google Scholar]
- 72.Huang H, Song TJ, Li X, Hu L, He Q, et al. 2009. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. PNAS 106:12670–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du KL, Ip HS, Li J, Chen M, Dandre F, et al. 2003. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol 23:2425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurdziel K, Vogt KR, Walton KD, Schneider GK, Gumucio DL. 2016. Transcriptome of the inner circular smooth muscle of the developing mouse intestine: evidence for regulation of visceral smooth muscle genes by the hedgehog target gene, cJun. Dev. Dyn 245:614–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kudo K, Gavin E, Das S, Amable L, Shevde LA, Reed E. 2012. Inhibition of Gli1 results in altered c-Jun activation, inhibition of cisplatin-induced upregulation of ERCC1, XPD and XRCC1, and inhibition of platinum-DNA adduct repair. Oncogene 31:4718–24 [DOI] [PubMed] [Google Scholar]
- 76.Ting-Berreth SA, Chuong CM. 1996. Sonic hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev. Dyn 207:157–70 [DOI] [PubMed] [Google Scholar]
- 77.Walton KD, Whidden M, Kolterud Å, Shoffner SK, Czerwinski MJ, et al. 2016. Villification in the mouse: Bmp signals control intestinal villus patterning. Development 143:427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turing AM. 1952. The chemical basis of morphogenesis. Philos. Trans. R. Soc. B 237:37–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walton KD, Mishkind D, Riddle MR, Tabin CJ, Gumucio DL. 2018. Blueprint for an intestinal villus: species-specific assembly required. Wiley Interdiscip. Rev. Dev. Biol 7:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, et al. 2013. Villification: how the gut gets its villi. Science 342:212–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. 2015. Bending gradients: how the intestinal stem cell gets its home. Cell 161:569–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freddo AM, Shoffner SK, Shao Y, Taniguchi K, Grosse AS, et al. 2016. Coordination of signaling and tissue mechanics during morphogenesis of murine intestinal villi: a role for mitotic cell rounding. Integr. Biol 8:918–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Affolter M, Caussinus E. 2008. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development 135:2055–64 [DOI] [PubMed] [Google Scholar]
- 84.Kim JE, Fei L, Yin WC, Coquenlorge S, Rao-Bhatia A, et al. 2020. Single cell and genetic analyses reveal conserved populations and signaling mechanisms of gastrointestinal stromal niches. Nat. Commun 11:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rao-Bhatia A, Zhu M, Yin WC, Coquenlorge S, Zhang X, et al. 2020. Hedgehog-activated Fat4 and PCP pathways mediate mesenchymal cell clustering and villus formation in gut development. Dev. Cell 52:647–58.e6 [DOI] [PubMed] [Google Scholar]
- 86.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. 1998. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat. Genet 20:54–57 [DOI] [PubMed] [Google Scholar]
- 87.van Dop WA, Uhmann A, Wijgerde M, Sleddens-Linkels E, Heijmans J, et al. 2009. Depletion of the colonic epithelial precursor cell compartment upon conditional activation of the hedgehog pathway. Gastroenterology 136:2195–203 [DOI] [PubMed] [Google Scholar]
- 88.van den Brink GR, Bleuming SA, Hardwick JCH, Schepman BL, Offerhaus GJ, et al. 2004. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet 36:277–82 [DOI] [PubMed] [Google Scholar]
- 89.Zacharias WJ, Li X, Madison BB, Kretovich K, Kao JY, et al. 2010. Hedgehog is an anti-inflammatory epithelial signal for the intestinal lamina propria. Gastroenterology 138:2368–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perreault N, Katz JP, Sackett SD, Kaestner KH. 2001. Foxl1 controls the Wnt/β-catenin pathway by modulating the expression of proteoglycans in the gut. J. Biol. Chem 276:43328–33 [DOI] [PubMed] [Google Scholar]
- 91.Wang CC, Biben C, Robb L, Nassir F, Barnett L, et al. 2000. Homeodomain factor Nkx2–3 controls regional expression of leukocyte homing coreceptor MAdCAM-1 in specialized endothelial cells of the viscera. Dev. Biol 224:152–67 [DOI] [PubMed] [Google Scholar]
- 92.Pabst O, Zweigerdt R, Arnold HH. 1999. Targeted disruption of the homeobox transcription factor Nkx2–3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development 126:2215–25 [DOI] [PubMed] [Google Scholar]
- 93.Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, et al. 2007. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development 134:1977–89 [DOI] [PubMed] [Google Scholar]
- 94.Lees CW, Zacharias WJ, Tremelling M, Noble CL, Nimmo ER, et al. 2008. Analysis of germline GLI1 variation implicates hedgehog signalling in the regulation of intestinal inflammatory pathways. PLOS Med. 5:1761–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Westendorp BF, Buller N, Karpus ON, van Dop WA, Koster J, et al. 2018. Indian Hedgehog suppresses a stromal cell-driven intestinal immune response. Cell. Mol. Gastroenterol. Hepatol 5:67–82.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee JJ, Rothenberg ME, Seeley ES, Zimdahl B, Kawano S, et al. 2016. Control of inflammation by stromal Hedgehog pathway activation restrains colitis. PNAS 113:E7545–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lees C, Howie S, Sartor RB, Satsangi J. 2005. The hedgehog signalling pathway in the gastrointestinal tract: implications for development, homeostasis, and disease. Gastroenterology 129:1696–710 [DOI] [PubMed] [Google Scholar]
- 98.Arimura S, Matsunaga A, Kitamura T, Aoki K, Aoki M, Taketo MM. 2009. Reduced level of smoothened suppresses intestinal tumorigenesis by down-regulation of Wnt signaling. Gastroenterology 137:629–38 [DOI] [PubMed] [Google Scholar]
- 99.Buller NV, Rosekrans SL, Metcalfe C, Heijmans J, van Dop WA, et al. 2015. Stromal Indian hedgehog signaling is required for intestinal adenoma formation in mice. Gastroenterology 148:170–80.e6 [DOI] [PubMed] [Google Scholar]
- 100.Greicius G, Virshup DM. 2019. Stromal control of intestinal development and the stem cell niche. Differentiation 108:8–16 [DOI] [PubMed] [Google Scholar]
- 101.Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, et al. 2016. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 15:911–18 [DOI] [PubMed] [Google Scholar]
- 102.van den Brink GR. 2007. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol. Rev 87:1343–75 [DOI] [PubMed] [Google Scholar]
- 103.Bai CB, Joyner AL. 2001. Gli1 can rescue the in vivo function of Gli2. Development 128:5161–72 [DOI] [PubMed] [Google Scholar]
- 104.Nakashima H, Nakamura M, Yamaguchi H, Yamanaka N, Akiyoshi T, et al. 2006. Nuclear factor-κB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 66:7041–49 [DOI] [PubMed] [Google Scholar]
- 105.Kasperczyk H, Baumann B, Debatin KM, Fulda S. 2009. Characterization of sonic hedgehog as a novel NF-κB target gene that promotes NF-κB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 23:21–33 [DOI] [PubMed] [Google Scholar]
- 106.Low JA, de Sauvage FJ. 2010. Clinical experience with hedgehog pathway inhibitors. J. Clin. Oncol 28:5321–26 [DOI] [PubMed] [Google Scholar]
- 107.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, et al. 2012. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med 366:2171–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nielsen CM, Williams J, van den Brink GR, Lauwers GY, Roberts DJ. 2004. Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab. Investig 84:1631–42 [DOI] [PubMed] [Google Scholar]


