Abstract
Background
Anopheles stephensi is an efficient vector of both Plasmodium falciparum and Plasmodium vivax in South Asia and the Middle East. The spread of An. stephensi to countries within the Horn of Africa threatens progress in malaria control in this region as well as the rest of sub-Saharan Africa.
Methods
The available malaria data and the timeline for the detection of An. stephensi was reviewed to analyse the role of An. stephensi in malaria transmission in Horn of Africa of the Eastern Mediterranean Region (EMR) in Djibouti, Somalia, Sudan and Yemen.
Results
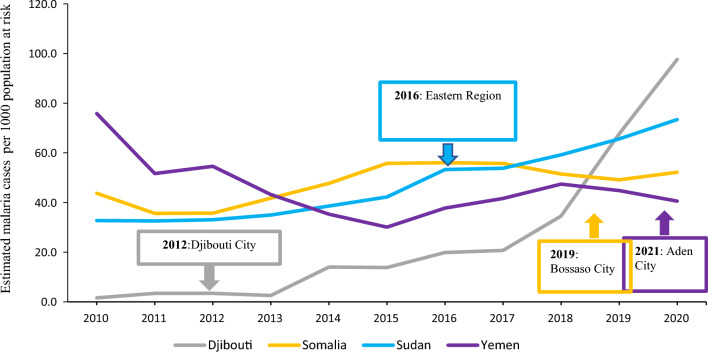
Malaria incidence in Horn of Africa of EMR and Yemen, increased from 41.6 in 2015 to 61.5 cases per 1000 in 2020. The four countries from this region, Djibouti, Somalia, Sudan and Yemen had reported the detection of An. stephensi as of 2021. In Djibouti City, following its detection in 2012, the estimated incidence increased from 2.5 cases per 1000 in 2013 to 97.6 cases per 1000 in 2020. However, its contribution to malaria transmission in other major cities and in other countries, is unclear because of other factors, quality of the urban malaria data, human mobility, uncertainty about the actual arrival time of An. stephensi and poor entomological surveillance.
Conclusions
While An. stephensi may explain a resurgence of malaria in Djibouti, further investigations are needed to understand its interpretation trends in urban malaria across the greater region. More investment for multisectoral approach and integrated surveillance and control should target all vectors particularly malaria and dengue vectors to guide interventions in urban areas.
Keywords: Anopheles stephensi, Invasive vector, Malaria, Vector surveillance, Eastern Mediterranean Region, Breeding sites
Background
The World Health Organization (WHO) estimated that, in 2021, 247 million cases of malaria and 619,000 deaths occurred worldwide, with 95% of cases and 96% of deaths in Africa [1]. In 2016, the WHO member states adopted the WHO Global Technical Strategy (GTS) for Malaria 2016–2030, committing to reduce malaria incidence and mortality by 90% by 2030 [2]. The World Malaria Report 2022 indicated that progress since 2015 has stalled, pointing to the need to analyse obstacles and increase efforts towards control and elimination [1]. The WHO estimated that in 2021, 6.2 million malaria cases occurred in the Eastern Mediterranean Region (EMR), an increase of 44% from 2015 [1]. In EMR, six countries are in the burden reduction phase (Afghanistan, Djibouti, Pakistan, Somalia, Sudan, and Yemen) and two countries are approaching elimination (Islamic Republic of Iran and Saudi Arabia). The remaining 14 countries are preventing malaria re-establishment. Two countries are WHO-certified malaria-free: United Arab Emirates in 2007 and Morocco in 2010 [1]. Sudan accounted for 54% of cases [1]. The Afrotropical Anopheles arabiensis, a member of the Anopheles gambiae species complex, is a major malaria vector in Djibouti, and the primary vector in Somalia, and Sudan, as well as Saudi Arabia and Yemen in the Arabian Peninsula. Both Plasmodium falciparum and Plasmodium vivax are transmitted in these countries, with P. falciparum contributing to the majority of the cases [1]. Anopheles arabiensis, which is mainly a rural malaria vector breeds in a variety of habitats in agricultural areas and in both permanent and transient ground pools, particularly in dry and semi-arid areas [3–7].
Anopheles stephensi is an efficient vector of both P. falciparum and P. vivax. Until 2011, its reported distribution was confined to certain countries in South Asia and large parts of the Arabian Peninsula excluding southwest in Saudi Arabia and Yemen [8–10]. Invasive An. stephensi was first reported in Djibouti in 2012 [11]. Since then, its presence has been reported from Ethiopia (2016) [12], Sri Lanka (2017) [13], Sudan (2016) [14] and Somalia (2019) [15, 16]. In 2019, a WHO Vector Alert recognized the threat of emergence and spread of An. stephensi outside its native geographical range, calling for vigilance and actions [8]. The presence of An. stephensi has since been reported from Nigeria (2020), Yemen (2021), and Kenya (2022) [16]. Anopheles stephensi appears to be better adapted in urban and peri-urban areas than An. arabiensis. It breeds mainly in water tanks, water storage containers, construction sites, desert coolers, wells and other human-made habitats [10, 17–22]. It is a major malaria vector in urban settings in India that successfully sustains malaria transmission even at low vector densities [23, 24]. Larval control through larviciding and distribution of larvivorous fish are mainly used to interrupt malaria transmission in urban areas in India. The organophosphate insecticide temephos and the bacteria Bacillus thuringiensis var israelensis (Bti) are commonly used larvicides [24]. In EMR, there is widespread resistance of An. stephensi, to the four major insecticide classes, organochlorines, pyrethroids, organophosphates and carbamates which include commonly used insecticides in the two-core malaria vector control interventions, insecticide-treated nets (ITNs) and indoor residual spraying (IRS) that target the adult stage of the vector [25, 26].
The emergence and spread of An. stephensi threatens progress in malaria control in the Horn of Africa and sub-Saharan Africa. Therefore, the data related to the role of An. stephensi in malaria transmission in the Eastern Mediterranean Region, specifically in the Horn of Africa and the southwestern part of the Arabian Peninsula was reviewed, including the distribution, genetic diversity and bionomics with focus on the type of vector breeding sites and the insecticide resistance in countries.
Methods
Scope and population
The situation of the four countries (Djibouti, Somalia, Sudan and Yemen) in the Eastern Mediterranean Region (EMR) where An. stephensi was detected for the first time between 2012 to 2021 was analysed.
Data sources
Epidemiological country reports to the World Health Organization
Two types of country reports to the WHO were examined: the national-level country data on malaria incidence and the sub-national data for the reported malaria cases from major cities. The national-level report data to the WHO on an annual basis through the WHO Integrated data platform (DHIS2) for the World Malaria Report. The sub-national data was obtained from national health facility-based disease surveillance systems in countries, including the national Health Management Information System (HMIS) in Somalia and Sudan and the national electronic Disease Early Warning System (eDEWS) in Yemen.
Entomological surveillance reports
Entomological surveillance reports were reviewed, which included detection of An. stephensi and detections included in Malaria Threats Map (https://apps.who.int/malaria/maps/threats/), an official WHO digital platform developed to track the spread of this vector. The platform includes detections reported in published scientific literature and detections reported to WHO by countries using a standard spreadsheet template. The template includes the geographical location of the detection, the development stage of the collected mosquitoes (adults or immature stages), the type of larval habitat (collection of immature stages) and the method of mosquito collection. The WHO checks the quality of unpublished country-reported data before uploading it to Malaria Threats Map.
Published studies
A literature review was conducted in the search engine PubMed (https://pubmed.ncbi.nlm.nih.gov/) for scientific publications on An. stephensi in the Horn of Africa and the Arabian Peninsula. The data for bionomics, insecticide resistance status and genetic diversity of the invasive vector populations was reviewed.
Data analysis
Estimated national malaria incidence in Djibouti, Somalia, Sudan and Yemen
The estimated malaria incidence was plotted for Djibouti, Somalia, Sudan and Yemen from 2010 to 2020 using the population denominator from the World Malaria Report 2021 for estimation of incidence (Annex 5 F). The year of the first reported detection of An. stephensi was indicated for each country.
Reported sub-national urban malaria incidence
The sub-national malaria incidence was plotted using reported confirmed cases divided by population at risk, over different time periods for the major cities where the An. stephensi was detected. These were Bossaso city (2012–2021) in Somalia, Khartoum City (2016–2021) in Sudan and Aden City (2015–2021) in Yemen. For Djibouti, malaria cases are highly concentrated and reported from Djibouti City, therefore the plotted estimated national malaria incidence also represented the urban malaria incidence.
Geographical distribution and population structure of Anopheles stephensi in Djibouti, Sudan, Somalia, and Yemen
The cumulative number of sites that detected An. stephensi was mapped overlaid with population density [27, 28] and created a timeline for reported detection of An. stephensi for each country. Previous findings from molecular surveillance and the results of investigations into the genetic diversity of An. stephensi were also summarized.
Bionomics and insecticide resistance status
The larval ecology and the insecticide resistance status of An. stephensi reported in Djibouti, Somalia, Sudan and Yemen using both unpublished country reports to the WHO and published literature were summarized. The types of larval habitats where studies and countries collected immature stages of An. stephensi were listed.
Results
Estimated national malaria incidence in Djibouti, Somalia, Sudan and Yemen
In the subregion of Horn of Africa and Yemen, the estimated incidence of malaria per 1000 population per year decreased from 44.9 per 1000 in 2010 to 41.6 per 1000 in 2015. However, it increased after 2015, reaching 61.5 cases per 1000 population in 2020. As of 2021, four countries of the region reported detection of An. stephensi. In Djibouti, where approximately 70% of the population lives in the capital city, the estimated malaria incidence per 1000 population was 1.6 per 1000 in 2010 and remained stable to 3.4 per 1000 until 2012 when An. stephensi was detected. Then incidence increased from 2.5 per 1000 in 2013 to 97.6 per 1000 in 2020 (an increase by 39-fold). In the three other countries where An. stephensi was detected, the association between detection and incidence at the national level was less clear. In Sudan, the estimated incidence per 1000 population increased regularly from 32.7 per 1000 in 2010 to 73.4 per 1000 in 2020 while An. stephensi was detected in 2016 [14]. In Somalia, the estimated incidence per 1000 population increased unevenly from 43.7 per 1000 in 2010 to 52.2 per 1000 in 2020 and An. stephensi was detected in 2019 [16]. In Yemen, the incidence per 1000 population decreased from 75.8 in 2010 to 40.6 in 2020, and An. stephensi was detected in 2021 [16] (Fig. 1).
Fig. 1.
Estimated malaria incidence and timeline of first reported detection of invasive An. stephensi, in 4 EMR countries, 2010–2020. Boxes indicate the date of first An. stephensi detection in each country
Reported malaria incidence in cities in EMR countries
Aden City, Yemen
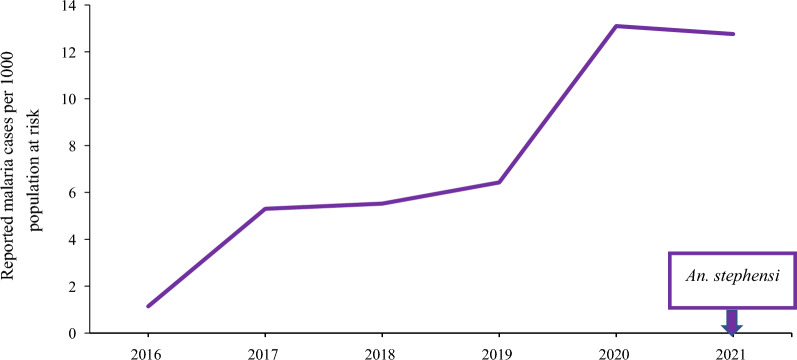
The reported malaria incidence in Aden City, Yemen, increased fivefold from 1.1 in 2016 to 5.3 cases per 1000 in 2017. By 2020 and 2021, the reported malaria incidence continued to rise, with 13.1 and 12.8 reported cases per 1000 population, respectively (Fig. 2).
Fig. 2.
Reported malaria incidence in Aden City, Yemen, 2016–2021. Box indicates the date of first An. stephensi detection in the country
Bossaso City, Somalia
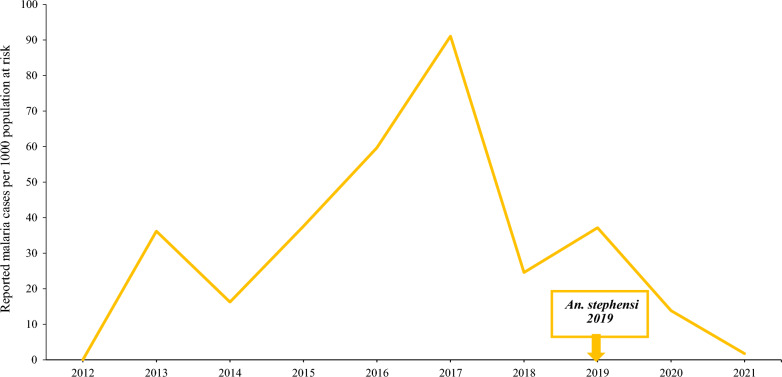
Malaria incidence in Bossaso City, Somalia was only 0.02 per 1000 population in 2012. The following year, the incidence reached a high of 36.2 per 1000, before dropping to 16.3 per 1000 in 2014. Malaria incidence increased again in 2017 to 91.1 per 1000, dropped to 24.6 per 1000 in 2018 and then rose in 2019 to 37.2 per 1000. However, after 2019, reported malaria cases decreased reaching an incidence of 1.8 per 1000 population in 2021 (Fig. 3).
Fig. 3.
Reported malaria incidence in Bossaso City, Somalia, 2012–2021. Box indicates the date of first An. stephensi detection in the country
Khartoum City, Sudan
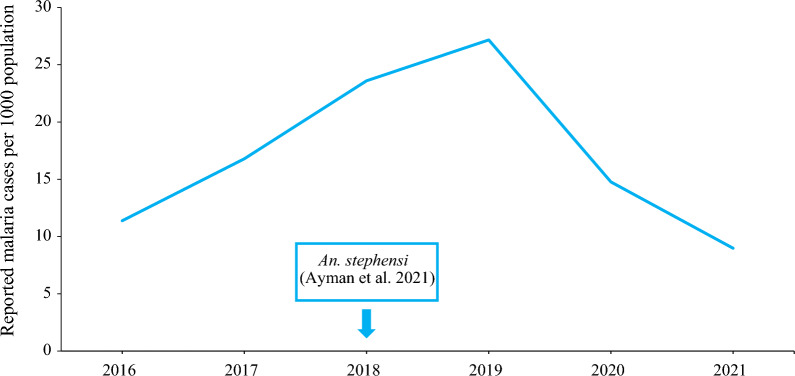
Khartoum State contains the largest city of Khartoum, the capital of Sudan. The reported malaria incidence per 1000 population was 11.4 in 2016, with an increase in the following years to reach 27.2 in 2019. In 2020 and 2021, the reported incidence gradually decreased to 9.0 per 1000 population (Fig. 4).
Fig. 4.
Reported malaria incidence in Khartoum City, Sudan, 2016–2021
Geographical distribution of An. stephensi in Djibouti, Sudan, Somalia, and Yemen
In 2012, Djibouti City reported invasive An. stephensi in one site, near the animal export and quarantine station located, approximately 14 km from Djibouti City and 4 km from the Somalian border [11]. Djibouti reported detecting An. stephensi in 6 sites in 2013 and 8 sites in 2014 [11] (Fig. 5).
Fig. 5.
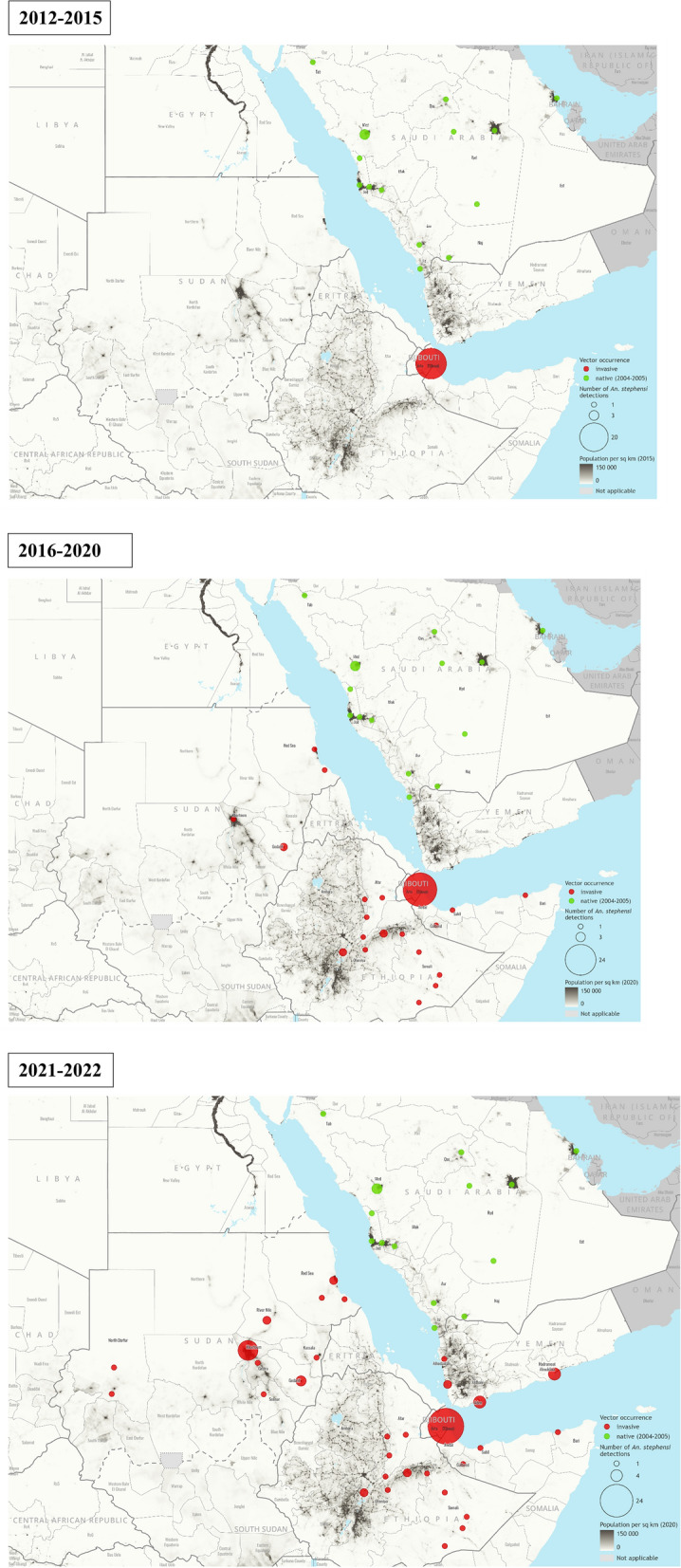
Detections of An. stephensi in countries in the Eastern Mediterranean Region in the Horn of Africa and the Arabian Peninsula reported to WHO, overlaid with population density, 2012–2021. Maps credit: WHO GIS Centre for Health
In 2019, Sudan reported An. stephensi from 4 sites in collections conducted from 2016 to 2018 [14, 16]: Port Sudan, Tokar, Abu Alnaja and Diam Bakur in Eastern States. A following report of collections in 2018 identified An. stephensi in Tuti Island in the capital Khartoum [29]. In 2021–2022, Sudan reported collections from 15 additional sites, which included sites in Khartoum, expanding to a total number of 20 sites, both urban and rural [16, 30].
In 2019, Bossaso City Somalia, was the first Somalian site to report identification of An. stephensi. Additional identification followed in 2020 in Hargeisa City, Berbera Port City, and Lawyo Ado, a rural site bordering Djibouti [15] (Fig. 5).
In 2021, Yemen reported the first identification of An. stephensi, from Aden City [31] in the south of the country. Additional entomological surveillance in 2021–2022 identified An. stephensi in Aden City (3 sites), Lahj governorate (1 site), Al Mukalla City (4 sites) and Broom Mafia (2 sites) in Hadramout governorate and Al Dahi (1 site) and Zabid City (1 site) in Al Hudaydah governorate [16] (Fig. 5).
In all four countries, most of the sites where An. stephensi were detected were urban which may be partially due to the fact that this is where national programmes conducted vector surveillance of the invasive An. stephensi.
Genetic diversity of the invasive An. stephensi
Genetic diversity studies based on mitochondrial DNA have been conducted for Djibouti, Somalia, and Sudan. Most of these studies utilize a common barcoding gene cytochrome c oxidase subunit 1, COI. These studies detected the presence of multiple haplotypes across this region. The populations of An. stephensi are genetically similar [15, 32, 33]. The predominant COI haplotypes in these countries that were first reported in Ethiopian An. stephensi [33] have been reported in South Asia but are absent in most of the long-established An. stephensi populations in the Arabian Peninsula. Work on the genetic diversity of new populations in Yemen is ongoing but initial evidence from Aden City indicates the presence of a Horn of Africa haplotype among Yemeni An. stephensi [31].
Bionomics
Available published studies describing the distribution of An. stephensi and reports of unpublished entomological surveys conducted by the national malaria control programmes point to common breeding sites of An. stephensi across countries (Table 1 and Fig. 6). Similar larval habitats include barrels, household water storage containers, and cement/metal/plastic tanks in Djibouti, Sudan and Yemen. In Djibouti City, in addition to the variety of human-made breeding habitats, water seepage inside houses creates productive breeding sites for both An. stephensi and Aedes aegypti (author’s field observation (Al-Eryani SM)). In Somalia, An. stephensi were collected only from locally made mud/cement water-storage reservoirs ‘Berkads’. Yemen also reported car tyres as An. stephensi larval habitats. These breeding sites are also the common breeding sites of Aedes aegypti, the primary dengue vector in the region.
Table 1.
Types of breeding sites from which positive (Presence) An. stephensi collections have been made, place and time
| Breeding sites where An. stephensi has been found | Sudan | Djibouti | Yemen | Somalia |
|---|---|---|---|---|
| Drum/barrel |
Present [30] |
Present [32], Identified by the author in 2019 |
Present [54] |
N/A |
| Bucket/other household water storage |
Present [30] |
Present [11] |
N/A | N/A |
| Construction water storage reservoir/cement tanks |
Present |
N/A |
Present [54] |
N/A |
| Metal/plastic water tank |
Present |
Present [32] |
N/A | N/A |
| Berkads | N/A | N/A | N/A |
Present [15] |
| Manhole |
Present [29] |
Present [32] |
N/A | N/A |
| Water leakage |
Present |
Present [32] |
N/A | N/A |
| Car wash runoff | N/A | N/A | N/A | N/A |
| Cemented floor | N/A | N/A | N/A | N/A |
| Desert cooler |
Present Identified by the author in 2019 |
N/A | N/A | N/A |
| Ditch | N/A |
Present [32] |
N/A | N/A |
| Water seepage inside houses | N/A |
Present Identified by the author in 2019 |
N/A | N/A |
| Puddle/pond | N/A | N/A | N/A | N/A |
| Septic tank/wastewater pit |
Present [29] |
N/A | N/A | N/A |
| Car tyre | N/A | N/A |
Present [31] |
N/A |
| Jerrycan | N/A | N/A |
Present [31] |
N/A |
References cited are presented in brackets
Fig. 6.
Common breeding sites for invasive Anopheles stephensi and Aedes aegypti in Djibouti (A–D), Somalia (G), Sudan (E, F) and Yemen (H–J), 2019–2022
Insecticide resistance
Few country reports were available on the susceptibility of the invasive An. stephensi to insecticides. Phenotypic insecticide resistance to the commonly used public health insecticides was reported in populations of An. stephensi in Djibouti and in Yemen. Anopheles stephensi populations were resistant to pyrethroids, organophosphates, carbamates and organochlorines (DDT) in Djibouti [34] and pyrethroids and carbamates in Yemen [35]. The presence of the knockdown resistance mutation (kdr) associated with resistance to pyrethroids and DDT was detected in specimens from Somalia [15].
Discussion
Since 2016, malaria progress has plateaued, and the world was off track to achieve the 2020 milestones of WHO’s global malaria strategy [1]. Four of the malaria endemic countries in EMR, Djibouti, Somalia, Sudan and Yemen have reported the detection of invasive An. stephensi, a new malaria vector in this part of the world.
In Djibouti City the available data suggests an impact of the invasive An. stephensi on malaria transmission in urban areas. The incidence started to increase in 2012 after detection of An. stephensi, with an overall increase by 39-fold by 2020. Anopheles stephensi may explain the resurgence of malaria in Djibouti City, including the major outbreaks in 2018–2020 [11, 32]. The evidence that associates An. stephensi as a cause of urban transmission in Djibouti City includes the detection of An. stephensi with P. falciparum (3.3%) collected from houses of malaria patients in March 2013 and a second collection of P. falciparum positive An. stephensi in November 2013 which coincided with the malaria outbreaks in 2013–2014 [11]. Furthermore, entomological data from 2019 reported An. stephensi to be the predominant Anopheles species breeding in a variety of widespread larval sites in Djibouti City [32].
Unlike in Djibouti, there was no association between malaria incidence and An. stephensi detections in Aden, Bossaso and Khartoum. Various causes might have led to the fluctuation of malaria cases reported in these cities. Malaria emerged in Bossaso City in 2013. In 2013 and 2014, long lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), were introduced in Bossaso [36]. These may have led to a decrease in reported malaria cases. In contrast, vector control activities were reduced in Djibouti City during this period [37]. Extreme climatic events in Somalia with heavy rains and floods in Bossaso occurred during 2016, with a major outbreak of dengue and chikungunya that year, followed by an increase of the reported malaria incidence up to a transmission peak in 2017. Concurrent malaria and dengue and chikungunya transmission is not often reported [38]. The epidemiological situation of malaria, dengue or their co-infections is not assessed in these cities that have weak health systems [39]. Other interventions in Bossaso city, including the introduction of active case detection, primaquine treatment for gametocyte carriers, implementation of larval source management such as larvivorous fish and ‘berkad’ modification in targeted areas and a drought since 2020 may have contributed to the reduction of cases from 2017 to 2021.
In Aden City, the upward trend of malaria incidence shares common features with the one in Djibouti City. In Yemen, there is moderate-to-low transmission in southern governorates forming the Aden region (Aden, Lahj, Taiz, Abyan and Al Dhale'e) [40]. During 2018–2020, 41% of the cases were reported from Aden City, accounting for 15% of the total reported malaria cases in Yemen. The increase of reported malaria cases in Aden was observed from 2016, one year following the civil war. The military conflicts in 2015 led to a collapse of the health system in Aden City. The lack of delivery of basic health services, including lack of piped water, influx of internally displaced persons and refugees from neighboring Ethiopia and Somalia contributed to unplanned urbanization. According to the available data, the presence of An. stephensi in Yemen was not reported prior to 2021, although it may have indeed been present. Prior to 2021, the focus of vector surveillance in Aden was for the dengue vector, Ae. aegypti and there was limited capacity for identification of An. stephensi. In 2022, Aden and other coastal cities like Mukalla City in the east of the country, where An. stephensi was also detected in 2021, were hosting the recent population displacement waves, both internal due to the ongoing conflict and refugees from the Horn of Africa. In addition, there is a trade route between Bossaso, Somalia and Mukulla Port City, Yemen [41]. These important city ports and trade routes might be used for the movement of An. stephensi between the Arabian Peninsula and the Horn of Africa.
Introduction of irrigation schemes in a number of States, which included Khartoum, might have contributed to the increase of the estimated malaria cases from 2016 to 2019. Irrigation schemes led to more intense and perennial malaria transmission in neighbouring countries in Ethiopia [42, 43] and Kenya [44]. The Khartoum Malaria-Free Initiative with larval-source management as the cornerstone of malaria control in Khartoum reduced malaria morbidity and mortality between 2000 and 2011 [45, 46]. However, the initiative was neglected in the following years, highlighted in the malaria programme review in 2018 [46]. Revitalization of the initiative including multisectoral approach for larval source management in Khartoum would be a first step for progress towards elimination. In 2020, the declining trend in malaria was likely due to the disruption of services and lockdown due to COVID-19 Pandemic, which reduced the number of attending patients for seeking health care at the health facilities that included malaria diagnosis. COVID-19 decreased outpatients’ consultation for malaria in Rwanda [47]. Covid-19 reduced in access to services in health facilities in 24 countries in Africa and 7 countries in Asia. Malaria diagnosis decreased 56% and 17% in the countries in Asia and Africa, respectively [48].
In the absence of more complete longitudinal data from all sites, genetic data has helped fill the knowledge gap on the origin of An. stephensi in the EMR. While genomic investigations are still underway, some clues have been derived from mitochondrial data generated to confirm species.
The work completed in Djibouti, Sudan, and Somalia illustrated the usefulness of the population genetic and phylogenetic analysis of An. stephensi populations to narrow the region of origin of the invasive species. The populations of An. stephensi are genetically similar [15, 32, 33] which suggests a common origin. On-going genome-wide analyses on An. stephensi in the EMR will provide more precise information about the connectivity between An. stephensi populations, the potential mode of introduction, and the environmental factors that influence its spread and adaptation. Genomic data will be particularly critical to establish the temporal relationship between An. stephensi populations in the Horn of Africa and those detected in Yemen that have yet to be resolved with single gene analysis. The genetic profiles of An. stephensi populations generated so far provide a helpful baseline to revisit with follow-up investigations.
The review of the larval habitat data on invasive An. stephensi populations pointed to characteristics shared with Ae. aegypti [11, 32, 49, 50]. The habitats of invasive An. stephensi were mainly in human-made containers, characteristic of native An. stephensi in India [20, 51]. In Djibouti City, water seepage inside houses also created productive breeding sites for both An. stephensi and Ae. aegypti (Al-Eryani, pers. commun.). Similarly, invasive Ae. aegypti was collected from typical breeding sites of An. stephensi in Iran [52, 53]. While An. stephensi was found in several different types of breeding sites in Djibouti [11, 32], Sudan [29, 30] and Yemen [54], only one type of locally human-made container (berkads) was reported as habitats for An. stephensi immatures collected in Somalia [15]. In neighbouring Ethiopia, breeding sites reported [12, 55, 56], included berkads and car tyres as in Somalia and Yemen, respectively. Car tyres provide ideal breeding sites for the dengue vector, Ae. aegypti. Aden City is one of the cities in Yemen reporting the highest number of dengue and chikungunya cases with frequent outbreaks [57]. Anopheles stephensi was coincidently detected for the first time during surveys for the control of Ae. aegypti [31]. The different types of breeding sites reported may reflect behavioural differences in An. stephensi between sites, differences in the type of breeding sites available at the sites, or the survey methods used.
Available data reported resistance in An. stephensi to pyrethroids, organochlorines, organophosphates and carbamates in sites where it has been monitored in Djibouti [34] and Yemen [35]. This is also consistent with the data reported in Ethiopia for 2016–2021 [50, 56] suggesting that An. stephensi is resistant to multiple classes of insecticides and to the data reported from other countries in Asia and the Arabian Peninsula [26]. The evidence of resistance to multiple insecticides highlights the need for alternative strategies involving new chemicals or non-chemical-based vector control.
The detection of An. stephensi in the geographic locations within these countries of the Horn of Africa, point to a risk of further expansion of An. stephensi across Africa, as evidenced by recent findings in Nigeria and Kenya [16]. Predictive maps showing suitable habitat for An. stephensi outside its native geographical range suggest that it will continue to spread, potentially exposing at least 126 million additional people at risk of malaria [58]. The WHO continues to track the vector in the WHO Malaria Threats Map, which was set up for this purpose and countries are encouraged to report detections as soon as they are confirmed [16].
There are several limitations to this review. First, data availability, analysis and interpretation of urban malaria data is often of limited quality. The limits of the “urban” area influence the data to determine the actual spread and distribution of An. stephensi. Mobility of human populations can complicate the establishment of an association between cases of malaria reported in urban areas and urban transmission. Second, there are also important challenges in understanding the spread of An. stephensi. Tracking species invasions is most accurately done when consistent surveillance systems have been in place. In the case of An. stephensi, surveillance methods and frequency varied from place to place, with an emphasis on urban and semi-urban areas. Habitats of An. stephensi in rural areas may differ from those in urban settings. The detection of An. stephensi in a city does not provide much information about when the invasion took place unless there has been rigorous and regular entomological monitoring. This is unfortunately not the case for the cities described here. In some cases, the first An. stephensi reports were often the first published report of any mosquito for that site [11, 15]. Thus, a detection of An. stephensi in 2019 in Bossaso Somalia, may not necessarily mean that An. stephensi first arrived in 2019. Similarly, even the first report from Djibouti does not mean that this port city was the site of introduction. Third, reports of positive detections of An. stephensi could constitute a publication bias. Knowing where An. stephensi has been looked for but not detected is also essential to understand conditions that prevent establishment of this invasive vector. Systematic data are needed to create a forecasting system that can predict, with confidence, the presence of An. stephensi in other areas. Finally, the insecticide resistance status, infectivity and behavior of invasive An. stephensi are not well understood. Important gaps remain to fully incriminate An. stephensi as an efficient malaria vector in these countries.
Conclusion
This analysis leads to four major points of conclusion. First, in Djibouti An. stephensi is well established and has likely been the driving force for the explosive increase in urban malaria. Second, outside of Djibouti, the impact of invasive An. stephensi on malaria trends remains unclear. Third, An. stephensi is resistant to many insecticides commonly used in public health, and this poses challenges to effective vector control options. Fourth, breeding sites for An. stephensi are often typical habitats for Ae. aegypti and the two vector species are frequently found together.
Based on the results of the review, a way forward can be proposed. The control of An. stephensi and Ae. aegypti should be aligned with the Global Vector Control Response (GVCR) 2017–2030 and EMR Regional plan for implementation of the GVCR 2019–2023, which provide strategic guidance to countries and development partners to strengthen vector control worldwide through increased capacity, improved surveillance, better national and international coordination and integrated action across sectors and diseases [59, 60]. Further to the regional advocacy, recent literature also emphasized an integrated vector control approach [61, 62].
More specifically, routine surveillance for An. stephensi is needed where it is currently reported and in sites at high risk of invasion. Research should better characterize the effectiveness of the current malaria vector control interventions, including novel approaches, on the invasive vector. Though molecular surveillance has been implemented in most countries at the point of initial detection, support for continued monitoring is necessary. Continued molecular surveillance of the population structure will be particularly useful for measuring the success of interventions such as larvicidal treatments. Further investigations are needed to understand the link between An. stephensi and urban malaria in new areas, particularly in the Afrotropical settings invaded by An. stephensi. Finally, available resources need to be used for integrated vector surveillance and control that can target urban disease vectors with intersectoral collaboration and community engagement. To adequately address the growing threat of invasive An. stephensi regular consistent monitoring and timely sharing of information can inform mitigation and containment efforts and aid in decreasing the risk for increased malaria in urban settings.
Acknowledgements
The authors would like to thank Dr. Ghasem Zamani (Regional Advisor WHO/EMRO-MVC) for the contribution in the conceptualization of the situation analysis, technical guidance and review during development of the manuscript. The authors would also like to thank Dr. Hoda Atta (Coordinator WHO/EMRO-HTM) for technical guidance and review during the development of the manuscript. The authors would like to thank WHO GIS Centre for Health for the development of the maps in the manuscript. Finally, the authors would like to thank the National Malaria Control Programmes in the countries for their continuing efforts in vector surveillance and control and thank donors, The Global Fund and King Salman Humanitarian Aid and Relief Center for continuing to support the countries in the fight against malaria.
Author contributions
SA, SI, TC and YH contributed to the conceptualization and development of the manuscript. SA compiled the data, preparation of the figures and finalizing the manuscript. AL, LF and AJ reviewed and provided input to contribute to completing the manuscript draft. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability statement
All available data are included in the manuscript and from publicaly available sources.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
None declare.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World malaria report 2022. Geneva, World Health Organization, 2022. https://apps.who.int/iris/handle/10665/365169
- 2.WHO. Global Malaria Programme. Global technical strategy for malaria, 2016–2030. Geneva, World Health Organization, 2015. https://www.who.int/publications/i/item/9789240031357
- 3.Shililu J, Mbogo C, Ghebremeskel T, Githure J, Novak R. Mosquito larval habitats in semiarid ecosystem in Eritrea: impact of larval habitat management on Anopheles arabiensis population. Am J Trop Med Hyg. 2007;76:103–110. doi: 10.4269/ajtmh.2007.76.103. [DOI] [PubMed] [Google Scholar]
- 4.Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Himeidan YE, Rayah EE. Role of some environmental factors on the breeding activity of Anopheles arabiensis in New Halfa town, eastern Sudan. East Mediterr Health J. 2008;14:252–259. [PubMed] [Google Scholar]
- 6.Al-Eryani S, Kelly-Hope L, Harbach RE, Briscoe AG, Barnish G, Azazy A, et al. Entomological aspects and the role of human behaviour in malaria transmission in a highland region of the Republic of Yemen. Malar J. 2016;15:130. doi: 10.1186/s12936-016-1179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eba K, Habtewold T, Yewhalaw D, Christophides GK, Duchateau L. Anopheles arabiensis hotspots along intermittent rivers drive malaria dynamics in semi-arid areas of Central Ethiopia. Malar J. 2021;20:154. doi: 10.1186/s12936-021-03697-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Vector alert: Anopheles stephensi invasion and spread: Horn of Africa, the Republic of the Sudan and surrounding geographical areas, and Sri Lanka: information note. Geneva, World Health Organization, 2019. https://apps.who.int/iris/handle/10665/326595
- 9.Al-Ghamdi K, Alikhan M, Mahyoub J, Afifi ZI. Studies on identification and population dynamics of anopheline mosquitoes from Jeddah Province of Saudi Arabia. Biosci Biotechnol Res Commun. 2008;1:19–24. [Google Scholar]
- 10.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti. Horn of Africa Acta Trop. 2014;139:39–43. doi: 10.1016/j.actatropica.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–186. doi: 10.1016/j.actatropica.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Gayan Dharmasiri AG, Perera AY, Harishchandra J, Herath H, Aravindan K, Jayasooriya HT, et al. First record of Anopheles stephensi in Sri Lanka: a potential challenge for prevention of malaria reintroduction. Malar J. 2017;16:326. doi: 10.1186/s12936-017-1977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed A, Pignatelli P, Elaagip A, Hamid MM, Alrahman OF, Weetman D. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016–2018. Emerg Infect Dis. 2021;27:2952. doi: 10.3201/eid2711.210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali S, Samake JN, Spear J, Carter TE. Morphological identification and genetic characterization of Anopheles stephensi in Somaliland. Parasit Vectors. 2022;15:247. doi: 10.1186/s13071-022-05339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Malaria Threats Map. Geneva, World Health Organization. https://apps.who.int/malaria/maps/threats/
- 17.Sharma RS. Urban malaria and its vectors Anopheles stephensi and Anopheles culicifacies (Diptera: Culicidae) in Gurgaon, India. Southeast Asian J Trop Med Public Health. 1995;26:172–176. [PubMed] [Google Scholar]
- 18.Rafinejad J, Vatandoost H, Nikpoor F, Abai MR, Shaeghi M, Duchen S, et al. Effect of washing on the bio-efficacy of insecticide-treated nets (ITNs) and long-lasting insecticidal nets (LLINs) against main malaria vector Anopheles stephensi by three bioassay methods. J Vector Borne Dis. 2008;45:143. [PubMed] [Google Scholar]
- 19.Sharma VP. Current scenario of malaria in India. Parassitologia. 1999;41:349–353. [PubMed] [Google Scholar]
- 20.Thomas S, Ravishankaran S, Justin JA, Asokan A, Mathai MT, Valecha N, et al. Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai. India Malar J. 2016;15:274. doi: 10.1186/s12936-016-1321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Sharma VP, Thavaselvam D, Sumodan PK. Control of Anopheles stephensi breeding in construction sites and abandoned overhead tanks with Bacillus thuringiensis var. israelensis. J Am Mosq Control Assoc. 1995;11:86–89. [PubMed] [Google Scholar]
- 22.Singh H, Gupta SK, Vikram K, Saxena R, Sharma A. The impact of mosquito proof lids of underground tanks “tanka” on the breeding of Anopheles stephensi in a village in western Rajasthan. India Malar J. 2021;20:412. doi: 10.1186/s12936-021-03939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma SN, Subbara SK, Choudhury DS. Role of An culicifacies and An stephensi in malaria transmission in urban Delhi. Indian J Malariol. 1993;30:155–168. [PubMed] [Google Scholar]
- 24.Subbarao SK, Nanda N, Rahi M, Raghavendra K. Biology and bionomics of malaria vectors in India: existing information and what more needs to be known for strategizing elimination of malaria. Malar J. 2019;18:396. doi: 10.1186/s12936-019-3011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Guidelines for malaria. Geneva, World Health Organization, 2022. Available from: https://apps.who.int/iris/handle/10665/354781
- 26.Enayati A, Hanafi-Bojd AA, Sedaghat MM, Zaim M, Hemingway J. Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO Eastern Mediterranean Region. Malar J. 2020;19:258. doi: 10.1186/s12936-020-03335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WorldPop. Open Spatial Demographic Data and Research. 2022. https://www.worldpop.org/
- 28.Linard C, Gilbert M, Snow RW, Noor AM, Tatem AJ. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS ONE. 2012;21(7):e31743. doi: 10.1371/journal.pone.0031743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed A, Khogali R, Elnour MA, Nakao R, Salim B. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State. Central Sudan Parasit Vectors. 2021;14:511. doi: 10.1186/s13071-021-05026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abubakr M, Sami H, Mahdi I, Altahir O, Abdelbagi H, Mohamed NS, et al. The phylodynamic and spread of the invasive Asian malaria vectors, Anopheles stephensi, in Sudan. Biology (Basel) 2022;11:409. doi: 10.3390/biology11030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allan R, Weetman D, Sauskojus H, Budge S, Hawail TB, Baheshm Y. Confirmation of the presence of Anopheles stephensi among internally displaced people’s camps and host communities in Aden City. Yemen Malar J. 2023;22:1. doi: 10.1186/s12936-022-04427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Santi VP, Khaireh BA, Chiniard T, Pradines B, Taudon N, Larréché S, et al. Role of Anopheles stephensi mosquitoes in malaria outbreak, Djibouti, 2019. Emerg Infect Dis. 2021;27:1697. doi: 10.3201/eid2706.204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter TE, Yared S, Getachew D, Spear J, Choi SH, Samake JN, et al. Genetic diversity of Anopheles stephensi in Ethiopia provides insight into patterns of spread. Parasit Vectors. 2021;14:602. doi: 10.1186/s13071-021-05097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Govoetchan R, Ibrahim MM, Sovi A, Omar HM, Boulhan AO, Darar HY. Anopheles stephensi: the emerging vector of malaria in the Republic of Djibouti. Horn of Africa Int J Biosci. 2023;22:8–17. [Google Scholar]
- 35.Ministry of Health Yemen. National Malaria Control Programme, Monitoring of Insecticide Resistance on Malaria vector in Bajil and Hodieda, Report September 2022.
- 36.Ministry of Health. Somalia Malaria Programme Review Report. 2020.
- 37.Ministry of Health. Djibouti Malaria Programme Review Report 2012–2018. 2018.
- 38.Wiwanitkit V. Concurrent malaria and dengue infection: a brief summary and comment. Asian Pacific J Trop Biomed. 2011;1:326–327. doi: 10.1016/S2221-1691(11)60053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdul-Ghani R, Mahdy MA, Alkubati S, Al-Mikhlafy AA, Alhariri A, Das M, et al. Malaria and dengue in Hodeidah city, Yemen: High proportion of febrile outpatients with dengue or malaria, but low proportion co-infected. PLoS ONE. 2021;25(16):e0253556. doi: 10.1371/journal.pone.0253556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ministry of Health Yemen. National Malaria Control Programme, Malaria Technical Review Report. 2021.
- 41.Dua J, Warsame A, Shire, A. Bosaso and the Gulf of Aden: changing dynamics of a land-sea network. Rift Valley Institute, 2020. https://riftvalley.net/publication/bosaso-and-gulf-aden-changing-dynamics-land-sea-network.
- 42.Kibret S, Wilson GG, Tekie H, Petros B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar J. 2014;13:360. doi: 10.1186/1475-2875-13-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haileselassie W, Zemene E, Lee MC, Zhong D, Zhou G, Taye B, et al. The effect of irrigation on malaria vector bionomics and transmission intensity in western Ethiopia. Parasit Vectors. 2021;14:516. doi: 10.1186/s13071-021-04993-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ondeto BM, Wang X, Atieli H, Orondo PW, Ochwedo KO, Omondi CJ, et al. Malaria vector bionomics and transmission in irrigated and non-irrigated sites in western Kenya. Parasitology Res. 2022;121:3529–3545. doi: 10.1007/s00436-022-07678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Khalifa SM, Mustafan IO, Wais M, Malik EM. Malaria control in an urban area: a success story from Khartoum, 1995–2004. East Mediterr Health J. 2008;14:206–215. [PubMed] [Google Scholar]
- 46.Republic of Sudan Federal Ministry of Health and WHO Regional Office for Eastern Mediterranean, Sudan. Malaria Programme Review covering the period 2013–2017. Khartoum, Sudan, 2018.
- 47.Hakizimana D, Ntizimira C, Mbituyumuremyi A, Hakizimana E, Mahmoud H, Birindabagabo P, et al. The impact of Covid-19 on malaria services in three high endemic districts in Rwanda: a mixed-method study. Malar J. 2022;21:48. doi: 10.1186/s12936-022-04071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Global Fund to Fight AIDS, Malaria, Tuberculosis. The impact of Covid-19 on HIV, Tb and malaria services and systems for health: a snapshot from 502 health facilities. 2021. https://www.theglobalfund.org/media/10776/covid-19_2020-disruption-impact_report_en.pdf
- 49.Yared S, Gebressielasie A, Damodaran L, Bonnell V, Lopez K, Janies D, et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar J. 2020;19:180. doi: 10.1186/s12936-020-03252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tadesse FG, Ashine T, Teka H, Esayas E, Messenger LA, Chali W, et al. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg Infect Dis. 2021;27:603–607. doi: 10.3201/eid2702.200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar AS, Thavaselvam D. Breeding habitats and their contribution to Anopheles stephensi in Panaji. Indian J Malariol. 1992;29:35–40. [PubMed] [Google Scholar]
- 52.Mehravaran A, Vatandoost H, Oshaghi MA, Abai MR, Edalat H, Javadian E, et al. Ecology of Anopheles stephensi in a malarious area, southeast of Iran. Acta Med Iranica. 2012;50:61–65. [PubMed] [Google Scholar]
- 53.Hanafi-Bojd AA, Azari-Hamidian S, Hassan V, Zabihollah C. Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pacific J Trop Med. 2011;4:498–504. doi: 10.1016/S1995-7645(11)60134-X. [DOI] [PubMed] [Google Scholar]
- 54.Ministry of Health Yemen. National Malaria Control Programme. Entomological surveillance reports. 2022.
- 55.Balkew M, Mumba P, Dengela D, Yohannes G, Getachew D, Yared S, et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 2020;13:35. doi: 10.1186/s13071-020-3904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar J. 2021;20:263. doi: 10.1186/s12936-021-03801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Garadi M. Epidemiological review of dengue fever in Yemen. Int J Adv Res. 2015;3:1578–1584. [Google Scholar]
- 58.Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–24908. doi: 10.1073/pnas.2003976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO. Global vector control response 2017–2030. Geneva, World Health Organization, 2017. Available from: https://www.who.int/publications/i/item/9789241512978
- 60.WHO. Regional plan of action 2019–2023 for implementation of the global vector control response 2017–2030. World Health Organization. Regional Office for the Eastern Mediterranean; 2019. Available from: https://apps.who.int/iris/handle/10665/325805
- 61.Mnzava A, Monroe AC, Okumu F. Anopheles stephensi in Africa requires a more integrated response. Malar J. 2022;21:156. doi: 10.1186/s12936-022-04197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allan R, Budge S, Sauskojus H. What sounds like Aedes, acts like Aedes, but is not Aedes? Lessons from dengue virus control for the management of invasive Anopheles. Lancet Glob Health. 2023;11:e165–e169. doi: 10.1016/S2214-109X(22)00454-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data are included in the manuscript and from publicaly available sources.