Abstract
Ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate (HFPO-DA) is a short chain member of per- and polyfluoroalkyl substances (PFAS). To better understand the relevance of histopathological effects seen in livers of mice exposed to HFPO-DA for human health risk assessment, histopathological effects were summarized from hematoxylin and eosin (H&E)-stained sections in several repeat-dose toxicity studies in mice. Findings across studies revealed histopathological changes consistent with peroxisomal proliferation, whereas two reports of steatosis could not be confirmed in the published figures. In addition, mechanisms of hepatocellular death were assessed in H&E sections as well as with the apoptotic marker cleaved caspase-3 (CCasp3) in newly cut sections from archived liver blocks from select studies. A comparison of serially CCasp3 immunolabeled and H&E-stained sections revealed that mechanisms of hepatocellular death cannot be clearly discerned in H&E-stained liver sections alone as several examples of putatively necrotic cells were positive for CCasp3. Published whole genome transcriptomic data were also reevaluated for enrichment of various forms of hepatocellular death in response to HFPO-DA, which revealed enrichment of apoptosis and autophagy, but not ferroptosis, pyroptosis, or necroptosis. These morphological and molecular findings are consistent with transcriptomic evidence for peroxisome proliferator-activated receptor alpha (PPARα) signaling in HFPO-DA exposed mice.
Keywords: liver, liver pathology, GenX, HFPO-DA, per- and polyfluoroalkyl substances (PFAS), peroxisome proliferator
Introduction
Per- and polyfluoroalkyl substances (PFAS) are anthropogenic organic compounds that have been used for decades in a wide variety of consumer and industrial products. The same physical properties that make these chemistries useful for commercial and industrial applications also make them resistant to biodegradation. 1 Due to their persistence and detection in the environment, there has been increasing scrutiny of their safety. One of the primary sites of action of PFAS in rodent safety studies is the liver,2,3 where there is considerable evidence for peroxisome proliferator-activated receptor alpha (PPARα) activation in both molecular and histopathological responses.4-7 Considering the structural diversity of PFAS (e.g., carbon chain length, interchain linkages), the liver pathology induced by individual members of this class of chemicals deserves scrutiny. In a recent meta-analysis of PFAS-induced liver pathology, legacy PFAS such as perfluorooctanoate (PFOA) and perfluorooctane sulfonic acid (PFOS) were described in detail, whereas newer replacement PFAS such as ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate (HFPO-DA) were given less attention. 2 Therein, HFPO-DA was described as inducing steatosis in mice, although several well-conducted studies on HFPO-DA have not observed this effect. As such, a more thorough accounting of mouse liver histopathological changes in response to HFPO-DA is warranted.
Several recent risk assessments on PFAS, including HFPO-DA, 3 have developed candidate toxicity values based on liver effects in mice. Despite strong evidence for these effects being mediated by PPARα signaling pathways, the US Environmental Protection Agency (EPA) concluded that evidence for minimal hepatocellular single-cell necrosis left open the possibility that HFPO-DA operated through a PPARα-independent cytotoxic mode of action (MOA). 3 Differences in the diagnosis of necrotic and apoptotic cell death by different pathologists have led to differing interpretations of the likely form of cell death and, therefore, the likely MOA3,4,6 as necrotic cell death might indicate a cytotoxic MOA, whereas apoptotic death can be consistent with other evidence for a PPARα MOA. Herein, data for HFPO-DA are used as a case study to evaluate the use of liver histopathology in mice for human health risk assessment. First, mouse liver histopathology findings in several HFPO-DA studies are summarized to gain a more accurate characterization of the liver changes. Second, cleaved caspase-3 (CCasp3) immunolabeling is used to further characterize apoptotic changes visible in hematoxylin and eosin (H&E)-stained sections and better inform the mechanism(s) of hepatocellular death. Third, recently published transcriptomic analyses are reanalyzed to specifically investigate mechanisms of hepatocellular death. Finally, these data are integrated to inform the likely MOA for liver effects in mice, which is the first step in assessing the relevance of such effects for human health risk assessment.
Methods
Literature Review
A comprehensive literature search was conducted to identify all primary peer-reviewed publications that examined the histopathological effects of HFPO-DA exposure in the livers of experimentally exposed mice. An independent search was performed in PubMed on June 9, 2022, using the same PubMed database search strings used by the US EPA in their final toxicity assessment of HFPO-DA. 3 The titles and abstracts of all results were screened for relevancy. Unoriginal research articles (e.g., reviews) and those studies not investigating liver pathology end points in mice were excluded.
Caspase-3 Immunohistochemical Labeling
Histopathological changes in the parental mouse livers, from DuPont Organization for Economic Cooperation and Development (OECD) Test Guideline (TG) 421, 8 served as the basis for the safety values for HFPO-DA developed by the US EPA. 3 Notably, the DuPont OECD TG 421 8 study was conducted in compliance with US EPA Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA; 40 Code of Federal Regulations [CFR] Part 160) and Toxic Substances Control Act (TSCA; 40 CFR Part 792) Good Laboratory Practice (GLP) Standards, the OECD Principles of GLP, and WIL Research’s standard operating procedures (SOPs; for activities conducted at WIL Research). The animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) at WIL Research Laboratories, accredited by AAALAC International. For this study, paraffin blocks of liver tissue from 5 animals per group per sex were selected at random from DuPont OECD TG 421, 8 microtomed at 4 to 5 µm and each section was mounted on a glass slide. Immunohistochemical (IHC) labeling for activated caspase-3 was performed according to routine methods (Primary antibody: CCasp3, Rabbit monoclonal, Cell Signaling, No. 9664; Secondary: Goat Anti-Rabbit IgG Antibody (H+L), Biotinylated, Vector Labs, BA-1000-1.5; Chromogen: Betazoid diaminobenzidine (DAB) Chromogen Kit, BioCare Medical, BDB2004; and Counterstain: Hematoxylin-2, Richard-Allen Scientific, 7231). Positive controls consisted of immunolabeled sections of thymus and intestine. Negative controls consisted of immunolabeled sections of thymus and intestine in which Rabbit IgG was substituted for the Caspase-3 primary antibody.
For Caspase-3 scoring, a single section of liver was processed and evaluated in its entirety. The degree of orange-brown DAB oxidation was graded semiquantitatively according to the following scale: grade 1 = pale to intense cytoplasmic labeling of Kupffer cells and occasional histiocytic macrophages; grade 2 = grade 1, plus additional finely granular cytoplasmic labeling of low numbers of hepatocytes; grade 3 = grade 2, plus additional punctate labeling of hepatocytes, occasional apoptotic bodies, and rare hepatocyte nuclei; and grade 4 = grade 3, plus frequent staining of hepatocyte nuclei. The slides were scored by an American College of Veterinary Pathologists (ACVP) board-certified veterinary pathologist (JW). Negative and positive controls for Caspase-3 immunostaining are shown in Supplemental Figure S1.
Serial Staining for CCasp3 and H&E
In a separate analysis, the same paraffin blocks from DuPont OECD TG 421 8 were sectioned at 4 to 6 µm and mounted on a glass slide. Immunohistochemical labeling for activated caspase-3 was performed as above. Slides were scanned at 40X, using the Hamamatsu NanoZoomer S360. Subsequently, sections were destained by removing the cover glass, hydrating sections, and performing heat-induced epitope retrieval (HIER) in a citrate-based antigen unmasking solution, Vector Labs, H-3300-250, inside the BioCare Medical Decloaking Chamber NxGen. The same slides were then stained by H&E and again scanned at 40X. The scanned images were evaluated by an ACVP board-certified veterinary pathologist (JC).
Assessment of Hepatocellular Death Through Whole-Transcriptome Sequencing
Hepatic transcriptomic data for HFPO-DA from repeat-dose studies in mice, DuPont OECD TG 421 8 and DuPont OECD TG 408, 9 were analyzed and published in Chappell et al 4 and Heintz et al, 5 respectively. Data from these studies were reanalyzed to specifically assess various forms of regulated and unregulated hepatocellular death in response to HFPO-DA: apoptosis, autophagy, ferroptosis, necroptosis, pyroptosis, and cytotoxicity. Significant differentially expressed genes (i.e., differentially expressed genes with an adjusted P value < 0.1) and dose-responsive gene sets (i.e., gene sets with a Fisher exact two-tailed test <0.1) involved in the regulation of hepatocellular death were determined using the methods and parameters described in Chappell et al 4 and Heintz et al. 5
Results
Results of Literature Search
A total of seven relevant published articles were identified. Three studies were removed for the following reasons. Chappell et al 4 was excluded as it is based on results from a mouse study that is included separately in this analyses. Cope et al 10 was excluded because the study investigated the effects of low- and high-fat diets that might confound comparison with the other studies. Guo et al 11 was removed as no histopathological data were reported. In addition to the remaining four published articles, three OECD TG studies conducted by DuPont were evaluated, including an OECD TG 407 Repeated Dose 28-day Oral Toxicity Study in Rodents, 12 an OECD TG 408 Repeated Dose 90-day Oral Toxicity Study in Rodents, 9 and an OECD TG 421 Reproduction/Developmental Toxicity Screening Study. 8 These DuPont studies are publicly available in US EPA’s Health & Environmental Research Online (HERO) database (https://hero.epa.gov).
Liver Pathology in HFPO-DA Repeat-Dose Studies
Table 1 summarizes histopathological findings as reported in repeat-dose studies conducted in mice. Three studies did not report incidence data, making it difficult to assess the magnitude of responses. Only two studies reported steatosis, which were inconsistent with one another: Wang et al 7 reported steatosis in mice exposed to 1 mg/kg/day HFPO-DA for 28 days, whereas Guo et al 13 reported steatosis at 10 mg/kg/day but not 2 or 0.4 mg/kg/day after 28 days of exposure. Both studies contained figures specifically indicating steatosis; however, review of the images did not support a diagnosis of steatosis in mice treated with HFPO-DA, based on standards promulgated by the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND). 14 Moreover, these two studies did not mention the use of any additional staining or microscopy approaches to further support the presence of steatosis. The main findings that were consistent among the studies were hepatocellular hypertrophy (or hepatocellular cytoplasmic alteration), hepatocyte death of any type, increased mitoses, and pigment accumulation. Blake et al 15 also reported hepatocellular vacuolation and peroxisomes, using transmission electron microscopy.
Table 1.
Summary of liver histopathology in HFPO-DA repeat-dose studies in mice.
| Dupont OECD 407 12 | ||||||||
|---|---|---|---|---|---|---|---|---|
| 28-day gavage | Male Crl: CD1(ICR) | Female Crl: CD1(ICR) | ||||||
| Dose (mg/kg/day): | 0 | 0.1 | 3 | 30 | 0 | 0.1 | 3 | 30 |
| Hepatocellular hypertrophy | 0/10 | 0/10 | 10/10 | 10/10 | 0/10 | 0/10 | 10/10 | 10/10 |
| Necrosis, single cell | 0/10 | 0/10 | 4/10 | 10/10 | 0/10 | 0/10 | 0/10 | 4/10 |
| Mitotic figures | 0/10 | 0/10 | 0/10 | 9/10 | 0/10 | 0/10 | 0/10 | 5/10 |
| Dupont OECD 408 9 | ||||||||
| 90-day gavage | Male Crl: CD1(ICR) | Female Crl: CD1(ICR) | ||||||
| Dose (mg/kg/day): | 0 | 0.1 | 0.5 | 5 | 0 | 0.1 | 0.5 | 5 |
| Hepatocellular hypertrophy | 0/10 | 0/10 | 8/10 | 10/10 | 0/10 | 0/10 | 0/10 | 10/10 |
| Hepatocellular single cell Necrosis | 0/10 | 0/10 | 0/10 | 10/10 | 0/10 | 0/10 | 0/10 | 1/10 |
| Mitotic figures | 0/10 | 0/10 | 0/10 | 9/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Increased pigment Kupffer cells | 0/10 | 0/10 | 0/10 | 10/10 | 0/10 | 0/10 | 0/10 | 1/10 |
| Dupont OECD 421 8 | ||||||||
| ~60-88 days, gavage | Male Crl: CD1(ICR) | Female Crl: CD1(ICR) | ||||||
| Dose (mg/kg/day): | 0 | 0.1 | 0.5 | 5 | 0 | 0.1 | 0.5 | 5 |
| Hepatocellular hypertrophy | 0/25 | 0/24 | 12/24 | 24/24 | 0/24 | 0/22 | 14/24 | 24/24 |
| Necrosis, single cell | 1/25 | 1/24 | 5/24 | 24/24 | 1/24 | 3/22 | 2/24 | 21/24 |
| Mitotic figures | 0/25 | 0/24 | 0/24 | 18/24 | 0/24 | 0/22 | 0/24 | 5/24 |
| Increased pigment Kupffer cells | 0/25 | 0/24 | 0/24 | 21/24 | 0/24 | 0/22 | 0/24 | 5/24 |
| Focal necrosis | 0/25 | 0/24 | 1/24 | 1/24 | 1/24 | 0/22 | 3/24 | 5/24 |
| Blake et al 15 | ||||||||
| Embryonic days 1.15-E17.5, gavage | Maternal CD-1, ED11.5 | |||||||
| Dose (mg/kg/day): | 0 | 2 | 10 | |||||
| Cytoplasmic alteration | 0/5 | 5/5 | 5/5 | |||||
| Cell death (apoptosis and necrosis) | 0/5 | 4/5 | 3/5 | |||||
| Increased mitotic figures | 0/5 | 3/5 | 4/5 | |||||
| Vacuolation | 0/5 | 0/5 | 5/5 | |||||
| Focal regions of classic necrosis | 1/5 | 0/5 | 1/5 | |||||
| Maternal CD-1, ED17.5 | ||||||||
| Dose (mg/kg/day): | 0 | 2 | 10 | |||||
| Cytoplasmic alteration | 0/5 | 5/5 | 5/5 | |||||
| Cell death (apoptosis and necrosis) | 0/5 | 0/5 | 5/5 | |||||
| Increased mitotic figures a | 5/5 | 0/5 | 0/5 | |||||
| Vacuolation | 0/5 | 0/5 | 5/5 | |||||
| Focal regions of classic necrosis | 0/5 | 1/5 | 1/5 | |||||
| Wang et al 7 | ||||||||
| 28-day gavage | Male ICR (incidence not reported) | |||||||
| Dose (mg/kg/day): | 0 | 1 | ||||||
| Swollen hepatocytes | X | |||||||
| Swollen nucleus | X | |||||||
| Necrosis | X | |||||||
| Mild steatosis | X | |||||||
| Inclusion bodies | X | |||||||
| Karyolysis | X | |||||||
| Guo et al 13 | ||||||||
| 28-day gavage | Male Balb/c (incidence not reported) | |||||||
| Dose (mg/kg/day): | 0 | 0.4 | 2 | 10 | ||||
| Steatosis | X | |||||||
| Xu et al 16 | ||||||||
| GD0-parturition, gavage | Maternal Balb/c (incidence not reported) | |||||||
| Dose (mg/kg/day): | 0 | 2 | ||||||
| Hepatocyte hypertrophy | X | |||||||
| Disarrangement | X | |||||||
| Cytoplasmic loss | X | |||||||
| Nuclear migration | X | |||||||
| Acidophil bodies | X | |||||||
| Inflammatory cell infiltration | X | |||||||
Abbreviations: GD, gestational day; HFPO-DA, ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate; OECD, Organization for Economic Cooperation and Development.
May be a reporting error in Supplemental Table S7 of Blake et al.
The various studies employed different terminologies for cell death. For example, the studies conducted by DuPont used the term “single cell necrosis,” which, at the time of their conduct, did not distinguish among different forms of cell death such as apoptosis and necrosis. Blake et al 15 specifically noted that cell death referred to both apoptotic and necrotic cell death without distinction. Efforts to better characterize single-cell necrosis as apoptotic or necrotic cell death through light microscopy in routine H&E-stained sections have been proposed. 17 Broadly speaking, necrotic cells tend to exhibit “cell and nuclear swelling and pale cytoplasm,” whereas apoptotic cells tend to be “smaller, shrunken hypereosinophilic.” 17 However, Elmore et al 17 also describes injured hepatocytes surrounded by inflammatory cells as likely to represent individual necrotic hepatocytes, in part because necrotic cells, unlike apoptotic cells, are believed to induce inflammation. Complicating matters, inflammatory foci can also induce apoptosis in some nearby cells (so-called “bystander effect”).
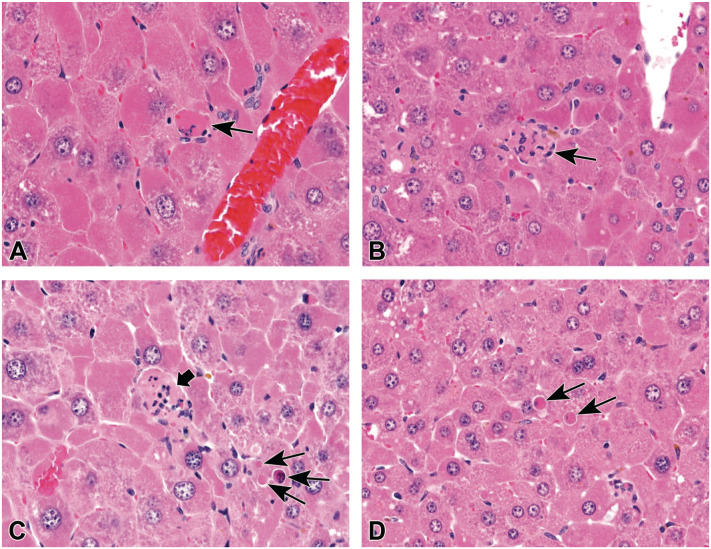
Depending on the criteria used, the form of cell death diagnosed in liver samples of mice exposed to HFPO-DA has differed. A review of DuPont OECD TG 408 9 revealed that the term “hepatocellular single-cell necrosis” was characterized as “. . . isolated eosinophilic bodies with occasional pyknotic nuclear fragments . . . thus was consistent with apoptosis.” Our review of histologic sections from DuPont OECD TG 408 9 and TG 421, 8 published in Thompson et al 6 and Chappell et al, 4 revealed very few, if any, cells exhibiting classic necrotic criteria and thus only apoptosis was diagnosed. In contrast, a reanalysis of H&E liver slides from DuPont OECD TG 408 9 and TG 421 8 conducted on behalf of the US EPA 3 concluded the presence of both apoptosis and necrosis (Table 2). Figure 1A-C contains examples that were considered necrotic cells. Figure 1A was considered necrotic despite being neither pale nor swollen; in fact, this could be an apoptotic cell based on some examples published in Elmore et al. 17 The necrotic cells in Figure 1B and C are inferred as such based on the presence of inflammatory cells. As suggested in Elmore et al, 17 additional techniques such as staining for activated/CCasp3 are needed to definitively distinguish necrosis and apoptosis.
Table 2.
Reevaluation of liver histopathology as reported in US EPA (2021).
| DuPont OECD 408 9 | ||||||||
|---|---|---|---|---|---|---|---|---|
| 90-day gavage | Male Crl: CD1(ICR) | Female Crl: CD1(ICR) | ||||||
| Dose (mg/kg/day): | 0 | 0.1 | 0.5 | 5 | 0 | 0.1 | 0.5 | 5 |
| Mixed cell infiltrate | 6/10 | 6/10 | 4/10 | 6/10 | 5/10 | 3/10 | 3/10 | 7/10 |
| Hepatocellular single cell necrosis a | 0/10 | 1/10 | 0/10 | 9/10 | 0/10 | 0/10 | 0/10 | 3/10 |
| Cytoplasmic alteration | 0/10 | 0/10 | 10/10 | 10/10 | 0/10 | 0/10 | 0/10 | 10/10 |
| Focal necrosis | 0/10 | 0/10 | 0/10 | 1/10 | 1/10 | 0/10 | 2/10 | 4/10 |
| Cytoplasmic vacuolization | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Extramedullary hematopoiesis | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Increased pigment | 0/10 | 0/10 | 0/10 | 10/10 | 0/10 | 0/10 | 0/10 | 4/10 |
| Hepatocellular apoptosis b | 0/10 | 0/10 | 0/10 | 10/10 | 0/10 | 0/10 | 0/10 | 3/10 |
| Increased mitotic figures | 0/10 | 0/10 | 0/10 | 7/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Bile duct hyperplasia | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| DuPont OECD 421 8 | ||||||||
| ~60-88 days, gavage | Male Crl: CD1(ICR) | Female Crl: CD1(ICR) | ||||||
| Dose (mg/kg/day): | 0 | 0.1 | 0.5 | 5 | 0 | 0.1 | 0.5 | 5 |
| Mixed cell infiltrate | 6/25 | 3/25 | 11/25 | 8/25 | 12/25 | 7/25 | 17/25 | 15/25 |
| Hepatocellular single cell necrosis a | 1/25 | 1/25 | 2/25 | 24/25 | 0/25 | 2/25 | 3/25 | 19/25 |
| Cytoplasmic alteration | 0/25 | 0/25 | 10/25 | 25/25 | 0/25 | 1/25 | 16/25 | 25/25 |
| Focal necrosis | 0/25 | 0/25 | 4/25 | 3/25 | 2/25 | 2/25 | 4/25 | 5/25 |
| Cytoplasmic vacuolization | 0/25 | 0/25 | 3/25 | 0/25 | 0/25 | 0/25 | 0/25 | 1/25 |
| Extramedullary hematopoiesis | 0/25 | 0/25 | 1/25 | 2/25 | 0/25 | 0/25 | 0/25 | 1/25 |
| Increased pigment | 0/25 | 0/25 | 0/25 | 21/25 | 0/25 | 0/25 | 0/25 | 3/25 |
| Hepatocellular apoptosis b | 0/25 | 0/25 | 0/25 | 22/25 | 0/25 | 0/25 | 0/25 | 10/25 |
| Increased mitotic figures | 0/25 | 0/25 | 0/25 | 17/25 | 0/25 | 0/25 | 0/25 | 2/25 |
| Oval cell hyperplasia | 0/25 | 0/25 | 0/25 | 4/25 | 0/25 | 0/25 | 0/25 | 0/25 |
| Inflammation granulomatous | 0/25 | 0/25 | 0/25 | 0/25 | 0/25 | 0/25 | 1/25 | 0/25 |
| Polyarteritis nodosa | 0/25 | 0/25 | 0/25 | 0/25 | 0/25 | 0/25 | 0/25 | 1/25 |
Abbreviations: EPA, Environmental Protection Agency; OECD, Organization for Economic Cooperation and Development.
All lesions were considered minimal severity (1-10 cells in ten 20X fields; US EPA 3 pp. D-5)
All lesions were considered minimal to mild in severity (1-10 cells or 11-40 cells in ten 20X fields; US EPA 3 pp. D-6).
Figure 1.
Examples of liver apoptotic and necrotic cell death in HFPO-DA exposed mice. (A) Necrotic cell (arrow). (B) Necrotic cell surrounded by inflammatory cells (arrow). (C) Necrotic cell (thick arrow) near apoptotic cells (thin arrows). (D) Apoptotic cells (arrows). Source: US EPA. 3 EPA, Environmental Protection Agency; HFPO-DA, ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate.
Cleaved Caspase-3 IHC Labeling for Assessing Apoptosis and Necrosis
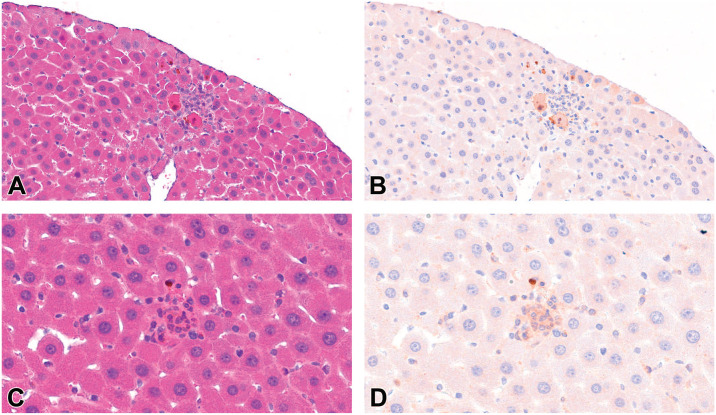
To assess the histologic characteristics and presence of apoptotic cell death, CCasp3 immunoreactivity was evaluated on newly cut liver sections from control and treated mice from DuPont OECD TG 421. 8 As shown in Table 3, CCasp3 immunoreactivity was elevated in liver sections at ≥0.5 mg/kg/day in both male in female mice. The staining was similar to that in our previous study in mice exposed to the same doses of HFPO-DA for a similar period of time. 4 Based on our review of H&E-stained mouse liver sections, the form of hepatocyte death appeared be limited to apoptosis. However, given the possible presence of nonclassical and/or inflammatory associated necrotic cells, such as those shown in Figure 1A-C, fresh liver sections cut from fixed tissue blocks of mice exposed to HFPO-DA were immunolabeled for CCasp3, scanned, stripped, and re-stained with H&E, and again scanned. Figure 2A is an H&E-stained section showing two cells that share some features with the necrotic cells depicted in Figure 1A; however, these cells clearly stain positive for CCasp3 (Figure 2B). Similarly, Figure 2C is an H&E-stained section showing a small region of inflammatory cells that might be diagnosed as necrotic based on the examples in Figure 1B and C; however, any cell that these inflammatory foci may be associated with appears to stain positive for CCasp3 (Figure 2D). This staining was considered specific to activated/CCasp3 as evidenced by the absence of similar staining in some inflammatory foci that might have been considered necrotic (Supplemental Figure S1). Taken together, these observations call into question the actual mechanism of hepatocyte death and the uncertainty of using H&E morphologic criteria alone to generate diagnoses of apoptosis versus necrosis.
Table 3.
Median score for CCasp3 immunoreactivity in mouse liver.
| DuPont OECD 421 8 | ||
|---|---|---|
| HFPO-DA (mg/kg/day) | Male | Female |
| 0 | 1 | 1 |
| 0.1 | 2 | 1 |
| 0.5 | 2 | 2 |
| 5 | 3 | 3 |
n = 5 mice per group/sex.
Abbreviations: CCasp3, cleaved caspase-3; HFPO-DA, ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate; OECD, Organization for Economic Cooperation and Development.
Figure 2.
Liver sections stained for CCasp3, stripped, and stained with H&E from a mouse treated with 5 mg/kg HFPO-DA. (A) Two possibly necrotic hepatocytes based on features in Figure 1A (H&E stain). (B) Same section stained for CCasp3. (C) A putative necrotic hepatocyte surrounded by inflammatory cells based on examples in Figure 1B-C (H&E stain). (D) Same section stripped and stained for CCasp3. Source: Mouse 5017 from DuPont OECD 421. 8 CCasp3, cleaved caspase-3; H&E, hematoxylin and eosin; HFPO-DA, ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate.
Assessment of Hepatocellular Death Through Whole Transcriptome Sequencing
Whole transcriptome responses to HFPO-DA have recently been published from two multi-dose toxicity studies in mice. Specifically, newly cut liver sections from archived formalin-fixed paraffin-embedded liver blocks from DuPont OECD TG 408 9 and TG 421 8 were analyzed and published in Chappell et al 4 and Heintz et al, 5 respectively. Both studies revealed evidence for PPARα signaling, apoptotic cell death, and cell proliferation, which were all consistent with the histopathologic results. To further assess the type(s) of hepatocellular death in the mouse liver following exposure to HFPO-DA, five forms of regulated hepatocellular death with known gene sets: apoptosis, autophagy, ferroptosis, necroptosis, and pyroptosis were evaluated using dose-response analysis. Table 4 shows the number of significantly enriched dose-responsive gene sets in the REACTOME pathway and Gene Ontology (GO) term databases for each form of regulated cell death. Apoptosis and autophagy were significantly enriched in both studies, whereas ferroptosis and pyroptosis were not enriched. Necroptosis also showed enrichment albeit for negative regulation of necroptosis. These data suggest that apoptosis and autophagy are the main forms of regulated cell death in these tissues.
Table 4.
Comparison of the number of significantly enriched dose-responsive gene sets for forms of regulated hepatocellular death in HFPO-DA-exposed mice.
| Regulated forms of cell death | Sex | DuPont OECD 408 9 (Chappell et al 4 ) | DuPont OECD 421 8 (Heintz et al 5 ) | ||
|---|---|---|---|---|---|
| Number of significantly enriched dose-responsive gene sets | Number of significantly enriched dose-responsive gene sets | ||||
| REACTOME | GO a | REACTOME | GO a | ||
| Apoptosis | Female | 0 | 12 | 2 | 21 |
| Male | 0 | 20 | 3 | 15 | |
| Autophagy | Female | 0 | 2 | 3 | 3 |
| Male | 0 | 0 | 0 | 2 | |
| Necroptosis | Female | 0 | 1 | 0 | 1 |
| Male | 0 | 2 | 0 | 2 | |
| Pyroptosis | Female | 0 | 0 | 0 | 0 |
| Male | 0 | 0 | 0 | 0 | |
| Ferroptosis b | Female | Not available | 0 | Not available | 0 |
| Male | Not available | 0 | Not available | 0 | |
Abreviations: GO, Gene Ontology; HFPO-DA, ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate; OECD, Organization for Economic Cooperation and Development.
Significant Gene Ontology(GO) terms presented are from level 7 of the GO term hierarchy.
Ferroptosis is not included in the REACTOME pathway database. Therefore, gene sets related to ferroptosis were not evaluated in dose-response analyses because BMDExpress software only includes REACTOME gene set collections. However, a gene set for ferroptosis is included in the WikiPathways database. Gene set enrichment analysis results from both Chappell et al 4 and Heintz et al 5 showed no significant enrichment of ferroptosis gene sets.
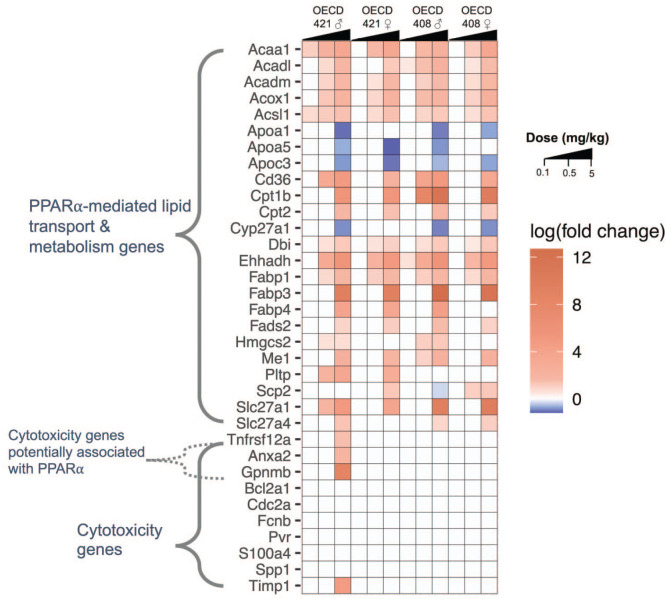
Although there are no gene sets for assessing necrosis in transcriptomic databases, a gene expression signature indicative of liver cytotoxicity has recently been developed from short-term toxicity studies in rats. 18 This cytotoxicity gene set has been applied by Corton et al 19 to identify rat carcinogens in short-term assays. Using the transcriptomic data from Chappell et al 4 and Heintz et al, 5 the expression of the 10 genes in this cytotoxicity gene set were compared with expression of PPARα-mediated genes in livers of mice exposed to HFPO-DA. The PPARα-regulated genes were induced at dose levels as low as 0.1 mg/kg/day HFPO-DA, whereas the genes associated/predictive of hepatic cytotoxicity were not significantly differentially expressed in male or female dose groups from either study, with the exception of four genes (Anxa2, Gpnmb, Timp1, and Tnfrsf12a) at 5 mg/kg/day in parental male mice from DuPont OECD TG 421 8 (Figure 3). The role(s) of these four genes are not fully known and some of the genes are likely altered downstream of PPARα activation (see “Discussion” section).
Figure 3.
Heatmap of hepatic gene expression of selected genes included in PPARα-mediated lipid transport and metabolism (KEGG PPAR Signaling Pathway) or cytotoxicity gene sets.18,19 Significant (FDR < 0.1) differentially expressed genes (rows) are indicated by warmer (i.e., red, upregulated genes) or cooler (i.e., blue, downregulated genes) colors. Intensity of colors is based on the log (fold change) value of each gene for each study, sex, and dose group. White cells indicate that gene was not significantly altered by HFPO-DA exposure compared with respective controls. Transcriptomic data included in this heatmap are from the DuPont OECD 408 9 and 421 8 studies. The HFPO-DA dose levels are indicated by right triangles at the top of the heatmap for each study, increasing from 0.1 to 5 mg/kg for each study and sex. Methods for transcriptomic analyses are described in Chappell et al 4 and Heintz et al. 5 FDR, false discovery rate; HFPO-DA, ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate; OECD, Organization for Economic Cooperation and Development; PPARα, peroxisome proliferator-activated receptor alpha.
Discussion
Several studies have reported liver effects in mice following oral exposure to HFPO-DA with varying levels of detail. Consistent findings among the studies were hepatocellular hypertrophy, hepatocyte death of various types, and increased mitoses. Only two studies reported liver steatosis; however, such effects were not evident in the published figures from those studies, nor did these studies employ staining or microscopy techniques to further support the presence of steatosis. Transcriptomic analyses indicate that HFPO-DA increases PPARα signaling in the mouse liver.4,5,7 It is important to accurately characterize the liver histopathology in HFPO-DA treated mice as claims of steatosis are already making their way into review articles, 2 risk assessments, 3 and discussion sections in primary research articles. 20
Consistent with PPARα activation, hepatocellular hypertrophy was evident in most of the studies (Table 1), and absolute and relative liver weights were increased 2-fold at 5 mg/kg/day (Supplemental Figure S2). This cellular hypertrophy leads to increased liver size and possibly focal necrosis due to compression against the capsule or adjacent organs that can cause focal hypoxia and cell death as the blood supply of the liver is limited just below the capsule. 14 As such, some of the diagnoses of focal necrosis in HFPO-DA treated mice can be secondary to hypertrophy, especially in the subcapsular region (see Figure 7 in Appendix D of US EPA) 3 .
The distinction between various forms of hepatocellular death may not be critical for routine histopathological evaluations but can be important in the context of human health risk assessment. Two independent reevaluations of “single cell necrosis” reported in the Dupont OECD TG 421 8 and DuPont OECD TG 408 9 studies, using more recent diagnostic criteria described in Elmore et al, 17 reached different conclusions regarding the presence of necrotic cell death in H&E-stained sections.3,4,6 One explanation for the different conclusions is that one group considered dying cells or debris surrounded by inflammatory cells as necrotic hepatocytes, whereas the other group focused on classic indicators of necrotic cells (i.e., swollen cytoplasm and swollen nuclei). The CCasp3 immunolabeling herein indicates that at least some putative necrotic hepatocytes appear, in fact, to be apoptotic. Apoptosis was supported by whole genome transcriptomic analyses along with evidence for autophagy. Other forms of regulated hepatocellular death (e.g., necroptosis) were not active. It should be recognized that increased apoptotic signaling has been noted in rodent livers following exposure to PPARα activators in a PPARα-dependent fashion, presumably as negative feedback for increased growth signaling.21,22
Although gene signatures for necrosis are not available in most curated databases, gene signatures for liver cytotoxicity have recently been proposed (note: the majority of liver tissue from which these signatures were derived were characterized as exhibiting degeneration/necrosis, with some exhibiting single cell necrosis).18,19 As shown in Figure 3, four of these liver cytotoxicity genes were activated in the highest dose group in only one of the four OECD TG studies. Among the four altered genes (Anxa2, Gpnmb, Timp1, and Tnfrsf12a), some might also be directly related to PPARα activation. For example, glycoprotein nonmetastatic melanoma protein B (Gpnmb) appears to be a hepatokine that is secreted by the liver to promote lipogenesis in white adipose tissue 23 and therefore might be related to PPARα-induced changes in lipid metabolism. Tumor necrosis factor receptor (Tnfrsf12a) codes for Fn14 that binds to tumor necrosis factor-like weak inducer of apoptosis (TWEAK), 24 which is potentially related to increased apoptosis present at 5 mg/kg/day; however, apoptosis was also observed at 5 mg/kg/day in other mice exposed to HFPO-DA that did not exhibit an increase in this gene. Anxa2 and Timp1 have been associated with fibrosis;26,25 however, no histopathological evidence of fibrosis has been observed in livers from mice exposed to HFPO-DA (Table 1). Increased Anxa2 expression has been observed in wild type but not humanized-PPARα mice treated with the PPARα-specific ligand WY-14,643, 27 suggesting mouse PPARα-mediated gene expression of Anxa2. If there is some cytotoxicity in the livers of mice exposed to 5 mg/kg/day HFPO-DA, it is likely secondary to the PPARα-related gene changes that are clearly shown in Figure 3 and described in detail elswhere.4,5,7
Despite the limited support for necrotic cell death, exposure to HFPO-DA has been associated with increases in serum liver enzymes, such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALKP), total bile acids, and others (Supplemental Figure S3). Notably, the classic PPARα activator WY-14,643 has been shown to increase serum ALT, ALKP, AST, and bile acids in mice several fold (indicative of cholestasis), and such effects are mitigated in mice null for the PPARα heterodimer partner retinoid X receptor α (RXRα)—indirectly indicating dependency on PPARα. 28 Increased bile acids was shown to be due to repression of the Na+-taurocholate cotransporting polypeptide (Ntcp) and the organic anion transporting polypeptide 1a1 (Oatp1a1) bile acid transporters in wild type mice fed WY-14,643. Notably, these genes are also repressed in mice exposed to HFPO-DA (Supplemental Figure S3), suggesting similar PPAR mediatedα- mediated responses between HFPO-DA and WY-14,643. These data indicate that changes in serum liver enzymes may not be a consequence of necrotic cell death, but rather a direct consequence of PPAR mediatedα- mediated gene expression changes. 29 Importantly, evidence suggests that PPARα-related induction of cholestasis in rodents does not occur in humans and nonhuman primates. 30 Additional in vivo studies in PPARα-null mice could provide important insight into the role and specificity of PPARα in the liver and serum effects observed in mice following exposure to HFPO-DA.
An important finding from the analyses herein is that mechanisms of hepatocellular death cannot be clearly discerned in H&E-stained liver sections alone, as multiple forms of putatively necrotic cells stain positive for CCasp3, indicating apoptotic cell death. As such, immunolabeling for activated/CCasp3 provides additional information, but other analyses may also be needed to inform the MOA of chemical toxicity in the liver. In this regard, multiple transcriptomic analyses have demonstrated PPARα signaling in mouse liver tissue following exposure to HFPO-DA as well as evidence for apoptotic and mitotic signaling.4,5,7 Importantly, transcriptomic analyses also allow for the assessment of various forms of hepatocellular death. Among regulated cell death mechanisms, only apoptosis and autophagy were evident, and both have been linked to PPARα signaling. Putative gene signatures for cytotoxicity/necrosis were minimally activated and only at the highest dose in one of four data sets. Overall, the data herein do not support a cytotoxic MOA in the livers of mice exposed to HFPO-DA. The extent to which these observations apply to other PFAS is beyond the scope of this review, but the case study herein provides a path forward for how liver changes induced by other chemistries might be evaluated.
Supplemental Material
Supplemental material, sj-tif-1-tpx-10.1177_01926233231159078 for Assessment of Mouse Liver Histopathology Following Exposure to HFPO-DA With Emphasis on Understanding Mechanisms of Hepatocellular Death by Chad M. Thompson, Melissa M. Heintz, Jeffrey C. Wolf, Roza Cheru, Laurie C. Haws and John M. Cullen in Toxicologic Pathology
Supplemental material, sj-tif-2-tpx-10.1177_01926233231159078 for Assessment of Mouse Liver Histopathology Following Exposure to HFPO-DA With Emphasis on Understanding Mechanisms of Hepatocellular Death by Chad M. Thompson, Melissa M. Heintz, Jeffrey C. Wolf, Roza Cheru, Laurie C. Haws and John M. Cullen in Toxicologic Pathology
Supplemental material, sj-tif-3-tpx-10.1177_01926233231159078 for Assessment of Mouse Liver Histopathology Following Exposure to HFPO-DA With Emphasis on Understanding Mechanisms of Hepatocellular Death by Chad M. Thompson, Melissa M. Heintz, Jeffrey C. Wolf, Roza Cheru, Laurie C. Haws and John M. Cullen in Toxicologic Pathology
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors include employees of ToxStrategies, LLC, a private consulting firm that provides services to private and public organizations for toxicology, epidemiology, and risk assessment issues. Authors also include scientists from Experimental Pathology Laboratories, a private contract research organization. Author JC served as an independent consultant. The work reported in this article was conducted during the normal course of employment. The authors (CT and LH) have presented study findings in meetings with regulators, including public meetings, on behalf of the Chemours Company FC, LLC.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Chemours Company FC, LLC. Chemours was given the opportunity to review the draft manuscript. The purpose of this review was for the authors to receive input on the clarity of the science presented but not on the interpretation of research results. The authors’ scientific conclusions and professional judgments were not subject to the funder’s control; the contents of this manuscript solely reflect the view of the authors.
ORCID iDs: Chad M. Thompson  https://orcid.org/0000-0002-2265-7420
https://orcid.org/0000-0002-2265-7420
Jeffrey C. Wolf  https://orcid.org/0000-0002-1901-0634
https://orcid.org/0000-0002-1901-0634
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J.Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366-394. [DOI] [PubMed] [Google Scholar]
- 2.Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect. 2022;130(4):46001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.USEPA. Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN 62037-80-3) Also Known as “GenX Chemicals.” Washington, DC: US Environmental Protection Agency Office of Water; 2021; EPA Document Number: 822R-21-010. [Google Scholar]
- 4.Chappell GA, Thompson CM, Wolf JC, Cullen JM, Klaunig JE, Haws LC.Assessment of the mode of action underlying the effects of GenX in mouse liver and implications for assessing human health risks. Toxicol Pathol. 2020;48(3):494-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heintz MM, Chappell GA, Thompson CM, Haws LC.Evaluation of transcriptomic responses in livers of mice exposed to the short-chain PFAS compound HFPO-DA. Front Toxicol. 2022;4:937168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson CM, Fitch SE, Ring C, Rish W, Cullen JM, Haws LC.Development of an oral reference dose for the perfluorinated compound GenX. J Appl Toxicol. 2019;39(9):1267-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Wang X, Sheng N, Zhou X, Cui R, Zhang H, et al. RNA-sequencing analysis reveals the hepatotoxic mechanism of perfluoroalkyl alternatives, HFPO2 and HFPO4, following exposure in mice. J Appl Toxicol. 2017;37(4):436-444. [DOI] [PubMed] [Google Scholar]
- 8.DuPont. An Oral (Gavage) Reproduction/Developmental Toxicity Screening Study of H-28548 in Mice (DuPont) [HERO ID: 4222148]. Ashland, OH: WIL Research Laboratories, LLC; 2010. [Google Scholar]
- 9.DuPont. H-28548: Subchronic Toxicity 90-day Gavage Study in Mice (DuPont) [HERO ID: 4229663]. Newark, DE: EI du Pont de Nemours and Company DuPont Haskell Global Centers for Health & Environmental Sciences; 2010. [Google Scholar]
- 10.Cope HA, Blake BE, Love C, McCord J, Elmore SA, Harvey JB, et al. Latent, sex-specific metabolic health effects in CD-1 mouse offspring exposed to PFOA or HFPO-DA (GenX) during gestation. Emerg Contam. 2021;7:219-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo H, Chen J, Zhang H, et al. Exposure to GenX and its novel analogs disrupts hepatic bile acid metabolism in male mice. Environ Sci Technol. 2022;56(10):6133-6143. [DOI] [PubMed] [Google Scholar]
- 12.DuPont. A 28-Day Oral (Gavage) Toxicity Study of H-28397 in Mice with a 28-Day Recovery (DuPont) [HERO ID: 4221053]. Ashland, OH: WIL Research Laboratories, LLC; 2008. [Google Scholar]
- 13.Guo H, Sheng N, Guo Y, Wu C, Xie W, Dai J.Exposure to GenX and its novel analogs disrupts fatty acid metabolism in male mice. Environ Pollut. 2021;291:118202. [DOI] [PubMed] [Google Scholar]
- 14.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38(7 Suppl.):5S-81S. [DOI] [PubMed] [Google Scholar]
- 15.Blake BE, Cope HA, Hall SM, et al. Evaluation of maternal, embryo, and placental effects in CD-1 mice following gestational exposure to perfluorooctanoic acid (PFOA) or hexafluoropropylene oxide dimer acid (HFPO-DA or GenX). Environ Health Perspect. 2020;128(2):27006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu LL, Chen YK, Zhang QY, Chen LJ, Zhang KK, Li JH, Liu JL, Wang Q, Xie XL. 2022. Gestational exposure to genx induces hepatic alterations by the gut-liver axis in maternal mice: A similar mechanism as pfoa. Sci Total Environ. 820:153281. [DOI] [PubMed] [Google Scholar]
- 17.Elmore SA, Dixon D, Hailey JR, Harada T, Herbert RA, Maronpot RR, et al. Recommendations from the INHAND apoptosis/necrosis working group. Toxicol Pathol. 2016;44(2):173-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaab WE, Holder D, He YD, et al. Universal toxicity gene signatures for early identification of drug-induced tissue injuries in rats. Toxicol Sci. 2021;181(2):148-159. [DOI] [PubMed] [Google Scholar]
- 19.Corton JC, Hill T, Sutherland JJ, Stevens JL, Rooney J.A set of six gene expression biomarkers identify rat liver tumorigens in short-term assays. Toxicol Sci. 2020;177(1):11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marques E, Pfohl M, Wei W, et al. Replacement per- and polyfluoroalkyl substances (PFAS) are potent modulators of lipogenic and drug metabolizing gene expression signatures in primary human hepatocytes. Toxicol Appl Pharmacol. 2022;442:115991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsman DS, Goldsworthy TL, Popp JA.Contrasting hepatocytic peroxisome proliferation, lipofuscin accumulation and cell turnover for the hepatocarcinogens Wy-14,643 and clofibric acid. Carcinogenesis. 1992;13(6):1011-1017. [DOI] [PubMed] [Google Scholar]
- 22.Xiao S, Anderson SP, Swanson C, Bahnemann R, Voss KA, Stauber AJ, et al. Activation of peroxisome proliferator-activated receptor alpha enhances apoptosis in the mouse liver. Toxicol Sci. 2006;92(2):368-377. [DOI] [PubMed] [Google Scholar]
- 23.Gong XM, Li YF, Luo J, Wang JQ, Wei J, Wang JQ, et al. Gpnmb secreted from liver promotes lipogenesis in white adipose tissue and aggravates obesity and insulin resistance. Nat Metab. 2019;1(5):570-583. [DOI] [PubMed] [Google Scholar]
- 24.Ratajczak W, Atkinson SD, Kelly C.The TWEAK/Fn14/CD163 axis-implications for metabolic disease. Rev Endocr Metab Disord. 2022;23(3):449-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zisser A, Ipsen DH, Tveden-Nyborg P.Hepatic stellate cell activation and inactivation in NASH-fibrosis-roles as putative treatment targets? Biomedicines. 2021;9(4):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang G, Duan J, Pu G, Ye C, Li Y, Xiu W, et al. 2022. The annexin a2-notch regulatory loop in hepatocytes promotes liver fibrosis in nafld by increasing osteopontin expression. Biochim Biophys Acta Mol Basis Dis. 1868(8):166413. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ.The PPAR alpha-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicol Sci. 2008;101(1):132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyamfi MA, Wan YJ.Mechanisms of resistance of hepatocyte retinoid X receptor alpha-null mice to WY-14,643-induced hepatocyte proliferation and cholestasis. J Biol Chem. 2009;284(14):9321-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng X, Maher J, Dieter MZ, Klaassen CD.Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33(9):1276-1282. [DOI] [PubMed] [Google Scholar]
- 30.Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, et al. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33(6):655-780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-tpx-10.1177_01926233231159078 for Assessment of Mouse Liver Histopathology Following Exposure to HFPO-DA With Emphasis on Understanding Mechanisms of Hepatocellular Death by Chad M. Thompson, Melissa M. Heintz, Jeffrey C. Wolf, Roza Cheru, Laurie C. Haws and John M. Cullen in Toxicologic Pathology
Supplemental material, sj-tif-2-tpx-10.1177_01926233231159078 for Assessment of Mouse Liver Histopathology Following Exposure to HFPO-DA With Emphasis on Understanding Mechanisms of Hepatocellular Death by Chad M. Thompson, Melissa M. Heintz, Jeffrey C. Wolf, Roza Cheru, Laurie C. Haws and John M. Cullen in Toxicologic Pathology
Supplemental material, sj-tif-3-tpx-10.1177_01926233231159078 for Assessment of Mouse Liver Histopathology Following Exposure to HFPO-DA With Emphasis on Understanding Mechanisms of Hepatocellular Death by Chad M. Thompson, Melissa M. Heintz, Jeffrey C. Wolf, Roza Cheru, Laurie C. Haws and John M. Cullen in Toxicologic Pathology