Abstract
Coronavirus Disease 2019 (COVID-19) was first identified in Wuhan province in China in late 2019. Around 15% of patients that develop severe acute respiratory syndrome from COVID-19 also develop severe COVID-19 pneumonia. Since the pandemic’s start, various treatments including remdesivir, dexamethasone, baricitinib, convalescent plasma, and tocilizumab have been approved by the Center for Disease Control (CDC). We present a case of a 62-year-old male hospitalized due to COVID-19 pneumonia and was initially treated with methylprednisolone and remdesivir, and later with tocilizumab. Soon after, he developed an abdominal perforation which was surgically treated. In terms of abdominal perforation, proposed mechanisms including the pathogenesis due to the presence of specific angiotensin-converting enzyme 2 (ACE-2) receptors located throughout the gastrointestinal tract, glucocorticoid steroid inflammatory suppression, in addition to the documented adverse effects from tocilizumab which has been previously reported. In summary, tocilizumab may increase the risk of abdominal perforation, especially when used in combination with steroids to treat COVID-19 because steroids may suppress clinical exam findings for abdominal perforation.
Keywords: COVID, tocilizumab use, GI perforation, immunosuppressive effects, masked presentation
Introduction
Studies examining SARS-CoV-2 shedding using serial respiratory sampling have found that peak viral load occurs within the first week of symptom onset; this increased viral replication leads to a rapid increase in proinflammatory cytokines, acute respiratory distress syndrome (ARDS), and organ failure.1, 2
IL-6 is a chemokine responsible for the elevation of inflammatory markers/acute phase reactants; inhibition of IL-6 production has been linked with autoimmunity and chronic inflammation. Elevated IL-6 levels in COVID-19 positive patients have been thought to be associated with the severe cytokine storm and inflammation during symptom onset. 3 Elevated levels of IL-6 also lead to endothelial dysfunction and vascular permeability; thus, the vascular dysfunction noted in COVID-19 might be related to IL-6 levels.
IL-6 is an important marker of inflammation in the body and has acted as an important predictive tool for COVID-19 severity. The importance of this IL-6 marker also suggests the potential treatment of COVID-19 through monoclonal antibodies targeting the IL-6 receptor. A drug of recent interest has been tocilizumab, a monoclonal antibody/antagonist that binds to the membrane and soluble forms of the interleukin (IL-6) receptor in the body. Tocilizumab has shown efficacy in treating diseases such as rheumatoid arthritis, cytokine release syndrome, and Castleman’s disease. 3 However, Rosas et al found that using tocilizumab did not significantly improve clinical status or lower mortality in COVID+ patients after a month.
Case Report
We present a 62-year-old male admitted with a past medical history of hypertension, glaucoma, ADHD. He came to the emergency room with concern for COVID-19. He reported that over the past 4 to 6 days before hospitalization, he felt increasingly worse with dyspnea, dry cough, fatigue, and intermittent fevers and chills. He also stated that he had decreased appetite and intake with associated loose bowel movements 2 to 3 times a day over the 4-day timeframe. He complained of body aches as well as pain in his chest when coughing. Denies any nausea, vomiting, headache, dysuria, hematuria, or flank pain.
In the ER, the patient was found to have a 4 L nasal cannula oxygen requirement to maintain saturations greater than 90%. A chest X-ray was obtained on admission and demonstrated evidence of multifocal infiltrates consistent with viral pneumonia. He was noted to have mild elevation in transaminases, and his CRP was 137. He was started on steroids with weight-based methylprednisolone 1 mg/kg every 12 hours and Remdesivir. Infectious Disease was consulted for help with the management of his case.
During the first week of hospitalization, mild transaminitis likely secondary to the virus in etiology was noted in addition to elevation of IL-6 (44.4 pg/mL). He aspirated, and oxygen requirements increased to 6 L. He continued to develop fatigue, body aches, and dyspnea which had become very winded with any movement. The initiation of 1 dose of tocilizumab was recommended.
Two days, it was noted that the patient had developed bilateral lower abdominal fullness and subcutaneous emphysema, with normal bowel function, without nausea or vomiting over the past 48-hours. A CT of the abdomen and pelvis with contrast showed diffuse colopathy of the sigmoid colon with a large amount of extraluminal air and fluid in the left retroperitoneum, tracking through the left perirenal space into the left upper quadrant abdomen as seen in Figure 1. While bowel perforations are considered intraperitoneal in nature, our patient developed subcutaneous emphysema secondary to a bowel perforation, explaining the retroperitoneal findings seen in Figure 1. There is also a 5.4 cm extraperitoneal abscess at the dome of the bladder. He then underwent CT-guided drainage of the pelvic abscess.
Figure 1.
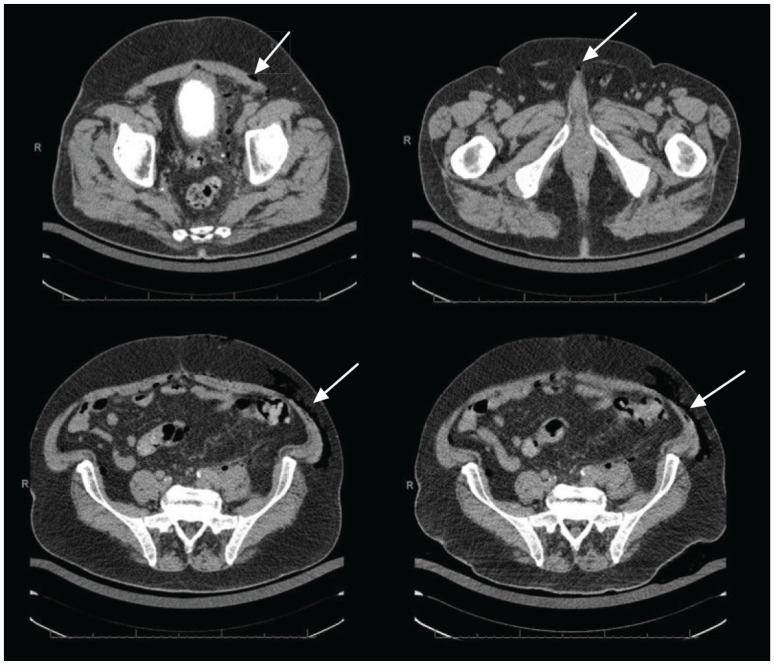
Axial CT of the abdomen and pelvis. Diffuse colonopathy of the sigmoid colon with large amount of extraluminal air and fluid in the left retroperitoneum, tracking through the left perirenal space into the left upper abdominal quadrant (white arrows).
Two days after undergoing CT guided drainage of the pelvic abscess, a repeat abdominal CT revealed a decreased size of pelvic abscess with drainage tube insertion. There was a persistent area of increased fluid and gas accumulation overlying the left iliac vessels. Gas tracking throughout the left retroperitoneum, left upper abdomen, and left abdominal wall was also noted. Two days later, a CT-guided placement of drain into the left pelvis was performed. Cultures were collected and positive for Enterococcus avium, Pseudomonas, E. coli, and Streptococcus constellatus, and he was started on piperacillin/tazobactam.
One week after starting piperacillin/tazobactam, he underwent an anterior resection, diverting loop ileostomy, open appendectomy, drainage of pelvic abscesses, omental pedicle flap, and a Jackson-Pratt drain placement. The operative findings were also significant for moderately severe hepatic cirrhosis. On the second day postoperatively, he was transferred to the surgical intensive care unit (SICU) due to an estimated blood loss of 400 cc. He was taken into surgery for an exploratory laparotomy to control the hemorrhage from the inferior mesenteric artery (IMA) stump.
Discussion
There are multiple proposed pathogenesis of perforation in COVID-19 patients. The first is the presence of angiotensin-converting enzyme (ACE 2) receptors in the GI tract, the second is the thromboembolic phenomenon leading to ischemia, the third is increased intraluminal pressure secondary to constipation, the fourth is the neuroinvasive propensity of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) leading to GI dilatation, and lastly a rare side effect of tocilizumab. 4 Anecdotally observed, many critically ill patients in the intensive care unit with COVID-19 were more likely to develop constipation and thus increase their intraluminal pressure. 5
Those critically ill patients with COVID-19 are typically kept “nothing by mouth” or “NPO,” and it’s proposed that many of these patients may develop ulceration due to stress-related mucosal damage. This stress, in addition to constipation, leads to an increase in intraluminal pressure. 4 With concerns to abdominal perforation in COVID-19 patients, tocilizumab, an approved treatment by the CDC, has been linked to such adverse effects. Asharaf and Papierniak 6 note a higher incidence for GI perforation in patients with previous corticosteroid use or preexisting diverticulitis. In addition, they state that increased expression of IL-6 stimulates epithelial proliferation, which was significantly reduced in patients at higher risk of intestinal perforation. 6 It is also important to mention that no adverse GI effects have been reported using convalescent plasma. 7
Tocilizumab, an IL-6 receptor antagonist, was initially approved by the Food and Drug Administration (FDA) for adults with moderate to severe rheumatoid arthritis and children ages 2 and old with either active Polyarticular Juvenile Idiopathic Arthritis or active Systemic Juvenile Idiopathic Arthritis. One of the critical safety warnings associated with tocilizumab is the reported cases of gastrointestinal perforation, which was first noted during the Phase 3 clinical trials. Of note, a history of lower GI perforation, diverticulitis, fistula, and abscess were all associated with the risk of abdominal perforation. This was also noted in patients who were currently taking methotrexate, nonsteroidal anti-inflammatory medication, and corticosteroids. 8 It has been noted that patients with Rheumatoid arthritis taking tocilizumab had twice the risk of developing gastrointestinal perforation than their equal counterparts taking certain biologics like tumor necrosis factor inhibitors (TNFis) and rituximab. 9 Monemi et al 10 states that nearly 85% of these gastric perforations occurred in the lower GI tract. Tocilizumab use has also been associated with the pathogenesis of cytomegalovirus colitis in patients with COVID-19 due to its immunosuppressive effects leading to potential reactivation of latent infection. 11
Throughout the GI tract, there are increased ACE 2 receptors lining the epithelium of the esophagus, stomach, duodenum, and rectum. Here, SARS-CoV-2 can readily replicate and wreak havoc in patient symptomatology ranging from diarrhea to constipation. 5 In those patients with constipation, the increase in the intraluminal pressure may go unnoticed if they are concurrently being treated with steroids. This is because glucocorticoid steroids can suppress the immune system, reduce wound healing time, suppress inflammation, increase the risk of peptic ulcers and tissue atrophy, and thus increase the risk for GI bleeding. Specifically, multiple studies have shown that prolonged steroid use may delay any symptom presentation of bowel perforation, abdominal pain, and peritonitis. Due to the delayed symptom presentation of gastrointestinal perforation, these patients have an increased morbidity and mortality rate. 12
In conclusion, the use of glucocorticoid steroids in critically ill COVID-19 increases the risk for gastrointestinal perforation and delays the diagnosis by mitigating the clinical presentation. Tocilizumab has also been linked to several cases of gastrointestinal perforation not only in patients receiving it for rheumatoid arthritis but those critically ill patients with COVID-19. Thus, if patients receive both glucocorticoid steroids and tocilizumab and suffer from persistent abdominal pain, gastrointestinal perforation must be ruled out. 13
Footnotes
Author Contributions: Each author added signifiicant contribution to the composition and editing of this manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2:e13-e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92:2283-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Argan RJ, Alqatari SG, Al Said AH, et al. Gastrointestinal perforation secondary to COVID-19: case reports and literature review. Medicine (Baltimore). 2021;100:e25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kangas-Dick A, Prien C, Rojas K, et al. Gastrointestinal perforation in a critically ill patient with COVID-19 pneumonia. SAGE Open Med Case Rep. 2020;8:2050313X20940570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asharaf A, Papierniak E. “Toci associated toxicity” – a report of tocilizumab associated bowel perforation in two critically ill patients with COVID-19 pneumonia. American Thoracic Society International Conference Meetings Abstracts. Accessed August 25, 2021. 10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2443 [DOI] [Google Scholar]
- 7.Shaikh DH, Patel H, Makker J, Badipatla K, Chilimuri S. Colonic ileus, distension, and ischemia due to COVID-19-related colitis: a case report and literature review. Cureus. 2021;13:e13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CiplaMed. ACTEMRA® IV injection. July2020. Accessed June 5, 2022. https://www.ciplamed.com/content/actemra-iv-injection
- 9.Rodriguez T.The risk of lower gastrointestinal perforation more than double with certain biologics. Rheumatology Advisor. January12, 2019. Accessed June 5, 2022. https://www.rheumatologyadvisor.com/home/topics/rheumatoid-arthritis/risk-of-lower-gastrointestinal-perforation-more-than-double-with-certain-biologics/
- 10.Monemi S, Berber E, Sarsour K, et al. Incidence of gastrointestinal perforations in patients with rheumatoid arthritis treated with tocilizumab from clinical trial, postmarketing, and real-world data sources. Rheumatol Ther. 2016;3:337-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taherifard E, Mortazavi R, Mokhtari M, Taherifard A, Kiani Salmi S, Taherifard E.Cytomegalovirus gastritis in a patient with severe acute respiratory syndrome coronavirus 2 infection: a case report and literature review. Respir Med Case Rep. 2022;37:101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro G, Ryan M, Franz M. Bowel perforation in COVID-19 patient treated with dexamethasone. Am J Surg Clin Case Rep. 2021;3:1-3. [Google Scholar]
- 13.Goethals L, Nieboer K, De Smet K, et al. Cortisone associated diverticular perforation. JBR-BTR. 2011;94:348-349. [DOI] [PubMed] [Google Scholar]


