Abstract
Objective
To undertake a network meta-analysis to compare the relative efficacy of a dual peroxisome proliferator-activated receptor (PPAR)α and PPARγ agonist, glucagon-like peptide-1 receptor agonists (GLP-1RAs) and metformin in patients with non-alcoholic fatty liver disease (NAFLD).
Methods
Electronic databases, including Embase®, PubMed® and The Cochrane Library, were searched systematically for eligible studies from inception to 20 July 2022. Randomized controlled trials (RCTs) that investigated aspartate aminotransferase, alanine aminotransferase (ALT) and triglyceride levels were considered for inclusion. Data were extracted using a standardized data collection table. A network meta-analysis was performed. Relative risk and 95% confidence interval were calculated for continuous data and I2 was used to assess the heterogeneity of studies.
Results
A total of 22 RCTs involving 1698 patients were eligible for inclusion in the analysis. Both direct analysis and indirect analysis showed that saroglitazar was significantly superior to GLP-1RAs in improving ALT levels. Metformin improved ALT levels, but the effect was not as good as saroglitazar.
Conclusion
Saroglizatar was the most effective drug for improving NAFLD.
INPLASY registration number: INPLASY202340066
Keywords: NAFLD, saroglizatar, glucagon-like peptide-1 receptor agonists, metformin
Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) is gradually increasing worldwide. 1 Non-alcoholic steatohepatitis (NASH) is the severe stage of NAFLD and may further progress to cirrhosis and hepatocellular carcinoma. 2 Currently, the management of NAFLD mainly focuses on lifestyle-related interventions, including healthy diets, caloric restriction and increased aerobic exercise. 3 However, the morbidity and mortality caused by NAFLD are still high in modern society. 4 Recently, several medications for NAFLD have been developed. 5
Hepatic lipid accumulation is one of the pathogenetic causes of NAFLD. 6 Peroxisome proliferator-activated receptor (PPAR) α and PPARγ are nuclear receptors that play key roles in modulating hepatic lipid metabolism. 7 Saroglitazar, a novel dual PPARα and PPARγ agonist, can improve alanine aminotransferase (ALT), aspartate aminotransferase (AST) and triglyceride (TG) levels in NAFLD patients. 8 Due to its efficacy, saroglitazar is now available in India as the first pharmacotherapy for NASH. 9 Glucagon-like peptide-1 (GLP-1) is a pleiotropic hormone secreted by the gut with broad pharmacological potential in lowering blood glucose levels. 10 GLP-1 receptor agonists (GLP-1RAs) have been widely applied in treating type 2 diabetes mellitus (T2DM). 11 Interestingly, several studies have shown that GLP-1RAs also have a potential therapeutic effect on preventing the development of NAFLD. 5 GLP-1Ras, including liraglutide and semaglutide, have a good effect on reducing ALT and AST levels in patients with NAFLD. 12 Metformin has been a classic glucose-lowering drug for patients with T2DM over the past 60 years. 13 More importantly, its ability to combat the disease process involved in the development of NAFLD has been identified in recent years. 13 Treatment with metformin for 72 weeks can improve ALT levels in patients with NAFLD. 14
Data directly comparing the efficacy of various drugs on NAFLD are lacking, which may limit their clinical application. This current study undertook a network meta-analysis to compare the relative efficacy of dual PPARα and PPARγ agonists, GLP-1RAs and metformin in patients with NAFLD.
Materials and methods
Search strategy
This network meta-analysis was conducted in accordance with the PRISMA guidelines. 15 Electronic databases, including Embase®, PubMed® and The Cochrane Library, were searched from inception to 20 July 2022 using the following keywords: (“liraglutide”[Mesh] OR “semaglutide” OR “dulaglutide” OR “saroglitazar” OR “metformin”) AND (“Non-alcoholic Fatty Liver Disease”[Mesh] OR “NAFLD” OR “Nonalcoholic Fatty Liver Disease”. The search was limited to human studies published in the English language. In error, this study was not prospectively registered, but it has been now registered retrospectively at INPLASY: registration number INPLASY202340066.
Eligibility criteria
Two reviewers (Z.Y.Z and Q.Y.) independently screened records according to the title/abstract and then screened the full text of the relevant records according to prespecified screening criteria. Disagreements during this process were resolved by consensus and a third reviewer (W.H.W.). The trials included in this network meta-analysis were selected based on the following criteria: (i) they were phase II, III or IV randomized controlled trials (RCTs); (ii) they included adults and adolescents patients with NAFLD, which was confirmed by biopsy or other testing; (iii) they compared dual PPARα and PPARγ agonist (saroglitazar) or GLP-1RAs (including semaglutide, liraglutide and dulaglutide) or metformin with placebo or each other; (iv) they had a follow-up duration of at least 4 months; (v) they reported the primary outcome of AST and ALT levels and/or the secondary outcome of serum TG levels.
Data extraction and quality assessment
Data from the included literature were extracted into a standardized table in Microsoft Excel 2022 (Microsoft, Redmond, WA, USA) and included the following: (i) study characteristics (author names, year of publication, research duration); (ii) participants characteristics (age, sex, body mass index [BMI], diabetes history); (iii) treatment characteristics (sample size, intervention time of each group); (iv) outcome assessments (changes in AST, ALT and TG levels from baseline in each treatment group).
The Cochrane Risk of Bias Tool was used to evaluate the risk of bias of each study, including the random generation sequence, concealment of assignments, patient and investigator blinding, outcome assessment blinding, incomplete outcome data, selective results reporting or other sources of bias. 16 Risk of bias was classified as low, high or unclear.
Statistical analyses
The comparison of different interventions was made by calculating alterations from baseline to the end of intervention of the primary/secondary outcomes. According to the data provided by the original literature, the changes of the mean ± SD were calculated. When median, range and size of a sample were used instead of the mean ± SD, a previously published method was used for the estimation. 17 A random-effects model was applied to estimate 95% confidence interval (CI) in the direct meta-analysis. Direct comparisons were performed using RevMan software (version 5.3; Cochrane Collaboration, Oxford, UK). I2 statistics were used as the main indicator of statistical heterogeneity, with values ≥50% indicating significant heterogeneity.
Due to the limited sample size, indirect comparisons were used to explore the difference in efficacy between two regimens. In order to evaluate the consistency of direct comparison and indirect comparison, a node splitting method was used to estimate the effect of indirect comparisons. A P-value <0.05 was considered statistically significant.
Results
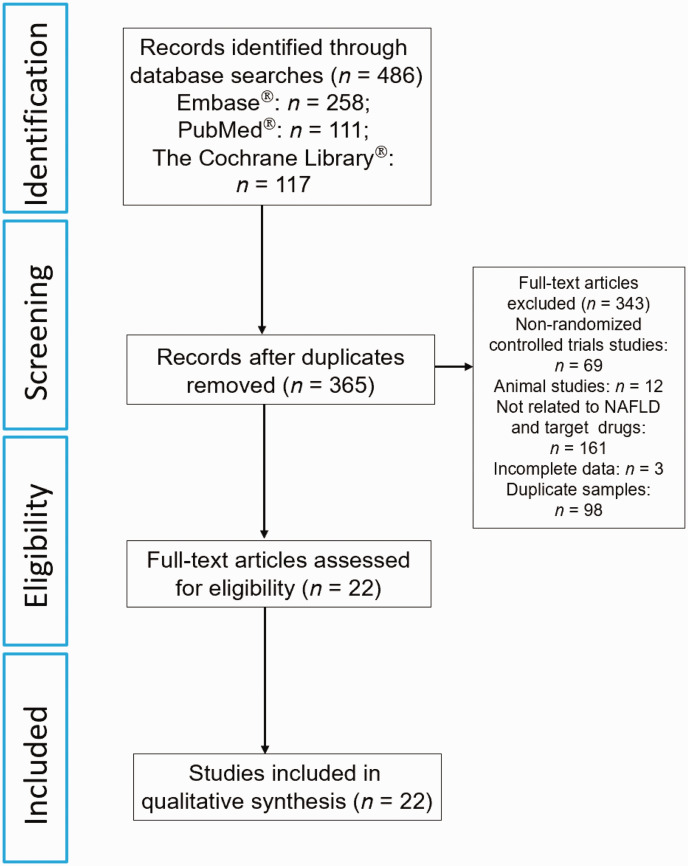
The initial database search identified 486 articles. Of these, 22 studies were selected for this meta-analysis (Figure 1).18–39 The characteristics of the included studies are summarized in Table 1.18–39 A total of 1698 patients with NAFLD were included in the 22 studies, with a mean age of 44.3 years and a median follow-up time of 12–96 weeks. Among the included studies, one study explored the efficacy of the dual PPARα and PPARγ agonist saroglitazar, 20 while seven studies assessed the effects of GLP-1RAs.18,19,21–23,38,39 The remaining 14 studies evaluated the efficacy of metformin.24–37 Twenty studies used placebo as the control;18–37 and two studies compared the effect between liraglutide and metformin.38,39 According to the Cochrane Collaboration’s tool for assessing risk of bias, all of these studies were at a low-to-moderate risk of bias (see supplementary materials, Supplementary Figure 1).
Figure 1.
Flow diagram of eligible studies showing the number of citations identified, retrieved and included in the final network meta-analysis to compare the relative efficacy of dual peroxisome proliferator-activated receptor (PPAR)α and PPARγ agonists, glucagon-like peptide 1 receptor agonists and metformin in patients with non-alcoholic fatty liver disease (NAFLD).
Table 1.
Baseline characteristics of the 22 studies included in the final network meta-analysis to compare the relative efficacy of dual peroxisome proliferator-activated receptor (PPAR)α and PPARγ agonists, glucagon-like peptide-1 receptor agonists and metformin in patients with non-alcoholic fatty liver disease (NAFLD).
| Authors | Study design | Study location | Follow-up duration, weeks | Intervention n = ITT | Control n = ITT | Main results |
|---|---|---|---|---|---|---|
| Newsome et al. 2021 18 | MC DB PC | Europe | 72 | 240 | 80 | Semaglutide was superior to placebo in liver steatosis and liver enzyme index. |
| Armstrong et al. 2016 19 | MC DB PC | UK | 48 | 26 | 26 | Compared with placebo, liraglutide improved with respect to histology and metabolic syndrome. |
| Gawrieh et al. 2021 20 | MC DB PC | USA | 17 | 78 | 28 | Saroglizatar significantly improved ALT, AST and insulin resistance. |
| Vedtofte et al. 2020 21 | DB PC | Denmark | 52 | 10 | 8 | No significant difference was found between the two treatment groups. |
| Kuchay et al. 2020 22 | NB | North India | 24 | 32 | 32 | Compared with the control group, the level of GGT was increased by dulaglutide, but AST and ALT had no significant changes. |
| Guo et al. 2020 23 | SC PC | China | 26 | 31 | 30 | In the liraglutide group, AST, ALT and intrahepatic content of lipid decreased significantly from baseline. |
| Lavine et al. 2011 24 | MC DB PC | USA | 96 | 57 | 58 | Metformin did not improve ALT and AST levels compared with the control group. |
| Sofer et al. 2016 25 | SC | Israel | 24 | 32 | 31 | Delta OPG was significantly improved in the metformin group compared with the placebo group. |
| Haukeland et al. 2009 26 | DB PC | Norway | 24 | 24 | 24 | Metformin was no better than placebo. |
| Hajiagha Mohammadi et al. 2022 27 | SC DB | Iran | 24 | 35 | 35 | Metformin was more potent in improving lipid profiles. |
| Homaei et al. 2022 28 | DB PC | Iran | 12 | 50 | 50 | Metformin was more effective in improving hepatic steatosis. |
| Anushiravani et al. 2019 29 | DB | Iran | 12 | 30 | 30 | Compared with placebo, BMI was improved, and ALT and AST levels were not statistically different. |
| Garinis et al. 2010 30 | SC | Italy | 24 | 20 | 25 | Compared with the control group, metformin improved liver steatosis and several metabolic indicators in NAFLD patients. |
| Handzlik et al. 2019 31 | SC NB | Poland | 20 | 21 | 21 | Metformin combined with diet was more effective in reducing liver steatosis and liver fibrosis than the control group. |
| Majnooni et al. 2021 32 | SC | Iran | 12 | 30 | 30 | Vitamin E combined with metformin has a beneficial effect on improving the complications of NAFLD patients compared with the control vitamin E group. |
| Omer et al. 2010 33 | SC NB | Turkey | 54 | 22 | 20 | Metformin was superior to placebo in decreasing ALT, AST, ALP and HOMA-IR. |
| Nobili et al. 2008 34 | SC | Italy | 96 | 28 | 29 | Compared with the control group, metformin did not significantly improve ALT, AST and steatosis in NAFLD patients. |
| Nar & Gedik 2009 35 | SC | Turkey | 24 | 19 | 15 | Both metformin and control groups could improve ALT and BMI levels. There was no significant difference in metformin between the two groups. |
| Zhang et al. 2017 36 | DB | China | 24 | 46 | 50 | Liraglutide was superior to placebo in decreasing bodyweight, BMI, waist circumference, FBG and 2 h-glucose. |
| Arslan et al. 2017 37 | SC | Turkey | 12 | 30 | 30 | Diet combined with metformin is better for NAFLD patients. |
| Feng et al. 2019 38 | SB | China | 24 | 29 | 29 | Liraglutide was superior to metformin in decreasing blood glucose. |
| Tian et al. 2018 39 | SB | China | 12 | 52 | 75 | Liraglutide was superior to metformin in improving AST, ALT, weight, BMI, C-reactive protein and adiponectin. |
ITT, intention-to-treat; MC, multi-centre; DB, double blind; PC, placebo controlled; ALT, alanine aminotransferase; ASP, aspartate aminotransferase; NB, non-blinded; GGT, gamma-glutamyl transferase; SC, single centre; OPG, osteoprotegerin; BMI, body mass index; ALP, alkaline phosphatase; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; FBG, fasting blood glucose; SB, single blind.
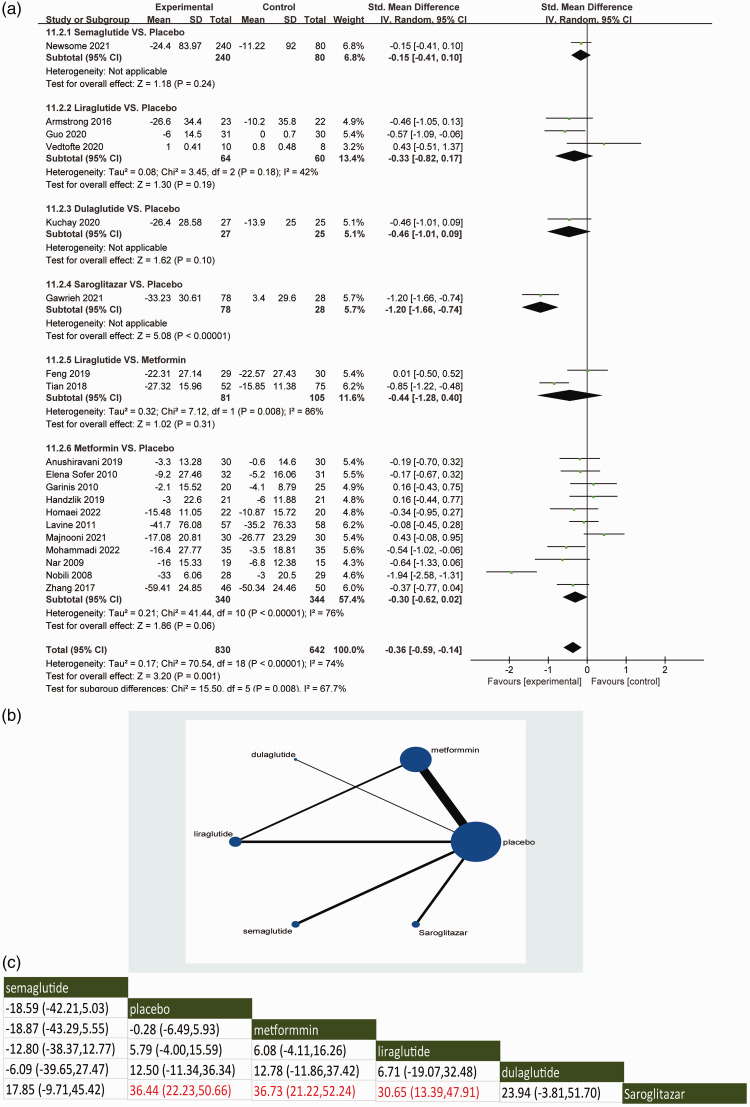
With regard to the primary outcome of lowering circulating ALT levels, the direct meta-analysis included 21 RCTs.18–36,38,39 Compared with placebo, saroglitazar was associated with an improvement in ALT levels in patients with NAFLD (95% CI: −1.66, −0.74) (Figure 2a). GLP-1RAs such as semaglutide, liraglutide and dulaglutide or metformin did not significantly improve ALT levels in NAFLD patients (Figure 2a).
Figure 2.
Direct meta-analysis (a), network graphs (b) and network meta-analysis (c) for the primary outcome of alanine aminotransferase levels. In the reticulation diagram (b), the size of the dots represents the number of patients with relevant interventions and the thickness of the wires represents the number of included studies.
With regard to the primary outcome of lowering circulating ALT levels, the indirect meta-analysis demonstrated the network comparisons of available treatments in assessing the effects in improving circulating ALT levels (Figure 2b). Consistent with the direct meta-analysis, saroglitazar significantly improved ALT levels in NAFLD patients when compared with placebo, metformin and liraglutide (P < 0.05) (Figure 2c). The Surface Under the Cumulative RAnking (SUCRA) score showed that saroglitazar ranked the highest in terms of improving ALT level, while the ranking for semaglutide was the lowest for the GLP-1RAs (see supplementary materials, Supplementary Figure 2A).
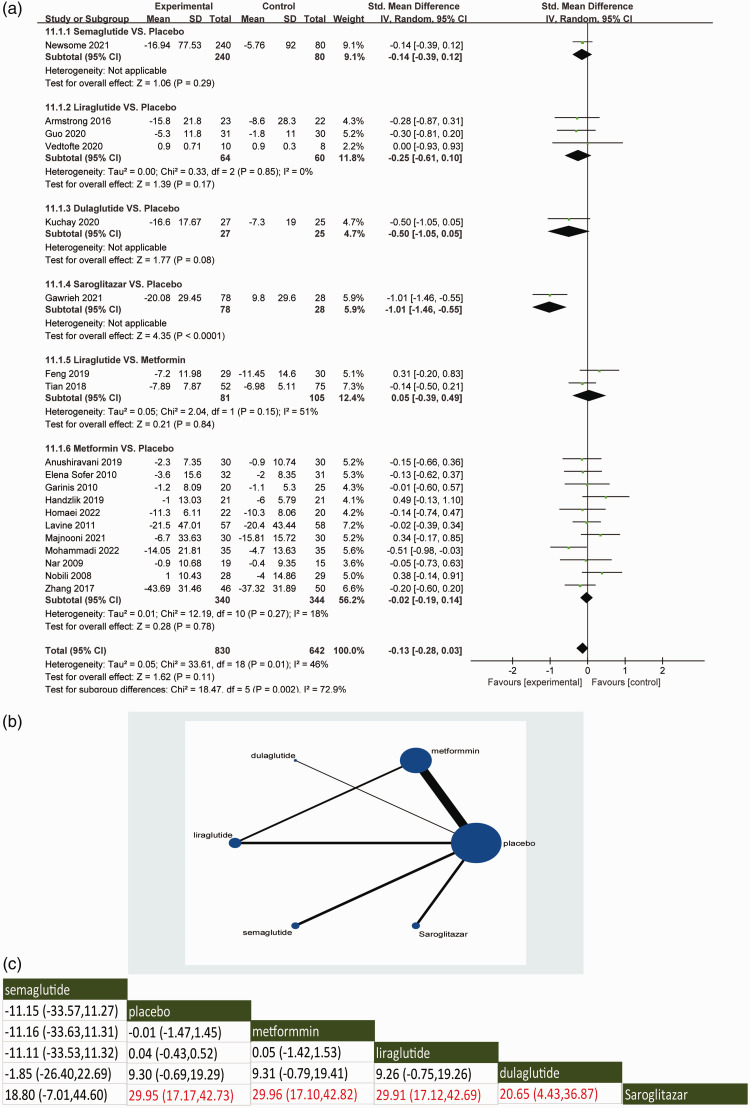
With regard to the primary outcome of lowering circulating AST levels, the direct meta-analysis demonstrated that when compared with placebo, saroglitazar were associated with a decrease in AST levels (95% CI: −1.46, −0.55) (Figure 3a). Several of the GLP-1RAs, including semaglutide, liraglutide and dulaglutide, did not show significant improvements in AST levels in patients with NAFLD compared with placebo. The effect of metformin on improving AST levels in NAFLD patients was not obvious. No significant differences in reducing circulating AST levels were observed between the other interventions.
Figure 3.
Direct meta-analysis (a), network graphs (b) and network meta-analysis (c) for the primary outcome of aspartate aminotransferase. In the reticulation diagram (b), the size of the dots represents the number of patients with relevant interventions and the thickness of the wires represents the number of included studies.
With regard to the primary outcome of lowering circulating AST levels, the indirect meta-analysis demonstrated the network comparisons of available treatments in assessing the effects of affecting circulating AST levels (Figure 3b). Saroglitazar was significantly superior to placebo in improving circulating AST levels (P < 0.05), which was consistent with the direct comparison. In addition, saroglitazar significantly improved AST levels compared with metformin or GLP-1RAs in the indirect comparison (P < 0.05). The SUCRA score showed that saroglitazar had the highest overall improvement in AST levels of these agents, followed by dulaglutide (see supplementary materials, Supplementary Figure 2B).
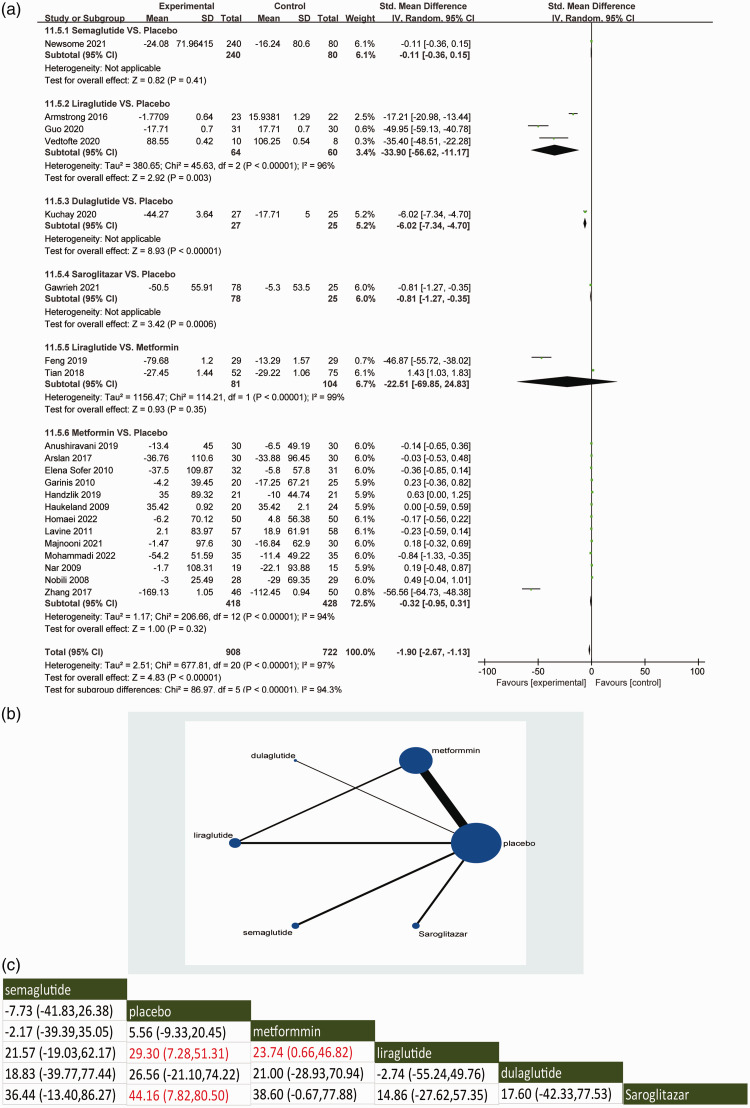
With regard to the secondary outcome of lowering circulating TG levels, the direct meta-analysis demonstrated that saroglitazar (95% CI: −1.27, −0.35) was associated with a reduction in TG levels in NAFLD patients compared with placebo (Figure 4a). Compared with placebo, liraglutide and dulaglutide could also improve the circulating levels of TG. Semaglutide did not reduce TG levels when compared with placebo. Liraglutide did not significantly improve TG levels when compared with metformin. In a controlled comparison with placebo, metformin was not superior to placebo in reducing TG levels.
Figure 4.
Direct meta-analysis (a), network graphs (b) and network meta-analysis (c) for the secondary outcome of triglycerides. In the reticulation diagram (b), the size of the dots represents the number of patients with relevant interventions and the thickness of the wires represents the number of included studies.
With regard to the secondary outcome of lowering circulating TG levels, the indirect meta-analysis demonstrated the network comparisons of available interventions evaluating the improvement of TG level (Figure 4b). In indirect comparisons, saroglitazar and liraglutide were consistent with the direct comparisons in improving TG levels compared with placebo. Semaglutide and dulaglutide did not significantly reduce TG levels compared with placebo (Figure 4c). Saroglitazar and liraglutide ranked first and second in terms of improving TG based on the SUCRA ranking system (see supplementary materials, Supplementary Figure 2C). The intervention of liraglutide was significantly better than metformin in lowering TG levels in the indirect comparison, which was inconsistent with the result of the direct comparison.
Discussion
This current network meta-analysis comprehensively evaluated the relative efficacy of different drugs, including GLP-1RAs, saroglitazar and metformin, in improving the development of NAFLD. The results indicated that the effects of saroglitazar on decreasing circulating ALT and AST levels were better than that of GLP-1RAs. However, there was no significant difference between saroglitazar and GLP-1RAs in improving TG levels. Metformin could also decrease circulating AST levels, but the effect was not as obvious as that of saroglizatar. Meanwhile, saroglitazar was more effective than GLP-1RAs and metformin in improving serum ALT and TG in NAFLD patients.
At present, there is increasing evidence that PPAR agonists and glucose-lowering drugs such as metformin and GLP-1RAs have potential value in the treatment of NAFLD. 40 However, there are few direct comparative studies on the effects of these drugs on NAFLD. Although the efficacy between PPAR agonists and GLP-1RAs has been discussed previously, the authors only analysed the mechanisms of these drugs. 36 A meta-analysis of the relevant clinical data was not performed. 41 The main purpose of this current network meta-analysis was to directly compare the effects of these drugs on the progression of NAFLD, especially with regard to liver enzymes and TG levels.
The development of NAFLD is a dynamic process, ranging from steatosis to fibrosis. 42 During this process, the levels of liver enzymes such as ALT and AST are closely associated with the severity of NAFLD. 43 If there are large amounts of enzyme in the cytoplasm of hepatocytes, ALT and AST can usually be detected in the serum of healthy people at a relatively low level. Once hepatocyte injury and apoptosis occur, the serum ALT and AST levels significantly increase. 44 Therefore, alterations of the ALT and AST levels are considered to be important indicators of NAFLD improvement. Serum ALT is independently correlated with liver TG content, therefore it is more suitable as a predictor of NAFLD than AST. 45 In Asian NAFLD patients, ALT levels are closely related to intrahepatic TG accumulation, steatosis, inflammation and fibrosis. 46
During the progression of NAFLD, in addition to the hepatic inflammatory response, there is also lipid accumulation in the liver. 42 Increased de novo lipogenesis leads to the accumulation of TG in the liver, accompanied by the production and secretion of TG-rich very low density lipoprotein particles. 7 Alterations in serum TG levels were analysed as a secondary outcome in the current network meta-analysis.
It has been reported that PPAR agonists have an important role in regulating several biological processes associated with NAFLD. 47 PPARα/γ ameliorates liver inflammation by reducing inflammatory cytokines and chemokines. 48 PPARα is mainly expressed in the liver and can promote the processes of fatty acid oxidation, ketogenesis, lipid transport and gluconeogenesis. 7 PPARγ is predominantly present in the liver and adipose tissue, and its activation increases insulin sensitivity. 49 Both oxidative stress and inflammation are involved in the process of NAFLD. 50 Saroglizatar is a novel PPAR agonist with predominantly PPARα and moderate PPARγ agonist activity. 47 The current results demonstrated that saroglizatar was the most effective drug in improving ALT and AST levels in NAFLD patients. In addition, saroglizatar was also superior to other drugs in decreasing TG levels.
Glucagon-like peptide-1, secreted by the gastrointestinal tract, has drawn considerable attention due to its glucose-lowering effects. For example, GLP-1 can regulate insulin secretion through GLP-1R in pancreatic β cells; 51 and it can also reduce blood glucose by inhibiting the secretion of glucagon in islet α cells. 52 GLP-1 can also reduce food intake by enhancing satiety through central mechanisms in the hypothalamus and brainstem. 53 In addition, the effects of GLP-1RA on improving hepatic lipotoxicity have been demonstrated in animal and human studies.54,55 However, based on this current network meta-analysis, the effect of GLP-1RAs in improving ALT and AST levels was not as obvious as that of saroglizatar. Liraglutide and dulaglutide were also effective in improving TG levels.
Metformin, a classic anti-diabetic drug, has an anti-inflammatory effect in the liver.56,57 In NAFLD patients, metformin increases hepatic β-oxidation and reduces gluconeogenesis. 58 In addition, metformin can also reduce caloric intake by inhibiting appetite, thereby reducing body weight and total body fat and visceral fat content. 59 In this current network meta-analysis, metformin could reduce serum ALT levels in NAFLD patients, although its effect is not superior to that of saroglizatar.
The current network meta-analysis had several limitations. First, the gold standard for NAFLD testing is liver biopsy, 60 which is difficult to achieve in clinical studies. The current analysis was based on the evaluation of the liver histology from a biochemical perspective. Secondly, due to the relatively small number of publications, the results of indirect and direct comparisons were not completely consistent. The inhibitory effects of GLP-1RAs, saroglizatar and metformin on ALT, AST and TG levels in the indirect comparison were supported by low-quality evidence. Therefore, larger randomized trials are needed to further verify these results.
In conclusion, GLP-1RAs and other drugs such as metformin and saroglizatar have some effects on improving the process of NAFLD. However, this network meta-analysis suggests that saroglizatar is the best one among these drugs in preventing the development of NAFLD. This network meta-analysis provides a focus for the future direction of the treatment of NAFLD and lays a foundation for subsequent drug research and development.
Supplemental Material
Supplemental material, sj-jpg-1-imr-10.1177_03000605231177191 for PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: A network meta-analysis by Zhuo-Ya Zhang, Qi Yan, Wen-Hao Wu, Yuan Zhao, Hua Zhang and Jin Li in Journal of International Medical Research
Supplemental material, sj-jpg-2-imr-10.1177_03000605231177191 for PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: A network meta-analysis by Zhuo-Ya Zhang, Qi Yan, Wen-Hao Wu, Yuan Zhao, Hua Zhang and Jin Li in Journal of International Medical Research
Acknowledgement
The authors thank Dr Huijie Zhang at Nanfang Hospital at Southern Medical University for their kind guidance and discussion.
Footnotes
Author contributions: Zhuo-Ya Zhang designed the research, wrote the manuscript and collected the data; Qi Yan conducted the research and data collection; Wen-Hao Wu analysed the data; Yuan Zhao and Hua Zhang modified the manuscript. Jin Li had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Funding: This work was supported by grants from the National Natural Science Foundation of China (no. 81970725, No. 82270915), Fok Ying Tong Education Foundation (no. 171031) and Shanxi Preferential Funding Projects for Scientific and Technological Activities of Returns (2019).
ORCID iD: Jin Li https://orcid.org/0000-0003-3926-7880
Supplementart materials
Supplemental material for this article is available online.
References
- 1.Younossi ZM.Non-alcoholic fatty liver disease – A global public health perspective. J Hepatol 2019; 70: 531–544. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Valenti L, Romeo S.Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018; 68: 268–279. [DOI] [PubMed] [Google Scholar]
- 3.Huang DQ, El-Serag HB, Loomba R.Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021; 18: 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022; 7: 851–861. [DOI] [PubMed] [Google Scholar]
- 5.Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med 2022; 292: 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagashimada M, Ota T.Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life 2019; 71: 516–522. [DOI] [PubMed] [Google Scholar]
- 7.Montagner A, Polizzi A, Fouché E, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016; 65: 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul U, Parmar D, Manjunath K, et al. New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: integrated analysis of the real world evidence. Cardiovasc Diabetol 2019; 18: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar S, Kumari D, Gupta SK, et al. Saroglitazar and Hepano treatment offers protection against high fat high fructose diet induced obesity, insulin resistance and steatosis by modulating various class of hepatic and circulating lipids. Biomed Pharmacother 2021; 144: 112357. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ.Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab 2018; 27: 740–756. [DOI] [PubMed] [Google Scholar]
- 11.Trujillo JM, Nuffer W.GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy 2014; 34: 1174–1186. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Xu J, Zhang D, et al. Efficacy and Safety of GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2021; 12: 769069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall SM.60 years of metformin use: a glance at the past and a look to the future. Diabetologia 2017; 60: 1561–1565. [DOI] [PubMed] [Google Scholar]
- 14.Doycheva I, Loomba R.Effect of metformin on ballooning degeneration in nonalcoholic steatohepatitis (NASH): when to use metformin in nonalcoholic fatty liver disease (NAFLD). Adv Ther 2014; 31: 30–43. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hozo SP, Djulbegovic B, Hozo I.Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med 2021; 384: 1113–1124. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016; 387: 679–690. [DOI] [PubMed] [Google Scholar]
- 20.Gawrieh S, Noureddin M, Loo N, et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021; 74: 1809–1824. [DOI] [PubMed] [Google Scholar]
- 21.Vedtofte L, Bahne E, Foghsgaard S, et al. One Year's Treatment with the Glucagon-Like Peptide 1 Receptor Agonist Liraglutide Decreases Hepatic Fat Content in Women with Nonalcoholic Fatty Liver Disease and Prior Gestational Diabetes Mellitus in a Randomized, Placebo-Controlled Trial. J Clin Med 2020; 9: 3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuchay MS, Krishan S, Mishra SK, et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: randomised controlled trial (D-LIFT trial). Diabetologia 2020; 63: 2434–2445. [DOI] [PubMed] [Google Scholar]
- 23.Guo W, Tian W, Lin L, et al. Liraglutide or insulin glargine treatments improves hepatic fat in obese patients with type 2 diabetes and nonalcoholic fatty liver disease in twenty-six weeks: A randomized placebo-controlled trial. Diabetes Res Clin Pract 2020; 170: 108487. [DOI] [PubMed] [Google Scholar]
- 24.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011; 305: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sofer E, Shargorodsky M.Effect of metformin treatment on circulating osteoprotegerin in patients with nonalcoholic fatty liver disease. Hepatol Int 2016; 10: 169–174. [DOI] [PubMed] [Google Scholar]
- 26.Haukeland JW, Konopski Z, Eggesbø HB, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol 2009; 44: 853–860. [DOI] [PubMed] [Google Scholar]
- 27.Hajiagha Mohammadi AA, Khajeh Jahromi S, Ahmadi Gooraji S, et al. Comparison of the Therapeutic Effects of Melatonin, Metformin and Vitamin E on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. J Adv Med Biomed Res 2022; 30: 232–240. [Google Scholar]
- 28.Homaei A, Alhadad M, Arad B, et al. Effect of Metformin or Vitamin E on Ultrasonographic Grade and Biochemical Findings of Children and Adolescents with Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. J Compr Ped 2022; 13: e123944. [Google Scholar]
- 29.Anushiravani A, Haddadi N, Pourfarmanbar M, et al. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol 2019; 31: 613–617. [DOI] [PubMed] [Google Scholar]
- 30.Garinis GA, Fruci B, Mazza A, et al. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond) 2010; 34: 1255–1264. [DOI] [PubMed] [Google Scholar]
- 31.Handzlik G, Holecki M, Kozaczka J, et al. Evaluation of metformin therapy using controlled attenuation parameter and transient elastography in patients with non-alcoholic fatty liver disease. Pharmacol Rep 2019; 71: 183–188. [DOI] [PubMed] [Google Scholar]
- 32.Majnooni MB, Ataee M, Bahrami G, et al. The effects of co-administration of artichoke leaf extract supplementation with metformin and vitamin E in patients with nonalcoholic fatty liver disease: a randomized clinical trial. Phytother Res 2021; 35: 6324–6334. [DOI] [PubMed] [Google Scholar]
- 33.Omer Z, Cetinkalp S, Akyildiz M, et al. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2010; 22: 18–23. [DOI] [PubMed] [Google Scholar]
- 34.Nobili V, Manco M, Ciampalini P, et al. Metformin use in children with nonalcoholic fatty liver disease: an open-label, 24-month, observational pilot study. Clin Ther 2008; 30: 1168–1176. [DOI] [PubMed] [Google Scholar]
- 35.Nar A, Gedik O.The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Acta Diabetol 2009; 46: 113–118. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Cheng K, Xu S, et al. Metformin and Diammonium Glycyrrhizinate Enteric-Coated Capsule versus Metformin Alone versus Diammonium Glycyrrhizinate Enteric-Coated Capsule Alone in Patients with Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Gastroenterol Res Pract 2017; 2017: 8491742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arslan I, Ulas T, Karakas EY, et al. Comparative effectiveness of diet alone and diet plus metformin treatment on omentin levels in type 2 diabetes patients with nonalcoholic fatty liver disease: A prospective randomized trial. Periodicum Biologorum 2017; 119: 9–15. [Google Scholar]
- 38.Feng WH, Bi Y, Li P, et al. Effects of liraglutide, metformin and gliclazide on body composition in patients with both type 2 diabetes and non-alcoholic fatty liver disease: a randomized trial. J Diabetes Investig 2019; 10: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian F, Zheng Z, Zhang D, et al. Efficacy of liraglutide in treating type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. Biosci Rep 2018; 38: BSR20181304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefan N, Häring HU, Cusi K.Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019; 7: 313–324. [DOI] [PubMed] [Google Scholar]
- 41.Mantovani A, Byrne CD, Targher G.Efficacy of peroxisome proliferator-activated receptor agonists, glucagon-like peptide-1 receptor agonists, or sodium-glucose cotransporter-2 inhibitors for treatment of non-alcoholic fatty liver disease: a systematic review. Lancet Gastroenterol Hepatol 2022; 7: 367–378. [DOI] [PubMed] [Google Scholar]
- 42.Alcoholic liver disease. Nat Rev Dis Primers2018; 4: 17. 10.1038/s41572-018-0019-2. [DOI] [PubMed]
- 43.Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018; 24: 908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sookoian S, Pirola CJ.Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol 2015; 21: 711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol 2007; 22: 778–787. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Han CK, Pan LL, et al. Serum alanine aminotransferase independently correlates with intrahepatic triglyceride contents in obese subjects. Dig Dis Sci 2014; 59: 2470–2476. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Cui Y, Wang XL, et al. PPARalpha/gamma agonists and antagonists differently affect hepatic lipid metabolism, oxidative stress and inflammatory cytokine production in steatohepatitic rats. Cytokine 2015; 75: 127–135. [DOI] [PubMed] [Google Scholar]
- 48.Christofides A, Konstantinidou E, Jani C, et al. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021; 114: 154338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawlak M, Lefebvre P, Staels B.Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol 2015; 62: 720–733. [DOI] [PubMed] [Google Scholar]
- 50.Peiseler M, Schwabe R, Hampe J, et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease – novel insights into cellular communication circuits. J Hepatol 2022; 77: 1136–1160. [DOI] [PubMed] [Google Scholar]
- 51.Campbell JE, Drucker DJ.Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 2013; 17: 819–837. [DOI] [PubMed] [Google Scholar]
- 52.Drucker DJ, Habener JF, Holst JJ.Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest 2017; 127: 4217–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Z, Liu L, Zhang J, et al. Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism. Sci Adv 2022; 8: eabn5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marra F, Svegliati-Baroni G.Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol 2018; 68: 280–295. [DOI] [PubMed] [Google Scholar]
- 55.Petit JM, Cercueil JP, Loffroy R, et al. Effect of Liraglutide Therapy on Liver Fat Content in Patients With Inadequately Controlled Type 2 Diabetes: The Lira-NAFLD Study. J Clin Endocrinol Metab 2017; 102: 407–415. [DOI] [PubMed] [Google Scholar]
- 56.Krakoff J, Clark JM, Crandall JP, et al. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity (Silver Spring) 2010; 18: 1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Y, Wang X, Yan C, et al. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Medicine (Baltimore) 2022; 101: e31437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Wagner LB, Rinella ME.The role of insulin-sensitizing agents in the treatment of nonalcoholic steatohepatitis. Therap Adv Gastroenterol 2011; 4: 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coll AP, Chen M, Taskar P, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020; 578: 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009; 49: 1017–1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-imr-10.1177_03000605231177191 for PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: A network meta-analysis by Zhuo-Ya Zhang, Qi Yan, Wen-Hao Wu, Yuan Zhao, Hua Zhang and Jin Li in Journal of International Medical Research
Supplemental material, sj-jpg-2-imr-10.1177_03000605231177191 for PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: A network meta-analysis by Zhuo-Ya Zhang, Qi Yan, Wen-Hao Wu, Yuan Zhao, Hua Zhang and Jin Li in Journal of International Medical Research