Abstract
Cancer-related stroke (CRS), referring to ischemic stroke occurring in cancer patients without other clear etiology, represents a clinical challenge, as it is associated with unfavorable clinical outcomes including high rates of recurrence and mortality. There are scarce international recommendations and limited consensus statements on CRS management. For this comprehensive overview, the available studies/reviews/meta-analyses on the use of acute reperfusion and secondary prevention treatments for cancer patients with ischemic stroke, focusing on antithrombotic agents, were collected and summarized. A practical management algorithm was designed per the available data. In short, acute reperfusion in the form of intravenous thrombolysis and mechanical thrombectomy appears to be safe in CRS and can be considered for eligible patients, though the functional outcomes are often poor, and mostly defined by the preexisting condition. Many patients carry indications for anticoagulation, in which case vitamin K antagonists are not preferred, while low-molecular weight heparins remain the treatment of choice; direct oral anticoagulants can be alternatively considered but are contraindicated for gastrointestinal malignancies. For patients without clear anticoagulation indications, no net benefit for anticoagulation compared to aspirin has been shown. Other targeted treatment options should be evaluated in an individualized approach, alongside the appropriate management of conventional cerebrovascular risk factors. Oncological treatment should be swiftly initiated/continued. In conclusion, acute CRS remains a clinical challenge, with many patients suffering recurrent stroke, despite preventive measures. More randomized-controlled clinical trials are urgently needed to pinpoint the most effective management options for this subset of stroke patients.
Keywords: cancer, cancer-related stroke, hypercoagulability, ischemic stroke, malignancy, stroke, tumor
Introduction
Cancer and stroke are two clinical entities that have been causally associated, with cancer patients presenting twice the risk of stroke compared to the general population. 1 The cancer types most commonly associated with ischemic stroke (IS) are lung, pancreas, breast, and prostate, 2 with metastatic cancer considerably increasing the mortality risk on the occurrence of stroke. 3 Cancer can predispose toward stroke through many different factors and mechanisms, directly and indirectly, with the oncological treatments also increasing the risk of cerebrovascular events.2,4 Cancer-related stroke (CRS) is an entity relatively recently introduced into the stroke literature. This term usually pertains to an IS that arises as a direct result of the malignancy itself, mainly due to cancer-induced hypercoagulability. 5 In most studies, it is defined as an IS in patients with a known malignancy, proof of hypercoagulability usually in the form of elevated D-dimers, ischemic lesions in multiple vascular territories in the magnetic resonance imaging (MRI), and no evidence of other ‘conventional’ stroke mechanisms; 6 these factors have all been repeatedly associated with ‘cryptogenic’ strokes in cancer patients. 7 Consequently, these strokes mostly fall into the ‘unknown/cryptogenic origin’ TOAST (Trial of Org 10172 in Acute Stroke Treatment) category 8 and are often described as ‘embolic stroke of unknown source’ (ESUS). It has also been suggested that stroke in cancer patients could eventually represent a separate category of IS5,9 since CRS represents a classification challenge. It may be at times referred to as ‘other determined etiology’ when the underlying hypercoagulability/Trousseau’s syndrome has been diagnosed, or even hide behind a ‘cardioembolic’ classification, for instance in cases of non-bacterial thrombotic endocarditis (NBTE), which is often detected in cancer patients and also falls into the cancer-associated hypercoagulability spectrum.2,10 The exact pathophysiology of CRS still remains elusive, 11 though hypercoagulability, with its multifaceted manifestations, 9 is still regarded as the main component of this clinical entity as well. 12 The activation of prothrombotic molecules and pathways, either via the cancer cells themselves or an indirect stimulation of the surrounding tissue cells, leads to the manifestation of disseminated intravascular coagulation, 13 described in both solid and hematological malignancies.13,14 Because of its high tissue factor and thromboplastin concentrations, 15 the brain may be susceptible to thrombotic events in settings of intravascular coagulopathy, and this may predispose to CRS.
It is also a well-documented fact that IS patients with concomitant cancer present an overall worse prognosis than patients without cancer, and high mortality rates (standardized mortality ratio of cancer patients with stroke: 2.17), 1 with almost half of them deceasing within 3 months and exhibiting poor functional outcomes, despite timely therapeutic interventions. 16 In addition, a cryptogenic origin of stroke in cancer patients, as is usually the case with CRS, has been linked to significantly worse prognosis and mortality. 17 Cancer patients also present high recurrence rates, 18 with up to 14% annual incidence of recurrent stroke, almost threefold compared to patients without a history of cancer; 19 advanced cancer, increased D-dimers, and ischemic lesions in multiple vascular territories are also more often encountered in cancer patients with recurrent strokes. 20 However, there is a growing debate on whether these patients profit from antiplatelet or anticoagulative therapies, with limited available randomized-controlled clinical trials.
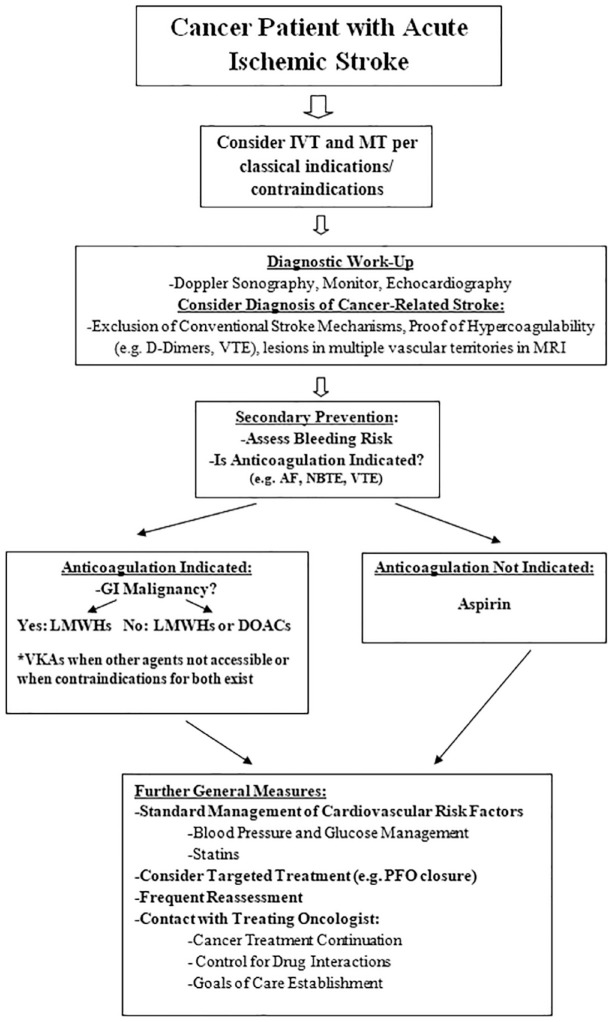
Evidently, it is crucial to find a preventive strategy that may prolong survival, reduce the risk of stroke recurrence, and improve functional outcomes. With the global population aging, the incidence of CRS increases exponentially, while their proper management remains unclear. In this narrative review, we sought to summarize the available data on acute management and secondary prophylactic treatment options in patients with CRS, with a focus on acute reperfusion and antithrombotics, attempting to present a management algorithm, based on the available data (Figure 1), and discussing future options.
Figure 1.
Proposed clinical algorithm for CRS. Abbreviations: AF, atrial fibrillation; DOACs, direct oral anticoagulants; GI, gastrointestinal; IVT, intravenous thrombolysis; LWMHs, low-molecular weight heparins; MRI, magnetic resonance imaging; MT, mechanical thrombectomy; NBTE, non-bacterial thrombotic endocarditis; PFO, patent foramen ovale; VKAs, vitamin K antagonists; VTE, venous thromboembolism.
Acute management
The mainstays of acute IS treatment are intravenous thrombolysis (IVT) and mechanical thrombectomy (MT).21–23 However, cancer patients have been traditionally excluded from the trials that proved the safety and efficacy of these treatments, and evidence of their applicability in this population came, and is still coming, with considerable delay, mostly through retrospective analyses of hospital records.
Intravenous thrombolysis
Unsurprisingly, contraindications for IVT, such as recent surgery, anticoagulation, and thrombocytopenia, arise more often in cancer patients. 18 However, as many studies and a subsequent meta-analysis have so far demonstrated, IVT appears to be similar in terms of safety (symptomatic intracranial hemorrhage: OR 2.12, 95% CI = 0.33–13.76; in-hospital mortality: OR 1.30, 95% CI = 0.93–1.81; and 3-month mortality: OR 1.00, 95% CI = 0.49–2.03) and efficacy (functional independence, expressed a modified Rankin Scale scores 0–2: OR 1.00, 95% CI = 0.51–1.97) in patients with and without cancer.24,25 As such, cancer per se is no contraindication for IVT, 26 and cancer patients should be assessed for IVT based on the existing eligibility criteria.21,27 In terms of guidelines, specific mentions of cancer have only so far emerged in the American Heart Association Guidelines, 21 where IVT can be considered in cancer patients with a reasonable life expectancy, with gastrointestinal (GI) and intra-axial brain neoplasms being named as contraindications. At least regarding the GI malignancies, the few numbers of these patients who did receive IVT did not show an increased risk of hemorrhagic complications; however, clinicians are still skeptic in administering the treatment in this specific cancer patient subgroup. 25 The contraindication for intra-axial brain tumors came from the studies associating malignant brain neoplasms and an intraparenchymal localization with higher mortality and intracranial hemorrhage rates after IVT.28,29 Specifically regarding CRS, no particular studies have been published, though mentions of cancer patients with IS of cryptogenic origin receiving IVT showed no increase in complication rates. 30
Mechanical thrombectomy
Moving on to MT, it is often considered the method of choice for cancer patients since its establishment in IS treatment, due to IVT contraindications, though studies assessing its applicability in cancer patients were also relatively scarce. A systematic review (18 studies) 31 and a meta-analysis (7 studies) 32 on this subject were recently published, and showed that MT is a safe option for cancer patients (symptomatic intracranial hemorrhage: OR 1.04, 95% CI = 0.59–1.85; in-hospital mortality rates: 4–43%), and even though the 3-month mortality was increased in some studies (OR 5.02, 95% CI = 2.90–8.69), 32 this was mostly attributed to the malignancy itself. 31 The efficacy assessments provided heterogeneous results (functional independence: OR 0.44, 95% CI = 0.32–0.60). Three studies had focused particularly on CRS patients,6,33,34 as defined above, and reported similar reperfusion and intracranial hemorrhage rates compared to other patient subgroups, albeit with consistent tendencies toward higher 3-month mortality and poor functional outcome rates.
It is also important to note that stroke can be the first manifestation of cancer and precede its diagnosis for several months. 35 As such, numerous patients may have received acute reperfusion therapies without the knowledge of the underlying cancer, with no large-scale systematic reports on unfavorable outcomes of increased complication rates. Consequently, both IVT and MT appear to be safe for cancer and CRS patients, when applicable. However, these patients, due to their significant disease burden, exhibit poor functional outcomes even after successful reperfusion, and treatment decisions should ultimately align with the patient’s wishes and best medical practice in an individualized approach.
Secondary prevention
Anticoagulation
As already mentioned, cancer patients are at risk of suffering recurrent strokes, whereas great uncertainty exists regarding the optimal antithrombotic treatment as a secondary prevention. Intuitively, knowing that hypercoagulability appears to represent the main underlying pathogenic mechanism in CRS, physicians hypothesize that anticoagulation may be indicated in these patients. This notion is reinforced by a growing number of studies examining elevated D-dimers, thrombin, and fibrinogen as markers in these patients,2,11,36 with D-dimers being independently associated with a worse prognosis. 37 Truly, some clinical entities pertaining to CRS, such as NBTE and paradoxical embolism, are indications for starting anticoagulants, 5 and many patients have already suffered from venous thromboembolism (VTE) in the near past and need to be anticoagulated. In this case, the available literature has been recently reviewed/meta-analyzed26,38 and translated into the respective guidelines for VTE. 39 It was concluded that other anticoagulants should be preferred over vitamin K antagonists (VKA) in cancer patients due to lower efficacy, high risk of bleeding, and difficulties in achieving coagulation times within the desired range;27,40,41 VKAs can be used when low-molecular weight heparins (LMWHs) and direct oral anticoagulants (DOACs) are not accessible, or when contraindications for both classes are present (for instance, clinically significant liver disease and advanced renal disease). 39 However, LMWHs and DOACs show similar efficacy in preventing thrombotic events, though DOACs exhibited higher bleeding rates in some of the studies, and especially regarding patients with GI malignancies.42,43 As such, most VTE guidelines now recommend LMWHs as first-line treatment and for patients with GI malignancies, while DOACs can also be considered for patients without GI malignancies.39,44 Of the available DOACs, rivaroxaban seems to carry a large body of evidence, showing a better efficacy profile than LMWHs, similar major bleeding rates, but increased rates of clinically relevant, non-major bleeding. 45 Increasing evidence supporting the use of edoxaban has also recently emerged, showing better effectiveness in VTE prevention than LMWHs, albeit with a slightly increased risk of major bleeding; 46 in the extended treatment beyond 6 months, edoxaban was also shown as safe and effective as dalteparin. 47 Interestingly, one study regarding Asian patients reported lower GI bleeding rates with DOACs; 48 this possibly highlights the role of pharmacogenetics and should be taken into careful consideration in the future, especially in the field of oncology, where genetic analyses are gaining more and more ground in leading clinical decisions.
Only a few reports have assessed anticoagulation in IS prevention for CRS. The OASIS-CANCER study reported that for CRS patients with elevated D-dimer levels who received LMWH or VKA, the decrease in D-dimer levels in the normal range within a week was associated with 1-year survival, though further elevation of D-dimers in follow-up was significantly associated with death within a month. 37 In a similar note, Seok et al. 49 reported that 3 of their 29 CRS patients who received anticoagulation had a recurrent stroke during their hospitalization, and these 3 showed persistently high D-dimer levels and embolic signals in transcranial Doppler sonography. Nam et al. 50 also reported very unfavorable outcomes for CRS patients despite anticoagulation (more than 50% 90-day mortality and new ischemic lesions), and no significant differences in efficacy and safety profiles of LMWH and DOACs.
Antiplatelet agents
On the other side, the crucial role of activated platelets has also been highlighted in cancer settings. Platelets are important mediators of clot formation and inflammation as well, 51 and aid in the induction of the cancer-associated hypercoagulable state. 52 In this sense, aspirin is thought to convey a multitude of benefits to cancer patients, and to even be able to prevent cancer in some cases.53,54 The protective effect of aspirin has also been showcased in the study of Navi et al., 55 which although prematurely terminated, documented that LMWH treatment is related to reduced compliance, and anticoagulation had no evident benefits compared to aspirin. This also reflects the overall experience with ESUS patients, where anticoagulation failed to prove its superiority in terms of efficacy compared to aspirin.56,57 Two randomized-controlled clinical trials comparing apixaban with aspirin (TEACH2) 58 and edoxaban with enoxaparin (ENCHASE, NCT03570281) in CRS have also been announced, and their results are awaited with great interest.
Management of cardiovascular risk factors
Standard management of conventional cardiovascular risk factors still pertains to the management of CRS, as per the international recommendations. 21 For statins, no particular indications and contraindications exist regarding cancer patients; it has been claimed, though, that they could carry oncological benefits, particularly for GI malignancies.59,60 As such, statins should be included in the management of CRS patients, especially in the presence of atherosclerosis or elevated low-density lipoprotein (LDL) levels. Blood pressure management also follows the guidelines of non-cancer patients, though particular attention is needed in the case of cancer therapy-related hypertension, which can lead to rebound hypotension on treatment cessation. 61 Similarly, standard glucose-lowering agents can be applied in cancer patients with diabetes and stroke, per the standard management algorithms. 62
Targeted treatment of rarer causes
The particular causes of IS in cancer patients also need to be more closely examined for targeted therapies to be offered. When paradoxical embolism is thought to be the underlying cause, with some preliminary data revealing increased right-to-left shunt rates in CRS patients, 63 a patent foramen ovale (PFO) closure could be offered for patients with prospects of full oncological recovery. However, no studies regarding IS prevention through PFO closure have included cancer patients and so data are limited. 64 The rates of inferior vena cava filter implantation have also risen in cancer patients, with studies showing that their implantation is safe even in patients with advanced disease, though their benefit in terms of quality of life improvement and survival prolongation is still a matter of discussion; patients in earlier disease stages seem to profit more, being made eligible for more interventions, though careful consideration is advised, alongside the timely removal of the filter (if retrievable). 65 However, their role in stroke prevention has not been elucidated. Moving on, surgical treatment for significant carotid stenosis by tumor compression or following radiation will most likely be necessary and should be offered, 66 which is also the case regarding the operative treatment of cardiac tumors. 67 For cancer-associated NBTE, a surgical treatment may be considered only in selected patients who do not respond to anticoagulation.5,68
Continuation of oncological treatment
Contact with the treating oncologist should be initiated soon after admitting the patient to establish common goals of care and carefully assess the patient’s medication since many chemotherapy agents predispose toward IS 2 and should be discontinued, while they may also interact with medications under consideration for the patient. Finally, all these antithrombotic approaches can be seen as ‘futile’ when the underlying pathology, that is, the cancer, remains untreated. As such, the oncological treatment must be prioritized and initiated as soon as possible. 58 The available treatment options should also be carefully examined, especially in terms of vasotoxicity and thrombogenic potential. It must be said, however, that performance status represents a major factor in the suitability of a patient for treatment, and on suffering a stroke, the treatment indication may not be present anymore. 69 Should there not be any possibly effective treatment options for the patient, then the focus should be shifted on antithrombotic agents and other symptomatic therapies, per the patient’s goals of care.
Future directions
The P2Y12 receptor antagonists, such as clopidogrel and ticagrelor, have been less studied in cancer patients, and some original concerns of possible carcinogenetic potential have not been confirmed in subsequent meta-analyses.70,71 In fact, ticagrelor possesses the ability to decrease P-selectin’s expression and thus platelet interaction with malignant cells. 72 Higher P-selectin levels have been reported in cancer patients with stroke, 11 and ticagrelor was also shown to reduce spontaneous platelet aggregation in metastatic cancer patients. 73 Similarly, clopidogrel was shown to be more effective in dissolving spontaneous thrombi than aspirin in an orthotopic pancreas cancer model. 52 As such, these agents merit much more attention in future studies of CRS patients. For other antiplatelet agents, for instance, GP IIb/IIIa antagonists, concerns regarding their clinical use have been raised (intravenous administration, high bleeding rates), 51 and are thus not considered the ideal research targets in this field as of now. Dual antiplatelet therapy has also not been assessed in this patient population yet, and neither has the combination of antiplatelet and anticoagulation agents. Positive results of studies, such as the COMPASS protocol, which combined aspirin and rivaroxaban for patients with concomitant atherosclerosis, 74 and the ongoing assessment of novel agents, such as the XIa inhibitors asundexian and milvexian, in combination with antiplatelets,75,76 provide noteworthy ideas. A similar combination of agents could theoretically benefit CRS patients, where the mechanisms of coagulopathy appear to be very complicated, and where atherosclerosis is also prevalent. 77 Both of these approaches will be particularly hard to examine though, given how cancer patients are particularly prone to bleeding complications and careful consideration of the risk–benefit ratio is needed.
Inflammatory mechanisms are also heavily implicated in cancer, 78 while recent evidence suggests that inflammatory molecules, such as interleukin-6, might increase stroke risk; 79 this has translated into efforts to apply immunomodulatory agents in stroke management, 80 with inconsistent results so far. However, applying an adjuvant anti-inflammatory treatment for CRS, where C-reactive protein is also consistently found elevated, 2 could potentially lead to better outcomes.
Finally, as medicine advances, the need for more personalized and specialized approaches increases. This also affects the field of stroke management, and especially CRS management. It would be oversimplifying to say that all CRS patients fall into the same category, when the underlying malignancies differ substantially in terms of prognosis and available therapies. As such, studies have also shown that different cancer types present different risks for IS, and different pathogenic mechanisms, which need to be more closely examined. 55 Solid tumors and especially adenocarcinomas, namely lung, pancreas, breast, colorectal, and prostate adenocarcinomas, appear to present the highest IS risks, 81 and mucins, for example, have often been incriminated as the underlying source of hypercoagulability.82,83 Though attempts to target mucins have not been succesful, 84 focusing on cancer-specific mechanisms could provide additional insight in optimizing the management of CRS.
Limitations of the available literature
The pitfalls of the available literature should also be addressed. One major point is the lack of homogeneity, as studies have so far provided different definitions of CRS and outcomes, and have demonstrated considerable design deviations, rendering the safe extraction of conclusions difficult. 31 As already mentioned, CRS could also be classified in various TOAST categories by healthcare practitioners, impairing its proper identification in retrospective analyses on the safety of reperfusion techniques. Patient numbers have also been relatively small; however, given the relative rarity of CRS, the publication of even small studies with an otherwise well-described group of CRS patients could prove valuable in accumulating data on these patients and allowing the conduction of a meta-analysis. Direct comparisons of antithrombotic agents have also not been widely available, so the superiority of one class of agents over the other regarding CRS management cannot be proven yet. In a similar note, other secondary preventive measures, for instance, statins and blood pressure management, as well as antithrombotic agent combinations have not been assessed particularly in CRS so far, so that recommendations can only be made indirectly, based on general experience with cancer and stroke patients.
Conclusion
CRS is a particularly challenging clinical entity, with relatively limited evidence on the available treatment options. Acute reperfusion therapies (IVT and MT) have been proven safe in cancer patients and should be considered in eligible patients. Regarding secondary prevention, anticoagulation often needs to be administered due to previous VTE or other entities that require full anticoagulation; though in CRS patients with no such clear indication, no obvious benefit of anticoagulation compared to antiplatelet therapy and aspirin has been shown. Clinical trials assessing and comparing all available alternatives are urgently required, while other options such as inflammatory molecules could also provide benefits, but have not been examined in this setting so far.
Acknowledgments
No further acknowledgments.
Footnotes
ORCID iDs: Athina-Maria Aloizou  https://orcid.org/0000-0001-9354-774X
https://orcid.org/0000-0001-9354-774X
Lina Palaiodimou  https://orcid.org/0000-0001-7757-609X
https://orcid.org/0000-0001-7757-609X
Georgios Tsivgoulis  https://orcid.org/0000-0002-0640-3797
https://orcid.org/0000-0002-0640-3797
Contributor Information
Athina-Maria Aloizou, Department of Neurology, St. Josef-Hospital, Ruhr University Bochum, Gudrunstr. 56, 44791 Bochum, Germany; Department of Neurology, Laboratory of Neurogenetics, University of Thessaly, University Hospital of Larissa, 41100 Larissa, Greece.
Lina Palaiodimou, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece.
Dimitra Aloizou, Department of Nursing, National and Kapodistrian University of Athens, Athens, Greece.
Efthimios Dardiotis, Department of Neurology, Laboratory of Neurogenetics, University of Thessaly, University Hospital of Larissa, Larissa, Greece.
Ralf Gold, Department of Neurology, St. Josef-Hospital, Ruhr University Bochum, Bochum, Germany.
Georgios Tsivgoulis, Second Department of Neurology, School of Medicine, ‘Attikon’ University Hospital, National and Kapodistrian University of Athens, Athens, Greece; Department of Neurology, The University of Tennessee Health Science Center, Memphis, TN, USA.
Christos Krogias, Department of Neurology, Evangelisches Krankenhaus Herne, Ruhr University Bochum, Bochum, Germany.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Athina-Maria Aloizou: Conceptualization; Investigation; Visualization; Writing – original draft.
Lina Palaiodimou: Investigation; Writing – review & editing.
Dimitra Aloizou: Investigation; Validation; Writing – review & editing.
Efthimios Dardiotis: Conceptualization; Writing – review & editing.
Ralf Gold: Conceptualization; Supervision; Writing – review & editing.
Georgios Tsivgoulis: Conceptualization; Writing – review & editing.
Christos Krogias: Conceptualization; Project administration; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Zaorsky NG, Zhang Y, Tchelebi LT, et al. Stroke among cancer patients. Nat Commun 2019; 10: 5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dardiotis E, Aloizou AM, Markoula S, et al. Cancer-associated stroke: pathophysiology, detection and management (Review). Int J Oncol 2019; 54: 779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pana TA, Mohamed MO, Mamas MA, et al. Prognosis of acute ischaemic stroke patients with cancer: a national inpatient sample study. Cancers (Basel) 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navi BB, Howard G, Howard VJ, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology 2018; 90: e2025–e2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YJ, Dong RG, Zhang MM, et al. Cancer-related stroke: exploring personalized therapy strategies. Brain Behav 2022; 12: e2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung S, Jung C, Hyoung Kim J, et al. Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke. Interv Neuroradiol 2018; 24: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 2010; 41: 798–801. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 9.Bang OY, Chung JW, Lee MJ, et al. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke 2020; 22: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikushima S, Ono R, Fukuda K, et al. Trousseau’s syndrome: cancer-associated thrombosis. Jpn J Clin Oncol 2016; 46: 204–208. [DOI] [PubMed] [Google Scholar]
- 11.Navi BB, Sherman CP, Genova R, et al. Mechanisms of ischemic stroke in patients with cancer: a prospective study. Ann Neurol 2021; 90: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012; 43: 3029–3034. [DOI] [PubMed] [Google Scholar]
- 13.Franchini M, Di Minno MN, Coppola A. Disseminated intravascular coagulation in hematologic malignancies. Semin Thromb Hemost 2010; 36: 388–403. [DOI] [PubMed] [Google Scholar]
- 14.Chalela JA, Raps EC, Kasner SE. Disseminated intravascular coagulation and stroke associated with cervical cancer. J Stroke Cerebrovasc Dis 1999; 8: 355–357. [DOI] [PubMed] [Google Scholar]
- 15.Tutwiler V, Peshkova AD, Andrianova IA, et al. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol 2017; 37: 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutting S, Wettengel M, Conners JJ, et al. Three-month outcomes are poor in stroke patients with cancer despite acute stroke treatment. J Stroke Cerebrovasc Dis 2017; 26: 809–815. [DOI] [PubMed] [Google Scholar]
- 17.Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke 2014; 45: 2292–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol 2018; 83: 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau KK, Wong YK, Teo KC, et al. Stroke patients with a past history of cancer are at increased risk of recurrent stroke and cardiovascular mortality. PLoS ONE 2014; 9: e88283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujinami J, Ohara T, Kitani-Morii F, et al. Cancer-associated hypercoagulation increases the risk of early recurrent stroke in patients with active cancer. Cerebrovasc Dis 2018; 46: 46–51. [DOI] [PubMed] [Google Scholar]
- 21.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 22.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turc G, Tsivgoulis G, Audebert HJ, et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischemic stroke and anterior circulation large vessel occlusion. J Neurointerv Surg 2022; 14: 209. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Lu X, Tang LV, et al. Efficacy and safety of intravenous thrombolysis for acute ischemic stroke in cancer patients: a systemic review and meta-analysis. Am J Transl Res 2020; 12: 4795–4806. [PMC free article] [PubMed] [Google Scholar]
- 25.Huang B, Qian F, Fan X, et al. Efficacy and safety of intravenous thrombolysis with alteplase for treating acute ischemic stroke at different time windows: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020; 99: e23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloizou AM, Siokas V, Mentis AA, et al. Advancements in the treatment of cerebrovascular complications of cancer. Curr Treat Options Neurol 2020; 22: 16. [Google Scholar]
- 27.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149: 315–352. [DOI] [PubMed] [Google Scholar]
- 28.Etgen T, Steinich I, Gsottschneider L. Thrombolysis for ischemic stroke in patients with brain tumors. J Stroke Cerebrovasc Dis 2014; 23: 361–366. [DOI] [PubMed] [Google Scholar]
- 29.Murthy SB, Moradiya Y, Shah S, et al. In-hospital outcomes of thrombolysis for acute ischemic stroke in patients with primary brain tumors. J Clin Neurosci 2015; 22: 474–478. [DOI] [PubMed] [Google Scholar]
- 30.Selvik HA, Naess H, Kvistad CE. Intravenous thrombolysis in ischemic stroke patients with active cancer. Front Neurol 2018; 9: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aloizou AM, Richter D, Charles James J, et al. Mechanical thrombectomy for acute ischemic stroke in patients with malignancy: a systematic review. J Clin Med 2022; 11: 4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caimano D, Letteri F, Capasso F, et al. Endovascular treatment in patients with acute ischemic stroke and cancer: systematic review and meta-analysis. Eur Stroke J 2022; 7: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon Y, Baik SH, Jung C, et al. Mechanical thrombectomy in patients with acute cancer-related stroke: is the stent retriever alone effective? J Neurointerv Surg 2021; 13: 318–323. [DOI] [PubMed] [Google Scholar]
- 34.Lee EJ, Bae J, Jeong HB, et al. Effectiveness of mechanical thrombectomy in cancer-related stroke and associated factors with unfavorable outcome. BMC Neurol 2021; 21: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navi BB, Reiner AS, Kamel H, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019; 133: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu Saadeh F, Langhe R, Galvin DM, et al. Procoagulant activity in gynaecological cancer patients; the effect of surgery and chemotherapy. Thromb Res 2016; 139: 135–141. [DOI] [PubMed] [Google Scholar]
- 37.Lee MJ, Chung JW, Ahn MJ, et al. Hypercoagulability and mortality of patients with stroke and active cancer: the OASIS-CANCER study. J Stroke 2017; 19: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobieraj DM, Baker WL, Smith E, et al. Anticoagulation for the treatment of cancer-associated thrombosis: a systematic review and network meta-analysis of randomized trials. Clin Appl Thromb Hemost 2018; 24(9_suppl): 182S–187S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streiff MB, Abutalib SA, Farge D, et al. Update on guidelines for the management of cancer-associated thrombosis. Oncologist 2021; 26: e24–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003; 349: 146–153. [DOI] [PubMed] [Google Scholar]
- 41.Rose AJ, Sharman JP, Ozonoff A, et al. Effectiveness of warfarin among patients with cancer. J Gen Intern Med 2007; 22: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018; 378: 615–624. [DOI] [PubMed] [Google Scholar]
- 43.Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018; 36: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 44.Lyman GH, Carrier M, Ay C, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv 2021; 5: 927–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed MFH, ElShafei MN, Ahmed MB, et al. The net clinical benefit of rivaroxaban compared to low-molecular-weight heparin in the treatment of cancer-associated thrombosis: systematic review and meta-analysis. Clin Appl Thromb Hemost 2021; 27: 1076029620940046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raskob GE, Buller HR, Segers A. Edoxaban for cancer-associated venous thromboembolism. N Engl J Med 2018; 379: 95–96. [DOI] [PubMed] [Google Scholar]
- 47.Di Nisio M, van Es N, Carrier M, et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE Cancer study. J Thromb Haemost 2019; 17: 1866–1874. [DOI] [PubMed] [Google Scholar]
- 48.Chen DY, Tseng CN, Hsieh MJ, et al. Comparison between non-vitamin K antagonist oral anticoagulants and low-molecular-weight heparin in Asian individuals with cancer-associated venous thromboembolism. JAMA Netw Open 2021; 4: e2036304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol 2010; 68: 213–219. [DOI] [PubMed] [Google Scholar]
- 50.Nam KW, Kim CK, Kim TJ, et al. Treatment of cryptogenic stroke with active cancer with a new oral anticoagulant. J Stroke Cerebrovasc Dis 2017; 26: 2976–2980. [DOI] [PubMed] [Google Scholar]
- 51.Bruno A, Dovizio M, Tacconelli S, et al. Antithrombotic agents and cancer. Cancers (Basel) 2018; 10: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palacios-Acedo AL, Mezouar S, Mège D, et al. P2RY12-inhibitors reduce cancer-associated thrombosis and tumor growth in pancreatic cancers. Front Oncol 2021; 11: 704945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol 2012; 9: 259–267. [DOI] [PubMed] [Google Scholar]
- 54.Patrignani P, Patrono C. Aspirin and cancer. J Am Coll Cardiol 2016; 68: 967–976. [DOI] [PubMed] [Google Scholar]
- 55.Navi BB, Marshall RS, Bobrow D, et al. Enoxaparin vs aspirin in patients with cancer and ischemic stroke: the TEACH pilot randomized clinical trial. JAMA Neurol 2018; 75: 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018; 378: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 57.Diener HC, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med 2019; 380: 1906–1917. [DOI] [PubMed] [Google Scholar]
- 58.Navi BB, Kasner SE, Elkind MSV, et al. Cancer and embolic stroke of undetermined source. Stroke 2021; 52: 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gachpazan M, Kashani H, Khazaei M, et al. The impact of statin therapy on the survival of patients with gastrointestinal cancer. Curr Drug Targets 2019; 20: 738–747. [DOI] [PubMed] [Google Scholar]
- 60.Vallianou NG, Kostantinou A, Kougias M, et al. Statins and cancer. Anticancer Agents Med Chem 2014; 14: 706–712. [DOI] [PubMed] [Google Scholar]
- 61.Cohen JB, Brown NJ, Brown SA, et al. Cancer therapy-related hypertension: a scientific statement from the American Heart Association. Hypertension 2023; 80: e46–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shahid RK, Ahmed S, Le D, et al. Diabetes and cancer: risk, challenges, management and outcomes. Cancers (Basel) 2021; 13: 5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iguchi Y, Kimura K, Kobayashi K, et al. Ischaemic stroke with malignancy may often be caused by paradoxical embolism. J Neurol Neurosurg Psychiatry 2006; 77: 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huber C, Wachter R, Pelz J, et al. Current challenges and future directions in handling stroke patients with patent foramen ovale – a brief review. Front Neurol 2022; 13: 855656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandhi MB, Desai KR, Ryu RK, et al. The role of inferior vena cava filters in cancer patients. Semin Intervent Radiol 2016; 33: 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adams HP., Jr.Cancer and cerebrovascular disease. Curr Neurol Neurosci Rep 2019; 19: 73. [DOI] [PubMed] [Google Scholar]
- 67.Baikoussis NG, Papakonstantinou NA, Dedeilias P, et al. Cardiac tumors: a retrospective multicenter institutional study. J BUON 2015; 20: 1115–1123. [PubMed] [Google Scholar]
- 68.Itzhaki Ben Zadok O, Spectre G, Leader A. Cancer-associated non-bacterial thrombotic endocarditis. Thromb Res 2022; 213(Suppl. 1): S127–S132. [DOI] [PubMed] [Google Scholar]
- 69.Takeshima S, Kawate N. Characteristics and management of a cancer patient with stroke: a case report. Prog Rehabil Med 2021; 6: 20210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotronias RA, Kwok CS, Wong CW, et al. Cancer event rate and mortality with thienopyridines: a systematic review and meta-analysis. Drug Saf 2017; 40: 229–240. [DOI] [PubMed] [Google Scholar]
- 71.Elmariah S, Doros G, Benavente OR, et al. Impact of clopidogrel therapy on mortality and cancer in patients with cardiovascular and cerebrovascular disease: a patient-level meta-analysis. Circ Cardiovasc Interv 2018; 11: e005795. [DOI] [PubMed] [Google Scholar]
- 72.Gareau AJ, Brien C, Gebremeskel S, et al. Ticagrelor inhibits platelet-tumor cell interactions and metastasis in human and murine breast cancer. Clin Exp Metastasis 2018; 35: 25–35. [DOI] [PubMed] [Google Scholar]
- 73.Wright JR, Chauhan M, Shah C, et al. The TICONC (Ticagrelor-Oncology) study: implications of P2Y12 inhibition for metastasis and cancer-associated thrombosis. JACC Cardiooncol 2020; 2: 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 75.Shoamanesh A, Mundl H, Smith EE, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 2022; 400: 997–1007. [DOI] [PubMed] [Google Scholar]
- 76.Sharma M, Molina CA, Toyoda K, et al. Rationale and design of the AXIOMATIC-SSP phase II trial: antithrombotic treatment with factor XIa inhibition to optimize management of acute thromboembolic events for secondary stroke prevention. J Stroke Cerebrovasc Dis 2022; 31: 106742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Zhao J, Lei Y, et al. Coronary atherosclerotic disease and cancer: risk factors and interrelation. Front Cardiovasc Med 2022; 9: 821267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454: 436–444. [DOI] [PubMed] [Google Scholar]
- 79.Papadopoulos A, Palaiopanos K, Bjorkbacka H, et al. Circulating interleukin-6 levels and incident ischemic stroke: a systematic review and meta-analysis of prospective studies. Neurology 2022; 98: e1002–e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aloizou AM, Siokas V, Pateraki G, et al. Thinking outside the ischemia box: advancements in the use of multiple sclerosis drugs in ischemic stroke. J Clin Med 2021; 10: 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Navi BB, Reiner AS, Kamel H, et al. Association between incident cancer and subsequent stroke. Ann Neurol 2015; 77: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 2011; 118: 4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahrenbrock M, Borsig L, Le D, et al. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest 2003; 112: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009; 9: 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]