Abstract
Background
Cancer is currently the second leading cause of death globally. There is much uncertainty regarding the comparative risks of new‐onset overall cancer and pre‐specified cancer for Type 2 diabetes mellitus (T2DM) patients on sodium‐glucose cotransporter 2 inhibitors (SGLT2I) versus DPP4I.
Methods
This population‐based cohort study patients included patients who were diagnosed with T2DM and administered either SGLT2 or DPP4 inhibitors between 1 January 2015 and 31 December 2020 in public hospitals of Hong Kong.
Results
This study included 60,112 T2DM patients (mean baseline age: 62.1 ± 12.4 years, male: 56.36%), of which 18,167 patients were SGLT2I users and 41,945 patients were dipeptidyl peptidase 4 inhibitor (DPP4I) users. Multivariable Cox regression found that SGLT2I use was associated with lower risks of all‐cause mortality (HR: 0.92; 95% CI: 0.84–0.99; p= 0.04), cancer‐related mortality (HR: 0.58; 95% CI: 0.42–0.80; p ≤ 0.001) and new diagnoses of any cancer (HR: 0.70; 95% CI: 0.59–0.84; p ≤ 0.001). SGLT2I use was associated with a lower risk of new‐onset breast cancer (HR: 0.51; 95% CI: 0.32–0.80; p ≤ 0.001), but not of other malignancies. Subgroup analysis on the type of SGLT2I, dapagliflozin (HR: 0.78; 95% CI: 0.64–0.95; p = 0.01) and ertugliflozin (HR: 0.65; 95% CI: 0.43–0.98; p = 0.04) use was associated with lower risks of new cancer diagnosis. Dapagliflozin use was also linked to lower risks of breast cancer (HR: 0.48; 95% CI: 0.27–0.83; p = 0.001).
Conclusion
Sodium‐glucose cotransporter 2 inhibitor use was associated with lower risks of all‐cause mortality, cancer‐related mortality and new‐onset overall cancer compared to DPP4I use after propensity score matching and multivariable adjustment.
Short abstract
SGLT2I was associated with lower risk of all‐cause mortality, cancer‐related mortality and new‐onset overall cancer compared to DPP4I after propensity score matching and multivariable adjustment.
1. INTRODUCTION
The burden of cancer incidence has drastically increased over the years and is currently the second leading cause of death globally. In 2020, the Global Cancer Observatory estimated a total of 19.3 million new cancer cases and 10 million cancer deaths. 1 Despite efforts to advance preventive interventions, the asymptomatic nature of the disease during its early stages poses a challenge for cancer diagnosis. 2 , 3 Although the aetiology of some cancer types still requires further exploration, currently established risk factors include but are not limited to Type 2 diabetes mellitus (T2DM), hypertension and smoking. 4 Numerous epidemiological studies have found supporting evidence for the association between T2DM and many different types of cancer, such as liver cancer, breast cancer and colorectal cancer. 5 , 6 As such, this has generated growing interest into anti‐diabetic medications as a potential adjuvant in the clinical management of cancer.
Metformin, in multiple pre‐clinical studies, has been described to be useful in the treatment of various types of malignancies. 7 , 8 , 9 However, current evidence presents conflicting results regarding the use of novel anti‐diabetic agents such as sodium‐glucose cotransporter 2 inhibitors (SGLT2I) and dipeptidyl peptidase 4 inhibitors (DPP4I). A systematic review and meta‐analysis revealed canagliflozin had protective effects against gastrointestinal cancers, while empagliflozin was found to have increased risks of bladder cancer. 10 Similarly, previous studies have reported increased risks of liver, kidney and bladder cancer and melanoma in T2DM patients using DPP4I. 11 In stark contrast, there is also evidence to suggest the absence of any association between these medications and malignancy, even when stratified by different subtypes of DPP4I. 12 Regarding SGLT2I, a retrospective study from Taiwan found SGLT2I usage was associated with lower risks of cancer‐related mortality relative to DPP4I. 13 Likewise, another investigation comparing the risk of urinary tract and haematological malignancies amongst SGLT2I and DPP4I users demonstrated superiority of the former. 14
Despite the aforementioned findings, there is still much uncertainty regarding the comparative associations between SGLT2I and DPP4I with different types of new‐onset overall cancer. 15 , 16 Given the prevalence with which these medications are used, the present study aims to assess the effects of SGLT2I versus DPP4I on the risk of new‐onset overall cancer and pre‐specified cancers in T2DM patients from Hong Kong.
2. METHOD
2.1. Study population
This population‐based, retrospective study has assessed integrated medical records of patients through the Clinical Data Analysis and Reporting System (CDARS), including disease diagnosis, laboratory results, past comorbidities, medication prescription details and clinical characteristics. The system has also been used by our team in previous epidemiological research in Hong Kong. 17 , 18 , 19 Patients who were diagnosed with T2DM and were administered either SGLT2 or DPP4 inhibitors, between 1 January 2015 and 31 December 2020, in centres under the Hong Kong Hospital Authority were included in the study cohort. The exclusion criteria for the cohort were as follows: (1) patients who died within 30 days after initial drug exposure; (2) patients under 18 years old; (3) patients with prior all‐cause malignancies; (4) patients with new‐onset all‐cause malignancies development less than 1 year after drug exposure; and (5) patients with both DPP4I and SGLT2I prescription. The study has received Ethics Approval from The Joint Chinese University of Hong Kong‐New Territories East Cluster Clinical Research Ethics Committee (Application reference: 2018.643, 2018.309).
2.2. Clinical and biochemical data collection
Biochemical and clinical data were extracted for this cohort. Patients' demographic information includes sex, baseline age and date of initial drug use. Past comorbidities include diabetes mellitus disease duration, hyperlipidaemia, obesity, hypertension, alcoholism, liver diseases, autoimmune diseases, HIV, carcinogen pathogens, previous irradiation, chronic obstructive pulmonary disease, gastrointestinal diseases, cardiovascular diseases, ischemic stroke, diabetic eye diseases and renal diseases. Moreover, Charlson's standard comorbidity index was also calculated. Renal function was calculated using the CKD‐EPI equation. 20
Moreover, anti‐diabetic and non‐SGLT2I/DPP4 medications and baseline laboratory data results were also extracted. Data on the following medications were extracted: sulphonylurea, insulin, metformin, thiazolidinedione, acarbose, glucagon‐like peptide‐1 receptor agonists, statins and fibrates, Angiotensin‐converting enzyme inhibitors, Angiotensin receptor blockers, anti‐depressant drugs, antihypertensive drugs, anti‐hepatitis drugs, anticoagulants, diuretics, nitrates, beta‐blockers, calcium channel blockers and non‐steroidal anti‐inflammatory drugs. The extracted laboratory data include lipid profiles, complete blood count, renal function test, biochemical test and glycaemic profiles.
2.3. Outcome and statistical analysis
The primary outcome of this study was new‐onset all‐cause cancer incidence, all‐cause cancer‐related mortality and all‐cause mortality. Mortality data were extracted from the Hong Kong Death Registry, an official government registry linked with CDARS that registers death records of all Hong Kong citizens. Study outcomes and comorbidities were documented using the ICD‐9 codes, whilst mortality outcomes were recorded using the ICD‐10 coding system. ICD‐10 codes C00‐C97 were used to identify all‐cause cancer mortality. The ICD‐9 and ICD‐10 codes are summarised in Table S1.
Descriptive statistics were used to summarise baseline characteristics for this cohort. Mean and standard deviation (SD) was used to represent continuous variables, while a number and percentage were used to represent categorical variables. Propensity score matching with a 1:1 ratio between SGLT2I and DPP4I users and patients with and without new‐onset overall cancer risk based on demographics, prior comorbidities, laboratory data, medication usage, Charlson comorbidity index and abbreviated modification of diet in renal disease were performed using the nearest neighbour strategy with the Calliper set at 0.1. Univariable and multivariable Cox proportional regressions were performed for both before and after matching to identify significant predictors of new‐onset all‐cause cancer occurrence and mortality. This is further corroborated by the inverse probability of treatment weighting using propensity scores and calculating incidence rate ratios. Cumulative incidence curves were also calculated to visually depict the difference in the time‐to‐adverse event by comparing the SGLT2I and DPP4I groups. p < 0.05 was considered statistically significant. Statistical analyses and propensity score matching was performed with RStudio software (version: 1.1.456) and Stata software (version 13.0), respectively.
3. RESULTS
3.1. Baseline characteristics
This study included 60,112 T2DM patients (mean baseline age: 62.1 ± 12.4 years, male: 56.36%, mean diabetes mellitus disease duration to baseline date: 640.6 ± 1264.0 days), of which 18,167 patients were SGLT2I users and 41,945 patients were DPP4I users. In the SGLT2I subgroup, the corresponding number of patients on individual SGLT2Is is as follows: 4523 (24.89%) on canagliflozin, 10,556 (58.10%) on dapagliflozin, 3780 (20.80%) on empagliflozin and 2527 (13.90%) on ertugliflozin. During the follow‐up period, 1533 patients developed new‐onset overall cancer, 3033 patients died from any cause, of which 506 patients died due to cancer‐related causes. Data on specific types of new‐onset overall cancers were also extracted: 249 patients developed new‐onset lung cancer, 817 patients developed new‐onset gastrointestinal cancer, 201 patients developed new‐onset breast cancer, 261 patients developed new‐onset genitourinary cancer and 97 patients developed new‐onset bladder cancer (Figure 1). The baseline characteristics for continuous and discrete variables of demographics, laboratory and medication histories for patients before and after matching are shown in Table 1, and Table S3A–C. The method of variability (standard deviation) calculation is shown in Table S2.
FIGURE 1.
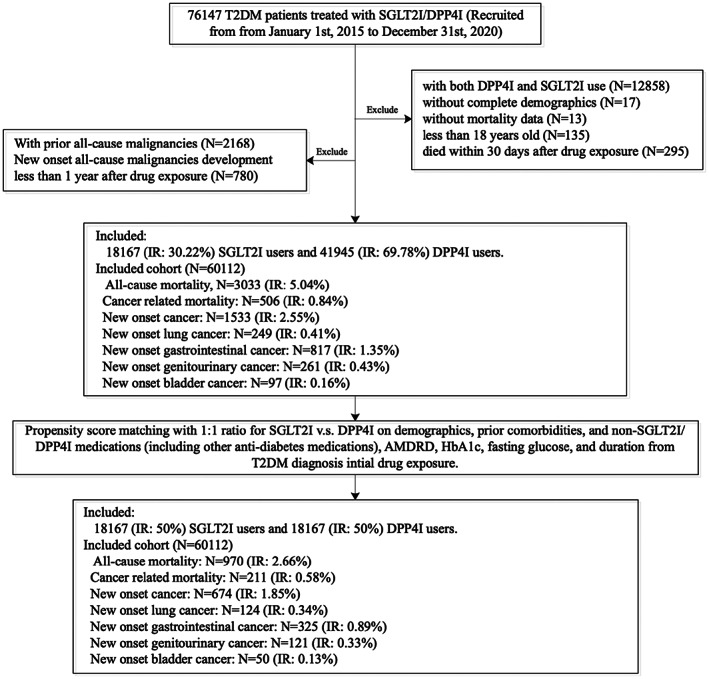
Procedures of data processing for the study cohort. DPP4I, dipeptidyl peptidase‐4 inhibitors; IR, incidence rate; SGLT2I, sodium‐glucose cotransporter‐2 inhibitors.
TABLE 1.
Baseline and clinical characteristics of patients with SGLT2I versus DPP4I use before and after propensity score matching (1:1).
| Before matching | After matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | All (N = 60,112) Mean (SD); N or count (%) | SGLT2I users (N = 18,167) Mean (SD); N or count (%) | DPP4I users (N = 41,945) Mean (SD); N or count (%) | SMD | All (N = 36,334) Mean (SD); N or count (%) | SGLT2I users (N = 18,167) Mean (SD); N or count (%) | DPP4I users (N = 18,167) Mean (SD); N or count (%) | SMD |
| Demographics | ||||||||
| Male gender | 33,883 (56.36%) | 11,138 (61.30%) | 22,745 (54.22%) | 0.14 | 22,288 (61.34%) | 11,147 (61.35%) | 11,141 (61.32%) | <0.01 |
| Female gender | 26,229 (43.63%) | 7029 (38.69%) | 19,200 (45.77%) | 0.14 | 14,046 (38.65%) | 7020 (38.64%) | 7026 (38.67%) | <0.01 |
| Baseline age, years | 62.1 (12.4); n = 60,112 | 57.8 (11.2); n = 18,167 | 63.9 (12.5); n = 41,945 | 0.51 a | 58.2 (11.0); n = 36,334 | 57.8 (11.2); n = 18,167 | 58.7 (10.9); n = 18,167 | 0.08 |
| 18–50 | 9057 (15.06%) | 3878 (21.34%) | 5179 (12.34%) | 0.24 a | 7212 (19.84%) | 3888 (21.40%) | 3324 (18.29%) | 0.08 |
| 50–60 | 17,550 (29.19%) | 6524 (35.91%) | 11,026 (26.28%) | 0.21 a | 13,285 (36.56%) | 6543 (36.01%) | 6742 (37.11%) | 0.02 |
| 60–70 | 18,029 (29.99%) | 5464 (30.07%) | 12,565 (29.95%) | <0.01 | 11,194 (30.80%) | 5457 (30.03%) | 5737 (31.57%) | 0.03 |
| 70–80 | 10,338 (17.19%) | 1883 (10.36%) | 8455 (20.15%) | 0.27 a | 3766 (10.36%) | 1870 (10.29%) | 1896 (10.43%) | <0.01 |
| >80 | 5145 (8.55%) | 421 (2.31%) | 4724 (11.26%) | 0.36 a | 881 (2.42%) | 412 (2.26%) | 469 (2.58%) | 0.02 |
| Past comorbidities | ||||||||
| Charlson standard comorbidity index | 1.9 (1.4); n = 60,112 | 1.5 (1.2); n = 18,167 | 2.1 (1.4); n = 41,945 | 0.45 a | 1.5 (1.2); n = 36,334 | 1.52 (1.17); n = 18,167 | 1.55 (1.14); n = 18,167 | 0.02 |
| Duration from earliest diabetes mellitus date to baseline date, day | 619.9 (1341.9); n = 60,112 | 616.9 (1352.3); n = 18,167 | 621.2 (1337.4); n = 41,945 | <0.01 | 590.9 (1323.1); n = 36,334 | 618.7 (1354.2); n = 18,167 | 563.1 (1290.6); n = 18,167 | 0.04 |
| Hypertension | 13,376 (22.25%) | 4284 (23.58%) | 9092 (21.67%) | 0.05 | 8325 (22.91%) | 4283 (23.57%) | 4042 (22.24%) | 0.03 |
| Hyperlipidaemia | 1620 (2.69%) | 66 2 (3.64%) | 958 (2.28%) | 0.08 | 1265 (3.48%) | 659 (3.62%) | 606 (3.33%) | 0.02 |
| Hypotension | 350 (0.58%) | 76 (0.41%) | 274 (0.65%) | 0.03 | 152 (0.41%) | 75 (0.41%) | 77 (0.42%) | <0.01 |
| Overweight, obesity and hyperalimentation | 432 (0.71%) | 297 (1.63%) | 135 (0.32%) | 0.13 | 576 (1.58%) | 300 (1.65%) | 276 (1.51%) | 0.01 |
| Gout | 1510 (2.51%) | 394 (2.16%) | 1116 (2.66%) | 0.03 | 782 (2.15%) | 394 (2.16%) | 388 (2.13%) | <0.01 |
| Heart failure | 1629 (2.70%) | 447 (2.46%) | 1182 (2.81%) | 0.02 | 874 (2.40%) | 447 (2.46%) | 427 (2.35%) | 0.01 |
| Acute myocardial infarction | 1521 (2.53%) | 616 (3.39%) | 905 (2.15%) | 0.08 | 1210 (3.33%) | 613 (3.37%) | 597 (3.28%) | <0.01 |
| Ischaemic heart disease | 5680 (9.44%) | 2342 (12.89%) | 3338 (7.95%) | 0.16 | 4466 (12.29%) | 2333 (12.84%) | 2133 (11.74%) | 0.03 |
| Peripheral vascular disease | 375 (0.62%) | 97 (0.53%) | 278 (0.66%) | 0.02 | 194 (0.53%) | 99 (0.54%) | 95 (0.52%) | <0.01 |
| Stroke/transient ischaemic attack | 1777 (2.95%) | 474 (2.60%) | 1303 (3.10%) | 0.03 | 940 (2.58%) | 477 (2.62%) | 463 (2.54%) | <0.01 |
| Atrial fibrillation | 1325 (2.20%) | 388 (2.13%) | 937 (2.23%) | 0.01 | 766 (2.10%) | 386 (2.12%) | 380 (2.09%) | <0.01 |
| Diabetic eye disease | 4045 (6.72%) | 1322 (7.27%) | 2723 (6.49%) | 0.03 | 2459 (6.76%) | 1321 (7.27%) | 1138 (6.26%) | 0.04 |
| Alcohol dependence | 119 (0.19%) | 22 (0.12%) | 97 (0.23%) | 0.03 | 44 (0.12%) | 23 (0.12%) | 21 (0.11%) | <0.01 |
| Chronic liver disease and cirrhosis | 1195 (1.98%) | 513 (2.82%) | 682 (1.62%) | 0.08 | 995 (2.73%) | 515 (2.83%) | 480 (2.64%) | 0.01 |
| Viral hepatitis | 629 (1.04%) | 209 (1.15%) | 420 (1.00%) | 0.01 | 414 (1.13%) | 211 (1.16%) | 203 (1.11%) | <0.01 |
| History of acute liver injury | 159 (0.26%) | 36 (0.19%) | 123 (0.29%) | 0.02 | 72 (0.19%) | 36 (0.19%) | 36 (0.19%) | <0.01 |
| Other liver disease | 612 (1.01%) | 163 (0.89%) | 449 (1.07%) | 0.02 | 324 (0.89%) | 160 (0.88%) | 164 (0.90%) | <0.01 |
| Autoimmune disease tissue | 620 (1.03%) | 198 (1.08%) | 422 (1.00%) | 0.01 | 386 (1.06%) | 200 (1.10%) | 186 (1.02%) | 0.01 |
| Infections by pathogens associated with cancer development (Helicobacterpylori, Human papillomavirus, Infectious mononucleosis) | 13 (0.02%) | 6 (0.03%) | 7 (0.01%) | 0.01 | 12 (0.03%) | 6 (0.03%) | 6 (0.03%) | <0.01 |
| Chronic obstructive pulmonary disease | 69 (0.11%) | 12 (0.06%) | 57 (0.13%) | 0.02 | 24 (0.06%) | 12 (0.06%) | 12 (0.06%) | <0.01 |
| Gastrointestinal disease | 1353 (2.25%) | 349 (1.92%) | 1004 (2.39%) | 0.03 | 690 (1.89%) | 350 (1.92%) | 340 (1.87%) | <0.01 |
| Medications | ||||||||
| SGLT2I vs. DPP4I | 18,167 (30.22%) | 18,167 (100.00%) | 0 (0.00%) | inf a | 18,167 (50.00%) | 18,167 (100.00%) | 0 (0.00%) | inf a |
| SGLT2I frequency | 7.4 (10.1); n = 18,167 | 7.4 (10.1); n = 18,167 | – | – | 7.3 (10.0); n = 18,167 | 7.3 (10.0); n = 18,167 | – | – |
| DPP4I frequency | 5.5 (7.2); n = 41,945 | – | 5.5 (7.2); n = 41,945 | – | 6.3 (8.0); n = 18,167 | – | 6.3 (8.0); n = 18,167 | – |
| SGLT2I duration, days | 538.1 (674.5); n = 18,167 | 538.1 (674.5); n = 18,167 | – | – | 538.5 (673.6); n = 18,167 | 538.5 (673.6); n = 18,167 | – | – |
| DPP4I duration, days | 528.9 (301.0); n = 41,945 | – | 528.9 (301.0); n = 41,945 | – | 575.3 (360.3); n = 18,167 | – | 575.3 (360.3); n = 18,167 | – |
| Metformin | 54,407 (90.50%) | 16,917 (93.11%) | 37,490 (89.37%) | 0.13 | 33,945 (93.42%) | 16,923 (93.15%) | 17,022 (93.69%) | 0.02 |
| Sulphonylurea | 46,521 (77.39%) | 12,899 (71.00%) | 33,622 (80.15%) | 0.21 a | 26,376 (72.59%) | 12,889 (70.94%) | 13,487 (74.23%) | 0.07 |
| Insulin | 29,349 (48.82%) | 9517 (52.38%) | 19,832 (47.28%) | 0.1 | 19,074 (52.49%) | 9509 (52.34%) | 9565 (52.65%) | 0.01 |
| Acarbose | 1566 (2.60%) | 751 (4.13%) | 815 (1.94%) | 0.13 | 1429 (3.93%) | 744 (4.09%) | 685 (3.77%) | 0.02 |
| Thiazolidinedone | 12,046 (20.03%) | 5130 (28.23%) | 6916 (16.48%) | 0.28 a | 9569 (26.33%) | 5134 (28.26%) | 4435 (24.41%) | 0.09 |
| Glucagon‐like peptide‐1 receptor agonists | 2044 (3.40%) | 1505 (8.28%) | 539 (1.28%) | 0.33 a | 2797 (7.69%) | 1506 (8.28%) | 1291 (7.10%) | 0.04 |
| ACEI/ARB | 18,249 (30.35%) | 11,149 (61.36%) | 7100 (16.92%) | 1.02 a | 22,352 (61.51%) | 11,152 (61.38%) | 11,200 (61.65%) | 0.01 |
| Antidepressants | 2742 (4.56%) | 1665 (9.16%) | 1077 (2.56%) | 0.28 a | 2971 (8.17%) | 1661 (9.14%) | 1310 (7.21%) | 0.07 |
| Antihypertensive drugs | 2311 (3.84%) | 1681 (9.25%) | 630 (1.50%) | 0.35 a | 3051 (8.39%) | 1680 (9.24%) | 1371 (7.54%) | 0.06 |
| Antihepatitis | 809 (1.34%) | 336 (1.84%) | 473 (1.12%) | 0.06 | 664 (1.82%) | 337 (1.85%) | 327 (1.79%) | <0.01 |
| Anticoagulants | 29,401 (48.91%) | 18,166 (99.99%) | 11,235 (26.78%) | 2.34 a | 36,332 (99.99%) | 18,166 (99.99%) | 18,166 (99.99%) | <0.01 |
| Antiplatelets | 10,115 (16.82%) | 5981 (32.92%) | 4134 (9.85%) | 0.59 a | 11,686 (32.16%) | 5975 (32.88%) | 5711 (31.43%) | 0.03 |
| Statins and fibrates | 34,307 (57.07%) | 13,773 (75.81%) | 20,534 (48.95%) | 0.58 a | 27,749 (76.37%) | 13,780 (75.85%) | 13,969 (76.89%) | 0.02 |
| Nitrates | 4652 (7.73%) | 2688 (14.79%) | 1964 (4.68%) | 0.35 a | 5278 (14.52%) | 2684 (14.77%) | 2594 (14.27%) | 0.01 |
| Non‐steroidal anti‐inflammatory drugs | 9719 (16.16%) | 5734 (31.56%) | 3985 (9.50%) | 0.57 a | 11,369 (31.29%) | 5730 (31.54%) | 5639 (31.03%) | 0.01 |
| Diuretics | 10,179 (16.93%) | 5633 (31.00%) | 4546 (10.83%) | 0.51 a | 11,095 (30.53%) | 5628 (30.97%) | 5467 (30.09%) | 0.02 |
| Beta‐blockers | 8075 (13.43%) | 4696 (25.84%) | 3379 (8.05%) | 0.49 a | 9261 (25.48%) | 4694 (25.83%) | 4567 (25.13%) | 0.02 |
| Calcium channel blockers | 14,154 (23.54%) | 8107 (44.62%) | 6047 (14.41%) | 0.70 a | 16,462 (45.30%) | 8102 (44.59%) | 8360 (46.01%) | 0.03 |
| Subclinical biomarkers | ||||||||
| Abbreviated MDRD, mL/min/1.73 m2 | 81.4 (27.9); n = 49,633 | 90.1 (23.8); n = 15,520 | 77.4 (28.8); n = 34,113 | 0.48 a | 88.6 (23.5); n = 29,687 | 90.1 (23.8); n = 15,514 | 86.9 (23.1); n = 14,173 | 0.14 |
| Most severe renal damage (<15 mL/min/1.73 m2) | 436.0 (0.72%) | 16.0 (0.08%) | 420.0 (1.00%) | 0.12 | 40.0 (0.11%) | 16.0 (0.08%) | 24.0 (0.13%) | 0.01 |
| Severe renal damage ([15, 30] mL/min/1.73 m2) | 1108.0 (1.84%) | 41.0 (0.22%) | 1067.0 (2.54%) | 0.2 | 111.0 (0.30%) | 39.0 (0.21%) | 72.0 (0.39%) | 0.03 |
| Moderate to severe renal damage ([30, 45] mL/min/1.73 m2) | 3548.0 (5.90%) | 265.0 (1.45%) | 3283.0 (7.82%) | 0.31 a | 657.0 (1.80%) | 261.0 (1.43%) | 396.0 (2.17%) | 0.06 |
| Mild to moderate renal damage ([45, 60] mL/min/1.73 m2) | 5816.0 (9.67%) | 999.0 (5.49%) | 4817.0 (11.48%) | 0.22 a | 2222.0 (6.11%) | 1000.0 (5.50%) | 1222.0 (6.72%) | 0.05 |
| Mild renal damage ([60, 90] mL/min/1.73 m2) | 19920.0 (33.13%) | 6788.0 (37.36%) | 13132.0 (31.30%) | 0.13 | 12997.0 (35.77%) | 6783.0 (37.33%) | 6214.0 (34.20%) | 0.07 |
| Chronic kidney disease (>90 mL/min/1.73 m2) | 18805.0 (31.28%) | 7411.0 (40.79%) | 11394.0 (27.16%) | 0.29 a | 13660.0 (37.59%) | 7415.0 (40.81%) | 6245.0 (34.37%) | 0.13 |
| Neutrophil‐to‐lymphocyte ratio | 3.4 (4.5); n = 23,978 | 3.0 (3.9); n = 8133 | 3.6 (4.8); n = 15,845 | 0.15 | 3.1 (3.8); n = 15,970 | 3.0 (3.9); n = 8146 | 3.2 (3.7); n = 7824 | 0.05 |
| Platelet‐to‐lymphocyte ratio | 142.0 (148.2); n = 23,976 | 133.5 (168.7); n = 8131 | 146.4 (136.3); n = 15,845 | 0.08 | 133.6 (137.8); n = 15,969 | 133.3 (168.5); n = 8144 | 133.9 (95.8); n = 7825 | <0.01 |
| Neutrophil‐to‐high‐density lipoprotein ratio | 0.3 (0.2); n = 21,968 | 0.27 (0.16); n = 7755 | 0.27 (0.2); n = 14,213 | 0.01 | 0.3 (0.2); n = 15,080 | 0.27 (0.16); n = 7770 | 0.27 (0.16); n = 7310 | 0.01 |
| Low density lipoprotein ratio‐to‐high density lipoprotein ratio | 2.1 (0.9); n = 46,116 | 2.14 (0.83); n = 14,654 | 2.1 (0.86); n = 31,462 | 0.05 | 2.1 (0.8); n = 27,933 | 2.14 (0.83); n = 14,653 | 2.13 (0.84); n = 13,280 | 0.01 |
| Triglyceride‐glucose index | 7.6 (0.7); n = 42,025 | 7.6 (0.7); n = 13,571 | 7.5 (0.7); n = 28,454 | 0.15 | 7.6 (0.7); n = 25,514 | 7.64 (0.72); n = 13,576 | 7.64 (0.73); n = 11,938 | 0.01 |
| Protein‐to‐creatinine ratio | 3.0 (1.5); n = 29,872 | 3.4 (1.5); n = 10,496 | 2.8 (1.5); n = 19,376 | 0.34 a | 3.3 (1.5); n = 19,746 | 3.4 (1.5); n = 10,502 | 3.2 (1.4); n = 9244 | 0.13 |
| Aspartate aminotransferase‐to‐alanine transaminase ratio | 1.1 (3.2); n = 8773 | 0.9 (1.0); n = 3284 | 1.2 (3.9); n = 5489 | 0.08 | 0.9 (0.8); n = 6377 | 0.92 (0.99); n = 3279 | 0.94 (0.5); n = 3098 | 0.03 |
| Complete blood counts, renal and liver functions | ||||||||
| Mean corpuscular volume, fL | 87.1 (7.5); n = 29,998 | 86.6 (7.2); n = 10,523 | 87.4 (7.6); n = 19,475 | 0.1 | 86.7 (7.2); n = 19,809 | 86.6 (7.2); n = 10,529 | 86.8 (7.3); n = 9280 | 0.03 |
| Potassium, mmol/L | 4.3 (0.5); n = 49,471 | 4.3 (0.4); n = 15,489 | 4.4 (0.5); n = 33,982 | 0.1 | 4.3 (0.4); n = 29,604 | 4.31 (0.43); n = 15,483 | 4.27 (0.45); n = 14,121 | 0.1 |
| Albumin, g/L | 41.9 (3.8); n = 37,720 | 42.5 (3.3); n = 13,085 | 41.5 (4.0); n = 24,635 | 0.27 a | 42.5 (3.3); n = 24,595 | 42.5 (3.3); n = 13,089 | 42.4 (3.4); n = 11,506 | 0.03 |
| Sodium, mmol/L | 139.3 (2.9); n = 49,494 | 139.1 (2.7); n = 15,492 | 139.3 (3.0); n = 34,002 | 0.07 | 139.3 (2.7); n = 29,612 | 139.1 (2.7); n = 15,486 | 139.4 (2.7); n = 14,126 | 0.08 |
| Urea, mmol/L | 6.4 (3.3); n = 49,483 | 5.7 (2.0); n = 15,486 | 6.8 (3.7); n = 33,997 | 0.34 a | 5.8 (2.1); n = 29,607 | 5.7 (2.0); n = 15,480 | 5.8 (2.2); n = 14,127 | 0.05 |
| Protein, g/L | 73.9 (5.4); n = 35,490 | 74.3 (4.9); n = 12,386 | 73.8 (5.6); n = 23,104 | 0.1 | 74.4 (5.0); n = 23,401 | 74.3 (4.9); n = 12,387 | 74.5 (5.0); n = 11,014 | 0.05 |
| Creatinine, μmol/L | 92.5 (71.5); n = 49,633 | 79.0 (28.9); n = 15,520 | 98.6 (83.2); n = 34,113 | 0.31 a | 80.4 (33.0); n = 29,687 | 79.0 (28.9); n = 15,514 | 82.0 (36.9); n = 14,173 | 0.09 |
| Alkaline phosphatase, U/L | 76.2 (30.1); n = 37,836 | 73.5 (26.1); n = 13,091 | 77.6 (31.9); n = 24,745 | 0.14 | 74.8 (27.2); n = 24,597 | 73.5 (26.1); n = 13,095 | 76.2 (28.3); n = 11,502 | 0.1 |
| Aspartate transaminase, U/L | 28.2 (53.4); n = 15,006 | 28.1 (26.9); n = 5161 | 28.2 (63.0); n = 9845 | <0.01 | 28.8 (30.5); n = 10,477 | 28.2 (26.9); n = 5162 | 29.4 (33.6); n = 5315 | 0.04 |
| Alanine transaminase, U/L | 29.4 (33.6); n = 32,037 | 32.1 (28.1); n = 11,145 | 28.0 (36.1); n = 20,892 | 0.13 | 32.3 (27.5); n = 20,438 | 32.1 (28.2); n = 11,145 | 32.4 (26.6); n = 9293 | 0.01 |
| Bilirubin, μmol/L | 11.3 (6.7); n = 37,645 | 11.4 (5.6); n = 13,060 | 11.2 (7.1); n = 24,585 | 0.04 | 11.4 (5.7); n = 24,545 | 11.4 (5.6); n = 13,064 | 11.3 (5.8); n = 11,481 | 0.01 |
| Lipid profiles | ||||||||
| Triglyceride, mmol/L | 1.7 (1.6); n = 46,943 | 1.8 (1.7); n = 14,910 | 1.7 (1.5); n = 32,033 | 0.06 | 1.8 (1.8); n = 28,423 | 1.8 (1.75); n = 14,910 | 1.82 (1.84); n = 13,513 | 0.01 |
| Low‐density lipoprotein, mmol/L | 2.4 (0.8); n = 46,121 | 2.37 (0.8); n = 14,658 | 2.4 (0.8); n = 31,463 | 0.04 | 2.4 (0.8); n = 27,937 | 2.37 (0.8); n = 14,657 | 2.39 (0.79); n = 13,280 | 0.03 |
| High‐density lipoprotein, mmol/L | 1.2 (0.3); n = 46,872 | 1.16 (0.31); n = 14,885 | 1.21 (0.33); n = 31,987 | 0.15 | 1.2 (0.3); n = 28,370 | 1.16 (0.31); n = 14,885 | 1.18 (0.32); n = 13,485 | 0.06 |
| Total cholesterol, mmol/L | 4.3 (1.0); n = 46,986 | 4.3 (1.0); n = 14,929 | 4.4 (1.0); n = 32,057 | 0.04 | 4.3 (1.0); n = 28,445 | 4.3 (1.0); n = 14,929 | 4.4 (1.0); n = 13,516 | 0.05 |
| Haemoglobin A1C, % | 8.1 (1.5); n = 48,920 | 8.3 (1.6); n = 15,353 | 8.0 (1.5); n = 33,567 | 0.21 a | 8.3 (1.6); n = 29,345 | 8.3 (1.57); n = 15,352 | 8.26 (1.61); n = 13,993 | 0.03 |
| Fasting glucose, mmol/L | 9.9 (3.3); n = 34,323 | 10.1 (3.2); n = 11,828 | 9.8 (3.4); n = 22,495 | 0.08 | 10.1 (3.3); n = 22,141 | 10.1 (3.2); n = 11,841 | 10.0 (3.5); n = 10,300 | 0.02 |
Abbreviations: ACEI/ARB, Angiotensin‐converting enzyme inhibitors Angiotensin receptor blockers; DPP4I, dipeptidyl peptidase‐4 inhibitor; MDRD, modification of diet in renal disease; SD, standard deviation; SGLT2I, sodium glucose cotransporter‐2 inhibitor.
For SMD ≥0.2.
The cumulative incidence of primary and secondary outcomes after propensity score matching is shown in Table 2A. The cumulative incidences of these outcomes stratified by initial drug exposure age, drug use, the combination of gender and drug exposure and combination of age and drug exposure effects are summarised by cumulative incidence curves (Figures 2B). Gender‐based and age‐based trends in the incidence of the different outcomes are shown in Figure S3A,B. Furthermore, summary figures of comparing annual incidence ratios with 95% CIs of different adverse events stratified by drug use are presented in Figure 3A.
TABLE 2A.
Annualised incidence rate (IR) per 1000 person‐years of primary and secondary cancer outcomes, all‐cause mortality and cancer related mortality in the cohort before and after 1:1 propensity score matching.
| Before matching | After 1:1 propensity score matching | ||||||
|---|---|---|---|---|---|---|---|
| All‐cause mortality | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.29 × 105 | 3033 | 9.2 [8.6–9.6] | 2.01 × 105 | 970 | 4.8 [4.5–5.1] | ||
| Cancer‐related mortality | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.29 × 105 | 506 | 1.5 [1.4–1.7] | 2.01 × 105 | 211 | 1.1 [0.9–1.2] | ||
| New‐onset all‐cause cancer | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.26 × 105 | 1533 | 4.7 [4.5–4.9] | 2.00 × 105 | 674 | 3.4 [3.1–3.6] | ||
| New‐onset lung cancer | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.28 × 105 | 249 | 0.8 [0.7–0.9] | 2.01 × 105 | 124 | 0.6 [0.5–0.7] | ||
| New‐onset gastrointestinal cancer | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.27 × 105 | 817 | 2.5 [2.3–2.7] | 2.00 × 105 | 325 | 1.6 [1.5–1.8] | ||
| New‐onset bladder cancer | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.29 × 105 | 97 | 0.3 [0.2–0.4] | 2.01 × 105 | 50 | 0.2 [0.2–0.3] | ||
| New‐onset genitourinary cancer | |||||||
| Overall | Person‐year | Events | IR [95% CI] | Overall | Person‐year | Events | IR [95% CI] |
| 3.28 × 105 | 261 | 0.8 [0.7–0.9] | 2.01 × 105 | 121 | 0.6 [0.5–0.7] | ||
FIGURE 2.
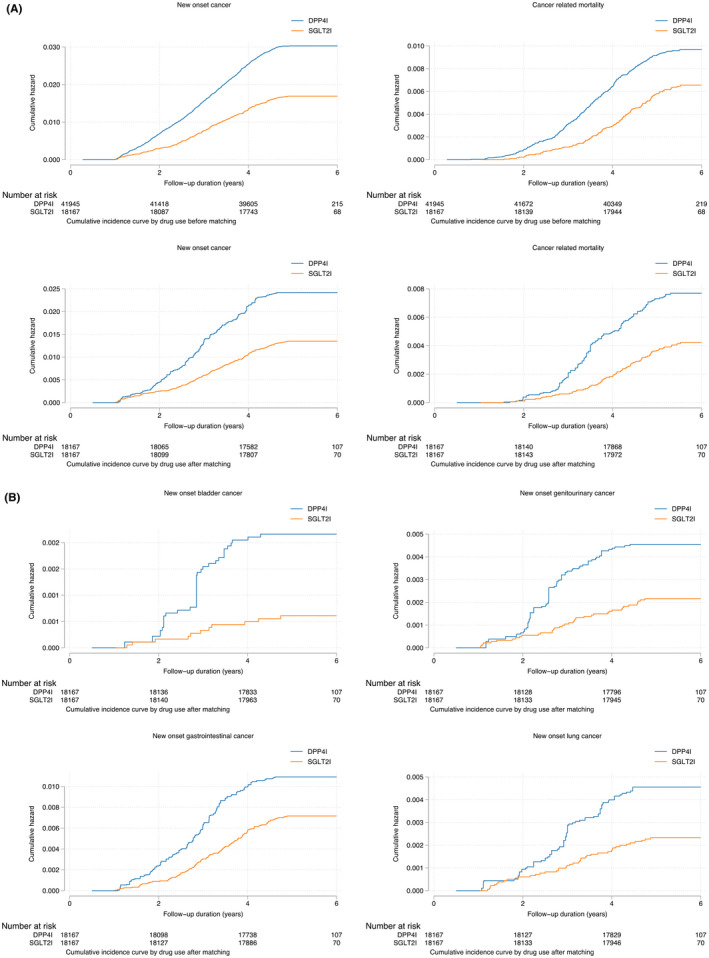
(A) Cumulative incidence curves for new‐onset cancer and cancer‐related mortality stratified by drug exposure effects of SGLT2I and DPP4I before and after propensity score matching (1:1). (B) Cumulative incidence curves for different new‐onset cancer outcomes stratified by drug exposure effects of SGLT2I and DPP4I in the matched cohort.
FIGURE 3.
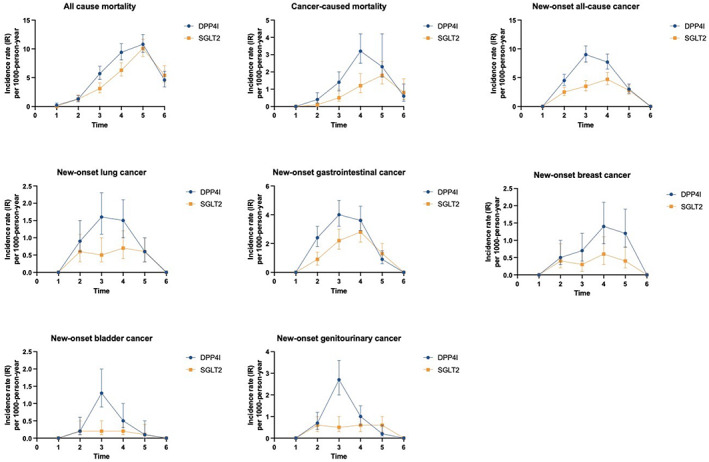
Summary figures of comparing annual incidence ratios with 95% CIs of different adverse events stratified by drug use.
3.2. Cox regression
The results of univariable Cox regression analysis for predicting general and pre‐specified cancer risk are displayed in Table S4A,B. Significant variables in univariable regression were subsequently incorporated into multivariable models to evaluate the relationship between SGLT2I and DPP4I with malignancy. As shown in Table 2B, after adjustment for significant demographics, past comorbidities, non‐SGLT2I/DPP4I medications, abbreviated MDRD, fasting glucose, HbA1c and duration from earliest diabetes mellitus date to initial drug exposure date, SGLT2I were associated with a comparatively decreased risk of all‐cause mortality (HR: 0.92; 95% CI: 0.84–0.99; p = 0.04), cancer‐related mortality (HR: 0.58; 95% CI: 0.42–0.80; p ≤ 0.001), as well as a 30% reduction in the risk of new‐onset overall cancer (HR: 0.70; 95% CI: 0.59–0.84; p = <0.001). When stratified by cancer subtype, SGLT2I were related to a lower risk of new‐onset breast cancer (HR: 0.51; 95% CI: 0.32–0.80; p = <0.001), but not with other malignancies. With subgroup analysis comparing DPP4I to different subtypes of SGLT2I, dapagliflozin (HR: 0.78; 95% CI: 0.64–0.95; p = 0.01) and ertugliflozin (HR: 0.65; 95% CI: 0.43–0.98; p = 0.04) both demonstrated superiority in relation to new‐onset overall cancer development, with the former also presenting with a relatively lower risk of breast cancer (HR: 0.48; 95% CI: 0.27–0.83; p = 0.001). There were no observable differences when comparing the use of either canagliflozin or empagliflozin with DPP4I in terms of overall or specific cancer risk.
TABLE 2B.
Multivariable Cox regression models with adjustments to predict new all‐cause cancers in the matched cohort.
| Characteristics | All‐cause mortality HR [95% CI]; p value | Cancer‐related mortality HR [95% CI]; p value | New‐onset cancer HR [95% CI]; p value | New‐onset lung cancer HR [95% CI]; p value | New‐onset gastrointestinal cancer HR [95% CI]; p value | New‐onset breast cancer HR [95% CI]; p value | New‐onset genitourinary cancer HR [95% CI]; p value | New‐onset bladder cancer HR [95% CI]; p value |
|---|---|---|---|---|---|---|---|---|
| Model 1 | ||||||||
| SGLT2I vs. DPP4I | 0.84 [0.74–0.96]; 0.0085** | 0.57 [0.43–0.75]; 0.0001*** | 0.58 [0.49–0.67]; <0.0001*** | 0.53 [0.37–0.77]; 0.0009*** | 0.68 [0.55–0.85]; 0.0008*** | 0.47 [0.31–0.71]; 0.0004*** | 0.48 [0.33–0.70]; 0.0002*** | 0.29 [0.15–0.56]; 0.0002*** |
| Dapagliflozin vs. DPP4I | 0.89 [0.77–1.03]; 0.1144 | 0.65 [0.46–0.91]; 0.0122* | 0.64 [0.53–0.77]; <0.0001*** | 0.69 [0.45–1.07]; 0.0952 | 0.65 [0.49–0.85]; 0.0016** | 0.42 [0.24–0.72]; 0.0018** | 0.71 [0.46–1.09]; 0.1215 | 0.41 [0.19–0.92]; 0.0302* |
| Empagliflozin vs. DPP4I | 0.75 [0.59–0.94]; 0.0143* | 0.53 [0.30–0.96]; 0.0346* | 0.75 [0.56–0.99]; 0.0423* | 0.59 [0.29–1.21]; 0.1512 | 0.86 [0.59–1.26]; 0.4304 | 0.68 [0.31–1.46]; 0.3180 | 0.70 [0.35–1.37]; 0.2966 | 0.53 [0.17–1.72]; 0.2933 |
| Canagliflozin vs. DPP4I | 0.94 [0.77–1.14]; 0.5218 | 0.82 [0.53–1.28]; 0.3897 | 0.72 [0.55–0.93]; 0.0123* | 0.47 [0.23–0.97]; 0.0406* | 0.90 [0.64–1.27]; 0.5531 | 1.14 [0.63–2.03]; 0.6683 | 0.30 [0.12–0.74]; 0.0092** | 0.14 [0.02–0.99]; 0.0485* |
| Ertugliflozin vs. DPP4I | 1.08 [0.85–1.37]; 0.5497 | 0.80 [0.45–1.44]; 0.4605 | 0.58 [0.40–0.84]; 0.0044** | 0.55 [0.22–1.35]; 0.1901 | 0.78 [0.48–1.25]; 0.2973 | 0.44 [0.14–1.40]; 0.1665 | 0.34 [0.11–1.07]; 0.0656 | 0.26 [0.04–1.88]; 0.1827 |
| Model 2 | ||||||||
| SGLT2I vs. DPP4I | 0.83 [0.73–0.94]; 0.0033** | 0.56 [0.42–0.74]; 0.0001*** | 0.57 [0.49–0.67]; <0.0001*** | 0.52 [0.36–0.76]; 0.0007*** | 0.68 [0.54–0.84]; 0.0006*** | 0.48 [0.31–0.72]; 0.0005*** | 0.48 [0.33–0.70]; 0.0001*** | 0.28 [0.14–0.55]; 0.0002*** |
| Dapagliflozin vs. DPP4I | 0.87 [0.75–1.01]; 0.0677 | 0.64 [0.46–0.90]; 0.0102* | 0.63 [0.52–0.77]; <0.0001*** | 0.68 [0.44–1.05]; 0.0833 | 0.64 [0.49–0.84]; 0.0013** | 0.42 [0.24–0.73]; 0.0020** | 0.71 [0.46–1.09]; 0.1163 | 0.41 [0.18–0.91]; 0.0294* |
| Empagliflozin vs. DPP4I | 0.75 [0.59–0.95]; 0.0169* | 0.53 [0.30–0.95]; 0.0322* | 0.74 [0.56–0.99]; 0.0392* | 0.59 [0.29–1.20]; 0.1469 | 0.86 [0.58–1.25]; 0.4232 | 0.68 [0.31–1.46]; 0.3193 | 0.70 [0.35–1.37]; 0.2949 | 0.54 [0.17–1.72]; 0.2946 |
| Canagliflozin vs. DPP4I | 0.93 [0.76–1.13]; 0.4572 | 0.82 [0.53–1.27]; 0.3762 | 0.71 [0.55–0.93]; 0.0111* | 0.47 [0.23–0.96]; 0.0380* | 0.90 [0.64–1.26]; 0.5387 | 1.14 [0.64–2.05]; 0.6495 | 0.30 [0.12–0.74]; 0.0087** | 0.13 [0.02–0.97]; 0.0465* |
| Ertugliflozin vs. DPP4I | 1.06 [0.83–1.34]; 0.6492 | 0.79 [0.44–1.42]; 0.4313 | 0.57 [0.39–0.84]; 0.0040** | 0.54 [0.22–1.33]; 0.1802 | 0.77 [0.48–1.24]; 0.2791 | 0.45 [0.14–1.42]; 0.1733 | 0.34 [0.11–1.07]; 0.0650 | 0.26 [0.04–1.88]; 0.1826 |
| Model 3 | ||||||||
| SGLT2I vs. DPP4I | 0.89 [0.79–0.98]; 0.0440* | 0.58 [0.44–0.77]; 0.0002*** | 0.59 [0.51–0.69]; <0.0001*** | 0.54 [0.37–0.79]; 0.0014** | 0.71 [0.57–0.89]; 0.0028** | 0.48 [0.31–0.73]; 0.0006*** | 0.49 [0.33–0.72]; 0.0002*** | 0.29 [0.15–0.56]; 0.0003*** |
| Dapagliflozin vs. DPP4I | 0.93 [0.80–1.07]; 0.3196 | 0.67 [0.48–0.94]; 0.0205* | 0.66 [0.54–0.79]; <0.0001*** | 0.71 [0.46–1.10]; 0.1267 | 0.67 [0.51–0.88]; 0.0044** | 0.43 [0.25–0.74]; 0.0024** | 0.72 [0.47–1.11]; 0.1354 | 0.42 [0.19–0.93]; 0.0319* |
| Empagliflozin vs. DPP4I | 0.77 [0.61–0.98]; 0.0316* | 0.53 [0.30–0.95]; 0.0334* | 0.75 [0.57–0.99]; 0.0436* | 0.60 [0.29–1.22]; 0.1564 | 0.88 [0.60–1.29]; 0.5186 | 0.66 [0.31–1.43]; 0.2935 | 0.70 [0.36–1.39]; 0.3086 | 0.52 [0.16–1.68]; 0.2764 |
| Canagliflozin vs. DPP4I | 0.96 [0.79–1.17]; 0.6902 | 0.83 [0.54–1.29]; 0.4157 | 0.72 [0.55–0.93]; 0.0132* | 0.47 [0.23–0.96]; 0.0384* | 0.91 [0.65–1.29]; 0.6062 | 1.11 [0.62–2.00]; 0.7155 | 0.31 [0.13–0.75]; 0.0097** | 0.14 [0.02–0.99]; 0.0486* |
| Ertugliflozin vs. DPP4I | 1.13 [0.89–1.44]; 0.2992 | 0.83 [0.46–1.48]; 0.5242 | 0.59 [0.40–0.86]; 0.0060** | 0.54 [0.22–1.32]; 0.1770 | 0.81 [0.50–1.30]; 0.3796 | 0.42 [0.13–1.32]; 0.1379 | 0.36 [0.11–1.12]; 0.0784 | 0.27 [0.04–1.93]; 0.1898 |
| Model 4 | ||||||||
| SGLT2I vs. DPP4I | 0.92 [0.84–0.999]; 0.0419* | 0.58 [0.42–0.80]; 0.0008*** | 0.70 [0.59–0.84]; 0.0001*** | 0.73 [0.47–1.13]; 0.1610 | 0.79 [0.62–1.01]; 0.0570 | 0.51 [0.32–0.80]; 0.0034** | 0.70 [0.46–1.08]; 0.1037 | 0.55 [0.26–1.14]; 0.1075 |
| Dapagliflozin vs. DPP4I | 1.02 [0.87–1.19]; 0.8491 | 0.72 [0.50–1.04]; 0.0775 | 0.78 [0.64–0.95]; 0.0136* | 0.91 [0.56–1.49]; 0.7141 | 0.76 [0.58–1.01]; 0.0631 | 0.48 [0.27–0.83]; 0.0095** | 1.02 [0.64–1.62]; 0.9387 | 0.77 [0.33–1.78]; 0.5352 |
| Empagliflozin vs. DPP4I | 0.72 [0.54–0.94]; 0.0174* | 0.46 [0.23–0.94]; 0.0323* | 0.88 [0.65–1.19]; 0.4045 | 0.83 [0.38–1.79]; 0.6293 | 1.01 [0.68–1.50]; 0.9800 | 0.73 [0.32–1.67]; 0.4542 | 0.79 [0.37–1.72]; 0.5565 | 0.95 [0.29–3.11]; 0.9271 |
| Canagliflozin vs. DPP4I | 1.01 [0.81–1.24]; 0.9582 | 0.89 [0.55–1.44]; 0.6435 | 0.84 [0.64–1.10]; 0.2098 | 0.59 [0.27–1.29]; 0.1867 | 1.00 [0.70–1.43]; 0.9893 | 1.28 [0.69–2.36]; 0.4329 | 0.42 [0.17–1.03]; 0.0581 | 0.21 [0.03–1.56]; 0.1282 |
| Ertugliflozin vs. DPP4I | 1.19 [0.91–1.55]; 0.2021 | 0.70 [0.34–1.43]; 0.3276 | 0.65 [0.43–0.98]; 0.0375* | 0.64 [0.23–1.75]; 0.3819 | 0.79 [0.47–1.33]; 0.3789 | 0.56 [0.18–1.77]; 0.3221 | 0.50 [0.16–1.59]; 0.2396 | 0.44 [0.06–3.25]; 0.4239 |
Note: Model 1 adjusted for significant demographics. Model 2 adjusted for significant demographics and past comorbidities. Model 3 adjusted for significant demographics, past comorbidities and non‐SGLT2I/DPP4I medications. Model 3 adjusted for significant demographics, past comorbidities and non‐SGLT2I/DPP4I medications. Model 4 adjusted for significant demographics, past comorbidities, non‐SGLT2I/DPP4I medications, abbreviated MDRD, fasting glucose, HbA1c and duration from earliest diabetes mellitus date to initial drug exposure date.
Abbreviations: CI, confidence interval; DPP4I, dipeptidyl peptidase‐4 inhibitor; HR, hazard ratio; SGLT2I: sodium glucose cotransporter‐2 inhibitor.
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001.
3.3. Sensitivity analysis
To assess the predictivity of the models, sensitivity analysis was conducted to evaluate the effect of matching on the results, namely with inverse probability of treatment weighting (Table S5). The findings confirmed those of univariable cox regression, that SGLT2I administration was still associated with a lower risk of all‐cause mortality, cancer‐related mortality, new‐onset overall cancer as well as all pre‐specified cancers (lung, breast, gastrointestinal, genitourinary and bladder) when compared to DPP4I usage.
4. DISCUSSION
To the best of our knowledge, this is the first territory‐wide study that does a direct comparison of the effect of SGLT2I and DPP4I on overall and pre‐specified cancer risk in a cohort of Asian patients. The main findings of this study are as follows: In comparison with DPP4I, (i) SGLT2I were associated with a lower risk of all‐cause mortality, cancer‐related mortality and new‐onset overall cancer; (ii) SGLT2I were related to a lower risk of new‐onset breast cancer; (iii) when stratified according to the medication subtype, dapagliflozin and ertugliflozin both demonstrated a reduced risk of new‐onset malignancy, with the former also presenting with a lower risk of breast cancer.
Anti‐diabetic medications are amongst the most commonly prescribed drugs in the world, with the indications of some expanding beyond T2DM to other non‐diabetic cardiovascular and chronic kidney conditions. 21 , 22 The clinical practicality of these medications, coupled with their multifaceted systemic effects, warrants a thorough assessment of the safety of their long‐term usage, which has raised some important concerns in recent years. This is of specific importance concerning the comparatively newer classes of oral hypoglycaemic drugs, namely DPP4I and SGLT2I, the first of which were marketed in 2006 (Sitagliptin) and 2013 (Canagliflozin), respectively. 23 Given the chronicity with which these medications are taken, a particularly significant outcome that is evaluated, unsurprisingly, is a cancer risk.
The majority of the comparative studies available in the existing literature have evaluated cancer risk across a wide range of anti‐diabetic drugs. Liu et al. performed a retrospective case‐controlled prognostic assessment for different anti‐diabetic medications, including metformin, thiazolidinediones, sulfonylureas, meglitinides, acarbose as well as insulin and its analogues, in turn revealing that apart from pioglitazone and insulin, the other therapies failed to show an association with cancer incidence. This relationship was maintained when stratifying outcomes by cancer type, namely for pancreatic, liver and lung cancer. 24 In addition to this, certain investigations have demonstrated the protective effect of some of the older classes against cancer, most notably with metformin, which has demonstrated either a reduced association with cancer 25 , 26 or a lower incidence of cancer on follow‐up relative to other anti‐diabetic medications. 27 Dąbrowski demonstrated that while some anti‐diabetic medications such as metformin and thiazolidinediones showed beneficial effects, the mitogenic effect of insulin could pose a harmful effect. 28 Interestingly, short‐term insulin use was found to be associated with increased risk of cancer but not for a longer duration use. Amongst diabetic patients, long‐term usage of oral diabetic medication correlated with reduced pancreatic cancer risk. 29 Incretin drugs and GLP‐1 receptor agonists supported a neutral association with cancer risk, with minimal preliminary evidence of its effect against various cancer types. 30
Despite this, it should be noted that there is much more uncertainty about the malignancy risk of the somewhat newer anti‐diabetic medications. Regarding DPP4I, a meta‐analysis compiled by Zhao et al. did not report any association between these medications and malignancy, even when stratified by different subtypes of DPP4I. 12 Similarly, the findings of another meta‐analysis lend further credence to this notion by not only failing to show a relationship with malignancy development but also purporting a potential protective effect of DPP4I against colorectal cancer. 31 Preliminary evidence suggests that DPP4I can alter our immune system through the activation of cytokines, reduction of cellular growth factors and systemic inflammatory responses. Suppression of the catalytic activity of chemokines stimulated by DPP4 can thereby inhibit tumour cell proliferation. In a pilot study, patients with colorectal cancer who took DPP4I and had improved cancer prognosis showed changes in post‐operative lymphocyte count, platelet count, prognostic nutritional index, neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio. 32 However, although these results may reflect much of the current school of thought concerning DPP4I, there have been some recent investigations that have suggested the possible existence of either a dose‐dependent or cancer‐type‐dependent correlation. As to the former, Chou et al. presented a higher incidence of colorectal cancer in patients on DPP4I who were receiving a high cumulative daily dose, but a corresponding lower risk of colorectal cancer amongst low cumulative dose users. 33 As it pertains to the latter, there is evidence to suggest that whilst a relationship between DPP4I and overall cancer risk may not exist, these drugs are associated with specific cancer types when categorised, namely bladder, kidney and liver cancer as well as melanoma. 11
Likewise, very much akin to that of DPP4I, the data centred around SGLT2I are also controversial. Most recently, a meta‐analysis performed by Benedetti et al. proposed a reduced cancer risk of SGLT2I when compared to placebo, with particular efficacy for dapagliflozin and ertugliflozin. 34 These results are in line with that of the present study, which also demonstrated the superiority of dapagliflozin and ertugliflozin in relation to cancer risk. Such findings are further emphasised by that of Pelletier et al, which also failed to display an increased cancer risk with SGLT2I users, regardless of cancer type. 35 Some researchers have identified regulatory functions of dapagliflozin on cell cycle and apoptosis, including an effect on reduced glucose uptake in CaKi‐1 cells. 36 Specifically, studies have identified that the drugs attenuate cancer cell proliferation through changing the mitochondrial membrane potential and various membrane transporters such as the sodium and glucose cotransporter. 37 Subsequently, the use of SGLT2I can reduce the viability and malignancy of carcinoma cells. The inhibitory effects of SGLT2I on glycolytic metabolism, cell cycle and intracellular ATP production in cancer cells are further supported in other research studies. 38 , 39 , 40 , 41 , 42 , 43 Alternatively, some animal studies demonstrate that dapagliflozin targets the reduction in glutathione metabolism, expression of pro‐inflammatory markers and the reversal of hyperinsulinemia to slow down tumour growth. 44 , 45 , 46 However, the obscurity in the findings concerning SGLT2I primarily resides in the fact that the malignancy risk varies depending on the SGLT2I and cancer subtypes. One study commented the overexpression of SGLT1 and SGLT2 on lung, colorectal, head, ovarian, oral and neck carcinomas, supporting the therapeutic approach of using SGLT2Is for early tumour detection. However, current findings in this research field require further verification as non‐specific SGLT antibodies were used. 47 Tang et al. showed that although the overall cancer incidence is lower with SGLT2I relative to other comparator drugs when analysing pre‐specified cancers, empagliflozin demonstrated a higher risk of bladder cancer whilst canagliflozin exhibited protective effects against gastrointestinal cancers. 10 The ambiguity regarding SGLT2I is further compounded by other contrarian evidence suggesting a reduced risk of malignancy with empagliflozin relative to other oral hypoglycaemic agents, but instead, an increased risk when compared to placebo. 48
Given the relatively newer status of DPP4I and SGLT2I, there is a paucity of literature comparing the non‐diabetic outcomes associated with these medications. Au et al. showcased a reduced incidence of pneumonia and pneumonia‐related mortality with SGLT2I relative to DPP4I in patients from Hong Kong. 49 In a Taiwanese cohort, SGLT2I similarly exhibited superiority in the risk of gout development when compared to DPP4I. 13 Moreover, one retrospective study in a Taiwanese cohort demonstrated that SGLT2I usage was associated with a lower risk of cancer‐related mortality compared to DPP4I, akin to the results to our investigations. 12 To date, in addition to our study, there is only one other that has directly assessed these two classes of medication and their respective cancer risks. The findings from this study indicated that the risk of a urinary tract and haematological malignancy with SGLT2I was half that of with DPP4I, albeit there were no other differences amongst other cancer subtypes. 14 These findings are supported by that of this study, which has likewise showcased that the use of SGLT2I is associated with a 30% reduction in new‐onset overall cancer risk in comparison with DPP4I, though there were no observable differences in genitourinary or bladder malignancy development between the two drug classes.
4.1. Limitations
There are certain limitations present in this population‐based study. First, due to the observational nature of this study, acquired results may be susceptible to information bias due to missing data, coding errors or under coding. Second, the retrospective nature of the study suggests that all derived findings regarding the relationship between SGLT2I, DPP4I and new‐onset overall cancer were correlational in nature. Third, information on drug exposure could not be directly obtained, and was instead determined indirectly through prescription refills, which may pose a liability concern. Fourth, as the drug exposure duration could not be standardised, this may have influenced the primary and secondary outcomes of the study. Finally, due to the lack of codes in CDARS, information regarding medical history, such as smoking status, were unattainable and could have been a confounding variable to cancer risk.
4.2. Conclusions
SGLT2I use was associated with lower risks of all‐cause mortality, cancer‐related mortality and new‐onset overall cancer compared to DPP4I use after propensity score matching and multivariable adjustment.
AUTHOR CONTRIBUTIONS
Cheuk To Chung: Conceptualization (equal); writing – original draft (equal); writing – review and editing (equal). Oscar Hou In Chou: Methodology (equal); validation (equal); visualization (equal). Teddy Tai Loy Lee: Investigation (equal); resources (equal); writing – review and editing (equal). Edward Christopher Dee: Conceptualization (equal); data curation (equal). Kenrick Ng: Resources (equal). Wing Tak Wong: Supervision (equal). Tong Liu: Investigation (equal); supervision (equal). Sharen Lee: Supervision (equal); writing – review and editing (equal). Bernard Cheung: Investigation (equal); supervision (equal). Qingpeng Zhang: Resources (equal). Jiandong Zhou: Formal analysis (equal); investigation (equal); methodology (equal); software (equal); visualization (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
None.
Chung CT, Lakhani I, Chou OHI, et al. Sodium‐glucose cotransporter 2 inhibitors versus dipeptidyl peptidase 4 inhibitors on new‐onset overall cancer in Type 2 diabetes mellitus: A population‐based study. Cancer Med. 2023;12:12299‐12315. doi: 10.1002/cam4.5927
Cheuk To Chung and Ishan Lakhani should be considered co‐first author.
Gary Tse and Jiandong Zhou should be considered co‐last author.
[Correction added on May 26, 2023 after first online publication. Author names Edward Christopher Dee and Kenrick Ng have been corrected in this version.]
Contributor Information
Gary Tse, Email: gary.tse@kmms.ac.uk.
Jiandong Zhou, Email: jiandong.zhou@ndm.ox.ac.uk.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Hanger S, Haria C, Li D, Mogal R. The burden of asymptomatic lung cancer. Eur Respiratory Soc. 2019;54:PA3038. [Google Scholar]
- 3. Inada R, Nagasaka T, Watanabe A, et al. Comparison of outcomes between symptomatic and asymptomatic patients with colorectal cancer: a propensity score‐matched analysis of surgical invasiveness, medical costs and oncological outcomes. BMJ Open Gastroenterol. 2017;4(1):e000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abudawood M. Diabetes and cancer: a comprehensive review. J Res Med Sci. 2019;24:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pan XF, He M, Yu C, et al. Type 2 diabetes and risk of incident cancer in China: a prospective study among 0.5 million Chinese adults. Am J Epidemiol. 2018;187(7):1380‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Y, Yang W, Song M, et al. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br J Cancer. 2018;119(11):1436‐1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi B, Hu X, He H, Fang W. Metformin suppresses breast cancer growth via inhibition of cyclooxygenase‐2. Oncol Lett. 2021;22(2):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao L, Liu M, Huang Y, et al. Metformin use and lung cancer risk in diabetic patients: a systematic review and meta‐analysis. Dis Markers. 2019;2019:6230162‐6230169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tseng CH. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: a retrospective cohort analysis. Diabetes Metab. 2017;43(5):438‐445. [DOI] [PubMed] [Google Scholar]
- 10. Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta‐analysis of randomised controlled trials. Diabetologia. 2017;60(10):1862‐1872. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Kim CH. Malignancies Associated with DPP4 Inhibitors and GLP1 Receptor Agonists: Data from a Large Real‐World Database. American Society of Clinical Oncology; 2020. [Google Scholar]
- 12. Zhao M, Chen J, Yuan Y, et al. Dipeptidyl peptidase‐4 inhibitors and cancer risk in patients with type 2 diabetes: a meta‐analysis of randomized clinical trials. Sci Rep. 2017;7(1):8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung MC, Hsu HT, Chang CH, et al. Association of SGLT2 inhibitors with lower incidence of death in type 2 diabetes mellitus and causes of death analysis. Sci Rep. 2022;12(1):10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rokszin G, Kiss Z, Sütő G, et al. Sodium‐glucose co‐transporter 2 inhibitors may change the development of urinary tract and hematological malignancies as compared with dipeptidyl peptidase‐4 inhibitors: data of the post‐hoc analysis of a nationwide study. Front Oncol. 2021;11:725465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chou OHI, Zhou J, Mui JV, et al. Lower risks of new‐onset acute pancreatitis and pancreatic cancer in sodium glucose cotransporter 2 (SGLT2) inhibitors compared to dipeptidyl peptidase‐4 (DPP4) inhibitors: a propensity score‐matched study with competing risk analysis. Diabetes Epidemiology and Management. 2023;9:100115. [Google Scholar]
- 16. Chan R, Chan RNF, Chou OHI, et al. Lower risks of incident colorectal cancer in SGLT2i users compared to DPP4i users: a propensity score‐matched study with competing risk analysis. Eur J Intern Med. 2023;110:125‐127. [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Zhou J, Leung KSK, et al. Comparison of sodium‐glucose Cotransporter‐2 inhibitor and dipeptidyl Peptidase‐4 inhibitor on the risks of new‐onset atrial fibrillation, stroke and mortality in diabetic patients: a propensity score‐matched study in Hong Kong. Cardiovasc Drugs Ther. 2022. 10.1007/s10557-022-07319-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou J, Wang X, Lee S, et al. Proton pump inhibitor or famotidine use and severe COVID‐19 disease: a propensity score‐matched territory‐wide study. Gut. 2021;70(10):2012‐2013. [DOI] [PubMed] [Google Scholar]
- 19. Chou OHI, Zhou J, Lee TTL, et al. Comparisons of the risk of myopericarditis between COVID‐19 patients and individuals receiving COVID‐19 vaccines: a population‐based study. Clin Res Cardiol. 2022;111(10):1098‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inker LA, Eneanya ND, Coresh J, et al. New creatinine‐and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yau K, Dharia A, Alrowiyti I, Cherney DZ. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022;7:2546‐2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bae JH, Kim S, Park EG, Kim SG, Hahn S, Kim NH. Effects of dipeptidyl peptidase‐4 inhibitors on renal outcomes in patients with type 2 diabetes: a systematic review and meta‐analysis. Endocrinol Metab (Seoul). 2019;34(1):80‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White JR Jr. A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu YC, Nguyen PA, Humayun A, et al. Does long‐term use of antidiabetic drugs changes cancer risk? Medicine (Baltimore). 2019;98(40):e17461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans JM, Donnelly LA, Emslie‐Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinn BJ, Kitagawa H, Memmott RM, Gills JJ, Dennis PA. Repositioning metformin for cancer prevention and treatment. Trends Endocrinol Metab. 2013;24(9):469‐480. [DOI] [PubMed] [Google Scholar]
- 27. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer‐related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254‐258. [DOI] [PubMed] [Google Scholar]
- 28. Dąbrowski M. Diabetes, antidiabetic medications and cancer risk in type 2 diabetes: focus on SGLT‐2 inhibitors. Int J Mol Sci. 2021;22(4):1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosetti C, Rosato V, Li D, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the international pancreatic cancer case‐control consortium. Ann Oncol. 2014;25(10):2065‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tseng CH, Lee KY, Tseng FH. An updated review on cancer risk associated with incretin mimetics and enhancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2015;33(1):67‐124. [DOI] [PubMed] [Google Scholar]
- 31. Dicembrini I, Nreu B, Montereggi C, Mannucci E, Monami M. Risk of cancer in patients treated with dipeptidyl peptidase‐4 inhibitors: an extensive meta‐analysis of randomized controlled trials. Acta Diabetol. 2020;57(6):689‐696. [DOI] [PubMed] [Google Scholar]
- 32. Ng L, Foo DC, Wong CK, Man AT, Lo OS, Law WL. Repurposing DPP‐4 inhibitors for colorectal cancer: a retrospective and single center study. Cancers (Basel). 2021;13(14):3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chou CL, Juan SH, Li CH, et al. Association between DPP‐4 inhibitors and events of colorectal and liver cancers in patients with diabetes receiving second‐line agents: a nested case‐control study. Front Oncol. 2022;12:840142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benedetti R, Benincasa G, Glass K, et al. Effects of novel SGLT2 inhibitors on cancer incidence in hyperglycemic patients: a meta‐analysis of randomized clinical trials. Pharmacol Res. 2022;175:106039. [DOI] [PubMed] [Google Scholar]
- 35. Pelletier R, Ng K, Alkabbani W, Labib Y, Mourad N, Gamble JM. The association of sodium‐glucose cotransporter 2 inhibitors with cancer: an overview of quantitative systematic reviews. Endocrinol Diabetes Metab. 2020;3(3):e00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuang H, Liao L, Chen H, Kang Q, Shu X, Wang Y. Therapeutic effect of sodium glucose co‐transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit. 2017;23:3737‐3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Komatsu S, Nomiyama T, Numata T, et al. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J. 2020;67(1):99‐106. [DOI] [PubMed] [Google Scholar]
- 38. Kaji K, Nishimura N, Seki K, et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142(8):1712‐1722. [DOI] [PubMed] [Google Scholar]
- 39. Zhou J, Zhu J, Yu SJ, et al. Sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother. 2020;132:110821. [DOI] [PubMed] [Google Scholar]
- 40. Jojima T, Wakamatsu S, Kase M, et al. The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non‐alcoholic steatohepatitis‐related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma. Int J Mol Sci. 2019;20(20):5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villani LA, Smith BK, Marcinko K, et al. The diabetes medication canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex‐I supported respiration. Mol Metab. 2016;5(10):1048‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saito T, Okada S, Yamada E, et al. Effect of dapagliflozin on colon cancer cell [Rapid Communication]. Endocr J. 2015;62(12):1133‐1137. [DOI] [PubMed] [Google Scholar]
- 43. Perry RJ, Shulman GI. Sodium‐glucose cotransporter‐2 inhibitors: understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem. 2020;295(42):14379‐14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nasiri AR, Rodrigues MR, Li Z, Leitner BP, Perry RJ. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 2019;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shiba K, Tsuchiya K, Komiya C, et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8(1):2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kato J, Shirakami Y, Ohnishi M, et al. Suppressive effects of the sodium‐glucose cotransporter 2 inhibitor tofogliflozin on colorectal tumorigenesis in diabetic and obese mice. Oncol Rep. 2019;42(6):2797‐2805. [DOI] [PubMed] [Google Scholar]
- 47. Vrhovac Madunić I, Madunić J, Breljak D, Karaica D, Sabolić I. Sodium‐glucose cotransporters: new targets of cancer therapy? Arh Hig Rada Toksikol. 2018;69(4):278‐284. [DOI] [PubMed] [Google Scholar]
- 48. Shi N, Shi Y, Xu J, et al. SGLT‐2i and risk of malignancy in type 2 diabetes: a meta‐analysis of randomized controlled trials. Front Public Health. 2021;9:668368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Au PCM, Tan KCB, Cheung BMY, Wong ICK, Wong Y, Cheung CL. Association between SGLT2 inhibitors vs DPP‐4 inhibitors and risk of pneumonia among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022;107(4):e1719‐e1726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


