Abstract
Objectives:
We examined the associations of the metabolic syndrome severity score (MSSS) and the metabolic syndrome (MetSyn) components with central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE).
Methods:
Participants in this cross-sectional study were 253 officers from the Buffalo Cardio-Metabolic Occupational Police Stress study (2011–2014). The MSSS is a z-score that represents the severity of MetSyn and was estimated using a sex/race-specific equation and the five MetSyn components. Associations of MSSS and the MetSyn with CRAE/CRVE were obtained using linear regression models or analysis of covariance.
Results:
For every 1-standard deviation of MSSS, CRAE decreased by 2.3 μm (SE = 1.2, P = 0.0262) and CRVE increased by 3.4 μm (SE = 1.6, P = 0.0308) after adjusting for confounders.
Conclusions:
Officers with higher MSSS had narrower (ie, worse) arteriolar diameters and wider (ie, worse) venular diameters.
Keywords: metabolic syndrome, retinal microvascular, CRAE, CRVE, police officers
The metabolic syndrome (MetSyn) is a cluster of three or more of the following conditions: abdominal obesity, hypertension, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, and glucose intolerance.1 Individuals diagnosed with MetSyn are at increased risk of heart attacks, strokes (related to fatty buildups in artery walls, ie, atherosclerosis),2 and type 2 diabetes mellitus.3 According to research studies that used data from the National Health and Nutrition Examination Survey, the prevalence of MetSyn was 36.9% among US adults in 2015–2016.4 Obesity was shown to be positively associated with MetSyn.5 Twenty percent of US workers had MetSyn in 1999–2004. “Miscellaneous food preparation and food service workers” and “farm operators, managers, and supervisors” had the highest prevalence of MetSyn (29.6% and 31.1%, respectively).6 Other studies show that police officers have a high prevalence of MetSyn, which may be partially due to the fact that police officers have unpredictable and stressful working environments and are often scheduled to work irregular shifts.7,8
Retinal photographic techniques and digital image analyses provide a noninvasive assessment of retinal microvascular caliber in vivo. Retinal microvascular abnormalities predict an increased risk of diabetes, hypertension, coronary heart disease, stroke, and mortality.9–12 Several studies also show the association between MetSyn and the retinal microvasculature,11,13–15 and verse versa.9,16 The Atherosclerosis Risk in Communities study showed that MetSyn was associated with microvascular changes in the retina.13 The Australian Heart Eye Study observed that MetSyn was associated with narrower retinal arterioles but not wider retinal venules among those at high risk of coronary artery disease.15 Most of these studies were population-based11,13 or included patients who were at high risk for diabetes or heart disease.14,15 None of the studies that investigated associations between MetSyn and retinal microvascular abnormalities were conducted on a specific occupational group.
Although all previous studies analyzed the MetSyn as a dichotomized variable (ie, present or absent), we used the continuous form of the MetSyn known as the metabolic syndrome severity score (MSSS). We also used the dichotomized version of MetSyn to compare those results to that of MSSS. In the present study, we examined the associations of MSSS and individual components of MetSyn with central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE) among police officers in Buffalo, New York.
METHODS
Study Design and Participants
The Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study was conducted to investigate associations between occupational stressors and subclinical cardiovascular disease in the high-stress occupation of police work. Additional details about the BCOPS study have been described by Violanti et al.17 The participants were police officers from the Buffalo Police Department, New York. The Center for Health Research, School of Public Health and Health Profession, the State University of New York at Buffalo (SUNY-Buffalo) conducted the data collection. Ethics approval for this study was obtained from the Internal Review Board of SUNY-Buffalo. All officers provided informed consent to participate in the study.
After the baseline BCOPS examination (2004–2009), the officers were invited to participate in two subsequent studies: a follow-up examination (2011–2014) and a microvascular study (2012–2016) in which retinal photos were collected. Funding for the microvascular study was approved after examinations for the follow-up visit had begun. Therefore, officers who had already been seen in the follow-up visit were invited back to participate in the microvascular study. In the follow-up examination, 300 officers participated and 253 of those officers consented to have retinal photography performed in the microvascular study. We merged all data from both examinations and retained only those officers who were active-duty and had complete information on the main variables of interest. The final sample size for this cross-sectional study included 253 police officers, 71 women and 182 men.
Assessment of Metabolic Syndrome Severity Score and Metabolic Syndrome
The MSSS is a z-score continuous range of values that represent the severity of the MetSyn. The sex- and race-specific equations for estimating MSSS were developed by Gurka and colleagues.18 It was developed to improve upon the traditional dichotomous categorization of MetSyn (ie, ≥3: present, ≤2:absent). The MSSS in this study was calculated based on the following variables: waist circumference (WC), systolic blood pressure (SBP), triglycerides, HDL cholesterol, fasting glucose, separately for each sex, and race/ethnicity combination.
The MetSyn was defined according to criteria by the Third Report of the National Cholesterol Education Program Adults Treatment Panel,1 which is the presence of three or more of the following five components:
WC ≥102 cm in men and ≥88 cm in women;
SBP ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg or reported physician-diagnosed hypertension and antihypertension treatment;
Triglyceride ≥150 mg/dL or reported treatment with nicotinic acid or fibrates;
HDL cholesterol <40 mg/dL in men and <50 mg/dL in women or reported treatment with nicotinic acid or fibrates; and
Fasting glucose ≥100 mg/dL or reported treatment for diabetes.
WC was measured twice at the midpoint between the lowest rib and the top point of the hip bone, and the average value was used in the analysis. BP was measured after the participants had rested in the supine position for about 5 minutes. Three BP measurements were taken 2 minutes apart. SBP and DBP were derived from the mean of the three respective measurements. Venous blood samples were drawn to provide biochemical data (triglyceride, HDL cholesterol, fasting glucose, etc) and analyzed by Kaleida Laboratory, Buffalo, NY. After collection at the clinic, the samples were packed in dry ice, picked up by a courier (from Kaleida Lab), and transported to the laboratory (approximately 3 miles away) every day. Blood samples were drawn by a trained phlebotomist at around 9:00 AM after the officers had fasted for a minimum of 8 hours. Also, blood was drawn after the officers had been off-duty for at least 3 days. We used the cut point of fasting glucose level ≥100 mg/dL, which was the diagnosis of diabetes mellitus established by the American Diabetes Association.19 Officers also met the criteria for hypertension or diabetes mellitus if they reported medication use for high BPor diabetes or physician’s diagnosis of the condition. Mean arterial blood pressure, used as a continuous variable of BP in the MetSyn components, was defined as the average arterial pressure in an individual during a single cardiac cycle: mean arterial blood pressure = 0.412 × SBP + 0.588 × DBP.20
Assessment of Retinal Vessel Caliber
Research associates in the BCOPS study were trained and certified to perform retinal photography by the University of Wisconsin Department of Ophthalmology personnel. A non-mydriatic ophthalmic digital imaging system was used to take two digital images per eye (four images total per participant) through a non-pharmacologically dilated pupil. Participants were seated in a windowless room with the lights turned off to allow the pupils to dilate naturally in preparation for the retinal imaging examination. One image was centered on the macula and the second on the optic nerve.
The digital images were sent to the University of Wisconsin Department of Ophthalmology Ocular Epidemiology Reading Center to be graded in a masked fashion using a standardized protocol described as follows. Retinal vessel diameters were measured at the Reading Center using a computer-assisted technique based on a standard protocol and using the Parr-Hubbard-Knudtson formula.21 Trained graders masked to participants’ characteristics and using a computer software program measured the diameters of all arterioles and venules coursing through a specified area one half to one disc diameter surrounding the optic disc. On average, between 7 and 14 arterioles and an equal number of venules were measured per eye. Individual arteriolar and venular measurements were combined into summary indices that reflected the average CRAE and CRVE based on the Parr-Hubbard-Knudtson formula.21,22
Covariates
Participants were given self-administered questionnaires to provide information about demographic characteristics (age, sex, race/ethnicity, education), lifestyle behaviors (smoking status, alcohol intake, physical activity, sleep duration), occupational characteristics (years of service, rank, shiftwork), medication use (antihypertension, antilipids), and inflammatory biomarkers (high sensitivity C-reactive protein [hs-CRP], interleukin, fibrinogen, tumor necrosis factor α). Age was defined at the time of the examination when retinal images were captured. Smoking status was reported as “current,” “former,” or “never” and included cigarette, cigar, and pipe smoking. Inflammatory biomarkers were analyzed by Kaleida Health, Buffalo, New York.
Statistical Analysis
Descriptive statistics are provided showing means (SD) (for continuous variables) and frequencies (%) (for categorical variables) for all officers, women, and men. Retinal microvascular parameters (ie, CRAE and CRVE) were analyzed as continuous variables. Pearson’s correlation coefficients and analysis of variance were used to investigate the associations of covariates with retinal variables. Potential confounders were selected based on their association with both the MetSyn variables or retinal vascular parameters in this study and whether they were reported as confounders in previous investigations. The selected confounders and CRAE risk factors were age, sex, smoking status, antihypertension medication, and CRVE. The selected confounders and CRVEriskfactorswereage,sex,smokingstatus,hs-CRP,antihypertension medication, and CRAE. Tests for interaction by age, sex, smoking status, physical activity, and antihypertension medication were performed by including interaction terms in the models. The criterion for significance in effect modification analyses was set at P < 0.1. No effect modification was identified in this study. We used analysis of variance and analysis of covariance to estimate mean levels of CRAE and CRVE for each dichotomized MetSyn status. Linear regression models were used to estimate slopes for CRAE and CRVE with the ordinal MetSyn variable (number of components of MetSyn), MSSS, and the continuous MetSyn components (triglycerides, HDL cholesterol, fasting glucose, mean arterial BP, WC). All P values presented were two-tailed, and statistical significance was set at P<0.05for all analyses, except for effect modification. Analyses were performed with the use of SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Table 1 shows descriptive statistics by sex for the study subjects. The study sample included 253 police officers (72% men) with an average 47.4 years old (SD = 9.3) and an average of 20 years (SD = 9.4) of police service. The alcohol intake was 5.0 drinks/week overall, 6.0 in male officers and 2.5 in female officers. Among all officers, 23.3% (n = 59) were taking antihypertensive medication. The prevalence of MetSyn was higher in males (34.1%) than in females (18.3%). The overall mean value of CRAE was 155.2 μm (SD = 13.4) and for CRVE, 223.1 μm (SD = 19.8).
TABLE 1.
Descriptive Statistics of the Study Population, BCOPS 2010–2014
| All (n = 253) |
Women (n = 71) |
Men (n = 182) |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
|
| |||
| Age, yrs | 47.4 (9.3) | 46.5 (8.8) | 47.7 (9.5) |
| Years of service | 19.9 (9.4) | 17.8 (8.7) | 20.8 (9.5) |
| Alcohol intake, drinks/wk | 5.0 (9.4) | 2.5 (3.2) | 6.0 (10.8) |
| Physical activity, hrs/wk | 7.5 (8.3) | 6.3 (4.8) | 7.9 (9.3) |
| Body mass index, kg/m2 | 29.3 (4.8) | 27.0 (5.0) | 30.2 (4.4) |
| Waist circumference, cm | 96.6 (13.7) | 85.3 (11.7) | 101.1 (11.8) |
| Systolic blood pressure, mmHg | 116.3 (l1.4) | 112.4(12.1) | 117.8 (10.8) |
| Diastolic blood pressure, mmHg | 78.0 (7.9) | 75.0 (7.2) | 79.1 (7.9) |
| Fasting glucose, mg/dL | 96.6 (20.0) | 91.5 (10.9) | 98.6 (22.3) |
| Triglyceride, mg/dL | 120.4 (86.0) | 93.1 (79.2) | 131.1 (86.3) |
| HDL cholesterol, mg/dL | 48.5 (13.7) | 58.4 (15.7) | 44.6 (10.5) |
| LDL cholesterol, mg/dL | 124.2 (34.0) | 125.6(31.4) | 123.7 (35.0) |
| Total cholesterol, mg/dL | 196.5 (37.3) | 201.9 (35.6) | 194.3 (37.8) |
| Hs-CRP, mg/L | 2.6 (2.9) | 3.1 (3.6) | 2.4 (2.6) |
| Interleukin 6, pg/mL | 2.0 (1.7) | 2.0 (1.9) | 2.0 (1.7) |
| Fibrinogen, mg/dL | 326.3 (76.3) | 339.6 (104.2) | 321.1 (61.7) |
| Tumor necrosis factor a, pg/mL | 3.7 (4.6) | 4.0 (8.1) | 3.5 (2.0) |
| Sleep duration (PSQI), hrs/24-hr | 6.1 (1.1) | 6.1 (1.4) | 6.1 (1.0) |
| Metabolic syndrome severity score (z-score) | 0.0 (0.8) | −0.6 (0.8) | 0.2 (0.7) |
| Central retinal arteriolar equivalent, pm | 155.2 (13.4) | 156.7(15.3) | 154.7 (12.6) |
| Central retinal venular equivalent, pm | 223.1 (19.8) | 224.7 (21.8) | 222.4 (18.9) |
|
| |||
| n (%) | n (%) | n (%) | |
|
| |||
| Race/ethnicity | |||
| White/Hispanic | 201 (79.5) | 49 (69.0) | 152 (83.5) |
| African American | 52 (20.5) | 22(31.0) | 30 (16.5) |
| Education | |||
| ≤HS/GED | 23 (9.1) | 3 (4.2) | 20 (11.0) |
| <4 yrs college | 126 (49.8) | 39 (54.9) | 87 (47.8) |
| ≥4 yrs college | 104(41.1) | 29 (40.9) | 75 (41.2) |
| Smoking status | |||
| Current | 27 (10.7) | 9 (12.9) | 18 (9.9) |
| Former | 76 (30.2) | 28 (40.0) | 48 (26.4) |
| Never | 149 (59.1) | 33 (47.1) | 116 (63.7) |
| Sleep quality | |||
| Good | 56 (23.0) | 15 (21.7) | 41 (23.6) |
| Poor | 187(77.0) | 54 (78.3) | 133 (76.4) |
| Rank | |||
| Patrol officer | 141 (56.6) | 45 (63.4) | 96 (53.9) |
| Sergeant/Lieut/Capt | 49 (19.7) | 13 (18.3) | 36 (20.2) |
| Det/Exec/other | 59 (23.7) | 13 (18.3) | 46 (25.9) |
| Shiftwork (entire career) | |||
| Day | 120 (47.8) | 49 (70.0) | 71 (39.2) |
| Afternoon | 82 (32.7) | 13 (18.6) | 69 (38.1) |
| Night | 49 (19.5) | 8(11.4) | 41 (22.7) |
| Hypertension medication | |||
| No | 194 (76.7) | 61 (85.9) | 133 (73.1) |
| Yes | 59 (23.3) | 10(14.1) | 49 (26.9) |
| Diabetes medication | |||
| No | 247 (97.6) | 71 (100.0) | 176 (96.7) |
| Yes | 6 (2.4) | 0 (0.0) | 6 (3.3) |
| Lipids medication | |||
| No | 197 (77.9) | 64(90.1) | 133 (73.1) |
| Yes | 56(22.1) | 7 (9.9) | 49 (26.9) |
| Metabolic syndrome | |||
| Yes (>3 components) | 75 (29.6) | 13 (18.3) | 62 (34.1) |
| No | 178 (70.4) | 58 (81.7) | 120 (65.9) |
Hs-CRP, high sensitivity C-reactive protein.
Table 2 shows the unadjusted associations for selected characteristics with CRAE and CRVE. Age and years of police service were significantly and inversely correlated with CRAE (r = −0.20, P = 0.0011; r = −0.25, P < 0.0001, respectively), but not with CRVE (r = −0.09, P = 0.1478; r = −0.06, P = 0.3693, respectively). The mean CRVE was significantly higher in African American than White/Hispanic officers (231.7 ± 20.8, 220.8 ± 18.9, respectively; P = 0.0004), whereas the mean CRAE was not significantly different between these two groups. Officers who were former smokers had the narrowest CRAE (152.0 ± 13.4), whereas current smokers had the widest CRVE (227.4 ± 20.4). Three inflammatory biomarkers were significantly and positively correlated with CRVE (hs-CRP: r = 0.22, P = 0.0004; interleukin 6: r = 0.32, P < 0.0001; fibrinogen: r = 0.15, P = 0.0171). None of the inflammatory biomarkers were significantly correlated with CRAE.
TABLE 2.
Association Between Selected Characteristics and Retinal Vessel Diameters, BCOPS 2010–2014
| CRAE, μm |
CRVE, μm |
|||
|---|---|---|---|---|
| Correlation | P | Correlation | P | |
|
| ||||
| Age, yrs | −0.20 | 0.0011 | −0.09 | 0.1478 |
| Years of service | −0.25 | <0.0001 | −0.06 | 0.3693 |
| Alcohol intake, drinks/wk | −0.09 | 0.1448 | −0.01 | 0.8252 |
| Physical activity, hrs/wk | 0.01 | 0.8321 | −0.06 | 0.3379 |
| Log of C-reactive protein | 0.05 | 0.4397 | 0.22 | 0.0004 |
| Log of interleukin 6 | 0.08 | 0.2218 | 0.32 | <0.0001 |
| Fibrinogen | 0.02 | 0.7078 | 0.15 | 0.0171 |
| Log of tumor necrosis factor α | −0.03 | 0.5908 | 0.07 | 0.2663 |
| Sleep duration (PSQI), hrs/24-hr | 0.03 | 0.6578 | 0.01 | 0.8429 |
|
| ||||
| Mean (SD) | P | Mean (SD) | P | |
|
| ||||
| Sex | 0.2928 | 0.4215 | ||
| Women | 156.7 (15.3) | 224.7 (21.8) | ||
| Men | 154.7 (12.6) | 222.4 (18.9) | ||
| Race/ethnicity | 0.1099 | 0.0004 | ||
| White/Hispanic | 154.5 (13.3) | 220.8 (18.9) | ||
| African American | 157.9 (13.7) | 231.7(20.8) | ||
| Education | 0.7535 | 0.5282 | ||
| ≤HS/GED | 156.7 (13.7) | 227.5 (20.7) | ||
| <4 yrs college | 154.7 (14.8) | 222.7 (21.3) | ||
| ≥4 yrs college | 155.6 (11.5) | 222.6 (17.5) | ||
| Smoking status | 0.0281 | 0.2154 | ||
| Current | 158.8 (16.1) | 227.4 (20.4) | ||
| Former | 152.0 (13.4) | 220.2 (20.2) | ||
| Never | 156.2 (12.7) | 223.8 (19.4) | ||
| Sleep quality | 0.4657 | 0.8100 | ||
| Good | 156.4 (12.1) | 222.3 (19.7) | ||
| Poor | 154.9 (14.0) | 223.0 (20.2) | ||
| Rank | 0.2835 | 0.4627 | ||
| Patrol officer | 155.9 (13.4) | 224.2 (20.7) | ||
| Sergeant/Lieut/Capt | 152.5 (11.3) | 220.1 (15.9) | ||
| Det/Exec/other | 156.0 (15.1) | 223.1 (20.8) | ||
| Shiftwork (entire career) | 0.1824 | 0.3733 | ||
| Day | 154.6 (14.7) | 223.4 (21.6) | ||
| Afternoon | 154.3 (13.0) | 220.9 (18.8) | ||
| Night | 158.4 (10.6) | 225.9(16.8) | ||
| Hypertension medication | 0.0722 | 0.6137 | ||
| No | 156.1 (13.4) | 223.4 (19.2) | ||
| Yes | 152.5 (13.3) | 221.9(21.6) | ||
| Lipids medication | 0.5557 | 0.6312 | ||
| No | 155.5 (13.8) | 222.7 (19.5) | ||
| Yes | 154.3 (12.0) | 224.2 (20.9) | ||
Associations between the MSSS and CRAE are presented in Table 3. With every 1-SD increase in MSSS, the CRAE decreased 2.26 μm (SE = 0.98, P = 0.0225) after adjusting for age, sex, smoking status, antihypertension medication, and the CRVE. The association between the number of the MetSyn components (range, 0–5) and the CRAE showed the same regression pattern as the association between the MSSS and CRAE (β = −1.40 ± 0.58, P = 0.0176). As the number of the MetSyn components increased by 1, the CRAE decreased by 1.40 μm (SE = 0.58, P = 0.0176). Two of the five MetSyn components were linearly associated with the CRAE: the higher the mean arterial BP or the WC, the narrower the CRAE (β = −0.48 ± 0.08, P < 0.0001; β = −0.13 ± 0.06, P = 0.0357, respectively). However, we did not observe a linear association between the blood biomarkers (triglycerides, HDL cholesterol, and fasting glucose) and the CRAE. Figure 1 presents the association between individual MetSyn components and the CRAE. Officers with elevated BP and increased WC had significantly narrower CRAE (P < 0.0001 and P = 0.0222, respectively).
TABLE 3.
Association Between Metabolic Syndrome Parameters and Central Retinal Arterioles Equivalent (μm), BCOPS 2010–2014
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
|
| ||||||
| Metabolic syndrome severity score (z-score) | −1.28 (1.02) | 0.2095 | −0.81 (1.12) | 0.4721 | −2.26 (0.98) | 0.0225 |
| Number of the MetSyn components (range: 0–5) | −1.20 (0.65) | 0.0482 | −0.79 (0.64) | 0.2152 | −1.40 (0.58) | 0.0176 |
| MetSyn Components (continuous scale) | ||||||
| Mean artery blood pressure | −0.03 (0.09) | <0.0001 | −0.53 (0.10) | <0.0001 | −0.48 (0.08) | <0.0001 |
| Waist circumference | −0.11 (0.06) | 0.0670 | −0.09 (0.07) | 0.2105 | −0.13 (0.06) | 0.0357 |
| Triglycerides | −0.01 (0.01) | 0.4268 | −0.01 (0.01) | 0.5618 | −0.01 (0.01) | 0.4070 |
| HDL cholesterol | −0.04 (0.06) | 0.5773 | −0.07 (0.07) | 0.3395 | 0.04 (0.06) | 0.4713 |
| Fasting glucose | 0.00 (0.04) | 0.9629 | 0.03 (0.04) | 0.5199 | −0.03 (0.04) | 0.5047 |
The values in boldface are statistically significant results.
Model 1 was unadjusted. Model 2 was adjusted for age, sex, and smoking status. Model 3 was adjusted for age, sex, smoking status, antihypertension medication, and CRVE.
FIGURE 1.
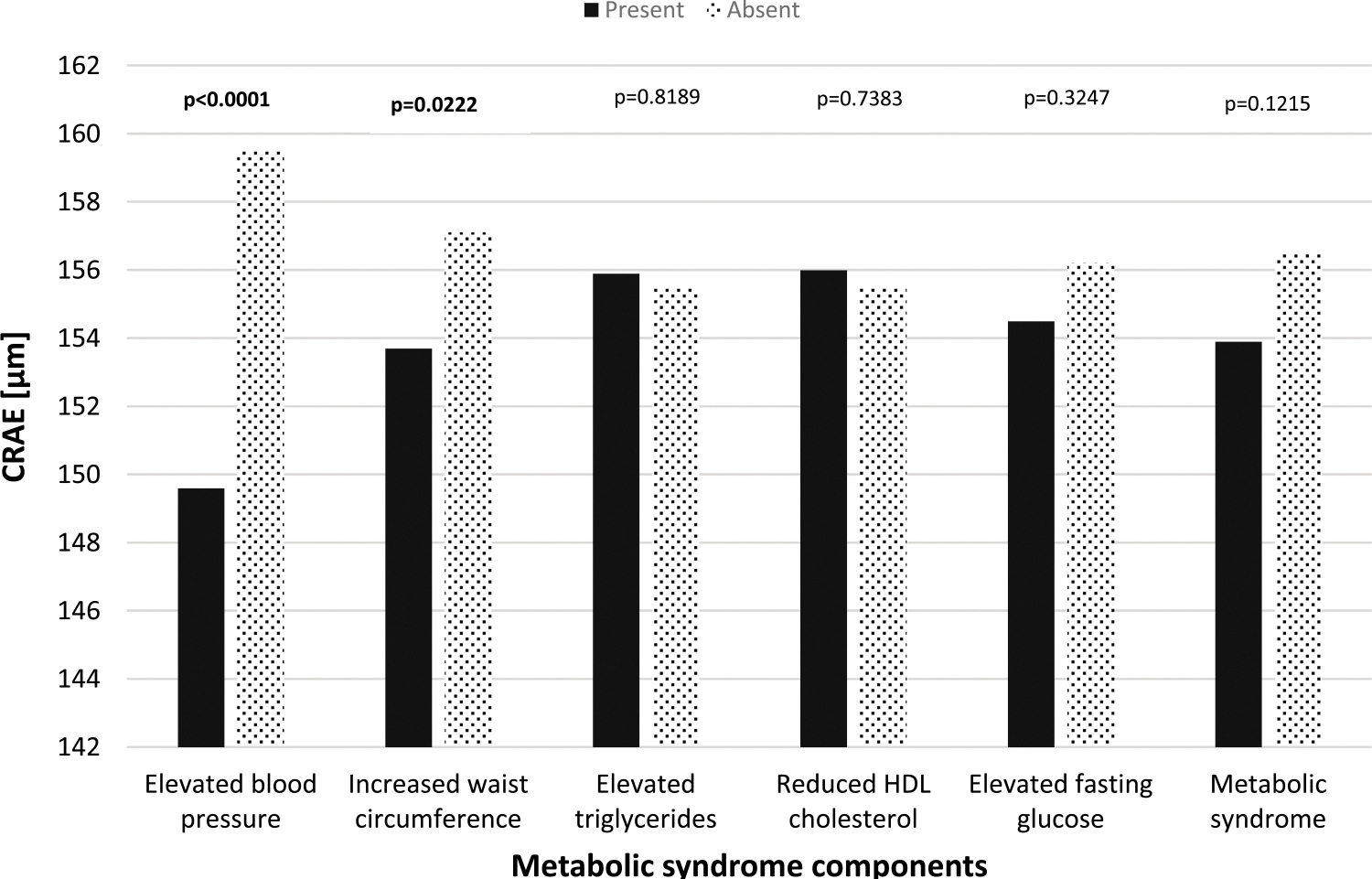
Solid dark bars: presence of the specific metabolic syndrome component. Dotted bars: absence of the specific metabolic syndrome component. The above models were adjusted for age, sex, smoking status, antihypertension medication, and CRVE.
Finally, we examined the association between the MSSS and the retinal venular calibers (CRVE) in Table 4.With every 1-SD increase in MSSS, CRVE increased by 3.74 μm (SE = 1.59, P = 0.0194) after controlling for age, sex, smoking status, hs-CRP, antihypertension medication, and CRAE. As the number of the MetSyn components increased by 1, the CRVE increased by 1.93 μm (SE= 0.94, P = 0.0411). HDL cholesterol and glucose were linearly associated with CRVE. As glucose levels increased, the CRVE also increased (β = 0.12 ± 0.06, P = 0.0309). As HDL cholesterol decreased, the CRVE increased (β = −0.21 ± 0.09, P = 0.0176). Figure 2 presents the comparison mean of CRVE by individual MetSyn components in dichotomous form. Officers with elevated BP had significantly wider CRVE (P = 0.0137), whereas the other components were not associated with CRVE.
TABLE 4.
Association Between Metabolic Syndrome Continuous Parameters and Central Retinal Venular Equivalent (μm), BCOPS 2010–2014
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
|
| ||||||
| Metabolic syndrome severity score (z-score) | 3.13 (1.50) | 0.0375 | 4.61 (1.67) | 0.0062 | 3.74 (1.59) | 0.0194 |
| Number of the MetSyn components (range: 0–5) | 1.42 (0.90) | 0.1147 | 2.04 (0.96) | 0.0340 | 1.93 (0.94) | 0.0411 |
| MetSyn Components (continuous scale) | ||||||
| Mean artery blood pressure | −0.14(0.14) | 0.3128 | −0.11 (0.15) | 0.4506 | 0.26 (0.14) | 0.0650 |
| Waist circumference | 0.07 (0.09) | 0.4301 | 0.16 (0.11) | 0.1325 | 0.08 (0.11) | 0.4403 |
| Triglycerides | 0.00 (0.02) | 0.7910 | 0.01 (0.01) | 0.6723 | 0.00 (0.01) | 0.9008 |
| HDL cholesterol | −0.23 (0.09) | 0.0102 | −0.32 (0.10) | 0.0017 | −0.21 (0.09) | 0.0176 |
| Fasting glucose | 0.14(0.06) | 0.0202 | 0.17 (0.06) | 0.0071 | 0.12(0.06) | 0.0309 |
Model 1 is unadjusted. Model 2 is adjusted for age, sex, and smoking status. Model 3 is adjusted for age, sex, smoking status, C-reactive protein, antihypertensive medication, and CRAE.
FIGURE 2.
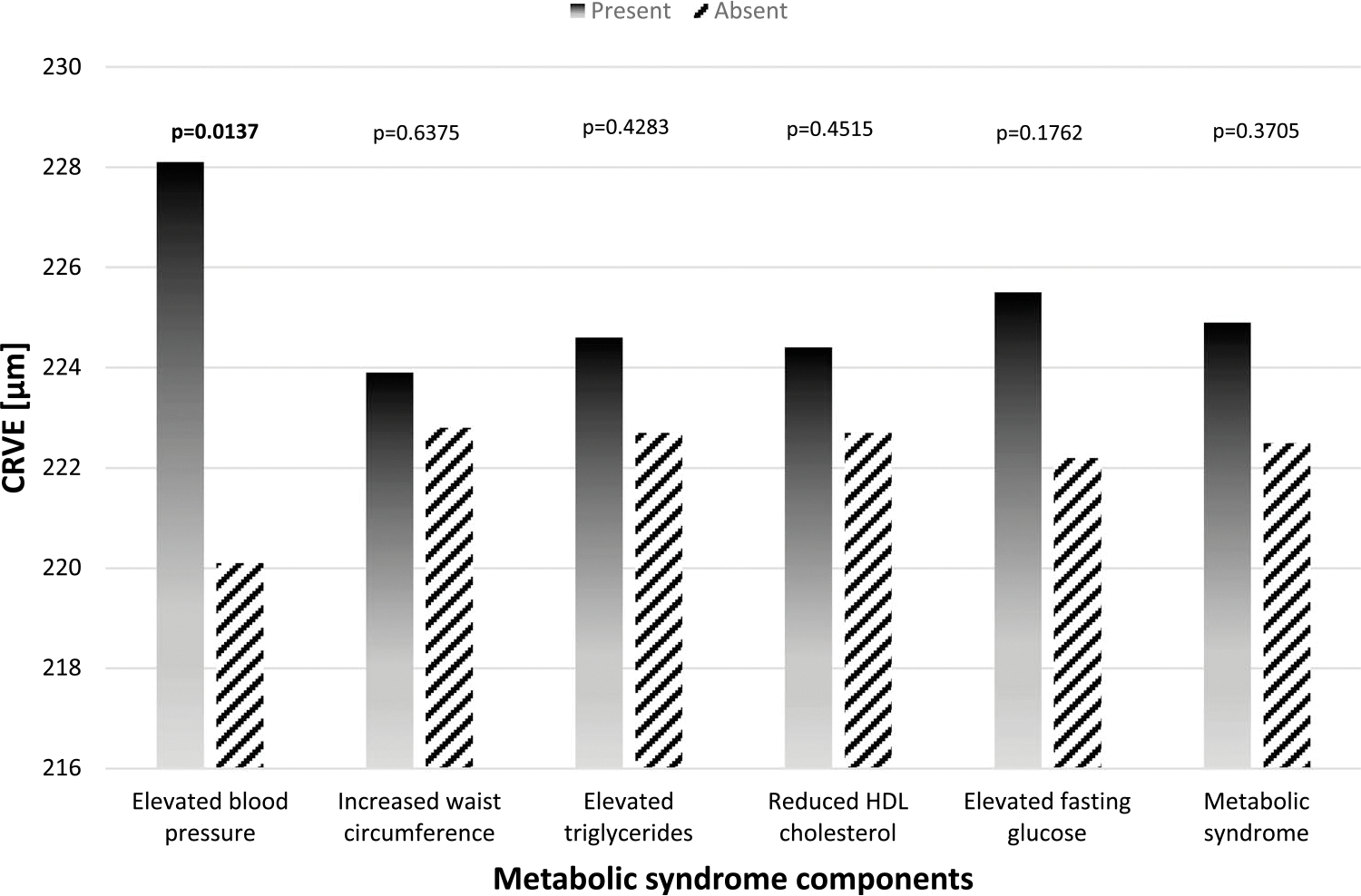
Solid bars: presence of the specific metabolic syndrome component. Diagonal bars: absence of the specific metabolic syndrome component. The above models were adjusted for age, sex, smoking status, C-creative protein, antihypertension medication, and CRAE.
DISCUSSION
Findings from previous studies showed significant associations between the MetSyn and the retinal microvascular diameters in the general population or in a group diagnosed with a certain disease,11,13,15,23 but not in an occupational group. In one of our earlier studies with this population of police officers, we focused on the association between BP, one of the components of MetSyn, and the retinal microvascular diameters. 24 The present study extended that investigation by addressing the association of the MetSyn with the retinal microvascular diameters.
The findings from our cross-sectional analysis indicated that the MSSS was significantly associated with retinal microvascular diameters. Higher values of MSSS were associated with narrowing of the retinal arteriolar diameter and widening of the retinal venular diameter. The use of the continuous form of MetSyn (ie, MSSS) is a novel feature of this investigation. In contrast, MetSyn in the dichotomous form was not associated with retinal vessel diameters. This result is not consistent with that of previous studies.11,13,15 The inconsistency may be due to age differences. The participants in this study were active-duty officers who were generally younger (average age was 47 years) than participants in the previous studies (average age was older than 60 years).
Higher BP andWCwere significantly associated with narrower retinal arteriolar diameters, but the three other clinical components (ie, triglycerides, HDL, and fasting glucose) were not associated with the CRAE. BP andWC (dichotomized versions)were also associated with the CRAE; officers diagnosed with hypertension and with elevated WC had narrower arteriolar diameters compared with their counterparts. Our results suggest that hypertension and abdominal obesity are associated with adverse conditions in the retinal arteriolar diameters. Results from the Austrian Heart Eye Study are consistent with our results.15
Lower HDL cholesterol and higher glucose values were significantly associated with wider venular diameters. These two components (HDL cholesterol and fasting glucose) were strongly associated with CRVE. In contrast, the Austrian Heart Eye Study showed that no MetSyn component was significantly associated with CRVE.15
Although hypertension was associated with retinal arteriolar narrowing and venular widening, we observed no significant associations between MetSyn status and the retinal microvascular diameters (Figs. 1, 2). Our result was different from those of previous studies, which showed that the mean retinal arteriolar diameter of those with MetSyn was significantly lower than those without MetSyn.11,13,15,23 One possible explanation for this disparity could be the healthy worker effect, in which workers tend to be healthier than those in the general population.25
Strengths and Limitations
A strength of this study is that it is the first study to investigate the association between MetSyn and retinal diameters using the MSSS variable and to do so using an occupational group. There are several limitations to our study. The participation rate of the study was relatively low (42% = 300/710), and the final target sample was only 253 officers (36%) because 47 officers did not participate in the retinal examination. Our results might be subject to selection bias, which may lead to an underestimate or overestimate of the observed associations. In addition, the cross-sectional study design prevents assessment of a causal relationship between MetSyn and the retinal microvascular diameters. Finally, The MetSyn components (continuous version) may be underestimated or overestimated because they included officers who took medications. For example, the mean arterial BP may be underestimated because of those who took antihypertensive medications medications, whereas HDL cholesterol may be overestimated because of those who took lipids medication
Conclusions
In conclusion, in this police cohort, the MSSS was found to be associated with narrower arteriolar diameter. This association was mainly driven by hypertension and elevated WC. In addition to being associated with wider venular diameter, HDL cholesterol and fasting glucose were mainly the link to the MSSS. The results of this study suggest that it may be worthwhile to further investigate the associations between the MSSS and the retinal microvascular in an occupational group with a larger sample. For example, an investigation of firefighters, who are also public safety workers, might be useful. A prospective study will be needed to evaluate causality between MetSyn and changes in the retinal microvascular diameters.
ACKNOWLEDGMENTS
The authors thank Dr. Desta Fekedulegn for deriving the variables of the metabolic syndrome and the metabolic syndrome severity score in the BCOPS study.
Disclosure of Grant Funding:
This work was supported by the National Institute for Occupational Safety and Health (contract no. 200-2012-50561 and grant no. 1R01OH 009640-01A1).
Footnotes
Conflict of Interest: None declared.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Ethics Review and Approval: All participants signed written informed consent. The Institutional Review Boards at the University at Buffalo approved the studies.
Contributor Information
Ja K. Gu, Bioanalytics Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, MS L-4050, 1095 Willowdale Rd, Morgantown, WV 26505-2888.
Luenda E. Charles, Bioanalytics Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, West Virginia.
Penelope Allison, Bioanalytics Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, West Virginia.
John M. Violanti, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, State University of New York at Buffalo, Buffalo, New York.
Michael E. Andrew, Bioanalytics Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, West Virginia.
REFERENCES
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.American Heart Association (AHA). About Metabolic Syndrome. Available at: https://www.heart.org/en/health-topics/metabolic-syndrome/about-metabolic-syndrome. Accessed June 25, 2021.
- 3.Regufe VMG, Pinto CMCB, Perez PMVHC. Metabolic syndrome in type 2 diabetic patients: a review of current evidence. Porto Biomed J. 2020;5:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;324:2526–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi TH, Wang B, Natarajan S. The influence of metabolic syndrome in predicting mortality risk among US adults: importance of metabolic syndrome even in adults with normal weight. Prev Chronic Dis. 2020;17:E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davila EP, Florez H, Fleming LE, et al. Prevalence of the metabolic syndrome among U.S. workers. Diabetes Care. 2010;33:2390–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartley TA, Burchfiel CM, Fekedulegn D, Andrew ME, Knox SS, Violanti JM. Associations between police officer stress and the metabolic syndrome. Int J Emerg Ment Health. 2011;3:243–256. [PMC free article] [PubMed] [Google Scholar]
- 8.Moline JM, McLaughlin MA, Sawit ST, et al. The prevalence of metabolic syndrome among law enforcement officers who responded to the 9/11 World Trade Center attacks. Am J Ind Med. 2016;59:752–760. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TT, Wong TY. Retinal vascular manifestations of metabolic disorders. Trends Endocrinol. 2006;17:262–268. [DOI] [PubMed] [Google Scholar]
- 10.Phan K, Mitchell P, Liew G, et al. Associations between retinal arteriolar and venular calibre with the prevalence of impaired fasting glucose and diabetes mellitus: a cross-sectional study. PLoS One. 2018;13:e0189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki R, Tielsch JM, Wang JJ, et al. The metabolic syndrome and retinal microvascular signs in a Japanese population: the Funagata study. Br J Ophthalmol. 2008;92:161–166. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65:1005–1009. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the atherosclerosis risk in communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Ikram MK, Vingerling JR, et al. Retinal vascular caliber and metabolic syndrome in a Chinese population. Intern Med J. 2011;1014–1022. [DOI] [PubMed] [Google Scholar]
- 15.Wang SB, Mitchell P, Plant AJH, et al. Metabolic syndrome and retinal microvascular calibre in a high cardiovascular disease risk cohort. Br J Ophthalmol. 2016;100:1041–1046. [DOI] [PubMed] [Google Scholar]
- 16.Saito K, Kawasaki Y, Nagao Y, Kawasaki R. Retinal arteriolar narrowing is associated with a 4-year risk of incident metabolic syndrome. Nutr Diabetes. 2015;5:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Violanti JM, Burchfiel CM, Miller DB, et al. The Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) pilot study: methods and participant characteristics. Ann Epidemiol. 2006;16:148–156. [DOI] [PubMed] [Google Scholar]
- 18.Gurka MJ, Lilly CL, Oliver MN, DeBoerMD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 20.Meaney E, Alva F, Moguel R, Meaney A, Alva J, Webel R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart. 2000;84:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr EyeRes. 2003;27:143–149. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Knudtson MD, Klein BE, et al. The relationship of retinal vessel diameter to changes in diabetic nephropathy structural variables in patients with type 1 diabetes. Diabetologia. 2010;53:1638–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Yang K, Wang F, et al. Associations between metabolic syndrome and syndrome components and retinal microvascular signs in a rural Chinese population: the Handan Eye Study. Graefes Arch Clin Exp Ophthalmol. 2012;250:1755–1763. [DOI] [PubMed] [Google Scholar]
- 24.Gu JK, Charles LE, Klein R, et al. Association between blood pressure and retinal vessel diameters among police officers in the US Northeast. J Occup Environ Med. 2018;60:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirkeleit J, Riise T, Bjørge T, Christiani DC. The Healthy worker effect in cancer incidence studies. Am J Epidemiol. 2013;177:1218–1224. [DOI] [PubMed] [Google Scholar]


