To the Editor: Non-Hodgkin lymphoma (NHL) is a common type of hematological malignancy. Although the development of targeted therapies has improved the survival of patients with aggressive NHL, autologous hematopoietic stem cell transplantation (HSCT) remains indispensable. There is no standard conditioning regimen for autologous stem cell transplantation (ASCT). Three common regimens are carmustine, etoposide, cytarabine, and melphalan (BEAM); carmustine, etoposide, cytarabine, and cyclophosphamide (BEAC); and total body irradiation. The 3-year progression-free-survival (PFS) rate associated with these regimens varies from 40% to 50%. Chidamide, a novel subtype-selective histone deacetylase inhibitor (HDACi), can directly inhibit tumor cell cycle progression, induce tumor cell apoptosis, and restore the sensitivity of drug-resistant tumor cells to drugs by loosening chromatin and exposing deoxyribonucleic acid (DNA). Clinical trials have shown that a combination of HDACi, cladribine, gemcitabine, and busulfan had a promising safety profile and efficacy as a pretransplant conditioning regimen in patients with relapsed/refractory NHL.[1] This phase II clinical trial aimed to evaluate the efficacy and safety of the chidamide-BEAC (Chi-BEAC) conditioning regimen with ASCT for treating high-risk or relapsed/refractory aggressive NHLs.
The eligibility and exclusion criteria are summarized in the Supplementary Materials, http://links.lww.com/CM9/B617. Briefly, patients with the following aggressive NHL types were enrolled: (1) high-risk diffuse large B-cell lymphoma (DLBCL) (defined as age-adjusted international prognostic index of ≥2) or double-hit lymphoma to receive frontline ASCT consolidation therapy, (2) relapsed/refractory DLBCL to receive salvage ASCT consolidation, (3) mantle cell lymphoma (MCL), and (4) peripheral T-cell lymphomas (PTCL) (except for those with anaplastic lymphoma kinase-positive anaplastic large cell lymphoma [ALCL]) or stage IV extranodal natural killer cell (NK)/T cell lymphoma (ENKTL). All patients had to achieve complete remission (CR) or partial remission (PR) before receiving ASCT. The protocol of this study was approved by the Institutional Review Board of Jiangsu Province Hospital and Anhui Provincial Cancer Hospital (No. 2017-SR-271). All patients provided written informed consent. This study was registered at ClinicalTrials.gov (NCT03629873).
The Chi-BEAC regimen was administered as follows: 30 mg of chidamide orally on day 7 before reinfusion of autologous stem cells, day 4 before reinfusion, the day of reinfusion (day 0), and day 3 after reinfusion; 300 mg/m2 of carmustine daily intravenously on day 6 before reinfusion; 150 mg/m2 of etoposide daily intravenously from day 5 to day 2 before reinfusion; 150 mg/m2 of cytarabine twice a day intravenously from day 5 to day 2 before reinfusion; and 1.0 g/m2 of cyclophosphamide daily intravenously from day 5 to day 2 before reinfusion.
Treatment response was assessed as CR, PR, stable disease (SD), or progressive disease (PD) according to the Lugano 2014 criteria. Follow-up assessments were planned every 3 months during the first year after ASCT and every 6 months during the second year after ASCT, including physical examination, laboratory tests, and computed tomography (CT) scans. All toxicities were defined using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0.
The primary objective was to assess the 2-year PFS. The secondary objectives were to assess the safety of Chi-BEAC, evaluate the 2-year overall survival (OS), and determine the CR rate in 3 months after ASCT. OS and PFS were estimated according to the Kaplan–Meier method. The log-rank test was used to assess the significance of differences for each prognostic factor in the univariate analysis. The Cox proportional hazards regression model was used to assess how the characteristics of the patients predict PFS and OS. Data were analyzed using the Statistical Package for the Social Sciences, version 13.0 (IBM Corp, Armonk, NY, USA), and GraphPad Prism 7.0 (Graphpad Software, Boston, MA, USA). Overall, 71 consecutive patients were recruited from August 2018 to November 2021; of these, 55 were recruited from Jiangsu Province Hospital and 16 from Anhui Provincial Hospital. One patient with MCL who was SD before ASCT was excluded. Finally, 70 patients were enrolled in the trial and underwent ASCT. One patient was lost to follow-up before the first response assessment; 69 patients completed ASCT and underwent at least one treatment response assessment. Among them, 6 patients withdrew informed consent and began maintenance therapy. Finally, 63 patients were included in the per-protocol set (PPS) [Supplementary Figure 1, http://links.lww.com/CM9/B617].
The characteristics of the 69 patients who underwent at least one treatment response assessment are listed in Supplementary Tables 1 and 2, http://links.lww.com/CM9/B617. Patients with DLBCL or MCL are referred to as B-NHLs, whereas those with PTCL or ENKTL are referred to as T-NHLs.
The median time of neutrophil engraftment was 10 days (range, 7–13 days), and the median time of platelet engraftment was 11 days (range, 6–22 days). Most non-hematological adverse events (AEs) were grade 1–2. Grade 3 AEs occurred in ≥5% of the patients and included febrile neutropenia (26/69, 37.7%), hyponatremia (17/69, 24.6%), hypokalemia (15/69, 21.7%), and increased γ-glutamyl transpeptidase (GGT) (5/69, 7.3%). Grade 4 AE was only observed in one patient, with increased GGT. No grade 3–4 cardiotoxicity was observed. The 100-day transplant-related mortality (TRM) was 0%. Two patients died of lung infection 6.3 months and 8.0 months after ASCT, respectively [Supplementary Table 3, http://links.lww.com/CM9/B617].
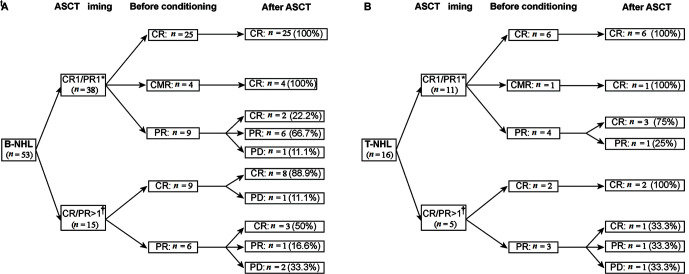
At the first follow-up assessment after treatment, the response rates were 79.7% (55/69) for CR, 13.0% (9/69) for PR, and 7.2% (5/69) for PD; 41.0% (9/22) of the patients who achieved PR before ASCT improved to CR after ASCT, including 5 with B-NHLs and 4 with T-NHLs [Figures 1A,1B].
Figure 1.
Treatment responses for NHL patients who were treated with Chi-BEAC conditioning regimen and ASCT for 53 B-NHL (A) and for 16 T-NHL (B). CR1/PR1*: Patients who underwent upfront ASCT; CR/PR >1†: Patients who underwent salvage ASCT. ASCT: Autologous stem cell transplantation; BEAC: Carmustine, etoposide, cytarabine, and cyclophosphamide; Chi-BEAC: Chidamide-BEAC; CMR: Complete metabolic remission; CR: Complete remission; NHL: Non-Hodgkin lymphoma; PD: Progressive disease; PR: Partial remission.
Survival analyses were performed for the PPS group. After a median follow-up of 25.2 months (range, 1.8–43.6 months), 55 (87.3%) of the patients were alive at the last follow-up, and 51 (81.0%) were disease-free. Eight patients died, in which six deaths were attributed to disease progression and two to lung infection. The median PFS and OS were not achieved at the end of the follow-up.
The 2-year PFS and OS rates were 81.1% and 86.1%, respectively [Supplementary Figures 2A, 2B, http://links.lww.com/CM9/B617]. No statistically significant differences in PFS or OS was observed between the disease groups [Supplementary Figures 2C, 2D, http://links.lww.com/CM9/B617]. Salvage therapy for patients with PD was shown in Supplementary Table 4, http://links.lww.com/CM9/B617. Stratified analysis revealed that, in patients with B-NHL who underwent upfront ASCT, the 2-year PFS was 79.9%, those with B-NHL who underwent salvage ASCT had a 2-year PFS of 78.8%, and those with T-NHL had a 2-year PFS of 84.8%. Patients who had achieved CR/CMR before transplantation tended to have better PFS and OS than those who had achieved PR (P = 0.158 and P = 0.115, respectively). No significant impact of stage, IPI, pathological subtype, or ASCT timing was found on the prognosis [Supplementary Table 5, http://links.lww.com/CM9/B617]. Survival analyses of all 69 patients (intention-to-treat group) are shown in Supplementary Figure 3, http://links.lww.com/CM9/B617. In the present study, we evaluated the efficacy and safety of chidamide with BEAC, a conventional conditioning regimen, in patients with high-risk NHLs. AEs were mild and manageable. We reached the primary endpoint with a 2-year PFS rate of 81.1%. Regarding tumor type and ASCT timing, the 2-year PFS was 79.9% in patients with B-NHL who underwent upfront ASCT, which is comparable to that reported in the SWOG9704 trial, which reported a 2-year PFS rate of 69.0%, and to the results of the DLBCL04 trial, which reported a 2-year PFS rate of 72.0% in the transplantation group. The 2-year PFS rate was 78.8% in patients with B-NHL who received salvage ASCT, which is an improvement compared with the previous data from the CORAL trial, showing a 3-year PFS rate of 53.0%. In the T-NHL group, the 2-year PFS rate was 84.8%, which outperforms previous studies showing long-term survival after ASCT of 50.0–70.0%.[2,3]
Of the 115 patients with T-NHL who underwent ASCT in the NLG-T-01 trial, 28 relapsed within 2 years. Among them, the prognosis of patients with angioimmunoblastic T-cell lymphoma (AITL) was worse than that of other patients. By contrast, in this study, 3 of the 4 patients with advanced-stage AITL remained disease-free after ASCT with Chi-BEAC conditioning at a median follow-up of 31.3 months (1 patient progressed at 43.6 months after ASCT). The follicular helper T-cell phenotype is an independent predictor of response to HDACi in PTCL. Although the phase III trial of romidepsin plus CHOP vs. CHOP in treatment-naive PTCLs failed to reach the primary endpoint, patients with AITL benefited from the addition of HDACi by achieving a median PFS of 19.5 months vs. 10.6 months in the CHOP arm.[4] In the present study, 4 of the 5 patients with stage IV ENKTL remained disease-free after ASCT with Chi-BEAC conditioning at a median follow-up of 25.7 months. The HEA-like subtype ENKTL (based on HDAC9, EP300, and ARID1A mutations), which is characterized by aberrant histone acetylation and activated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and T cell receptor (TCR) signaling pathways, has been reported to be highly sensitive to chidamide both in vitro and in vivo. Accordingly, chidamide with anti-programmed cell death protein 1 achieved an objective response rate of 58.3% and a CR rate of 44.4% in treating advanced and relapsed/refractory ENKTL.[5] The aforementioned data from preclinical studies and clinical trials have confirmed the effectiveness of HDACi in PTCL (especially AITL and ENKTL). Although longer follow-up and more cases are needed, these outcomes are encouraging because most relapses in these high-risk populations occur early after transplantation.
This study has several limitations. First, this study was a phase II trial that included only a few patients and was non-randomized. Second, the histological types and timing of ASCT varied. Third, the genetic features of the tumors were unclear, hampering further stratified analyses. Finally, the inclusion of chidamide in pre-ASCT conditioning was less effective in patients with B-NHL and may give way to chimeric antigen receptor T-cell (CAR-T). However, the outcomes in patients with T-NHLs are encouraging.
In conclusion, the Chi-BEAC conditioning regimen before ASCT was found to be a well-tolerated and effective treatment for high-risk or relapsed NHL. Adding HDACi to ASCT conditioning regimen may be an important treatment option, particularly for patients with T-cell lymphomas.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Nos.81770166, 81700193, 82170186 and 81720108002), Jiangsu Province's Medical Elite Programme (No. ZDRCA2016022), Project of National Key Clinical Specialty, Jiangsu Provincial Special Program of Medical Science (No.BE2017751), National Science and Technology Major Project (No.2018ZX09734007), China Postdoctoral Science Foundation (No. 2021M691336), Jiangsu Postdoctoral Science Foundation (No. 2021K083A), and Translational Research Grant of NCRCH (No. 2020ZKZB01).
Conflicts of interest
None.
Supplementary Material
Footnotes
Yi Xia, Li Wang, and Kaiyang Ding contributed equally to this work.
How to cite this article: Xia Y, Wang L, Ding KY, Wu JZ, Yin H, Hu MG, Shen HR, Liang JH, Chen RZ, Li Y, Zhu HY, Li JY, Xu W. Chidamide-BEAC plus autologous stem cell transplantation in high-risk non-Hodgkin lymphoma: A phase II clinical trial. Chin Med J 2023; 136:1491–1493. doi: 10.1097/CM9.0000000000002636
References
- 1.Ji J Liu Z Kuang P Dong T Chen X Li J, et al. . A new conditioning regimen with chidamide, cladribine, gemcitabine and busulfan significantly improve the outcome of high-risk or relapsed/refractory non-Hodgkin's lymphomas. Int Journal Cancer 2021;149: 2075–2082. doi: 10.1002/ijc.33761. [DOI] [PubMed] [Google Scholar]
- 2.Reimer P Rudiger T Geissinger E Weissinger F Nerl C Schmitz N, et al. . Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: Results of a prospective multicenter study. J Clin Oncol 2009;27: 106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 3.d'Amore F Relander T Lauritzsen GF Jantunen E Hagberg H Anderson H, et al. . Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol 2012;30: 3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 4.Bachy E Camus V Thieblemont C Sibon D Casasnovas RO Ysebaert L, et al. . Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T-cell lymphoma: Results of the Ro-CHOP phase III study (conducted by LYSA). J Clin Oncol 2022;40: 242–251. doi: 10.1200/JCO.21.01815. [DOI] [PubMed] [Google Scholar]
- 5.He X, Gao Y, Li Z, Huang H. Review on natural killer/T-cell lymphoma. Hematol Oncol 2021. doi: 10.1002/hon.2944. [DOI] [PubMed] [Google Scholar]