Abstract
The intestinal mucus layer is a barrier that separates intestinal contents and epithelial cells, as well as acts as the "mucus layer-soil" for intestinal flora adhesion and colonization. Its structural and functional integrity is crucial to human health. Intestinal mucus is regulated by factors such as diet, living habits, hormones, neurotransmitters, cytokines, and intestinal flora. The mucus layer's thickness, viscosity, porosity, growth rate, and glycosylation status affect the structure of the gut flora colonized on it. The interaction between "mucus layer-soil" and "gut bacteria-seed" is an important factor leading to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Probiotics, prebiotics, fecal microbiota transplantation (FMT), and wash microbial transplantation are efficient methods for managing NAFLD, but their long-term efficacy is poor. FMT is focused on achieving the goal of treating diseases by enhancing the "gut bacteria-seed". However, a lack of effective repair and management of the "mucus layer-soil" may be a reason why "seeds" cannot be well colonized and grow in the host gut, as the thinning and destruction of the "mucus layer-soil" is an early symptom of NAFLD. This review summarizes the existing correlation between intestinal mucus and gut microbiota, as well as the pathogenesis of NAFLD, and proposes a new perspective that "mucus layer-soil" restoration combined with "gut bacteria-seed" FMT may be one of the most effective future strategies for enhancing the long-term efficacy of NAFLD treatment.
Keywords: Non-alcoholic fatty liver disease, Gut microbiome, Fecal microbiota transplantation, Intestinal barrier, Intestinal mucus layer
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and is frequently associated with metabolic risk factors including obesity, type 2 diabetes (T2D), hyperlipidemia, and hypertension.[1] As the global obesity epidemic worsens metabolic conditions, NAFLD has become the most prevalent chronic liver disease globally, affecting approximately 1 billion people.[2,3] Among them, non-alcoholic steatohepatitis (NASH) has become a common indication for liver transplantation in the United States.[4] However, the pathogenesis of NAFLD remains unclear, with the intestinal microenvironment possibly playing a role.[5]
The intestinal mucus layer is the substrate for the adhesion and colonization of many microorganisms, as well as a key entry point for bacteria and bacterial products to enter the circulation ectopically. In addition to playing a crucial role as an immune barrier, the intestinal mucus layer aids in nutrient absorption and prevents the invasion of various organisms. Recent studies have shown that the intestinal mucus layer is a part of the gut–liver axis, a complex mechanism whose crosstalk with microbiota is closely associated with the incidence and progression of NAFLD.[6] The repair of the mucus layer may be beneficial to the adhesion and colonization of probiotics and could be the key to enhancing the long-term efficacy of biotherapy, making it a novel avenue to explore for the clinical treatment of NAFLD.
Gut Microbes and NAFLD
There is a large number of symbiotic bacteria in the human intestinal tract. These symbiotic bacteria together contain approximately 100-fold more genes than that found in humans. The intestinal tract of mammals is sterile at birth, then gets colonized by symbiotic bacteria.[7] The gut microbiota and its active metabolites play an important role in the host[8,9] by maintaining the immune level of the intestinal environment, participating in the intestinal defense against pathogenic microbial colonization and in metabolic syndrome.[10] As an independent organ, the liver receives blood supply from the portal vein and the hepatic artery, which are pooled by the mesenteric vein. The portal vein contains the nutrients that come from the intestinal tract and the microbial products of the intestinal tract. Thus, the liver is directly affected by changes in the gut microecology.[11,12]
Compared to individuals with a high gut bacterial abundance, those with a low bacterial abundance show more overall obesity, insulin resistance, and dyslipidemia, and a more pronounced inflammatory phenotype over time; furthermore, obese individuals in populations with low bacterial abundance are more likely to gain weight.[13] Two bacterial phyla, namely Bacteroidetes and Firmicutes, predominate in the human gut and account for 92.6% of all 16S ribosomal RNA (rRNA) sequences; however, the abundance of Bacteroidetes is relatively reduced in an obese patient population.[14] Based on 16S rRNA gene sequencing results, the abundance of Bacteroidetes in the feces of ob/ob obese mice decreased by nearly 50%, whereas the proportion of Firmicutes increased.[15] Moreover, the abundance of Bacteroidetes is associated with the percentage of weight loss.[14] In a 16S rRNA test of gut microbes in monozygotic or dizygotic twins, obese individuals were found to contain significantly less number of Bacteroidetes (P =0.003) and more Actinobacteria (P =0.002), with no significant difference in the abundance of Firmicutes (P =0.090), compared to those in lean individuals.[16]
Gut microbes and liver lipid de novo synthesis
Changes in gut microecology have a direct impact on liver physiology and pathology.[11] Gut microbes influence the liver lipid de novo synthesis of NAFLD in the following main ways. First, gut microbes can break down indigestible foods. Gut microbes contain abundant glycoside hydrolases and polysaccharide lyases, which help to extract energy from indigestible polysaccharides in the diet and convert them into monosaccharides and short-chain fatty acids (SCFAs), which can enter the liver through the portal vein to synthesize triglycerides (TG).[11] Second, gut microbes promote the absorption of lipids. The adipocyte hypertrophy in conventionally fed mice was 40% greater than that in germ-free (GF) mice when fed the same polysaccharide diet,[7] despite the fact that the conventionally fed mice ate less than the GF mice. This may be due to gut microbes converting indigestible polysaccharides in the food to absorb energy.[17] Third, intestinal microbial metabolism can affect liver pathophysiology directly through the portal vein. SCFAs fermented by gut microbes stimulate de novo hepatic lipid synthesis while also serving as carbon donors.[18] Further, commensal colonization increases glucose uptake (GU) in the host gut, leading to elevated blood glucose and insulin levels.[7]
Pathogenic bacteria induces NAFLD
Escherichia/Shigella bacteria significantly increase in patients with NAFLD compared to that in healthy controls.[19] Furthermore, lipid accumulates in the liver after transplantation of Escherichia/Shigella in host rats as the bacteria secrete small RNA (msRNA) 23487, which inhibits hepatic lipid β-oxidation and peroxisome proliferator-activated receptor-α, thereby promoting liver lipid de novo synthesis.[19] Colonoscopy biopsy revealed that the relative abundance of Escherichia coli NF73-1 was significantly increased in the mucosa of patients with NAFLD or even NASH. Through the Toll-like receptor 2-nuclear factor kappa B/NOD-like receptor protein 3-caspase-1 signaling pathway, Escherichia coli NF73-1 increased M1 macrophages in the NASH mouse livers, exacerbating high fat diet (HFD)-induced steatosis in mice.[20]
Administration of an HFD to GF mice after transplantation of Enterobacter cloacae B29 can induce obesity and insulin resistance in the mice, possibly through the production of lipopolysaccharide (LPS) endotoxin, which can aggravate inflammatory reactions and induce obesity in mice.[21] Mice colonized with Enterobacter cloacae B29 show significantly enhanced scores of liver steatosis and NAFLD activity than HFD-fed mice alone.[22] These results confirm that pathogenic bacteria contribute independently to the occurrence and development of NAFLD.
Probiotics, prebiotics, and fecal microbiota transplantation (FMT) are an effective way to manage NAFLD
There are no Food and Drug Administration (FDA)-approved drugs for treating NAFLD, and probiotics, prebiotics, and FMT are regarded as effective methods for treating NAFLD. In general, prebiotics improve gut bacterial composition, modulate host energy metabolism, and improve the intestinal barrier to ameliorate metabolic disease.[23] Furthermore, prebiotics are also involved in insulin signaling, by which they improve NAFLD outcomes; for example, they improve insulin resistance,[24] reduce hepatic collagen accumulation,[25] and reduce lipid peroxidation.[26]
Probiotics affect the body by regulating the gut microbiota, enhancing intestinal barrier function, increasing competitive adhesion to mucous membranes and epithelium, and regulating the gut-associated lymphatic immune system.[27] Lactobacillus plantarum NCU116 improves NAFLD by upregulating the expression of genes related to lipolysis and fatty acid (FA) oxidation and downregulating the expression of adipogenesis genes.[28] Lactobacillus rhamnosus GG and Lactobacillus plantarum WCFS1 co-administration can reduce rat serum TG, total cholesterol (TC), free fatty acids (FFAs), and liver fat deposition levels by upregulating cholesterol 7a-hydroxylase (CYP7A1) expression.[29] Providing hamsters HFD with heat-killed Lactobacillus reuteri GMNL-263 reportedly improves lipid and cholesterol metabolism, which may improve liver health.[30]
In a recent randomized, triple-blind clinical trial, probiotic capsules (comprising Lactobacillus acidophilus ATCC B3280, Bifidobacterium bifidum ATCC SD6576, Bifidobacterium lactis DSMZ 32269, and Lactobacillus rhamnosus DSMZ 21690) administered to children with NAFLD for 12 weeks improved serum alanine aminotransferase (ALT) and lipid levels, but this had no influence on the body mass index (BMI).[31] A meta-analysis that included four randomized controlled trials involving 134 patients with NASH evaluated the effects of various probiotic therapies on NAFLD and showed that using probiotic therapy significantly reduced serum aminotransferase, TC, high-density lipoprotein (HDL), and tumor necrosis factor-α (TNF-α) levels. These findings suggest a potential role for probiotic treatment in modulating gut microbiota to treat NAFLD.[32] Another meta-analysis that evaluated prebiotics and probiotics for the treatment of NAFLD retrieved nine articles on prebiotics, 11 articles on probiotics, and seven articles on microbiota transplantation, involving a total of 1309 patients, and found that microbial therapy significantly reduced the BMI, serum liver enzyme levels such as of ALT, aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GT), serum TC, low-density lipoprotein cholesterol (LDL)-c, and TG. This meta-analysis supports microbial therapy's potential role in the treatment of NAFLD.[33] Furthermore, FMT administered to NAFLD mice for eight weeks could increase the abundance of beneficial bacteria such as Christensenellaceae and Lactobacillus, increase the expression of the intestinal tight junction protein zonula occludens-1 (ZO-1), and improve liver steatosis.[34] In a clinical study that recruited 20 patients with NAFLD, administration of FMT as a continuous intervention for six months was found to prevent gut microbiota from returning to baseline, improve liver magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) values, and reduce serum AST levels.[35] In another randomized controlled clinical trial that recruited 21 patients with NAFLD who received once either an allogenic or an autologous FMT treatment, and the follow-up after six months found that FMT did not improve insulin resistance and liver MRI-PDFF but did have the potential to reduce small intestinal permeability of NAFLD patients in six weeks[36] [Supplementary Table 1, http://links.lww.com/CM9/B569]. Therefore, FMT has a certain effect on NAFLD in a short time range, but the long-term efficacy is not good, which may be related to the degree of colonization of healthy intestinal bacteria, and so increasing the frequency or the colonization rate of intestinal microorganisms of FMT is the key to future research on FMT therapy.
Mucus Layer Regulates Gut Microbiota Structure
The gut microbiome primarily colonizes the mucus layer of the gastrointestinal tract, and their interaction with the mucus layer plays an important role in regulating the immune system and host health.[37] The intestinal mucus layer serves as a substrate for the adhesion and colonization of many microorganisms, as well as an important entry point for bacteria and bacterial products. In addition to its important role as an immune barrier, the intestinal mucus layer aids in nutrient absorption and prevents the invasion of various organisms.[6]
Composition of the intestinal mucus layer
The intestinal mucus layer is a thin, aqueous, and viscoelastic secretion composed of multiple components: water (90%–95%),[38,39] electrolytes, lipids (1%–2%), mucus proteins, and others, with mucin (MUC) serving as the main structural and functional component in mucus. At a concentration of 1%–5%, MUC forms a complex layered, large-scale polymer network as the skeleton of the mucus layer, attached to the intestinal epithelial cell surface.[40] The colon has two mucus layers, with the outer mucus layer serving as a habitat for colonic gut microbes.[41] The thickness of the mucus layer and growth rate varies along with different parts of the gut as well different bacterial species.[42]
Mucus layer affects gut microbiome structure
MUC2 is a family of highly glycosylated proteins secreted by goblet cells in the gut. Its O-glycans are used as ligands for bacterial adhesins,[43–45] and serve as bacterial attachment points to promote bacterial growth and colonization[46] and to maintain the homeostasis and immune response of the intestinal environment. Secretory and transmembrane MUC both provide interaction and attachment sites, and the microorganisms' ability to bind to MUC determines their colonization ability.[47] In addition to providing attachment sites, MUC glycans can also serve as nutrients for microbes known as "mucolytic bacteria."[48]
The mucosa-associated microbiota bacterial species use glycans as carbon sources. Altered glycan availability alters the composition of the microbiota.[49] The mucin-deficient Muc2-/-mouse model shows impaired epithelial barrier, and colonoscopy in these mice showed erosion of the mucosal surface.[50] Thus, Muc2-/- mouse were more prone to developing colitis and rectal cancer.[51] Furthermore, gut dysbiosis was observed in the Muc2-/-mice, with pro-inflammatory microbiota colonization, such as increased abundance of Clostridiales and decreased abundance of Lactobacillaceae.[50] Due to differences in the mucus layer, the small intestine and colon have different gut microbiomes. The small intestine contains immunoglobulin A (IgA) and antimicrobial peptide (AMP), which are secreted into the mucus layer by plasma cells and Paneth cells within the lamina propria, respectively, making the small intestine relatively unsuitable for bacterial growth.[52,53] The colon has fewer Paneth cells, resulting in less IgA and AMP secretion on the colon, coupled with a large amount of mucus and thicker mucus on the colon, making the colon more conducive to the growth of microorganisms.[39]
Mucus layer prevents colonization by pathogenic microorganisms
Mucus, a component of the innate intestinal mucosal barrier, is responsible for reducing antigen exposure and bacterial exposure to the immune system under the intestinal epithelium, serving as the first line of immune defense against potentially harmful compounds.[54] Mucus also serves as a surface-cleaning agent, eliminating foreign pathogens.[55] The intestinal mucosa helps maintain the intestinal barrier by secreting AMPs produced by Paneth cells for innate immunity (e.g., α-defensins, lysozyme C, phospholipases, C-type lectin, and RegIIIγ). Additionally, the antimicrobial-rich mucosal system establishes and maintains a steady-state relationship between the host and its colonizing microbiota.[56,57] It is due to its interaction with the immune system that the mucus layer provides protection. Secretory IgA (SIgA) is the major globulin on the mucosal surface of humans and many other mammals, and its function is immune rejection, which restricts the entry of pathogenic microorganisms and mucosal antigens through the mucosal barrier.[53] SIgA may prevent the colonization of harmful microorganisms by recognizing multiple antigenic epitopes on the surface of viral and pathogen proteins. Additionally, SIgA can recognize and control the homeostasis of commensal bacteria in the host.[58] In Muc2-/- mice, the Firmicutes phylum is enriched while the abundance of the Bacteroidetes phylum is reduced, which might potentially lead to pathogen colonization, causing spontaneous colitis or even colorectal cancer.[59]
Intestinal mucosa is affected by microbial colonization and dietary structure
The regulation of the mucus layer is highly complex and is influenced by multiple factors, including external factors (e.g., pathogens, probiotics, diet, food additives or pollutants, antibiotics) and host factors (e.g., hormones, cytokines, neurotransmitters, lipids).[60] Many pathogenic microorganisms, including Vibrio cholerae, the protozoa Giardia lamblia in the small intestine, proteases produced by Escherichia coli (E. coli), the protozoan Entamoeba histolytica, or the nematode Trichuris muris in the large intestine, have the ability to degrade mucus proteins and alter the mucus layer.[61] Studies have shown that probiotic transplantation helps increase the expression of MUC genes and increases the thickness of the mucus layer. Beneficial bacteria can prevent pathogens from invading by increasing mucus production and occupying available binding sites on MUCs, thereby preventing pathogens from adhering.[62] Studies have also shown that feeding a western diet supplemented with Bifidobacterium longum for 4 weeks can restore mucus growth.[63] Lactobacillus spp. transplantation can promote the expression of MUC genes, which can stimulate the expression of MUC3 and the production and secretion of MUC2 in human intestinal epithelial cells.[64,65] Lactobacillus reuteri bacteria expresses adhesins such as mucus-binding proteins CmbA and MUB,which increased binding to human colon carcinoma HT-29 (ATCC HTB-38) and LS174T cells (ATCC CL-188). Therefore, Lactobacillus reuteri binding to the mucus layer resulted in decreased Escherichia coli adherence ability to small intestinal biopsy epithelium.[66] Therefore, Lactobacillus reuteri transplantation protected mice against dextran sulfate-induced inflammatory bowel disease and increased mucus layer thickness.[67]
After seven days of treatment with a probiotic mixture of Lactobacilli, Bifidobacteria, and Streptococci, the mucin content in the colon of rats increased by 60%.[68] Akkermansia muciniphila derived extracellular vesicles as delivery vehicles can reportedly improve HFD-induced mucosal crypt depth and thickness, increase intestinal permeability, and upregulate tight junction protein (Zo-1, Occludin, and Claudin-1) expression, thereby improving obesity.[69] These findings support the development of therapeutic strategies based on human mucus colonizers, where probiotic transplantation prevents or treats obesity and its associated metabolic disorders by improving the mucus layer.
Dietary structure can affect the mucus layer. In mouse ileal cells induced by HFD, Ctfr gene expression was reduced, mucus synthesis and secretion were hindered, and the mucus layer became thinner and less dense.[70] Decreased expression of Muc2 protein in mucus samples from western diet-fed mice resulted in decreased mucus layer density, increased mucus layer permeability, and a reduced mucus layer growth rate.[63] A western diet containing 40.5% kcal from fat (41% saturated, 52% monounsaturated) and 40.5% kcal from carbohydrates (sucrose 18.0%, corn starch 16.0%, maltodextrin 12.0%, cellulose 4.0% [w/v]) was fed to mice for three days, which results in a decrease in SCFA and in the thickness of the mucus layer, and an increase in permeability in the colon of mice.[71] Regular consumption of dietary fiber can also help protect the mucus layer.[72] In mice on a microbiota-accessible carbohydrate-deficient diet, a study found reduced mucus thickness in the colon, bacteria were localized close to the intestinal epithelium, and sparser bacterial localization was found in the lumen.[73] Considering fiber polysaccharides are the primary source of energy for gut microbes, mucus increases the number of degrading bacteria when fiber polysaccharides are absent in the diet.[61]
Furthermore, some widely used food additives, such as carboxymethyl cellulose and polysorbate, can reduce the thickness of the mucus layer.[74] MUC2 expression is also influenced by a number of cytokines.[75] TNF-α stimulates MUC2 transcription via the nuclear factor-kappa B (NF-κB)-induced kinase and phosphatidylinositol 3-kinase (PI3K)/serine/threonine kinase 2 (Akt 2) pathways, and inhibits MUC2 transcription via the c-jun amino terminal kinase (JNK) pathway. However, NF-κB transcriptional activation can counteract the JNK pathway inhibition.[76] Interleukin(IL)-1β can upregulate the expression of MUC2 through the protein kinase C (PKC)/mitogen-activated extracellular signal-regulated kinase (MEK)/extracellular regulated protein kinases (ERK)/PI3K signaling pathway.[76] IL-4 and IL-13 increase Muc2 gene expression by activating MAPK phosphorylation.[77] IL-10 promotes MUC2 secretion by improving MUC2 folding in goblet cells and preventing endoplasmic reticulum stress.[78]
The current review included studies describing the regulation of the mucus layer mainly focusing on external factors (microbiota, diet, etc.). However, the data on the regulation of the mucus layer by host factors remain limited, especially studies on the regulation of the mucus layer by host factors in biological disorders, intestinal motility dysfunction, and stress. Given that these factors are linked to the incidence of gut dysbiosis disorders and enteric neurohormonal secretion, more research is needed in the future to acquire complete data.
Interaction between mucus layer and gut bacteria is a key factor of NAFLD
The mucous layer is related to the occurrence of diseases. The regulation of its chemical composition and synthesis and the process of its synthesis and degradation are subject to pathological and physiological regulation. In metabolic diseases such as T2D, obesity, and NAFLD, the mucus layer is altered during the development and growth of the gut microbiota, and the mucus layer is impaired prior to the disease phenotype.[79,80] Furthermore, microbial invasion of the medial mucus layer is associated with insulin resistance and the bacterial-epithelial distance is inversely related to the BMI, blood glucose, and hemoglobin levels in people with metabolic syndrome.[81]
The mucus–bacteria interaction is disrupted in chronic inflammation or metabolic syndrome, allowing bacteria to penetrate the mucus layer and reach epithelial cells.[74] In the mucin-deficient Muc2–/– mice, bacteria were found not only in direct contact with intestinal epithelial cells but also deep in the crypts, even below the epithelial nucleus; moreover, Muc2–/– mice developed colitis at 7 weeks of age and colon cancer at 6–12 months.[82] Disruption of the intestinal epithelial barrier leading to the translocation of microbial products to the portal vein can induce liver inflammation. High-fat pork protein diet intake can reduce the number of mucus goblet cells and inhibit the expression of Muc2 protein while reducing the key ZO-1 and E-cadherin proteins to increase intestinal permeability and induce host obesity.[83] A high-fat, high-sugar, and high-cholesterol diet produces more severe liver fibrosis in knockout mice lacking the gene encoding junctional adhesion molecule-A/F11 Receptor (JAM-A/F11R), whereas antibiotics can improve liver tissue fibrosis.[84]
In another study,[85] Muc2–/– mice were found to have less alcoholic liver damage and steatosis than wild-type mice when alcoholic liver was induced in mice by the Tsukamoto–French method (comprising continuous gavage of an isocaloric diet or alcohol). Additionally, the LPS content in serum was lower in Muc2–/– mice than that in wild-type mice, which may be due to the expression of regenerating insulin-derived 3β and γ AMP proteins in the jejunum of the Muc2–/– mice, which protects against alcohol-associated microorganisms' growth and reduces transfer of bacterial products such as LPS into the circulation, thereby reducing alcoholic liver damage. The same studies have shown that the intestinal mucosa of Muc2–/– mice is impaired, and transcriptomic studies have shown that it plays a role in maintaining ileal homeostasis by enhancing the interleukin-22 (IL-22) signal transducer and activator of transcription 3 (STAT3) signaling pathway, thereby reducing Toll receptors and downregulating immune factors and chemokine signaling pathways.[86]
Mucus layer and hepatic de novo lipid synthesis
The intestinal mucosa serves not only as a barrier to microbial invasion of tissues but also as a conduit for the uptake of nutrients from food sources and metabolites from microbiome sources. Food consumption rapidly activates a population of intestinal neurons expressing vasoactive intestinal peptide (VIP) 4, enhancing the growth of epithelial-associated commensal microbes, such as segmented filamentous bacteria and increasing lipid absorption.[87] Additionally, the levels of epithelial cell-derived AMPs are reduced but the expression of lipid-binding proteins and transporters is enhanced.[88]
There is growing evidence that dietary fat disrupts the intestinal barrier, negatively regulating intestinal mucus composition, increasing luminal content penetration into the mucosa and submucosa of adjacent immune cells, and promoting inflammatory responses.[70]
A protein with a molecular weight of 12,000 that binds to unsaturated fat more readily than FA and medium-chain FAs has been found in tissues such as the intestinal mucosa; this protein may be involved in the absorption of FA by the mammalian intestinal mucosa.[89] The secretion of bile acids promotes the entry of FA and cholesterol into the intestinal mucosa.[90] During fat absorption, the release of mast cells from the intestinal mucosa is promoted.[91] Additionally, butyrate infusion or exercise-induced SCFAs could stimulate mucosal growth and increase mucosal DNA content in rats.[92] These results suggest that intestinal mucus layer, which affects the absorption of lipids in the intestine, may be the primary factor affecting de novo hepatic fat synthesis in NAFLD.
Mucus layer and insulin resistance
Insulin resistance is closely associated with NAFLD and its progression through regulation of adipose tissue breakdown, changes in de novo lipogenesis rates, and mitochondrial FA-β oxidation.[93] In patients with T2D, however, the mucus layer is damaged and thinned, the microbiota-epithelial distance is reduced by nearly three-fold, and fasting blood glucose and hemoglobin levels are positively correlated with the degree of mucus layer damage. This suggests that microbial invasion of epithelial cells is a cause or feature of T2D.[81] The gut is the primary site of glucose absorption and a major cause of insulin resistance induction. Glucose uptake (GU) in the gut and skeletal muscle, measured using [18F]-fluoro-2-deoxyglucose and positron emission tomography, revealed that insulin-stimulated skeletal muscle GU was attenuated in obese diabetic and non-diabetic patients compared with that in healthy controls, but had no effect on intestinal GU stimulation. Intestinal GU is associated with systemic insulin sensitivity after bariatric surgery in obese patients, suggesting that the intestinal mucosa may mediate systemic glycemic status and insulin resistance.[94] This suggests that intestinal insulin resistance is an early feature of obese individuals. Targeting the intestinal mucus layer may be the key to preventing T2D and reducing early insulin resistance.
Mucus layer and obesity
Obesity appears to play a role in the pathogenesis of NAFLD by reducing FA uptake in subcutaneous adipose tissue and causing excess circulating FFAs to accumulate ectopically and insulin resistance in the liver in the obese state.[95] There is mounting evidence linking obesity and the mucus barrier. We performed a genetic analysis in the mucus layer of obese and non-obese stomachs and found 929 differential genes between the two groups. Oxidative stress and inflammatory responses contribute to gastric mucus injury in obese patients, with TNF-α and IL-6 expression showing significant upregulation.[96] Interestingly, mucus layer defects are associated with obesity, with obese mice exhibiting greater mucus permeability and a decreased mucus growth rate compared to lean mice, while if co-housing the obese and lean mice, the intestinal permeability and mucosal growth rate of obese mice can be restored to some extent. Mucus growth rate in mice suggests that altered gut microbiota structure in obese mice may be responsible for obesity-related mucosal defects.[97]
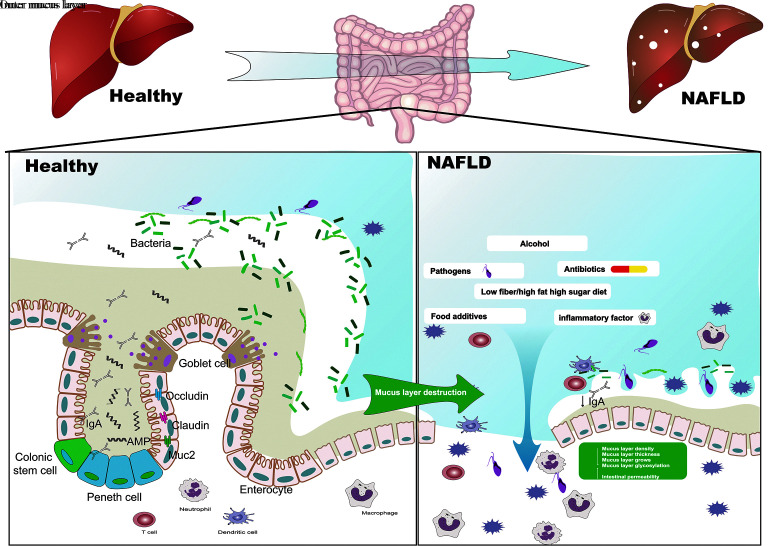
In conclusion, the interaction between gut microbiota and the mucus layer is crucial to the occurrence and development of gut microbiota disorders. We believe that factors affecting NAFLD (such as changes in microbiota, HFD, neurotransmitters, cytokines) may also affect the thickness, viscosity, porosity, growth rate, and glycosylation changes in the host's "mucus layer-soil," which would then in a feedback manner influence the composition and colonization of the intestinal microbiota. After pathogenic bacteria enter the mucus, they alter the internal environment of the intestinal mucosa "soil," weaken the protective effect of the mucus layer, and affect the colonization of the flora, resulting in a decline in intestinal biodiversity and an imbalance in the ratio of harmful bacteria to beneficial bacteria, which is one of the primary causes of NAFLD [Figure 1].
Figure 1.
The interaction between the "mucus layer-soil" and intestinal bacteria is a key factor in the occurrence and development of NAFLD. Changes in the intestinal microbiome, HFD, neurotransmitters, cytokines, etc. affect the thickness, viscosity, porosity, growth rate, and glycosylation changes in the host "mucus layer-soil" and negative feedback affects the gut. Disturbance of the intestinal microflora and colonization of the mucus layer by pathogenic bacteria will change the internal environment of the intestinal mucosa "soil", further weakening the protective effect of the mucus layer and affecting the colonization of the commensal bacteria. This will result in the decline of intestinal biodiversity and a harmful bacteria/beneficial bacteria ratio imbalance, which is one of the key factors leading to the occurrence of NAFLD. NAFLD: Non-alcoholic fatty liver disease; HFD: High-fat diet; IgA: Immunoglobulin A; AMP: Adenosine 5′-monophosphate.
Intestinal Mucus Layer-mediated Regulation of NAFLD
Microbial diversity strengthens the mucus barrier
Commensal microbes rely on undigested polysaccharides and the host's endogenous glycans as an energy source and produce thousands of carbohydrate-active enzymes, leading to the production of SCFAs, bile acids, and organic acids, which in turn creates a favorable substrate for the growth of commensal bacteria.[98] Studies have shown that as the microbiome becomes more diverse, the mucus layer in the colon in particular, becomes more viscous. Mice housed in different breeding rooms on the same pathogen-free (SPF) breeding platform exhibit different intestinal microbiota structures, and different breeding rooms and different intestinal microbiota structures can cause mice to exhibit different mucosal layer characteristics.[99] While GF mice had an impaired mucus barrier, it was restored after wild mice fecal transplantation into GF mice.[100] On day 7 of colonization in GF mice, the mucus layer permeability was similar to that of wild mice, with reduced systemic bacterial antigen exposure and reduced susceptibility to intestinal injury.[101] These results suggest that the mucus layer thickness can be improved by increasing the abundance of gut microbes.
Repairing the mucus layer helps probiotic colonization improve NAFLD
Probiotics have been shown to be an effective way to manage NAFLD. In NAFLD models, the mucus layer tends to be disrupted, and repairing the mucus layer is more effective than probiotic colonization in managing NAFLD. Nuciferine can enhance the intestinal barrier by enhancing the expression of occludin, goblet cells, and MUC2, as well as altering the relative abundance of mucus-associated microbiota (Akkmensia muciniphila, Ruminococcaceae) and LPS-producing microbiota (Desulfovibrionaceae) for weight loss and steatosis.[102] Administration of laver degradant can improve a series of problems such as HFD-induced obesity by increasing the growth of goblet cells and mucus in the mucous membrane of mice, increasing the expression level of lysozyme, and stimulating the secretion of SIgA.[103] HFD-induced obesity and T2D are characterized by altered gut microbiota, inflammation, and gut barrier disruption.[104] Similarly, probiotic colonization improved NAFLD by repairing mucus layer damage caused by HFD. HFD induced a 46% reduction in mucus layer thickness in mice, and colonization with live Akkermansia muciniphila favored increased endocannabinoid intestinal peptide secretion and restored the mucus layer thickness reduced by HFD to treat hyperlipidemia. Moreover, Akkermansia muciniphila degraded MUC by upregulating mucolytic activity (protease, glycosyl hydrolase, and sulfatase) to facilitate self-colonization and increase the abundance of the butyrate-producing commensal Anaerostipes caccae.[105]
"Seeds and soil" may be new strategies to treat NAFLD
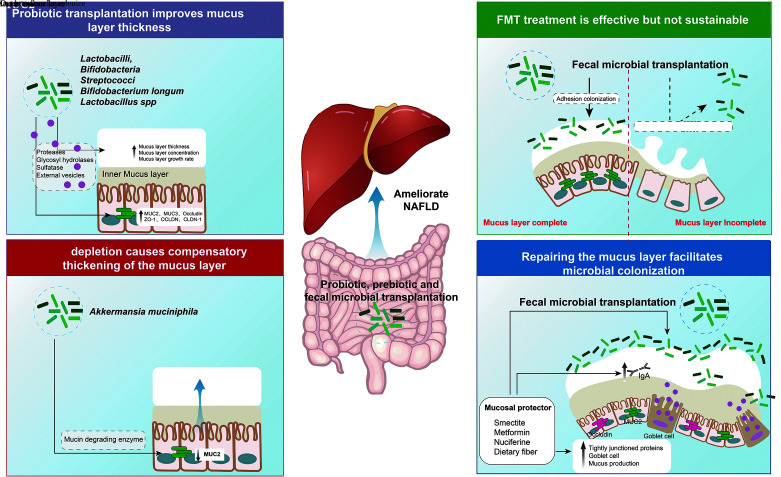
Growing evidence suggests that restoring barrier function can improve the clinical manifestations of gastrointestinal or systemic disease. Probiotics, FMT, and washed microbiota transplantation are thought to be effective treatments for NAFLD, but their long-term efficacy is questionable. All of these clinical treatment strategies achieve the purpose of treating diseases by changing the "gut bacteria-seeds," but there is a lack of strengthening/repair and management strategies for the "mucus layer-soil," which may be the reason why the probiotic "seeds" cannot be transplanted very well. Because the thinning and destruction of the "mucus layer-soil" is an early symptom of NAFLD, repairing the "mucus layer-soil" is critical to the colonization of probiotics and intestinal bacteria after FMT, and may be the basis for the long-term prognosis of FMT. Smectite is a naturally occurring silicate that has mucus layer-stabilizing properties. In a monosodium glutamate-induced NAFLD rat model, we provided the multi-probiotic and the multi-probiotic + smectite combination, respectively, and the results showed that the multi-probiotic complex + smectite combination was associated with a more pronounced reduction in leaflet inflammation. The stabilizing effect of smectite on the mucus layer could enhance the effect of probiotics and improve lobular inflammation.[106] These findings suggest that "soil" remediation combined with "seed" transplantation (such as FMT) may be one of the effective strategies to improve the long-term efficacy of NAFLD treatment in the future [Figure 2].
Figure 2.
New clinical treatment strategies to improve the long-term efficacy and prognosis of NAFLD. Along with transplantation of "gut microbiota-seeds", such as FMT, the expression of mucin is promoted to repair the broken and thin "mucin layer-soil," which can prevent the direct contact between intestinal microbes and epithelial cells and promote the adhesion and colonization of probiotics in the intestine. Therefore, improving the intestinal microecology in terms of "soil" and "seed" is the key to future treatment of NAFLD. FMT: Fecal microbiota transplantation; MUC: Mucin; NAFLD: Non-alcoholic fatty liver disease; IgA: Immunoglobulin A.
Conclusion
Changes in the gut microbiota and microbiome have been extensively reported to affect bacterial translocation, both in patients and experimental models of NAFLD. Increased levels of plasma endotoxin and bacterial DNA are associated with gut bacterial overgrowth in patients. Bacterial overgrowth in the small intestine causes significant liver inflammation in rats.[107] NAFLD was found in the liver and intestine, and intestinal bacteria are the key factors in the occurrence and development of NAFLD. FMT transplantation of probiotics, prebiotics, and healthy intestinal microbiota is an emerging approach to manage NAFLD. FMT has been shown to have a significant short-term effect on NAFLD, but the long-term prognosis is poor, possibly due to the return of intestinal bacteria to baseline levels. The mucus layer in the intestine not only acts as the defense line of the intestinal barrier but also provides a nutrient matrix for the adhesion and colonization of intestinal commensal bacteria, which is essential for affecting the structure of the intestinal bacteria and maintaining homeostasis. The thinning and breakage of the mucus layer is an early characteristic event of metabolic diseases such as NAFLD, and the thin "mucus layer-soil" is not conducive to the colonization and growth of FMT "seeds," which is an important reason for the weakening of the long-term effect of FMT. Therefore, improving the intestinal mucus "soil" is the key to the future management of NAFLD with probiotics, prebiotics, and FMT, which can not only prevent direct contact between intestinal bacteria and epithelial cells but also improve intestinal insulin resistance. Meanwhile the repaired "mucus layer-soil" also facilitates the adhesion and colonization of FMT beneficial bacteria, providing long-term therapeutic effects for NAFLD. Future strategies to improve the long-term efficacy and prognosis of NAFLD treatment may include "soil" remediation in conjunction with "seed" FMT.
Funding
This work was supported by grants from the National Natural Science Funds of China (Nos. 82204827, 81970545, and 82170609); Zhejiang Provincial Basic Public Welfare Research Project (Nos. LQ23H270016 and LGF20H030010); Hangzhou Major Science and Technology Plan Project (No. 20172016A02); and Major Projects of Hangzhou Medical and Health Science and Technology Plan (No. 0020191059).
Conflicts of interest
None.
Supplementary Material
Footnotes
Binbin Zhang, Jie Li, and Jinlong Fu contributed equally to this work.
How to cite this article: Zhang BB, Li J, Fu JL, Shao L, Yang LP, Shi JP. Interaction between mucus layer and gut microbiota in non-alcoholic fatty liver disease: Soil and seeds. Chin Med J 2023;136:1390–1400. doi: 10.1097/CM9.0000000000002711
References
- 1.Liu J Tian Y Fu X Mu C Yao M Ni Y, et al. Estimating global prevalence, incidence, and outcomes of non-alcoholic fatty liver disease from 2000 to 2021: Systematic review and meta-analysis. Chin Med J 2022;135: 1682–1691. doi: 10.1097/cm9.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64: 73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Li J Zou B Yeo YH Feng Y Xie X Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4: 389–398. doi: 10.1016/s2468-1253(19)30039-1. [DOI] [PubMed] [Google Scholar]
- 4.Le MH Yeo YH Li X Li J Zou B Wu Y, et al. 2019 global NAFLD prevalence: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022;20: 2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Hou K Wu ZX Chen XY Wang JQ Zhang D Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther 2022;7: 135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandl K, Kumar V, Eckmann L. Gut-liver axis at the frontier of host-microbial interactions. Am J Physiol Gastrointest Liver Physiol 2017;312: G413–G419. doi: 10.1152/ajpgi.00361.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bäckhed F Ding H Wang T Hooper LV Koh GY Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101: 15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Wang H, Chen X, Xie P. Gut microbiota: A new insight into neurological diseases. Chin Med J 2022. Jul 14. doi: 10.1097/cm9.0000000000002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan R Yang L Yao G Geng S Ge Q Bo S, et al. Features of gut microbiota in patients with anorexia nervosa. Chin Med J 2022;135: 1993–2002. doi: 10.1097/cm9.0000000000002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang PX, Deng XR, Zhang CH, Yuan HJ. Gut microbiota and metabolic syndrome. Chin Med J 2020;133: 808–816. doi: 10.1097/cm9.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444: 1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 12.Duseja A, Chawla YK. Obesity and NAFLD: The role of bacteria and microbiota. Clin Liver Dis 2014;18: 59–71. doi: 10.1016/j.cld.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Le Chatelier E Nielsen T Qin J Prifti E Hildebrand F Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013;500: 541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 14.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature 2006;444: 1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102: 11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbaugh PJ Hamady M Yatsunenko T Cantarel BL Duncan A Ley RE, et al. A core gut microbiome in obese and lean twins. Nature 2009;457: 480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamanaka M, Nomura T, Kametaka M. Influence of intestinal microbes on heat production in germ-free, gnotobiotic and conventional mice. J Nutr Sci Vitaminol (Tokyo) 1977;23: 221–226. doi: 10.3177/jnsv.23.221. [DOI] [PubMed] [Google Scholar]
- 18.Rolandelli RH, Koruda MJ, Settle RG, Leskiw MJ, Stein TP, Rombeau JL. The effect of pectin on hepatic lipogenesis in the enterally-fed rat. J Nutr 1989;119: 89–93. doi: 10.1093/jn/119.1.89. [DOI] [PubMed] [Google Scholar]
- 19.Xin FZ Zhao ZH Liu XL Pan Q Wang ZX Zeng L, et al. Escherichia fergusonii promotes nonobese nonalcoholic fatty liver disease by interfering with host hepatic lipid metabolism through its own msRNA 23487. Cell Mol Gastroenterol Hepatol 2022;13: 827–841. doi: 10.1016/j.jcmgh.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y Jiang W Xu J Wu N Wang Y Lin T, et al. E. coli NF73-1 isolated from NASH patients aggravates NAFLD in mice by translocating into the liver and stimulating M1 polarization. Front Cell Infect Microbiol 2020;10: 535940. doi: 10.3389/fcimb.2020.535940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 2013;7: 880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monga Kravetz A Testerman T Galuppo B Graf J Pierpont B Siebel S, et al. Effect of gut microbiota and PNPLA3 rs738409 variant on nonalcoholic fatty liver disease (NAFLD) in obese youth. J Clin Endocrinol Metab 2020;105: e3575–e3585. doi: 10.1210/clinem/dgaa382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact 2011;10 Suppl 1: S10. doi: 10.1186/1475-2859-10-s1-s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mencarelli A Cipriani S Renga B Bruno A D'Amore C Distrutti E, et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One 2012;7: e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velayudham A Dolganiuc A Ellis M Petrasek J Kodys K Mandrekar P, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology 2009;49: 989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loguercio C Federico A Tuccillo C Terracciano F D'Auria MV De Simone C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 2005;39: 540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 27.Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int J Mol Sci 2016;17: 928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C Nie SP Zhu KX Ding Q Li C Xiong T, et al. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct 2014;5: 3216–3223. doi: 10.1039/c4fo00549j. [DOI] [PubMed] [Google Scholar]
- 29.Mei L Tang Y Li M Yang P Liu Z Yuan J, et al. Co-administration of cholesterol-lowering probiotics and anthraquinone from cassia obtusifolia L. ameliorate non-alcoholic fatty liver. PLoS One 2015;10: e0138078. doi: 10.1371/journal.pone.0138078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting WJ Kuo WW Hsieh DJ Yeh YL Day CH Chen YH, et al. Heat killed Lactobacillus reuteri GMNL-263 reduces fibrosis effects on the liver and heart in high fat diet-hamsters via TGF-β suppression. Int J Mol Sci 2015;16: 25881–25896. doi: 10.3390/ijms161025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. J Pediatr Gastroenterol Nutr 2017;64: 413–417. doi: 10.1097/mpg.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 32.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J Gastroenterol 2013;19: 6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr Rev 2018;76: 822–839. doi: 10.1093/nutrit/nuy031. [DOI] [PubMed] [Google Scholar]
- 34.Zhou D Pan Q Shen F Cao HX Ding WJ Chen YW, et al. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 2017;7: 1529. doi: 10.1038/s41598-017-01751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong VW Won GL Chim AM Chu WC Yeung DK Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol 2013;12: 256–262. doi: 10.1016/S1665-2681(19)31364-X. [PubMed] [Google Scholar]
- 36.Craven L Rahman A Nair Parvathy S Beaton M Silverman J Qumosani K, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: A randomized control trial. Am J Gastroenterol 2020;115: 1055–1065. doi: 10.14309/ajg.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 37.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9: 799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 38.Hansson GC. Mucins and the microbiome. Annu Rev Biochem 2020;89: 769–793. doi: 10.1146/annurev-biochem-011520-105053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016;16: 639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bansil R, Turner BS. The biology of mucus: Composition, synthesis and organization. Adv Drug Deliv Rev 2018;124: 3–15. doi: 10.1016/j.addr.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 2001;280: G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 42.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis 2011;17: 362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 43.Juge N. Microbial adhesins to gastrointestinal mucus. Trends Microbiol 2012;20: 30–39. doi: 10.1016/j.tim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Johansson ME, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res 2009;8: 3549–3557. doi: 10.1021/pr9002504. [DOI] [PubMed] [Google Scholar]
- 45.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci U S A 2011;108(Suppl 1): 4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergstrom KS, Xia L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013;23: 1026–1037. doi: 10.1093/glycob/cwt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelaseyed T, Hansson GC. Membrane mucins of the intestine at a glance. J Cell Sci 2020;133: jcs240929. doi: 10.1242/jcs.240929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H Limenitakis JP Fuhrer T Geuking MB Lawson MA Wyss M, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun 2015;6: 8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouwerkerk JP, de Vos WM, Belzer C. Glycobiome: Bacteria and mucus at the epithelial interface. Best Pract Res Clin Gastroenterol 2013;27: 25–38. doi: 10.1016/j.bpg.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Van der Sluis M De Koning BA De Bruijn AC Velcich A Meijerink JP Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006;131: 117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Velcich A Yang W Heyer J Fragale A Nicholas C Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002;295: 1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 52.Mukherjee S Partch CL Lehotzky RE Whitham CV Chu H Bevins CL, et al. Regulation of C-type lectin antimicrobial activity by a flexible N-terminal prosegment. J Biol Chem 2009;284: 4881–4888. doi: 10.1074/jbc.M808077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corthésy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 2013;4: 185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corfield AP. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta 2015;1850: 236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Hansson GC. Mucus and mucins in diseases of the intestinal and respiratory tracts. J Intern Med 2019;285: 479–490. doi: 10.1111/joim.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011;9: 356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 57.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010;10: 159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 58.Mestecky J, Russell MW, Elson CO. Intestinal IgA: Novel views on its function in the defence of the largest mucosal surface. Gut 1999;44: 2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu M, Wu Y, Li J, Bao Y, Guo Y, Yang W. The dynamic changes of gut microbiota in Muc2 deficient mice. Int J Mol Sci 2018;19: 2809. doi: 10.3390/ijms19092809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 2013;10: 352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 2018;16: 457–470. doi: 10.1038/s41579-018-0036-x. [DOI] [PubMed] [Google Scholar]
- 62.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2016;14: 20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schroeder BO Birchenough GMH Ståhlman M Arike L Johansson MEV Hansson GC, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018;23: 27–40.e7. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bron PA Kleerebezem M Brummer RJ Cani PD Mercenier A MacDonald TT, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 2017;117: 93–107. doi: 10.1017/s0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sicard JF, Le Bihan G, Vogeleer P, Jacques M, Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front Cell Infect Microbiol 2017;7: 387. doi: 10.3389/fcimb.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walsham AD MacKenzie DA Cook V Wemyss-Holden S Hews CL Juge N, et al. Lactobacillus reuteri inhibition of enteropathogenic Escherichia coli adherence to human intestinal epithelium. Front Microbiol 2016;7: 244. doi: 10.3389/fmicb.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin NR Lee JC Lee HY Kim MS Whon TW Lee MS, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014;63: 727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 68.Caballero-Franco C, Keller K, De Simone C, Chadee K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 2007;292: G315–G322. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 69.Ashrafian F Shahriary A Behrouzi A Moradi HR Keshavarz Azizi Raftar S Lari A, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front Microbiol 2019;10: 2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rohr MW, Narasimhulu CA, Rudeski-Rohr TA, Parthasarathy S. Negative effects of a high-fat diet on intestinal permeability: A review. Adv Nutr 2020;11: 77–91. doi: 10.1093/advances/nmz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birchenough G, Schroeder BO, Bäckhed F, Hansson GC. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes 2019;10: 246–250. doi: 10.1080/19490976.2018.1513765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desai MS Seekatz AM Koropatkin NM Kamada N Hickey CA Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016;167: 1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Earle KA Billings G Sigal M Lichtman JS Hansson GC Elias JE, et al. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 2015;18: 478–488. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chassaing B Koren O Goodrich JK Poole AC Srinivasan S Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519: 92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015;3: e982426. doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim YD Jeon JY Woo HJ Lee JC Chung JH Song SY, et al. Interleukin-1beta induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J Korean Med Sci 2002;17: 765–771. doi: 10.3346/jkms.2002.17.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwashita J, Sato Y, Sugaya H, Takahashi N, Sasaki H, Abe T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol 2003;81: 275–282. doi: 10.1046/j.1440-1711.2003.t01-1-01163.x. [DOI] [PubMed] [Google Scholar]
- 78.Hasnain SZ Tauro S Das I Tong H Chen AC Jeffery PL, et al. IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2013;144: 357–368.e9. doi: 10.1053/j.gastro.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 79.Cani PD Bibiloni R Knauf C Waget A Neyrinck AM Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57: 1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 80.Cani PD. Gut microbiota - at the intersection of everything? Nat Rev Gastroenterol Hepatol 2017;14: 321–322. doi: 10.1038/nrgastro.2017.54. [DOI] [PubMed] [Google Scholar]
- 81.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 2017;4: 205–221. doi: 10.1016/j.jcmgh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008;105: 15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hussain M Umair Ijaz M Ahmad MI Khan IA Brohi SA Shah AU, et al. Meat proteins in a high-fat diet have a substantial impact on intestinal barriers through mucus layer and tight junction protein suppression in C57BL/6J mice. Food Funct 2019;10: 6903–6914. doi: 10.1039/c9fo01760g. [DOI] [PubMed] [Google Scholar]
- 84.Rahman K Desai C Iyer SS Thorn NE Kumar P Liu Y, et al. Loss of junctional adhesion molecule a promotes severe steatohepatitis in mice on a diet high in saturated fat, fructose, and cholesterol. Gastroenterology 2016;151: 733–746.e12. doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartmann P Chen P Wang HJ Wang L McCole DF Brandl K, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology 2013;58: 108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sovran B Loonen LM Lu P Hugenholtz F Belzer C Stolte EH, et al. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier. Inflamm Bowel Dis 2015;21: 531–542. doi: 10.1097/mib.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 87.Talbot J, Hahn P, Kroehling L, Nguyen H, Li D, Littman DR. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 2020;579: 575–580. doi: 10.1038/s41586-020-2039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mao K Baptista AP Tamoutounour S Zhuang L Bouladoux N Martins AJ, et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 2018;554: 255–259. doi: 10.1038/nature25437. [DOI] [PubMed] [Google Scholar]
- 89.Ockner RK, Manning JA, Poppenhausen RB, Ho WK. A binding protein for fatty acids in cytosol of intestinal mucosa, liver, myocardium, and other tissues. Science 1972;177: 56–58. doi: 10.1126/science.177.4043.56. [DOI] [PubMed] [Google Scholar]
- 90.Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest 1976;58: 97–108. doi: 10.1172/jci108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji Y Sakata Y Yang Q Li X Xu M Yoder S, et al. Activation of rat intestinal mucosal mast cells by fat absorption. Am J Physiol Gastrointest Liver Physiol 2012;302: G1292–G1300. doi: 10.1152/ajpgi.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kripke SA, Fox AD, Berman JM, Settle RG, Rombeau JL. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. JPEN J Parenter Enteral Nutr 1989;13: 109–116. doi: 10.1177/0148607189013002109. [DOI] [PubMed] [Google Scholar]
- 93.Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 2019;70: 711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 94.Mäkinen J Hannukainen JC Karmi A Immonen HM Soinio M Nelimarkka L, et al. Obesity-associated intestinal insulin resistance is ameliorated after bariatric surgery. Diabetologia 2015;58: 1055–1062. doi: 10.1007/s00125-015-3501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019;92: 82–97. doi: 10.1016/j.metabol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 96.Wen X Su B Gao M Chen J Zhou D You H, et al. Obesity-associated up-regulation of lipocalin 2 protects gastric mucosa cells from apoptotic cell death by reducing endoplasmic reticulum stress. Cell Death Dis 2021;12: 221. doi: 10.1038/s41419-021-03512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schroeder BO Birchenough GMH Pradhan M Nyström EEL Henricsson M Hansson GC, et al. Obesity-associated microbiota contributes to mucus layer defects in genetically obese mice. J Biol Chem 2020;295: 15712–15726. doi: 10.1074/jbc.RA120.015771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai R, Cheng C, Chen J, Xu X, Ding C, Gu B. Interactions of commensal and pathogenic microorganisms with the mucus layer in the colon. Gut Microbes 2020;11: 680–690. doi: 10.1080/19490976.2020.1735606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jakobsson HE Rodríguez-Piñeiro AM Schütte A Ermund A Boysen P Bemark M, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 2015;16: 164–177. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johansson ME Jakobsson HE Holmén-Larsson J Schütte A Ermund A Rodríguez-Piñeiro AM, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 2015;18: 582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayes CL Dong J Galipeau HJ Jury J McCarville J Huang X, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep 2018;8: 14184. doi: 10.1038/s41598-018-32366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan J, Sun J, Li T, Yan X, Jiang Y. Nuciferine prevents hepatic steatosis associated with improving intestinal mucosal integrity, mucus-related microbiota and inhibiting TLR4/MyD88/NF-κB pathway in high-fat induced rats. J Funct Foods 2022;88: 104859. doi: 10.1016/j.jff.2021.104859. [Google Scholar]
- 103.Li C, Cheng X, Cao W, Wang Y, Xue C, Tang Q. Enzymatic hydrolysate of porphyra enhances the intestinal mucosal functions in obese mice. J Food Biochem 2022;46: e14175. doi: 10.1111/jfbc.14175. [DOI] [PubMed] [Google Scholar]
- 104.Everard A Belzer C Geurts L Ouwerkerk JP Druart C Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013;110: 9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chia LW Hornung BVH Aalvink S Schaap PJ de Vos WM Knol J, et al. Deciphering the trophic interaction between Akkermansia muciniphila and the butyrogenic gut commensal Anaerostipes caccae using a metatranscriptomic approach. Antonie Van Leeuwenhoek 2018;111: 859–873. doi: 10.1007/s10482-018-1040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobyliak N, Abenavoli L, Falalyeyeva T, Beregova T. Efficacy of probiotics and smectite in rats with non-alcoholic fatty liver disease. Ann Hepatol 2018;17: 153–161. doi: 10.5604/01.3001.0010.7547. [DOI] [PubMed] [Google Scholar]
- 107.Lichtman SN, Sartor RB, Keku J, Schwab JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology 1990;98: 414–423. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]