Abstract
Ferroptosis is an iron-dependent cell death pathway that is different from apoptosis, pyroptosis, and necrosis. The main characteristics of ferroptosis are the Fenton reaction mediated by intracellular free divalent iron ions, lipid peroxidation of cell membrane lipids, and inhibition of the anti-lipid peroxidation activity of intracellular glutathione peroxidase 4 (GPX4). Recent studies have shown that ferroptosis can be involved in the pathological processes of many disorders, such as ischemia–reperfusion injury, nervous system diseases, and blood diseases. However, the specific mechanisms by which ferroptosis participates in the occurrence and development of acute leukemia still need to be more fully and deeply studied. This article reviews the characteristics of ferroptosis and the regulatory mechanisms promoting or inhibiting ferroptosis. More importantly, it further discusses the role of ferroptosis in acute leukemia and predicts a change in treatment strategy brought about by increased knowledge of the role of ferroptosis in acute leukemia.
Keywords: Acute lymphoblastic leukemia, Acute myeloid leukemia, Ferroptosis, Oxidative damage, Antioxidant defense, Lipid peroxidation
Introduction
The concept of ferroptosis, which is defined as iron-dependent regulated cell death, was put forward in 2012, and the main causes of ferroptosis are lipid peroxidation and plasma membrane rupture.[1–3] Ferroptosis is different from the common forms of regulated cell death previously discovered, such as apoptosis, pyroptosis, and necroptosis.[4] Apoptosis is mediated by molecules such as caspases 3/6/7/8/9, poly (adenosine diphosphate [ADP]-ribose) polymerases (PARP), B-cell lymphoma-2 (BCL2), BCL2 associated X (BAX), and BCL2 antagonist/killer 1 (BAK1). Pyroptosis also depends on the role of caspases, of which caspases 1/4/5/11 play a major role. In addition, pyroptosis is also regulated by gasdermin D (GSDMD), interleukin-1 beta (IL-1B), and interleukin-18 (IL-18). Necroptosis does not depend on the function of caspases; instead, receptor-interacting protein kinase 1 (RIPK1), RIPK, and mixed lineage kinase domain-like protein (MLKL) mediate its effects.[2,5] Studies have found that dozens of key cytokines, mainly including nuclear receptor coactivator 4 (NCOA4), arachidonate 15-lipoxygenase (ALOX15), voltage-dependent anion-selective channel protein 2 (VDAC2/3), solute carrier family 7 member 11 (SLC7A11), solute carrier family 3 member 2 (SLC3A2), glutathione peroxidase 4 (GPX4), transferrin receptor (TFR), transferrin (TF), and ferritin, are involved in the regulation of ferroptosis.
The main characteristics of ferroptosis are iron accumulation and excessive lipid peroxidation.[1,6,7] In recent years, there have been an increasing number of in-depth studies on the mechanism of ferroptosis. The system Xc- cystine/glutamate antiporter and GPX4 have been found to be involved in ferroptosis, and using the compounds erastin and an inhibitor of GPX4 (RSL3), respectively, to inhibit these two molecules can induce ferroptosis.[1,8–10] Uncontrolled lipid peroxidation is a sign of ferroptosis. Some studies have found that acyl-coenzyme A (acyl-CoA) synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are important factors driving ferroptosis.[11–13] Furthermore, certain lipoxygenases (LOX) can directly oxidize polyunsaturated fatty acids (PUFAs) on biofilms and thus are also considered to have a great potential to mediate ferroptosis.[14] An increasing number of studies have revealed the regulatory mechanism of ferroptosis, providing a sufficient theoretical basis for ferroptosis to be used to enhance treatment strategies in the future.
Leukemia is a group of heterogeneous hematopoietic stem cell (HSC) malignant tumors. Leukemic cells are abnormally aggregated and undifferentiated primordial cells, which can proliferate unrestrictedly in the bone marrow and interfere with the production of normal blood cells. Leukemia can be divided into acute and chronic leukemia.Acute leukemia (AL) contains acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). For now, there are many ways to treat leukemia clinically, but because of drug resistance or relapse, leukemia patients still need more treatment strategies.[15–18] A number of experiments and epidemiological studies have shown that iron metabolism disorders are related to the occurrence and development of AL.[19–21] The occurrence of leukemia involves many genes related to iron metabolism, including hemochromatosis (HFE) gene, transferrin receptor 1 gene, and other genes involved in iron metabolism. Leukemic cells showed an increase in iron uptake and a decrease in iron efflux, resulting in an increase in cellular iron levels.[7,22]
At present, the main methods for the clinical treatment of leukemia are chemotherapy and bone marrow transplantation.[23,24] However, traditional chemotherapy drugs not only kill leukemia cells but also cause damage to healthy cells, causing huge side effects, and bone marrow transplantation is not suitable for all leukemia patients for various reasons. Although the development of chimeric antigen receptor (CAR)-T therapy provides a new hope for the treatment of leukemia, it is still not widely used because of its high price, troublesome preparation, and other limitations.[25] Leukemic cells are more easily affected by iron depletion than normal cells. Therefore, targeting the iron metabolic pathway may provide a good strategy for the treatment of AL. Ferroptosis is a newly discovered way of cell death. The rational use of ferroptosis will provide a new dawn for the treatment of AL.[26]
History of Ferroptosis
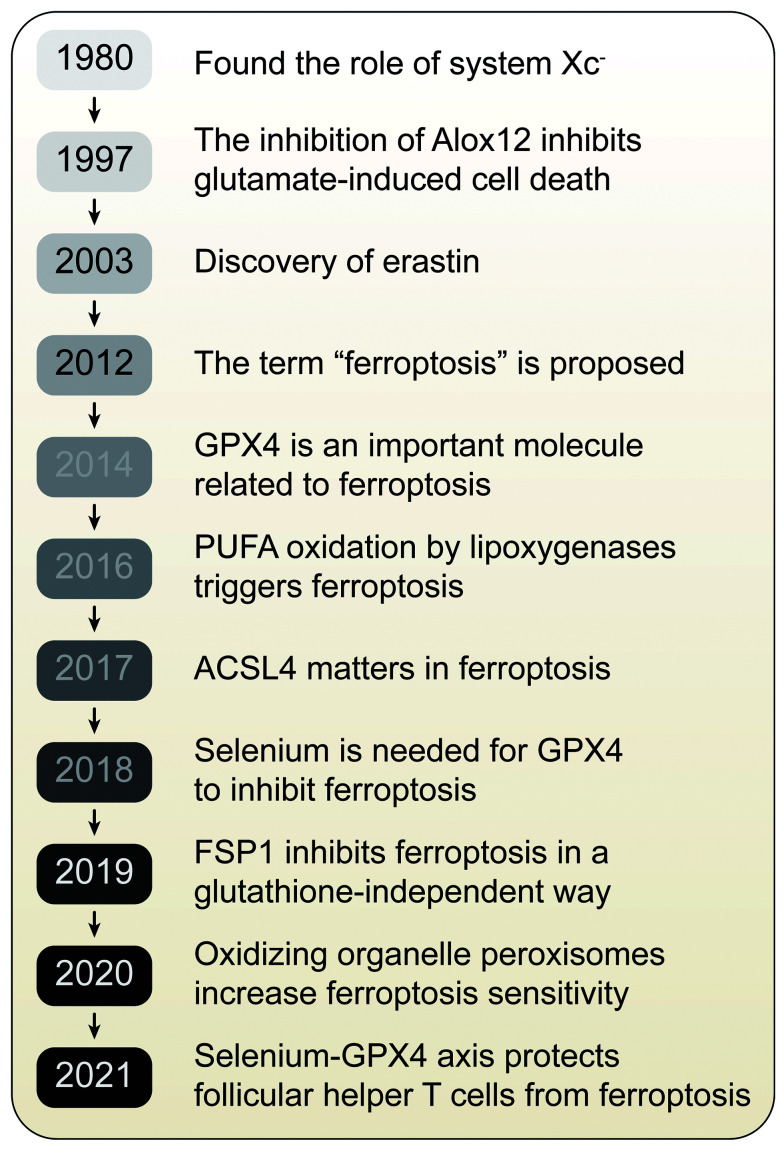
As early as 1980, it was found that system Xc- could transport cystine into cells in exchange for glutamate.[27] It has been reported that inhibition of cystine transport can induce glutamate toxicity in nerve cells and produce oxidative stress.[28] In 1997, studies showed that inhibition of arachidonate 12-lipoxygenase (Alox12) could inhibit glutamate-induced cell death in the hippocampal cell line HT22 and primary cortical neurons.[29] In 2003, erastin, an inducer of ferroptosis, was discovered in high-throughput small-molecule screening studies. Erastin have lethal effects on rat sarcoma (RAS)-mutant tumor cells.[30] In 2012, the concept of ferroptosis was formally proposed, and studies have found that erastin induces cell death by inhibiting cystine uptake via system Xc-.[1] Soon after in 2014, another important molecule related to ferroptosis, GPX4, was discovered. Yang et al[8] reported that GPX4 can prevent ferroptosis by reducing phospholipid hydrogen peroxide, thereby inhibiting LOX-mediated lipid peroxidation. Two years later, it was reported that PUFA oxidation by LOX triggers ferroptosis.[31] In 2017, studies showed that ACSL4 is a biomarker of and plays a key role in ferroptosis and is necessary to produce the PUFAs needed for ferroptosis.[12] In 2018, a study of GPX4 showed that selenium was necessary for GPX4 to inhibit ferroptosis.[32] Recently, studies have shown that the coenzyme Q10 (CoQ10) reductase ferroptosis suppressor protein 1 (FSP1) can inhibit ferroptosis in a glutathione (GSH)-independent manner, providing a new pathway for the study of ferroptosis.[33,34] In a 2020 study to identify ferroptosis-sensitive genes, we found that oxidizing organelle peroxisomes can increase ferroptosis sensitivity through the synthesis of polyunsaturated fatty ether phospholipids.[35] With more in-depth research, it has been found that there is a strong relationship between ferroptosis and the immune system. Follicular helper T (Tfh) cells are a specialized subset of cluster of differentiation 4 (CD4) T cells that essentially support germinal center responses generating high-affinity and long-lived humoral immunity. The regulation of T cell survival remains unclear. Kuhn's findings reveal the central role of the selenium–GPX4–ferroptosis axis in regulating Tfh homeostasis, which can be targeted to enhance T cells' function in infection and following vaccination [Figure 1].
Figure 1.
Timeline of the discovery of important molecules related to ferroptosis. Based on the findings of a large number of basic studies, the formal definition of "ferroptosis" was proposed in 2012. Since then, many studies have revealed the important molecules and mechanisms related to ferroptosis. ACSL4: Acyl-CoA synthetase long-chain family member 4; AlOX12: Arachidonate 12-lipoxygenase; FSP1: Ferroptosis suppressor protein 1; GPX4: Glutathione peroxidase 4; PUFA: Polyunsaturated fatty acid.
Characteristics of Ferroptosis
Ferroptosis is different from other forms of regulated cell death, such as apoptosis, pyroptosis, and necroptosis, in terms of morphological characteristics, biochemical immune characteristics, and genetic regulation. Ferroptosis is characterized by the following morphological features: (1) a significantly decreased mitochondrial crest, increased membrane density, and rupture of the membrane, leading to atrophy of mitochondria; (2) a normal nucleus; and (3) increased cell membrane density and a disintegrated cell membrane. Ferroptosis is characterized by the following biochemical and immune characteristics: (1) apparently decreased mitochondrial membrane potential; (2) significantly increased intracellular iron ion level; (3) markedly increased reactive oxygen species (ROS) level; (4) enhanced lipid peroxidation; (5) release of injury-related molecular factors that promote the inflammatory response; and (6) caspase independence.[1,5,36–38]
Mechanism of Ferroptosis
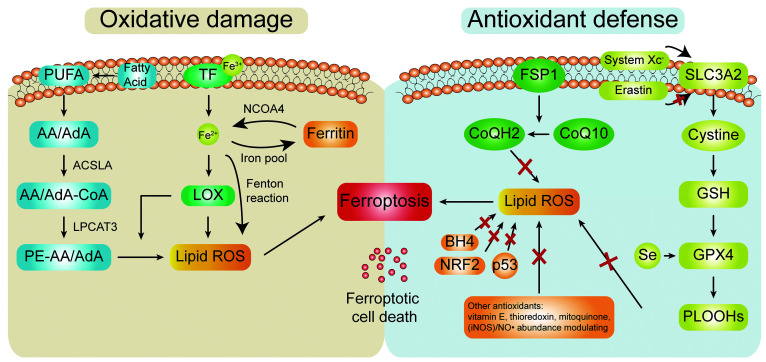
The main features of ferroptosis are increased iron and the accumulation of lipid peroxides on the membrane.[2] The core function of ferroptosis is to balance oxidative damage and antioxidant defense[3,39] [Figure 2].
Figure 2.
The function of the ferroptosis mechanism is to balance oxidative damage and antioxidant defense. The mechanism of ferroptosis mainly involves two parts: oxidative damage and antioxidant defense. When the physiological state of the body changes and oxidative damage increases, ferroptosis and ferroptotic cell death are induced; in contrast, overexuberant antioxidant defense hinders the occurrence of ferroptosis. AA: Arachidonic acid; ACSL4: Acyl-CoA synthetase long-chain family member 4; AdA: Adrenic acid; BH4: Tetrahydrobiopterin; CoQ10: Coenzyme Q10; CoQ12: Coenzyme Q12; FSP1: Ferroptosis suppressor protein 1; GPX4: Glutathione peroxidase 4; GSH: Glutathione; iNOS: Inducible nitric oxide synthase; LOX: Lipoxygenase; NCOA4: Nuclear receptor coactivator 4; NO: Nitric oxide; NRF2: Nuclear factor erythroid 2-related factor 2; PE-AA: Arachidonic acid-phosphatidylethanolamine; PLOOHs: Phospholipid hydroperoxides; PUFA: Polyunsaturated fatty acid; ROS: Reactive oxygen species; SLC3A2: Solute carrier family 3 member 2; TF: Transferrin.
Oxidative damage
Lipid peroxidation
Lipid peroxidation refers to the loss of hydrogen atoms by lipids under the action of free radicals or lipid peroxidases, which leads to the oxidation, breaking, and shortening of lipid carbon chains and the production of cytotoxic substances such as lipid free radicals, lipid hydroperoxides, and active aldehydes, resulting in cell damage.[40] PUFAs, an important component of the cell membrane, are the main substrate of lipid peroxidation during ferroptosis. PUFAs are long-chain fatty acids with more than two double bonds that regulate many important physiological activities, such as growth, proliferation, differentiation, senescence, immunity, and inflammation. PUFAs are easily oxidized, and the more double bonds they contain, the higher their oxidation sensitivity.[41–46] Some studies have shown that ROS mainly attacks PUFAs located on the cell membrane. Lipid peroxidation of PUFAs begins with the formation of lipid free radicals. Subsequently, lipid free radicals interact with oxygen molecules to form lipid peroxidation free radicals. Lipid peroxidation free radicals can continuously participate in the oxidation of PUFAs; as such, the lipid peroxidation of PUFAs occurs via a cascade reaction. The PUFAs that participate in the induction of ferroptosis are mainly arachidonic acid (AA) and adrenic acid (AdA).[47] Furthermore, ACSL4 and lysophosphatidylcholine acyltransferase 3 (LPCAT3) also play an important role in this process. It has been reported that reducing ACSL4 can reduce the sensitivity of cells to iron-related death, while upregulation of ACSL4 expression after inhibition of the nuclear factor E2-related factor 2 (NRF2)-yes1 associated transcriptional regulator (YAP) pathway can promote ferroptosis.[48–50] LPCAT3 targets acetylated AA and esterifies CoA-AA intermediates to form arachidonic acid-phosphatidylethanolamine PE-AA, which ultimately leads to ferroptosis under the action of LOX.[13,46,51] In recent years, because LOX can oxidize PUFAs located on the cell membrane, researchers have begun to explore whether LOX can mediate ferroptosis.[14] It has been reported that ferroptosis induced by 12-LOX can promote the inhibition of p53-dependent tumors.[52] The application of LOX inhibitors can inhibit ferroptosis.[29,53] However, since more experimental evidence is needed to determine whether LOX is directly related to ferroptosis, we cannot conclude that LOX can mediate ferroptosis.
Role of iron
Iron is a necessary nutrient for living creatures. The level of iron in cells is a result of the balance between the absorption, output, utilization, and storage of iron. When ferroptosis occurs, a large amount of free divalent Fe2+ accumulates in cells, which mainly comes from the binding of TF with Fe3+ to TFR on the plasma membrane. TF enters the cell, and Fe3+ dissociates from TF and is reduced to Fe2+ or combines with ferritin to be stored in the iron pool. Additionally, ferritin in the iron pool can be degraded, which releases a large amount of divalent iron ions, in a process mediated by nuclear receptor coactivator 4 (NCOA4), serving as another source of free Fe2+.[54–56] Current studies suggest that excess iron can promote lipid peroxidation and then induce ferroptosis by two main mechanisms, namely, the generation of ROS via the iron-dependent Fenton reaction and the activation of iron-containing enzymes (e.g., LOX).[2,57] In the Fenton reaction, extremely oxidative free Fe2+ easily reacts with H2O2 to produce hydroxyl radicals that can cause oxidative damage to deoxyribonucleic acid (DNA), proteins, and membrane lipids, promoting lipid peroxidation, damaging the cell membrane, and eventually leading to cell death. Fenton's reaction formula is Fe2+ + H2O2 → Fe3+ + (OH)- + OH·.[38] In addition, studies have shown that the enzymes LOX and nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome P450 reductase (POR), which are related to phospholipid peroxidation, require iron catalysis to participate in ferroptosis.[10,58]
Antioxidant defense
GPX4-dependent pathway
GPX4 is a selenium-containing cysteine and GSH-dependent enzyme belonging to the GSH peroxidase family (including GPX1-8). It is a necessary enzyme that scavenges lipid hydrogen peroxide by reducing it to the corresponding ethanol or reducing H2O2 to free H2O2 and water. As such, GPX4 plays an important role in preventing ferroptosis.[59–61] In recent years, studies have shown that the cystine-importing GSH-GPX4 machinery plays an important role in the prevention of ferroptosis, and it has been proven that phospholipid hydroperoxide (PLOOH) is a substrate of GPX4 and might be an executor of ferroptosis.[62] GSH is synthesized by the catalysis of the cytosolic enzymes glutamate cysteine ligase (GCL) and glutathione synthetase (GSS) and participates in the regulation of ferroptosis.[63] GSH can produce glutathione disulfide (GSSG), and GSSG regenerates GSH through glutathione reductase (GR). In this process, GSH can act as an electron donor, so GSH is necessary for the GPX4 catalytic reaction and an important factor for maintaining the activity of GPX4. Because GSH participates in maintaining the activity of GPX4, GPX4 can prevent ferroptosis. In addition, cystine is the most restricted amino acid in the process of GSH synthesis, so the cystine-importing GSH-GPX4 machinery is the most critical signaling pathway for preventing ferroptosis. It has been reported that the cystine/glutamate antiporter system Xc- can promote ferroptosis by inhibiting cysteine import.[1,64,65] Additionally, erastin can inhibit cysteine input and inactivate GPX4 indirectly, resulting in the accumulation of PLOOH, a substrate of GPX4, and the accumulation of PLOOH can quickly initiate the Fenton reaction; in addition, the amount of PLOOH can be rapidly increased, resulting in ferroptotic cell death. Selenium is an essential element for organisms and participates in many important physiological processes in the body.[34] In addition to the cystine-importing GSH-GPX4 signaling pathway, selenium can also affect the expression of GPX4 and induce ferroptosis. It has been suggested that selenium can regulate GPX4 expression by increasing ribosome transfer RNA (tRNA) density downstream of UGA-Sec codons and selenium incorporation efficiency in part by affecting the degree of Sec-tRNA[Ser]SecUm34 methylation. Sec-tRNA must first be activated by the addition of an isopentenyl lipid group, a product of the mevalonate (MVA) pathway. This phenomenon may explain how disruption of the MVA pathway by statins leads to reduced GPX4 expression and increased ferroptosis in certain cells.[66–69]
GPX4-independent pathway
To date, the most important signaling axis known to regulate ferroptosis is dependent on GPX4. Inhibition of GPX4 to induce ferroptosis is a new therapeutic strategy for the treatment of tumors. However, different cell lines have different sensitivities to GPX4 inhibitors. Therefore, we speculate that there are other mechanisms regulating ferroptosis in the body that do not depend on GPX4.[11] Through CRISPR/cas9 screening, Doll et al[33] found that FSP1 is an anti-ferroptosis gene.[34] Other studies on FSP1 have proven that FSP1 is a powerful ferroptosis inhibitor independent of GPX4, GSH, or ACSL4.[34] FSP1 was initially reported to be involved in p53-mediated apoptosis because its amino acid sequence is similar to that of human apoptosis-inducing factor (AIF), so it is also called AIF-like mitochondrion-associated inducer of death (AMID).[70–72] Many studies have shown that FSP1 can regulate the ferroptosis sensitivity of cells. FSP1 is an oxidoreductase that can reduce CoQ10 to ubiquinol, which is a lipophilic free radical-trapping antioxidant that can prevent lipid peroxidation. Therefore, FSP1 inhibits ferroptosis through this process.[34]
The study of Chen et al[73] shows that the inhibition of ferroptosis by FSP1 is independent of GPX4, and the myristoylation of FSP1 mediates the recruitment of FSP1 to the cell membrane, which plays an important role in the inhibition of ferroptosis by FSP1. In addition to FSP1 and GPX4 playing an important role in the inhibition of ferroptosis, a large number of studies have shown that natural antioxidants such as vitamin E, thioredoxin, and mitoquinone can also participate in the inhibition of ferroptosis.[74–76] Recent research discovered that inducible nitric oxide synthase (iNOS)/NO∙ abundance modulates susceptibility to ferroptosis in macrophages/microglia.[77] The free radical-capturing antioxidant tetrahydrobiopterin (BH4) inhibits ferroptosis by reducing lipid peroxidation.[78] Furthermore, NRF2 can play an important role in ferroptosis through many different pathways.[79–85]
Abnormal Iron Metabolism and Leukemic Cells
Cancer cells will change the normal mechanism of iron metabolism for proliferation, and abnormal iron metabolism is closely related to the occurrence and development of leukemia.[86] Patients with leukemia need a large number of red blood cell transfusions due to disturbance of erythropoiesis and anemia caused by chemotherapy, so iron overload often occurs. Abnormal iron metabolism will seriously affect normal hematopoiesis. The expression of TFR1 in leukemic cells is generally higher than that in normal controls, and its level may be directly related to the differentiation of AML.[87] However, iron depletion caused by TFR1 inhibition can damage the proliferation and differentiation of hematopoietic progenitor cells and reduce the regeneration potential of HSCs.[88] TFR2 is also upregulated in AML subtypes (AML1, AML2, and AML6). Some studies have shown that the expression of TFR2 α subtypes may be positively correlated with a good prognosis.[89–91] Excess iron and ROS catalytic products promote the malignant transformation of HSCs through nicotinamide adenine dinucleotide phosphate oxidase (NOX) and subsequent GSH depletion.[92] At the same time, ROS may also promote the transformation process of AML.[93] In monocyte AML, the increase of ROS can induce apoptosis of adjacent natural killer (NK) cells and CD4 and CD8 T cells through poly-ADP-ribose polymerase-1 (PARP1), and invalidate the subsequent anti-leukemia response.[94] Serum ferritin is also frequently increased in patients with leukemia and is associated with poor prognosis in patients undergoing chemotherapy[95–98] and patients receiving allogeneic stem cell transplantation.[99] In patients with AML, overexpression of ferritin heavy chain (FTH) leads to chemotherapy resistance through nuclear factor-kappa B (NF-κB) pathway and pro-oxidative pathway.[95] It is worth noting that the low expression of ferroportin in AML seems to be associated with improved prognosis and higher chemosensitivity.[100]
Recent Advances of Ferroptosis in AL
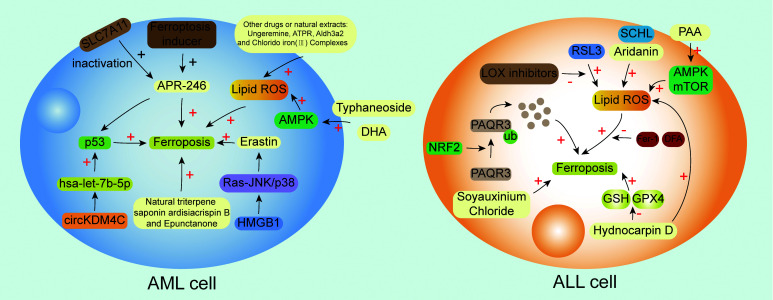
In recent years, studies related to ferroptosis have emerged, and many studies have shown that ferroptosis is closely related to the occurrence and development of tumors.[26,38,101–104] Here, we focus on AL and report that ferroptosis affects the occurrence and development of AL through various pathways, including various signaling pathways, natural extracts, drugs, compounds, ferroptosis-related molecules, and so on [Figure 3 and Table 1].
Figure 3.
Summary of the mechanisms related to ferroptosis in AML and ALL cells. The interaction of various molecules forms a network of molecular pathways related to ferroptosis. In AML cells, the upregulation of p53 expression by circKDM4C via hsa-let-7b-5p can reduce ferroptosis; also, APR-246 can target the mutant protein p53 in AML and promote the binding of mutant p53 to the target site of DNA to regain its transcriptional activity, the combination of APR-246 with ferroptosis inducers, or the inactivation of SLC7A11 has synergistic anti-leukemic activity in vitro; HMGB1 can regulate Erastin-induced ferroptosis through Ras-JNK/p38 signal pathway; additionally, some drugs or natural abstracts can also induce ferroptosis and help to fight AML. In ALL cells, the ubiquitination of PAQR3 by NRF2 can induce ferroptosis; RSL3 can induce ferroptosis by enhancing the production of ROS and this process can be blocked by LOX inhibitors or ferroptosis inhibitors; HD induces ferroptosis via reducing GSH and GPX4. ALL: Acute lymphoblastic leukemia; AML: Acute myeloid leukemia; AMPK: Adenosine monophosphate-activated protein kinase; APR-246: Eprenetapopt; ATLL: Adult T cell leukemia/lymphoma; ATPR: 4-Amino-2-trifluoromethyl-phenyl retinate; DFA: Deferoxamine; DHA: Dihydroartemisinin; DNA: Deoxyribonucleic acid; Fer-1: Ferrostain-1; GPX4: Glutathione peroxidase 4; GSH: Glutathione; HD: Hydnocarpin D; HMGB1: High mobility group protein 1; KDM4C: Lysine demethylase 4c; LOX: Lipoxygenases; NRF2: Nuclear factor erythroid 2-related factor 2; PAQR3: Progestin and adipoq receptor family member 3; Ras-JNK: Rat sarcoma-jun N-terminal kinase; ROS: Reactive oxygen species; RSL3: An inhibitor of glutathione peroxidase 4; SLC7A11: Solute carrier family 7 member 11; ub: Ubiquitination.
Table 1.
Ferroptosis-related compounds, natural extracts, and drugs for acute leukemia and their mechanisms.
| Compound/drug/natural extract | Disease | Mechanism |
|---|---|---|
| APR-246 | AML | Deplete intracellular GSH and induce lipid peroxide production |
| DHA | AML | Induce autophagy by regulating the activity of AMPK/mTOR/70 kDa ribosomal protein S6 kinase (p70S6K) signaling pathway, accelerate the degradation of ferritin, increase the labile iron pool, promote the accumulation of cellular ROS |
| ATPR | AML | ROS-autophagy-lysosomal pathway |
| Aldh3a2 | AML | Aldh3a2 knockout changes the redox state of cells, affects lipid metabolism, and combines with GPX4 inhibition to activate ferroptosis |
| Sulfasalazine | AML | Inhibit cystine importer SLC7A11/xCT and lead to depletion of GSH bank and oxidative stress-dependent cell death |
| Typhaneoside | AML | Induce autophagy by promoting the activation of the AMPK signal pathway and then increase the labile iron pool and promote the accumulation of cellular ROS |
| Chlorido[N,N΄-disalicylidene-1,2-phenylenediamine] iron(III) complexes | AML | Increase the production of ROS |
| Ungeremine | AML | Activate caspase, change metalloproteinases, increase ROS production |
| Epunctanone | AML | The addition of deferoxamine or ferrostatin-1 decreased its cytotoxicity |
| Natural triterpene saponin ardisiacrispin B | AML | The addition of deferoxamine or ferrostatin-1 decreased its cytotoxicity |
| HD | ALL | Increase the accumulation of ROS and decrease GSH and GPX4 |
| SCHL | ALL | The addition of deferoxamine or ferrostatin-1 decreased its cytotoxicity |
| Aridanin | ALL | The addition of deferoxamine or ferrostatin-1 decreased its cytotoxicity |
| PAA | T-ALL | Increase ROS production, induce autophagy through AMPK/mTOR and LC3 signaling pathways, and cause GSH downregulation |
Aldh3a2: aldehyde dehydrogenase 3a2; ALL: Acute lymphocytic leukemia; AML: Acute myeloid leukemia; AMPK: Adenosine monophosphate-activated protein kinase; ATPR: 4-Amino-2-trifluoromethyl-phenyl retinate; DHA: Dihydroartemisinin; GPX4: Glutathione peroxidase 4; GSH: Glutathione; HD: Hydnocarpin D; LC3: Microtubule-associated protein 1 light chain 3; mTOR: Mechanistic target of rapamycin; PAA: Poricoic acid A; ROS: Reactive oxygen species; SCHL: Soyauxinium chloride; SLC7A11: Solute carrier family 7 member 11; T-ALL: T cell ALL; xCT: System Xc- consists of light chain subunit SLC7A11.
AML
Long non-coding ribonucleic acids (lncRNAs) are transcripts of >200 nucleotides and usually do not encode proteins. They play important physiological roles in the body and can regulate epigenetics, the cell cycle, and cell differentiation. Previous studies have shown that lncRNAs can participate in the process of iron-related death in tumors.[105–108] Zheng et al[109] identified seven lncRNAs (AP001266.2, AC133961.1, AF064858.3, AC007383.2, AC008906.1, AC026771.1, and KIF26B-AS1) associated with ferroptosis. Those lncRNAs are related to GSH metabolism and tumor immunity and have been proven to be able to predict the prognosis of AML, providing insights for the development of new AML treatment strategies. Wang et al[110] found a nuclear lncRNA (LINC00618) with low expression in leukemia but high expression after vincristine (VCR) treatment. LINC00618 can accelerate ferroptosis by increasing the levels of lipid ROS and iron, reducing the expression of solute carrier family 7 member 11 (SLC7A11) and inhibiting the expression of lymphoid-specific helicase (LSH) (LSH inhibits iron-related death by enhancing transcription after recruitment to the promoter region of SLC7A11). In addition, LINC00618 can promote ferroptosis and apoptosis induced by VCR, and LINC00618 can accelerate the occurrence of iron-related death through apoptosis.
Bioinformatics databases play an increasingly important role in cancer research. Chen et al[11] used ferroptosis-related genes to distinguish two subtypes in the the cancer genome atlas (TCGA) cohort. From the differentially expressed gene (DEG) analysis of these two subtypes, they established an AML prediction model containing 13 genes (ATG3, FAM106A, KLHL9, LCMT2, LRRC40, LZTR1, NCR2, PAFAH2, PCMTD2, PLA2G5, SCARB1, TK1, ZNF576), which was verified in an independent AML cohort. At the same time, the model can also provide a reference for identifying the sensitivity of chemotherapy drugs and ferroptosis inducers in high-risk groups and low-risk groups. It has the potential value for clinical transformation. In conclusion, these works have revealed the specific relationship between ferroptosis and AML, which may help clinical treatment in the future.
p53 is a well-known tumor suppressor and plays an important role in regulating ferroptosis.[1,112] Some studies have found that the circular RNAs of lysine-specific demethylase 4C (circKDM4C) is downregulated in AML and inhibits the proliferation, migration, and invasion of AML cells. In addition, the upregulation of p53 expression by circKDM4C via hsa-let-7b-5p can reduce ferroptosis.[113] As a promising drug, eprenetapopt (APR-246) can target the mutant protein p53 in AML and promote its binding to target DNA to restore p53 transcriptional activity.[114–118] Birsen et al[119] have shown that AML cell death induced by APR-246 can be inhibited by iron-chelating agents, lipophilic antioxidants, and lipid peroxidation inhibitors, indicating that APR-246 can induce ferroptosis of AML cells. The combination of APR-246 and ferroptosis inducers has synergistic anti-leukemic activity in vitro, and inactivation of the SLC7A11 gene and APR-246 treatment have synergistic anti-leukemic activity in vivo.[119]
The GPX family of enzymes has peroxidase activity. The abnormal expression of GPXs is related to the occurrence and development of tumors.[120,121] Functional enrichment analysis showed that cells with differential expression of GPXs were mainly enriched in oxidative stress, immune regulation, and inflammation, in addition to GSH metabolism and ferroptosis.[122] GPX1 is an important member of the GPX family and is closely related to the occurrence and development of tumors.[123,124] Studies have shown that GPX1 is highly expressed in AML, and high GPX1 expression is associated with poor prognosis. In AML, GPX1 is involved in signal transduction processes such as GSH metabolism and ferroptosis.[125]
By querying the metabolic pathways related to leukemia stem cells and survival rate in multiple AML data sets, Itzykson et al[126] determined that SLC7A11 is related to the poor prognosis of AML, and SLC7A11 can promote the survival of AML cells by encoding xCT cystine importer. The drug sulfasalazine with xCT inhibitory activity has anti-leukemia activity, which can lead to depletion of the GSH bank and oxidative stress-dependent cell death (partially related to ferroptosis). More importantly, in vivo and in vitro experiments confirmed that the combination of anthracycline daunorubicin and sulfasalazine has the best anti-AML effect, indicating that targeting cystine import can be used for AML treatment, and sulfasalazine combined with chemotherapy is a promising treatment strategy for AML patients in the future.[126]
The human immunodeficiency virus type I enhancer binding protein (HIVEP) family has been proven to be involved in many biological processes, such as cell survival, tumor necrosis factor (TNF) signal transduction, and tumor formation. However, its expression pattern, prognostic relevance, and functional significance in AML remain unclear. Ma's team found in the database that the increased transcription level of HIVEP3 in AML patients is an independent factor affecting the poor prognosis of AML patients, and is related to AML subtype, age, cytogenetic risk, and disease-related molecules.[127] The HIVEP3 co-expression gene cluster is rich in functional pathways related to AML leukemia. Further cell experiments showed that HIVEP3 messenger RNA (mRNA) and protein levels were abnormally expressed in leukemia cells and primitive cells in bone marrow tissue, and HIVEP3 participated in the iron cell apoptosis signal pathway. The research shows that HIVEP3 can be used as an independent prognostic indicator for AML patients. Targeting HIVEP3 to affect the iron death signal pathway of AML may provide a new scheme for AML treatment.[127]
Erastin is a quinazolinone derivative that was found when attempting to identify small synthetic lethal molecules to be used for cells with RAS oncogene expression. In addition, erastin can induce ferroptosis in tumor cells.[1,30] Yu et al[128] found that erastin-induced ferroptosis in HL-60 cells in a dose-dependent manner and enhanced the sensitivity of AML cells to chemotherapeutic drugs such as cytarabine and doxorubicin in a RAS-independent manner, providing a possible solution for AML drug resistance. Previous reports have shown that the transcription factor high mobility group protein 1 (HMGB1) plays an important role in the pathogenesis and chemotherapy resistance of leukemia.[129–133] Ye et al[134] showed that HMGB1 can regulate erastin-induced ferroptosis in HL-60/NRASQ61L cells through the Ras- jun N-terminal kinase (JNK)/p38 signaling pathway, informing the mechanism by which HMGB1 modulates ferroptosis in leukemia.
The resistance of tumor cells to existing anticancer drugs hinders the success of tumor chemotherapy. Many natural products show cytotoxicity against tumor cells. Ungeremine induces ferroptosis by activating caspases, changing the mitochondrial membrane potential (MMP), and increasing ROS production.[135] Typhanthin (typhneoside, TYP) is the main flavonoid in the extract of pollen typhae. In AML, TYP can activate the adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway, induce autophagy, accelerate the accumulation of ferritin, promote the accumulation of ROS, and eventually lead to ferroptosis.[136] Mbaveng et al[137] showed that epunctanone induces apoptosis and ferroptosis in CCRF-CEM cells by changing the MMP and increasing ROS production.
The naturally occurring oleanane-type triterpene saponin ardisiacrispin B, isolated from the fruit of Ardisia kivuensis Taton (Myrsinaceae), induces apoptosis and ferroptosis of CCRF-CEM cells by activating promoter caspases 8 and 9 and effector caspases 3 and 7, modulating the MMP, and increasing the production of ROS.[138]
In addition to some natural products that can induce ferroptosis in AML, some compounds, molecules, and artificially designed drugs can also be useful. One study has shown that the expression level of adrenomedullin (ADM) is related to the expression of genes involved in leukemogenesis, cell proliferation regulation, and ferroptosis, providing insights for further exploration of new therapeutic strategies for AML.[139] Dihydroartemisinin (DHA) has been reported to inhibit the growth of lymphocytic leukemia.[140] However, the mechanism of its action in AML is not clear. Du et al[141] found that DHA can activate autophagy, promote ferritin-related lysis, and increase intracellular ROS accumulation by affecting the activity of the AMPK/mTOR (mTOR: Mechanistic target of rapamycin)/70 kDa ribosomal protein S6 kinase (p70S6K) signaling pathway, ultimately leading to ferroptotic cell death. 4-Amino-2-trifluoromethyl-phenyl retinate (ATPR) is an all-trans retinoic acid (ATRA) derivative designed by Li et al[142] and has been shown to be more effective than ATRA in many tumors. ATPR has also been found to cause autophagy-induced ferroptosis in vivo and in vitro in studies targeting AL.[142–148] Leukemic cells prevent oxidative damage by oxidizing long-chain aliphatic aldehydes via aldehyde dehydrogenase 3a2 (Aldh3a2). The study of Yusuf et al[149] shows that knocking down Aldh3a2 can affect the lipid metabolism and redox state of AML cells and induce ferroptosis. Importantly, ferroptosis caused by Aldh3a2 depletion have a synergistic effect with the inhibition of GPX4, which may provide a theoretical basis for the combined application of Aldh3a2 inhibitors and cytotoxic therapy.[149] In recent years, iron-based nanodrugs have become a new type of ferroptosis inducer in leukemia.[150–152] Luo et al[153] studied the effects of two kinds of iron nanoparticles, 2,3-dimercaptosuccinic acid (DMSA)-coated Fe3O4 nanoparticles (FeNPs) as a ROS inducer and Prussian blue nanoparticles (PBNPs) as a ROS scavenger, on the transcripts of two kinds of leukemic cells (KG1a AML cells and HL60 acute promyelocytic leukemia cells). According to the results, there were 14 common upregulated genes and 4 common downregulated genes; of these genes, FTL, DNM1, and transferrin receptor (TRFC) play an important role in iron metabolism, indicating that the two drugs may cause cytotoxicity to leukemic cells through ferroptosis. It has been reported that the chloro [N-methylethyl] iron(III) complex produces lipid-based ROS and induces ferroptotic cell death in leukemic and neuroblastoma cell lines, suggesting that it can be used as a novel inducer of ferroptosis in leukemic and neuroblastoma cell lines.[154]
ALL
ALL is a common hematological tumor characterized by the malignant proliferation of lymphoid progenitor cells. Many important factors are involved in the regulation of ALL.[155,156] It has been reported that the progestin and adipoq receptor family member 3 (PAQR3), a tumor suppressor, inhibits the proliferation and induces apoptosis of human leukemia cells, but its effect on cell proliferation and iron-related death and related regulatory mechanisms needs to be further studied.[157] Jin et al[158] showed that low expression of PAQR3 in ALL inhibits the proliferation of ALL cells and aggravates ferroptosis. Further experiments showed that PAQR3 binds to NRF2 and regulates its expression in ALL through ubiquitination, thus affecting cell proliferation and ferroptosis. ALL is closely related to ferroptosis. Marcotte's team performed whole-genome CRISPR/Cas deletion screening on a panel of seven B-ALL cell lines.[159] The results showed that compared with other cancers, the survival of ALL cell lines depended on several unique metabolic pathways, including sensitivity to GPX4 depletion and induction of iron cell apoptosis. Detailed molecular analysis showed that B-ALL cells showed high steady-state oxidative stress potential, low buffer capacity, and GPX4-independent secondary lipid peroxidation detoxification pathway, suggesting that they were sensitive to ferroptosis. Finally, Marcotte used samples from B-ALL patients to verify that B-ALL is indeed sensitive to iron-related apoptosis.[159]
Ferroptosis is characterized by the generation of lipid-based ROS and lipid peroxidation.[160] Several ROS-generating enzymes contain iron or iron derivatives as essential cofactors for their proper function, such as LOX, nicotinamide adenine dinucleotide phosphate hydride (NADPH) oxidase (NOX), xanthine oxidase, and cytochrome P450 enzymes.[6] LOXs are key enzymes that catalyze the oxygenation of polyunsaturated fatty acyl groups to lipid hydroperoxides,[161] while GPX family members are responsible for the reduction of hydrogen and lipid peroxides.[162] It is also reported that the regulatory effect of LOXs on RSL3-induced ferroptosis in ALL cells. Their research indicates that RSL3, an inhibitor of GPX4, triggers lipid peroxidation, ROS generation, and cell death in ALL cells. All of these events were prevented by the presence of iron statin-1 (Fer-1), a small molecular inhibitor of lipid peroxidation. Importantly, LOX inhibitors, including the selective LOX12/15 inhibitor baicalein and the pan-LOX inhibitor nordihydroguaiaretic acid (NDGA), protect cells from RSL3-stimulated lipid peroxidation, ROS production, and cell death, suggesting that LOXs contribute to ferroptosis.[163]
Previously, many natural extracts were shown to be involved in the induction of ferroptosis in AML. In ALL, some compounds and drug components are also associated with ferroptosis. Hydnocarpin D (HD) is a bioactive flavonoid lignin with good antitumor activity.[164,165] HD inhibits the proliferation of the T cell ALL (T-ALL) cell lines Jurkat and Molt-4 in vitro by inducing cell cycle arrest, which results in apoptosis. In addition, HD increases the level of microtubule-associated protein light chain 3 (LC3)-II, a marker of autophagy, and the formation of autophagic lysosomal vacuoles. The inhibitory effect of ATG5/7 gene knockout or 3-MA pretreatment on autophagy partially enhanced the apoptosis induced by HD, suggesting that autophagy enhances the influence of HD. Moreover, this cytotoxic autophagy caused ferroptosis, which was characterized by the accumulation of lipid ROS and a decrease in GSH and GPX4, and conversely, inhibition of autophagy hindered cell ferroptosis.[166] Soyauxinium chloride (SCHL) is a naturally occurring indole-quinazoline-type alkaloid. Some studies found that SCHL induced cytotoxicity of CCRF-CEM leukemia cells through caspase 3, 7, 8, and 9 activation, MMP alteration, and increased ROS generation, while the presence of Fer-1 (ferrostain-1) or DFA (deferoxamine; an iron-related death inhibitor) decreased SCHL cytotoxicity by 7.11- and 4.64-fold, respectively, indicating that SCHL is involved in ferroptosis.[167] Similarly, a naturally occurring N-acetylated oleanolic acid glycoside (aridanin) was cytotoxic to CCRF-CEM leukemia cells, and when nec-1 (necrosis inhibitor), Fer-1 (iron-related death inhibitor), and DFA (iron-related death inhibitor) were added, allicin was 2.74-, 1.61-, and 2.70-fold less cytotoxic, respectively, indicating its ability to induce necrosis and ferroptosis in CCRF-CEM cells.[168] Poricoic acid A (PAA) is the main chemical component of the surface layer of mushroom Poria cocos, which has a protective effect on various diseases. Sun et al[169] found that PAA can trigger apoptosis of T-ALL cells by increasing ROS production, induce autophagy through AMPK/mTOR and LC3 signaling pathways, and cause GSH downregulation and malonaldehyde (MDA) upregulation, leading to ferroptosis. The in vitro experiments also confirmed the inhibitory effect of PAA on T-ALL, indicating that PAA may be a new therapeutic strategy for patients with T-ALL.
Adult T cell leukemia/lymphoma (ATLL)
ATLL is an invasive disease caused by human T cell leukemia virus type 1 (HTLV-1) infection. The therapeutic effect in patients is limited by chemotherapy resistance. Therefore, there is an urgent need to develop new and effective strategies.[170,171] Artesunate (ART) is a widely used antimalarial compound that has been proven to have cytotoxicity.[172] However, the mechanism of its action in ATLL is not clear. Ishikawa et al[173] showed that ART has a cytotoxic effect on ATLL cells, which can be partially reversed by treatment with ROS scavengers, iron-chelating agents, and inhibitors of necrosis or ferroptosis, indicating that ART can induce ferroptosis in ATLL cells and kill tumor cells.
Others
There are currently fewer studies on ferroptosis in chronic leukemia than in AL. Although it has been proven that typhanthin and Chlorido[N,N'-disalicylidene-1,2-phenylenediamine]iron(III) complexes can induce ferroptotic cell death in the K562 cell line (CML cells), sufficient experimental evidence is lacking. In the future, a large number of studies are needed to explain the role of ferroptosis in chronic lymphocytic leukemia (CLL) and chronic myelocytic leukemia (CML).[136,154]
Conclusions and Perspectives
In this review, we provided the definition, basic characteristics, and history of ferroptosis, briefly described the mechanism of ferroptosis, illustrated the relationship between abnormal iron metabolism and leukemia illustrated the relationship between abnormal iron metabolism and leukemia, and focused on recent progress in the understanding of the role of ferroptosis in leukemia. At present, leukemia is one of the most common malignant tumors and major disorders affecting human health. In recent years, with advances in radiotherapy, chemotherapy, HSC transplantation, and the emergence of new treatments such as targeted therapy, biotherapy, and cell therapy, the prognosis of patients with leukemia has been greatly improved. However, due to various factors, the overall survival time of patients with leukemia is still very short, and new treatment strategies to improve the survival time of patients still need to be developed. Ferroptosis is a form of oxidative cell death characterized by accumulated iron and excessive lipid peroxidation. Previous studies of ferroptosis have shown outstanding antitumor effects. The tumor-inhibitory effect of ferroptosis in fibrosarcoma, prostate cancer, and osteosarcoma has been confirmed. Targeting ferroptosis may become another innovative therapeutic strategy for the treatment of tumors. There has been much research to reveal the mechanism and utility of ferroptosis in hematological malignant tumors. Many natural drug extracts show great cytotoxicity against leukemic cells by inducing ferroptosis. Some lncRNAs and circular RNAs (circRNAs) also show the ability to induce ferroptosis in hematological malignant tumors, and some molecules participate in ferroptosis by regulating key molecules such as TP53, NRF2, GPX4, and GSH, which regulate ferroptotic cell death. Moreover, in addition inducing leukemic cell death, inducing ferroptosis can also assist in treatment by increasing sensitivity to drugs and chemotherapy. However, we have only just begun to understand the role of ferroptosis in leukemia. The results of studies on the role of ferroptosis in leukemia are still deficient. In the future, more in-depth research on the mechanism of ferroptosis in leukemia will contribute to the development of effective anticancer drugs. There is still great potential for the study of ferroptosis in leukemia.
Conflicts of interest
None.
Footnotes
Tianxin Lyu and Xudong Li contributed equally to this work.
How to cite this article: Lyu TX, Li XD, Song YP. Ferroptosis in acute leukemia. Chin Med J 2023;136:886–898. doi: 10.1097/CM9.0000000000002642
References
- 1.Dixon SJ Lemberg KM Lamprecht MR Skouta R Zaitsev EM Gleason CE, et al. . Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012;149: 1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stockwell BR Friedmann Angeli JP Bayir H Bush AI Conrad M Dixon SJ, et al. . Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017;171: 273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: The role of ferroptosis in cancer. Nat Rev Clin Oncol 2021;18: 280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 4.Gong L, Huang D, Shi Y, Liang Z, Bu H. Regulated cell death in cancer: From pathogenesis to treatment. Chin Med J 2022. doi: 10.1097/cm9.0000000000002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res 2019;29: 347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol 2014;10: 9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 7.Wang F Lv H Zhao B Zhou L Wang S Luo J, et al. . Iron and leukemia: New insights for future treatments. J Exp Clin Cancer Res 2019;38: 406. doi: 10.1186/s13046-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WS SriRamaratnam R Welsch ME Shimada K Skouta R Viswanathan VS, et al. . Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156: 317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon SJ Patel DN Welsch M Skouta R Lee ED Hayano M, et al. . Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 2014;3: e02523. doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Stockwell BR, Conrad M. Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 2021;22: 266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou Y Palte MJ Deik AA Li H Eaton JK Wang W, et al. . A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun 2019;10: 1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doll S Proneth B Tyurina YY Panzilius E Kobayashi S Ingold I, et al. . ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 2017;13: 91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon SJ Winter GE Musavi LS Lee ED Snijder B Rebsamen M, et al. . Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 2015;10: 1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 2015;1851: 308–330. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y Zhang Y Jiang Q Meng L Li W Liu B, et al. . Safety of SARS-CoV-2 vaccines in patients with chronic myeloid leukemia: A multicenter survey in China. Chin Med J 2022;135: 1498–1499. doi: 10.1097/cm9.0000000000001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y Bai L Cheng Y Lu A Wang Y Wu J, et al. . Haploidentical hematopoietic stem cell transplantation may improve long-term survival for children with high-risk T-cell acute lymphoblastic leukemia in first complete remission. Chin Med J 2022;135: 940–949. doi: 10.1097/cm9.0000000000001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Y Tang H Miao Y Zhu H Wang L Fan L, et al. . Overexpression of c-Myc-dependent heterogeneous nuclear ribonucleoprotein A1 promotes proliferation and inhibits apoptosis in NOTCH1-mutated chronic lymphocytic leukemia cells. Chin Med J 2022;135: 920–929. doi: 10.1097/cm9.0000000000002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao LQ Zhou Y Liu YR Xu LP Zhang XH Wang Y, et al. . A risk score system for stratifying the risk of relapse in B cell acute lymphocytic leukemia patients after allogenic stem cell transplantation. Chin Med J 2021;134: 1199–1208. doi: 10.1097/cm9.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benadiba J Rosilio C Nebout M Heimeroth V Neffati Z Popa A, et al. . Iron chelation: An adjuvant therapy to target metabolism, growth and survival of murine PTEN-deficient T lymphoma and human T lymphoblastic leukemia/lymphoma. Leuk Lymphoma 2017;58: 1433–1445. doi: 10.1080/10428194.2016.1239257. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy AE Kamdar KY Lupo PJ Okcu MF Scheurer ME Baum MK, et al. . Examination of HFE associations with childhood leukemia risk and extension to other iron regulatory genes. Leuk Res 2014;38: 1055–1060. doi: 10.1016/j.leukres.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Hagag AA, Badraia IM, Abdelmageed MM, Hablas NM, Hazzaa SME, Nosair NA. Prognostic value of transferrin receptor-1 (CD71) expression in acute lymphoblastic leukemia. Endocr Metab Immune Disord Drug Targets 2018;18: 610–617. doi: 10.2174/1871530318666180605094706. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Zhang NJ, Zhang LJ. Oxidative stress in leukemia and antioxidant treatment. Chin Med J 2021;134: 1897–1907. doi: 10.1097/cm9.0000000000001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gale RP. Chronic lymphocytic leukemia in China. Chin Med J 2022;135: 883–886. doi: 10.1097/cm9.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan M Wang Y Lin R Lin T Huang F Fan Z, et al. . Haploidentical transplantation has a superior graft-versus-leukemia effect than HLA-matched sibling transplantation for Ph–high-risk B-cell acute lymphoblastic leukemia. Chin Med J 2022;135: 930–939. doi: 10.1097/cm9.0000000000001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q Xu H Xue L Wang M Pan G Zhang X, et al. . CD19-targeted chimeric antigen receptor-modified T cells induce remission in patients with relapsed acute B lymphoblastic leukemia after umbilical cord blood transplantation. Chin Med J 2021;135: 98–100. doi: 10.1097/cm9.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan HF Zou T Tuo QZ Xu S Li H Belaidi AA, et al. . Ferroptosis: Mechanisms and links with diseases. Signal Transduct Target Ther 2021;6: 49. doi: 10.1038/s41392-020-00428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannai S, Kitamura E. Transport interaction of L-cystine and L-glutamate in human diploid fibroblasts in culture. J Biol Chem 1980;255: 2372–2376. doi: 10.1016/S0021-9258(19)85901-X. [PubMed] [Google Scholar]
- 28.Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989;2: 1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 1997;19: 453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 30.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 2003;3: 285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 31.Zilka O Shah R Li B Friedmann Angeli JP Griesser M Conrad M, et al. . On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci 2017;3: 232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingold I Berndt C Schmitt S Doll S Poschmann G Buday K, et al. . Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018;172: 409–422.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Doll S Freitas FP Shah R Aldrovandi M da Silva MC Ingold I, et al. . FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019;575: 693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 34.Bersuker K Hendricks JM Li Z Magtanong L Ford B Tang PH, et al. . The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019;575: 688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou Y Henry WS Ricq EL Graham ET Phadnis VV Maretich P, et al. . Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020;585: 603–608. doi: 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y Hou W Song X Yu Y Huang J Sun X, et al. . Ferroptosis: Process and function. Cell Death Differ 2016;23: 369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vande Walle L, Lamkanfi M. Pyroptosis. Curr Biol 2016;26: R568–R572. doi.org/10.1016/j.cub.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: Molecular mechanisms and health implications. Cell Res 2021;31: 107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuang F, Liu J, Tang D, Kang R. Oxidative damage and antioxidant defense in ferroptosis. Front Cell Dev Biol 2020;8: 586578. doi: 10.3389/fcell.2020.586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014: 360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill I, Valivety R. Polyunsaturated fatty acids, part 2: Biotransformations and biotechnological applications. Trends Biotechnol 1997;15: 470–478. doi: 10.1016/S0167-7799(97)01077-9. [DOI] [PubMed] [Google Scholar]
- 42.Gill I, Valivety R. Polyunsaturated fatty acids, part 1: Occurrence, biological activities and applications. Trends Biotechnol 1997;15: 401–409. doi: 10.1016/S0167-7799(97)01076-7. [DOI] [PubMed] [Google Scholar]
- 43.Porter NA, Wolf RA, Yarbro EM, Weenen H. The autoxidation of arachidonic acid: Formation of the proposed SRS-A intermediate. Biochem Biophys Res Commun 1979;89: 1058–1064. doi: 10.1016/0006-291x(79)92115-6. [DOI] [PubMed] [Google Scholar]
- 44.Yin H, Xu L, Porter NA. Free radical lipid peroxidation: Mechanisms and analysis. Chem Rev 2011;111: 5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 45.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev 2003;103: 2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 46.Frank CE. Hydrocarbon autoxidation. Chem Rev 1950;46: 155–169. doi: 10.1021/cr60143a003. [DOI] [PubMed] [Google Scholar]
- 47.Kagan VE Mao G Qu F Angeli JP Doll S Croix CS, et al. . Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017;13: 81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown CW, Amante JJ, Goel HL, Mercurio AM. The α6β4 integrin promotes resistance to ferroptosis. J Cell Biol 2017;216: 4287–4297. doi: 10.1083/jcb.201701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y Feng D Wang Z Zhao Y Sun R Tian D, et al. . Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 2019;26: 2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 2016;478: 1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 51.Küch EM Vellaramkalayil R Zhang I Lehnen D Brügger B Sreemmel W, et al. . Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim Biophys Acta 2014;1841: 227–239. doi: 10.1016/j.bbalip.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Chu B Kon N Chen D Li T Liu T Jiang L, et al. . ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 2019;21: 579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seiler A Schneider M Förster H Roth S Wirth EK Culmsee C, et al. . Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 2008;8: 237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012;51: 5705–5724. doi: 10.1021/bi300752r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014;509: 105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grignano E, Birsen R, Chapuis N, Bouscary D. From iron chelation to overload as a therapeutic strategy to induce ferroptosis in leukemic cells. Front Oncol 2020;10: 586530. doi: 10.3389/fonc.2020.586530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 2016;113: E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conrad M, Pratt DA. The chemical basis of ferroptosis. Nat Chem Biol 2019;15: 1137–1147. doi: 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 59.Chen JJ, Galluzzi L. Fighting resilient cancers with iron. Trends Cell Biol 2018;28: 77–78. doi: 10.1016/j.tcb.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Tang D, Kroemer G. Ferroptosis. Curr Biol 2020;30: R1292–R1297. doi: 10.1016/j.cub.2020.09.068. [DOI] [PubMed] [Google Scholar]
- 61.Wang H Cheng Y Mao C Liu S Xiao D Huang J, et al. . Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther 2021;29: 2185–2208. doi: 10.1016/j.ymthe.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater 2019;31: e1904197. doi: 10.1002/adma.201904197. [DOI] [PubMed] [Google Scholar]
- 63.Forcina GC, Dixon SJ. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019;19: e1800311. doi: 10.1002/pmic.201800311. [DOI] [PubMed] [Google Scholar]
- 64.Comporti M, Saccocci C, Dianzani MU. Effect of CCl-4 in vitro and in vivo on lipid peroxidation of rat liver homogenates and subcellular fractions. Enzymologia 1965;29: 185–204. [PubMed] [Google Scholar]
- 65.Yagoda N von Rechenberg M Zaganjor E Bauer AJ Yang WS Fridman DJ, et al. . RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 2007;447: 864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howard MT, Carlson BA, Anderson CB, Hatfield DL. Translational redefinition of UGA codons is regulated by selenium availability. J Biol Chem 2013;288: 19401–19413. doi: 10.1074/jbc.M113.481051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warner GJ, Berry MJ, Moustafa ME, Carlson BA, Hatfield DL, Faust JR. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J Biol Chem 2000;275: 28110–28119. doi: 10.1074/jbc.M001280200. [DOI] [PubMed] [Google Scholar]
- 68.Kryukov GV Castellano S Novoselov SV Lobanov AV Zehtab O Guigó R, et al. . Characterization of mammalian selenoproteomes. Science 2003;300: 1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 69.Viswanathan VS Ryan MJ Dhruv HD Gill S Eichhoff OM Seashore-Ludlow B, et al. . Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017;547: 453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horikoshi N, Cong J, Kley N, Shenk T. Isolation of differentially expressed cDNAs from p53-dependent apoptotic cells: Activation of the human homologue of the Drosophila peroxidasin gene. Biochem Biophys Res Commun 1999;261: 864–869. doi: 10.1006/bbrc.1999.1123. [DOI] [PubMed] [Google Scholar]
- 71.Wu M, Xu LG, Li X, Zhai Z, Shu HB. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem 2002;277: 25617–25623. doi: 10.1074/jbc.M202285200. [DOI] [PubMed] [Google Scholar]
- 72.Gong M, Hay S, Marshall KR, Munro AW, Scrutton NS. DNA binding suppresses human AIF-M2 activity and provides a connection between redox chemistry, reactive oxygen species, and apoptosis. J Biol Chem 2007;282: 30331–30340. doi: 10.1074/jbc.M703713200. [DOI] [PubMed] [Google Scholar]
- 73.Chen L, Xie J. Ferroptosis-suppressor-protein 1: A potential neuroprotective target for combating ferroptosis. Mov Disord 2020;35: 400. doi: 10.1002/mds.27990. [DOI] [PubMed] [Google Scholar]
- 74.Carlson BA Tobe R Yefremova E Tsuji PA Hoffmann VJ Schweizer U, et al. . Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol 2016;9: 22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Llabani E Hicklin RW Lee HY Motika SE Crawford LA Weerapana E, et al. . Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat Chem 2019;11: 521–532. doi: 10.1038/s41557-019-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jelinek A Heyder L Daude M Plessner M Krippner S Grosse R, et al. . Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med 2018;117: 45–57. doi: 10.1016/j.freeradbiomed.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 77.Kapralov AA Yang Q Dar HH Tyurina YY Anthonymuthu TS Kim R, et al. . Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol 2020;16: 278–290. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soula M Weber RA Zilka O Alwaseem H La K Yen F, et al. . Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 2020;16: 1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 2003;278: 12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 80.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal 2018;29: 1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasaki H Sato H Kuriyama-Matsumura K Sato K Maebara K Wang H, et al. . Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002;277: 44765–44771. doi: 10.1074/jbc.M208704200. [DOI] [PubMed] [Google Scholar]
- 82.Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 2011;123: 590–600. doi: 10.1093/toxsci/kfr183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee C. Collaborative power of Nrf2 and PPARgamma activators against metabolic and drug-induced oxidative injury. Oxid Med Cell Longev 2017;2017: 1378175. doi: 10.1155/2017/1378175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol 2017;11: 254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rothe T Gruber F Uderhardt S Ipseiz N Rössner S Oskolkova O, et al. . 12/15-Lipoxygenase-mediated enzymatic lipid oxidation regulates DC maturation and function. J Clin Invest 2015;125: 1944–1954. doi: 10.1172/JCI78490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torti SV, Torti FM. Iron and cancer: More ore to be mined. Nat Rev Cancer 2013;13: 342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Q Wang M Hu Y Xing H Chen X Zhang Y, et al. . Significance of CD71 expression by flow cytometry in diagnosis of acute leukemia. Leuk Lymphoma 2014;55: 892–898. doi: 10.3109/10428194.2013.819100. [DOI] [PubMed] [Google Scholar]
- 88.Wang S He X Wu Q Jiang L Chen L Yu Y, et al. . Transferrin receptor 1-mediated iron uptake plays an essential role in hematopoiesis. Haematologica 2020;105: 2071–2082. doi: 10.3324/haematol.2019.224899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawabata H, Nakamaki T, Ikonomi P, Smith RD, Germain RS, Koeffler HP. Expression of transferrin receptor 2 in normal and neoplastic hematopoietic cells. Blood 2001;98: 2714–2719. doi: 10.1182/blood.v98.9.2714. [DOI] [PubMed] [Google Scholar]
- 90.Kollia P Samara M Stamatopoulos K Belessi C Stavroyianni N Tsompanakou A, et al. . Molecular evidence for transferrin receptor 2 expression in all FAB subtypes of acute myeloid leukemia. Leuk Res 2003;27: 1101–1103. doi: 10.1016/s0145-2126(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 91.Nakamaki T Kawabata H Saito B Matsunawa M Suzuki J Adachi D, et al. . Elevated levels of transferrin receptor 2 mRNA, not transferrin receptor 1 mRNA, are associated with increased survival in acute myeloid leukaemia. Br J Haematol 2004;125: 42–49. doi: 10.1111/j.1365-2141.2004.04866.x. [DOI] [PubMed] [Google Scholar]
- 92.Hole PS Zabkiewicz J Munje C Newton Z Pearn L White P, et al. . Overproduction of NOX-derived ROS in AML promotes proliferation and is associated with defective oxidative stress signaling. Blood 2013;122: 3322–3330. doi: 10.1182/blood-2013-04-491944. [DOI] [PubMed] [Google Scholar]
- 93.Rassool FV Gaymes TJ Omidvar N Brady N Beurlet S Pla M, et al. . Reactive oxygen species, DNA damage, and error-prone repair: A model for genomic instability with progression in myeloid leukemia? Cancer Res 2007;67: 8762–8771. doi: 10.1158/0008-5472.Can-06-4807. [DOI] [PubMed] [Google Scholar]
- 94.Aurelius J Thorén FB Akhiani AA Brune M Palmqvist L Hansson M, et al. . Monocytic AML cells inactivate antileukemic lymphocytes: Role of NADPH oxidase/gp91(phox) expression and the PARP-1/PAR pathway of apoptosis. Blood 2012;119: 5832–5837. doi: 10.1182/blood-2011-11-391722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bertoli S Paubelle E Bérard E Saland E Thomas X Tavitian S, et al. . Ferritin heavy/light chain (FTH1/FTL) expression, serum ferritin levels, and their functional as well as prognostic roles in acute myeloid leukemia. Eur J Haematol 2019;102: 131–142. doi: 10.1111/ejh.13183. [DOI] [PubMed] [Google Scholar]
- 96.Ihlow J Gross S Sick A Schneider T Flörcken A Burmeister T, et al. . AML: High serum ferritin at initial diagnosis has a negative impact on long-term survival. Leuk Lymphoma 2019;60: 69–77. doi: 10.1080/10428194.2018.1461860. [DOI] [PubMed] [Google Scholar]
- 97.Lebon D Vergez F Bertoli S Harrivel V De Botton S Micol JB, et al. . Hyperferritinemia at diagnosis predicts relapse and overall survival in younger AML patients with intermediate-risk cytogenetics. Leuk Res 2015;39: 818–821. doi: 10.1016/j.leukres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Tachibana T Andou T Tanaka M Ito S Miyazaki T Ishii Y, et al. . Clinical significance of serum ferritin at diagnosis in patients with acute myeloid leukemia: A YACHT multicenter retrospective study. Clin Lymphoma Myeloma Leuk 2018;18: 415–421. doi: 10.1016/j.clml.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 99.Artz AS Logan B Zhu X Akpek G Bufarull RM Gupta V, et al. . The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016;101: 1426–1433. doi: 10.3324/haematol.2016.145847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gasparetto M, Pei S, Minhajuddin M, Stevens B, Smith CA, Seligman P. Low ferroportin expression in AML is correlated with good risk cytogenetics, improved outcomes and increased sensitivity to chemotherapy. Leuk Res 2019;80: 1–10. doi: 10.1016/j.leukres.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer 2022;22: 381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol Cancer 2022;21: 47. doi: 10.1186/s12943-022-01530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li H, Lin Y, Zhang L, Zhao J, Li P. Ferroptosis and its emerging roles in acute pancreatitis. Chin Med J 2022;135: 2026–2034. doi: 10.1097/cm9.0000000000002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang YQ Li HZ Gong WW Chen YY Zhu C Wang L, et al. . Cancer incidence and mortality in Zhejiang province, southeast China, 2016: A population-based study. Chin Med J 2021;134: 1959–1966. doi: 10.1097/cm9.0000000000001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dai C Zhao C Xu M Sui X Sun L Liu Y, et al. . Serum lncRNAs in early pregnancy as potential biomarkers for the prediction of pregnancy-induced hypertension, including preeclampsia. Mol Ther Nucleic Acids 2021;24: 416–425. doi: 10.1016/j.omtn.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luo H Zhu G Xu J Lai Q Yan B Guo Y, et al. . HOTTIP lncRNA promotes hematopoietic stem cell self-renewal leading to AML-like disease in mice. Cancer Cell 2019;36: 645–659.e8. doi: 10.1016/j.ccell.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang N, Zhang X, Gu X, Li X, Shang L. Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discov 2021;7: 30. doi: 10.1038/s41420-021-00407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang M Mao C Ouyang L Liu Y Lai W Liu N, et al. . Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ 2019;26: 2329–2343. doi: 10.1038/s41418-019-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng Z Wu W Lin Z Liu S Chen Q Jiang X, et al. . Identification of seven novel ferroptosis-related long non-coding RNA signatures as a diagnostic biomarker for acute myeloid leukemia. BMC Med Genomics 2021;14: 236. doi: 10.1186/s12920-021-01085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Z Chen X Liu N Shi Y Liu Y Ouyang L, et al. . A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol Ther 2021;29: 263–274. doi: 10.1016/j.ymthe.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cui Z Fu Y Yang Z Gao Z Feng H Zhou M, et al. . Comprehensive analysis of a ferroptosis pattern and associated prognostic signature in acute myeloid leukemia. Front Pharmacol 2022;13: 866325. doi: 10.3389/fphar.2022.866325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang L Kon N Li T Wang SJ Su T Hibshoosh H, et al. . Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015;520: 57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dong LH Huang JJ Zu P Liu J Gao X Du JW, et al. . CircKDM4C upregulates P53 by sponging hsa-let-7b-5p to induce ferroptosis in acute myeloid leukemia. Environ Toxicol 2021;36: 1288–1302. doi: 10.1002/tox.23126. [DOI] [PubMed] [Google Scholar]
- 114.Maslah N Salomao N Drevon L Verger E Partouche N Ly P, et al. . Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2020;105: 1539–1551. doi: 10.3324/haematol.2019.218453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nahi H Merup M Lehmann S Bengtzen S Möllgård L Selivanova G, et al. . PRIMA-1 induces apoptosis in acute myeloid leukaemia cells with p53 gene deletion. Br J Haematol 2006;132: 230–236. doi: 10.1111/j.1365-2141.2005.05851.x. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Q, Bykov VJN, Wiman KG, Zawacka-Pankau J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis 2018;9: 439. doi: 10.1038/s41419-018-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sallman DA DeZern AE Garcia-Manero G Steensma DP Roboz GJ Sekeres MA, et al. . Phase 2 results of APR-246 and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (AML). Blood 2019;134: 676. doi: 10.1182/blood-2019-131055. [Google Scholar]
- 118.Cluzeau T Sebert M Rahmé R Cuzzubbo S Walter-petrich A Lehmann che J, et al. . APR-246 combined with azacitidine (AZA) in TP53 mutated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). A phase 2 study by the groupe francophone des myélodysplasies (GFM). Blood 2019;134: 677. doi: 10.1182/blood-2019-125579. [Google Scholar]
- 119.Birsen R Larrue C Decroocq J Johnson N Guiraud N Gotanegre M, et al. . APR-246 induces early cell death by ferroptosis in acute myeloid leukemia. Haematologica 2022;107: 403–416. doi: 10.3324/haematol.2020.259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Takebe G Yarimizu J Saito Y Hayashi T Nakamura H Yodoi J, et al. . A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem 2002;277: 41254–41258. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 121.Jiao Y, Wang Y, Guo S, Wang G. Glutathione peroxidases as oncotargets. Oncotarget 2017;8: 80093–80102. doi: 10.18632/oncotarget.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei J Xie Q Liu X Wan C Wu W Fang K, et al. . Identification the prognostic value of glutathione peroxidases expression levels in acute myeloid leukemia. Ann Transl Med 2020;8: 678. doi: 10.21037/atm-20-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lubos E, Loscalzo J, Handy DE. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2011;15: 1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brigelius-Flohé R, Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 2009;1790: 1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 125.Wei R Qiu H Xu J Mo J Liu Y Gui Y, et al. . Expression and prognostic potential of GPX1 in human cancers based on data mining. Ann Transl Med 2020;8: 124. doi: 10.21037/atm.2020.02.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pardieu B Pasanisi J Ling F Dal Bello R Penneroux J Su A, et al. . Cystine uptake inhibition potentiates front-line therapies in acute myeloid leukemia. Leukemia 2022;36: 1585–1595. doi: 10.1038/s41375-022-01573-6. [DOI] [PubMed] [Google Scholar]
- 127.Zhang X Zhang X Liu K Li W Wang J Liu P, et al. . HIVEP3 cooperates with ferroptosis gene signatures to confer adverse prognosis in acute myeloid leukemia. Cancer Med 2022;11: 5050–5065. doi: 10.1002/cam4.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu Y Xie Y Cao L Yang L Yang M Lotze MT, et al. . The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol 2015;2: e1054549. doi: 10.1080/23723556.2015.1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yu Y, Xie M, He YL, Xu WQ, Zhu S, Cao LZ. Role of high mobility group box 1 in adriamycin-induced apoptosis in leukemia K562 cells (in Chinese). Chin J Cancer 2008;27: 929–933. [PubMed] [Google Scholar]
- 130.Kang R, Tang DL, Cao LZ, Yu Y, Zhang GY, Xiao XZ. High mobility group box 1 is increased in children with acute lymphocytic leukemia and stimulates the release of tumor necrosis factor-alpha in leukemic cell (in Chinese). Chin J Pediatr 2007;45: 329–333. [PubMed] [Google Scholar]
- 131.Xie M Kang R Yu Y Zhu S He YL Xu WQ, et al. . Enhancive effect of HMGB1 gene silence on adriamycin-induced apoptosis in K562/A02 drug resistance leukemia cells (in Chinese). Chin J Hematol 2008;29: 549–552. [PubMed] [Google Scholar]
- 132.Yang L Yu Y Kang R Yang M Xie M Wang Z, et al. . Up-regulated autophagy by endogenous high mobility group box-1 promotes chemoresistance in leukemia cells. Leuk Lymphoma 2012;53: 315–322. doi: 10.3109/10428194.2011.616962. [DOI] [PubMed] [Google Scholar]
- 133.Liu L Yang M Kang R Wang Z Zhao Y Yu Y, et al. . HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia 2011;25: 23–31. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- 134.Ye F Chai W Xie M Yang M Yu Y Cao L, et al. . HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRASQ61L cells. Am J Cancer Res 2019;9: 730–739. [PMC free article] [PubMed] [Google Scholar]
- 135.Mbaveng AT, Bitchagno GTM, Kuete V, Tane P, Efferth T. Cytotoxicity of ungeremine towards multi-factorial drug resistant cancer cells and induction of apoptosis, ferroptosis, necroptosis and autophagy. Phytomedicine 2019;60: 152832. doi: 10.1016/j.phymed.2019.152832. [DOI] [PubMed] [Google Scholar]
- 136.Zhu HY, Huang ZX, Chen GQ, Sheng F, Zheng YS. Typhaneoside prevents acute myeloid leukemia (AML) through suppressing proliferation and inducing ferroptosis associated with autophagy. Biochem Biophys Res Commun 2019;516: 1265–1271. doi: 10.1016/j.bbrc.2019.06.070. [DOI] [PubMed] [Google Scholar]
- 137.Mbaveng AT Fotso GW Ngnintedo D Kuete V Ngadjui BT Keumedjio F, et al. . Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine 2018;48: 112–119. doi: 10.1016/j.phymed.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 138.Mbaveng AT Ndontsa BL Kuete V Nguekeu YMM Çelik I Mbouangouere R, et al. . A naturally occurring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 2018;43: 78–85. doi: 10.1016/j.phymed.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 139.Simonetti G Angeli D Petracci E Fonzi E Vedovato S Sperotto A, et al. . Adrenomedullin expression characterizes leukemia stem cells and associates with an inflammatory signature in acute myeloid leukemia. Front Oncol 2021;11: 684396. doi: 10.3389/fonc.2021.684396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singh H, Shelat AA, Singh A, Boulos N, Williams RT, Guy RK. A screening-based approach to circumvent tumor microenvironment-driven intrinsic resistance to BCR-ABL+ inhibitors in Ph+ acute lymphoblastic leukemia. J Biomol Screen 2014;19: 158–167. doi: 10.1177/1087057113501081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Du J Wang T Li Y Zhou Y Wang X Yu X, et al. . DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med 2019;131: 356–369. doi: 10.1016/j.freeradbiomed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 142.Li Y Li G Wang K Xie YY Zhou RP Meng Y, et al. . Autophagy contributes to 4-amino-2-trifluoromethyl-phenyl retinate-induced differentiation in human acute promyelocytic leukemia NB4 cells. Toxicol Appl Pharmacol 2017;319: 1–11. doi: 10.1016/j.taap.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 143.Feucht MJ Kühle J Bode G Mehl J Schmal H Südkamp NP, et al. . Arthroscopic transtibial pullout repair for posterior medial meniscus root tears: A systematic review of clinical, radiographic, and second-look arthroscopic results. Arthroscopy 2015;31: 1808–1816. doi: 10.1016/j.arthro.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 144.Liu H, Chen F, Zhang L, Zhou Q, Gui S, Wang Y. A novel all-trans retinoic acid derivative 4-amino-2-trifluoromethyl-phenyl retinate inhibits the proliferation of human hepatocellular carcinoma HepG2 cells by inducing G0/G1 cell cycle arrest and apoptosis via upregulation of p53 and ASPP1 and downregulation of iASPP. Oncol Rep 2016;36: 333–341. doi: 10.3892/or.2016.4795. [DOI] [PubMed] [Google Scholar]
- 145.Xia Q Zhao Y Wang J Qiao W Zhang D Yin H, et al. . Proteomic analysis of cell cycle arrest and differentiation induction caused by ATPR, a derivative of all-trans retinoic acid, in human gastric cancer SGC-7901 cells. Proteomics Clin Appl 2017;11: 7–8. doi: 10.1002/prca.201600099. [DOI] [PubMed] [Google Scholar]
- 146.Du Y Li LL Chen H Wang C Qian XW Feng YB, et al. . A novel all-trans retinoic acid derivative inhibits proliferation and induces apoptosis of myelodysplastic syndromes cell line SKM-1 cells via up-regulating p53. Int Immunopharmacol 2018;65: 561–570. doi: 10.1016/j.intimp.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 147.Ju J, Wang N, Wang J, Wu F, Ge J, Chen F. 4-Amino-2-trifluoromethyl-phenyl retinate inhibits proliferation, invasion, and migration of breast cancer cells by independently regulating CRABP2 and FABP5. Drug Des Devel Ther 2018;12: 997–1008. doi: 10.2147/DDDT.S151029. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 148.Du Y Bao J Zhang MJ Li LL Xu XL Chen H, et al. . Targeting ferroptosis contributes to ATPR-induced AML differentiation via ROS-autophagy-lysosomal pathway. Gene 2020;755: 144889. doi: 10.1016/j.gene.2020.144889. [DOI] [PubMed] [Google Scholar]
- 149.Yusuf RZ Saez B Sharda A van Gastel N Yu VWC Baryawno N, et al. . Aldehyde dehydrogenase 3a2 protects AML cells from oxidative death and the synthetic lethality of ferroptosis inducers. Blood 2020;136: 1303–1316. doi: 10.1182/blood.2019001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sang M Luo R Bai Y Dou J Zhang Z Liu F, et al. . Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics 2019;9: 6209–6223. doi: 10.7150/thno.36283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang R Li Y Wang X Yan J Pan D Xu Y, et al. . Doxorubicin loaded ferritin nanoparticles for ferroptosis enhanced targeted killing of cancer cells. RSC Adv 2019;9: 28548–28553. doi: 10.1039/C9RA04478G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song J Lin L Yang Z Zhu R Zhou Z Li ZW, et al. . Self-assembled responsive bilayered vesicles with adjustable oxidative stress for enhanced cancer imaging and therapy. J Am Chem Soc 2019;141: 8158–8170. doi: 10.1021/jacs.8b13902. [DOI] [PubMed] [Google Scholar]
- 153.Luo T, Gao J, Lin N, Wang J. Effects of two kinds of iron nanoparticles as reactive oxygen species inducer and scavenger on the transcriptomic profiles of two human leukemia cells with different stemness. Nanomaterials (Basel) 2020;10: 10. doi: 10.3390/nano10101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sagasser J Ma BN Baecker D Salcher S Hermann M Lamprecht J, et al. . A new approach in cancer treatment: Discovery of chlorido[N, N'-disalicylidene-1,2-phenylenediamine]iron(III) complexes as ferroptosis inducers. J Med Chem 2019;62: 8053–8061. doi: 10.1021/acs.jmedchem.9b00814. [DOI] [PubMed] [Google Scholar]
- 155.Onciu M. Acute lymphoblastic leukemia. Hematol Oncol Clin North Am 2009;23: 655–674. doi: 10.1016/j.hoc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 156.Gregory S. Adult acute lymphoblastic leukemia: Treatment and management updates. Semin Oncol Nurs 2019;35: 150951. doi: 10.1016/j.soncn.2019.150951. [DOI] [PubMed] [Google Scholar]
- 157.Xu Y, Deng N, Wang X, Chen Y, Li G, Fan H. RKTG overexpression inhibits proliferation and induces apoptosis of human leukemia cells via suppression of the ERK and PI3K/AKT signaling pathways. Oncol Lett 2017;14: 965–970. doi: 10.3892/ol.2017.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jin L, Tong L. PAQR3 inhibits proliferation and aggravates ferroptosis in acute lymphoblastic leukemia through modulation Nrf2 stability. Immun Inflamm Dis 2021;9: 827–839. doi: 10.1002/iid3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lalonde ME Sasseville M Gélinas AM Milanese JS Béland K Drouin S, et al. . Genome-wide CRISPR screens identify ferroptosis as a novel therapeutic vulnerability in acute lymphoblastic leukemia. Haematologica 2023;108: 382–393. doi: 10.3324/haematol.2022.280786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang WS, Stockwell BR. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol 2016;26: 165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol 2015;6: 297–310. doi: 10.1016/j.redox.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 2013;1830: 3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 163.Probst L, Dachert J, Schenk B, Fulda S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharmacol 2017;140: 41–52. doi: 10.1016/j.bcp.2017.06.112. [DOI] [PubMed] [Google Scholar]
- 164.Reddy SV, Tiwari AK, Kumar US, Rao RJ, Rao JM. Free radical scavenging, enzyme inhibitory constituents from antidiabetic Ayurvedic medicinal plant Hydnocarpus wightiana blume. Phytother Res 2005;19: 277–281. doi: 10.1002/ptr.1491. [DOI] [PubMed] [Google Scholar]
- 165.Sharma DK, Hall IH. Hypolipidemic, anti-inflammatory, and antineoplastic activity and cytotoxicity of flavonolignans isolated from Hydnocarpus wightiana seeds. J Nat Prod 1991;54: 1298–1302. doi: 10.1021/np50077a010. [DOI] [PubMed] [Google Scholar]
- 166.Lou S Hong H Maihesuti L Gao H Zhu Z Xu L, et al. . Inhibitory effect of hydnocarpin D on T-cell acute lymphoblastic leukemia via induction of autophagy-dependent ferroptosis. Exp Biol Med (Maywood) 2021;246: 1541–1553. doi: 10.1177/15353702211004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mbaveng AT Noulala CGT Samba ARM Tankeo SB Abdelfatah S Fotso GW, et al. . The alkaloid, soyauxinium chloride, displays remarkable cytotoxic effects towards a panel of cancer cells, inducing apoptosis, ferroptosis and necroptosis. Chem Biol Interact 2021;333: 109334. doi: 10.1016/j.cbi.2020.109334. [DOI] [PubMed] [Google Scholar]
- 168.Mbaveng AT Chi GF Bonsou IN Abdelfatah S Tamfu AN Yeboah EMO, et al. . N-acetylglycoside of oleanolic acid (aridanin) displays promising cytotoxicity towards human and animal cancer cells, inducing apoptotic, ferroptotic and necroptotic cell death. Phytomedicine 2020;76: 153261. doi: 10.1016/j.phymed.2020.153261. [DOI] [PubMed] [Google Scholar]
- 169.Chen L Fang W Liu J Qi X Zhao L Wang Y, et al. . Poricoic acid A (PAA) inhibits T-cell acute lymphoblastic leukemia through inducing autophagic cell death and ferroptosis. Biochem Biophys Res Commun 2022;608: 108–115. doi: 10.1016/j.bbrc.2022.03.105. [DOI] [PubMed] [Google Scholar]
- 170.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 2014;15: e517–e526. doi: 10.1016/S1470-2045(14)70202-5. [DOI] [PubMed] [Google Scholar]
- 171.Katsuya H Ishitsuka K Utsunomiya A Hanada S Eto T Moriuchi Y, et al. . Treatment and survival among 1594 patients with ATL. Blood 2015;126: 2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 172.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol 2017;46: 65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 173.Ishikawa C, Senba M, Mori N. Evaluation of artesunate for the treatment of adult T-cell leukemia/lymphoma. Eur J Pharmacol 2020;872: 172953. doi: 10.1016/j.ejphar.2020.172953. [DOI] [PubMed] [Google Scholar]