Progression of IgA nephropathy varies on the basis of clinical and histological variables at onset, response to treatment, and ethnicity. Evaluation of the prognosis of IgA nephropathy has been recently simplified by an international risk-prediction tool that uses clinical and histological parameters to predict a 50% decline in eGFR or kidney failure.1 While this tool has been validated in multiethnic cohorts across the globe and is easily accessible (www.qxmd.com), it cannot reliably predict kidney failure risk after 5–7 years from kidney biopsy because of the limited follow-up duration of the derivation cohorts. Given that IgA nephropathy predominantly affects young adults, information on its course over the expected lifetimes of patients is crucial to understand the real story. Furthermore, after the success of early phases of clinical trials testing novel therapies for IgA nephropathy,2 more than a dozen randomized clinical trials (RCTs) are in progress or are in the pipeline for the near future. Because RCTs are planned for the short term, evaluating the ability of short-term surrogate end points to predict disease progression over one's lifetime seems an interesting unmet need.
Proteinuria has been frequently used as a surrogate end point in observational studies and clinical trials of IgA nephropathy. Our current perception of IgA nephropathy is that in most patients, this is a relatively slowly progressive disease. A slow and steady approach with a liberal target of proteinuria reduction to <1 g/d3 is also perceived to be associated with favorable outcomes. However, we also know that higher baseline proteinuria is known to be associated with a higher risk of adverse kidney outcomes during the long term.4–6 Time-average proteinuria during follow-up is increasingly recognized to be more relevant in predicting disease progression than baseline proteinuria. In a single-center study of 130 patients in Malaysia7 having a baseline CKD stage 1–2 in 64% and a baseline proteinuria of 2.4 g/d (approximately 44% having nephrotic-range proteinuria), time-average proteinuria was the only factor associated with a 50% decline in eGFR or kidney failure during follow-up. Time-average proteinuria <0.5 g/d was noted in only 28% of patients. The difference in kidney survival between 0.5 g/d and 0.5–1 g/d groups was noted after 20 years of follow-up on Kaplan–Meier curves. The annual decline of eGFR was –2 ml/min per 1.73 m2 in this cohort, and 30-year kidney survival was 65%. Of note, time-average proteinuria was reduced to 1.26 g/d in patients with nephrotic-range proteinuria at baseline; most were treated with immunosuppressive agents. The eGFR decline (−2.6 ml/min per 1.73 m2) in this group was statistically similar to the eGFR decline (−2.1 ml/min per 1.73 m2) in the group with subnephrotic proteinuria having a time-average proteinuria of 0.5 g/day. In a Chinese registry database8 of 1155 patients with IgA nephropathy with a median follow-up of 5.4 years, approximately 80% had CKD stage 1–2 at baseline, baseline proteinuria was 0.89 g/d, and 45% had time-average proteinuria <0.5 g/d during follow-up. The rate of kidney function decline was −1.7 ml/min per 1.73 m2, and 20-year kidney survival was 67%. Patients with time-average proteinuria <0.5 g/d had the best kidney outcomes, followed by 0.5–1 g/d and >1 g/d. The group with time-average proteinuria <0.5 g/d had better outcomes than that with 0.5–1 g/d.8
Pitcher and colleagues9 reported on the long-term (over expected lifetimes) outcomes of IgA nephropathy in a predominantly White cohort in this issue of CJASN. Both adults and children (5.7%) were included, and the median follow-up was 5.9 years. The median eGFR was lower (47.6 ml/min per 1.73 m2) than in previous similar studies; most patients (approximately 65%) had CKD stage 3 or below at baseline. Baseline proteinuria was 1.72 g/d. Kidney failure or death occurred in approximately 50% of patients. The mean eGFR decline during follow-up was −3.6 ml/min per 1.73 m2. CKD stage was the strongest baseline predictor of eGFR slope and 10-year kidney survival. Interestingly, time-average proteinuria over the first 2 years and the median follow-up, respectively, were noted to be a significant predictor of kidney failure, irrespective of baseline proteinuria and after adjusting for baseline CKD stage. Patients with time-average proteinuria >0.88 g/g had a higher risk of progression to kidney failure or death than those with <0.88 g/g. Of note, even a time-average proteinuria of <0.88 g/g (i.e., protein excretion of <1 g/d), which is considered a low risk for disease progression,3 was associated with significantly lowered kidney survival (almost reduced by 50%) when followed over 15 years. Furthermore, time-average proteinuria <0.44 g/g was observed in 24% of patients, and this cutoff of time-average proteinuria was associated with a slower eGFR decline (−0.3 ml/min per 1.73 m2) than that of 0.44 to <0.88 g/g (−1.6 ml/min per 1.73 m2). What is striking to note is that almost all patients were observed to be at risk of kidney failure within their expected lifetime unless eGFR decline was maintained at <1 ml/min per 1.73 m2. In the high-risk (proteinuria >1 g/d, eGFR 30 ml/min per m2) RCT representative population, even an 80% reduction in proteinuria from baseline was not associated with an eGFR slope of <1 ml/min per 1.73 m2, implying the need for a more aggressive target of proteinuria reduction to achieve such an eGFR slope target. Missing information on treatment, BP control, and kidney biopsy findings limits drawing any inferences from this study for guiding patient care. Moreover, generalizability is limited because the study included mainly a White population. Most importantly, it highlights the long-term risks associated with the perceived ideal target (<1 g/d proteinuria). Stricter proteinuria control to <0.5 g/d seemed to improve kidney survival. In addition, almost all patients are at risk of kidney failure within expected lifetimes unless an eGFR slope of <1 ml/min per 1.73 m2 is sustained. While these observations are insightful, the target eGFR slope seems arduous to achieve with the current patient care approach. However, aiming to get closer to such a target eGFR slope with modifications in the current approach, such as better proteinuria and BP control with therapies targeting specific pathogenic pathways, can improve patient outcomes (Figure 1).
Figure 1.
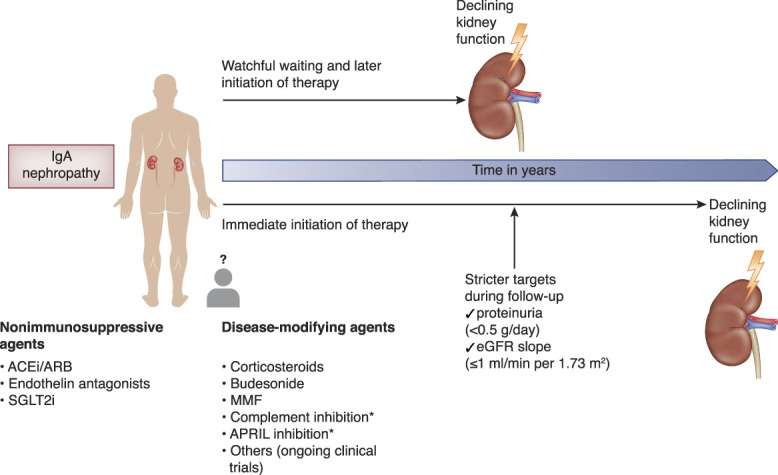
Choosing a novel aggressive approach over the conventional liberal approach in treating IgA nephropathy might avoid kidney failure in patient's expected lifetime. *In clinical trials, not approved yet. ACEi, angiotensin‐converting enzyme inhibitor; APRIL, a proliferation-inducing ligand; ARB, angiotensin receptor blocker; MMF, mycophenolate mofetil; SGLT2i, sodium‐glucose cotransporter 2 inhibitor.
What modifications must be made in the management approach of IgA nephropathy to reach a stringent eGFR slope target of approximately 1 ml/min per 1.73 m2? Will treating the typical patient with IgA nephropathy with targeted therapies early on, instead of waiting 3–6 months to optimize nonspecific supportive therapies,3 move us toward meeting the target? This leads to the next question, “which factors should decide early treatment?” and the following question, “which agent(s) should be used for a particular patient?” With the background of handy tools for prognostication and promising novel agents to treat the disease, we surely are in the right place at the right time to aim for zero kidney failures from IgA nephropathy. IgA nephropathy may not be that slow progressive disease we once believed it was. Treating aggressively up front may matter in our patients. Further work on applying the components of the tools, i.e., clinical and biopsy variables, to decide on one or more agents from the therapeutic armamentarium is necessary to lead us to the road toward our goal.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
See related article, “Long-Term Outcomes in IgA Nephropathy,” on pages 727–738.
Disclosures
K.D. Jhaveri is a founder and co-president of the American Society of Onco-Nephrology, serves as the US co-national lead for the IgA Nephropathy trial—VISIONARY, and reports employment with Northwell Health. K.D. Jhaveri reports consultancy agreements with Calliditas, George Clinical, PMV Pharmaceuticals, and Secretome; reports honoraria from the American Society of Nephrology and UpToDate.com; reports serving on the editorial boards of the American Journal of Kidney Diseases, CJASN, the Clinical Kidney Journal, the Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; and reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Kenar D. Jhaveri.
Data curation: Joyita Bharati, Kenar D. Jhaveri.
Formal analysis: Kenar D. Jhaveri.
Investigation: Kenar D. Jhaveri.
References
- 1.Barbour SJ Coppo R Zhang H et al.. Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179(7):942–952. doi: 10.1001/jamainternmed.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barratt J Hour B Kooienga L et al.. POS-109 Interim results of phase 1 and 2 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of BION-1301 in patients with IgA nephropathy. Kidney Int Rep. 2022;7(2):S46. doi: 10.1016/j.ekir.2022.01.121 [DOI] [Google Scholar]
- 3.Rovin BH Adler SG Barratt J et al.. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 4.Ibels LS, Györy AZ. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine. 1994;73(2):79–102. doi: 10.1097/00005792-199403000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Maixnerova D Bauerova L Skibova J et al.. The retrospective analysis of 343 Czech patients with IgA nephropathy–one centre experience. Nephrol Dial Transplant. 2012;27(4):1492–1498. doi: 10.1093/ndt/gfr482 [DOI] [PubMed] [Google Scholar]
- 6.Moriyama T Tanaka K Iwasaki C et al.. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014;9(3):e91756. doi: 10.1371/journal.pone.0091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohd R, Mohammad Kazmin NE, Abdul Cader R, et al. Long term outcome of immunoglobulin A (IgA) nephropathy: a single center experience. PLoS One. 2021;16(4):e0249592. doi: 10.1371/journal.pone.0249592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le W Liang S Hu Y et al.. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479–1485. doi: 10.1093/ndt/gfr527 [DOI] [PubMed] [Google Scholar]
- 9.Pitcher D Braddon F Hendry B et al.. Long-term outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2023;18(6):727–738. doi: 10.2215/CJN.0000000000000135 [DOI] [PMC free article] [PubMed] [Google Scholar]


