Keywords: process evaluation, renal dialysis, nephrology, nurse-led intervention, qualitative, care bundle
Abstract
Key Points
Health professionals resisted practice change in environments of low infection where the perception of a need to change is small.
Standardizing care of central venous catheters for hemodialysis requires breaking down silos of practice to benefit all patients.
Knowledge of and adherence to guidelines, formal change management, and ongoing facilitation are required to implement standardized care.
Background
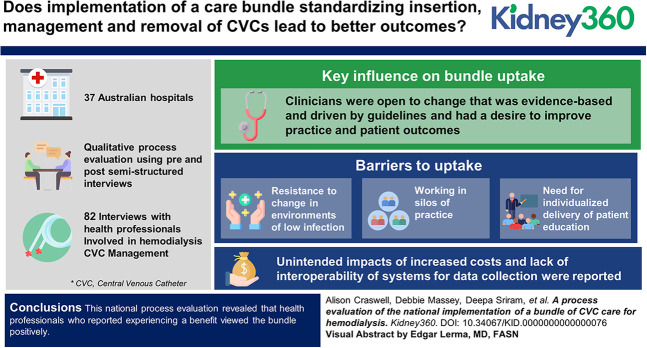
Implementation of a care bundle standardizing insertion, management, and removal practices to reduce infection related to central venous catheters (CVCs) used for hemodialysis was evaluated in a stepped wedge, cluster randomized controlled trial conducted at 37 Australian hospitals providing kidney services, with no reduction in catheter-related blood stream infection detected. This process evaluation explored the barriers, enablers, and unintended consequences of the implementation to explain the trial outcomes.
Methods
Qualitative process evaluation using pre-post semistructured interviews with 38 (19 nursing and 19 medical) and 44 (25 nursing and 19 medical) Australian health professionals involved in hemodialysis CVC management. Analysis was guided by the process implementation domain of the Consolidated Framework for Implementation Research.
Results
Key influences on bundle uptake were that clinicians were open to change that was evidence-based and driven by guidelines and had a desire to improve practice and patient outcomes. However, resistance to change in environments of low infection, working in silos of practice, and a need for individualized delivery of patient education created barriers to uptake. Unintended effects of increased costs and lack of interoperability of systems for data collection were reported. Because the trial was in progress at the time of qualitative data collection, perceptions of the bundle may have been influenced by the fact that practices of participants were being observed as a part of the trial.
Conclusion
This national process evaluation revealed that health professionals who reported experiencing a benefit viewed the bundle positively. Those who already provided most of the recommended care or perceived that their patient population was not included in the research evidence that underpinned the interventions, resisted the implementation of the bundle. Potentially, formal change management processes using facilitation may improve implementation of evidence-based practice.
Clinical Trial registry name and registration number:
Australian New Zealand Clinical Trials Registry, ACTRN12616000830493.
Introduction
Hemodialysis using a central venous catheter (CVC) for access is associated with high risk of infections such as central line–associated bloodstream infections (CLABSIs), exit-site infections, and subcutaneous tunnel infections, all of which adversely impact patient outcomes.1–3 Although the rates of infections are dropping in some jurisdictions, the effect of CLABSI on patient outcomes and health services remains significant. The management of such CVCs is complex, and relevant guidelines aimed at reducing infection rates4 have not been shown to reduce the large variations in the care of CVCs.5–8 This complexity and a lack of standardization in catheter care makes it important to gain a granular understanding of the factors that influence attempts to reduce variation and improve patient outcomes. Translation of health research into clinical practice is crucial to an improvement in patient outcomes.9
Care bundles are a grouping of care elements for a particular symptom, procedure, or treatment that are performed collectively and reliably to standardize practice and improve health care quality outcomes.9 The effect of such bundles is influenced by the associated implementation processes including the need to change multiple staff behaviors within complex clinical organizational systems.10,11 The evidence base for the effectiveness of such bundles is not strong, with meta-analyses of the mostly nonrandomized trials suggesting small or no effects.11 In addition, our previous work has suggested that substantial clinical variation exists in catheter management in Australia.12
The REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach (REDUCCTION) project tested the effect of a standardized care bundle (Supplemental Appendix 1) on dialysis CLABSIs across Australian dialysis units in a stepped wedge cluster randomized design.13,14 This large, randomized trial reported no effect on the bloodstream infection rate from the care bundle. A third of sites did change significant aspects of catheter care (dressing types and locking solutions), with demonstrated high rates of adherence during the intervention phase. Although many elements of the intervention were part of existing clinical practice at services, certain key elements such as designated clinical leaders, the ability to access real-time rates of catheter-related bloodstream infection at a service and national level, and the education tools for patients and clinicians were not available before this study.13
Understanding how such bundles affect complex health services, especially when implemented on a broad scale such as in REDUCCTION, is important in shaping further research and the implementation of findings. Elements such as the nature of the desired change in practice, the specific features of the setting, the professionals involved, and the inherent barriers to use of the evidence within the implementation context11,15 are central to this understanding. Accordingly, this process evaluation aimed to understand the findings from the trial through exploration of the barriers, enablers, and unintended consequences of the implementation of the evidence-based interventions.
Methods
The REDUCCTION trial began with a baseline phase in December 2016 and ended in March 2020 after a 12-month observational phase after the intervention phase at all sites. The multifaceted intervention encompassed the entirety of catheter care, with elements applied at the time of insertion, maintenance, and at the time of removal (Supplemental Appendix 1). There was variability in the changes that kidney services needed to make to their catheter practices. This study encompassed 37 kidney services and collected data on 6364 unique participants, deriving 1,146,265 catheter days of follow-up.13,14 This qualitative, process evaluation is guided by the process implementation domain of the Consolidated Framework for Implementation Research (CFIR).16
Process evaluation is as an important element to enable understanding of how interventions are implemented and translated into the clinical setting.17,18 Aligned with the process implementation domain of the CFIR, we describe a priori categories of (1) enablers and barriers to implementation experienced by staff (planning and executing) and (2) unintended consequences of care bundle implementation on clinical practice/clinical service provision (evaluating and refining).19 The process evaluation was conducted independent of the larger REDUCCTION research team to maximize open and honest communication from the site staff.
Ethical approval was granted as part of the REDUCCTION study ethics approval, Concord Repatriation general Hospital (HREC/16/CRGH/76), and by the University of the Sunshine Coast Human Research Ethics Committee (A171023), and the study adheres to the Declaration of Helsinki.20 Site-specific governance for each hospital and health service was granted before data collection. This manuscript was prepared using the COREQ guideline.21
Participant Recruitment and Selection
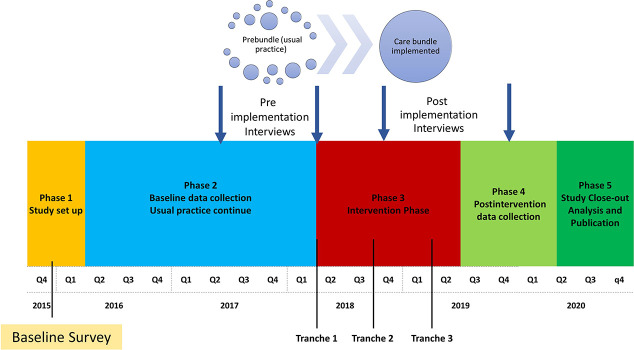
A purposive sampling technique was used to select eight of the 37 sites participating in the larger trial to interview. Australia has a broad geography, and we tried to ensure that the participating sites were geographically spread and included a mixture of regional, rural urban, high/low proportion of First Nations people, sites which were expected to have challenges with the trial as well as those who did not. A member of the research team contacted the nursing and medical leaders at selected sites by e-mail and invited them to participate in this study. The site nursing and physician leaders from each unit were encouraged to recommend other potential interviewees who were contacted by the researchers and invited to take part in this study. Interviews were conducted at two time points, before (October 2017-February 2018) and after (July-September 2019) implementation of the bundle (Figure 1). Participants were given an information sheet, and written informed consent was obtained from all participants.
Figure 1.
Randomized controlled trial study timeline highlighting when pre-post process evaluation was undertaken.
Data Collection
Qualitative, semistructured, individual, face-to-face interviews were conducted with Australian health professionals, involved in the management of hemodialysis CVCs at each site. An interview guide, developed using the constructs from the process domain of the CFIR, was used to elicit information on practices preimplementation and postimplementation of the REDUCCTION care bundle, staff attitudes to change of practice and the implementation process, and barriers and enablers during the process of implementation (Supplemental Appendix 1). Questions were focused on the multifaceted care bundle (Supplemental Appendix 2) and the role and experiences of the participants in implementation and practice change. In brief, the care bundle included directions for insertion, maintenance, and removal of the CVC, including information about patient education and when to provide this (Supplemental Appendix 2). One-on-one interviews were conducted by female authors (D.S. and A. Craswell) who were not from the REDUCCTION trial team and with no prior relationship with participants. Both were experienced in qualitative interviewing. Interviews took place in a private setting in each workplace, digitally recorded, and transcribed verbatim. Interviews continued until all selected sites were represented, and no new information was gathered. Transcripts were managed using NVIVO 11 software (QSR International, Melbourne, VIC).
Data Analysis
A qualitative content analysis approach was used on the basis of a priori categories. Formative process evaluation aimed to identify what worked and why, across multiple contexts to complement outcomes.18 Reflexive thematic analysis was used to analyze the data.22 Two researchers, A. Craswell and D.M., separate from the REDUCCTION team independently undertook iterative theme development within the a priori categories. Diversity of meaning within topic themes was managed by reflective engagement with the identified data to fully develop understanding and knowledge within each area.22 Independent review and analysis by researchers enhanced the rigor of the data analysis.
Results
Health professionals from eight sites participated in interviews, 38 (19 nursing and 19 medical) preintervention and 44 (25 nursing and 19 medical) postintervention. They were an experienced cohort (median years of experience 15.4, interquartile range 15) and were of a mean age of 47 years, Figure 2. The services covered six states and territories and were a combination of small, medium, and large sites servicing different types of populations. Participants included nephrologists, vascular surgeons, interventional radiologists, radiology suite nurses, junior and senior registered nurses, and nurse educators in kidney services. Interviews lasted between 15 and 30 minutes. To the best of our knowledge, no participants refused to take part in this study. The findings are presented under the a priori categories of (1) enablers and barriers to process change and (2) unintended effect of process change on service provision.
Figure 2.
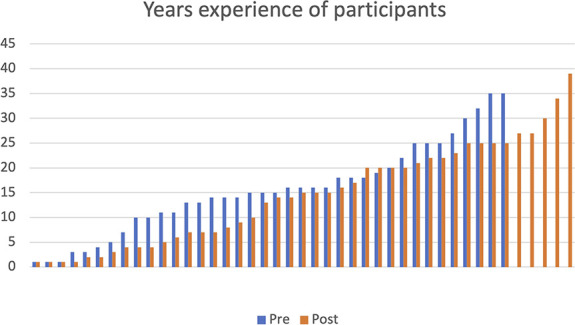
Participants' years of experience in kidney services.
Enablers and Barriers of Process Change Postimplementation
Enablers
Preinterviews and postinterviews revealed several factors considered relevant to practice change with potential effect on adoption of the proposed bundle. These included working as a team with a bundle champion, learning through education and others, and perceived benefits of the bundle. These were comprehensively expressed by a participant:
“You need a local champion and they need to buy-in to what you are selling. And then you need to have good education to the staff…There needs to be some kind of reward to do it…hopefully reduced bacteremia and better patient outcomes, and people need to buy-in to that as a plausible outcome” P16, Nurse Unit Manager (NUM)
Working as a Team with a Bundle Champion
Participants spoke highly of their bundle champion, most commonly the nurse coordinating REDUCCTION at that site or the nurse educator, stating: “(the champion is) keeping us all in line” P42, Clinical Nurse Consultant (CNC). In addition to care bundle champions identified at each site, participants recognized the importance of a culture that supported and embraced sustainable change.
Providing evidence and setting goals were also seen as important to assist the change process during the implementation. The desire to benchmark against other sites through the national shared data on infection rates was expressed by many and seemed to reflect a spirit of healthy competition.
Learning through Education and Others
A desire to improve practice and patient outcomes and a need to learn from other sites were identified as important by the participants. Preimplementation education provided by videos was identified as an important enabler.
“I think the YouTube videos were really good, especially for the satellite staff, they could have a look at them later on just as a reminder because they don’t have catheters all of the time. So, we’re sending you somebody with a catheter. I’d get them to revisit the video.” P18, Nurse Educator
Perceived Benefits
For some sites, elements of the care bundle reduced the staff workload. Such improvements to practice were viewed as positive outcomes of the practice change. Similarly, participants reported that patients had an improved response to the new dressings with fewer adverse reactions, Table 1.
Table 1.
Representative quotes for all themes
| Theme | Participant | Representative Quote |
|---|---|---|
| Working as a team with a bundle champion | P31, Nurse Unit Manager (NUM) | “I think we've tried really hard when we have a change of practice that we remind each other what we're doing, helping with that process change. It was really a team effort.” |
| P35, Registered Nurse (RN), renal access coordinator | “We, all around the place, keep our own data and don't share it and all think we're doing well. I think it would be good to see at the end what actually comes out of it and see, is there something somebody else is doing better? Or are we doing better, or not so well at things?” | |
| Perceived benefits | P10, Clinical Nurse (CN) | “So, to have (a dressing) that's only required as a weekly change (rather than every session) is much more time effective for nurses on the floor.” |
| P7, Nurse Practitioner (NP) | “Surprisingly, since we've changed from our previous dressings to the chlorhexidine gel dressing, very few of the patients have been allergic to it. I've actually expected a lot more having redness and rashes like they had for alcoholic chlorhexidine swabs or other dressings, Betadine and the like. Actually, it's been really well received.” | |
| Resistence to change | P1, Nephrologist | “There are two interventions that we were asked to do. One was to use an antimicrobial lock. There's no evidence, really. There are many studies that show that they work but we have data that shows that actually, our results are better than any of those treatment arms. So, why would we…we had no justification to do that. Secondly, the use of antimicrobial patches has only been demonstrated to be of benefit in central venous lines used in the intensive care unit, not in the outpatient unit, not with dialysis lines. There was no evidence to do that. Now we would obviously choose, and some patients who we think were grubby or whatever, that we would use those sort of things. Patients who've had catheter related bacteremia, we may use antimicrobial locks. Again, there's no evidence for it but there are studies that suggest that may preserve the catheter or prevent subsequent infections but to use it to everybody there's no evidence, so why should we do it?” |
| P31, NUM | “I think it just confirmed that we were probably doing some quite good things in the first place but we could have been better with the patient education, making sure that they're understanding of how to care for a catheter. “ | |
| P34, RN | “We're giving them a copy of the care sheet but I think living in the tropics as we do, it's okay at the moment - we're having a bit of a nice bit of weather recently, but it's going to get stinking hot and telling people not to have that shower for three days is actually quite a challenge if they were living out of the hospital, is what I'm saying. It's a big ask if you lived here in wet season.” | |
| Working in silos of practice | P3, Nephrologist | “Sometimes you get vascaths which are put in in ICU [intensive care unit] and we actually remove them completely because the site of insertion, or the site of entry, which happens in ICU, is not permissible for us to replace the catheter. They go high jugular and we go low jugular. Many times, I just remove it, leave it for 24 to 48 hours and reinsert the catheter.” “So, if something goes wrong with some new patient, I've got to put a catheter in. I'm always trying to get it in, get out, get to what I've got to do next and then the nurses do the education. And to be perfectly frank, nurses are better at doing that sort of routine stuff. They're more disciplined to do that than doctors that tend to be less thorough, maybe.” |
| Need for an individualized approach to patient education. | P19, RN | “That's probably the only fall down with the care bundle, is it's not necessarily aimed at our particular cohort of patients. We have quite a number of illiterate patients, we have a fair number of patients with a fairly low education standard. And I think sometimes wordy documents … they just don't even bother to look. So, in that way, yes. It's probably not the most appropriate document for every patient that we have here.” |
| Unintended impact of implementation on care provision | P34, RN | “One of the things that Indigenous patients' find is that all of the resources that are given out usually have lovely white faces on them. There are not a lot of black faces, people that they can relate to.” P34 |
| P17, Clinical Nurse Consultant (CNC) | “…because they know that I'm collecting data and I'm submitting it they will be more careful. …It's Australia and New Zealand wide. So, if we have a few infections, they will see it straight away and it doesn't look good for us, does it?” | |
| P16, NUM | “I shouldn't say that there was minimal impact because I also saw an impact on my budget … because the Tegaderm CHG and the Duralock, were buy-ins. So, I saw an increase in my budget.” | |
| P35, RN | “…my biggest issue has been the fact of initially we were told that the data could be extracted from our own database, and that was not the case. So, it has actually been very time consuming for us keeping two databases and double data entry.” |
Barriers to Process Change Postimplementation
Participants identified barriers to the uptake of standardized practice, including resistance to change, working in silos of practice, the need for individualized patient education, and a lack of awareness of the need for education at insertion.
Resistance to Change
Kidney services had a widespread perception that CVC care provided preimplementation matched the current evidence, current guidelines, and the care bundle once implemented. Yet, when shown the bundle, participants recognized and described differences. Instances where participants' interpretation of the evidence conflicted with the standardized bundle suggests some resistance to use, see participant quotes in Table 1.
Some sites believe that for them, change was not required because their infection rates were already very low, but they recognized the care bundle as a reminder that there was always room to improve. There were rare situations where clinicians deviated from the bundle or patient actions made complying with the bundle impossible.
Working in Silos of Practice
At most sites medical and nursing staff spoke highly of their kidney team and the care they provided. However, postimplementation, it was clear that silos of practice persisted and that effecting change outside the kidney unit (i.e. interventional radiology or intensive care unit) could be difficult, see representative participant quotes in Table 1.
There were difficulties getting people to work outside their silos of practice. As an example, interventional radiology staff were not aware of REDUCCTION, the care bundle, or the requirement for education at insertion. Preinsertion education, and that those patients should receive a copy of the REDUCCTION catheter care sheet, are recommended in the bundle. However, respondents from several sites said they did very little to no education of patients at or before insertion because it was not part of their role or they were time pressured.
Need for an Individualized Approach to Patient Education
As part of the care bundle, a dialysis catheter care sheet was produced on the basis of current evidence and reviewed by clinicians and consumers to standardize information given to patients. Although most participants believed that the care sheet was a concise summary of necessary information, it was considered the most challenging element of the bundle because it potentially conflicted with an individualized approach to patient education, Table 1.
This led to some sites giving out both the recommended care sheet and extra localized information or simply reverting to their own information handouts. Language and culture were not addressed in the standardized care sheet creating barriers for patients with poor literacy or who were non-English speaking. The timing of the care sheet being given to patients was problematic with some reporting uncertainty about whose role it was and when to provide it to patients. This increased the potential of it being missed.
Unintended Effect of Implementation on Care Provision
Participants reported the presence of the bundle as a reminder suggesting that, regardless of the content, the bundle stimulated vigilance. This may have inadvertently impacted established practice; see exemplar participant quotes in Table 1.
Improvements to practice through reduced dressing changes, the increased access to national data, made possible through the implementation, were positively received, particularly by sites with the lowest infection rates, because it provided the ability to benchmark against other sites. Sites where care bundle implementation represented a specific change in practice or resource use reported negative effects, including increased costs related to changes in recommended equipment, lack of interoperability of systems of data collection, and issues with supply of specific resources, that is, dressings. A summary of the challenges faced and potential solutions is presented in Table 2.
Table 2.
Summary of challenges and potential solutions
| Challenges Identified | Potential Solutions |
|---|---|
| Low motivation to change practice when infection rates already low | Increased staff education for importance of continual monitoring of practice Continual practice improvement for optimal outcomes The use of recognized key performance indicators to promote practice change |
| Individual interpretation of evidence conflicts with bundle recommendations | Publish practice guidelines by groups such as Caring for Australian and New Zealander's with Kidney Impairment (CARI) on the basis of the evidence used to develop the bundle The use of bundle champion to promote and encourage behaviour change |
| Silos of practice inhibit all patients benefiting from care bundle | Engage all professionals involved in CVC delivery in roll out of change as part of a vascular access team Provide educational resources to ensure all health providers involved in CVC delivery provide a consistent approach to practice |
| Standardizing education conflicts with individualized patient education, cultural considerations, and levels of health literacy | Increase funding in research projects available for individualizing education materials for different cultural groups; areas providing care for different populations Engage consumers in development of all educational materials |
| Cost of the bundle | Cost-benefit analysis of the bundle and catheter-related blood stream infections |
| Lack of interoperability of data systems | Develop a single national data system or data linkage |
CVC, central venous catheter.
Discussion
This article presents the qualitative process evaluation of a national project implementing a care bundle for insertion, maintenance, and removal of CVCs for hemodialysis finding that the key enablers to adoption were site champions and the ability to benchmark infection rates with readily available national data. Inhibitors to practice change included resistance to standardizing practice for all patients, particularly if the site infection rates were already low, the need for education resources to be more patient-centered, and challenges in communication between different acute care areas who manage catheters.
Participants in our study responded positively to the bundle elements that they perceived decreased their workload or improved the patient experience, such as decreased reactions to dressings or access to national-level benchmarking data. In line with previous reports, when participants were able to recognize the benefits of the bundle, they were more likely to accept and implement all its elements.23,24 Participants suggested initiatives which improved uptake of the interventions included having a bundle champion, the use of videos for ongoing education, and a culture that embraced change and valued evidence. This is consistent with reports of the critical role of the physician and nursing champions contributing to site and team ownership and culture shift.25 This role requires support from leadership of the organization to be successful.26 The atypical situations where clinicians deviated from standardized practice may reflect broader social determinants of health of patients requiring hemodialysis through CVC in addition to geographical location and climate.
A key finding of our study was the need for individualized patient education, which challenges the underpinning criteria of standardization implied by care bundles. Although the evidence-based education summary was well received, individualization of the delivery of education to meet the needs of patients with different socioeconomic status, language, cultural background, health literacy, and local site contact information is required. The Institute for Healthcare Improvement states that for any element to be included into a care bundle it should (1) have robust evidence for the clinical change, (2) have little or no controversy concerning its efficacy, and (3) have a high degree of acceptance.27 Participants in our study identified that the education element of the bundle was problematic to implement. Preinsertion education did not always occur as recommended by the bundle. Timing of and how best to approach insertion management education for patients may requires further investigation. In addition, participants often found themselves reverting to providing their own information in a style appropriate to their patients' preferred modes of communication, language, and levels of health literacy, potentially creating confusion for patients and highlighting the importance of codesign of patient facing materials with patients.23,28,29 Patient education is a pivotal element of kidney care, and engaging with consumers to ensure concordance with all elements of their care is essential to improve outcomes.30,31
The cost of implementing the bundle concerned several participants from different sites. Researchers reviewing care bundles have identified similar concerns related to bundle adoption and translation,32 and upfront costs are highlighted as a potential preimplementation barrier in Diffusion of Innovation Theory.33 Our participants understood that the bundle to “dictate practice” rather than allow clinicians to make individualized decisions about the use of more expensive items for only those patients thought to be at high risk of infection. This was contrary to the original aim of the bundle, which enabled clinician choice, yet highlights the risk of misinterpretation when formal implementation of the bundle was not funded as part of the larger study. Regardless, there is no tool or algorithm to identify patients at a high risk of CVC infection, and thus, we would advocate the use of evidence-based practice for all patients, and having standardized policies are likely to encourage this. In an environment of perceived low infection rates, this increased upfront cost could be perceived as unnecessary, but given the high financial and patient cost of catheter-related bloodstream infections, any reduction in infections is likely to represent cost savings.34,35
Limitations and Recommendations
A strength of this study is its breadth of engagement using a purposeful sample from a large, multisite study. In addition, we engaged multidisciplinary stakeholders from beyond just the kidney services themselves, which provides a wider and more applicable understanding of the factors driving the intervention uptake. Process evaluation of complex interventions, such as this, remains uncommon yet enable a deeper understanding of challenges of implementation of change into clinical practice. It is important to bear in mind that the randomized controlled trial was in progress at the time of data collection, so perceptions of the bundle may have been influenced by the fact that practices of participants were being observed as a part of the trial. Limitations of this study include that the sites selected for the process evaluation may not represent the entirety of REDUCCTION site experiences because some sites were invited to participate predominantly as they were expected to experience challenges with the implementation and the REDUCCTION team wished to understand the barriers to sustainable implementation. The interview questions were developed to understand perceptions and attitudes that influence the decision to change. The findings are limited in understanding the level to which these (perceptions and attitudes) affected implementation of the care bundle. In future clinical intervention studies, proper costing to include formal implementation methods are recommended.
CVCs are used globally as access for hemodialysis in people with end-stage kidney failure. Standardizing practice using care bundles aims to be proactive in preventing infection from CVC use and reducing adverse events. This national process evaluation revealed that health professionals involved in hemodialysis CVC use viewed the implemented care bundle positively when they experienced some benefit, such as reduced infection rates or improved patient outcomes, and that a site champion was crucial. If their infection rate was perceived to be low or they were concerned regarding the application of some aspects of a standardized bundle of care to their local patient population, participants were ambivalent. The patient education element was consistently identified as needing a broader consideration of health literacy and culture for patient education. The implementation of evidence care bundles is not without cost and can add to the complexity of care provision. Understanding the barriers may help clinicians be more selective about which situations will benefit most from the introduction of bundled care approaches. Implementing an evidence-based intervention is challenging, and the enablers identified in our study should be incorporated into future implementation projects.
Supplementary Material
Acknowledgments
We submit this on behalf of the REDUCCTION investigators (detailed in Supplemental Appendix 4).
We acknowledge the participants in this research for their time and contribution to extending knowledge in the care of patients requiring hemodialysis.
Contributor Information
Collaborators: Senthil Kumar Balakrishnan, David Fernandes, Hemant Kulkarni, Casey Light, Jo Ryan, Emma Marsh, David Semple, Omar Tombocon, Rowan Walker, Scott Wilson, Vilma Lleva, Lucy Mwangi, Peter Mount, Maree Ross-Smith, Marieke Veenendaal, Vicki Smith, Christine Somerville, Shaun Davidson-West, Natalie Grainer, Stella Green, Murty Mantha, Kati Thiessen, Girish Talaulikar, Alison Winsbury, Irene Yao, Emily Neville, Pamela Lopez-Vargas, Khalilah Marquez, Mona Razavian, Lisa Tienstra, Glenn Stewart, Cathy Chan, Peta McLean, Lawrence McMahon, Matthew Roberts, Dong Wang, Monika Chang, Anna Chiam, Duncan Wright, Orla O'Brien, Ramyasuda Swaminathan, Samadhi Wimalasena, Harish Puttagunta, Jeffrey Barbara, Amanda Luke, Margaret Pummeroy, Kim Torpey, Gemma Nicholls, Amy Swinbank, Thomas Titus, Peter Choi, Ginger Chu, Leanne Garvey, Alastair Gillies, Shilpa Jesudason, Josephine Chow, Imelda De Guzman, Jeanny Gando, Jeffrey Wong, Richard Nguyen, Roy Cherian, Raye Gillard, Rachel James, Michael Burke, Leanne Glancy, Shimbie Lewis, Richard Baer, Sophie Wade, Kate Fitt, Peter Kerr, Kevan Polkinghorne, Mechelle Seneviratne, Muralikrishna Komala, Junie McCourt, Craig Lawlor, Julia Bell, David Johnson, Michaela Kelleher, Sradha Kotwal, Amanda Coburn, Sarah Guo, David Johnson, Joanna Sudak, Diana Leary, Jenny Anderson, Thin Han, Tresna Titmarsh, Emily Adam, Bronwyn Hockley, Jenny Latte, Yvonne Matthew, Stephen McDonald, Chen Au Peh, Rebecca Taylor, David McIntyre, Sharadchandra Ratanjee, Karolynn Maurice, Fiona Rettie, Madhivanan Sundaram, Naomi Grimshaw, Matthew Jose, Gail Read, Jayne Amy, Patricia Coutts, Maria Presno, Nigel Toussaint, Debbie Knagge, Colleen Van Senden, Linh Pham, Muh Geot Wong, Jane Nicholson, Paul Snelling, Neil Boudville, Alison Farmer, Ingrid Holmes, Victoria Link, Vivien Perreau, Nicole Warnecke, Sunil Badve, Yanella Martinez-Smith, Jayson Catiwa, Frank Ierino, Emmet O'flaherty, Nicholas Gray, Gerald Hilder, Kaylene Wadd, Andrea Pollock, Stanley Searle, Cheryl Wertheim, Stephen May, Jill Telfer, Martin Gallagher, Sradha Kotwal, Sarah Coggan, Casey Yates, Kathryn Higgins, Earl James, Alison Coenen, Kris Rogers, Gian Luca Di Tanna, Jayanthi Mysore, Joseph Alvin Santos, Benjamin Talbot, Katrina Thistlethwaite, Bruce Neal, Elizabeth Coroneos, Shannon Nugent, Ian Fox, Sree Venuthurupalli, Jennifer Connor, Ruth Thachaw, Sandra Crikis, Pauline Byrne, Karumathil Murali, Hicham Cheikh Hassan, Lin Huang, Romiereeza Dizon, Deepika Joshi, Jon Mendoza, Bishi Augustine, Disha Balraj, Vincent Lee, and David O'Donnell
Disclosures
M. Gallagher reports the following: Research Funding: Bayer Pharmaceuticals; Advisory or Leadership Role: Ellen Medical Devices Pty Ltd; and Other Interests or Relationships: The George Institute for Global Health. N. Gray reports the following: Ownership Interest: ANZ Bank, Neuren Pharmaceuticals, Origin Energy, QBE insurance, and Westpac Bank; and Honoraria: Astrazeneca. S. Kotwal reports the following: Consultancy: Chinook Pharmaceuticals and Dimerix Pharmaceuticals; Research Funding: The George Institute and its affiliated entities work with numerous health and pharmaceutical companies in the design, implementation and analyses of clinical research and clinical trials. It is possible that some of these companies have products relevant to the clinical space covered in this analysis, but Dr S. Kotwal is not aware of any possible conflicts arising from this work; and Advisory or Leadership Role: Dimerix Pharmaceuticals and Chinook Pharmaceuticals (steering committee member). G. Talaulikar reports the following: Consultancy: Spouse—Amgen, Beigene, Eusa, Janssen-Cilag, and Roche; Research Funding: Spouse—Janssen and Roche; Honoraria: Spouse—as listed above; and Advisory or Leadership Role: Australia NZ Society of Nephrology and Member Finance Committee; Spouse—Scientific Board—Beigene, Janssen, and Roche. All remaining authors have nothing to disclose.
Funding
The REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach (REDUCCTION) trial was supported by a National Health and Medical Research Council Partnership Grant (APP1103241), Department of Health and Human Services (Victoria), Queensland Health, and 19 other partners contributing in-kind and financial support (see Supplemental Appendix 4). S. Kotwal was supported by a Medical Research Future Fund Next Generation TRIP Fellowship (MRF1150335). The funding bodies had no input into the design or conduct of, or decision to publish, the study.
Author Contributions
Conceptualization: Alan Cass, Alison Craswell, Martin Gallagher, Nicholas Gray, Sradha Kotwal, Kevan Polkinghorne, Girish Talaulikar, Marianne Wallis.
Data curation: Alison Craswell, Deepa Sriram.
Formal analysis: Alison Craswell, Debbie Massey.
Funding acquisition: Alan Cass, Martin Gallagher, Nicholas Gray, Sradha Kotwal, Kevan Polkinghorne, Girish Talaulikar, Marianne Wallis.
Investigation: Alison Craswell.
Methodology: Alison Craswell, Sradha Kotwal, Marianne Wallis.
Project administration: Alison Craswell, Sradha Kotwal, Deepa Sriram.
Writing – original draft: Alison Craswell.
Writing – review & editing: Alan Cass, Alison Craswell, Martin Gallagher, Nicholas Gray, Sradha Kotwal, Debbie Massey, Kevan Polkinghorne, Deepa Sriram, Girish Talaulikar, Marianne Wallis.
Data Availability Statement
The individual patient data generated in the trial can be shared in accordance with the trial's data sharing policy and in accordance with the local regulatory and ethical approval for the trial.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A317.
Supplemental Appendix 1. REDUCCTION Interventions.
Supplemental Appendix 2. FACT SHEET: Caring for your dialysis catheter.
Supplemental Appendix 3. Interview Guide.
Supplemental Appendix 4. REDUCCTION investigators.
References
- 1.Almasri J, Alsawas M, Mainou M, et al. Outcomes of vascular access for hemodialysis: a systematic review and meta-analysis. J Vasc Surg. 2016;64(1):236–243. doi: 10.1016/j.jvs.2016.01.053 [DOI] [PubMed] [Google Scholar]
- 2.Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79(6):587–598. doi: 10.1038/ki.2010.471 [DOI] [PubMed] [Google Scholar]
- 3.Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013;24(3):465–473. doi: 10.1681/ASN.2012070643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polkinghorne KR, Chin GK, MacGinley RJ, et al. KHA-CARI Guideline: vascular access—central venous catheters, arteriovenous fistulae and arteriovenous grafts. Nephrology. 2013;18(11):701–705. doi: 10.1111/nep.12132 [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Vargas PA, Craig JC, Gallagher MP, et al. Barriers to timely arteriovenous fistula creation: a study of providers and patients. Am J Kidney Dis. 2011;57(6):873–882. doi: 10.1053/j.ajkd.2010.12.020 [DOI] [PubMed] [Google Scholar]
- 6.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115 [DOI] [PubMed] [Google Scholar]
- 7.Ferrara P, Albano L. The adherence to guidelines for preventing CVC-related infections: a survey among Italian health-care workers. BMC Infect Dis. 2018;18(1):606. doi: 10.1186/s12879-018-3514-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson ME, Pilhammar E, Khalaf A, Willman A. Registered nurses' adherence to clinical guidelines regarding peripheral venous catheters: a structured observational study. Worldviews Evid Based Nurs. 2008;5(3):148–159. doi: 10.1111/j.1741-6787.2008.00105.x [DOI] [PubMed] [Google Scholar]
- 9.Lavallée JF, Gray TA, Dumville J, Russell W, Cullum N. The effects of care bundles on patient outcomes: a systematic review and meta-analysis. Implement Sci. 2017;12(1):142. doi: 10.1186/s13012-017-0670-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson MJ, May CR. Promoting professional behaviour change in healthcare: what interventions work, and why? A theory-led overview of systematic reviews. BMJ Open. 2015;5(9):e008592. doi: 10.1136/bmjopen-2015-008592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luker JA, Bernhardt J, Graham ID, et al. Interventions for the uptake of evidence‐based recommendations in acute stroke settings. Cochrane Database Syst Rev. 2017;2017:CD012520. doi: 10.1002/14651858.CD012520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smyth B, Kotwal S, Gallagher M, Gray NA, Polkinghorne K. Dialysis catheter management practices in Australia and New Zealand. Nephrology. 2019;24(8):827–834. doi: 10.1111/nep.13507 [DOI] [PubMed] [Google Scholar]
- 13.Kotwal S, Cass A, Coggan S, et al. ; on behalf of the REDUCCTION investigators. Multi-faceted intervention to reduce haemodialysis catheter-related bloodstream infections: REDUCCTION stepped wedge, cluster randomised trial. BMJ. 2022;377:e069634. doi: 10.1136/bmj-2021-069634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotwal S, Coggan S, McDonald S, et al. REDUcing the burden of dialysis Catheter ComplicaTIOns: a National approach (REDUCCTION)—design and baseline results. Kidney360. 2020;1(8):746–754. doi: 10.34067/KID.0001132020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francke AL, Smit MC, de Veer AJE, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8(1):38. doi: 10.1186/1472-6947-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oakley A, Strange V, Bonell C, Allen E, Stephenson J. Process evaluation in randomised controlled trials of complex interventions. BMJ. 2006;332(7538):413–416. doi: 10.1136/bmj.332.7538.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts S, McInnes E, Bucknall T, Wallis M, Banks M, Chaboyer W. Process evaluation of a cluster-randomised trial testing a pressure ulcer prevention care bundle: a mixed-methods study. Implement Sci. 2017;12(1):18. doi: 10.1186/s13012-017-0547-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbanski MA, Wilk AS, Escoffery C, Patzer RE. Dissemination and implementation science: a primer and applications in nephrology. Kidney360. 2022;3(1):185–189. doi: 10.34067/Kid.0005662021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 310(20), 2013. 2191–2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 22.Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exerc Health. 2019;11(4):589–597. doi: 10.1080/2159676X.2019.1628806 [DOI] [Google Scholar]
- 23.Gilhooly D, Green SA, McCann C, Black N, Moonesinghe SR. Barriers and facilitators to the successful development, implementation and evaluation of care bundles in acute care in hospital: a scoping review. Implement Sci. 2019;14(1):47. doi: 10.1186/s13012-019-0894-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts S, McInnes E, Wallis M, Bucknall T, Banks M, Chaboyer W. Nurses' perceptions of a pressure ulcer prevention care bundle: a qualitative descriptive study. BMC Nurs. 2016;15:64–10. doi: 10.1186/s12912-016-0188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gramlich LM, Sheppard CE, Wasylak T, et al. Implementation of Enhanced Recovery after Surgery: a strategy to transform surgical care across a health system. Implement Sci. 2017;12(1):67. doi: 10.1186/s13012-017-0597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renolen Å, Hjälmhult E, Høye S, Danbolt LJ, Kirkevold M. Creating room for evidence-based practice: leader behavior in hospital wards. Res Nurs Health. 2020;43(1):90–102. doi: 10.1002/nur.21981 [DOI] [PubMed] [Google Scholar]
- 27.Resar R, Griffin F, Haraden C, Nolan T. Using Care Bundles to Improve Health Care Quality. IHI Innovation Series White Paper. Institute for Healthcare Improvement; 2012. [Google Scholar]
- 28.Fernandes Agreli H, Murphy M, Creedon S, et al. Patient involvement in the implementation of infection prevention and control guidelines and associated interventions: a scoping review. BMJ Open. 2019;9(3):e025824. doi: 10.1136/bmjopen-2018-025824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert G, Cornwell J, Locock L, Purushotham A, Sturmey G, Gager M. Patients and staff as codesigners of healthcare services. BMJ. 2015;350:g7714. doi: 10.1136/bmj.g7714 [DOI] [PubMed] [Google Scholar]
- 30.Alikari V, Tsironi M, Matziou V, et al. The impact of education on knowledge, adherence and quality of life among patients on haemodialysis. Qual Life Res. 2019;28(1):73–83. doi: 10.1007/s11136-018-1989-y [DOI] [PubMed] [Google Scholar]
- 31.Narva AS, Norton JM, Boulware LE. Educating patients about CKD: the path to self-management and patient-centered care. Clin J Am Soc Nephrol. 2016;11(4):694–703. doi: 10.2215/CJN.07680715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ladbrook E, Khaw D, Bouchoucha S, Hutchinson A. A systematic scoping review of the cost-impact of ventilator-associated pneumonia (VAP) intervention bundles in intensive care. Am J Infect Control. 2021;49(7):928–936. doi: 10.1016/j.ajic.2020.11.027 [DOI] [PubMed] [Google Scholar]
- 33.Rogers E. Diffusion of Innovations, 5th ed. Free Press; 2003. [Google Scholar]
- 34.Hymes JL, Mooney A, Van Zandt C, Lynch L, Ziebol R, Killion D. Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard HD Antimicrobial Barrier Cap. Am J Kidney Dis. 2017;69(2):220–227. doi: 10.1053/j.ajkd.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 35.Kosa SD, Lok CE. The economics of hemodialysis catheter-related infection prophylaxis. Semin Dial. 2013;26(4):482–493. doi: 10.1111/sdi.12115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual patient data generated in the trial can be shared in accordance with the trial's data sharing policy and in accordance with the local regulatory and ethical approval for the trial.