Visual Abstract
Keywords: chronic kidney disease, transplantation
Abstract
Background
CKD affects 850 million people worldwide and is associated with high risk of kidney failure and death. Existing, evidence-based treatments are not implemented in at least a third of eligible patients, and there is socioeconomic inequity in access to care. While interventions aiming to improve delivery of evidence-based care exist, these are often complex, with intervention mechanisms acting and interacting in specific contexts to achieve desired outcomes.
Methods
We undertook realist synthesis to develop a model of these context-mechanism-outcome interactions. We included references from two existing systematic reviews and from database searches. Six reviewers produced a long list of study context-mechanism-outcome configurations based on review of individual studies. During group sessions, these were synthesized to produce an integrated model of intervention mechanisms, how they act and interact to deliver desired outcomes, and in which contexts these mechanisms work.
Results
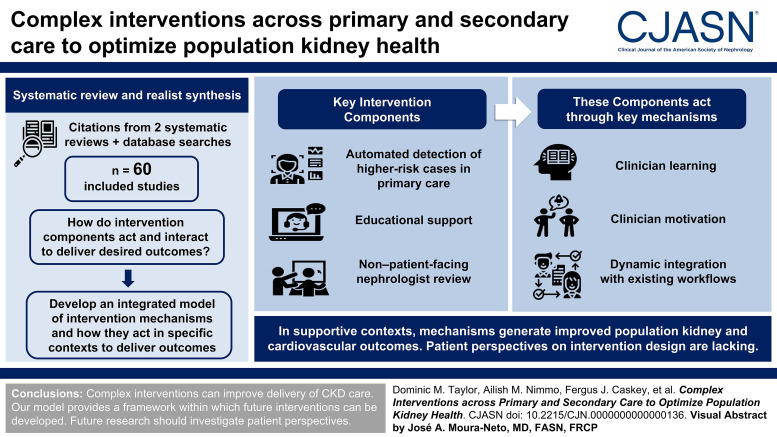
Searches identified 3371 relevant studies, of which 60 were included, most from North America and Europe. Key intervention components included automated detection of higher-risk cases in primary care with management advice to general practitioners, educational support, and non–patient-facing nephrologist review. Where successful, these components promote clinician learning during the process of managing patients with CKD, promote clinician motivation to take steps toward evidence-based CKD management, and integrate dynamically with existing workflows. These mechanisms have the potential to result in improved population kidney disease outcomes and cardiovascular outcomes in supportive contexts (organizational buy-in, compatibility of interventions, geographical considerations). However, patient perspectives were unavailable and therefore did not contribute to our findings.
Conclusions
This systematic review and realist synthesis describes how complex interventions work to improve delivery of CKD care, providing a framework within which future interventions can be developed. Included studies provided insight into the functioning of these interventions, but patient perspectives were lacking in available literature.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_05_08_CJN_POD_EP11_050823.mp3
Introduction
CKD affects 850 million people worldwide1 and is increasing in prevalence as populations age and rates of diabetes increase.2 Compared with general populations, people with CKD have at least three times the risk of cardiovascular disease, kidney failure, and death.3,4 Progression of CKD results in progressively higher risk: cardiovascular risk is up to 15 times higher in those with severe CKD compared with general populations.5 Quality of life also reduces progressively.6
Most people with CKD are treated by general practitioners, with secondary care referral indicated by severe or rapidly progressive disease. Evidence-based treatments that reduce the risk of CKD progression and cardiovascular events include targeted BP reduction, renin-aldosterone-angiotensin system (RAAS) blockade, statins, and newly available sodium-glucose transporter inhibitors (SGLT2is).7–11 These treatments are recommended in international guidance12 but are not well implemented: Only 50% of those with CKD stages 3–5 meet BP targets, 67% are offered statins,13 and 55% are prescribed RAAS blockers.14 Rollout of SGLT2i treatment to eligible patients is slow.15 Furthermore, access to secondary care services for those meeting referral criteria is incomplete and inequitable: In the United Kingdom, 66% of patients meeting criteria for nephrology review are not referred.13 Those from deprived areas undergo less-frequent monitoring16 and are referred with more advanced CKD,17 despite higher CKD prevalence,18 more rapid CKD progression,19 higher rates of established kidney failure,20 and higher mortality.16
In an attempt to improve delivery of equitable, evidence-based population CKD care, complex interventions have been developed and implemented.21,22 Individual centers report successes, but these interventions have not been systematically developed and optimized according to established guidance.23 Two previous systematic reviews describing these interventions21,22 found evidence was “critically lacking,” existing interventions “poorly designed,” and effects on clinical outcomes were inconsistent. Neither review synthesized and analyzed intervention components and mechanisms. Future intervention development would benefit from knowledge of which aspects of existing interventions are likely to lead to positive outcomes.
In this review, we used methods informed by realist synthesis and intervention component analysis24 to describe features of complex interventions aiming to improve delivery of evidence-based CKD treatment in primary care. We aimed to (1) synthesize reports of existing complex interventions to establish the mechanisms by which intervention components act in specific contexts and (2) develop an integrated model linking components to these context-mechanism-outcome configurations.
Methods
This systematic review is registered with the international prospective register of systematic reviews (PROSPERO) (ref: CRD42021278693) and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance.25
Study Identification
A scoping review identified five categories of components in existing interventions (Figure 1).
Risk screening of primary care patient databases—searching electronic patient records to identify patients with high risk of CKD progression or cardiovascular disease
CKD management recommendations, delivered to clinicians based on patients' risk, often through automated clinical systems
Prompts to refer to secondary care, often delivered automatically
Non–patient-facing nephrologist review—clinical data are reviewed by a nephrologist without direct patient contact, and clinical recommendations reported to primary care to be discussed with the patient
Primary care educational interventions supporting other intervention components
Figure 1.
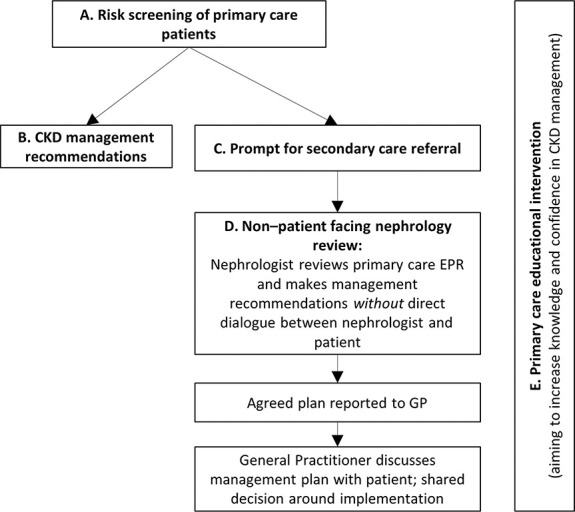
Key components of identified interventions identified in initial scoping review. EPR, electronic patient record; GP, general practitioner.
Our study-identification strategy aimed to identify complex interventions including these components.
Eligibility Criteria
We included English-language articles or conference abstracts (excluding commentaries), using any method to analyze or describe interventions delivered at primary care practice or regional level aimed at improving delivery of evidence-based treatment for CKD in primary care for adults aged 18 years or older (excluding those receiving KRT) in any country.
Study-Identification Strategy
Our study-identification strategy had three stages.
-
We searched the Cochrane and the PROSPERO databases for CKD-related systematic reviews. We found that evidence for interventions that include components A, B, C, and E was summarized in:
All referenced studies from these reviews were considered for inclusion.
2. To identify evidence focusing on the use of component D, we searched Ovid MEDLINE, Embase, EmCare, and the Cochrane Databases (Systematic Reviews and Register of Controlled Trials) from inception to July 2021. Search terms are in Supplemental Table 1.
Study titles and abstracts were reviewed independently by D.M.T. and A.M.N. to identify studies reporting the use of component D.
3. We performed forward and backward citation tracking of included studies from 1. and 2. using “citation chaser.”26 The results were reviewed independently by D.M.T. and A.M.N. against the eligibility criteria, and agreement on inclusion or exclusion reached after full-text review and discussion if required.
This iterative approach is typical of search strategies in realist reviews.27
Study Description and Quality Appraisal
Characteristics of included studies were summarized in a table, including study year, country, design, setting, patient characteristics, and reported outcomes. Studies were appraised and scored by G.J.M.T. and D.M.T. on a “−/+/++” scale, both for their relevance to the current review (considering intervention complexity and setting) and for study quality (considering study type and methodology).
Analysis
We opted to use realist synthesis methodology to develop an explanatory model describing how successful interventions work, alongside contextual influences. This explanatory model also allows identification of key evidence gaps. Alternative methodologies include logic modeling, which does not readily consider context, and implementation frameworks, which aim to describe implementation strategies rather than how interventions work. Our methods were informed by intervention components analysis28 and realist synthesis,29 drawing on principles of grounded theory.30
Included studies were allocated randomly to the six authors (D.M.T., A.M.N., F.J.C., R.J., M.P., G.J.M.T.). Each author independently reviewed studies allocated to them and used an “open coding” approach to propose context-mechanism-outcome configurations relevant to intervention strategies described. Proposal of context-mechanism-outcome configurations was informed by all data contained in study articles, including quantitative outcome data, qualitative study results, descriptions of interventions, responses to surveys, or “informal evidence” such as authors' descriptions of perceived strengths and weaknesses of intervention components.28 In a series of “data sessions,” we created a long list of context-mechanism-outcome configurations and attendant intervention strategies linked to these. We then used axial coding to synthesize this long list into higher-level context-mechanism-outcome configurations that were relevant and analytically generalizable across multiple studies. Higher-level context-mechanism-outcome configurations were then integrated into a model of interacting mechanisms, and contexts required allow these mechanisms to result in desired outcomes in the presence of specific intervention components. The results were presented as a flow diagram of context-mechanism-outcome configurations and their interactions, a narrative description, and a summary table/glossary.
Results
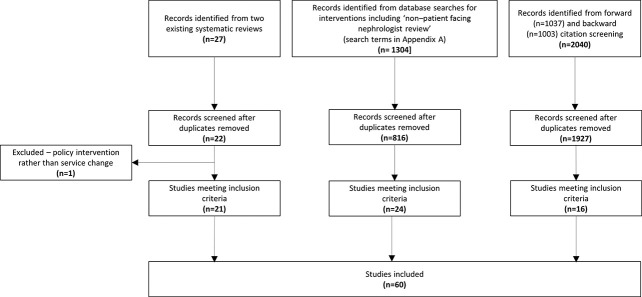
The study selection process (Figure 2) identified 60 studies31–90 for inclusion, including 56 original articles31–36,38–51,53–55,57–63,65–90 and four abstracts.37,52,56,64 One study91 included in an existing systematic review22 was excluded because it related to a national policy intervention. Study characteristics and quality appraisal scores are in Supplemental Table 2 and the PRISMA checklist in Supplemental Table 3.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart showing study selection.
Of 60 studies, 21 were service reports,33,36–38,45,49,52,56–58,60–62,64,65,69–71,78,84,90 20 were randomized controlled trials or their protocols,31,34,40–44,46,48,51,55,63,67,68,73,74,79,85,86,89 nine were before-and-after studies,32,39,47,66,75,77,81,87,88 four quality improvement projects,53,54,59,83 three survey or focus group reports,52,76,82 two implementation studies or their protocols,72,80 and one was a cohort study.50 one were from the United States,31,37,40–43,45,48,52–54,63,64,66,70,71,73,74,76,82,85 15 from the United Kingdom,46,49,56–60,67,69,75,77,81,83,84,88 12 from Canada,32,34–36,39,47,50,55,62,65,68,72 seven from continental Europe,33,38,51,78,79,86,87 two from South America,44,90 one from Australia,61 one from Japan,89 and one from China.80
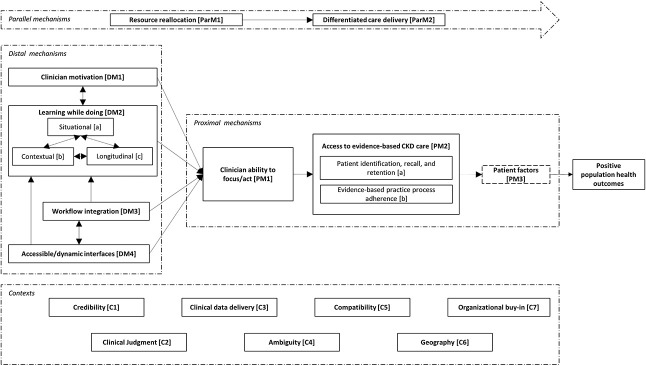
Figure 3 shows a schematic diagram of an integrated model of interventions in primary care aiming to improve delivery of evidence-based CKD treatment. The following narrative description, intended to be read with reference to Figure 3, summarizes the mechanisms by which intervention components act, the contexts within which these mechanisms are salient, interactions between mechanisms, and the route to the desired outcome(s) of these interventions, summarized as “positive population health outcomes.” Mechanisms are grouped and coded as “distal mechanisms” (furthest from the outcome, “DM1-4”), “proximal mechanisms” (closest to the outcome, “PM1-3”), and parallel mechanisms (contemporaneous, and equally necessary mechanisms, “ParM1-2”). Contexts are coded as “C1-6.” Supplemental Table 4 contains a glossary of contexts, mechanisms, and outcomes, with illustrative examples.
Figure 3.
Schematic diagram of an integrated model of interventions aiming to improve delivery of evidence-based CKD treatment.
Distal Mechanisms
Through clinician motivation [DM1], clinicians have the impetus to learn about CKD management and manage patients' CKD proactively. This includes primary care clinicians' motivation to learn or act while engaging with a novel model of CKD care. Clinician motivation [DM1] may be promoted by financial incentives,59 other incentives (lunch53), or competition with other care providers46 but also by clinician confidence and perceived credibility [C1] of the care model to the clinician. For instance, general practitioners engaging with an intervention that used automated detection of high-risk patients and non–patient-facing nephrologist review reported increased motivation to treat CKD, driven by increased CKD awareness and confidence.84 This is an example of clinician motivation [DM1] increasing through learning while doing [DM2]. While patient motivation is also a key mechanism promoting uptake of evidence-based care, which may facilitate clinicians' ability to focus/act [PM1], included studies did very little to address patient motivation (or other patient characteristics influencing care delivery, such as activation,92 deprivation,16 and associated literacy93 and health literacy94) or how interventions might promote (or reduce) patients' motivation. One exception was a study providing patients with access to test results and management plans,80 which could increase patient motivation.
Learning while doing [DM2] describes clinicians' development of knowledge and skills during the process of caring for patients with CKD. This applies to both primary and secondary care clinicians and comprises situational learning [DM2a], contextual learning [DM2b], and longitudinal learning [DM2c].
Situational learning [DM2a] occurs when clinicians engage in problem-solving for individual patients, for instance general practitioners' learning through responding to automated alerts or to nephrologists' advice.58,78,84 The provision of rapid responses from nephrologists to general practitioners' requests for management advice reportedly helped develop general practitioners' CKD knowledge, empowering them to apply this knowledge when managing other patients. Nephrologists may also experience situational learning [DM2a] when delivering non–patient-facing consultations, which, for many, would be a new experience.
Another example of situational learning [DM2a] illustrates its effectiveness in contrast to more didactic learning methods: A UK study46 tested an “audit-based education” process whereby patients with CKD registered at each primary care practice were identified, alongside markers of evidence-based care. This process was effective in achieving BP targets, whereas the comparator (didactic provision of guidelines and prompts to treat) was not effective. Other didactic methods of clinician education32,44,76 seemed to improve clinical knowledge without significant effect on clinical outcomes.
Contextual learning [DM2b] describes clinicians applying knowledge of CKD care in new contexts. For example, general practitioners may opportunistically apply CKD management principles (learned when engaging with a non–patient-facing consultation service) without having been prompted or advised to do so. Longitudinal learning [DM2c] describes applying knowledge to populations and over time. This highlights the potential benefit of promoting situational learning for individual patients (non–patient-facing nephrology review, automated individualized management advice based on guidelines) in combination with promotion of contextual and longitudinal learning (point-of-care electronic patient record prompts, whole-practice audit-based review of CKD management). Several interventions also included direct support for general practitioners from specialist clinicians (nephrologists, specialist nurses) to facilitate patient data review and management and therefore promote learning while doing [DM2].34,53,67,88 Through their interdependence, the components of learning while doing promote clinician confidence and motivation, enhancing clinicians' ability to focus and act [PM1] to implement appropriate, evidence-based care. This ability is promoted by sound clinical judgment [C2], which allows implementation of guidance while considering patients' needs and preferences.
The mechanisms of learning while doing [DM2] by clinicians and associated clinician motivation [DM1] are promoted by two features of intervention structure or design. Workflow integration [DM3] describes designing interventions that can be integrated into existing patterns of work. Examples include “point-of-care” electronic patient record alert systems where alerts are presented when relevant to the task at hand and therefore more likely to result in clinician action.58,78,82 Similarly, accessible and dynamic interfaces [DM4] describe the mode of delivery of interventions that allow access to timely, appropriate information and guidance, provided in a clinical context. Examples include intuitive electronic patient record systems delivering automated alerts82 or portals allowing two-way dialogue between nephrologists and general practitioners.72,78,84 The function of these interfaces requires adequate clinical data delivery [C3]: clinical systems that provide data required for clinical decision making, including kidney function expressed as eGFR, risk estimation by GFR and albuminuria categorization,12 CKD risk predictors including the kidney failure risk calculation,95 and BP readings.
Key to the effectiveness of learning while doing [DM2] in enhancing clinicians' ability to focus/act [PM1] are credibility [C1] and ambiguity [C4]. For clinical advice to be effective in influencing patient care, primary care teams must see the advice as credible and worthwhile. Intervention components must be readily applicable to clinical encounters between clinicians and patients and therefore lacking ambiguity [C4].
Workflow integration [DM3] and accessible and dynamic interfaces [DM4] are interdependent mechanisms. For instance, an IT system that is user friendly may not be effective if its use cannot become embedded within daily practice (one non–patient-facing referral service was implemented but underutilized and costly so was closed33), while a system that is integrated into practice may be ignored by clinicians if it is cumbersome to use. Together, these mechanisms promote clinicians' ability to focus and act [PM1] and contribute to intervention success by promoting learning while doing [DM2]. Effective workflow integration [DM3] and accessible/dynamic interfaces [DM4] depend on compatibility [C5]: the way in which interventions are compatible with existing work structures. Compatibility [C5] is influenced by outside factors within primary care organizations: for instance, parallel introduction of other novel work patterns may reduce the effectiveness of a CKD intervention by overwhelming clinicians with new processes.
Proximal Mechanisms
Clinicians' ability to focus and act [PM1] describes the knowledge, skills, motivation, time, and opportunity to work with patients to deliver effective, evidence-based CKD care, affected by distal mechanisms as discussed above. Effective focus and action results in patients having effective access to evidence-based CKD care [PM2]. Over burden of general practitioners could result in impaired ability to focus and act [PM1].
Access to evidence-based CKD care [PM2] is driven by two factors. Patient identification, recall, and retention [PM2a] describes processes whereby CKD is diagnosed and recorded (or registered), patients are recalled to clinical interactions, and retained within this system, avoiding loss to follow-up. The success of these processes within health care systems can be influenced by geography [C6], for example, the rurality of the region being served by the intervention. While it might be expected that more rural areas would benefit specifically from interventions delivering CKD care primarily through general practitioners (electronic alerts; non–patient-facing nephrologist review),35,36 included studies also report that implementation can be hampered by “unique challenges experienced when treating patients in rural locations”47 such as lack of internet access, long travel times (even to primary care centers), and the higher prevalence of other barriers to care. Evidence-based practice process adherence [PM2b] describes patients identified and retained within this structure being managed with reference to the evidence base. We can be confident that these processes would result in improved population health outcomes because of the strong evidence base for CKD interventions themselves (BP control, RAAS blockade, statins, SGLT2is).7–11 What is less clear is the effect of patient factors [PM3] on concordance with interventions and with proposed treatments.
Patient factors [PM3] describes any aspect of patient characteristics, circumstances, behavior, or access to care that influence the likelihood of patients receiving and implementing management recommendations. As noted above, the influence of patient factors is a relative unknown in included studies, yet patients' responses to aspects of interventions may be key to their success. For instance, non–patient-facing nephrologist review may be seen as helpful and convenient for general practitioners and nephrologists but may not be as successful as patient-facing nephrologist review if the treatment recommendations lack credibility for the patient or result in patient dissatisfaction because of the loss of direct patient-nephrologist interaction.
Parallel Mechanisms
Intervention success depends on two parallel mechanisms. Differentiated care delivery [ParM2] describes broadening the ways in which CKD care can be delivered, beyond established structures of primary care management with deference to secondary care for management of more severe or complex cases. For example, the use of non–patient-facing nephrologist review is shown to reduce the requirement for patient-facing nephrologist review for patients with milder CKD.78,86 This change in the mode of care delivery can improve cost-effectiveness.53,88 However, changing the mode of care delivery requires resource reallocation [ParM1]: prioritization of CKD care within health systems with appropriate funding, clinician time, and IT resource to support new activities. Resource reallocation [ParM1] in primary and secondary care organizations promotes differentiated care delivery [ParM2] and hence the success of interventions. Of course, resources cannot be reallocated based only on the enthusiasm or drive from individual clinicians to change models of care delivery. Instead, this requires organizational buy-in [C7]: agreement from primary and secondary care organizations to reallocate resources and alter structures of care. The financial and governance structures within which health care systems operate could promote (or restrict) organizational buy-in: payment arrangements between primary and secondary care that are structured around outdated models of care or resource reallocation without corresponding alteration of payment models could result in loss of income to primary or secondary care organizations. Organizational buy-in also describes involvement in the process from supporting staff and from clinicians, cited as a reason for success in one registry-based intervention.85
Discussion
This review synthesizes data from descriptions and evaluations of existing complex interventions that aim to improve primary care delivery of evidence-based CKD care. An integrated model of these interventions describes how these components act. The mechanisms of clinician motivation and learning while doing, supported by parallel mechanisms of workflow integration and accessible/dynamic interfaces, combine to promote clinician focus and action in treating CKD. Within successful interventions, these processes can realize positive patient (and population) outcomes by successful identification, recall, and retention of patients within processes that deliver evidence-based treatments. These processes are supported by key contexts, including the credibility of interventions, how they are compatible within the structure of health care organizations, and how these organizations adapt to changes in models of care delivery by reallocating resource and allowing care to be delivered by varied means. Our model identifies core elements of successful interventions in this area and represents a step toward development of optimized interventions most likely to result in the desired outcomes.23 However, important data describing interventions' acceptability to patients and how patient factors influence the delivery of these interventions were not available, which may explain the variability in success of the interventions in improving patient outcomes. Patient views are urgently required before further interventions of this type are implemented.
This review differs in focus from previous reviews in this area21,22 because it considers intervention mechanisms, intermechanism relationships, and the contexts within which mechanisms work, rather than only considering intervention components and quantitative outcome markers. A large number of studies were identified, and evidence from multiple countries was considered, although the majority of studies were from North America and Europe. The relevant diversity of expertise of our research team is a key strength of this work, with representative expertise in nephrology and primary care (research and active clinical practice), combined with expertise in realist review and associated methodologies. There are some limitations. While we feel confident that included studies broadly represent the evidence in this area, it is possible that our search processes missed some existing interventions, notably non–English-language studies. Identified evidence chiefly represents interventions within health care services in countries with high levels of health care resource, so our findings are less applicable to resource-poor settings. Realist methods are also fundamentally inductive, blending methods from qualitative analysis with an accounting of intervention effectiveness. While this is not a limitation per se, it is possible that a different analysis team may have reached a different account of contexts, mechanisms, and outcomes. In addition, as with all realist syntheses, findings from our analysis are tentative, with the possibility of confirmation, contradiction, or nuancing as additional research becomes available and contexts of care change. Finally, our analysis may or may not generalize beyond the specific class of interventions we examined here.
Early detection of CKD followed by intervention to optimize kidney and cardiovascular outcomes is a global kidney health priority.96 This study assimilates published knowledge from a range of predominantly high-income settings on context, mechanism, and outcome to move forward the process of complex intervention development in this area and focus on future steps in successful intervention development. These include (1) research into how patient factors and perspectives could influence success of these interventions; (2) focusing future evaluation on the key outcomes of evidence-based care delivery (rather than attempts to measure clinical outcomes) to demonstrate interventions' benefit in delivering treatments that are already known to be effective, without unnecessarily using vital research resource to demonstrate effect on hard clinical end points (with a risk of null results related to limited statistical power); and (3) the development of tailored interventions with consideration of the identified contexts.
Supplementary Material
Acknowledgments
Many thanks to Ms. Sarah Rudd, Medical Librarian, North Bristol NHS Trust for search strategy formulation.
Disclosures
F.J. Caskey reports research funding from National Institute for Health Research and serving advisory or leadership roles for International Society of Nephrology (Treasurer, Honorary Secretary, Executive Committee member), all of which are unpaid. M. Pippias reports serving as an ISN SharE-RR fellow. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Fergus J. Caskey, Rachel Johnson, G.J. Melendez-Torres, Dominic M. Taylor.
Data curation: Ailish M. Nimmo, Dominic M. Taylor.
Formal analysis: G.J. Melendez-Torres, Ailish M. Nimmo, Maria Pippias, Dominic M. Taylor.
Investigation: Rachel Johnson, G.J. Melendez-Torres, Ailish M. Nimmo, Maria Pippias, Dominic M. Taylor.
Methodology: Fergus J. Caskey, G.J. Melendez-Torres, Dominic M. Taylor.
Project administration: Dominic M. Taylor.
Supervision: Fergus J. Caskey, G.J. Melendez-Torres.
Writing – original draft: G.J. Melendez-Torres, Dominic M. Taylor.
Writing – review & editing: Fergus J. Caskey, Rachel Johnson, G.J. Melendez-Torres, Ailish M. Nimmo, Maria Pippias, Dominic M. Taylor.
Data Sharing Statement
All data are included in the manuscript and/or supporting materials.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B658.
Supplemental Table 1. Search strategy for records addressing component D (Medline example).
Supplemental Table 2. Description of included studies, with relevance and quality scoring.
Supplemental Table 3. PRISMA checklist.
Supplemental Table 4. Glossary of contexts, mechanisms, and outcomes with examples from included studies.
References
- 1.Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96(5):1048–1050. doi: 10.1016/j.kint.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 2.Public Health England. Chronic Kidney Disease Prevalence Model, London, Public Health England Publications, 2014. [Google Scholar]
- 3.Astor BC Matsushita K Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–1340. doi: 10.1038/ki.2010.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsushita K van der Velde M Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/s0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 6.Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes. 2012;10(1):71. doi: 10.1186/1477-7525-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv J Ehteshami P Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185(11):949–957. doi: 10.1503/cmaj.121468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y He D Zhang W, et al. ACE inhibitor benefit to kidney and cardiovascular outcomes for patients with non-dialysis chronic kidney disease stages 3-5: a network meta-analysis of randomised clinical trials. Drugs. 2020;80(8):797–811. doi: 10.1007/s40265-020-01290-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baigent C Landray MJ Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkovic V Jardine MJ Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 11.Heerspink HJL Stefánsson BV Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 12.(KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:4. doi: 10.1038/kisup.2012.76 [DOI] [PubMed] [Google Scholar]
- 13.Kim LG, Cleary F, Wheeler DC, Caplin B, Nitsch D, Hull SA. How do primary care doctors in England and Wales code and manage people with chronic kidney disease? Results from the National Chronic Kidney Disease Audit. Nephrol Dial Transplant. 2017;33(8):1373–1379. doi: 10.1093/ndt/gfx280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jameson K, Jick S, Hagberg KW, Ambegaonkar B, Giles A, O'Donoghue D. Prevalence and management of chronic kidney disease in primary care patients in the UK. Int J Clin Pract. 2014;68(9):1110–1121. doi: 10.1111/ijcp.12454 [DOI] [PubMed] [Google Scholar]
- 15.Farmer RE Beard I Raza SI, et al. Prescribing in type 2 diabetes patients with and without cardiovascular disease history: a descriptive analysis in the UK CPRD. Clin Ther. 2021;43:320–335. doi: 10.1016/j.clinthera.2020.12.015 [DOI] [PubMed] [Google Scholar]
- 16.Sawhney S Blakeman T Blana D, et al. Care processes and outcomes of deprivation across the clinical course of kidney disease: findings from a high-income country with universal healthcare. Nephrol Dial Transplant. 2022:gfac224. doi: 10.1093/ndt/gfac224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bello AK, Peters J, Rigby J, Rahman AA, El Nahas M. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol. 2008;3(5):1316–1323. doi: 10.2215/CJN.00680208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser SDS Roderick PJ Aitken G, et al. Chronic kidney disease, albuminuria and socioeconomic status in the Health Surveys for England 2009 and 2010. J Public Health. 2013;36(4):577–586. doi: 10.1093/pubmed/fdt117 [DOI] [PubMed] [Google Scholar]
- 19.Merkin SS, Coresh J, Diez Roux AV, Taylor HA, Powe NR. Area socioeconomic status and progressive CKD: the Atherosclerosis risk in Communities (ARIC) study. Am J Kidney Dis. 2005;46(2):203–213. doi: 10.1053/j.ajkd.2005.04.033 [DOI] [PubMed] [Google Scholar]
- 20.Judge A Caskey FJ Welton NJ, et al. Inequalities in rates of renal replacement therapy in England: does it matter who you are or where you live? Nephrol Dial Transplant. 2012;27(4):1598–1607. doi: 10.1093/ndt/gfr466 [DOI] [PubMed] [Google Scholar]
- 21.Galbraith L, Jacobs C, Hemmelgarn BR, Donald M, Manns BJ, Jun M. Chronic disease management interventions for people with chronic kidney disease in primary care: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(1):112–121. doi: 10.1093/ndt/gfw359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath CC Dobler CC McCoy RG, et al. Improving blood pressure management in primary care patients with chronic kidney disease: a systematic review of interventions and implementation strategies. J Gen Intern Med. 2020;35(S2):849–869. doi: 10.1007/s11606-020-06103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skivington K Matthews L Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ. 2021;374:n2061. doi: 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukumbang FC, Marchal B, Van Belle S, van Wyk B. A realist approach to eliciting the initial programme theory of the antiretroviral treatment adherence club intervention in the Western Cape Province, South Africa. BMC Med Res Methodol. 2018;18(1):47. doi: 10.1186/s12874-018-0503-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ McKenzie JE Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddaway NR, Grainger MJ, Gray CT. Citationchaser: an R package for forward and backward citations chasing in academic searching. Res Synth Methods. 2022;13(4):533–545. doi: 10.5281/zenodo.4543513 [DOI] [PubMed] [Google Scholar]
- 27.Booth A, Briscoe S, Wright JM. The “realist search”: a systematic scoping review of current practice and reporting. Res Synth Methods. 2020;11(1):14–35. doi: 10.1002/jrsm.1386 [DOI] [PubMed] [Google Scholar]
- 28.Sutcliffe K, Thomas J, Stokes G, Hinds K, Bangpan M. Intervention Component Analysis (ICA): a pragmatic approach for identifying the critical features of complex interventions. Syst Rev. 2015;4(1):140. doi: 10.1186/s13643-015-0126-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Pol. 2005;10(suppl l):21–34. doi: 10.1258/1355819054308530 [DOI] [PubMed] [Google Scholar]
- 30.Strauss ACJ. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Sage Publications, Inc.; 1998. [Google Scholar]
- 31.Abdel-Kader K, Fischer GS, Li J, Moore CG, Hess R, Unruh ML. Automated clinical reminders for primary care providers in the care of CKD: a small cluster-randomized controlled trial. Am J Kidney Dis. 2011;58(6):894–902. doi: 10.1053/j.ajkd.2011.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akbari A Swedko PJ Clark HD, et al. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med. 2004;164(16):1788–1792. doi: 10.1001/archinte.164.16.1788 [DOI] [PubMed] [Google Scholar]
- 33.Alamartine E Thibaudin D Maillard N, et al. Telemedicine: an unfruitful experience of tele-expertise in nephrology. Presse Medicale. 2010;39(5):e112–e116. doi: 10.1016/j.lpm.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 34.Barrett BJ Garg AX Goeree R, et al. A nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1241–1247. doi: 10.2215/CJN.07160810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bello AK Molzahn A Girard L, et al. Patient and provider perspectives on the design and implementation of an electronic consultation system for kidney care delivery in Canada: a focus group study. BMJ Open. 2017;7(3):e014784. doi: 10.1136/bmjopen-2016-014784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bello AK Zaidi D Braam B, et al. Electronic advice request system for nephrology in Alberta: pilot results and implementation. Can J Kidney Health Dis. 2019;6:205435811987977. doi: 10.1177/2054358119879778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betancourt JDS, Torres CA, Arencibia YO, Venkat VN, Soberon DJ, Avellaneda MAL. Telenephrology care for veterans in the covid-19 pandemic. J Am Soc Nephrol. 2020;31:224–225. [Google Scholar]
- 38.Boom VE van der Kamp LT van Zuilen AD, et al. Ongoing effects of eConsultation in nephrology on hospital referral rates: an observational study. J Telemed Telecare. 2020;28(6):423–428. doi: 10.1177/1357633x20942037 [DOI] [PubMed] [Google Scholar]
- 39.Brimble KS Boll P Grill AK, et al. Impact of the KidneyWise toolkit on chronic kidney disease referral practices in Ontario primary care: a prospective evaluation. BMJ Open. 2020;10(2):e032838. doi: 10.1136/bmjopen-2019-032838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll JK Pulver G Dickinson LM, et al. Effect of 2 clinical decision support strategies on chronic kidney disease outcomes in primary care: a cluster randomized trial. JAMA Netw Open. 2018;1(6):e183377. doi: 10.1001/jamanetworkopen.2018.3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter BL Coffey CS Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8(3):235–243. doi: 10.1161/circoutcomes.114.001283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang AR Evans MA Yule C, et al. Using pharmacists to improve risk stratification and management of stage 3A chronic kidney disease: a feasibility study. BMC Nephrol. 2016;17(1):168. doi: 10.1186/s12882-016-0383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooney D Moon H Liu Y, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrol. 2015;16(1):56. doi: 10.1186/s12882-015-0052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortés-Sanabria L Cabrera-Pivaral CE Cueto-Manzano AM, et al. Improving care of patients with diabetes and CKD: a pilot study for a cluster-randomized trial. Am J Kidney Dis. 2008;51(5):777–788. doi: 10.1053/j.ajkd.2007.12.039 [DOI] [PubMed] [Google Scholar]
- 45.Crowley ST Belcher J Choudhury D, et al. Targeting access to kidney care via telehealth: the VA experience. Adv Chronic Kidney Dis. 2017;24(1):22–30. doi: 10.1053/j.ackd.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 46.de Lusignana S Gallagher H Jones S, et al. Audit-based education lowers systolic blood pressure in chronic kidney disease: the Quality Improvement in CKD (QICKD) trial results. Kidney Int. 2013;84(3):609–620. doi: 10.1038/ki.2013.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donald M Smekal MD Elliott MJ, et al. Online clinical pathway for chronic kidney disease management in primary care: a retrospective cohort study. BMC Nephrol. 2021;22(1):332. doi: 10.1186/s12882-021-02533-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drawz PE, Miller RT, Singh S, Watts B, Kern E. Impact of a chronic kidney disease registry and provider education on guideline adherence—a cluster randomized controlled trial. BMC Med Inform Decis Mak. 2012;12(1):62. doi: 10.1186/1472-6947-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elsayed I, Khwaja A, Siddall S, Mortimer F. A novel approach to managing chronic kidney disease: remote monitoring. Nephrol Dial Transplant. 2013;28(4):972–981. doi: 10.1093/ndt/gfs552 [DOI] [PubMed] [Google Scholar]
- 50.Ennis J Gillen DL Rubenstein AH, et al. Clinical decision support improves physician guideline adherence for laboratory monitoring of chronic kidney disease: a matched cohort study. BMC Nephrol. 2015;16(1):163. doi: 10.1186/s12882-015-0159-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erler A Beyer M Petersen JJ, et al. How to improve drug dosing for patients with renal impairment in primary care—a cluster-randomized controlled trial. BMC Fam Pract. 2012;13(1):91. doi: 10.1186/1471-2296-13-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldstein D, Arndt B, Hetzel S, Smith P, Wiegmann D, Gabert T. A novel clinical decision support tool improves primary care treatment of chronic kidney disease. J Gen Intern Med. 2011;26:386–392. doi: 10.1007/s11606-010-1523-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox CH, Swanson A, Kahn LS, Glaser K, Murray BM. Improving chronic kidney disease care in primary care practices: an upstate New York practice-based research Network (UNYNET) study. J Am Board Fam Med. 2008;21(6):522–530. doi: 10.3122/jabfm.2008.06.080042 [DOI] [PubMed] [Google Scholar]
- 54.Haley WE Beckrich AL Sayre JJ, et al. Improving care coordination between nephrology and primary care: a quality improvement Initiative using the renal physicians association toolkit. Am J Kidney Dis. 2015;65(1):67–79. doi: 10.1053/j.ajkd.2014.06.031 [DOI] [PubMed] [Google Scholar]
- 55.Hamarneh YNA Tsuyuki RT Jones C, et al. Effectiveness of pharmacist interventions on cardiovascular risk in patients with CKD: a subgroup analysis of the randomized controlled RxEACH trial. Am J Kidney Dis. 2017;71:42–51. doi: 10.1053/j.ajkd.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 56.Hardy D, Winocour P, Moore-Haines K, Currie A, Solomon A, Renshaw C. ENHIDE telehealth primary care support of adults with diabetes and chronic kidney disease. Future Healthc J. 2019;6(suppl 1):143. doi: 10.7861/futurehosp.6-1-s143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harnett P, Jones M, Kunnath V, Ballasubramaniam G, Almond M. A virtual clinic to improve long-term outcomes in chronic kidney disease. Clin Med. 2018;18(5):356–363. doi: 10.7861/clinmedicine.18-5-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hull SA Rajabzadeh V Thomas N, et al. Do virtual renal clinics improve access to kidney care? A preliminary impact evaluation of a virtual clinic in East London. BMC Nephrol. 2020;21(1):10. doi: 10.1186/s12882-020-1682-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humphreys J, Harvey G, Hegarty J. Improving CKD diagnosis and blood pressure control in primary care: a tailored multifaceted quality improvement programme. Nephron Extra. 2017;7(1):18–32. doi: 10.1159/000458712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones C, Roderick P, Harris S, Rogerson M. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47(1):103–114. doi: 10.1053/j.ajkd.2005.09.020 [DOI] [PubMed] [Google Scholar]
- 61.Katz IJ Pirabhahar S Williamson P, et al. iConnect CKD—virtual medical consulting: a web-based chronic kidney disease, hypertension and diabetes integrated care program. Nephrology (Carlton). 2018;23(7):646–652. doi: 10.1111/nep.13070 [DOI] [PubMed] [Google Scholar]
- 62.Keely E, Li J, Magner P, Afkham A, Liddy C. Nephrology eConsults for primary care providers: original investigation. Can J Kidney Health Dis. 2018;5:205435811775361–2054358117753619. doi: 10.1177/2054358117753619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khoong EC Karliner LS Lo L, et al. A pragmatic cluster randomized trial of an electronic clinical decision support system to improve chronic kidney disease management in primary care: design, rationale, and implementation experience. JMIR Res Protoc. 2019;8(6):e14022. doi: 10.2196/14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy D, Grewal R, Liebman SE, Le TH. Nephrology econsultation: the 'curb side' consult for the 21st century. J Am Soc Nephrol. 2020;31:225. [Google Scholar]
- 65.Liddy C, Maranger J, Afkham A, Keely E. Ten steps to establishing an e-consultation service to improve access to specialist care. Telemed J E Health. 2013;19(12):982–990. doi: 10.1089/tmj.2013.0056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Litvin CB, Hyer JM, Ornstein SM. Use of clinical decision support to improve primary care identification and management of chronic kidney disease (CKD). J Am Board Fam Med. 2016;29(5):604–612. doi: 10.3122/jabfm.2016.05.160020 [DOI] [PubMed] [Google Scholar]
- 67.Major RW Brown CA Shepherd D, et al. The primary-secondary care partnership to improve outcomes in chronic kidney disease (PSP-CKD) study: a cluster randomized trial in primary care. J Am Soc Nephrol. 2019;30(7):1261–1270. doi: 10.1681/ASN.2018101042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manns BJ Tonelli M Culleton BF, et al. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7(4):565–572. doi: 10.2215/CJN.12391211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mark DA, Fitzmaurice GJ, Haughey KA, O'Donnell ME, Harty JC. Assessment of the quality of care and financial impact of a virtual renal clinic compared with the traditional outpatient service model. Int J Clin Pract. 2011;65(10):1100–1107. doi: 10.1111/j.1742-1241.2011.02750.x [DOI] [PubMed] [Google Scholar]
- 70.Mendu ML Ahmed S Maron JK, et al. Development of an electronic health record-based chronic kidney disease registry to promote population health management. BMC Nephrol. 2019;20(1):72. doi: 10.1186/s12882-019-1260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mendu ML, McMahon GM, Licurse A, Solomon S, Greenberg J, Waikar SS. Electronic consultations in nephrology: pilot implementation and evaluation. Am J Kidney Dis. 2016;68(5):821–823. doi: 10.1053/j.ajkd.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 72.Ong SW, Kaushal A, Chan C, Pariser P. An integrated kidney care eConsult practice model: results from the iKinect project. Am J Nephrol. 2019;50(4):262–271. doi: 10.1159/000502602 [DOI] [PubMed] [Google Scholar]
- 73.Peralta CA Livaudais-Toman J Lo L, et al. Electronic decision support for management of CKD in primary care: a pragmatic randomized trial. Am J Kidney Dis. 2020;76(5):636–644. doi: 10.1053/j.ajkd.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peralta CA Robinson AD Livaudais-Toman J, et al. Pragmatic cluster-randomized trial of an electronic clinical decision support system (ECDSS) to improve CKD management in primary care. J Am Soc Nephrol. 2019;30:13–14.30545982 [Google Scholar]
- 75.Rayner HC Baharani J Dasgupta I, et al. Does community-wide chronic kidney disease management improve patient outcomes? Nephrol Dial Transplant. 2014;29(3):644–699. doi: 10.1093/ndt/gft486 [DOI] [PubMed] [Google Scholar]
- 76.Regan ME. Implementing an evidence-based clinical decision support tool to improve the detection, evaluation, and referral patterns of adult chronic kidney disease patients in primary care. J Am Assoc Nurse Pract. 2017;29(12):741–753. doi: 10.1002/2327-6924.12505 [DOI] [PubMed] [Google Scholar]
- 77.Richards N Harris KPG Whitfield M, et al. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23(2):549–555. doi: 10.1093/ndt/gfm857 [DOI] [PubMed] [Google Scholar]
- 78.Scherpbier-de Haan ND, van Gelder VA, Van Weel C, Vervoort GMM, Wetzels JFM, de Grauw WJC. Initial implementation of a Web-based consultation process for patients with chronic kidney disease. Ann Fam Med. 2013;11(2):151–156. doi: 10.1370/afm.1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scherpbier-de Haan ND Vervoort GM van Weel C, et al. Effect of shared care on blood pressure in patients with chronic kidney disease: a cluster randomised controlled trial. Br J Gen Pract. 2013;63(617):e798–e806. doi: 10.3399/bjgp13X675386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen H van der Kleij R van der Boog PJM, et al. Development and evaluation of an eHealth self-management intervention for patients with chronic kidney disease in China: protocol for a mixed-method hybrid type 2 trial. BMC Nephrol. 2020;21(1):495. doi: 10.1186/s12882-020-02160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stoves J Connolly J Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. BMJ Qual Saf. 2010;19(5):e54. doi: 10.1136/qshc.2009.038984 [DOI] [PubMed] [Google Scholar]
- 82.Strait A Velasquez A Handley MA, et al. Acceptability of a multilevel intervention to improve blood pressure control among patients with chronic kidney disease in a public health care delivery system. Clin Kidney J. 2017;11(4):540–548. doi: 10.1093/ckj/sfx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas N, Gallagher H, Jain N. A quality improvement project to improve the effectiveness and patient-centredness of management of people with mild-to-moderate kidney disease in primary care. BMJ Qual Improv Rep. 2014;3(1):u201337.w825. doi: 10.1136/bmjquality.u201337.w825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas N, Rajabzadeh V, Hull S. Using chronic kidney disease trigger tools for safety and learning: a qualitative evaluation in East London primary care. Br J Gen Pract. 2019;69(687):e715–e723. doi: 10.3399/bjgp19x705497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuot DS McCulloch CE Velasquez A, et al. Impact of a primary care CKD registry in a US public safety-net health care delivery system: a pragmatic randomized trial. Am J Kidney Dis. 2018;72(2):168–177. doi: 10.1053/j.ajkd.2018.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Gelder VA Scherpbier-de Haan ND van Berkel S, et al. Web-based consultation between general practitioners and nephrologists: a cluster randomized controlled trial. Fam Pract. 2017;34(4):430–436. doi: 10.1093/fampra/cmw131 [DOI] [PubMed] [Google Scholar]
- 87.Via-Sosa MA, Lopes N, March M. Effectiveness of a drug dosing service provided by community pharmacists in polymedicated elderly patients with renal impairment—a comparative study. BMC Fam Pract. 2013;14(1):96. doi: 10.1186/1471-2296-14-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu G, Major RW, Shepherd D, Brunskill NJ. Making an IMPAKT; improving care of chronic kidney disease patients in the community through collaborative working and utilizing information technology. BMJ Qual Improv Rep. 2017;6(1):u207671.w4577.doi: 10.1136/bmjquality.u207671.w4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamagata K Makino H Iseki K, et al. Effect of behavior modification on outcome in early- to moderate-stage chronic kidney disease: a cluster-randomized trial. PLoS One. 2016;11(3):e0151422. doi: 10.1371/journal.pone.0151422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zuniga C, Muller H, Riquelme C, Astorga C, Vergara G, Espinoza M. Using telenephrology to improve access to nephrologist and global kidney management of CKD primary care patients. Kidney Int Rep. 2020;5(6):920–923. doi: 10.1016/j.ekir.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karunaratne K Stevens PE Irving J, et al. The impact of pay for performance on the control of blood pressure in people with chronic kidney disease stage 3–5. Nephrol Dial Transplant. 2013;28(8):2107–2116. doi: 10.1093/ndt/gft093 [DOI] [PubMed] [Google Scholar]
- 92.Magadi W Lightfoot CJ Memory KE, et al. Patient activation and its association with symptom burden and quality of life across the spectrum of chronic kidney disease stages in England. BMC Nephrol. 2022;23(1):45. doi: 10.1186/s12882-022-02679-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Irwin SK, Jungeblut A, Jenkins L, Kolstad A. Adult Literacy in America: A First Look at the Findings of the National Adult Literacy Survey. US Department of Education: Office of Educational Research and Improvement; 1993. [Google Scholar]
- 94.Taylor DM, Fraser SD, Oniscu GC, Tomson C, Ravanan R, Roderick PJ. Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol Dial Transplant. 2018;33(9):1545–1558. doi: 10.1093/ndt/gfx293 [DOI] [PubMed] [Google Scholar]
- 95.Tangri N Grams ME Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shlipak MG Tummalapalli SL Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34–47. doi: 10.1016/j.kint.2020.10.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript and/or supporting materials.