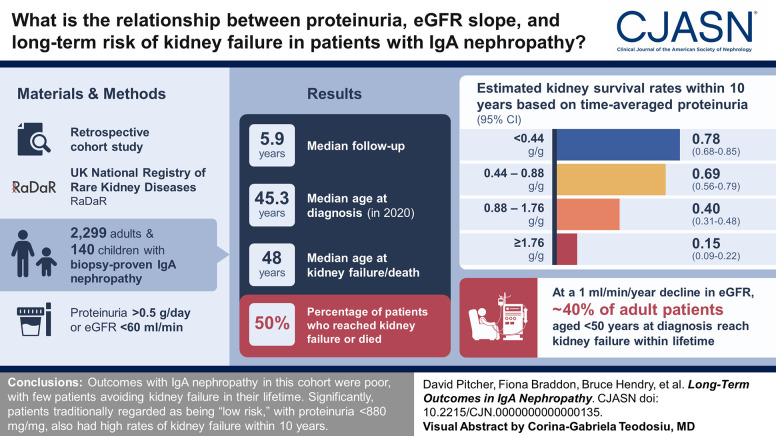
Visual Abstract
Keywords: chronic kidney disease, chronic kidney failure, end-stage kidney disease (ESKD), end-stage renal disease (ESRD), IGA nephropathy, kidney disease, nephropathy, proteinuria
Abstract
Background
IgA nephropathy can progress to kidney failure, and risk assessment soon after diagnosis has advantages both for clinical management and the development of new therapeutics. We present relationships among proteinuria, eGFR slope, and lifetime risks for kidney failure.
Methods
The IgA nephropathy cohort (2299 adults and 140 children) of the UK National Registry of Rare Kidney Diseases (RaDaR) was analyzed. Patients enrolled had a biopsy-proven diagnosis of IgA nephropathy plus proteinuria >0.5 g/d or eGFR <60 ml/min per 1.73 m2. Incident and prevalent populations and a population representative of a typical phase 3 clinical trial cohort were studied. Analyses of kidney survival were conducted using Kaplan–Meier and Cox regression. eGFR slope was estimated using linear mixed models with random intercept and slope.
Results
The median (Q1, Q3) follow-up was 5.9 (3.0, 10.5) years; 50% of patients reached kidney failure or died in the study period. The median (95% confidence interval [CI]) kidney survival was 11.4 (10.5 to 12.5) years; the mean age at kidney failure/death was 48 years, and most patients progressed to kidney failure within 10–15 years. On the basis of eGFR and age at diagnosis, almost all patients were at risk of progression to kidney failure within their expected lifetime unless a rate of eGFR loss ≤1 ml/min per 1.73 m2 per year was maintained. Time-averaged proteinuria was significantly associated with worse kidney survival and more rapid eGFR loss in incident, prevalent, and clinical trial populations. Thirty percent of patients with time-averaged proteinuria of 0.44 to <0.88 g/g and approximately 20% of patients with time-averaged proteinuria <0.44 g/g developed kidney failure within 10 years. In the clinical trial population, each 10% decrease in time-averaged proteinuria from baseline was associated with a hazard ratio (95% CI) for kidney failure/death of 0.89 (0.87 to 0.92).
Conclusions
Outcomes in this large IgA nephropathy cohort are generally poor with few patients expected to avoid kidney failure in their lifetime. Significantly, patients traditionally regarded as being low risk, with proteinuria <0.88 g/g (<100 mg/mmol), had high rates of kidney failure within 10 years.
Introduction
IgA nephropathy is the most common form of primary glomerulonephritis and a major cause of CKD and kidney failure worldwide.1,2 Most patients are diagnosed before age 40 years.3–5 Life expectancy in countries where IgA nephropathy is commonly encountered varies from 70 to 85 years.6 Current perceptions of risk of progression are based on outcome data typically spanning 10 or 15 years,5,7 a relatively short period that represents less than half the remaining lifespan of the typical patient with IgA nephropathy.
To facilitate investment in new therapies, a focus of recent research has been to identify surrogate end points that predict long-term clinical outcomes.8,9 Reduction in proteinuria has recently been accepted by regulatory authorities as a reasonably likely surrogate end point for IgA nephropathy.10 The design of ongoing phase 2 and 3 randomized controlled trials (RCTs) focuses on generating data over a 1- to 2-year period for proteinuria and rate of eGFR loss. After the inception of this approach, more than 15 phase 2 or 3 RCTs for new therapeutic approaches for IgA nephropathy are in progress in 2022.
Outstanding questions are first, the extent to which such short-term proteinuria and eGFR data predict the long-term rate of eGFR loss and risk of kidney failure in IgA nephropathy, and second, the degree of proteinuria reduction that would be associated with slowing eGFR decline so that kidney failure is not reached during a patient's lifetime. According to the Kidney Disease Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases,11 a reduction of proteinuria to <1 g/d is considered as a reasonable treatment target in patients with IgA nephropathy. However, long-term outcomes for patients with proteinuria <1 g/d need to be better understood.
To address these issues, we studied clinical data from patients with IgA nephropathy enrolled into the UK National Registry of Rare Kidney Diseases (RaDaR), a national registry with validated end points and no withdrawals from follow-up. We analyzed the characteristics and outcomes of patients with IgA nephropathy and delineated the burden of disease progression by age at diagnosis to gain insight into the magnitude of proteinuria change and rate of eGFR loss required of new treatments to prevent kidney failure in a patient's lifetime. We assessed the relationships among key parameters including baseline and time-averaged proteinuria, rate of eGFR loss, and kidney survival. Finally, we assessed the value of short-term changes in proteinuria and eGFR slope, typically measured in IgA nephropathy RCTs, for predicting longer-term clinical outcomes. Through these analyses, we aim to improve understanding of current outcomes and begin discussion on how lifetime kidney failure risk should be evaluated and management strategies developed such that no patient with IgA nephropathy reaches kidney failure.
Methods
Data Source
This retrospective cohort study used data from the RaDaR IgA nephropathy cohort, for which enrollment began in 2013. Patients eligible for enrollment must have a biopsy-proven diagnosis of IgA nephropathy plus proteinuria >0.5 g/d or eGFR <60 ml/min per 1.73 m2 at any time in the history of their disease. All forms of secondary IgA nephropathy are excluded. Further description of the data source is included in the Supplemental Methods.
Study Population and Definitions
Summaries of the eligibility criteria, patient disposition, and study attrition are provided in Supplemental Figure 1.
Urinary protein-creatinine ratio (UPCR) measurements were only available at diagnosis for 23% of adult patients in the full-analysis population (Table 1). Four adult subpopulations were, therefore, developed to facilitate focused analysis of proteinuria versus eGFR slope and clinical outcomes. Population 1 is a representative incident population examining time-averaged proteinuria over follow-up without requirement for a baseline UPCR at diagnosis. Populations 2 and 3 were selected to be representative of incident and prevalent cases, respectively, and allow assessment of baseline and time-averaged proteinuria. Population 4 is a prevalent population selected using criteria representative of a typical phase 3 RCT. Supplemental Figure 1 and Supplemental Table 1 provide detailed summaries of the four subpopulations. Patients could be in multiple subpopulations (Supplemental Figure 2); the only restriction was that population 2 was a subset of population 1.
Table 1.
Demographic and clinical characteristics at diagnosis and clinical outcomes during follow-up (full-analysis population)
| Category | Overall | Adult | Pediatric |
|---|---|---|---|
| Age at diagnosis, No. (%) | 2439 (100) | 2299 (100) | 140 (100) |
| Mean, yrs (SD) | 41 (15) | 42 (14) | 13 (5) |
| Median, yrs (Q1, Q3) | 40 (29, 51) | 41 (31, 52) | 14 (10, 17) |
| Sex, No. (%) | 2439 (100) | 2299 (100) | 140 (100) |
| Female | 716 (29) | 674 (29) | 42 (30) |
| Male | 1723 (71) | 1625 (71) | 98 (70) |
| Ethnicity, No. (%) | 2439 (100) | 2299 (100) | 140 (100) |
| Asian | 228 (9) | 221 (10) | 7 (5) |
| Black | 32 (1) | 32 (1) | 0 (0) |
| Mixed | 14 (1) | 12 (1) | 2 (1) |
| Others | 36 (1) | 36 (2) | 0 (0) |
| White | 1885 (77) | 1768 (77) | 117 (84) |
| Not stated/missing | 244 (10) | 230 (10) | 14 (10) |
| BMI at diagnosis, No. (%) | 328 (13) | 313 (14) | 15 (11) |
| Mean, (SD) | 28 (8.5) | 29 (8.3) | 20 (7.9) |
| Median, (Q1, Q3) | 28 (24.1, 31.5) | 28 (24.2, 31.6) | 21 (16.2, 24.6) |
| Systolic BP at diagnosis, No. (%) | 299 (12) | 287 (12) | 12 (9) |
| Mean, (SD) | 138 (24) | 139 (24) | 118 (16) |
| Median, (Q1, Q3) | 137 (124, 152) | 138 (125, 153) | 113 (108, 127) |
| UPCR at diagnosis, No. (%) | 545 (22) | 526 (23) | 19 (14) |
| Mean, g/ga (SD) | 2.42 (3.57) | 2.41 (2.72) | 2.75 (2.78) |
| Median, g/g (Q1, Q3) | 1.51 (0.64, 3.13) | 1.51 (0.66, 3.09) | 2.10 (0.42, 4.04) |
| Nephrotic range proteinuria (>2.64 g/g) | 169 (7) | 161 (7) | 8 (6) |
| eGFR at diagnosis, No. (%) | 896 (37) | 880 (38) | 16 (11) |
| Mean, ml/min per 1.73 m2 (SD) | 55 (29) | 55 (29) | 78 (33) |
| Median, ml/min per 1.73 m2 (Q1, Q3) | 48 (32, 75) | 48 (32, 75) | 76 (53, 108) |
| CKD stage at diagnosis, No. (%) | 896 (37) | 880 (38) | 16 (11) |
| Stage 1 | 141 (16) | 136 (15) | 5 (31) |
| Stage 2 | 197 (22) | 191 (22) | 6 (38) |
| Stage 3 | 366 (41) | 361 (41) | 5 (31) |
| Stage 4 | 178 (20) | 178 (20) | 0 (0) |
| Stage 5 | 14 (2) | 14 (2) | 0 (0) |
| Length of follow-up, No. (%) | 2439 (100) | 2299 (100) | 140 (100) |
| Mean, yrs (SD) | 8.0 (7.3) | 7.7 (6.7) | 13.4 (12.4) |
| Median, yrs (Q1, Q3) | 5.9 (3.0, 10.5) | 5.8 (2.9, 10.1) | 8.2 (5.3, 17.0) |
| Kidney failure or death event, No. (%) | 2439 (100) | 2299 (100) | 140 (100) |
| Yes | 1210 (50) | 1156 (50) | 54 (39) |
| No | 1229 (50) | 1143 (50) | 86 (61) |
| First event, No. (%) | 1210 (50) | 1156 (50) | 54 (39) |
| Death | 21 (2) | 21 (2) | 0 (0) |
| Dialysis | 298 (25) | 277 (24) | 21 (39) |
| Transplant | 95 (8) | 86 (7) | 9 (17) |
| eGFR <15 ml/min per 1.73 m2 | 796 (66) | 772 (67) | 24 (44) |
| Time to first event, No. (%) | 1210 (50) | 1156 (50) | 54 (39) |
| Mean, yrs (SD) | 6.9 (7.1) | 6.6 (6.6) | 13.6 (12.3) |
| Median, yrs (Q1, Q3) | 4.5 (1.9, 9.6) | 4.3 (1.8, 9.3) | 10.2 (6.1, 16.1) |
| Age at first event, No. (%) | 1210 (50) | 1156 (50) | 54 (39) |
| Mean, yrs (SD) | 48 (15) | 49 (14) | 27 (10) |
| Median, yrs (Q1, Q3) | 48 (37, 58) | 49 (38, 59) | 24 (21, 32) |
| Survival rate, estimate (95% CI) | 2439 (100) | 2299 (100) | 140 (100) |
| 5-year | 0.72 (0.70 to 0.74) | 0.71 (0.69 to 0.73) | 0.91 (0.85 to 0.95) |
| 10-year | 0.54 (0.51 to 0.56) | 0.52 (0.50 to 0.55) | 0.76 (0.66 to 0.83) |
| 15-year | 0.40 (0.37 to 0.43) | 0.38 (0.36 to 0.41) | 0.62 (0.52 to 0.72) |
| 20-year | 0.29 (0.27 to 0.32) | 0.28 (0.25 to 0.31) | 0.52 (0.41 to 0.62) |
| Quartile survival estimate, yr (95% CI) | 2439 (100) | 2299 (100) | 140 (100) |
| 75% | 4.2 (3.9 to 4.7) | 4.0 (3.7 to 4.4) | 10.9 (7.3 to 12.4) |
| 50% | 11.4 (10.5 to 12.5) | 10.8 (10.0 to 12.0) | 21.6 (15.9 to NE) |
| 25% | 24.3 (21.8 to 25.8) | 22.9 (19.6 to 25.5) | NE (NE to NE) |
| eGFR slope, total, No. (%) | 1863 (76) | 1795 (78) | 68 (49) |
| Mean, ml/min per 1.73 m2/yr (SD) | −3.6 (8.2) | −3.7 (7.4) | −2.2 (19.6) |
| Median, ml/min per 1.73 m2/yr (Q1, Q3) | −2.4 (−5.7, −0.6) | −2.4 (−5.6, −0.6) | −3.6 (−8.3, −0.9) |
Q, quartile; BMI, body mass index; UPCR, urinary protein-creatinine ratio; CI, confidence interval; NE, not estimable.
0.0088 g/g=1 mg/mmol.
Diagnosis was the earliest of either biopsy date or primary kidney diagnosis date recorded in RaDaR. Kidney failure was defined as the first occurrence of either long-term KRT, a confirmed12 eGFR <15 ml/min per 1.73 m2, or CKD stage 5 recorded in RaDaR. Kidney survival was defined as the absence of either kidney failure or death. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2021 formula13 in adults and the bedside Schwartz equation14 for patients younger than 18 years.
Annualized eGFR slopes were calculated using linear regression to fit a straight line through patients' mean eGFR values for each 3-month period of follow-up. Slopes were calculated for total follow-up and 6–30 months after baseline.
UPCR values were recorded and presented in g/g with urinary albumin-creatinine ratio (UACR) values converted to UPCR using UPCR=UACR/0.7.15–17 For comparison with other studies, a UPCR of 0.88 g/g (100 mg/mmol) may be considered comparable with protein excretion of 1 g/d.18 Time-averaged proteinuria was defined as the time-weighted averages for UPCR (Supplemental Methods).5
Life expectancy was based on World Bank year of birth and sex estimates.
Statistical Analyses
Continuous variables were reported as mean (SD) and median (interquartile range [IQR]) and categorical variables as frequencies and percentages. Kidney survival times were calculated from baseline to first kidney failure event, death from any cause, or end of follow-up for those with no event. Kaplan–Meier plots display kidney survival estimates; log-rank tests were used for comparison where appropriate. Analyses of kidney survival were conducted using Cox regression. eGFR slope was estimated using linear mixed models with random intercept and slope.
eGFR slope and kidney survival analyses were adjusted for age, sex, ethnicity, baseline CKD stage, baseline UPCR category, time-averaged proteinuria category, and other factors found to have significant associations with outcome during univariable analysis. Linear mixed models were used to analyze the association between eGFR slope and log (% change) in proteinuria, calculated from baseline value to both 6–12-month and 6–24-month time-averaged proteinuria. Data were analyzed using SAS 9.4. A two-sided P value of <0.05 was considered statistically significant with no correction for multiple comparisons.
Results
Characteristics, Outcomes, and Lifetime Kidney Failure Risk
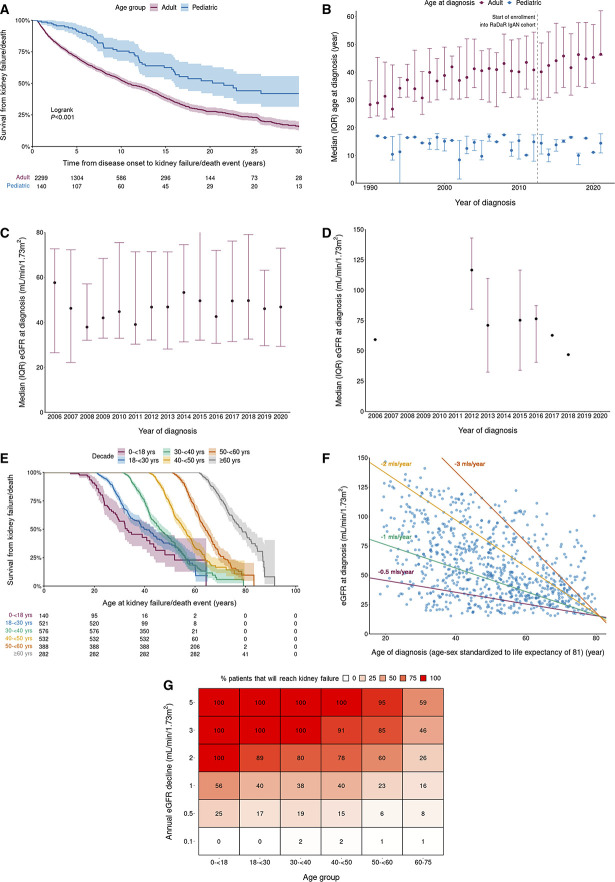
The full-analysis population included 2439 patients: 2299 adults and 140 children. Table 1 presents demographic and clinical characteristics at diagnosis. The cohort had long follow-up (median [Q1, Q3]; 5.9 [3.0, 10.5] years), and 50% of patients reached kidney failure or died within that time. The median (95% confidence interval [CI]) kidney survival was 11.4 (10.5 to 12.5) years with a mean (SD) age at kidney failure/death of 48 (15) years. The median (Q1, Q3) time to first event in patients diagnosed while pediatric was over double than that for adults (10.2 [6.1, 16.1] years versus 4.3 [1.8, 9.3] years). Kaplan–Meier survival analysis of pediatric versus adult patients demonstrated that patients diagnosed at younger than 18 years had significantly longer median kidney survival than adults (log-rank P < 0.001) (Figure 1A). In the adult cohort, baseline CKD stage was the strongest predictor of 10-year kidney survival and eGFR slope in unadjusted and multivariable analyses (Supplemental Table 2). Asian ethnicity was also associated with higher risk of disease progression (Asian versus White, hazard ratio [HR] [95% CI]: 1.36 [1.12 to 1.64]).
Figure 1.
Outcomes and characteristics for the full-analysis population. (A) Kaplan–Meier survival curves of time to kidney failure/death event in adult versus pediatric patients. (B) Age at diagnosis by decade in adult versus pediatric patients. Dotted line highlights first year of recruitment of patients with IgA nephropathy into RaDaR (2013). (C) eGFR at diagnosis by year in adult patients. (D) eGFR at diagnosis by year in pediatric patients. (E) Kaplan–Meier survival curves of time to kidney failure/death event on the basis of age at diagnosis. (F) Scatter plot of eGFR at diagnosis against age at diagnosis for patients with IgA nephropathy. Reference lines showing rates of decline that reach eGFR=15 by age-sex standardized life expectancy of 81 years. Patients below a reference line will reach an eGFR of 15 ml/min per 1.73 m2 before 81 years at the reference line rate of loss of eGFR. (G) Percentage of patients who will reach kidney failure during life expectancy on the basis of their eGFR at diagnosis. Life expectancy is based on year of birth and sex. IQR, interquartile range; RaDaR, UK National Registry of Rare Kidney Diseases.
The median (IQR) age at diagnosis in adults increased from 40 (30–50) years in 2013 to 45 (36–57) years in 2020 (Figure 1B), with a significant yearly increase of 0.7 years (0.2–1.1), P = 0.002 across this period. Data before 2013 may be affected by survivor bias because of RaDaR recruitment dates. The median age at which pediatric patients were diagnosed remained relatively stable although patient numbers were small (Figure 1B). Median eGFR at diagnosis remained relatively stable for both adult and pediatric patients between 2006 and 2020 (Figure 1, C and D).
Kaplan–Meier survival analysis by decade of age at diagnosis showed that most patients progressed to kidney failure within 10–15 years in all age groups (Figure 1E). On the basis of eGFR and age at diagnosis, almost all patients were at risk of kidney failure within their expected lifetime unless a rate of eGFR loss ≤1 ml/min per 1.73 m2 per year was maintained from diagnosis (Figure 1F). An annual eGFR decline of 3 ml/min per 1.73 m2, if sustained, would result in 100% of patients younger than 40 years at diagnosis reaching kidney failure within their expected lifetime (Figure 1G). Strikingly, a decline of as little as 1 ml/min per 1.73 m2 per year would still result in approximately 40% of adult patients younger than 50 years at diagnosis reaching kidney failure within their expected lifetime.
Relationship between Proteinuria and Kidney Failure
Baseline characteristics and clinical outcomes for all subpopulations are presented in Supplemental Table 3.
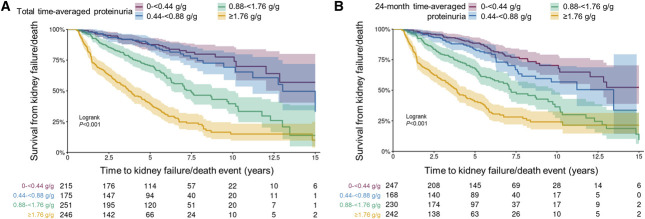
Time-averaged proteinuria from diagnosis over total follow-up (median [Q1, Q3], 4.5 [2.5, 6.8] years) (Supplemental Table 3) was analyzed as a categorical variable in population 1, with higher time-averaged proteinuria significantly associated with worse kidney survival and more rapid eGFR loss (Table 2; Supplemental Table 4). Similar results were seen with time-averaged proteinuria over 0–24 months. Accompanying Kaplan–Meier survival analyses calculated from both total and 0–24-month time-averaged proteinuria showed that patients with time-averaged proteinuria >0.88 g/g (>100 mg/mmol) were likely to progress to kidney failure or death more quickly than patients with time-averaged proteinuria <0.88 g/g (Figure 2).
Table 2.
Clinical outcomes of proteinuria analysis in population 1
| Proteinuria Analysis Population 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time-Averaged Proteinuriaa Duration | Total | 0–24 Months | |||||||
| Time-Averaged Proteinuria Category | Overall | <0.44 g/g | 0.44 to <0.88 g/g | 0.88 to <1.76 g/g | ≥1.76 g/g | <0.44 g/g | 0.44 to <0.88 g/g | 0.88 to <1.76 g/g | ≥1.76 g/g |
| Survival rate, estimate (95% CI) | n=887 | n=215b | n=175b | n=251 | n=246 | n=247b | n=168b | n=230 | n=242 |
| 10-yr | 0.46 (0.41 to 0.51) | 0.78 (0.68 to 0.85)b | 0.69 (0.56 to 0.79)b | 0.40 (0.31 to 0.48) | 0.15 (0.09 to 0.22) | 0.70 (0.61 to 0.78)b | 0.57 (0.44 to 0.68)b | 0.36 (0.26 to 0.47) | 0.24 (0.17 to 0.32) |
| Kidney failure risk (10-yr), Cox regression, HR (95% Wald CL) | |||||||||
| Unadjusted | N/A | Referenceb | 1.26 (0.78 to 2.01)b | 3.08 (2.09 to 4.5) | 7.00 (4.81 to 10.09) | Referenceb | 1.42 (0.94 to 2.14)b | 2.81 (2.00 to 3.95) | 5.46 (3.95 to 7.55) |
| Adjusted | N/A | Referenceb | 1.07 (0.64 to 1.79)b | 2.73 (1.78 to 4.16) | 7.66 (5.09 to 11.52) | Referenceb | 1.32 (0.85 to 2.04)b | 2.65 (1.79 to 3.92) | 5.73 (3.92 to 8.38) |
| eGFR slope, total (ml/min per 1.73 m2 per year), mean (SD) | n=887 | n=215b | n=175b | n=251 | n=246 | n=247b | n=168b | n=230 | n=242 |
| Mean (SD) | −3.7 (8.1) | −0.0 (7.3)b | −1.1 (5.7)b | −3.8 (5.5) | −9.5 (9.4) | −0.6 (7.1)b | −1.9 (5.9)b | −3.8 (6.3) | −8.7 (9.6) |
| Median (IQR) | −2.6 (−6.0 to −0.4) | −0.3 (−1.8 to 1.2)b | −1.6 (−2.9 to −0.1)b | −3.3 (−5.7 to −1.3) | −7.3 (−12.3 to −3.4) | −0.9 (−2.6 to 0.7)b | −1.7 (−3.9 to −0.1)b | −3.3 (−6.0 to −1.2) | −6.4 (−12.2 to −2.7) |
CI, confidence interval; HR, hazard ratio; CL, confidence limit; NA, not available; IQR, interquartile range.
0.44 g/g=50 mg/mmol (approximately 0.5 g/d); 0.88 g/g=100 mg/mmol (approximately 1 g/d); 1.76 g/g=200 mg/mmol (approximately 2 g/d).
Represent patients who would be classified as low-risk disease progression (proteinuria of <1 g/d) as termed by Kidney Disease Improving Global Outcomes.11
Figure 2.
Kaplan–Meier survival curves of time to kidney failure/death event in population 1. (A) Using total follow-up time-averaged proteinuria. (B) Using 24-month time-averaged proteinuria. 0.44 g/g=50 mg/mmol; 0.88 g/g=100 mg/mmol; 1.76 g/g=200 mg/mmol.
Considering time-averaged proteinuria over total follow-up, this analysis demonstrates that 30% of patients with time-averaged proteinuria of 0.44 to <0.88 g/g (50 to <100 mg/mmol) and approximately 20% of patients with time-averaged proteinuria <0.44 g/g developed kidney failure within 10 years (Table 2). Time-averaged proteinuria calculated over 0–24 months showed similar results. Kaplan–Meier analysis demonstrated that kidney survival probability decreased by almost half over the 15-year follow-up among patients with time-averaged proteinuria of 0.44 to <0.88 g/g (Figure 2). KDIGO guidelines describe reduction in proteinuria to <1 g/d (approximately equivalent to UPCR <0.88 g/g) as a reasonable treatment target in patients who remain at high risk of progressive CKD.11 However, this analysis indicated poor long-term outcomes for many patients at time-averaged proteinuria levels typically perceived as low risk. Incident (population 2) and prevalent (population 3) populations were used to assess the relationship between time-averaged proteinuria and clinical outcomes among patients commonly perceived as high or low risk on the basis of baseline UPCR values (≥0.88 versus <0.88 g/g). Among patients in population 3 with baseline UPCR ≥0.88 g/g, the probability of kidney survival at 5, 10, and 15 years was lower with higher time-averaged proteinuria (Table 3). The same association of poorer kidney survival with higher time-averaged proteinuria was evident among patients with baseline UPCR <0.88 g/g, highlighting the strong predictive value of proteinuria, even in patients currently perceived to be at low risk of progression. Similar results were observed among newly diagnosed patients in population 2 (Supplemental Table 5).
Table 3.
Estimated survival rate as a clinical outcome of proteinuria analysis in population 3
| Proteinuria Analysis Population 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline UPCR | <0.88 g/g | ≥0.88 g/g | |||||||||
| Total Time-Averaged Proteinuriaa Category | Overall | Combined | <0.44 g/g | 0.44 to <0.88 g/g | 0.88 to <1.76 g/g | ≥1.76 g/g | Combined | <0.44 g/g | 0.44 to <0.88 g/g | 0.88 to <1.76 g/g | ≥1.76 g/g |
| n=1153 | n=638 | n=302b | n=150b | n=141 | n=45 | n=515 | n=16b | n=77b | n=210 | n=212 | |
| % | Reference | 47.3%b | 23.5%b | 22.1% | 7.1% | Reference | 3.1%b | 15.0%b | 40.8% | 41.2% | |
| Survival rate, estimate (95% CI) | |||||||||||
| 5-yr | 0.72 (0.69 to 0.75) | 0.85 (0.81 to 0.88) | 0.90 (0.85 to 0.93)b | 0.94 (0.89 to 0.97)b | 0.75 (0.66 to 0.82) | 0.59 (0.41 to 0.72) | 0.58 (0.53 to 0.63) | 1.00 (1.00 to 1.00)b | 0.79 (0.66 to 0.87)b | 0.67 (0.59 to 0.73) | 0.40 (0.32 to 0.47) |
| 10-yr | 0.50 (0.45 to 0.54) | 0.64 (0.58 to 0.69) | 0.81 (0.74 to 0.87)b | 0.76 (0.62 to 0.86)b | 0.39 (0.28 to 0.50) | 0.26 (0.11 to 0.43) | 0.32 (0.26 to 0.39) | 0.50 (0.01 to 0.91)b | 0.60 (0.42 to 0.74)b | 0.35 (0.24 to 0.46) | 0.19 (0.11 to 0.27) |
| 15-yr | 0.31 (0.24 to 0.38) | 0.44 (0.33 to 0.54) | 0.75 (0.63 to 0.84)b | 0.41 (0.17 to 0.64)b | 0.20 (0.08 to 0.36) | 0.00 (0.00 to 0.00) | 0.16 (0.08 to 0.25) | NE (NE to NE)b | 0.00 (0.00 to 0.00)b | 0.15 (0.05 to 0.30) | 0.11 (0.04 to 0.22) |
UPCR, urinary protein-creatinine ratio; CI, confidence interval; NE, not estimable.
0.44 g/g=50 mg/mmol (approximately 0.5 g/d); 0.88 g/g=100 mg/mmol (approximately 1 g/d); 1.76 g/g=200 mg/mmol (approximately 2 g/d).
Represent patients who would be classified as low-risk disease progression (proteinuria of <1 g/d) as termed by Kidney Disease Improving Global Outcomes.11
Clinical Outcomes in the RCT-Representative Population 4
Population 4 was selected to be representative of a typical phase 3 RCT: age 18 years or older, baseline UPCR ≥0.88 g/g (considered comparable with protein excretion ≥1 g/d),18 and eGFR ≥30 ml/min per 1.73 m2, thereby excluding patients commonly perceived not to be high risk. This population was used to determine how changes in proteinuria may be associated with near- and long-term rate of eGFR loss and kidney survival. In population 4, comparisons of kidney survival and rate of eGFR loss were made between time-averaged proteinuria over 6–24 months (representing a full RCT duration) and 6–12 months (representing an interim analysis).
In both the 6–12- and 6–24-month analyses, higher time-averaged proteinuria was associated with higher risk of kidney failure and greater mean eGFR loss (Table 4). Greater separation of outcomes for time-averaged proteinuria over 6–24 months suggested that there was a value in longer follow-up for estimating long-term outcomes, although time-averaged proteinuria over 6–12 months produced similar results, allowing reliable prediction of longer-term outcomes. Additional outcomes for population 4 are presented in Supplemental Table 6.
Table 4.
Clinical outcomes of proteinuria analysis in population 4
| Proteinuria Analysis Population 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time-Averaged Proteinuriaa Duration | 6–12 Months | 6–24 Months | |||||||
| Time-Averaged Proteinuria Category | Overall | Combined | <0.88 g/g | 0.88 to <1.76 g/g | ≥1.76 g/g | Combined | <0.88 g/g | 0.88 to <1.76 g/g | ≥1.76 g/g |
| Survival rate, estimate (95% CI) | n=535 | n=410 | n=125 | n=158 | n=127 | n=509 | n=152 | n=200 | n=157 |
| 5-yr | 0.71 (0.66 to 0.75) | 0.70 (0.64 to 0.75) | 0.83 (0.73 to 0.89) | 0.75 (0.66 to 0.82) | 0.50 (0.40 to 0.60) | 0.70 (0.65 to 0.75) | 0.88 (0.80 to 0.93) | 0.71 (0.62 to 0.78) | 0.53 (0.43 to 0.61) |
| 10-yr | 0.42 (0.35 to 0.50) | 0.38 (0.29 to 0.48) | 0.50 (0.32 to 0.66) | 0.44 (0.26 to 0.60) | 0.22 (0.11 to 0.36) | 0.43 (0.35 to 0.51) | 0.65 (0.49 to 0.77) | 0.45 (0.32 to 0.58) | 0.21 (0.11 to 0.34) |
| Kidney failure risk (10-year), HR (95% CI) | |||||||||
| Unadjusted | NA | NA | Reference | 1.38 (0.86 to 2.24) | 3.30 (2.10 to 5.19) | N/A | Reference | 2.03 (1.26 to 3.27) | 4.18 (2.64 to 6.63) |
| Adjusted | NA | NA | Reference | 1.43 (0.87 to 2.33) | 3.57 (2.24 to 5.70) | N/A | Reference | 2.01 (1.24 to 3.26) | 4.49 (2.80 to 7.21) |
| eGFR slope, 6–30 months (ml/min per 1.73 m2 per year) | n=501 | n=388 | n=120 | n=150 | n=118 | n=477 | n=144 | n=186 | n=147 |
| Mean (SD) | −5.0 (8.6) | −5.0 (8.8) | −1.9 (6.6) | −3.7 (7.1) | −9.7 (10.7) | −4.9 (8.5) | −1.4 (5.9) | −3.8 (6.9) | −9.7 (10.2) |
| Median (IQR) | −3.7 (−8.5 to −0.2) | −3.7 (−8.5 to −0.2) | −2.1 (−5.2 to 1.4) | −3.2 (−7.3 to 0.0) | −7.5 (−14.1 to −3.7) | −3.7 (−8.5 to −0.2) | −1.8 (−4.3 to 1.7) | −3.3 (−7.6 to −0.4) | −7.5 (−14.1 to −3.7) |
| eGFR slope, total (ml/min per 1.73 m2 per year) | n=510 | n=392 | n=121 | n=151 | n=120 | n=484 | n=147 | n=187 | n=150 |
| Mean (SD) | −5.3 (7.3) | −5.3 (7.6) | −2.9 (4.3) | −4.2 (6.2) | −8.9 (10.1) | −5.3 (7.3) | −2.2 (4.2) | −4.6 (5.6) | −9.1 (10.2) |
| Median (IQR) | −3.7 (−7.2 to −1.4) | −3.8 (−7.4 to −1.3) | −2.2 (−4.7 to −0.6) | −3.2 (−6.2 to −1.3) | −6.0 (−12.0 to −3.3) | −3.8 (−7.2 to −1.4) | −1.9 (−4.2 to −0.1) | −3.7 (−6.9 to −1.6) | −6.2 (−11.4 to −3.2) |
CI, confidence interval; HR, hazard ratio; NA, not available; IQR, interquartile range.
0.44 g/g=50 mg/mmol (approximately 0.5 g/d); 0.88 g/g=100 mg/mmol (approximately 1 g/d); 1.76 g/g=200 mg/mmol (approximately 2 g/d).
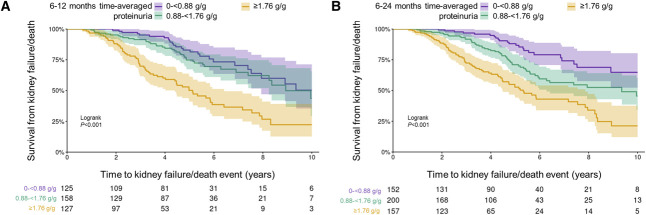
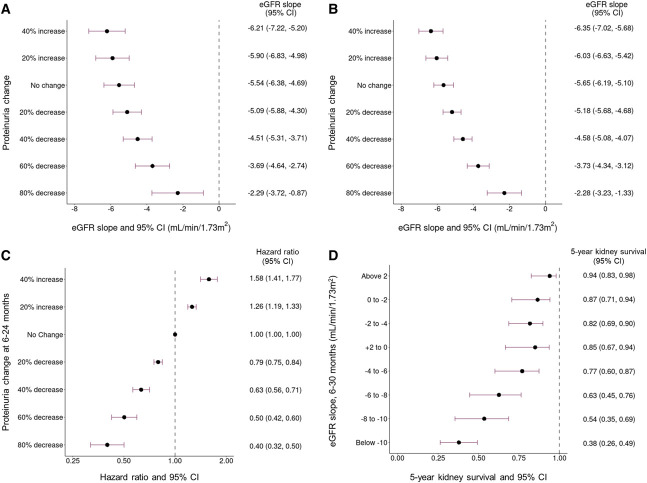
Kaplan–Meier survival analysis demonstrates that median kidney survival was significantly lower at time-averaged proteinuria ≥1.76 g/g using either the 6–12- or 6–24-month time-averaged proteinuria windows compared with lower time-averaged proteinuria (log-rank P < 0.001) (Figure 3). A decrease from baseline to 6–12-month time-averaged proteinuria was associated with a slower rate of eGFR loss; 40% and 60% reductions were estimated to reduce the rate of eGFR loss over 6–30 months from 5.5 ml/min per 1.73 m2 per year to 4.5 and 3.7 ml/min per 1.73 m2 per year, respectively (Figure 4A). The same pattern was observed in the total rate of eGFR loss when proteinuria was measured over 6–24 months (Figure 4B). Each 10% decrease in proteinuria from baseline was estimated to be associated with a hazard ratio (95% CI) for kidney failure/death of 0.89 (0.87 to 0.92) after adjusting for age, sex, baseline eGFR, and time from diagnosis to baseline (Figure 4C). Five-year kidney survival rates were lower with greater eGFR loss (6–30 months) (Figure 4D). Patients with an eGFR slope of 0–2 ml/min per 1.73 m2 per year had a 5-year kidney survival (95% CI) of 0.87 (0.71 to 0.94), compared with 0.54 (0.35 to 0.69) for those with a slope of 8–10 ml/min per 1.73 m2 per year.
Figure 3.
Kaplan–Meier survival curves of time to kidney failure/death event in population 4. (A) Using 6–12-month time-averaged proteinuria. (B) Using 6–24-month time-averaged proteinuria. 0.88 g/g=100 mg/mmol; 1.76 g/g=200 mg/mmol.
Figure 4.
Forest plots of population 4. (A) Percentage of change in proteinuria at 6–12-month time-averaged proteinuria versus 6–30-month eGFR slope. (B) Percentage of change in proteinuria at 6–24-month time-averaged proteinuria versus total eGFR slope. (C) Percentage of change in proteinuria at 6–24-month time-averaged proteinuria versus hazard ratio of kidney failure/death event. (D) 6–30-month eGFR slope versus 5-year kidney survival rate. CI, confidence interval.
Discussion
We assessed a large cohort of patients with biopsy-proven IgA nephropathy enrolled into RaDaR; 77% were White and 94% were adult, with a mean (SD) age at diagnosis (among adults) of 42 (14) years (Table 1). Using representative incident and prevalent populations, we provide insight into the burden of IgA nephropathy, lifetime kidney failure risk, and value of short-term proteinuria and eGFR slope assessments for predicting long-term outcomes.
Outcomes for both adult and pediatric cohorts were poor, with a median (95% CI) kidney survival of 10.8 (10.0 to 12.0) years and mean (SD) age at kidney failure of only 49 (14) years for adults and 21.6 (15.9 to not estimable) years and 27 (10) years, respectively, for those diagnosed while children (Table 1). When assessing adult incident (population 1) and prevalent (population 3) populations, we show that higher time-averaged proteinuria was associated with a greater likelihood of progressing to kidney failure more quickly. These findings in our predominantly White cohort are aligned with findings in other White cohorts7,19 and an Asian cohort.5 Also consistent with published literature,1,10 Asian ethnicity was found to be a risk factor for progression (Supplemental Tables 2 and 7).
Phase 2 and 3 RCTs in IgA nephropathy currently use proteinuria as a surrogate end point and typically recruit patients with baseline proteinuria >1 g/d (approximately equivalent to 0.88 g/g), which is perceived as high risk in accordance with KDIGO.11 However, focusing on patients with proteinuria >1 g/d means that long-term outcomes for those with lower proteinuria measurements remain largely unexplored. We show that in an incident population (population 1), approximately 20% of patients with time-averaged proteinuria <0.44 g/g and 30% with time-averaged proteinuria 0.44 to <0.88 g/g progressed to kidney failure within 10 years of diagnosis. A similar pattern was observed in a prevalent population (population 3) among patients with baseline UPCR <0.88 g/g, with higher time-averaged proteinuria associated with significantly poorer outcomes. These results demonstrate that IgA nephropathy cannot be considered a benign condition, even when proteinuria is <1 g/d. There was substantial improvement associated with time-averaged proteinuria <0.44 g/g on rate of eGFR loss and lifetime kidney failure risk in patients commonly perceived as being at low risk of progression (Table 3; Supplemental Table 5). This brings into question the appropriateness of using the KDIGO threshold of <1 g/d as a treatment target in IgA nephropathy.11 This is the first time that the substantial risk of kidney failure among patients currently perceived as low risk has been demonstrated in a predominantly White population. In a Chinese cohort, Le et al.5 demonstrated that proteinuria of 0.5 g/d could be a more appropriate risk threshold than 1 g/d.
Age at diagnosis steadily increased over the past 30 years (Figure 1B). This likely reflects the increasing threshold for kidney biopsy in the United Kingdom, driven by the perception that people with suspected IgA nephropathy and proteinuria <1 g/d are at low risk for progression and, irrespective of the biopsy findings, would not be eligible for therapy beyond renin-angiotensin-system inhibition, BP control, and lifestyle management. As our results show, this approach fails to identify patients with IgA nephropathy who are at significant risk of kidney failure, where early intervention could affect their lifetime kidney failure risk. In particular, disease-modifying therapies that specifically target the immune system are more likely to be effective early in the natural history of IgA nephropathy, before the kidneys accumulate significant irreversible fibrosis.
A striking observation in our cohort was that almost all patients with IgA nephropathy were expected to progress to kidney failure within their lifetime, regardless of age or eGFR at diagnosis. We show that eGFR decline of 3 ml/min per 1.73 m2 per year would result in 100% of patients diagnosed before 40 years of age reaching kidney failure. Even a decline of as little as 1 ml/min per 1.73 m2 per year would result in around 40% of patients diagnosed before 50 years of age reaching kidney failure. An eGFR decline of <1 ml/min per 1.73 m2 per year must be the target if patients are to avoid kidney failure. It is clear that current therapies do not provide a rate of eGFR loss even approaching this target: −3.5 ml/min per 1.73 m2 per year (dapagliflozin) and −4.7 ml/min per 1.73 m2 per year (control) (Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease [DAPA-CKD]),20 −2.5 ml/min per 1.73 m2 per year (methylprednisolone) and −5.0 ml/min per 1.73 m2 per year (control) (TESTING).21 We must, therefore, consider what can be done to reach this target rate of eGFR loss (e.g., earlier diagnosis, intervention at lower levels of proteinuria, new drugs, and new combinations) and how these approaches can be assessed in future RCTs. Short-term assessment of proteinuria in our RCT-representative population (population 4) predicted longer-term outcomes with a relatively high degree of certainty, supporting the validity of short-term proteinuria changes in RCTs as a surrogate end point. However, we also demonstrate that a more than 80% reduction in time-averaged proteinuria would be needed to achieve the target rate of eGFR decline in that population.
The results of this study must be considered in the context of several limitations inherent in registry analyses. The cohort was representative of patients managed in the United Kingdom and of the genetic and ethnic makeup of the UK population, and its generalizability to populations in other countries is unknown. Recruitment into RaDaR was initiated in 2013; survivor bias may have affected assessment of patients diagnosed earlier than 2013, while ascertainment bias may have reduced the chance of recruiting individuals presenting before 2013 whose clinical course was benign. Availability of patient medication and BP data was a limiting factor in this study. Future analysis of these crucial parameters will be essential to strengthen recommendations regarding the modification of existing patient care approaches.
To achieve the aspiration that no patient with IgA nephropathy should progress to kidney failure, the current approach to patient care needs to be re-evaluated. This will require a lower threshold for biopsy allowing earlier diagnosis and initiation of treatment before extensive, irreversible damage has occurred. We must also consider the use of combination therapies to maximize effectiveness. A lower proteinuria target for assessing treatment response and inclusion in clinical trials of patients presenting with proteinuria levels below 0.88 g/g (100 mg/mmol) should also be considered.
Disclosures
J. Barratt reports consultancy for Alnylam Pharmaceuticals, Argenx, Astellas, BioCryst, Calliditas, Chinook Therapeutics, Dimerix, Galapagos, GSK, Novartis, Omeros, Travere Therapeutics, UCB, Vera Therapeutics, and Visterra; research funding from Argenx, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Travere Therapeutics, and Visterra; and advisory or leadership roles on the Editorial Boards of CJASN, Clinical Science, Glomerular Diseases, and Kidney International. F. Braddon reports employment with UK Kidney Association and UK Renal Registry. D.P. Gale reports consultancy for Alexion, Britannia, Calliditas, Judo Bio, Novartis, Reata Inc, and Travere Therapeutics, Inc.; research funding from Novartis, Pfizer, Sanofi, and Travere; and other interests or relationships as Trustee for AlportUK and Chair of UK Kidney Association Rare Diseases Committee. B. Hendry reports employment with Travere Therapeutics, stock in Travere Therapeutics, an advisory or leadership role for South West Thames Institute for Renal Research (Board Chair), and other interests or relationships as Emeritus Professor, King's College London. A. Mercer reports employment with JAMCO Pharma Consulting AB and consultancy for Travere Therapeutics, Inc and Vera Therapeutics, Inc. K. Osmaston reports employment with UK Kidney Association. D. Pitcher reports employment with UK Kidney Association and current ongoing work as part of employment with UK Kidney Association to analyze data held in the UK National Registry of Rare Kidney Diseases (RaDaR) with Travere Therapeutics, Pfizer, and Sanofi. M.A. Saleem reports employment with University of Bristol; consultancy for Confo Therapeutics, Mission Therapeutics, Pfizer, Purespring Therapeutics, Retrophin, and Travere Therapeutics, Inc.; ownership interest in Purespring Therapeutics; research funding from Evotec, Retrophin, and UCB; honoraria from Purespring Therapeutics as Director and Chief Scientific Officer; patents or royalties from Purespring Therapeutics and University of Bristol; and advisory or leadership role as Director, Purespring Therapeutics. R. Steenkamp reports employment with UK Kidney Association, UK Renal Registry. A.N. Turner reports consultancy for Purespring 2021–current, Enyo Pharma from 2022, and Calliditas Therapeutics from 2023; advisory board/speaker for Shire-Takeda (2017–20) and Sanofi-Genzyme (2019–current); speakers bureau for staff education, Chiesi 2022; and other interests or relationships as Trustee and supporter of the patient-led charity Alport UK. K. Wang reports employment with and stock in Travere. K. Wong reports employment with UK Kidney Association.
Supplementary Material
Acknowledgments
RaDaR was established with funding from the MRC, Kidney Research UK, and Kidney Care UK.
Medical writing support was provided by Eve Hunter-Featherstone and David Cork, employees of Genesis Research (Newcastle upon Tyne, UK), which received funding from Travere Therapeutics, Inc. Expert statistical advice was provided by Kevin Carroll (KJC Statistics Ltd, Cheshire, UK).
Footnotes
See related editorial, “Prognosis of IgA Nephropathy: A Lifetime Story,” on pages 699–701.
Contributor Information
David Pitcher, Email: david.pitcher@renalregistry.nhs.uk.
Fiona Braddon, Email: f.braddon@gmail.com.
Bruce Hendry, Email: bruce.hendry@travere.com.
Alex Mercer, Email: alex.mercer@travere.com.
Kate Osmaston, Email: Kate.osmaston@ukkidney.org.
Moin A. Saleem, Email: m.saleem@bristol.ac.uk.
Retha Steenkamp, Email: retha.steenkamp@renalregistry.nhs.uk.
Katie Wong, Email: katie.wong@nhs.net.
A. Neil Turner, Email: Neil.Turner@ed.ac.uk.
Kaijun Wang, Email: kwang8535@gmail.com.
Daniel P. Gale, Email: d.gale@ucl.ac.uk.
Funding
Travere Therapeutics, Inc.
Author Contributions
Conceptualization: Jonathan Barratt, Daniel P. Gale, Bruce Hendry, Alex Mercer, A. Neil Turner.
Data curation: Fiona Braddon, Kate Osmaston, David Pitcher, Retha Steenkamp, Katie Wong.
Formal analysis: Fiona Braddon, Alex Mercer, Kate Osmaston, David Pitcher, Retha Steenkamp, Katie Wong.
Funding acquisition: Jonathan Barratt, Daniel P. Gale.
Investigation: Jonathan Barratt, Fiona Braddon, Daniel P. Gale, Bruce Hendry, Alex Mercer, Kate Osmaston, David Pitcher, Moin A. Saleem, Retha Steenkamp, A. Neil Turner, Kaijun Wang, Katie Wong.
Methodology: Jonathan Barratt, Daniel P. Gale, Bruce Hendry, Alex Mercer, David Pitcher, Moin A. Saleem, A. Neil Turner, Kaijun Wang.
Project administration: Fiona Braddon, Alex Mercer, Kate Osmaston, Retha Steenkamp, Katie Wong.
Supervision: Fiona Braddon.
Validation: David Pitcher, Kaijun Wang.
Writing – original draft: Jonathan Barratt, Alex Mercer.
Writing – review & editing: Jonathan Barratt, Fiona Braddon, Daniel P. Gale, Bruce Hendry, Alex Mercer, Kate Osmaston, David Pitcher, Moin A. Saleem, Retha Steenkamp, A. Neil Turner, Kaijun Wang, Katie Wong.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B758.
Supplemental Figure 1. Study population disposition.
Supplemental Figure 2. Venn diagram displaying the degree of overlap between proteinuria subpopulations.
Supplemental Table 1. Description of populations 1–4.
Supplemental Table 2. Univariable and multivariable analysis of 10-year survival and eGFR slope (full-analysis population).
Supplemental Table 3. Demographic and clinical characteristics at diagnosis and clinical outcomes during follow-up (populations 1–4).
Supplemental Table 4. Clinical outcomes of proteinuria analysis in population 1.
Supplemental Table 5. Clinical outcomes of proteinuria analysis in populations 2 and 3.
Supplemental Table 6. Clinical outcomes of proteinuria analysis in population 4.
Supplemental Table 7. Univariable and multivariable analysis of 10-year survival and eGFR slope (populations 1–4).
References
- 1.Canney M Barbour SJ Zheng Y, et al. . Quantifying duration of proteinuria remission and association with clinical outcome in IgA nephropathy. J Am Soc Nephrol. 2021;32(2):436–447. doi: 10.1681/ASN.2020030349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. doi: 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 3.Hastings MC Bursac Z Julian BA, et al. . Life expectancy for patients from the southeastern United States with IgA nephropathy. Kidney Int Rep. 2018;3(1):99–104. doi: 10.1016/j.ekir.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarrick S Lundberg S Welander A, et al. . Mortality in IgA nephropathy: a nationwide population-based cohort study. J Am Soc Nephrol. 2019;30(5):866–876. doi: 10.1681/ASN.2018101017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le W Liang S Hu Y, et al. . Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012;27(4):1479–1485. doi: 10.1093/ndt/gfr527 [DOI] [PubMed] [Google Scholar]
- 6.Global Health Observatory data repository. Life Expectancy and Healthy Life Expectancy Data by Country. World Health Organization. 2020. Accessed August 26, 2022. https://apps.who.int/gho/data/node.main.SDG2016LEX?lang=en [Google Scholar]
- 7.Reich HN, Troyanov S, Scholey JW, Cattran DC. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177–3183. doi: 10.1681/ASN.2007050526 [DOI] [PubMed] [Google Scholar]
- 8.Inker LA Heerspink HJL Tighiouart H, et al. . Association of treatment effects on early change in urine protein and treatment effects on GFR slope in IgA nephropathy: an individual participant meta-analysis. Am J Kidney Dis. 2021;78(3):340–349.e1. doi: 10.1053/j.ajkd.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inker LA Mondal H Greene T, et al. . Early change in urine protein as a surrogate end point in studies of IgA nephropathy: an individual-patient meta-analysis. Am J Kidney Dis. 2016;68(3):392–401. doi: 10.1053/j.ajkd.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 10.Thompson A Carroll K A Inker L, et al. . Proteinuria reduction as a surrogate end point in trials of IgA nephropathy. Clin J Am Soc Nephrol. 2019;14(3):469–481. doi: 10.2215/CJN.08600718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovin BH Adler SG Barratt J, et al. . KDIGO 2021 clinical Practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–s276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 12.Levin A Agarwal R Herrington W, et al. . International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int. 2020;98(4):849–859. doi: 10.1016/j.kint.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS Stevens LA Schmid CH, et al. . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ Muñoz A Schneider MF, et al. . New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins RC, Briganti EM, Zimmet PZ, Chadban SJ. Association between albuminuria and proteinuria in the general population: the AusDiab Study. Nephrol Dial Transplant. 2003;18(10):2170–2174. doi: 10.1093/ndt/gfg314 [DOI] [PubMed] [Google Scholar]
- 16.Collier G, Greenan MC, Brady JJ, Murray B, Cunningham SK. A study of the relationship between albuminuria, proteinuria and urinary reagent strips. Ann Clin Biochem. 2009;46(3):247–249. doi: 10.1258/acb.2009.008189 [DOI] [PubMed] [Google Scholar]
- 17.Methven S, MacGregor MS, Traynor JP, O'Reilly DS, Deighan CJ. Assessing proteinuria in chronic kidney disease: protein-creatinine ratio versus albumin-creatinine ratio. Nephrol Dial Transplant. 2010;25(9):2991–2996. doi: 10.1093/ndt/gfq140 [DOI] [PubMed] [Google Scholar]
- 18.Methven S, Traynor JP, Hair MD, O’Reilly DSJ, Deighan CJ, MacGregor MS. Stratifying risk in chronic kidney disease: an observational study of UK guidelines for measuring total proteinuria and albuminuria. QJM. 2011;104(8):663–670. doi: 10.1093/qjmed/hcr026 [DOI] [PubMed] [Google Scholar]
- 19.Coppo R Troyanov S Bellur S, et al. . Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86(4):828–836. doi: 10.1038/ki.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler DC Toto RD Stefánsson BV, et al. . A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100(1):215–224. doi: 10.1016/j.kint.2021.03.033 [DOI] [PubMed] [Google Scholar]
- 21.Lv J Wong MG Hladunewich MA, et al. . Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2022;327(19):1888–1898. doi: 10.1001/jama.2022.5368 [DOI] [PMC free article] [PubMed] [Google Scholar]