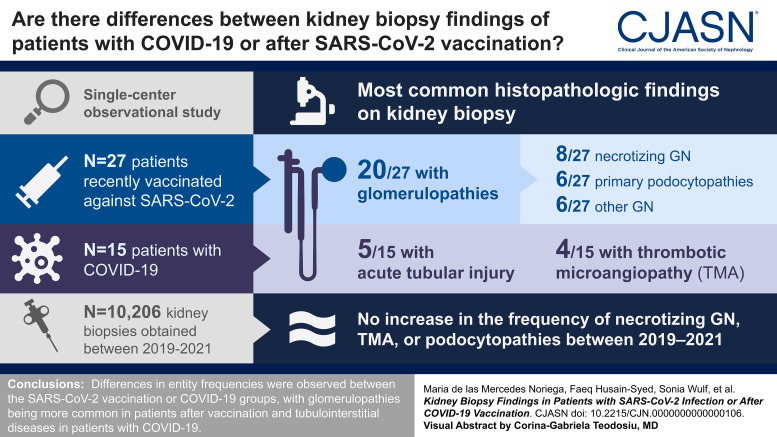
Visual Abstract
Keywords: vaccine, necrotizing vasculitis, pauci-immune, podocytopathy, thrombotic microangiopathy, COVID-19, SARS-CoV-2
Abstract
Background
Emerging case series described a temporal association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and de novo or relapsing kidney diseases. We aimed to further understand vaccination- and coronavirus disease 2019 (COVID-19)–associated kidney diseases.
Methods
We present findings from native kidney biopsies of patients recently vaccinated against SARS-CoV-2 (n=27) and those with COVID-19 (n=15), reviewed at a single German center. Diagnoses were compared among all native kidney biopsies (n=10,206) obtained between the prepandemic (2019), pandemic (2020), and vaccination periods (2021) to determine whether there was an increase in kidney diseases in the observed periods.
Results
Biopsy indication was increased serum creatinine and/or new-onset proteinuria. Glomerulopathies (20/27, 74%) were more common than tubulointerstitial diseases in postvaccination patients, with necrotizing GN (8/27, 30%) and primary podocytopathies and other GN types (6/27, 22% each) the most common forms. Acute tubular injury was the most common kidney disease in patients with COVID-19, followed by thrombotic microangiopathy (TMA) and necrotizing GN. The postvaccination and COVID-19 infection groups had similar kidney function recovery rates (69% and 73%, respectively). Furthermore, the frequencies of necrotizing GN, pauci-immune GN, TMA, or primary podocytopathies at our center did not increase between 2019 and 2021.
Conclusions
We observed differences in entity frequencies between the SARS-CoV-2 vaccination or COVID-19 groups, with glomerulopathies being more common in patients after vaccination and tubulointerstitial diseases in patients with COVID-19. Cases of TMA were observed only in the COVID-19 group. We detected no increase in the frequency of necrotizing GN, TMA, or podocytopathies between 2019 and 2021.
Clinical Trial registry name and registration number:
Kidney Histopathology After COVID-19 and SARS-CoV-2 Vaccination, NCT05043168
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_05_08_CJN_POD_EP_10_050823.mp3
Introduction
Kidney involvement is a part of the coronavirus disease 2019 (COVID-19) clinical spectrum and is associated with higher morbidity and mortality.1 Native kidney biopsy case series of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–infected patients have shown a wide spectrum of kidney manifestations, with acute tubular injury (ATI), thrombotic microangiopathy (TMA), myoglobin cast nephropathy, collapsing glomerulopathy, and proliferative GN with monoclonal immune deposits as the predominant findings.2–5
As SARS-CoV-2 vaccination rolled out worldwide, with more individuals being vaccinated than at any period in the past,6 emerging reports described a temporal association between the vaccines and heightened off-target immune responses potentially leading to de novo onset of kidney diseases or exacerbation of preexisting immunological disorders. Reported manifestations in native kidney biopsies include minimal change disease, noncollapsing FSGS, collapsing glomerulopathy, immunoglobulin A (IgA) nephropathy, ANCA-associated GN, antiglomerular basement membrane GN, membranous nephropathy, lupus nephritis, and interstitial nephritis (IN) (for individual frequencies, see Supplemental Figure 1). Although the exact mechanism of infection-related and vaccination-related exacerbation of kidney diseases is unknown, endothelial injury and complement activation might play a role.7 Furthermore, it remains unknown whether the frequency and the percentage distribution of biopsy findings differ between those vaccinated against SARS-CoV-2 and those with COVID-19.
In this study, we provide a series of 42 native kidney biopsy cases of patients in temporal association with SARS-CoV-2 vaccination (n=27) or diagnosed with COVID-19 (n=15), reviewed at a single center. We describe the clinical course and disease severity based on the patients' charts. We also sought to determine whether the observed kidney manifestations during the COVID-19 pandemic differed from the pre–COVID-19 era.
Methods
Study Design
This single-center study identified all native kidney biopsies from adult patients with the onset and/or progression of kidney disease potentially associated with SARS-CoV-2 vaccination or infection accessioned by the Nephropathology Section of the University Medical Center Hamburg-Eppendorf from September 1, 2020, to October 21, 2021. We analyzed 42 kidney biopsies (including one previously published8). The biopsies originated from 24 institutions in Germany (the number of biopsies per institution is provided in the Supplemental Methods). Inclusion criteria were onset and/or progression of kidney disease after SARS-CoV-2 vaccination or confirmed COVID-19 diagnosis by real-time reverse transcription urine protein-to-creatinine ratio as indicated by the submitting nephrologist. Cases in which the biopsy was collected before COVID-19 diagnosis or after recovery from COVID-19 were excluded.
The protocol was approved by the Ethics Committees of the University Medical Center Hamburg-Eppendorf (2021-100682-BO-ff) and Justus-Liebig-University Giessen (AZ-173/21) and complied with the tenets of the Declaration of Helsinki. Participants provided written informed consent. When written informed consent could not be obtained (e.g., the patient could not be reached or had died), we used the clinical data submitted with the biopsy samples following the national legislation (§12 Hamburgisches Krankenhausgesetz). This study was prospectively registered at ClinicalTrials.gov (Identifier: NCT05043168).
We used our kidney biopsy database from January 1, 2019, to December 31, 2021, to compare disease frequency of overall necrotizing GN, pauci-immune GN, TMA, and podocytopathies between the prepandemic (2019), pandemic (2020), and vaccination periods (2021). The Hamburg Nephropathology Section receives biopsies from all over Germany and is likely representative of the biopsied population in Germany.
Histologic Processing of Kidney Biopsies
Core needle biopsies were processed by standard techniques and divided for light and electron microscopy and immunohistochemistry (Supplemental Material and Supplemental Table 1).
Histopathologic Evaluation
Three nephropathologists evaluated all cases for the following parameters. Light microscopic parameters, including the presence of crescents, segmental sclerosis, and microangiopathic changes. Tubulointerstitial parameters included the degree of ATI and interstitial fibrosis and tubular atrophy (IF/TA). Vascular parameters, including arterial intimal fibrosis and arteriolar hyalinosis, were graded on a trace-to-3+ scale. Immunhistochemistry of fibrinogen, IgA, immunoglobulin G, immunoglobulin M, fibrinogen, C3, and C1q was graded on a trace-to-3+ scale, noting the deposit character and compartment. Electron microscopy assessment included the presence of deposits (subepithelial, subendothelial, or mesangial) and the degree of podocyte foot process effacement.
Clinical Evaluation
Once written consent was obtained, we contacted the submitting nephrologists and internists to obtain patient characteristics, and clinical, laboratory, and follow-up data, using predefined questionnaires (Supplemental Tables 2 and 3). Unresolved queries were directly communicated. SARS-CoV-2 vaccination regimen (BNT162b2 [Pfizer/BioNTech], mRNA-1273 [Moderna Biotech], ChAdOx1 nCoV-19 [Oxford/AstraZeneca], or Ad26.COV.S [Johnson & Johnson]), including date of vaccination and kidney biopsy, were also provided (see Supplemental Methods and Supplemental Tables 4 and 5 for detailed information on data collection). Furthermore, primary care physicians/nephrologists were contacted to obtain clinical and laboratory findings from before vaccination or SARS-CoV-2 infection and, if applicable, additional follow-up data not available at the index hospital. Finally, participants were recontacted to confirm or retrieve missing clinical data (a summary of the missing data is provided in Supplemental Tables 6 and 7). Follow-up duration was not prespecified; however, we aimed to obtain data covering the longest possible time during the study period.
Indications for kidney biopsy were recorded as any combination of AKI, AKI superimposed on CKD, nephrotic-range proteinuria, nephrotic syndrome, nephritic urinary sediment, or rapidly progressive GN, as previously described.9 AKI and CKD diagnosis was based on the 2012 Kidney Disease Improving Global Outcomes guidelines (Supplemental Methods).10,11 Definitions of kidney recovery, nephrotic syndrome remission, and baseline serum creatinine are provided in the Supplemental Material. An adjudication committee comprising three expert nephrologists (Christian F. Krebs, Horst-Walter Birk, and Claudio Ronco) blinded to clinical data reviewed all cases to confirm the baseline serum creatinine, AKI, and CKD diagnosis. Disagreements were resolved by discussion. Laboratory parameters, depending on availability, included serum creatinine and quantitative proteinuria, albuminuria, and α1-microglobulin excretion and hematuria (dipstick or sediment analyses; Supplemental Methods).
Statistical Analyses
Descriptive statistics are expressed as median (interquartile range [IQR]) or mean (range) for continuous variables and n (%) for categorical variables. Among cases with range of days from vaccination to the onset of symptoms, the average value was considered. Proportions of GN, TMA, and podocytopathies in 2019, 2020, and 2021 were compared using the chi-squared test.
Results
Postvaccination Cases
This study group included 27 patients (median age, 54 [IQR, 28–68] years; 56% male) recruited between March 2021 and October 2021 (Table 1 and Supplemental Table 4). Most patients were White (26/27, 96%). Fourteen had one or more comorbidities, including hypertension (HTN) (n=14) and diabetes (n=4). Five patients had CKD, in three of which it was biopsy-proven as nephrosclerosis/diabetic kidney disease (DKD), IgA nephropathy, and proteinase 3 (PR3)-ANCA–associated pauci-immune GN. In three patients, the CKD status could not be determined.
Table 1.
Coronavirus disease 2019–vaccinated patient characteristics, clinical information, and laboratory findingsa
| Pt. | Age, yr | Female Sex, n (%) | CKD Status, n (%) | Vaccine Regimen (First to Second), n (%) | Dose, n (%) | Days from Vaccination to Onset of Symptoms | Kidney Presentation/Biopsy Indication, n (%) | Days from Vaccination to Kidney Biopsy | Peak Serum Creatinine (mg/dl)b | Proteinuria (mg/g Creatinine)/Hematuria, n (%) | Serology/Laboratory Findings, n (%) | Treatment, n (%) | Outcome, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Necrotizing GN | |||||||||||||
| V1–V8 | 76 (62–82) | 3 (38) | Stable CKD: 2 (25); including one patient with biopsy-proven ANCA-associated GN | PB–PB: 5 (63) AZ–Mod: 1 (13) J&J: 1 (13) PB: 1 (13) |
Second: 6 (75) | 3 (2–6) | AKI with or without positive ANCA serology: 6 (75) Suspected MPO-ANCA–positive vasculitis/increased proteinuria: 1 (13) Increased PR3-ABs/nephritic urinary sediment: 1 (13) |
31 (14–47) | 4.4 (1.4–10.8) | 2767 (657–4703)/ 8 (100) |
Positive anti–MPO-ABs: 3 (38) Positive anti–PR3-ABs: 2 (25) Negative anti–MPO/-PR3-ABs: 3 (38) |
Cs+CYP: 6 (75) Cs: 1 (13) None: 1 (13) |
Maintenance dialysis: 3 (38) Short-term KRT; PKR: 1 (13) Progressive kidney disease: 1 (13) PKR: 2 (25) Clinical improvement: 1 (13) |
| Podocytopathies | |||||||||||||
| V9–V14 | 43 (26–56) | 3 (50) | Stable CKD (IgA nephropathy): 1 (17) | PB–PB: 5 (83) J&J: 1 (17) |
Second: 5 (83) | 8 (2–28) | Nephrotic syndrome: 5 (83) Nephrotic syndrome, AKI on CKD: 1 (17) |
69 (35–110) | 0.9 (0.8–1.9) | 7547 (4574–8216)/ 3 (50) |
None: 5 (83) Antinuclear antibodies 1:80 (anti-Sjögren's syndrome A/Rho positive); positive MPO-ABs: 1 (17) |
ACEi, Cs: 4 (67) ACEi, Cs+Tac: 1 (17) ACEi: 1 (17) |
Maintenance dialysis: 1 (17) Partial remission: 3 (50) Complete remission: 2 (33) |
| Other GN types | |||||||||||||
| V15–V20 | 32 (22–67) | 2 (33) | Stable CKD: 1 (17) | PB–PB: 2 (33) AZ–Mod: 1 (17) PB: 1 (17) J&J: 1 (17) AZ: 1 (17) |
Second: 2 (33); in both patients mild symptoms after first | 3 (2–5) | Nephrotic syndrome: 3 (50) CKD G2A1 of unknown etiology, nephritic urinary sediment: 1 (17) AKI, elevated proteinuria: 1 (17) AKI: 1 (17) |
59 (17–87) | 1.0 (0.6–1.8) | 1234 (587–8837)/ 6 (100) |
Positive serum anti–PLA2R-ABs: 2 (33) Negative serum anti–PLA2R-ABs: 1 (17) Serum anti–PLA2R-ABs not tested: 1 (17) Staphylococcus aureus bacteremia, positive ASO titer: 1 (17) None: 1 (17) |
ACEi, SGLT2i: 1 (17) ACEi: 3 (50) Cs: 1 (17) N/A: 1 (17) |
Progressive kidney disease: 1 (17) Stable disease: 2 (33) Partial remission: 1 (17) Clinical improvement: 1 (17) N/A: 1 (17) |
| ATI | |||||||||||||
| V21–V24 | 32 (20–57) | 2 (50) | Stable CKD (nephrosclerosis/DKD): 1 (25) | PB–PB: 2 (50) AZ: 1 (25) PB: 1 (25) |
Second: 2 (50) | 5 (1–12) | AKI: 3 (75) AKI on CKD: 1 (25) |
18 (6–46) | 5.0 (3.5–13.2) | 1200 (N/A in three patients)/ 1 (25; N/A in one patient) |
None: 3 (75; N/A in one patient) | None: 4 (100) | Short-term KRT; PKR: 1 (25) PKR: 2 (50) CKR: 1 (25) |
| IN | |||||||||||||
| V25–V27 | 54 (20–81) | 2 (67) | No CKD: 3 (100) | PB–PB: 2 (67) PB: 1 (33) |
Second: 2 (67) | 20 (2–50) | AKI: 3 (100) | 43 (22–98) | 4.0 (2.6–6.7) | 714 (508–920; N/A in one patient)/ 1 (33) |
None: 3 (100) | Cs: 2 (67) None: 1 (33) |
PKR: 3 (100) |
Values are median (interquartile range) or n (%). Pt., patient; PB, BNT162b2 (Pfizer/BioNTech); AZ, ChAdOx1 nCoV-19 (Oxford/AstraZeneca); Mod, mRNA-1273 (Moderna Biotech); J&J, Ad26.COV.S (Johnson & Johnson); MPO, myeloperoxidase; PR3, proteinase 3; ABs, antibodies; Cs, corticosteroids; CYP, cyclophosphamide; PKR, partial kidney recovery; IgA, immunoglobulin A; ACEi, angiotensin-converting enzyme inhibitor; Tac, tacrolimus; anti–PLA2R-AB, anti–M-type phospholipase A2 receptor antibody; ASO, antistreptolysin-O; SGLT2i, sodium-glucose cotransporter 2 inhibitor; N/A, not available; ATI, acute tubular injury; DKD, diabetic kidney disease; CKR, complete kidney recovery; IN, interstitial nephritis.
Detailed clinical and laboratory data on the investigated cases postvaccination are presented in Supplemental Table 4.
To convert the serum creatinine values to μmol/L, multiply by 88.4.
Necrotizing GN was the most common kidney disease in this group (8/27, 30%), followed by primary podocytopathies and other GN types (6/27, 22% each), ATI (4/27, 15%), and IN (3/27, 11%; Figure 1A and Table 2). Figure 2 and Supplemental Figure 2 show representative kidney biopsy findings of the postvaccination cases.
Figure 1.
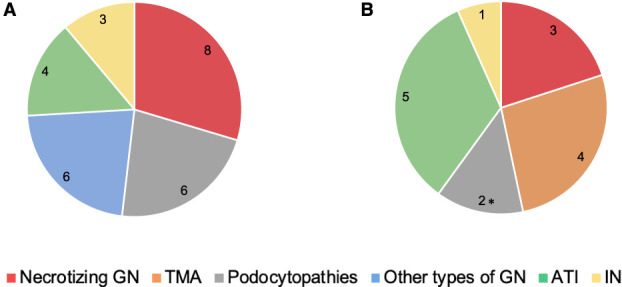
Frequencies of diagnosis in postvaccination and COVID-19 kidney biopsies. The figure illustrates biopsy-based kidney disease frequencies in patients with a temporal association to SARS-CoV-2 vaccination (A) or COVID-19 infection (B). *Including one case of collapsing glomerulopathy. ATI, acute tubular injury; COVID-19, coronavirus disease 2019; IN, interstitial nephritis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMA, thrombotic microangiopathy.
Table 2.
Pathology findings in kidney biopsies of coronavirus disease 2019–vaccinated patients
| Pt. | Diagnosis, n (%) | Other Findings, n (%) | Clinically Relevant Data, n (%) | Light Microscopy | Immunohistochemistry | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Glom | No. GS (% No. Glom) | No. SS (% No. Glom) | Fibrous Crescents (% No. Glom) | Cellular/Fresh Necrosis (% No. Glom) | ATI | IF/TA | AIF | AH | IgA | IgG | IgM | Fibrinogen | C3 | C1q | ||||
| Necrotizing GN | ||||||||||||||||||
| V1–V8 | Pauci-immune GN: 7 (88) Postinfectious/Parainfectious GN: 1 (13) |
Pauci-immune GN (biopsy-proven in 2014): 1 (13) | HTN: 5 (63) HTN/DM II: 2 (25) |
18 (8–34) | 4 (0%–75%) | 2 (0%–6%) | 2 (0%–5%) | 4 (5%–65%) | 46% (20%–90%) | 35% (0%–75%) | 2 (0–3); N/A in two patients | 1 (0–2) | 0 (0–1) | 0 (0–1) | 1 (0–1) | 0 (0–2) | 1 (0–3) | 1 (0–1) |
| Podocytopathies | ||||||||||||||||||
| V9–V14 | Minimal change disease: 4 (67) FSGS: 2 (33) |
IgA nephropathy: 2 (33) (with one patient biopsy-proven in 1991) | HTN: 1 (17) | 17 (4–35) | 1 (0%–14%) | 1 (0%–50%) | 0 (0%–50%) | 0 (0%) | 8% (0%–40%) | 11% (0%–50%) | 1 (0–2) | 1 (0–2) | 0 (0–1) | 1 (0–0) | 1 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–1) |
| Other types of GN | ||||||||||||||||||
| V15–V20 | IgA nephropathy: 1 (17) Membranous nephropathy, stages I–III versus II–IV, PLA2R1-AB–positivea: 2 (33) Membranous nephropathy, stages I–III versus stage not determinable, PLA2R1, THSD7A, and NELL1-AB–negativea: 2 (33) Postinfectious/parainfectious GN: 1 (17) |
ATI: 1 (17) | HTN: 2 (33) HTN/DM II: 1 (17) |
11 (6–16) | 1 (0%–20%) | 0 (0%–10%) | 0 (0%) | 0 (0%) | 0% (0%–70%) | 0% (0%–25%) | 0 (0–2); N/A in one patient | 1 (0–0) | 1 (0–3) | 1 (0–2) | 1 (0–1) | 1 (0–2) | 1 (0–2) | 1 (0–1) |
| ATI | ||||||||||||||||||
| V21–V24 | ATI: 4 (100) | IgA nephropathy: 1 (25) Nephrosclerosis/DKD (biopsy-proven in 2016): 1 (25) |
HTN/DM II: 1 (25) | 16 (3–25) | 3 (0%–48%) | 1 (0%–12%) | 0 (0%) | 0 (0%) | 65% (20%–90%) | 11% (0%–30%) | 2 (0–3) | 1 (0–2) | 0 (0–1) | 0 (0–0) | 1 (0–1) | 0 (0–1) | 0 (0–1) | 1 (0–1) |
| IN | ||||||||||||||||||
| V25–V27 | IN: 3 (100) | — | HTN: 2 (67) | 8 (4–5) | 0 (0%–7%) | 0 (0%) | 0 (0%) | 0 (0%) | 10% (10%) | 27% (0%–70%) | 0; N/A in two patients | 1 (0–2) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
Values are mean (range) or n (%). The immunohistology, arterial intimal fibrosis, and arteriolar hyalinosis findings are presented using a semiqualitative scoring system, ranging between 0 and 3, with a higher score indicating a more extensive staining. Pt., patient; Glom, glomeruli; GS, global sclerosis; SS, segmental sclerosis; ATI, acute tubular injury; IF/TA, interstitial fibrosis and tubular atrophy; AIF, arterial intimal fibrosis; AH, arteriolar hyalinosis; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; C3, complement C3; C1q, complement C1q; HTN, hypertension; DM, diabetes mellitus; DM II, type 2 DM; N/A, not assessable; PLA2R1-AB, M-type phospholipase A2 receptor 1 antibody; THSD7A, thrombospondin type I domain-containing 7A; NELL1, neural epidermal growth factor–like 1 protein; AB, antibody; DKD, diabetic kidney disease; IN, interstitial nephritis.
Membranous nephropathy stages were based on the localization of the electron-dense deposits according to the Churg and Ehrenreich classification.12
Figure 2.
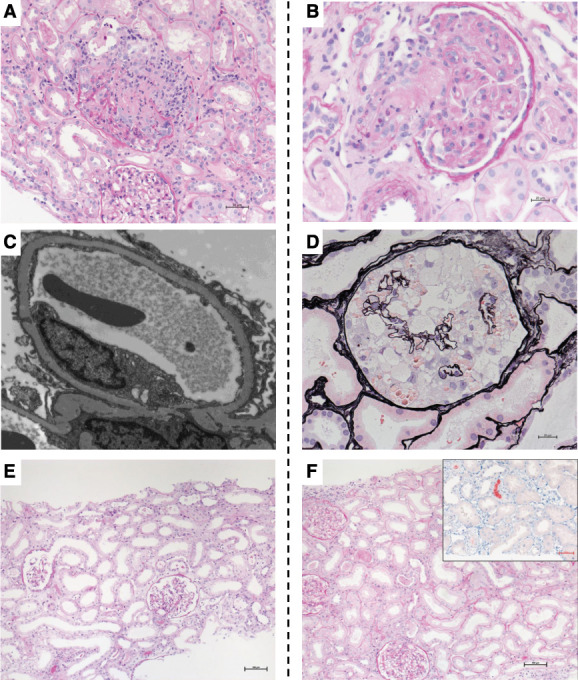
Representative kidney biopsy findings of postvaccination case and those with COVID-19. The figure illustrates representative kidney histopathology findings in patients after SARS-CoV-2 vaccination (panels A, C, E) or with COVID-19 (panels B, D, F). (A) Light microscopy image showing ANCA-associated necrotizing GN and ATI in case V1. PAS staining. Magnification, ×200. (B) Light microscopy image showing TMA in case C7. PAS staining. Magnification, ×200. (C) Diffuse foot process effacement (minimal changes) in case V11. Transmission electron microscopy. Magnification, ×8000. (D) Light microscopy image showing a collapsing glomerulopathy lesion characterized by glomerular epithelial cell hyperplasia and underlying glomerular capillary collapse in case C8 of African ancestry. Jones methenamine silver staining. Magnification, ×400. (E) Light microscopy image showing ATI with dilatation, flattened epithelium, and brush border defects in case V21 that had ATI. PAS staining. Magnification, ×100. (F) Light microscopy image showing ATI and myoglobin casts (inset shows myoglobin immunohistochemistry staining) in case C12. PAS. Magnification, ×100. PAS, periodic acid–Schiff.
Most cases (22/27, 82%) received an mRNA-based vaccine regimen (at least one mRNA-based vaccine dose). Patients receiving a vector-based regimen (i.e., Oxford/AstraZeneca or Johnson & Johnson) were more likely to present after the first dose (6/7, 86%), while most patients receiving an mRNA-based vaccine (i.e., Pfizer/BioNTech or Moderna Biotech) presented after the second dose (17/18, 95%). The median follow-up time was 20 (IQR, 15–31) days. For one patient, there was no available data beyond the day of biopsy.
Clinical Characteristics and Follow-Up by Disease
Necrotizing GN
Eight cases had necrotizing GN, seven newly diagnosed and one relapse. Of the cases, six had a de novo pauci-immune GN (three were myeloperoxidase [MPO]-ANCA–positive, one was PR3-ANCA–positive, and two were ANCA-negative), one had a relapse of PR3-ANCA–positive GN, and one had a postinfectious/parainfectious GN (Supplemental Figures 2B and 3). Kidney biopsy in all eight cases showed cellular/fresh necrosis, six of eight cases with fibrous crescents, while in two of eight, only cellular/fresh necrosis was seen.
Postvaccination symptoms included weakness, generalized pain, muscle weakness, fatigue, fever, and edema. All patients with de novo pauci-immune GN except case V3 received high-dose corticosteroids and cyclophosphamide, while case V8 with postinfectious/parainfectious GN received corticosteroids only. Four patients required acute KRT, with three ultimately referred to maintenance dialysis.
Podocytopathies
Six cases had de novo primary podocytopathies, including four with minimal change disease (Figure 2C) and two with FSGS (Supplemental Figure 2A). The median intervals from vaccination to symptom onset and kidney biopsy were 8 (IQR, 2–28) and 69 (IQR, 35–110) days, respectively.
Other Forms of GN
Six cases had other types of GN. Among these, four were diagnosed with membranous nephropathy (two PLA2R1-positive and two were negative for PLA2R1, THSD7A, and NELL1; Supplemental Figure 2, D and E), one with post/parainfectious GN, and one with IgA nephropathy. The median time from vaccination to symptom onset was 3 (IQR, 2–5) days. While the median time from vaccination to kidney biopsy in patients with nephrotic syndrome was 20 (IQR, 9–70) days, it was considerably longer (71 [IQR, 48–136] days) in the others.
Acute Tubular Injury
Four cases had ATI (one with stable CKD secondary to biopsy-proven nephrosclerosis/DKD).
Confounding factors that could represent additional triggers for ATI were present in two cases, including nonsteroidal anti-inflammatory drug (NSAID) use and intraocular anti-vascular endothelial growth factor injection (a treatment for diabetic macular edema established over a year before vaccination with no recent dosage or product change). Three patients experienced partial kidney recovery (PKR) (one required short-term KRT given the advanced AKI status with a peak serum creatinine of 15.6 mg/dl) and one experienced complete kidney recovery.
Interstitial Nephritis
Three cases had IN, with considerably long median times from vaccination to symptom onset and kidney biopsy (20 [IQR, 2–50] and 43 [IQR, 22–98] days, respectively). AKI was the main indication for biopsy. NSAID use and antibiotics were identified as confounding factors for ATI in two patients. None of the patients had a history of CKD, and all displayed PKR (two were treated with corticosteroids).
Patients with COVID-19
This is a study group of 15 patients (median age, 50 [IQR, 35–72] years), 47% male, recruited between September 2020 and October 2021 (Table 3 and Supplemental Table 5). None of the patients was vaccinated against SARS-CoV-2. All patients except one were White (14/15, 93%). The predominant comorbidities included HTN (n=8) and diabetes (n=3). Four patients had CKD, one of which with biopsy-proven FSGS; CKD status could not be determined in four patients. The median follow-up time was 13 (IQR, 8–34) days.
Table 3.
Characteristics, clinical information, and laboratory findings of patients with coronavirus disease 2019 who underwent kidney biopsya
| Pt. | Age, yr | Female Sex, n (%) | HTN/DM Present, n (%) | CKD Status, n (%) | Kidney Presentation/Biopsy Indication, n (%) | Peak Serum Creatinine (mg/dl)b | UPCR (mg/g Creatinine) | Dipstick Hematuria | Serology/Laboratory Findings, n (%) | Risk Factors for AKI (Other Than COVID-19), n (%) | Treatment, n (%) | Outcome, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Necrotizing GN | ||||||||||||
| C1–C3 | 81 (75–81) | 2 (69) | HTN/DM II: 2 (67) | CKD: 1 (33) | AKI with positive ANCA serology: 3 (100) | 4.2 (4.1–5.9) | 2778 (1915 –3640) |
2+ (1+ to 3+) | Positive anti–MPO-ABs: 2 (67) Positive anti–PR3-ABs: 1 (33) |
Respiratory failure due to pulmonary-renal syndrome: 1 (33) Acute decompensated heart failure: 1 (33) Sepsis, vasopressor use: 1 (33) |
Cs (for vasculitis)+RTx: 1 (33) Cs (for COVID-19 and vasculitis)+CYP: 1 (33) None: 1 (33) |
Short-term KRT; ultimately death: 1 (33) Short-term KRT; PKR: 1 (33) PKR: 1 (33) |
| TMA | ||||||||||||
| C4–C7c | 41 (28–50) | 3 (75) | HTN: 1 (25) | Stable CKD: 1 (25) | Postpartum AKI, suspected DIC/TMA: 2 (50) AKI with nephrotic syndrome, suspected TMA: 1 (25) AKI on CKD, suspected TMA: 1 (25) |
5.0 (3.5–7.6) | 8160 (1600 –14,720); N/A in one patient |
3+ (2+ to 3+); N/A in one patient | Thrombocytopenia, hemolytic anemia, positive fragmentocytes; no mutations in the complement regulators: 1 (25) Thrombocytopenia, hemolytic anemia: 1 (25) Heterozygous variant c.2792G>A p.(Cys931Tyr) (chr1:g.196709758G>A) in complement factor H: 1 (25) None: 1 (25) |
Peripartum hemorrhage with massive blood transfusion and clotting factor replacement; mechanical ventilation: 1 (25) Postpartum sepsis with multiple organ failure including cardiomyopathy, suspected HELLP syndrome/DIC, rhabdomyolysis, mechanical ventilation, vancomycin therapy: 1 (25) None: 2 (50) |
Plasmapheresis, eculizumab, Cs for COVID-19: 1 (25) Plasmapheresis, eculizumab, Cs for COVID-19: 1 (25) None: 2 (50) |
Maintenance dialysis: 1 (25) Short-term KRT; outpatient eculizumab treatment; CKR: 1 (25) Short-term KRT; PKR: 2 (50) |
| Podocytopathies | ||||||||||||
| C8–C9 | 49 (35–62) | 1 (50) | HTN/DM II: 1 (50) | CKD: 1 (50) | AKI on CKD: 1 (50) Nephrotic syndrome: 1 (50) |
2.8 (0.6–5.1) | 7000 and dipstick 3+ | 2+: 2 (100) | None: 2 (100) | (Patient of African origin): 1 (50) None: 1 (50) |
Cs for COVID-19: 1 (50) Cs for minimal change disease: 1 (50) |
PKR: 2 (100) |
| ATI | ||||||||||||
| C10–C14 | 50 (29–62) | 2 (40) | HTN: 4 (80) | Stable CKD (primary FSGS): 1 (20) | AKI on CKD, nephrotic syndrome: 1 (20) AKI, suspected TMA: 1 (20) AKI, NRP: 1 (20) AKI: 2 (40) |
11.5 (6.1–14.4) | 3551 (2175–22,970) | 3+ (1+ to 3+) | Positive circulating immune complexes: 1 (20) None: 4 (80) |
Noninvasive ventilation, critical illness: 2 (40) Piperacillin/tazobactam therapy; rhabdomyolysis: 1 (20) Frequent preadmission NSAID use; piperacillin-tazobactam therapy: 1 (20) None: 1 (20) |
Cs for COVID-19: 2 (40) None: 3 (60) |
Maintenance dialysis: 2 (40) Short-term KRT; PKR: 2 (40) PKR: 1 (20) |
| IN | ||||||||||||
| C15 | 32 | M | None | Unknown | AKI on suspected CKD | 7.7 | 460 | Negative dipstick | None | None | Cs for IN | PKR |
Values are median (interquartile range) or n (%). Pt., patient; HTN, hypertension; DM, diabetes mellitus; UPCR, urine protein-to-creatinine ratio; COVID-19, coronavirus disease 2019; DM II, type 2 DM; MPO, myeloperoxidase; ABs, antibodies; PR3, proteinase 3; Cs, corticosteroids; RTx, rituximab; CYP, cyclophosphamide; PKR, partial kidney recovery; TMA, thrombotic microangiopathy; DIC, disseminated intravascular coagulopathy; N/A, not available; HELLP, hemolysis, elevated liver enzymes, and low platelets; CKR, complete kidney recovery; ATI, acute tubular injury; NRP, nephrotic-range proteinuria; NSAID, nonsteroidal anti-inflammatory drug; IN, interstitial nephritis; M, male.
Detailed clinical and laboratory data on the investigated cases with COVID-19 are provided in Supplemental Table 5.
To convert the values for serum creatinine to μmol/L, multiply by 88.4.
The information provided on case C7 has been published in part.8
ATI was the most common kidney disease in this group (5/15, 33%), followed by TMA (4/15, 22%), necrotizing GN (3/15, 20%), primary podocytopathies (2/15, 13%), and IN (1/15, 7%; Table 4). Figure 2 and Supplemental Figure 2 show representative kidney biopsy histopathological findings in patients with COVID-19. We detected no viral particles in electron microscopy of any of our cases.
Table 4.
Pathology findings in kidney biopsies of patients with coronavirus disease 2019
| Pt. | Diagnosis, n (%) | Other Findings, n (%) | Clinically Relevant Data, n (%) | Light Microscopy | Immunohistochemistry | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Glom | No. GS (% No. Glom) | No. SS (% No. Glom) | Fibrous Crescents (% No. Glom) | Cellular/Fresh Necrosis (% No. Glom) | ATI | IF/TA | AIF | AH | IgA | IgG | IgM | Fibrinogen | C3 | C1q | Myoglobin | ||||
| Necrotizing GN | |||||||||||||||||||
| C1–C3 | Pauci-immune GN: 3 (100) | — | HTN/DM II: 2 (67) | 10 (11–43) | 10 (6%–58%) | 5 (6%–45%) | 4 (0%–45%) | 3 (0%–100%) | 50% (30%–80%) | 40% (10%–60%) | 2 (1–3) | 2 (1–3) | 0 (0–0) | 0 (0–1) | 1 (1–2) | 0 (0–0) | 0 (0–1) | 1 (0–2) | 0 (0–0) |
| TMA | |||||||||||||||||||
| C4–C7a | TMA: 4 (100) | ATI: 2 (50) ATI, myoglobin cast nephropathy: 1 (25) |
HTN: 1 (25) | 9 (5–13) | 2 (0%–6%) | 1 (0%–18%) | 0 (0%) | 0 (0%) | 65% (40%–80%) | 18% (5%–40%) | 1 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–1) | 2 (1–2) | 0 (0–1) | 1 (0–1) | 2 (1–3) | 0 (0–1) |
| Podocytopathies | |||||||||||||||||||
| C8–C9 | Collapsing FSGS: 1 (50) Minimal change disease: 1 (50) |
ATI: 1 (50) IgA nephropathy: 1 (50) |
HTN/DM II: 1 (50) | 17 (12–22) | 6 (17%–45%) | 2 (0%–14%) | 0 (0%) | 0 (0%) | 43% (10%–75%) | 15% (5%–25%) | 2 (1–3) | 1 (0–1) | 1 (0–1) | 0 (0–0) | 2 (1–2) | 1 (0–1) | 0 (0–0) | 2 (1–2) | 0 (0–0) |
| ATI | |||||||||||||||||||
| C10–C14 | ATI: 5 (100) | FSGS (biopsy-proven in 1991): 1 (20) Myoglobin cast nephropathy: 1 (20) IN: 1 (20) |
HTN: 4 (80) | 7 (3–13) | 1 (0%–67%) | 1 (0%–67%) | 0 (0%) | 0 (0%) | 70% (30%–90%) | 24% (5%–60%) | 1 (0–3) | 1 (0–2) | 0 (0–0); N/A in one case | 0 (0–0); N/A in one case | 1 (0–2); N/A in one case | 0 (0–0); N/A in one case | 0 (0–1); N/A in one case | 1 (0–2); N/A in one case | 0 (0–1) |
| IN | |||||||||||||||||||
| C15 | IN | — | — | 14 | 4 (29%) | 0 (0%) | 0 (0%) | 0 (0%) | 15% | 70% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
Values are mean (range) or n (%). The immunohistology, arterial intimal fibrosis, and AH findings are presented using a semiqualitative scoring system, ranging between 0 and 3, with a higher score indicating a more extensive staining. Pt., patient; Glom, glomeruli; GS, global sclerosis; SS, segmental sclerosis; ATI, acute tubular injury; IF/TA, interstitial fibrosis and tubular atrophy; AIF, arterial intimal fibrosis; AH, arteriolar hyalinosis; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; C3, complement C3; C1q, complement C1q; HTN, hypertension; DM, diabetes mellitus; DM II, type 2 DM; TMA, thrombotic microangiopathy; N/A, not assessable; IN, interstitial nephritis.
The information provided for case C7 has been published in part.8
Clinical Characteristics and Follow-Up by Disease
Thrombotic Microangiopathy
Four cases had TMA (one with an underlying CKD likely due to nephrosclerosis). The main indications for kidney biopsy were severe AKI necessitating acute KRT/suspected TMA (n=4) and nephrotic syndrome (n=1). Two of these cases had postpartum AKI with PKR at hospital discharge.
Necrotizing GN
Three cases had newly diagnosed necrotizing GN, two MPO-ANCA–positive and one PR3-ANCA–positive. The main indications for biopsy were AKI and suspected ANCA-positive rapidly progressive GN. All three patients had severe COVID-19 with multiple risk factors potentially associated with AKI; two patients required acute KRT, and one died after discontinuation of therapy following the patient's request. Notably, kidney biopsy in cases C1 and C2 showed advanced chronic changes, with fibrous crescents and 50%–60% IF/TA, suggesting, in part, the presence of vasculitis before the COVID-19 onset. Case C3 had only fresh glomerular lesions.
Podocytopathies
Two patients had primary podocytopathies; case C8, a patient of African origin, was diagnosed with collapsing glomerulopathy (Figure 2D), while case C9 had minimal change disease, with IgA nephropathy as a secondary finding.
Acute Tubular Injury and Interstitial Nephritis
Five cases had ATI, one of which with a stable CKD secondary to biopsy-proven FSGS. Other than case C10, who also had nephrotic syndrome secondary to an underlying FSGS, AKI was the main indication for biopsy. All patients except case C11 had severe COVID-19 with multiple risk factors potentially associated with AKI. Four patients required acute KRT, of which two ultimately underwent maintenance dialysis. Notably, case C12 also had associated myoglobin cast nephropathy (Figure 2F).
Case C15 presented with ANCA-negative severe AKI. The kidney biopsy showed IN with noncaseating non-necrotizing granulomatous components and advanced IF/TA (Supplemental Figure 2F). The causative factor for the granulomatous components and advanced changes was not determined (Supplemental Table 5). The kidney function partially recovered under corticosteroid treatment.
Frequency of Kidney Biopsy Diagnoses between 2019 and 2021
We did not detect an increase in the frequency of overall necrotizing GN, pauci-immune GN, TMA, and primary podocytopathies among all kidney biopsies obtained at our center between the prepandemic (2019), pandemic (2020), and vaccination periods (2021) (Table 5).
Table 5.
Annual frequency of necrotizing GN, pauci-immune GN, and podocytopathies between 2019 and 2021
| Final Diagnosis, n (% of Total Native Kidney Biopsies) | Year | |||
|---|---|---|---|---|
| 2019 | 2020 | 2021a | P Value | |
| Total number of native kidney biopsiesb | 3625 (100%) | 3280 (100%) | 3301 (100%) | |
| Non–pauci-immune necrotizing GN | 307 (9%) | 263 (8%) | 257 (8%) | 0.568 |
| Pauci-immune necrotizing GN | 337 (9%) | 299 (9%) | 246 (8%) | 0.012 |
| TMA | 57 (2%) | 56 (2%) | 63 (2%) | 0.561 |
| Podocytopathies (minimal change disease/primary FSGS) | 207 (6%) | 162 (5%) | 165 (5%) | 0.272 |
The table shows the annual frequencies of overall necrotizing GN, pauci-immune GN, TMA, podocytopathies, immunoglobulin A nephropathy, and membranous nephropathy among all kidney biopsies analyzed at the study center in 2019, 2020, and 2021. TMA, thrombotic microangiopathy.
All biopsies from January 1, 2021, to December 31, 2021, were considered.
Total number of native kidney biopsies included immunoglobulin A nephropathy (year 2019/2020/2021: 608 [17%]/503 [15%]/445 [14%]), membranous nephropathy (2019/2020/2021: 251 [7%]/217 [7%]/150 [5%]), and other entities (e.g., diabetic nephropathy, nephrosclerosis, membranoproliferative GN, C3-associated GN, light chain nephropathy, lymphoma-associated nephropathy, interstitial nephritis, and hereditary and congenital kidney diseases).
Discussion
As mass vaccination against COVID-19 is pursued worldwide, safety data continue to report GN and nephrotic syndrome cases after vaccination.13,14 Many cases of kidney disease in association with SARS-CoV-2 vaccination have been reported.15 There are also sporadic reports linking kidney diseases (e.g., ANCA-associated GN, minimal change disease, membranous nephropathy, IgA nephropathy) with other vaccines, particularly the influenza vaccine and also meningococcal C-conjugate vaccine.16–24
Our single-center clinicopathologic study in Germany included native kidney biopsies of patients with kidney diseases occurring in temporal association with SARS-CoV-2 vaccination or COVID-19 infection. The biopsy indication in all patients was increased serum creatinine and/or new-onset proteinuria. Glomerulopathies were more common than tubulointerstitial diseases in postvaccination patients. Among the glomerulopathies, there were eight cases of necrotizing GN, five ANCA-associated pauci-immune forms, and six with podocytopathies. Patients with COVID-19 infection showed a different frequency distribution, with ATI being the commonest kidney disease, followed by TMA, necrotizing GN, primary podocytopathies, and IN.
The case number was larger in our postvaccination group than our COVID-19 group; however, many more people were vaccinated than infected during the sampling period, so the relative frequency of kidney disease after vaccination is likely much lower after SARS-CoV-2 infection. We did not detect an increase in the frequencies of necrotizing GN, pauci-immune GN, TMA, or primary podocytopathies between the prepandemic (2019), pandemic (2020), and vaccination periods (2021). Notably, the total number of kidney biopsies was decreased in 2020–2021 (with a significant drop in pauci-immune GN in 2021 compared with 2019), which likely reflects lockdown measures and fewer medical visits during the pandemic when potential kidney disease could be identified in the population.25 These observations indicate that if there is any association between vaccination and autoimmune kidney disease, it is exceedingly rare, given the millions of people vaccinated. Our results are consistent with those of a recent multicenter Swiss study, in which no increase in the incidence of new-onset GN was detected after SARS-CoV-2 vaccination.26
Other studies have reported IgA nephropathy as the most frequent GN after SARS-CoV-2 vaccination.15,27 Conversely, we found only one patient with IgA nephropathy in our vaccination group. It has been reported that COVID-19 can trigger an IgA response in the bronchial mucosa.28 However, it is unclear how a nonmucosal vaccine could trigger this response. Nevertheless, gross hematuria after SARS-CoV-2 vaccination in patients with IgA nephropathy has been described29–31 and seems unrelated to the SARS-CoV-2 antispike IgG antibody response.32 Notably, patients with IgA nephropathy have previously been reported to have a stronger IgA1 response to intramuscular influenza vaccine than healthy participants.22
The temporal relationship between the SARS-CoV-2 vaccines and de novo or relapsing kidney diseases, if any, has been explained by various mechanisms, including molecular mimicry between the SARS-CoV-2 spike protein and self-antigens in the kidney,15 T cell–mediated podocyte injury,33 polyclonal activation, or a transient systemic proinflammatory cytokine response, which might provoke and/or unmask underlying autoimmune diseases in genetically predisposed individuals.34 Reports on adverse effects of SARS-CoV-2 vaccines are analogous to recent observations that infection with SARS-CoV-2 could be associated with flares of underlying autoimmune glomerular diseases.3 It is noteworthy that most of the patients in our study with pauci-immune GN had fibrous crescents, suggesting that an immune response to vaccination or COVID-19 could trigger an exacerbation of preexisting (subclinical) necrotizing GN. ANCA-associated vasculitis has been seen with certain medications such as hydralazine,35 infections, and cancers. There is also evidence that ANCA-activated neutrophils, for example by cytokines, can attack small vessels.36 However, although the timing of vaccination and new-onset kidney disease suggests an association, recent studies on SARS-CoV-2 vaccination indicate that these events are uncommon and likely coincidental, with attribution bias.26 Overall, given that existing reports involve both mRNA-based and vector-based vaccines, the potential mechanism of vaccine-associated kidney disease, if any, probably does not involve a direct effect of the vaccine but rather a nonspecific activation of the immune system.
The groups in our series showed similar kidney function recovery rates (postvaccination, 69%; COVID-19 infection, 73%). However, we observed some frequency differences between the groups, especially in TMA, present in the infection group but not the vaccination group. These might point to differences in the pathogenesis of kidney disease after vaccination and after COVID-19 infection, for example, heightened endothelial injury, microvascular thrombosis, and stronger complement activation after infection.38 Direct infection of podocytes and the proximal tubular epithelium with SARS-CoV-2 has also been suggested to play a role,39–41 but the literature on this topic is controversial.3,41–44 We did not detect viral particles in electron microscopy of any of our cases.
Although the presence of APOL1 risk alleles confers a higher risk of collapsing glomerulopathy,45 we did not systematically genotype our cohort given that it was a mainly White German population. Notably, however, one of the two patients with COVID-19 and FSGS had a collapsing glomerulopathy and was of African descent, making the APOL1 high-risk genotype plausible in that patient.
Our study has some limitations. First, variation in biopsy practice among centers and nephrologists limits the generalizability of our results. In particular, the COVID-19 pandemic may have disrupted care delivery, including the postponement of kidney biopsies25; this likely contributed to the lower number of kidney biopsy referrals as seen in our results. Second, it is noteworthy that the first kidney biopsy linked with COVID-19 was submitted in September 2020—approximately 6 months after the beginning of the pandemic. Although there are various possible reasons for this delay, we hypothesize that it can be attributed to an abundance of caution with anti-infection precautions at the onset of the pandemic, scheduling hurdles, and the initial lack of knowledge about COVID-19–associated kidney disease. To our knowledge, there is no German kidney biopsy guideline to assist clinicians and standardize care for patients with COVID-19. Third, our assumption of kidney disease in temporal association with vaccination was based on clinical data submitted with the biopsy samples, thus making selection bias likely. Fourth, we stated that there was no increase in the percentage of various kidney diagnoses on biopsy between 2019 and 2021. However, this may understate any possible link, considering this percentage is of a shrinking denominator (i.e., fewer kidney biopsies). Fifth, owing to the limited follow-up time and small cohorts, we were unable to identify histologic findings associated with kidney outcome. Finally, given the observational study design, information on SARS-CoV-2–specific humoral immune response and cytokine production after vaccination was only available in some cases.
In conclusion, we provide a series of native kidney biopsy cases of patients in temporal association with SARS-CoV-2 vaccination or the COVID-19 pandemic. We observed differences in entity frequencies between the two groups, with glomerulopathies being more common in patients after vaccination and tubulointerstitial diseases in patients with COVID-19. Cases of TMA were observed only in the COVID-19 group. We detected no change in the frequency of necrotizing GN, pauci-immune GN, TMA, or primary podocytopathies between the prepandemic, COVID-19 pandemic, and SARS-CoV-2 vaccination periods. Although the timing of vaccination and new-onset kidney disease suggests an association, epidemiologic surveys suggest that these events are uncommon and likely coincidental. However, although definitive causality is difficult to establish, greater awareness of potential adverse effects of vaccination is called for, and their frequencies should be determined. Important questions remain regarding the utility and risks of immunosuppressants for autoimmune kidney diseases or subsequent vaccine doses. The authors caution that isolated reports and small case series should not lead to vaccine hesitation during this pandemic because the benefits of vaccination greatly outweigh its potential risks. The risk of developing or exacerbating kidney diseases is likely much higher during SARS-CoV-2 infection than after vaccination.
Supplementary Material
Acknowledgments
The authors declare that the results presented in this paper except case C7 have not been published previously in whole or part.
The authors thank the nursing staff and physicians of all participating centers for their hard work and commitment to patient well-being. Without their support, this work would not have been possible.
We acknowledge the individual contributions of the CoV-Kidney Investigators (the respective affiliations are provided in the Supplemental Material): Provided care for study patients and collected and/or analyzed data: Frank Aedtner, MD, Susanne Bartel-Kuss, MD, Alexander Bauer, MD, Bernhard Böll, MD, Philipp Brylka, MD, Wolfgang Clasen, MD, Maria-Magdalena Eicher, MD, Amr Elnaggar, MD, Alexander Farid, MD, Johannes Francois, MD, Jens Gerth, MD, Birgit Jennert, MLA, Christoph Jüttner, MD, Kazuhiro Kobayashi, MD, Monika Koop, MD, Kamil Kuczkowski, MD, Martin Loyen, MD, Cornelia Marczynski, MD, Martin Nitschke, MD, Norbert Schleucher, MD, Diana Schmerler, MD, Elisa Alba Schmidt, MD, Michael Schmitz, MD, Lorenz Sellin, MD, Fabian Srugies, MD, Agnieszka Swiecicka, Sibylle von Vietinghoff, MD, Clemens Weinberg, MD, Ulrich Wenzel, MD, and Stephan Christian Werth, MD. Provided care for study patients: Mustafa Bačinović, MD, Joachim Beige, MD, Horst-Walter Birk, MD, Süha Dasdelen, MD, Annette Helmke, MD, Helen Hepburn, MD, Markus Hollenbeck, MD, Tobias B. Huber, MD, Akel Khaled, MD, Maida Mahmud, MD, Tobias N. Meyer, MD, Michael A. Reiter, MD, Rüdiger Schmidt, MD, Werner Seeger, MD, and Jochen Selbach, MD. Adjudicated kidney function: Horst-Walter Birk, MD and Claudio Ronco, MD. Served as scientific advisor: Claudio Ronco, MD.
Footnotes
The list of nonauthor contributors is extensive and has been provided in Supplemental Material.
M.d.l.M.N. and F.H.-S. are joint first authors.
See related Patient Voice, “Mississippi View of COVID-19 and Kidney Failure,” on pages 549–550.
Contributor Information
Collaborators: Frank Aedtner, Mustafa Bačinović, Susanne Bartel-Kuss, Alexander Bauer, Joachim Beige, Horst-Walter Birk, Bernhard Böll, Philipp Brylka, Wolfgang Clasen, Süha Dasdelen, Maria-Magdalena Eicher, Amr Elnaggar, Alexander Farid, Johannes Francois, Jens Gerth, Annette Helmke, Helen Hepburn, Markus Hollenbeck, Tobias B. Huber, Birgit Jennert, Christoph Jüttner, Akel Khaled, Kazuhiro Kobayashi, Monika Koop, Kamil Kuczkowski, Martin Loyen, Maida Mahmud, Cornelia Marczynski, Tobias N. Meyer, Martin Nitschke, Michael A. Reiter, Claudio Ronco, Norbert Schleucher, Diana Schmerler, Elisa Alba Schmidt, Rüdiger Schmidt, Michael Schmitz, Werner Seeger, Jochen Selbach, Lorenz Sellin, Fabian Srugies, Agnieszka Swiecicka, Sibylle von Vietinghoff, Clemens Weinberg, Ulrich Wenzel, and Stephan Christian Werth
Disclosures
W.J. Jabs reports honoraria from Alexion, Sanofi-Genzyme, StadaPharm, and VIFOR PHARMA, all unrelated to this work. T. Wiech reports honoraria from Alexion, Bayer, GlaxoSmithKline GmbH, and Novartis; speaker's fees from Alexion, Bayer, GlaxoSmithKline GmbH, and Novartis; patent EP 3771468 (unrelated to this work); and advisory or leadership roles for Novartis and Retrophin. T. Wiech is supported by a grant from the Deutsche Forschungsgemeinschaft as part of the collaborative research program “Immune-Mediated Glomerular Diseases” SFB 1192, Projects B6 and C1 as well as the Bundesministerium für Bildung und Forschung (BMBF), Netzwerk Universitätsmedizin (DEFEAT PANDEMIcs). S. Wulf reports patent EP 3771468, unrelated to this work. P.F. Zipfel reports consultancy agreements with Alexion, Bayer, eleva GMBH, Generic Assays, Novartis, Samsung Bioepis, and Vifor Fresenius Medical Care Renal Pharma; research funding from Bayer Health Care, Collaborative Research Programm, DAAD, Deutsche Forschungsgemeinschaft, DFG, Eleva GMBH, German Academic Exchange Service, German Research Council, Kidneeds, Iowa City, USA, Leibniz Institute für Naturstoff Forschung und Infektionsbiologie, and SFB 1192; honoraria from Alexion Pharmaceuticals, Bayer Health Care, Eleva GMBH, Generic Assays, Novartis, and Vifor Fresenius Medical Care; patents or royalties from CSL Behring and Leibniz Institute for Natural Product Research; advisory or leadership roles for Novartis and Samsung Bioepis; speakers bureau for Alexion Pharmaceuticals, Bayer Health Care, eleva GMBH, Novartis, and Vifor Fresenius Medical Care; and other interests or relationships with Patient Advocacy Group in Germany. All remaining authors have nothing to disclose.
Funding
Deutsche Forschungsgemeinschaft (Grant/Award Number: “CRC 1192 [project B6]”), Netzwerk Universitätsmedizin (DEFEAT PANDEMIcs) (Grant/Award Number: “01KX2021”).
Author Contributions
Conceptualization: H.-J. Gröne, F. Husain-Syed, M. de las Mercedes Noriega, T. Wiech.
Data curation: B. Csala, H.-J. Gröne, F. Husain-Syed, W.J. Jabs, C.F. Krebs, M. de las Mercedes Noriega, T. Wiech, S. Wulf, P.F. Zipfel.
Formal analysis: H.-J. Gröne, F. Husain-Syed, M. de las Mercedes Noriega, T. Wiech, S. Wulf.
Investigation: B. Csala, H.-J. Gröne, F. Husain-Syed, W.J. Jabs, C.F. Krebs, M. de las Mercedes Noriega, T. Wiech, S. Wulf, P.F. Zipfel.
Methodology: B. Csala, H.-J. Gröne, F. Husain-Syed, W.J. Jabs, C.F. Krebs, M. de las Mercedes Noriega, T. Wiech, P.F. Zipfel.
Project administration: F. Husain-Syed, M. de las Mercedes Noriega, T. Wiech.
Resources: T. Wiech.
Supervision: T. Wiech.
Visualization: F. Husain-Syed, M. de las Mercedes Noriega, T. Wiech.
Writing – original draft: H.-J. Gröne, F. Husain-Syed, M. de las Mercedes Noriega, T. Wiech.
Writing – review & editing: B. Csala, H.-J. Gröne, F. Husain- Syed, W.J. Jabs, C.F. Krebs, M. de las Mercedes Noriega, T. Wiech, S. Wulf, P.F. Zipfel.
Data Sharing Statement
The data used for the work are available from the corresponding author on reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B640.
Supplemental Table 1. Immunohistochemistry staining protocols.
Supplemental Table 2. Questionnaire on patients with COVID-19 vaccination.
Supplemental Table 3. Questionnaire on patients with SARS-CoV-2 infection.
Supplemental Table 4. Demographic data, clinical information, and laboratory findings of patients who underwent kidney biopsy after receiving COVID-19 vaccination.
Supplemental Table 5. Demographic data, clinical information, and laboratory findings of patients with SARS-CoV-2 infection who underwent kidney biopsy.
Supplemental Table 6. Summary of missing data for patients with COVID-19 vaccination.
Supplemental Table 7. Summary of missing data for patients with SARS-CoV-2 infection.
Supplemental Figure 1. Biopsy-based kidney disease frequencies reported in the literature in patients with a temporal association to SARS-CoV-2 vaccination.
Supplemental Figure 2. Kidney histopathological findings seen in patients after SARS-CoV-2 vaccination (A–E) or with COVID-19 (F).
Supplemental Figure 3. A postvaccinated patient with maculopapular rash and palpable purpura indicative of leukocytoclastic vasculitis before corticosteroid treatment (A) and displaying clinical improvement during treatment with corticosteroids (B).
References
- 1.Hirsch JS Ng JH Ross DW, et al. . Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P Uppal NN Wanchoo R, et al. . COVID-19-Associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958. doi: 10.1681/ASN.2020050699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudose S Batal I Santoriello D, et al. . Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akilesh S Nast CC Yamashita M, et al. . Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77(1):82–93.e1. doi: 10.1053/j.ajkd.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May RM Cassol C Hannoudi A, et al. . A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID-19). Kidney Int. 2021 10;100(6):1303–1315. doi: 10.1016/j.kint.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Coronavirus disease (COVID-19) dashboard. Accessed November 14, 2021. https://covid19.who.int.
- 7.Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol. 2022;22(2):77–84. doi: 10.1038/s41577-021-00665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufeld JK Reinhardt M Schroder C, et al. . Atypical hemolytic and uremic syndrome triggered by infection with SARS-CoV2. Kidney Int Rep. 2021;6(10):2709–2712. doi: 10.1016/j.ekir.2021.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choung HYG Bomback AS Stokes MB, et al. . The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int. 2019;95(3):647–654. doi: 10.1016/j.kint.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(suppl 1):1–138. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. doi: 10.1038/kisup.2012.73 [DOI] [Google Scholar]
- 12.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3(3):905–919. doi: 10.2215/CJN.04321007 [DOI] [PubMed] [Google Scholar]
- 13.European Medicines Agency. COVID-19 Vaccine Safety Update October 6, 2021. COMIRNATY BioNTech Manufacturing GmbH. Accessed November 21, 2021. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-6-october-2021_en.pdf. [Google Scholar]
- 14.European Medicines Agency. COVID-19 Vaccine Safety Update October 6, 2021. SPIKEVAX Moderna Biotech Spain, S.L. Accessed November 21, 2021. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-spikevax-previously-covid-19-vaccine-moderna-6-october-2021_en.pdf. [Google Scholar]
- 15.Caza TN Cassol CA Messias N, et al. . Glomerular disease in temporal association with SARS-CoV-2 vaccination: a series of 29 cases. Kidney360. 2021;2(11):1770–1780. doi: 10.34067/KID.0005372021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffs LS, Peh CA, Jose MD, Lange K, Hurtado PR. Randomized trial investigating the safety and efficacy of influenza vaccination in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2015;20(5):343–351. doi: 10.1111/nep.12416 [DOI] [PubMed] [Google Scholar]
- 17.Kielstein JT, Termuhlen L, Sohn J, Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol. 2000;54(3):246–248. [PubMed] [Google Scholar]
- 18.Gutiérrez S Dotto B Petiti JP, et al. . Minimal change disease following influenza vaccination and acute renal failure: just a coincidence? Nefrologia. 2012;32(3):414–415. doi: 10.3265/Nefrologia.pre2012.Feb.11370 [DOI] [PubMed] [Google Scholar]
- 19.Kutlucan A, Gonen I, Yildizhan E, Aydin Y, Sav T, Yildirim U. Can influenza H1N1 vaccination lead to the membranous glomerulonephritis? Indian J Pathol Microbiol. 2012;55(2):239–241. doi: 10.4103/0377-4929.97893 [DOI] [PubMed] [Google Scholar]
- 20.Shah H, Patel C. Membranous nephropathy and severe acute kidney injury following influenza vaccination. Saudi J Kidney Dis Transpl. 2015;26(6):1289–1293. doi: 10.4103/1319-2442.168676 [DOI] [PubMed] [Google Scholar]
- 21.Banerjee S, Dissanayake PV, Abeyagunawardena AS. Vaccinations in children on immunosuppressive medications for renal disease. Pediatr Nephrol. 2016;31(9):1437–1448. doi: 10.1007/s00467-015-3219-y [DOI] [PubMed] [Google Scholar]
- 22.van den Wall Bake AW Beyer WE Evers-Schouten JH, et al. . Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest. 1989;84(4):1070–1075. doi: 10.1172/JCI114269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel C, Shah HH. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl. 2019;30(5):1002–1009. doi: 10.4103/1319-2442.270254 [DOI] [PubMed] [Google Scholar]
- 24.Abeyagunawardena AS, Goldblatt D, Andrews N, Trompeter RS. Risk of relapse after meningococcal C conjugate vaccine in nephrotic syndrome. Lancet. 2003;362(9382):449–450. doi: 10.1016/s0140-6736(03)14072-x [DOI] [PubMed] [Google Scholar]
- 25.Hakroush S, Tampe D, Korsten P, Tampe B. Impact of the COVID-19 pandemic on kidney diseases requiring renal biopsy: a single center observational study. Front Physiol. 2021;12:649336. doi: 10.3389/fphys.2021.649336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diebold M Locher E Boide P, et al. . Incidence of new onset glomerulonephritis after SARS-CoV-2 mRNA vaccination is not increased. Kidney Int. 2022;2538(22):00697–704. doi: 10.1016/j.kint.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bomback AS, Kudose S, D'Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78(4):477–480. doi: 10.1053/j.ajkd.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan Ali O Bomze D Risch L, et al. . Severe coronavirus disease 2019 (COVID-19) is associated with elevated serum immunoglobulin (Ig) A and antiphospholipid IgA antibodies. Clin Infect Dis. 2021;73(9):e2869–e2874. doi: 10.1093/cid/ciaa1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negrea L, Rovin BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99(6):1487. doi: 10.1016/j.kint.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahim SEG, Lin JT, Wang JC. A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination. Kidney Int. 2021;100(1):238. doi: 10.1016/j.kint.2021.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park K, Miyake S, Tai C, Tseng M, Andeen NK, Kung VL. Letter regarding: “A case of gross hematuria and IgA nephropathy flare-up following SARS-CoV-2 vaccination.” Kidney Int Rep. 2021;6(8):2246–2247. doi: 10.1016/j.ekir.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrin P, Bassand X, Benotmane I, Bouvier N. Gross hematuria following SARS-CoV-2 vaccination in patients with IgA nephropathy. Kidney Int. 2021;100(2):466–468. doi: 10.1016/j.kint.2021.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332–345. doi: 10.2215/CJN.05000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeffs LS, Nitschke J, Tervaert JWC, Peh CA, Hurtado PR. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin Rheumatol. 2016;35(4):943–951. doi: 10.1007/s10067-015-3073-0 [DOI] [PubMed] [Google Scholar]
- 35.Pendergraft WF, III, Niles JL. Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol. 2014;26(1):42–49. doi: 10.1097/bor.0000000000000014 [DOI] [PubMed] [Google Scholar]
- 36.Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol. 2017;12(10):1680–1691. doi: 10.2215/CJN.02500317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Legrand M Bell S Forni L, et al. . Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–764. doi: 10.1038/s41581-021-00452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun F, Huber TB, Puelles VG. Proximal tubular dysfunction in patients with COVID-19: what have we learnt so far? Kidney Int. 2020;98(5):1092–1094. doi: 10.1016/j.kint.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braun F Lutgehetmann M Pfefferle S, et al. . SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597–598. doi: 10.1016/s0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puelles VG Lutgehetmann M Lindenmeyer MT, et al. . Multiorgan and renal tropism of SARS-CoV-2. New Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westhoff TH Seibert FS Bauer F, et al. . Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant. 2020;20(11):3216–3220. doi: 10.1111/ajt.16223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR. COVID-19-Associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020 10;2(4):493–497. doi: 10.1016/j.xkme.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu H Larsen CP Hernandez-Arroyo CF, et al. . AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020 10;31(8):1688–1695. doi: 10.1681/ASN.2020050558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shetty AA Tawhari I Safar-Boueri L, et al. . COVID-19-Associated glomerular disease. J Am Soc Nephrol. 2021;32(1):33–40. doi: 10.1681/ASN.2020060804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the work are available from the corresponding author on reasonable request.