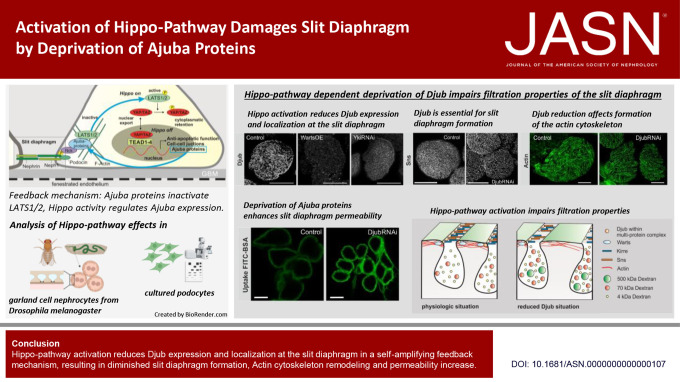
Keywords: podocyte, cell signaling, cell survival, FSGS, glomerular filtration barrier, nephrin, cytoskeleton
Abstract
Significance Statement
Nuclear exclusion of the cotranscription factor YAP, which is a consequence of activation of the Hippo signaling pathway, leads to FSGS and podocyte apoptosis. Ajuba proteins play an important role in the glomerular filtration barrier by keeping the Hippo pathway inactive. In nephrocytes from Drosophila melanogaster, a well-established model system for podocyte research, Ajuba proteins ensure slit diaphragm (SD) formation and function. Hippo pathway activation leads to mislocalization of Ajuba proteins, decreased SD formation, rearrangement of the actin cytoskeleton, and increased SD permeability. Targeting the kinases of the Hippo pathway with specific inhibitors in the glomerulus could, therefore, be a promising strategy for therapy of FSGS.
Background
The highly conserved Hippo pathway, which regulates organ growth and cell proliferation by inhibiting transcriptional cofactors YAP/TAZ, plays a special role in podocytes, where activation of the pathway leads to apoptosis. The Ajuba family proteins (Ajuba, LIM domain-containing protein 1 (LIMD1) and Wilms tumor protein 1–interacting protein [WTIP]) can bind and inactivate large tumor suppressor kinases 1 and 2, (LATS1/2) two of the Hippo pathway key kinases. WTIP, furthermore, connects the slit diaphragm (SD), the specialized cell-cell junction between podocytes, with the actin cytoskeleton.
Methods
We used garland cell nephrocytes of Drosophila melanogaster to monitor the role of Ajuba proteins in Hippo pathway regulation and structural integrity of the SD. Microscopy and functional assays analyzed the interplay between Ajuba proteins and LATS2 regarding expression, localization, interaction, and effects on the functionality of the SD.
Results
In nephrocytes, the Ajuba homolog Djub recruited Warts (LATS2 homolog) to the SD. Knockdown of Djub activated the Hippo pathway. Reciprocally, Hippo activation reduced the Djub level. Both Djub knockdown and Hippo activation led to morphological changes in the SD, rearrangement of the cortical actin cytoskeleton, and increased SD permeability. Knockdown of Warts or overexpression of constitutively active Yki prevented these effects. In podocytes, Hippo pathway activation or knockdown of YAP also decreased the level of Ajuba proteins.
Conclusions
Ajuba proteins regulate the structure and function of the SD in nephrocytes, connecting the SD protein complex to the actin cytoskeleton and maintaining the Hippo pathway in an inactive state. Hippo pathway activation directly influencing Djub expression suggests a self-amplifying feedback mechanism.
Introduction
The Hippo signaling pathway is a highly conserved and complex network of proteins, controlling activity of cotranscription factors YAP (Yes-associated transcriptional regulator) and TAZ (WW domain-containing transcription regulator protein 1). YAP/TAZ enhances expression of genes supporting cell growth and proliferation and suppresses ones that promote apoptosis (reviewed in1). Hippo pathway activation results in phosphorylation of YAP/TAZ, their export from the nucleus, and degradation. Therefore, the Hippo pathway is usually inactive in proliferating/growing cells and becomes active under conditions where further growth and proliferation of the cells should be prevented. Interestingly, for podocytes, which are highly differentiated, postmitotic cells forming a central part of the glomerular filtration barrier, we have shown that nuclear/active YAP/TAZ is essential for cell viability.2 Loss of nuclear YAP decreases transcription of anti-apoptotic genes culminating in cell death. In mice, podocyte-specific YAP deletion resulted in FSGS.3
While the Hippo pathway is regulated by a large network of proteins, the central event of Hippo pathway activation is phosphorylation of large tumor suppressor kinases 1 and 2 (LATS1/2), the most downstream kinases of the pathway, which phosphorylate YAP/TAZ.1 Ajuba proteins, Ajuba (LIM domain-containing protein Ajuba), Wilms tumor protein 1–interacting protein (WTIP), and LIM domain-containing protein 1 (LIMD1), are actin-binding and bundling proteins that localize to focal adhesions and cell-cell junctions.4,5 All three family members bind LATS1/2.6,7. It was also reported that Ajuba proteins recruit LATS to the cell membrane, and knockdown or knockout of Ajuba proteins leads to activation of the Hippo pathway in mammalian cell lines8,9 as well as in the eyes or wings of Drosophila melanogaster (D. melanogaster).7,10 This confirms that these mechanisms are highly homologous in D. melanogaster.
In podocytes, WTIP has been shown to recruit LATS1 to the protein complex of the slit diaphragm (SD).11 The SD between interdigitating podocyte foot processes forms a fine-tuned, size-selective part of the glomerular filtration barrier. As shown for WTIP,11 all Ajuba proteins may indirectly interact with Nephrin, one main component of the SD protein complex, which raises the question of whether Ajuba proteins regulate formation of SD by suppressing Hippo pathway activation. On the other hand, our previous report showed that activation of LATS2 reduced mRNA levels of WTIP and Ajuba, suggesting reciprocal regulation of Ajuba proteins by the Hippo pathway.2
In this article, we studied the reciprocal relationship between Ajuba proteins and the Hippo pathway and their role in the structural integrity of SD. To achieve this, we used garland cell nephrocytes (GCN) from D. melanogaster, an established in vivo model for SD analysis. Djub, Ajuba ortholog in D. melanogaster, partly colocalized with Warts (fly ortholog of LATS1/2) at the SD in GCN. Djub knockdown in nephrocytes led to rearrangement of Warts, Hippo pathway activation, and apoptosis. More importantly, both activation of the Hippo pathway and Djub knockdown reduced SD formation and affected filtration properties of the nephrocytes, suggesting that Ajuba proteins are a structural link suppressing the Hippo pathway and are essential for SD formation and function.
Methods
Drosophila Stocks and Genetics
Drosophila stocks were maintained under normal conditions (at 25°C on cornmeal agar). For nephrocyte-specific overexpression or knockdown of proteins, a nephrocyte-specific sns:GAL4 driver line12 was crossed (at 25°C for 3 days) to responder lines expressing specific RNAi and overexpression constructs. Larvae were then shifted at 29°C to increase transgene expression. The following fly lines were used: UAS:Djub-RNAi (Upstream activating sequence, Bloomington Drosophila Stock Center, IN, #41938, #32923), UAS:Djub-RNAi, sns:GAL4, myc-Warts (Bloomington Drosophila Stock Center #56809), UAS:Yorkie-RNAi (Bloomington Drosophila Stock Center #34067), UAS:KirreRNAi (Vienna Drosophila Resource Center, Vienna, Austria #27227), UAS:Djub-green fluorescent protein (GFP) (Bloomington Drosophila Stock Center #56806), UAS:Djub-GFP, myc-Warts, UAS:Yorkie-V5 (Bloomington Drosophila Stock Center #28819), UAS:WartsRNAi (Bloomington Drosophila Stock Center #34064), and death-associated inhibitor of apoptosis (DIAP):LacZ (Bloomington Drosophila Stock Center #545).
Nephrocyte Immunofluorescence
Drosophila third instar larvae were dissected to isolate GCNs and were collected in HL3.1 saline.13 Fixation occurred by 30 seconds of incubation in boiling heat fix saline (0.03% Triton X-100 in HL3.1 saline). For staining with phalloidin or Djub antibody, nephrocytes were fixed for 10 minutes in 4% paraformaldehyde (PFA) in HL3.1 saline (stated in figure legends). After washing steps in PBS+0.1% Triton X-100 (PBS-T) and blocking in 10% bovine serum albumin (BSA) for 1 hour, incubation with primary antibodies for 2 hours followed. After washing with PBS-T, secondary antibodies were added for 1 hour. Nephrocytes were mounted with Mowiol 4-88 (Sigma-Aldrich). Primary antibodies used were Djub (rabbit, 1:100, kindly provided by G. Longmore), myc (mouse, 1:100 Developmental Studies Hybridoma Bank, DSHB, clone 9E10), Sns (chicken, 1:500, described in14) or Sns (rabbit, 1:200, described in15), GFP (rabbit, Clontech, 632381), Polychaetoid (Pyd) (mouse, 1:100, DSHB PYD2), and β-Galactosidase (mouse, 1:25, DSHB clone JIE7). Secondary antibodies were goat anti-mouse, goat anti-rabbit, and goat anti-chicken conjugated to Alexa Fluor 488, Alexa Fluor 568, or Alexa Fluor 647 (1:1000, Thermo Fisher Scientific), respectively. Staining with phalloidin-Alexa-Fluor 594 (Thermo Fisher Scientific A12381, 1:1000), WGA-Alexa-Flour 555 (WGA: Wheat germ agglutinin; Invitrogen, Invitrogen, Waltham, MA W32464, 1:1000), and DAPI was performed in combination with the secondary antibody incubation step. For imaging, a Leica SP8 confocal microscope was used. Images were processed by the Leica LIGHTNING detection concept and analyzed using ImageJ. Quantification of SD/µm was performed by counting the strands crossing an orthogonal 5-µm line; data points are mean values of three different measurements per nephrocyte. For the determination of Sns-positive signal on the nephrocyte surface, the percentage area of Sns in images of each single cell was quantified after thresholding using ImageJ.
Expansion Microscopy
Nephrocytes were prepared with modifications as originally described by Unnerjö-Jess et al.16,17 Staining was performed using the protocol described above, but two-fold concentrated primary and four-fold concentrated secondary antibodies were used. After staining, instead of mounting with Mowiol 4-88 (Sigma-Aldrich), nephrocytes were further processed by incubation with 1 mM methacrylic acid N-hydroxysuccinimide ester (MA-NHS) (Sigma-Aldrich) in PBS for 1 hour at room temperature. Next, nephrocytes were incubated in a monomer solution (2 M NaCl, 8.625% [w/v] sodium acrylate, 2.5% [w/v] acrylamide, 0.15% [w/v] N, N′-methylenebisacrylamide, ×1 PBS). Gelation was initiated by addition of 0.2% Tetramethylethylenediamine (TEMED), 0.01% 4-hydroxy-(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl (TEMPO), and 0.2% ammonium persulfate (APS) to the monomer solution. Samples were transferred into an Ibidi chamber and covered with a coverslip on top for planar polymerization. After polymerization for 2 hours at 37°C, the samples were digested with Proteinase K (16 units/mL) in digestion buffer (50 mM Tris [pH 8], 1 mM EDTA, 0.5% Triton X-100, 0.8 M guanidine HCl) overnight at 37°C without a coverslip. Finally, an expansion step followed by washing the sample with ddH2O three times for 20 minutes each. For imaging, the Leica SP8 confocal microscope with HC PL APO CS2 ×63/1.40 oil was used. Images were processed by Leica LAS X Lightning and analyzed using ImageJ.
Terminal Deoxynucleotidyl Transferase–Mediated Digoxigenin-Deoxyuridine Nick-End Labeling Assay
After dissection as described above, nephrocytes were fixed with 4% PFA in HL3.1 saline for 10 minutes and permeabilized with PBS-T for 20 minutes. A terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assay was performed following the manufacturer's instructions of the In Situ Cell Death Detection Kit (Roche). For imaging, the Leica SP8 confocal microscope was used. Images were processed by Leica LAS X Lightning and analyzed using ImageJ.
WGA Staining of Adult Nephrocytes
Drosophila adult flies were dissected to isolate GCNs and collected in HL3.1 saline.13 After washing with PBS, nephrocytes were fixed for 10 minutes with 4% PFA in HL3.1 saline. Subsequently nephrocytes were washed, stained with WGA Alexa Fluor 555 (Invitrogen W32464, 1:1000) and DAPI, and mounted with Mowiol 4-88 (Sigma-Aldrich).
Transmission Electron Microscopy (TEM)
Nephrocytes were dissected and fixed with 2.5% glutaraldehyde in Sörensen phosphate buffer (SÖP: 100 mM Na2HPO4/KH2PO4, pH 7.3) overnight at 4°C. After washing with SÖP, incubation with osmium tetroxide (1% in SÖP) for 1 hour, and another three washing steps with SÖP, the samples were dehydrated by incubation in aqua bidest/ethanol mixtures with increasing ethanol concentration (30% for 5 minutes and then 50%, 70%, 90%, and 100% ethanol for 10 minutes each). Next, samples were embedded in a resin epon single mix (EPS) containing 16.6 g Glycidether 100 (SERVA Electrophoresis GmbH, Heidelberg, Germany), 8.6 g dodecenyl succinic anhdride, 8 g methyl nadic anhydride, and 0.4 g 2,4,6-tri(dimethylaminoethyl)-phenol (Agar Scientific Ltd., Essex, United Kingdom). Embedding is performed by incubation in EPS in solutions with increasing concentration of EPS in propylene oxide up to 100% EPS (incubation overnight) and finally polymerized in fresh EPS for 36 hours at 60°C. Seventy-nanometer sections on Cu grids coated with pioloform (Plano GmbH, Wetzlar, Germany) were double contrasted with uranyl acetate (2% aqua bidest, 20 minutes) and lead citrate (3% Reynolds) for 90 seconds. For imaging, Philips CM10 transmission electron microscope with camera TemCam F416 (Tietz Video and Image Processing Systems) was used. Images were further processed by EM Measure (Tietz Video and Image Processing Systems, Gauting, Germany).
Uptake Assays
After dissection as described before, live nephrocytes were incubated in HL3.1 saline with 1 mg/ml FITC-BSA (Sigma-Aldrich A9771) for 1 minute or with 1 mg/ml FITC-Dextran (4 kDa [Sigma-Aldrich, FD4]; 70 kDa [Sigma-Aldrich, FD70S], 500 kDa [Sigma-Aldrich, FD500S]) for 15 minutes. After washing with PBS, nephrocytes were fixed for 10 minutes with 4% PFA in HL3.1 saline. Subsequently nephrocytes were washed, stained with WGA Alexa Fluor 555 (Invitrogen W32464, 1:1000) and DAPI, and mounted with Mowiol 4-88 (Sigma-Aldrich). WGA staining was used to identify nephrocyte cortexes during quantification (not shown in figures). For imaging, the Leica SP8 confocal microscope was used. Uptake efficiency was quantified as average fluorescence signal within the nephrocytes, the cortex marked by WGA, using ImageJ and normalized to control of each experiment.
Cell Culture
Human immortalized podocytes (AB8/13) (kindly provided by M Saleem) and HEK293T (Thermo Scientific, Waltham, MA) were cultivated as described earlier.18,19 In brief, AB8/13 cells were grown in a standard RPMI 1640 medium containing 10% FCS with added penicillin, streptomycin, insulin, transferrin, and selenite (Sigma-Aldrich St. Louis, MO) at 33°C in 5% CO2. HEK293T cells were cultivated in a standard medium (Dulbecco modified Eagle medium supplemented with 10% FCS and 1% antibiotics [Pen/Strep]).20 All cells were tested negative for mycoplasma contamination by using PCR. For transient transfection, HEK293T cells were transfected by using the calcium phosphate method as described earlier.20 AB8/13 cells were transiently transfected using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer's instructions. For experiments with inhibitors, cells were incubated with 100 nM bortezomib (ApexBio, Houston, TX), 100 nM bafilomycin (Sigma-Aldrich), 20 µg/ml 3 MA (Westlake Village, CA), 10 µM leupeptin (Sigma-Aldrich), or 1 µM MG132 (Sigma-Aldrich).
CRISPaint
Base-specific gene tagging of Ajuba with C-terminal TagGFP was performed according to Schmid-Burgk et al.21 AB8/13 cells were transiently transfected with pCRISPaint-TagGFP2-PuroR (Addgene plasmid #80970), pCAS9-mCherry-Frame +1 (Addgene plasmid #66940) (both part of the CRISPaint Gene Tagging Kit), and px458 (Addgene plasmid #48138) with Ajuba-specific guide RNA (gRNA) (GATTGCAGCTCAGATATAGT) inserted into a gRNA scaffold sequence. After 2 days of recovery, the cells were selected with 2 μg/ml puromycin for 4 days.
Constructs and Cloning
For inducible overexpression of WTIP, wild-type form of LATS2 (LATS2-WT), LATS2 T1041E, and LATS2-T1041A fused to a fluorescent protein tag, the coding sequences of full-length human WTIP were amplified from a human immortalized podocyte library and the coding sequences for LATS2 variants from the vectors created before.2 Amplified DNA was inserted into pENTR-D/TOPO vector (Invitrogen, Waltham, MA) according to the manufacturer's instructions. For the expression of proteins with an N-terminal fluorescent tag, the coding DNAs were subcloned in frame using AscI/PacI restriction enzymes into pENTR-D/TOPO vectors containing the coding sequences of red fluorescent protein (RFP) or GFP. The constructs were then shuttled into a modified pINDUCER21 vector-containing puromycin resistance by LR-clonase reaction (Invitrogen).19 The doxycycline-inducible lentiviral vectors, pTRIPZ-EGFP-LIMD1 and pTRIPZ-EGFP-Ajuba (EGFP: enhanced green fluorescent protein), were a kind gift from Kenneth D. Irvine (Department of Molecular Biology and Biochemistry, Rutgers University, Piscataway). For stable expression of LIMD1 with a C-terminal EGFP tag, the coding sequence of LIMD1 was amplified excluding the stop codon from the pTRIPZ-EGFP-LIMD1 vector and inserted using XbaI/AgeI restriction enzymes into pLenti CMV Zeo GFP (Addgene plasmid #17449). All constructs were verified by DNA sequencing.
Generation of Stable Cell Lines
The generation of stable, doxycycline-inducible podocytes that overexpress LATS2-WT, LATS2-T1041E, or LATS2-T1041A or cells that overexpress short hairpin RNA against YAP was described before.2 All additional stable cell lines created in the course of this study were generated according to the same protocol. Briefly, in the case of lentiviral vectors, HEK293T cells were simultaneously transfected with pINDUCER21 (for WTIP and RFP-LATS2), pTRIPZ (for Ajuba and LIMD1), or pLenti (for LIMD1-GFP) construct DNA with the respective insert and helper plasmids psPAX2 (Addgene plasmid #12260) and pMD2.G (Addgene plasmid #12259) by using the calcium phosphate method.20 The virus-containing supernatant was collected and filtered through a sterile 0.45-μm syringe-driven filter unit (Millipore, Schwalbach am Taunus, Germany). Subsequently, AB8/13 cells were infected for 24 hours using one volume of fresh Dulbecco modified Eagle medium and one volume of the virus-containing filtrate supplemented with polybrene (Sigma-Aldrich, final concentration 8 μg/ml). After a regeneration period of 24 hours, the infection procedure was repeated. Transduced cells were selected by puromycin (InvivoGen, Toulouse France, 2 μg/ml for pINDUCER21 and pTRIPZ) or Zeocin (InvivoGen, 3 µg/ml for pLenti). Protein expression was induced by doxycycline (125 ng/ml). The overexpression of target proteins was verified by Western blot analysis. In the case of retroviral vector pQCXIP, viral particles were generated following the same protocol, with the exception that pUMVC (Addgene plasmid #8449) and pCMV-VSV-G (Addgene plasmid #8454) were cotransfected (instead of psPAX2 and pMD2.G).
Western Blot
Cells were grown on dishes (9.5 cm2) and harvested in RIPA buffer (Thermo Fisher Scientific) with protease inhibitor cocktail (Roche Basel, Switzerland) and phosphatase inhibitor cocktail 2/3 (Sigma-Aldrich). Cells were lysed by incubation on ice for 30 minutes and sonication for 10 minutes, followed by centrifugation. After centrifugation, supernatants were mixed with Laemmli buffer (final concentration: 4% SDS, 5% 2-mercaptoethanol, 10% glycerol, 0.002% bromophenol blue, 0.0625 M Tris-HCl; pH 6.8) and boiled at 95°C for 5 minutes. Lysates were separated by SDS-PAGE (10%–15%, BioRad, Hercules, CA), and proteins were transferred on the polyvinylidene fluoride (PVDF) membrane, (Millipore, Burlington, MA) blocked (1 hour in 5% skim milk powder in TBS-containing 0.05% Tween-20 [Tris-buffered saline, TBS-T] at room temperature (RT), and incubated with a primary antibody solution overnight at 4°C or for 1 hour at RT. The following primary antibodies were used (all diluted 1:1000 unless otherwise stated): GFP (Clontech #632381), RFP (Chromotek, Planegg, Germany, #6G6), YAP (#14074), LATS2 (#5888), Ajuba (#34648), LIMD1 (#13245), Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (#2118), Lamin B2 (#12255) (all from Cell Signaling, Danvers, MA), WTIP (1:250, Sigma Aldrich, SAB1411766/HC151), LC3 (1:4000, Novus Biologicals, Germany, NB100-2220), and α Actinin-4 (ENZO, Farmingdale, NY, ALX-210-356). After washing with TBS-T, the membrane was incubated with horseradish peroxidase–coupled anti-rabbit (Dianova, 111-0335-144) or anti-mouse (Dianova, 115-035-068) secondary antibodies (both diluted 1:2000) for 45 minutes at RT. After another washing step, the signal was detected using one of the following Western blot substrates: Clarity or Clarity Max ECL Western Blotting Substrates (BioRad) or Lumi-Light or Lumi-LightPLUS (Roche).
Immunofluorescence
AB8/13 cells cultured on glass coverslips were washed with PBS and fixed with 4% paraformaldehyde for 10 minutes at RT. After washing with PBS, cells were quenched with 50 mM NH4Cl and permeabilized with PBS containing 0.2% gelatin and 0.2% TritonX-100 (PBS-TG). Cells were blocked for 20 minutes with 10% goat serum diluted in PBS-TG. Immunofluorescence staining was performed by incubation with primary antibodies diluted in PBS-TG containing 2% goat serum for one. Antibodies, described in the Western blot section, and ZO-1 (Invitrogen, 33-9100) or Paxillin (Santa Cruz, sc-365174) were diluted in a 1:100 ratio. Afterward, coverslips were washed in PBS-TG and incubated with fluorochrome-conjugated secondary antibodies (dilution 1:1000), coupled with Alexa-Fluor 488/594/647 (anti-rabbit, anti-mouse, Thermo Fisher Scientific). To visualize polymerized actin phalloidin, Alexa Fluor 488 or 594 (Thermo Fisher Scientific, A12379/A12381; 1:1000) was used. Nuclei were stained with DAPI (dilution 1:5000, Roche). Cells on coverslips were mounted in Mowiol. For imaging, Zeiss Observer Z1 equipped with an EC Plan Apochromat ×63/1.40 Oil M27 objective and Apotome technology was used. Images were analyzed using ImageJ (http://rsbweb.nih.gov/ij/).
Quantification of the Pearson Coefficient to Analyze Colocalization
Immunofluorescence images of nephrocyte surfaces stained with antibodies against GFP to visualize GFP-Djub, myc to visualize myc-Warts, and Sns were analyzed using the JACoP plugin tool in ImageJ.22 Colocalization levels were evaluated by the determination of the Pearson coefficient comparing two channels of the same microscope image. Each data point results from the evaluation of one nephrocyte. A value of 1 implies complete positive correlation, a value of 0 implies no correlation, and a value of −1 implies complete negative correlation.
Live Cell Imaging
AB8/13 cells were grown on an Ibidi chamber with glass bottom (Ibidi GmbH, Martinsried, München). Imaging was performed using Leica SP8 equipped with a temperature control incubation chamber and using the HC PL APO ×40/1.10 W motCORR CS2 objective. Four hours before imaging, the medium was replaced with a L-15 medium (Sigma) containing 10% FCS and, if required, 125 ng/ml doxycycline to induce expression of fluorescent proteins and was left in the microscope incubation chamber at 37°C. To monitor redistribution of the proteins within the cell, 3D stacks were acquired every 20 minutes. Deconvolution of acquired images was performed with LAS X Lightning software and presented as maximum projections.
Quantification and Statistical Analyses
Signals derived from immunoblot and immunofluorescence were quantified densitometrically using ImageJ (http://rsbweb.nih.gov/ij/). Data points of densitometric quantification of Western blot results were received from at least three different experiments. Graphical and statistical analyses (one-way ANOVA with the Tukey multiple-comparisons test) were performed using the GraphPad Prism 9 software: NS: P > 0.05; *P ≤ 0.05. For graphical visualization, box plots and super plots were used.23 The results from densitometrical analyses of microscope images were visualized by super plots. Small colored dots correspond to single data points; big colored dots are mean values of each experiment. Each data point results from an individual cell. The color-coding shows the affiliation of the individual data points to the various and individual experimental replicates.
Results
In D. Melanogaster Nephrocytes, Djub Colocalizes with Warts at the SD
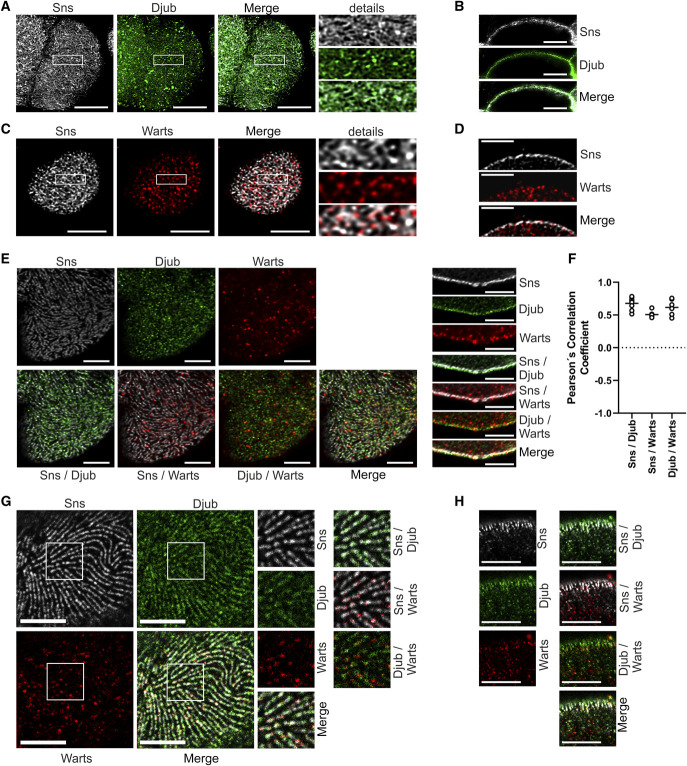
Our finding that an activation of the Hippo pathway led to a loss of Ajuba protein transcription in podocytes,2 combined with the knowledge that WTIP binds to Nephrin by the adapter protein Nck,11 led to the assumption that Hippo pathway activation could have an effect on SD functionality. Because cultured podocytes do not establish functional SD, we used GCN from D. melanogaster for SD analysis. The Drosophila genome encodes a single Ajuba ortholog, the Drosophila Jub (Djub). In a first experiment, localization of Djub at SD in wild-type nephrocytes was confirmed by costaining of endogenous Djub and the Drosophila Nephrin ortholog Sticks and stones (Sns), which marks SD. Staining for Sns revealed the typical fingerprint-like structure on the surface of nephrocytes. Djub colocalized or intertwined with Sns structures (Figure 1A).
Figure 1.
Djub and Warts colocalize with Sns at nephrocytes SD. (A) Microscopy of wild-type third instar larvae garland nephrocytes stained with antibodies against Sns (gray) and Djub (green) after PFA fixation. The staining with Sns shows the typical fingerprint-like structure of SDs on the nephrocyte cortex. Djub staining shows a very similar structure with partial colocalization of Sns and Djub at SD. (B) Cross-sections of nephrocytes as used in (A) confirm partial colocalization of Sns and Djub at the cortex. (C) Microscopy of third instar larvae garland nephrocytes overexpressing myc-Warts stained with antibodies against Sns (gray) and myc (red) after fixation in a heat fix solution. Warts staining partly overlapped with the typical fingerprint-like staining pattern, confirming partial SD localization of Warts. (D) Cross-sections of nephrocytes as used in (C) proved partial SD localization of Warts. An additional staining of Warts was detected within the cytoplasm of the nephrocytes, which may be an effect of myc-WartsOE. (E) Microscopy of third instar larvae garland nephrocytes overexpressing GFP-Djub and myc-Warts stained with antibodies against Sns (gray), GFP (green), and myc (red) show colocalization between Djub and Sns in the fingerprint-like staining pattern on the nephrocyte surface (left). Warts staining at SD is less than Djub staining but, where detected, Warts colocalizes with Djub and Sns. Cross-sections through the nephrocytes confirm that Djub strongly and Warts partly colocalizes with Sns at SD (right). When overexpressed, Djub and Warts additionally localize within the cytoplasm. In the case of Djub, this is an effect of overexpression (compare B). Lack of Warts-specific antibody makes this proof impossible for Warts. (F) Evaluation of colocalization between the proteins Sns and Djub, Sns and Warts, and Djub and Warts by determination of Pearson coefficients confirmed strong correlation between localization of all three proteins (mean values between 0.5 and 1). (G) Expansion microscopy of nephrocytes as used in (E). Surface analyses confirm very similar structures and partial colocalization between Djub and Sns at SD. Warts also colocalizes with SD structures. (H) Cross-sections of nephrocytes as shown in (E) using expansion microscopy confirm colocalization of Sns and Djub at SD and, to a smaller amount, colocalization with Warts. Scale bars (A–F) 5 µm and (G and H) 20 µm. Figure 1 can be viewed in color online at www.jasn.org.
Images obtained through the equatorial plane of the nephrocytes (Figure 1B) showed also partial colocalization of Sns and Djub at the cortex.
Because no antibody against Warts was available, myc-tagged Warts were overexpressed in nephrocytes and costained with Sns to analyze Warts localization. The analyses showed that Warts staining partly overlapped with Sns staining confirming partial SD localization of Warts (Figure 1C). Images obtained through the equatorial plane of the nephrocytes proved this observation (Figure 1D), but in addition showed Warts localization within the cytoplasm of the nephrocytes, eventually because of overexpression.
Djub recruits Warts to adherens junctions of Drosophila wing disks.10 We, therefore, expected colocalization of Warts and Djub with Sns also at nephrocyte SD. Because overexpression of Warts influences the expression and localization of Djub, most likely because of an imbalance in the Warts/Djub ratio (explained later), both proteins were coexpressed for these experiments (Figure 1E). The stainings confirmed colocalization of Djub with Sns-stained SD. Warts staining also partly overlapped with Sns and Djub staining. Partial colocalization of the respective proteins was confirmed by estimation of Pearson coefficients (Figure 1F). Colocalization of Djub, Sns, and Warts at SD was also confirmed by immunostaining combined with expansion microscopy, which provided much higher spatial resolution. The results suggest recruitment of Warts by Djub to nephrocyte SD similar to its recruitment to adherens junctions in Drosophila wings10 (Figure 1, G and H).
Ajuba Protein Localization and Hippo Pathway Activity are Mutually Dependent
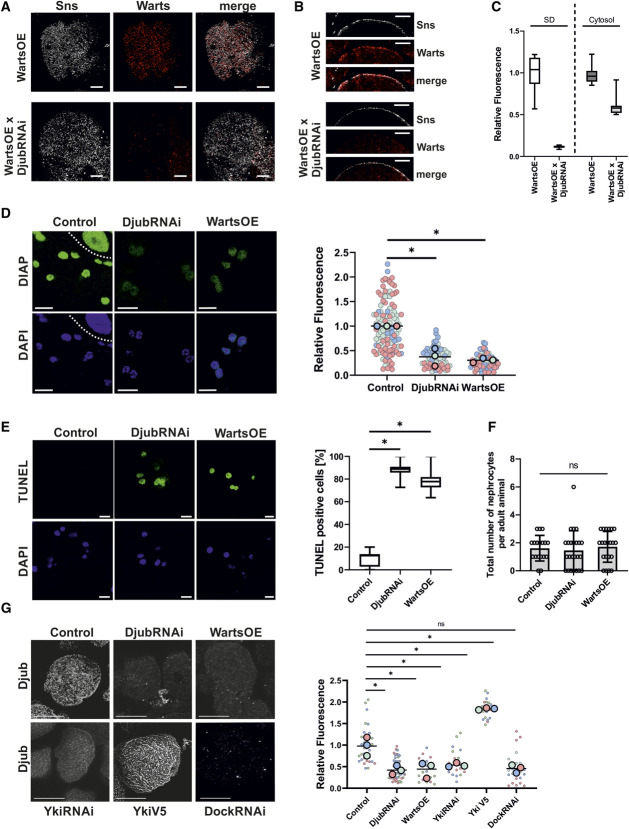
Myc-Warts partly localized near SD in nephrocytes with an endogenous level of Djub (Figure 1, C and D). To test whether Djub is required to recruit Warts to SD, Warts localization was analyzed in nephrocytes with reduced Djub expression (DjubRNAi). The SD-associated level of Warts was strongly reduced (Figure 2A). This indicates that Djub is essential for localization of Warts at SD. Sections through the equatorial plane of the cells confirmed this result (Figure 2B). Furthermore, a quantification of Warts staining proved that reduction of Djub expression also led to reduced expression of cytosolic Warts, which suggests an enhanced degradation of Warts (Figure 2C).
Figure 2.
Ajuba protein localization and Hippo pathway activity depend on each other. (A). Immunostaining of Sns (gray) and myc (red) in third instar larvae garland nephrocytes overexpressing myc-Warts (WartsOE) with and without additional Djub knockdown (WartsOE versus WartsOE×DjubRNAi) demonstrates decreased expression of Warts in Djub-knockdown nephrocytes. (B) Cross-section images of nephrocytes as used in (A) show reduced Warts signal at the cortex of the nephrocyte stained with Sns. (C) Densitometric quantification of myc-Warts signal on the nephrocyte surface and in the cytoplasm from nephrocytes as used in (A). Djub knockdown results in significantly reduced Warts signal on the surface, approximately 10% of the Warts signal at endogenous Djub level. Cytoplasmic Warts signal was also significantly decreased after DjubRNAi, but the level was only halved. This indicates that Djub is essential for Warts localization at SD and that a loss of SD association increases degradation of Warts. (D) Djub knockdown and WartsOE both activate the Hippo pathway, resulting in decreased transcription of the Yki target gene DIAP; the cell above the dotted line in Control is part of the stomach. Densitometric quantification of DIAP signal in the nucleus shows significantly reduced Yki activity in DjubRNAi and WartsOE nephrocytes (n>60). (E) TUNEL assays indicate apoptotic processes in DjubRNAi and Warts overexpression garland nephrocytes (>12 individual larvae per genotype). Statistical analyses show a significant increase of TUNEL-positive nephrocytes for Djub knockdown and Warts overexpression. (F) Quantification of the total nephrocyte number in adult flies (>3 days older than larvae used for other nephrocyte studies) shows no significant reduced number of nephrocyte caused by Djub knockdown or Warts overexpression, despite induction of apoptosis shown in (E). For visualization, nephrocytes were stained with WGA. (G) Immunostaining with antibody against Djub in third instar larvae garland nephrocytes. In comparison with control, the Djub signal was strongly decreased in nephrocytes with knockdown of Djub or Yki (DjubRNAi, YkiRNAi) and WartsOE. Overexpression of Yki-V5 increases Djub surface expression. Knockdown of Dock (DockRNAi) led to a similar decrease in SD-associated Djub and suggests Dock as a molecular connector between Djub and the SD protein complex. Densitometric quantification of the Djub signal on the GCN surface from mutants shown in (D) confirms significance of the changes in Djub surface expression (n>20). *P < 0.05. Scale bars (A and B) 5 µm and (D–F) 10 µm. Figure 2 can be viewed in color online at www.jasn.org.
We further observed that nephrocytes with Djub knockdown were smaller than wild-type nephrocytes (Supplemental Figure 1). A possible explanation for this observation is Djub-reduction dependent Hippo pathway activation,7 despite reduction of total Warts expression in Djub-knockdown nephrocytes. Hippo activation consequently leads to loss of nuclear Yorkie (Yki, Drosophila ortholog of YAP/TAZ) as a cell growth regulating cotranscription factor. In fact, transcription of the Yki target diap124 was strongly reduced in Djub-knockdown GCNs and in Warts-overexpressing GCNs (Figure 2D). These results support the hypothesis that Ajuba proteins are able to sequester LATS/Warts at SD in an inactive form.7,11
Both Hippo pathway activation by Warts overexpression or Djub depletion seem to provoke apoptotic processes as detected by the TUNEL assay (Figure 2E). However, because the number of nephrocytes in adult flies was the same in control as in Djub-knockdown or Warts-overexpression flies (Figure 2F) and comparable with published data25 and because nephrocytes are postmitotic cells, these processes obviously did not lead to acute cell death or were even reversed at later time points. Even more important for our study was that the effects of Djub knockdown or Warts overexpression on SD were the same both in TUNEL-positive and -negative cells (described below).
What we show here for the first time is that Hippo pathway activation reciprocally influences Djub localization at SD, which suggests a self-amplifying feedback mechanism. In nephrocytes overexpressing Warts or with Yki knockdown, the amount of Djub at SD was decreased to the level observed under knockdown of Djub itself (Figure 2G). Confirming Yki-dependent regulation of Djub, YkiV5 overexpression led to an increase in the Djub level. This result shows that Djub not only regulates Hippo pathway activity, but itself is a subject of regulation by the Hippo pathway. This observation matches the finding that the mammalian Ajuba proteins, WTIP and Ajuba, were described as transcriptional targets of YAP in breast cancer cells.26
In podocytes, WTIP binds to the SD protein Nephrin by Nck.11 Knockdown of the Nck ortholog Dock in nephrocytes severely decreased Djub localization at the surface, which indicated that Dock is required to localize Djub at SD (Figure 2G).
LATS2 Affects Ajuba Protein Expression and Localization in Human Podocytes
Next, we analyzed whether Hippo-dependent regulation of Ajuba proteins is conserved in human podocytes. Overexpression of LATS2-WT diminished the amount of all three Ajuba family proteins—LIMD1, WTIP, and Ajuba (Figure 3A). To study effects of LATS activity on the level of Ajuba proteins, previously described LATS2 mutants were used.2 Overexpression of constitutively active LATS2-TE, which phosphorylates and excludes YAP from the nucleus, decreased the protein levels of all three Ajuba proteins similarly to the overexpression of LATS-WT (Figure 3B). On the contrary, overexpression of the inactive LATS2-TA mutant, which does not induce nuclear export and inactivation of YAP, resulted in an only slight decrease in the levels of LIMD1 and WTIP and increase in the Ajuba protein level (Figure 3C). This suggests that LATS activity, and by extension, inactivation of YAP, is sufficient to decrease the levels of Ajuba proteins. To validate that YAP is indeed involved in the regulation of Ajuba proteins in podocytes, YAP knockdown was performed. In cells expressing YAP-shRNA, the protein levels of Ajuba, LIMD1, and WTIP were decreased (Figure 3D).
Figure 3.
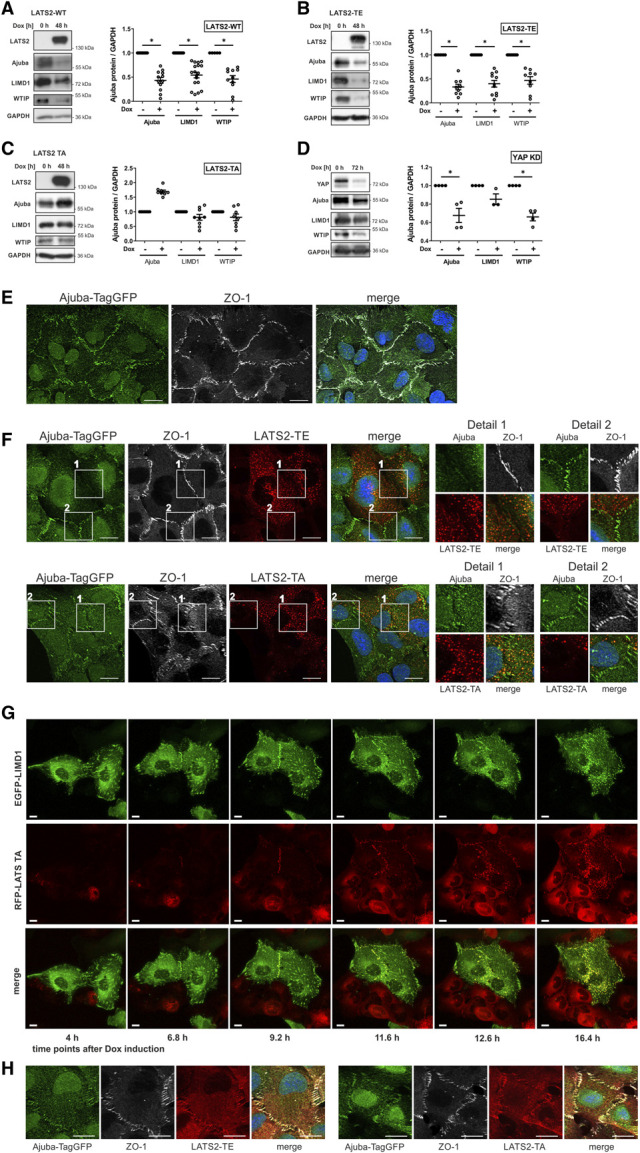
LATS2 affects Ajuba protein localization and expression in human podocytes. (A) Western blott (WB) analysis of Ajuba proteins after induction of LATS2-WT expression in AB8/13 cells shows reduced expression levels of all three Ajuba proteins. (B and C) The reduction of protein levels of Ajuba proteins partly depends on kinase activity of LATS2. An increase in kinase active LATS2-TE strongly reduces protein levels of all three Ajuba proteins (B); an increase in kinase inactive LATS2-TA slightly decreases LIMD1 and the WTIP level and increases the Ajuba level (C). (D) Knockdown of YAP in AB8/13 cells reduces protein expression levels of Ajuba proteins. (E) AB8/13 cells with TagGFP inserted in frame in the last Ajuba exon using a gene-tagging technique of CRISPaint,21 which allows visualization of Ajuba expressed on an endogenous level. Ajuba-TagGFP (green) mainly localized at cell-cell contacts, which were marked with an antibody against tight junction protein ZO-1. Scale bars 20 µm. (F) AB8/13 cells expressing Ajuba-TagGFP on the endogenous level as used in (E) were additionally stably transduced with inducible RFP-tagged LATS2-TE or LATS2-TA. LATS2 expression was induced by Dox treatment for 24 hours. Because not all cells were successfully transduced, a comparison between LATS2-expressing cells and cells without LATS2 expression was possible. Ajuba localization was diminished at cell-cell junctions between neighboring cells, both overexpressing LATS2. Furthermore, overexpression of LATS2 led to punctate structures in the cytoplasm, which colocalize with Ajuba (see also detail 1). If one of the neighboring cells does not overexpress LATS2, Ajuba-TagGFP remains enriched at cell-cell junctions where it colocalizes with ZO-1 (see also detail 2). Scale bars 20 µm. (G) Live cell imaging of AB8/13 cells stably overexpressing LIMD1-GFP (green) and inducibly expressing RFP-LATS2-TA (red). Time specifications indicate time after addition of Dox. Cells without LIMD1 expression show unspecific cytoplasmic LATS2-TA localization. In cells with LIMD1 expression, during the first phase of doxycycline induction (until 11.6 hours), LATS2-TA is recruited to cell-cell junctions and focal adhesions, where it colocalizes with LIMD1. When protein expression further increases (from 12.6 hours after induction), LIMD1/LATS2-TA aggregates appear in the cytoplasm. Usage of the kinase inactive LATS2-TA mutant for this experiment proves that this process is independent of kinase activity. Scale bars 10 µm. (H) AB8/13 cells expressing Ajuba-TagGFP on the endogenous level as used in (E) were additionally stably transduced with inducible RFP-tagged LATS2-TE or LATS2-TA. LATS2 expression was induced by Dox treatment for 4 hours. After this short induction period, both LATS2 variants were enriched at cell-cell junctions, where they colocalized with Ajuba and ZO-1. Figure 3 can be viewed in color online at www.jasn.org.
Analogous to nephrocytes, in which overexpression of Warts alters the localization of Djub. In cultured human podocytes, overexpressed EGFP-tagged Ajuba proteins colocalized with paxillin at the end of actin bundles marking focal adhesions (Supplemental Figure 2, A–C). Furthermore, Ajuba proteins localized at cell-cell junctions and colocalized there with LATS2, which was described earlier,27–29 and could also be demonstrated for overexpressed WTIP with endogenous LATS2 (Supplemental Figure 2D). Interestingly, overexpression of LATS2 (LATS-WT, active LATS2-TE or inactive LATS2-TA) resulted in redistribution of Ajuba proteins to punctate structures (Supplemental Figure 3, A–C) without affecting focal adhesions or actin cytoskeleton (Supplemental Figure 4D). We could not further characterize these structures, but they do not seem to be either early recycling or late endosomes because no colocalization with corresponding markers—EEA1, Rab11, or Rab7—has been observed (data not shown). Because redistribution of Ajuba proteins occurred independently from YAP localization (Supplemental Figure 4), we concluded that this process was independent of LATS2 and YAP activity.
To control for overexpression effects, we inserted TagGFP in the last exon of the Ajuba gene to generate a cell line expressing Ajuba-TagGFP at the endogenous level.21 Besides nuclear localization, Ajuba-TagGFP predominantly localized at cell-cell junctions, colocalizing with the tight junction protein ZO-1 used to mark cellular junctions (Figure 3E). In cells co-expressing LATS2-TE or LATS2-TA and Ajuba-TagGFP, Ajuba localization at cell-cell junctions was diminished if both cells expressed LATS2 (Figure 3F, compare details 1 and 2). Furthermore, LATS2/Ajuba-TagGFP–positive punctate structures were observed in the cytoplasm of these cells. This confirms that Ajuba redistribution is independent from LATS2 kinase activity. Previous studies revealed two potential Ajuba-interacting regions within LATS2, both outside of the kinase domain.6 We postulate that overexpressed LATS2 accumulates in the cytoplasm and reduces junction-bound Ajuba proteins by direct interaction. A similar mechanism will be responsible for Warts-dependent Djub reduction at SD in nephrocytes.
To gain better understanding of the redistribution process, we performed live cell experiments after induction of RFP-LATS2-TA expression in a mixed-cell culture with cells stably expressing LIMD1-GFP and others without LIMD1-GFP overexpression. Before expression of LATS2-TA in LIMD1–expressing cells, LIMD1 was distributed at focal adhesions, cell-cell contacts, and diffusely around the nucleus (Figure 3G; 4 hours). Expression of LATS2-TA in these cells led to the appearance of RFP-LATS2-TA at cell-cell junctions (Figure 3G; 6.8 and 9.2 hours). At later time points, both LATS2-TA and LIMD1 redistributed to punctate structures and the junctional localization of the proteins seemed to be increasingly lost, especially between those cells that were both double-positive for LATS-TA and LIMD1 (Figure 3G; 12.6 and 16.4 hours, film in Supplemental Video 1). Junctional localization of LATS-TA or punctate structures were hardly observed in the LIMD1-negative cells, indicating that not only redistribution of LIMD1 is regulated by LATS2 but also localization and redistribution of LATS2 itself depend on the presence of LIMD1 (compare LIMD1–positive and negative cells in Figure 3G). Junctional localization of LATS2, evident by colocalization with ZO-1, was also seen in Ajuba-TagGFP cells overexpressing LATS2-TE or -TA after a short induction period (Figure 3H) before this localization disappeared after long time induction (Figure 3F).
Loss of Djub and Hippo Pathway Activation Damage the Nephrocyte Slit Diaphragm
In nephrocytes, we analyzed whether a loss of Ajuba protein at SD by Djub knockdown or mimicking Hippo pathway activation by Warts overexpression or Yki knockdown affects SD formation. Immunostainings of Sns in wild-type nephrocytes in comparison with nephrocytes with Djub or Yki knockdown or Warts overexpression showed decreased SD density and disturbances of the fine SD structure (Figure 4, A and B, Supplemental Figure 5). Nephrocytes with knockdown of Dock, as a connector between Djub and the SD protein complex, showed a similar effect. However, none of the above manipulations led to a complete absence of the SD as the knockdown of Kirre, a Drosophila ortholog of KIRREL1 (kirre-like nephrin family adhesion molecule 1), did (Figure 4, A and B). The disturbances of the SD structure do not appear to depend on apoptotic processes because TUNEL-negative and TUNEL-positive nephrocytes showed comparable SD structures under Djub knockdown or Warts overexpression (Figure 4C). To test whether Yki regulates SD downstream of Djub, we performed Warts knockdown (Warts-RNAi) or Yki overexpression (YkiV5) simultaneous to Djub knockdown. Both WartsRNAi and YkiV5 partially rescued the effect of DjubRNAi on SD (Figure 4, D and E), indicating that indeed Yki regulated SD formation downstream of Djub.
Figure 4.
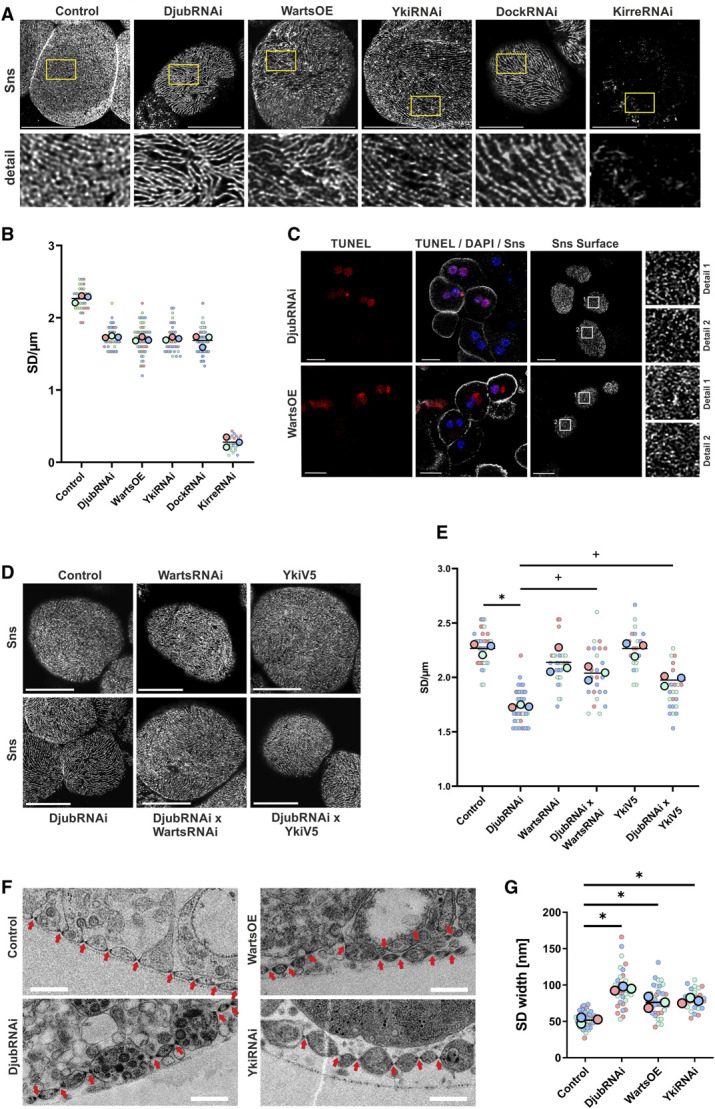
Knockdown of Djub and activation of Hippo pathway damage nephrocyte SD. (A) Immunostaining of Sns in third instar larvae garland nephrocytes as a SD marker. Knockdown of Djub, Yki, or Dock (DjubRNAi, YkiRNAi, DockRNAi) and WartsOE change the fingerprint-like structure of Sns on the surface, leading to increased distances between SDs with gaps in the structure. Scale bar 10 µm. (B) The statistical analysis of the number of Sns stained SDs crossing a 5-µm long line, drawn vertical to SDs, proves increased distances between SDs for mutants shown in (A) (>30 nephrocytes/genotype with three lines each). (C) Immunostaining of Sns in combination with TUNEL assays in nephrocytes with knockdown of Djub (DjubRNAi) and Warts OE after PFA fixation, which results in a somewhat shriveled surface. Red stained nuclei show TUNEL-positive nephrocytes; only DAPI (blue) stained nuclei show TUNEL-negative nephrocytes. The Sns-stained SD structures on the surface are completely comparable in TUNEL-positive and negative nephrocytes, which demonstrates that SD disturbances are independent from apoptotic processes. (D) Immunostaining of Sns in third instar larvae garland nephrocytes as a SD marker. As shown in (A), knockdown of Djub (DjubRNAi) changes the fingerprint-like structure of Sns on the surface, leading to increased distances between SDs. Warts knockdown or Yki overexpression simultaneous to Djub knockdown (DjubRNAi×WartsRNAi; DjubRNAi×YkiV5) partly rescues this effect. Warts knockdown (WartsRNAi) or Yki-V5 overexpression (YkiV5) alone has no effect. Scale bar 10 µm. (E) Statistical analysis of the number of Sns-stained SDs crossing a 5-µm long line, drawn vertical to SDs in nephrocyte mutants as shown in (C). Simultaneous Warts knockdown (DjubRNAi×WartsRNAi) or Yki-V5 overexpression (DjubRNAi×YkiV5) rescues the reduction of SD/µm observed in Djub-knockdown GCN. Warts knockdown (WartsRNAi) or Yki-V5 overexpression (YkiV5) alone had no effect on the number of SDs in comparison with control (n>15). (F) Transmission electron microscopy (TEM) of third instar larvae garland nephrocytes. Wild-type nephrocytes (control) show a regular arrangement of SDs (arrows). In DjubRNAi and YkiRNAi as well as Warts-overexpression nephrocytes, the distances between SDs are partly increased. In addition, in Warts overexpression, the arrangement of SDs seems to be partly disordered as sometimes a second row of SDs appears behind the front line. Scale bars 500 nm. (G) Quantification of SD width using TEM images of nephrocytes as shown in (E). *P < 0.05 compared with control, +P < 0.05 compared with DjubRNAi. Figure 4 can be viewed in color online at www.jasn.org.
TEM images revealed increased distances between SDs in Djub, Warts overexpression (WartsOE), or Yki knockdown nephrocytes (Figure 4F). In addition, in WartsOE nephrocytes, double layers of SDs were present. These results suggest that Hippo pathway inactivation is required for proper SD formation in nephrocytes. Moreover, not only distances between SDs increased but also the width of the SD itself seemed to be enlarged in mutant fly lines (Figure 4G). Together, these results underline the importance of Djub expression and Hippo pathway repression for the SD structure.
Loss of Djub and Hippo Pathway Activation Affect Actin Cytoskeleton
The actin cytoskeleton is required for SD formation and maintenance.30 Because Ajuba proteins directly interact with F-actin,5 we hypothesized that Djub may play a role in connecting F-actin to SD structures. For unknown reasons, a costaining with phalloidin and Sns did not work well enough; therefore, we stained the SD with an antibody against the ZO-1 ortholog Pyd, which is also a marker of nephrocyte SD.31 Costaining for F-actin, GFP-Djub, and Pyd revealed that F-actin fibers were often located orthogonally to Djub and Pyd-stained SD or partially colocalized with Djub- and Pyd-positive structures (Figure 5A). This indicates that Djub may serve as a link between actin cytoskeleton and SD. Djub knockdown, Yki or Dock knockdown, or Warts overexpression resulted in both structural reorganization of actin fibers (Figure 5B) and a decrease in total actin density at the surface (Figure 5C), confirming the importance of Djub for integrity of the cortical cytoskeleton.
Figure 5.
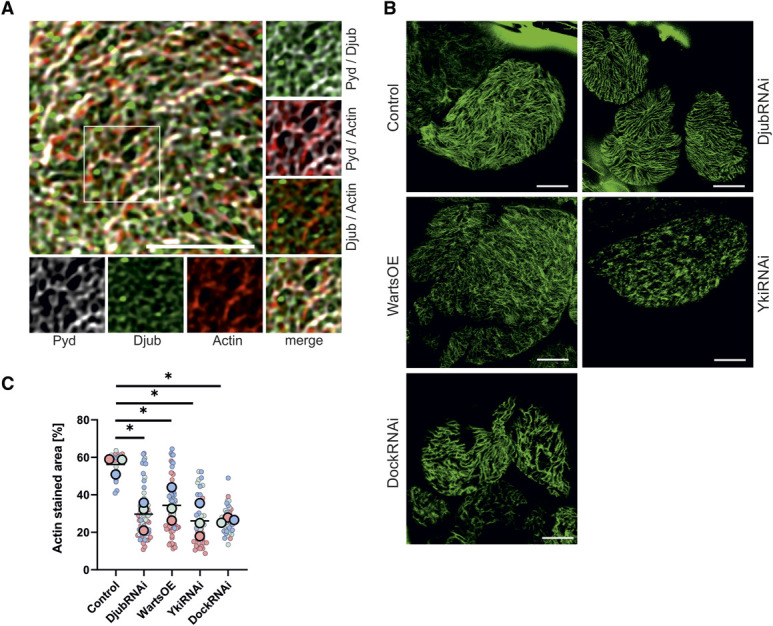
Djub connects the SD of nephrocytes with the cytoskeleton. (A) Immunostaining of third instar larvae garland nephrocytes with antibodies against Djub (green) and Pyd (gray). F-actin was stained using phalloidin (red). Details show actin filaments crossing Djub and Pyd-stained SDs or partial colocalization at intersections. (B) Actin staining with phalloidin (green) of third instar larvae garland nephrocytes after knockdown of Djub, Yki, or Dock (DjubRNAi, YkiRNAi, DockRNAi) or Warts overexpression showed a reduction of actin strains on the surface. (C) Densitometric quantification of F-actin by measurement of the relative coverage on the surface, normalized to control, illustrates this reduction caused by DjubRNAi, YkiRNAi, DockRNAi, or Warts overexpression (n>30). Scale bars 5 µm. Figure 5 can be viewed in color online at www.jasn.org.
Loss of Djub and Hippo Pathway Activation Increase SD Permeability
The function of Drosophila nephrocytes is to sequester toxic material. Uptake processes are regulated by (1) filtration across SDs with a size cutoff of around 70 kDa, (2) accumulation of filtrated material in the lacunae, and (3) consecutive endocytosis.12,32,33 Djub knockdown, Warts overexpression or Yki knockdown led to a significantly enhanced accumulation of fluorescently tagged BSA in the lacunae while Kirre knockdown abolished uptake nearly completely (Figure 6, A and B). Thus, effects of Djub downregulation on SD differ from the effects of downregulation of structural SD components.32 Different accumulation was also observed during incubation at 4°C, which virtually excludes enhanced endocytosis processes as a cause for increased FITC-BSA uptake (Supplemental Figure 6, A and B) and rather indicates increased accumulation of the tracer in the lacunae. In control nephrocytes, uptake of fluorescently tagged dextran demonstrated clear size selectivity with a high uptake of 4 kDa, an intermediate uptake of 70 kDa, and virtually no uptake of 500 kDa dextran (Supplemental Figure 7). In nephrocytes with a reduction of SD-bound Djub (DjubRNAi, WartsOE, YkiRNAi), dextrans of all analyzed sizes accumulated in the lacunae (Figure 6C, Supplemental Figure 8). This shows that filtration of small molecules like 4 kDa Dextran, which should freely pass SD, is not a completely unobstructed process in normal nephrocytes. In nephrocytes with reduced SD-bound Djub, the largest increase was observed for 70 kDa dextran. This became especially evident when we normalized the uptake of 70 and 500 kDa dextran to the uptake of 4 kDa under the same conditions (Supplemental Figure 9), indicating that while the filtration barrier persists and restricts the entry of large molecules, its filtration properties are affected most likely because of widening of SD (Figure 4G). Importantly, Warts knockdown or YkiV5 overexpression rescued the effects of Djub knockdown on dextran uptake (Figure 6C, Supplemental Figure 9) confirming that Djub exerts its effect on SD by suppressing the Hippo pathway. The model in Figure 6D illustrates our results.
Figure 6.
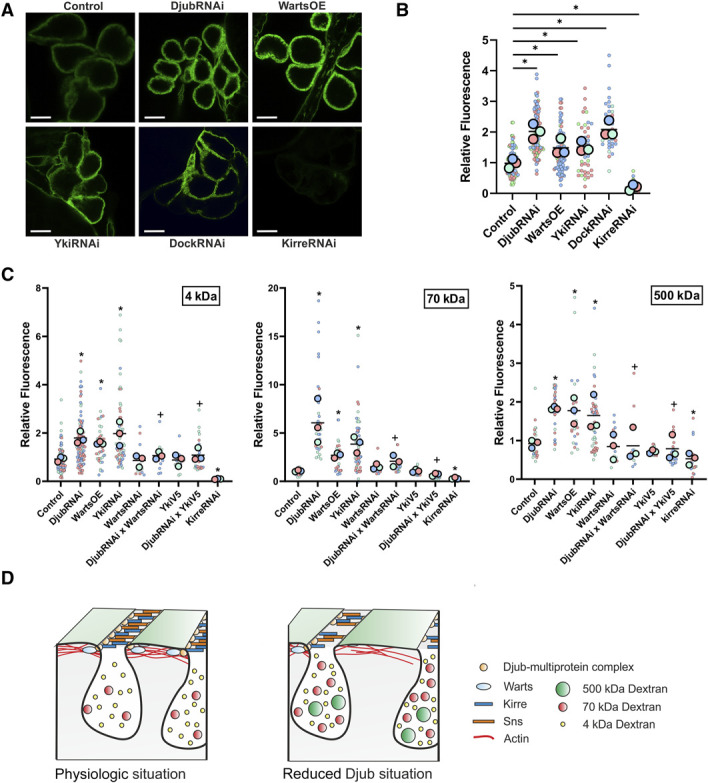
SD permeability increases because of loss of Djub and Hippo pathway activation. (A) Uptake assays with FITC-BSA (1 mg/ml, 1 minute incubation) at RT show increased FITC signal within the lacunae of the nephrocytes with Djub, Yki, or Dock knockdown (DjubRNAi, YkiRNAi, DockRNAi) and Warts overexpression while Kirre knockdown prevents uptake of FITC-BSA nearly completely. (B) Quantification of BSA uptake as shown in (A) confirms this observation (n>60). (C) Measurement of the uptake efficiency of FITC-dextran with molecular sizes of 4, 70, and 500 kDa. Djub or Yki knockdown (DjubRNAi, YkiRNAi) and WartsOE increase the uptake of all three FITC-dextrans. Enhancement of uptake efficiency is strongest for 70 kDa dextran. Simultaneous Warts knockdown (DjubRNAi×WartsRNAi) or Yki-V5 overexpression (DjubRNAi×YkiV5) rescues this effect. Kirre knockdown (KirreRNAi) shows almost no uptake of FITC-dextran (n>20). (D) Scheme: Under physiologic conditions, the SD proteins Sns and Kirre form a filtration barrier with a cutoff size of approximately 70 kDa, covering the nephrocytes labyrinthine channels. Djub as part of a multiprotein complex is localized at the SD and recruits Warts to SDs, which ensures Hippo pathway inactivity. A loss of Djub destabilizes the SD complex, which leads to fewer functional SDs and an increased permeability. Activation of the Hippo pathway enhances this effect. *P < 0.05 compared with control, +P < 0.05 compared with DjubRNAi. Figure 6 can be viewed in color online at www.jasn.org.
Discussion
In this study, we have demonstrated that Ajuba family proteins regulate SD formation by controlling the Hippo pathway while being themselves a subject to regulation by the Hippo pathway. These results provide new insight into regulation of structural and functional features of nephrocytes and potentially podocytes.
YAP is active in podocytes both in vivo and in vitro,2 which is very unusual for postmitotic cells, because a well-known function of YAP is to regulate cell growth and proliferation. This raises two questions: (1) What is the importance of YAP in podocytes and (2) how is the Hippo pathway, which inactivates YAP in other postmitotic cells, kept silent in podocytes? Our previous report has already revealed one important function of YAP in podocytes—to prevent apoptotic cell death.2 In this study, we have demonstrated that YAP plays another important role by supporting formation and integrity of SD. This was demonstrated using Drosophila GCNs, a well-established model to study SD formation. In GCNs, knockdown of Yki, Drosophila homolog of YAP, or Warts overexpression, kinase-inactivating Yki led to structural and functional impairments of SD, such as (1) reduction in the number of SD, (2) increase in SD width, (3) misorganization of the actin cytoskeleton, and (4) increased permeability. In contrast to a plethora of proteins, knockdown of which results in reduced/abolished permeability,32 Djub knockdown has an opposite effect indicating that Djub plays a modulatory role in fine-tuning of the SD structure and function. The overall structure of SDs remains intact, but widened SDs enable increased uptake. Effects similar to ours were demonstrated if GSK3 was knocked down or inhibited by LiCl.34
We have also demonstrated that Djub is essential for YAP activity in nephrocytes. First, knockdown of Djub in nephrocytes led to a decrease in Yki-dependent gene expression. Second, defects in SD induced by Djub knockdown could be rescued by the expression of a constitutively active YkiV5 mutant. It is also important that Djub was required for localization of Warts to SD. This is in line with previous reports that Djub sequesters Warts at cell-cell contacts in Drosophila wings and Djub knockdown leads to activation of the Hippo pathway.7,10
Interestingly, Ajuba proteins are themselves a subject of regulation by the Hippo pathway. We could show that Hippo activation influences Djub localization at SD in nephrocytes. The reciprocal relationship between Ajuba proteins and the Hippo pathway could be revealed in molecular detail in cultured human podocytes. While these cells do not form SD and, therefore, cannot be used to study SD formation and function, they preserve dependence on YAP activity for survival2 and can, therefore, be used to investigate podocyte-specific regulation of YAP. Overexpression of active or wt LATS2, the most downstream kinase of the Hippo pathway, which phosphorylates and inactivates YAP, as well as YAP knockdown led to decreased levels of all three members of the Ajuba family. WTIP and Ajuba were described as transcriptional targets of YAP in breast cancer cells26; we could confirm transcriptional regulation of both by LATS2 activity in cultured human podocytes.2 Even more importantly, Ajuba proteins and LATS2 regulate localization and redistribution of each other in podocytes. Ajuba proteins interact with LATS2/Warts7–10. Indeed, localization and redistribution of Ajuba/LATS2 depend on the expression of LATS2 or Ajuba proteins but not on LATS2 activity because even inactive mutant LATS2-TA caused this redistribution.
Combining our results from cultured human podocytes and Drosophila nephrocytes, we propose the following hypothesis about the role of Ajuba proteins in the regulation of SD functionality. Interaction with Ajuba proteins sequesters LATS2 at the SD and prevents it from phosphorylating YAP. In this way, YAP can be maintained actively, which is essential for podocyte survival and formation of SD. Ajuba proteins also regulate the organization of the SD-associated cytoskeleton.4,5,11,27 In its turn, actin remodeling also affects Hippo pathway activity in podocytes.35 These roles of Ajuba proteins indicate that their misregulation under pathological conditions may lead to a runaway effect culminating in the failure of the podocyte function. Nuclear exclusion of YAP, which is observed during FSGS,36 first could be restrained by Ajuba protein sequestering LATS and ensuring sufficient YAP activity for podocytes survival and function. However, prolonged or excessive activity of Hippo would lead to a decrease in the level of Ajuba proteins. This, in turn, would result in unhindered inhibition of YAP by LATS, further decreasing Ajuba proteins, and, because of diminished SD formation and increased permeability, impaired podocyte function even before cell death because of the lack of YAP activity.
Taken together, the results of our study identified a feedback mechanism between the Hippo pathway and Ajuba proteins and established Ajuba proteins as an essential link connecting Hippo pathway regulation to formation of SD in podocytes. By restraining the activity of the Hippo pathway and controlling the integrity of the SD, Ajuba proteins play an essential role in podocyte function and survival. Because the level of Ajuba proteins in podocytes can be regulated pharmacologically,34 Ajuba proteins may be a potential target for FSGS treatment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrea Ricker for excellent technical assistance. We also thank the Bloomington Drosophila Stock Center at the University of Indiana (United States), the Vienna Drosophila Resource Center (Austria), and the Developmental Studies Hybridoma Bank at the University of Iowa (United States) for providing reagents.
Disclosures
H.-J. Pavenstädt reports Research Funding: Sanofi; and Advisory or Leadership Role: Deutsche Forschungsgemeinschaft (DFG). B.A. Vollenbröker reports spouse Employer: Klosterfrau Healthcare Group. The remaining authors have nothing to disclose.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (Grant Numbers: CRC1348‐A05, KR3901/8‐1, VO 2312/2-1, WE 5823/3-1) and IZKF of the University of Muenster (Grant Number: Kr-A-031.21).
Author Contributions
Conceptualization: Kevin Gilhaus, Michael P. Krahn, Pavel I. Nedvetsky, Beate A. Vollenbröker, Dirk O. Wennmann.
Data curation: Claudia Cepok, Jana Emich, Kevin Gilhaus, David Kamm, Harald Nüsse, Katharina Saatkamp, Alina Sundukova, Beate Surmann.
Formal analysis: Claudia Cepok, Jana Emich, Kevin Gilhaus, David Kamm, Katharina Saatkamp, Alina Sundukova, Beate Surmann.
Funding acquisition: Michael P. Krahn, Beate A. Vollenbröker, Dirk O. Wennmann.
Methodology: Kevin Gilhaus, Harald Nüsse.
Project administration: Beate A. Vollenbröker.
Resources: Britta George, Jürgen Klingauf, Hermann-Joseph Pavenstädt.
Supervision: Michael P. Krahn, Hermann-Joseph Pavenstädt, Beate A. Vollenbröker.
Validation: Pavel I. Nedvetsky.
Visualization: Kevin Gilhaus, Beate A. Vollenbröker.
Writing – original draft: Kevin Gilhaus, Pavel I. Nedvetsky, Beate A. Vollenbröker.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E385 and http://links.lww.com/JSN/E386.
Supplemental Figure 1. Djub knockdown decreases the diameter of nephrocytes.
Supplemental Figure 2. Localization of Ajuba proteins at focal adhesions and cell-cell contacts in podocytes.
Supplemental Figure 3. Increased LATS2 expression results in recruitment of Ajuba proteins in punctate structures in the cytoplasm independent of kinase activity.
Supplemental Figure 4. Redistribution of Ajuba proteins occurs independently from YAP localization.
Supplemental Figure 5. Second DjubRNAi line shows comparable SD phenotype.
Supplemental Figure 6. Djub knockdown increases the uptake of FITC-BSA.
Supplemental Figure 7. Uptake efficiency of FITC-Dextran depends on molecular size.
Supplemental Figure 8. Representative images of Dextran uptake experiments.
Supplemental Figure 9. Increased uptake efficiency, especially of 70 kDa FITC-Dextran, after Djub knockdown or Hippo activation demonstrates changes in size selectivity.
Supplemental Video 1. Live cell imaging after induction of RFP-LATS2-TA expression (red) in a mixed AB8/13 cell culture with cells stably expressing EGFP-LIMD1 (green) and others without EGFP-LIMD1 overexpression.
References
- 1.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi: 10.1038/ncb2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonse J, Wennmann DO, Kremerskothen J, et al. Nuclear YAP localization as a key regulator of podocyte function. Cell Death Dis. 2018;9(9):850. doi: 10.1038/s41419-018-0878-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartzman M, Reginensi A, Wong JS, et al. Podocyte-specific deletion of yes-associated protein causes FSGS and progressive renal failure. J Am Soc Nephrol. 2016;27(1):216–226. doi: 10.1681/ASN.2014090916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nola S, Daigaku R, Smolarczyk K, et al. Ajuba is required for Rac activation and maintenance of E-cadherin adhesion. J Cell Biol. 2011;195(5):855–871. doi: 10.1083/jcb.201107162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marie H, Pratt SJ, Betson M, et al. The LIM protein ajuba is recruited to cadherin-dependent cell junctions through an association with α-catenin. J Biol Chem. 2003;278(2):1220–1228. doi: 10.1074/jbc.M205391200 [DOI] [PubMed] [Google Scholar]
- 6.Abe Y, Ohsugi M, Haraguchi K, Fujimoto J, Yamamoto T. LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 2006;580(3):782–788. doi: 10.1016/j.febslet.2005.12.096 [DOI] [PubMed] [Google Scholar]
- 7.Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol. 2010;20(7):657–662. doi: 10.1016/j.cub.2010.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibar C, Kirichenko E, Keepers B, Enners E, Fleisch K, Irvine KD. Tension-dependent regulation of mammalian Hippo signaling through LIMD1. J Cell Sci. 2018;131(5):jcs214700. doi: 10.1242/jcs.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagannathan R, Schimizzi GV, Zhang K, et al. AJUBA LIM proteins limit Hippo activity in proliferating cells by sequestering the Hippo core kinase complex in the cytosol. Mol Cell Biol. 2016;36(20):2526–2542. doi: 10.1128/MCB.00136-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. Cytoskeletal tension inhibits Hippo signaling through an Ajuba–Warts complex. Cell. 2014;158(1):143–156. doi: 10.1016/j.cell.2014.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyvani Chahi A, Martin CE, Jones N. Nephrin suppresses Hippo signaling through the adaptor proteins Nck and WTIP. J Biol Chem. 2016;291(24):12799–12808. doi: 10.1074/jbc.M116.724245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang S, Shao H, Guo F, Trimble R, Pearce E, Abmayr SM. Sns and Kirre, the Drosophila orthologs of Nephrin and Neph1, direct adhesion, fusion and formation of a slit diaphragm-like structure in insect nephrocytes. Development. 2009;136(14):2335–2344. doi: 10.1242/dev.031609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Ueda A, Wu C-F. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18(2):377–402. doi: 10.1080/01677060490894522 [DOI] [PubMed] [Google Scholar]
- 14.Hochapfel F, Denk L, Mendl G, et al. Distinct functions of Crumbs regulating slit diaphragms and endocytosis in Drosophila nephrocytes. Cell Mol Life Sci. 2017;74(24):4573–4586. doi: 10.1007/s00018-017-2593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dlugos CP, Picciotto C, Lepa C, et al. Nephrin signaling results in integrin β1 activation. J Am Soc Nephrol. 2019;30(6):1006–1019. doi: 10.1681/ASN.2018040362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unnersjö-Jess D, Scott L, Sevilla SZ, Patrakka J, Blom H, Brismar H. Confocal super-resolution imaging of the glomerular filtration barrier enabled by tissue expansion. Kidney Int. 2018;93(4):1008–1013. doi: 10.1016/j.kint.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 17.Heiden S, Siwek R, Lotz ML, et al. Apical-basal polarity regulators are essential for slit diaphragm assembly and endocytosis in Drosophila nephrocytes. Cell Mol Life Sci. 2021;78(7):3657–3672. doi: 10.1007/s00018-021-03769-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630–638. doi: 10.1681/ASN.v133630 [DOI] [PubMed] [Google Scholar]
- 19.Schulze U, Vollenbröker B, Kühnl A, et al. Cellular vacuolization caused by overexpression of the PIKfyve-binding deficient Vac14 L156R is rescued by starvation and inhibition of vacuolar-ATPase. Biochim Biophys Acta Mol Cell Res. 2017;1864(5):749–759. doi: 10.1016/j.bbamcr.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 20.Duning K, Schurek E-M, Schlüter M, et al. KIBRA modulates directional migration of podocytes. J Am Soc Nephrol. 2008;19(10):1891–1903. doi: 10.1681/ASN.2007080916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid-Burgk JL, Höning K, Ebert TS, Hornung V. CRISPaint allows modular base-specific gene tagging using a ligase-4-dependent mechanism. Nat Commun. 2016;7(1). doi: 10.1038/ncomms12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(3):213–232. doi: 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- 23.Lord SJ, Velle KB, Mullins RD, Fritz-Laylin LK. SuperPlots: communicating reproducibility and variability in cell biology. J Cell Biol. 2020;219(6):e202001064. doi: 10.1083/JCB.202001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi: 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 25.Gerstner L, Chen M, Kampf LL, et al. Inhibition of endoplasmic reticulum stress signaling rescues cytotoxicity of human apolipoprotein-L1 risk variants in Drosophila. Kidney Int. 2022;101(6):1216–1231. doi: 10.1016/j.kint.2021.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanconato F, Forcato M, Battilana G, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17(9):1218–1227. doi: 10.1038/ncb3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srichai MB, Konieczkowski M, Padiyar A, et al. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem. 2004;279(14):14398–14408. doi: 10.1074/jbc.M314155200 [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Konieczkowski M, Mukherjee A, et al. Podocyte injury induces nuclear translocation of WTIP via microtubule-dependent transport. J Biol Chem. 2010;285(13):9995–10004. doi: 10.1074/jbc.M109.061671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Mukherjee A, Madhavan SM, Konieczkowski M, Sedor JR. WT1-interacting protein (Wtip) regulates podocyte phenotype by cell-cell and cell-matrix contact reorganization. Am J Physiol Renal Physiol. 2012;302(1):F103–F115. doi: 10.1152/ajprenal.00419.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17(9):428–437. doi: 10.1016/j.tcb.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 31.Carrasco-Rando M, Prieto-Sánchez S, Culi J, Tutor AS, Ruiz-Gómez M. A specific isoform of Pyd/ZO-1 mediates junctional remodeling and formation of slit diaphragms. J Cell Biol. 2019;218(7):2294–2308. doi: 10.1083/jcb.201810171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermle T, Braun DA, Helmstädter M, Huber TB, Hildebrandt F. Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J Am Soc Nephrol. 2017;28(5):1521–1533. doi: 10.1681/ASN.2016050517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weavers H, Prieto-Sánchez S, Grawe F, et al. The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature. 2009;457(7227):322–326. doi: 10.1038/nature07526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurcombe JA, Hartley P, Lay AC, et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat Commun. 2019;10(1):403. doi: 10.1038/s41467-018-08235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wennmann DO, Vollenbröker B, Eckart AK, et al. The Hippo pathway is controlled by Angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis. 2014;5(11). doi: 10.1038/cddis.2014.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meliambro K, Wong JS, Ray J, et al. The Hippo pathway regulator KIBRA promotes podocyte injury by inhibiting YAP signaling and disrupting actin cytoskeletal dynamics. J Biol Chem. 2017;292(51):21137–21148. doi: 10.1074/jbc.M117.819029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available in this article.