Keywords: genetics, ADPKD, hypertension, neurovascular, intracranial aneurysms, autosomal polycystic kidney disease, aneurysmal locations
Abstract
Key Points
IAs location distribution in patients with ADPKD differ from the ones in non-ADPKD patients
IAs in patients with ADPKD are more commonly located in the anterior circulation and in large caliber arteries
Because of IA multiplicity and singular IA distribution, patients with ADPKD represent a special population who need to be closely followed
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most common genetic condition associated with intracranial aneurysms (IAs). The associated pathophysiology remains unknown, but an association with wall shear stress is suspected. Cerebral arterial location is the principal factor influencing IA natural history. This study aims to compare IA location-specific distribution between ADPKD and non-ADPKD patients.
Methods
The ADPKD group comprised data from a systematic review of the literature (2016–2020, N=7) and three cohorts: integrated biomedical informatics for the management of cerebral aneurysms, Novosibirsk, and Unruptured Cerebral Aneurysms Study. The non-ADPKD group was formed from the integrated biomedical informatics for the management of cerebral aneurysms, Unruptured Cerebral Aneurysms Study, International Stroke Genetics Consortium, and the Finnish cohort from the literature. Patients and IAs characteristics were compared between ADPKD and non-ADPKD groups, and a meta-analysis for IA locations was performed.
Results
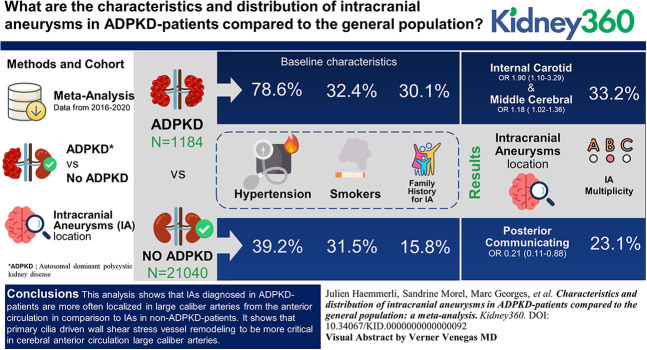
A total of 1184 IAs from patients with ADPKD were compared with 21,040 IAs from non-ADPKD patients. In total, 78.6% of patients with ADPKD had hypertension versus 39.2% of non-ADPKD patients. A total of 32.4% of patients with ADPKD were smokers versus 31.5% of non-ADPKD patients. In total, 30.1% of patients with ADPKD had a positive family history for IA versus 15.8% of the non-ADPKD patients. Patients with ADPKD showed a higher rate of IA multiplicity (33.2% versus 23.1%). IAs from patients with ADPKD showed a significant predominance across the internal carotid and middle cerebral arteries. Posterior communicating IAs were more frequently found in the non-ADPKD group. The meta-analysis confirmed a predominance of IAs in the patients with ADPKD across large caliber arteries (odds ratio [95% confidence interval]: internal carotid artery: 1.90 [1.10 to 3.29]; middle cerebral artery: 1.18 [1.02–1.36]). Small diameter arteries, such as the posterior communicating, were observed more in non-ADPKD patients (0.21 [0.11–0.88]).
Conclusion
This analysis shows that IAs diagnosed in patients with ADPKD are more often localized in large caliber arteries from the anterior circulation in comparison with IAs in non-ADPKD patients. It shows that primary cilia driven wall shear stress vessel remodeling to be more critical in cerebral anterior circulation large caliber arteries.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by several extrarenal manifestations, such as intracranial aneurysms (IAs).1–10 The incidence of IAs in patients with ADPKD is higher than the one reported in the general population (4%–40% versus 3%–5%, respectively).2–4,7,11 Patients with ADPKD carry a mutation in polycystin-1 or polycystin-2 genes coding for proteins found in the cerebrovascular endothelial layer and in the lamina muscularis.2,12,13 One hypothesis regarding the development of IAs in patients with ADPKD is that mutated polycystins affect the expression or the function of the primary cilia present in vascular cells leading to an abnormal vascular fragility.14 In arterial endothelial cells, primary cilia are sensors of wall shear stress (WSS), an hemodynamic factor well known to be part of IA formation.15–17
Risks factors for IAs rupture have been extensively analyzed in patients not suffering of non-ADPKD.3,18–20 Observations from these studies highlight that IA location is the strongest factor associated with IA formation and rupture.18–22 More recently, location-based hemodynamic and computed fluid dynamic models have been conducted to understand the appearance and evolution of IA and have demonstrated different patterns of WSS in the cerebral arterial tree.23–26 Considering the importance of WSS sensing in aneurysmal disease, we hypothesized that IAs distribution and the diameter of affected arteries could differ between ADPKD and non-ADPKD patients. To investigate differences between demographic data of ADPKD and non-ADPKD patients and differences between characteristics of their IAs, we analyzed combined data from six studies from the literature and three independent cohorts.
Materials and Methods
Review of the Literature and PRISMA Statements
A systematic review of the literature was conducted in three databases (PubMed/MEDLINE and Embase). Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram used for the purpose of this study.27 The literature was searched using the following sequence on PubMed/MEDLINE “(((intracranial aneurysms [Title/Abstract]) OR cerebral aneurysms)) AND ((autosomal polycystic kidney disease [Title/Abstract]) OR polycystic kidney disease [Title/Abstract]).” Search on Embase was performed using the sequence “('intracranial aneurysms':ab,ti OR 'cerebral aneurysms':ab,ti) AND [2016-2020]/py”. Inclusion criteria were (1) series or systematic reviews reporting patients with ADPKD with IA from November 2016 to February 2020, (2) studies on IA treatment of patients with ADPKD, (3) comparison of non-ADPKD versus ADPKD patients with IAs, and (4) articles in English/French/German. Exclusion criteria were (1) case reports or collected data on <3 patients, (2) review, (3) studies reporting patients with ADPKD without detailed information on IAs, (4) studies on IAs without detailed information on patients with ADPKD, and (5) genetic studies. For case-control studies of patients with ADPKD, only patients with ADPKD and IA data were included. No patients with other cystic genetic condition associated with intracranial aneurism were included within the ADPKD group.
Figure 1.
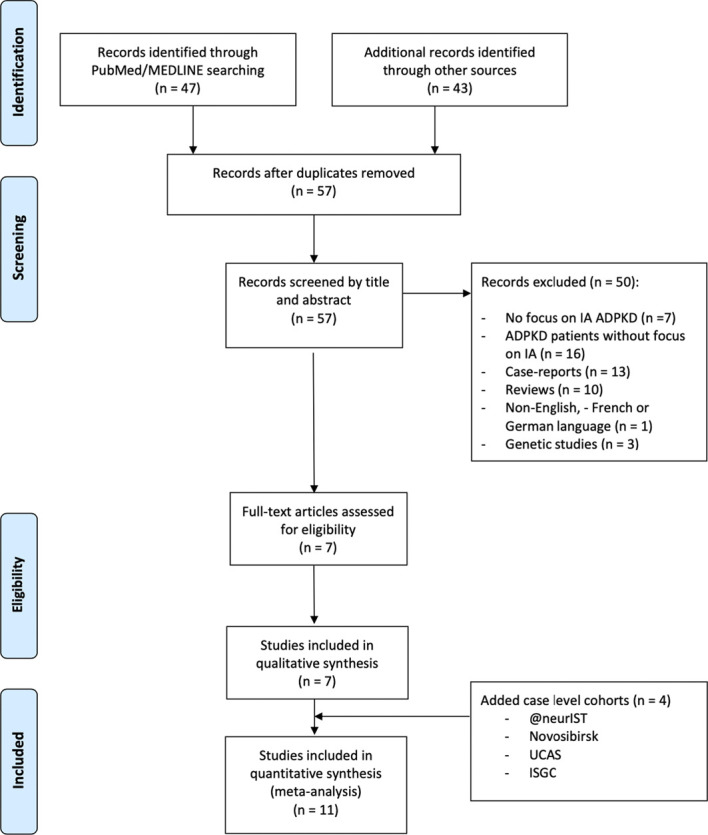
PRISMA flow diagram. @neurIST, integrated biomedical informatics for the management of cerebral aneurysms; ADPKD, autosomal polycystic kidney disease; IA, intracranial aneurysms; ISGC, International Stroke Genetics Consortium; UCAS, Unruptured Cerebral Aneurysms Study.
Prospective Data
Data on ADPKD and non-ADPKD patients were collected from three cohorts of patients diagnosed with at least one IA and with a known ADPKD status. Patients with other cystic genetic condition were excluded from all prospective cohorts.
The integrated biomedical informatics for the management of cerebral aneurysms (@neurIST) cohort of the Geneva University Hospitals is a prospective and consecutive collection of clinical, radiologic, and pathologic information on patients with IAs.12 Inclusion criteria were (1) newly diagnosed IA between 2006 and 2017 on the basis of angiographic imaging (digital subtraction angiography, magnetic resonance angiogram, or computed tomography with angiogram sequence); (2) patients older than 18 years; (3) presence or absence of ADPKD: the diagnosis of ADPKD was previously made based on enlarged kidney size, the presence of bilateral renal cysts and/or familial history, and made by a nephrologist; and (4) acceptance and signature of consent forms. This study was approved by the Ethical Committee of the Geneva University Hospitals, Geneva, Switzerland, as part of the @neurIST study (PB_2018-0073, previously NAC 07-056). All patients gave their consent and approved for the use of clinical and radiologic data, as well as biologic samples in the field of cerebrovascular research.
The Novosibirsk cohort is a prospective collection of consecutive data only on patients with ADPKD. The applied inclusion criteria were the same as for the @neurIST cohort. This study was approved by the local ethical committee (N°11, researchers Anatoliy V. Bervitskiy and Jamil Rzaev).
The Unruptured Cerebral Aneurysms Study (UCAS) cohort is a prospective collection of data already used in 2012 to study the natural history of aneurysms in the Japanese population. The ADPKD status of patients was recorded and not yet analyzed nor reported.19 In accordance with the authors and the local ethical committee, we included the patients with ADPKD from this cohort to the ADPKD group.
The systematic review lacks information on non-ADPKD cases to allow an ADPKD versus non-ADPKD comparison. Only the study by Nurmonen et al.5 compared ADPKD and non-ADPKD patients. To allow comparing ADPKD patient with non-ADPKD patient characteristics, data collected globally in the context of the International Stroke Genetics Consortium (ISGC) IA working group (International Stroke Genetics Consortium Intracranial Aneurysm Group) was used.22 To avoid duplicates, Geneva @neurIST cases were removed from the ISGC dataset for this analysis. All patients from the ISGC, Nurmonen et al. study, @neurIST, and UCAS cohorts who were not affected by ADPKD formed the non-ADPKD group. The non-ADPKD group was used as reference group for all comparison when data on non-ADPKD were missing (Table 1).
Table 1.
Distribution of nonautosomal polycystic kidney disease intracranial aneurysmss per locations from the Finnish, integrated biomedical informatics for the management of cerebral aneurysms, Unruptured Cerebral Aneurysms Study, and International Stroke Genetics Consortium cohorts
| Study | ICA N (%) |
Acom N (%) |
MCA N (%) |
Perical. N (%) |
Pcom N (%) |
PCir N (%) |
Other N (%) |
Total N |
|---|---|---|---|---|---|---|---|---|
| Nurmonen et al.5 | 446 (7) | 1332 (21) | 2612 (40) | 320 (5) | 904 (14) | 569 (9) | 276 (4) | 6459 |
| @neurIST | 185 (18) | 211 (20) | 359 (35) | 41 (4) | 87 (8) | 74 (7) | 74 (7) | 1031 |
| UCAS19 | 1244 (19) | 1034 (15) | 2415 (36) | 267 (4) | 1034 (15) | 565 (8) | 116 (2) | 6675 |
| ISGC22 | 1045 (15) | 1926 (28) | 1709 (25) | 238 (3) | 1084 (16) | 794 (12) | 79 (1) | 6875 |
| All non-ADPKD | 2920 (14) | 4503 (21) | 7095 (34) | 866 (4) | 3109 (15) | 2002 (10) | 545 (3) | 21,040 |
ICA, internal carotid artery; Acom, anterior communicating artery; MCA, middle cerebral; Peri, pericallosal artery; Pcom, posterior communicating; PCir, posterior circulation; @neurIST, integrated biomedical informatics for the management of cerebral aneurysms; UCAS, Unruptured Cerebral Aneurysms Study; ISGC, International Stroke Genetics Consortium; ADPKD, autosomal dominant polycystic kidney disease.
Studied Parameters
The following demographic parameters were searched for both ADPKD and non-ADPKD groups: (1) age, (2) sex, (3) positive family history of IA, (4) high blood pressure (hypertension), (5) smoking status, and (6) multiplicity of IA.
The following radiologic parameters were searched for both groups: (1) number of IA, (2) IA maximal diameter, and (3) IA location. On the basis of the current literature,19,21,28 locations were defined as internal carotid artery (ICA, including ophthalmic and bifurcation segments), anterior communicating artery (Acom), middle cerebral artery (MCA, including M1 segment, bifurcation, and M2 segment), posterior communicating artery (Pcom), pericallosal artery (Peri), posterior circulation (PCir, including posterior inferior cerebellar artery, basilar, and it branches, posterior cerebral artery), and other locations (other).
Aneurysm rupture status was not evaluated for the purpose of this study. In addition, concerning the ADPKD group, neither the renal function at time of IA diagnosis nor the renal transplant status were not recorded as these data were missing in the included literature.
Outcomes
Basic characteristics of patients with ADPKD with IAs were compared with non-ADPKD patients with IAs. The primary aim was to compare and potentially identify differences in distribution across locations of IAs between the ADPKD and the non-ADPKD cohorts. Secondary outcomes were the demographic data analysis and IA characteristics between the ADPKD and the non-ADPKD populations.
Statistics and Analysis
RStudio was used for the purpose of the analysis (RStudio, version 1.2.5019 running R version 3.6.1). Binary variables were expressed in proportions and analyzed using a chi-squared test and a Fisher exact test. Ordinal variables were expressed in median and quartiles and were compared using the Fisher exact test or Mann-Whitney U test when appropriate. Continuous variables were reported for means and SD and were compared using two-tailed nonpaired t test. The assessments of differences in distribution among groups were performed using a Kruskal-Wallis test. Hypothesis testing was considered significant for P value <0.05. Distribution of IA according to locations was expressed using Pearson residuals, with value ≥2 considered as significant (P<0.001). The meta-analysis included all available data. Distribution of IA among locations in the ADPKD group was homogenous between all data sources. Distribution of IA among location in the non-ADPKD group was heterogeneous. It was decided to perform in addition to the pooled data analysis presented in Figure 2 and Table 2, a meta-analysis comparing the distribution of IAs among locations in each study where the data were available and use the data of the non-ADPKD group if non-ADPKD data were missing in this study. For the purpose of the meta-analysis according to locations, odds ratios (ORs) and relative risks were calculated per locations comparing ADPKD-IAs and non–ADPKD-IAs. A fixed effect model was retained when the calculated heterogeneity I2 was ≥70%, and a random effect model was accepted for a I2 >70%.29,30 An OR above 1.0 represents an overrepresentation in the ADPKD group.
Figure 2.
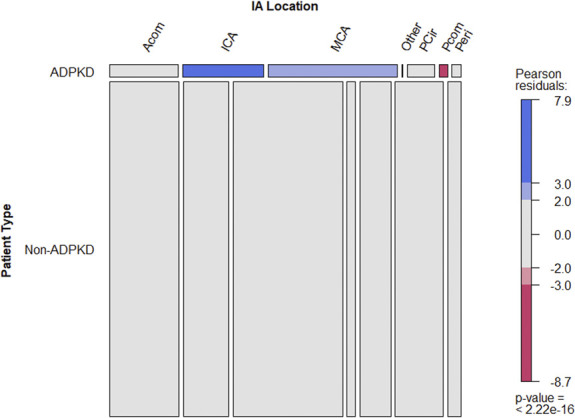
Mosaic plot depicting the number of IAs in the ADPKD and non-ADPKD groups for each IA location. Exact numbers and relative proportions regarding IAs by locations groups between Autosomal dominant polycystic kidney disease (ADPDK) and non-ADPKD patients are presented in Table 2. Acom, anterior communicating artery; ADPKD, autosomal dominant polycystic kidney disease; IA, intracranial aneurysms; ICA, internal carotid artery; MCA, middle cerebral artery; PCir, posterior circulation; Pcom, posterior communicating artery; Peri, pericallosal artery.
Table 2.
Patients and IAs characteristics in the ADPKD and non-ADPKD groups
| COLUMN HEADING | ADPKD | Non-ADPKD | P value |
|---|---|---|---|
| Patients characteristics | |||
| Total of patients | 894 | 17,864 | |
| Age, yr (mean±SD) | 51.32±4.0 | 56.9±4.9 | |
| Female | 608 (68%) | 11,575 (65%) | |
| Tobacco | 84/259 (32.4%) | 5627 (31.5%) | 0.74 |
| Hypertension | 703 (78.6%) | 6946 (38.9%) | <0.001 |
| IA multiplicity | 81/244 (33.2%) | 4131 (23.1%) | <0.001 |
| IA-positive family history | 78/259 (30.1%) | 2831 (15.8%) | <0.001 |
| IAs characteristics | |||
| Total of aneurysms | 1184 | 21,040 | |
| Size, mm (mean±SD) | 5.1±0.99 | 6.2±0.62 | |
| Total of IA with location | 795 (67.1%) | 21,040 (100%) | |
| Location | |||
| MCA | 317 (39.9%) | 7095 (33.7%) | <0.001 |
| ICA | 198 (24.9%) | 2920 (13.9%) | <0.001 |
| Acom | 168 (21.1%) | 4503 (21.4%) | 0.89 |
| Pcom | 21 (2.6%) | 3109 (14.8%) | <0.001 |
| Peri | 22 (2.8%) | 866 (4.1%) | 0.06 |
| PCir | 66 (8.3%) | 2002 (9.5%) | 0.27 |
| Other | 3 (0.4%) | 545 (2.62%) | <0.001 |
IA, intracranial aneurysm; ADPKD, autosomal dominant polycystic kidney disease; MCA, middle cerebral artery; ICA, internal carotid artery; Acom, anterior communicating artery; Pcom, posterior communicating artery; Peri, pericallosal artery; PCir, posterior circulation.
Results
Literature Review and Additional Cohorts
The search strategy is presented in Figure 1. A total of 90 studies published between November 2016 and February 2020 reporting patients with ADPKD with IAs were identified. After removal of duplicates and according to inclusion and exclusion criteria, seven studies were included (Table 3). In addition, patients with ADPKD from the @neurIST (21 patients with ADPKD with 45 IAs), Novosibirsk (5 patients with ADPKD with nine IAs), and UCAS (18 patients with ADPKD with 22 IAs) cohorts were included (Table 3). Nurmonen et al. conducted a prospective analysis including Finnish patients from a local databank. The authors compared demographic and radiographic data on IAs among ADPKD and non-ADPKD patients from Finland.5
Table 3.
Characteristics of the included studies
| Study | Article Type | Origin | Total of Patients | Total of Non-ADPKD Patients | Total of ADPKD-Patients | Total of IAs | Total of Non–ADPKD-IAs | Total of ADPKD-IAs |
|---|---|---|---|---|---|---|---|---|
| Included studies from systematic review of the literature (2016–2020) | ||||||||
| Nurmonen et al.5 | Cohort | Finland | 4436 | 4383 | 53 | 6554 | 6459 | 95 |
| Cagnazzo et al.4 | Systematic review | International | 563 | 0 | 563 | 679 | 0 | 679 |
| Sorenson et al.6 | Case-control | USA | 42 | 0 | 42 | 61 | 0 | 61 |
| Sanchis et al.11 | Cohort | USA | 75 | 0 | 75 | 94 | 0 | 94 |
| Kim et al.7 | Cohort | Korea | 23 | 0 | 23 | 43 | 0 | 43 |
| Wilkinson et al.31 | Cohort | USA | 45 | 0 | 45 | 71 | 0 | 71 |
| Yoshida et al.10 | Cohort | Japan | 49 | 0 | 49 | 65 | 0 | 65 |
| Additional included cohorts | ||||||||
| @neurIST | Cohort | Switzerland | 925 | 904 | 21 | 1076 | 1031 | 45 |
| Novosibirsk | Cohort | Russia | 5 | 0 | 5 | 9 | 0 | 9 |
| UCAS19 | Cohort | Japan | 5720 | 5702 | 18 | 6697 | 6675 | 22 |
| ISGC22 | Cohort | International | 0 | 6875 | 6875 | 0 | ||
| Total | 11,883 | 10,989 | 894 | 15,349 | 14,165 | 1184 | ||
ADPKD, autosomal polycystic kidney disease; IA, intracranial aneurysms; @neurIST, integrated biomedical informatics for the management of cerebral aneurysms; UCAS, Unruptured Cerebral Aneurysms Study; ISGC, International Stroke Genetics Consortium.
Non-ADPKD patients from the @neurIST, UCAS, ISGC cohorts, and the Finnish cohort were compared for IA distribution. Differences were found between the cohorts that are averaged out by pooling the data in a non-APDKD group used as a surrogate to impute for missing values. Consequently, a total of 10,989 patients with 14,165 IAs were included in the non-ADPKD group (Table 3).
Patients and IAs Characteristics in the ADPKD and Non-ADPKD Groups
A total of 894 patients with ADPKD with 1184 IAs were compared with 17,864 non-ADPKD patients with 21,040 IAs. Table 2 presents the demographic comparison between the non-ADPKD and the ADPKD groups. No difference was found regarding sex and age at diagnosis. A total of 32.4% of patients in the ADPKD group were smokers versus 31.5% in the non-ADPKD group (P = 0.74). At the time of IA diagnosis, uncontrolled or poorly controlled arterial hypertension was more frequently observed in the ADPKD group (78.6%) than in the non-ADPKD group (38.9%, P < 0.001). In total, 30.1% of ADPKD patients had a positive family history for IA against 15.8% in the non-ADPKD group (P < 0.001). Furthermore, multiple IAs were more frequently found in patients with ADPKD (33.2%) than in the non-ADPKD patients (23.1%, P < 0.001).
Concerning IAs characteristics between ADPKD and non-ADPKD patients (Table 2), no difference was found regarding IA size at diagnosis between the two groups (5.1±0.99 mm versus 6.2±0.62 mm, respectively). All IA locations were reported for the non-ADPKD group; 795 IA locations were described in the ADPKD group (67.1%). IAs distribution by location in the non-ADPKD group was as follows: MCA 33.7%, ICA 13.9%, Acom 21.4%, Pcom 14.8%, PCir 9.5%, Peri 4.1%, and other 2.6%. In the ADPKD group, in comparison with the non-ADPKD patients, IAs were more frequently found at ICA (24.9%, P < 0.001) and MCA (39.9%, P < 0.001) and less frequently at Pcom (2.6%, P < 0.001). No significant difference was found regarding the Acom location (21.1%, P = 0.89) and the Peri location (2.8%, P = 0.06). Interestingly, no significant difference was found concerning the PCir location (8.3%, P = 0.27). Figure 2 presents the mosaic plot of the distribution of IAs per location using the Pearson residual to assess the strength of the differences observed.
ORs of IAs Distribution by Location: Meta-Analysis
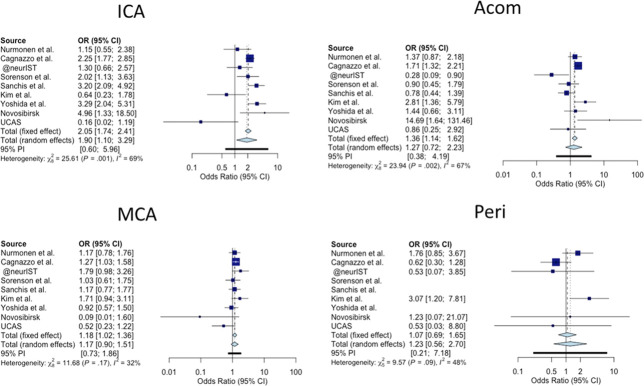
To investigate further the difference in IA locations between ADPKD and non-ADPKD patients, we have compared IA locations between these two groups for each study/cohort (Figures 3 and 4). The study conducted by Wilkinson et al. did not report IA location and has not been considered for this analysis.31 Regarding the anterior circulation locations (Figure 3), the meta-analysis revealed an OR in favor of patients with APDKD regarding the ICA location (OR: 1.90, 95% confidence interval [CI]: 1.10 to 3.29 in the random effect model). The same founding was observed for the MCA location (OR 1.18, 95% CI: 1.02 to 1.36, fixed effect model). No significant association was found the for Acom and Peri locations.
Figure 3.
Forest plot of the IA location distribution at the anterior circulation level. A fixed effect model was chosen for an interstudies heterogeneity I2≤70% and a random effect model for an I2>70%. An odds ratio >1 favors the location to Autosomal dominant polycystic kidney disease (ADPDK) condition. Acom, anterior communicating artery; CI, confidence interval; IA, intracranial aneurysms; ICA, internal carotid artery; MCA, middle cerebral artery; OR, odds ratio; Peri, pericallosal artery; UCAS, Unruptured Cerebral Aneurysms Study.
Figure 4.
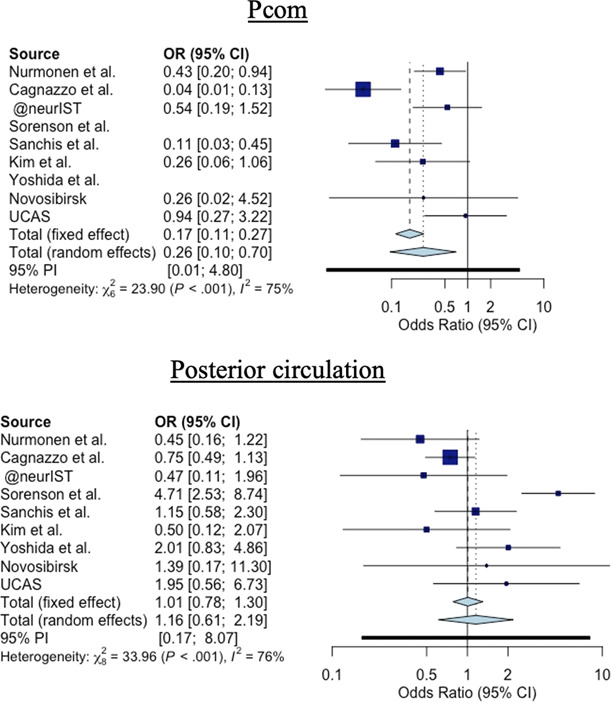
Forest plot of the IA location distribution at the posterior circulation level. A fixed effect model was chosen for an interstudies heterogeneity I2≤70% and a random effect model for a I2>70%. An odds ratio >1 favors the location to Autosomal dominant polycystic kidney disease (ADPDK) condition. CI, confidence interval; OR, odds ratio; Pcom, posterior communicating artery; UCAS, Unruptured Cerebral Aneurysms Study.
Concerning the Pcom and posterior locations (Figure 4), IAs in the non-ADPKD group were more associated with the Pcom location than IAs in the ADPKD group (OR: 0.31, 95% CI: 0.11 to 0.88, random effect model). Finally, the PCir location showed no significant result for ADPKD-IAs compared with non–ADPKD-IAs, with an OR 0.95 (95% CI, 0.74 to 1.22, fixed effect model).
Discussion
We present here a meta-analysis comparing IAs location of patients affected or not by ADPKD. Data were extracted from seven articles published between 2016 and 2020 and combined to data from 3 additional cohorts: @neurIST (Switzerland), Novosibirsk (Russia), and UCAS (Japan). The ADPKD group contained more smokers, patients presenting hypertension, patients having multiple IAs, or having a positive family history for IAs than the non-ADPKD group. We showed that in patients affected by ADPKD, the frequency of IAs location in MCA, ICA, and Acom is higher in comparison with non-ADPKD patients. Thus, patients with ADPKD presented less IAs located on Pcom or Peri.
It has previously been described that IAs in patients with ADPKD patients are more frequently found within the anterior circulation.32–34 Our analysis confirms such observations and emphasizes that IAs in patients with ADPKD are more particularly found on large caliber arteries of the anterior circulation, mostly ICA and MCA, in comparison with non-ADPKD patients. Concerning the PCir, 14.3% of the IAs observed in non-ADPKD patients are located at the Pcom, which is in accordance with the UCAS19 and the International Study of Unruptured Intracranial Aneurysms cohorts.20 Interestingly, in our cohort of patients with ADPKD, only 2.6% of the IAs are diagnosed at this location. Finally, a larger proportion of IA was observed in the Acom, the last large caliber artery of the anterior cerebral trunk, for the ADPKD group, however, without reaching the level of significance.
The clinical relevance of these findings resides in the threshold after which a treatment should be proposed. Thus, the threshold at which non–ADPKD-IA located across large caliber arteries of the anterior circulation are considered at risk of rupture has been set at 7 mm.28 Because ADPKD-IAs present the tendency to rupture at smaller diameters5,11,35 and to show more IA multiplicity, the threshold should probably set at a lower size in patients with ADPKD.
IA development is influenced by several factors, an important one being WSS.15–17 Recent studies showed that IAs predominate in arteries with high velocity and at bifurcations where WSS is high.36–39 Arterial hypertension is a well-known major risk factor for IA development and rupture.18,19,40 Indeed, high WSS conditions favor leukocytes recruitment leading to internal elastic lamina disruption and formation of IAs. Because of the progressive renal dysfunction, patients with ADPKD present a higher rate of hypertension than the general population (Table 2). However, as Niemczyk et al.40 pointed out, it is very unlikely that arterial hypertension is the only and major factor explaining the higher frequency of IA development compared with the general population. The authors analyzed the blood pressure pattern between ADPKD patients with and without IA as well as the blood pressure variation during the day and the night. They concluded that arterial hypertension and high variation in blood pressure may influence IA development but should not be considered as a necessary factor for IA development. In patients with ADPKD, IAs are more frequently found in large caliber arteries with rare small bifurcations where WSS is low. In this sense, high blood pressure in patients with ADPKD represents more a marker of the severity of the systemic cardiovascular dysfunction than a potent inducer of IA development. One sensor of WSS in endothelial cells is primary cilia whom the expression and function are controlled in part by the polycystin-1 and polycystin-2 proteins. However, their exact roles for the physiology of the cerebrovascular tree and for pathologic disorders affecting cerebral arteries remain unclear. Extrapolating their mechanosensing role in kidney cilia, it can be postulated that primary cilia absence or dysfunction in patients with ADPKD favors wall fragility of the cerebral arteries.14 In a recent study, Diagbouga et al.41 conducted a histopathologic analysis in which they compared aneurysm domes located on MCA between ADPKD and non-ADPKD patients. They showed that in comparison with non–ADPKD-IA domes, ADPKD-IA domes were thinner, contained less collagen, and had a higher frequency of extremely thin thrombosis-lined hypocellular wall which are characteristics of severe IA wall deterioration.41
Because of the singular IAs distribution and characteristics of patients with ADPKD, the questions of which specific follow-up patients with ADPKD should receive and when their IAs should be treated have to be answered. The actual guidelines for IA screening in patients with ADPKD are controversial and differ between specialist societies.1,11,31,42,43 However, recent publications have highlighted a benefit of a presymptomatic screening in patients with ADPKD. Sanchis et al., a nephrologic study group, reviewed 3010 patients with ADPKD from 1989 to 2017 of whom 812 patients without neurologic symptoms were screened with magnetic resonance angiography.11 They showed that 9.2% of the patients with ADPKD presented at least one IA and 1.7% had an IA ≤2 mm. The follow-up with magnetic resonance angiography allowed them to detect 1.07 de novo IAs per 100 patient-years and aneurysm growth in 13% of cases.11 The authors concluded that a presymptomatic screening was useful in their cohort. Similarly, Flahault et al. analyzed the cost-effectiveness of a presymptomatic screening in an ADPKD population.44 They concluded that a systematic presymptomatic screening was cost-effective and provided a gain of 0.68 quality-adjusted life years compared with targeted screening. The singular location distribution of IA among patients with ADPKD and their tendency to rupture at smaller diameter, we advocate for large IA screening and close imaging follow-up in all patients with ADPKD.
Limitations
The main limitation of this study resides in the heterogeneity observed between studies (Figures 3 and 4) as assessed by a moderate to high I2 value.29,45 To overcome this limitation, a prospective observational study on patients with ADPKD with IA should be conducted. Assessing the IA rupture rate in patients with ADPKD in a followed up cohort might be highly biased by patient selection and ethically compromised.
Another limitation is the lack of information in the literature of the renal function at the time of IA diagnosis in patients with ADPKD, as well as the transplant status. A prospective study on patients with ADPKD harboring IAs should be conducted, with close monitoring of the renal function.
Finally, as patients with ADPKD represent a small proportion of the general population,46 our study compares a relative low number of patients with ADPKD harboring IA to a higher number of non-ADPKD patients.
In this study, we showed that IAs location distribution in patients with ADPKD differ from the ones in non-ADPKD patients. IAs in patients with ADPKD are more commonly located in the anterior circulation and in large caliber arteries. The ADPKD population more often presents with multiple IAs compared with the non-ADPKD individuals. Because of IA multiplicity and singular IA location distribution, patients with ADPKD represent a special neurovascular population who need to be closely followed.
Supplementary Material
Acknowledgment
We thank all the study nurses, physicians, nurses, patients, and family members who have taken part in this study and helped create the different cohorts. We would like to acknowledge the ISGC-IA study group (participant list in Supplemental Table 1) for giving us access to the patient-level ISGC dataset. Consortium members were involved in the @neurIST project.
Footnotes
Due to the number of contributing authors, the affiliations are listed at the end of this article.
Disclosures
F. Chebib reports the following: Research Funding: Research grant—Otsuka pharmaceuticals and Advisory or Leadership Role: PKD foundation—Chair of Education Advisory Panel. All remaining authors have nothing to disclose.
Funding
The @neurIST project was supported by the sixth framework program of the European Commission (FP6-IST-2004-027,703). Geneva data collection was part of the AneuX project supported by the SwissSystemsX.chinitiative, evaluated by the Swiss National Science Foundation, and which also funded the SyBIT project. Dutch studies (YMR) have received funding from the European Research Council under the European Union's Horizon 2020 research and innovation program (PRYSM, Grant Agreement No. 852173). MKB and YMR were supported by the Netherlands Cardiovascular Research Initiative: An initiative with support of the Dutch Heart Foundation, CVON2015-08 ERASE. North American cohort (DW) was supported by NIH Funding. French data (RB) was supported by the French Regional Council of Pays-de-la-Loire (VaCaRMe program) and the Agence Nationale de la Recherche (ANR-15-CE17-0008-01 to G.L). HD and RB were supported by the French Ministry of Health (clinical trial NCT02848495 to HD), the Genavie Foundation, the Société Française de Radiologie, and the Société Française de Neuroradiologie. Spanish data collection (JJC and ECG) was supported in part by Spain's Ministry of Health (Instituto de Salud Carlos III Fondo de Investigaciones sanitarias P19/00011 and by “RICORS-ICTUS RD21/0006/0021). Canadian data collection (GAR) was supported by the Canadian Institutes of Health Research. Finnish data (MN) collection was supported by the Helsinki University Central Hospital EVO Grant No. TYH2018316. UK data (JB, GOSH study) collection was funded by the Stroke Association. Regarding the Japanese data (AM, UCAS study), the study was supported by the Ministry of Health, Labor, and Welfare in Japan; the National Cerebral and Cardiovascular Center in Japan; and the Japan Brain Foundation.
Author Contributions
Conceptualization: Philippe Bijlenga, Julien Haemmerli
Data curation: Anatoliy Bervitskiy, Marc Georges, Julien Haemmerli, Sandrine Morel, Akio Morita
Formal analysis: Philippe Bijlenga, Julien Haemmerli, Sandrine Morel
Investigation: Julien Haemmerli
Methodology: Philippe Bijlenga, Julien Haemmerli, Fadi Haidar, Sandrine Morel
Project administration: Julien Haemmerli
Resources: Philippe Bijlenga, Julien Haemmerli
Software: Julien Haemmerli
Supervision: Philippe Bijlenga, Karl Schaller
Validation: Anatoliy Bervitskiy, Philippe Bijlenga, Fouad Chebib, Julien Haemmerli, Fadi Haidar, Sandrine Morel, Akio Morita, Kazuhiko Nozaki, Jamil Rzaev, Karl Schaller, Teiji Tominaga
Visualization: Philippe Bijlenga, Julien Haemmerli, Fadi Haidar, Sandrine Morel, Kazuhiko Nozaki, Jamil Rzaev, Teiji Tominaga
Writing – original draft: Julien Haemmerli
Writing – review and editing: Anatoliy Bervitskiy, Philippe Bijlenga, Fouad Chebib, Julien Haemmerli, Fadi Haidar, Sandrine Morel, Akio Morita, Karl Schaller
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A329.
Supplemental Table 1. International Stroke Genetic Consortium—Aneurysm Group.
References
- 1.Wilkinson DA, Burke JF, Nadel JL, et al. A large database analysis of rates of aneurysm screening, elective treatment, and subarachnoid hemorrhage in patients with polycystic kidney disease. Neurosurgery. 2019;85(2):E266-E274. doi: 10.1093/neuros/nyy551 [DOI] [PubMed] [Google Scholar]
- 2.Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2015;11(10):589-598. doi: 10.1038/nrneph.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. 2017;13(2):126. doi: 10.1038/nrneurol.2017.14 [DOI] [PubMed] [Google Scholar]
- 4.Cagnazzo F, Gambacciani C, Morganti R, Perrini P. Intracranial aneurysms in patients with autosomal dominant polycystic kidney disease: prevalence, risk of rupture, and management. A systematic review. Acta Neurochir (Wien). 2017;159(5):811-821. doi: 10.1007/s00701-017-3142-z [DOI] [PubMed] [Google Scholar]
- 5.Nurmonen HJ, Huttunen T, Huttunen J, et al. Polycystic kidney disease among 4,436 intracranial aneurysm patients from a defined population. Neurology. 2017;89(18):1852-1859. doi: 10.1212/wnl.0000000000004597 [DOI] [PubMed] [Google Scholar]
- 6.Sorenson TJ, Brinjikji W, Jagani M, Wald JT, Lanzino G. Aneurysm morphology in patients with autosomal dominant polycystic kidney disease: a case-control study. J Clin Neurosci. 2019;69:220-223. doi: 10.1016/j.jocn.2019.07.048 [DOI] [PubMed] [Google Scholar]
- 7.Kim JY, Jung SC, Ko Y, et al. Intracranial aneurysms in patients receiving kidney transplantation for autosomal dominant polycystic kidney disease. Acta Neurochir. 2019;161(11):2389-2396. doi: 10.1007/s00701-019-04060-7 [DOI] [PubMed] [Google Scholar]
- 8.Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393(10174):919-935. doi: 10.1016/s0140-6736(18)32782-x [DOI] [PubMed] [Google Scholar]
- 9.Niemczyk M, Gradzik M, Fliszkiewicz M, Kulesza A, Gołębiowski M, Pączek L. Natural history of intracranial aneurysms in autosomal dominant polycystic kidney disease. Neurol Neurochir Pol. 2017;51(6):476-480. doi: 10.1016/j.pjnns.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida H, Higashihara E, Maruyama K, et al. Relationship between intracranial aneurysms and the severity of autosomal dominant polycystic kidney disease. Acta Neurochir (Wien). 2017;159(12):2325-2330. doi: 10.1007/s00701-017-3316-8 [DOI] [PubMed] [Google Scholar]
- 11.Sanchis IM, Shukoor S, Irazabal MV, et al. Presymptomatic screening for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2019;14(8):1151-1160. doi: 10.2215/CJN.14691218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel S, Diagbouga MR, Dupuy N, et al. Correlating clinical risk factors and histological features in ruptured and unruptured human intracranial aneurysms: the Swiss AneuX study. J Neuropathol Exp Neurol. 2018;77(7):555-566. doi: 10.1093/jnen/nly031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diagbouga MR. Histological Characterization and the Role of Biomechanical Forces in Intracranial Aneurysm Disease [Internet]. 2019. https://archive-ouverte.unige.ch/unige:129927 [Google Scholar]
- 14.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci USA. 2000;97(4):1731-1736. doi: 10.1073/pnas.040550097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajabzadeh-Oghaz H, Siddiqui AH, Asadollahi A, Kolega J, Tutino VM. The association between hemodynamics and wall characteristics in human intracranial aneurysms: a review. Neurosurg Rev. 2022;45(1):49-61. doi: 10.1007/s10143-021-01554-w [DOI] [PubMed] [Google Scholar]
- 16.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35(7):1254-1262. doi: 10.3174/ajnr.a3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diagbouga MR, Morel S, Bijlenga P, Kwak BR. Role of hemodynamics in initiation/growth of intracranial aneurysms. Eur J Clin Invest. 2018;48(9):e12992. doi: 10.1111/eci.12992 [DOI] [PubMed] [Google Scholar]
- 18.Backes D, Vergouwen MDI, Tiel Groenestege AT, et al. PHASES score for prediction of intracranial aneurysm growth. Stroke. 2015;46(5):1221-1226. doi: 10.1161/strokeaha.114.008198 [DOI] [PubMed] [Google Scholar]
- 19.The UCAS Japan Investigators, Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474-2482. doi: 10.1056/nejmoa1113260 [DOI] [PubMed] [Google Scholar]
- 20.International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339(24):1725-1733. doi: 10.1056/NEJM199812103392401 [DOI] [PubMed] [Google Scholar]
- 21.Rousseau O, Karakachoff M, Gaignard A, et al. ICAN Investigators: location of intracranial aneurysms is the main factor associated with rupture in the ICAN population. J Neurol Neurosurg Psychiatry. 2020;92(2):122-128. doi: 10.1136/jnnp-2020-324371 [DOI] [PubMed] [Google Scholar]
- 22.Morel S, Hostettler IC, Spinner GR, et al. Intracranial aneurysm classifier using phenotypic factors: an international pooled analysis. J Personalized Med. 2022;12(9):1410. doi: 10.3390/jpm12091410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geers AJ, Morales HG, Larrabide I, Butakoff C, Bijlenga P, Frangi AF. Wall shear stress at the initiation site of cerebral aneurysms. Biomech Model Mechanobiol. 2017;16(1):97-115. doi: 10.1007/s10237-016-0804-3 [DOI] [PubMed] [Google Scholar]
- 24.Riccardello GJ, Changa AR, Al-Mufti F, et al. Hemodynamic impingement and the initiation of intracranial side-wall aneurysms. Interv Neuroradiol. 2018;24(3):288-296. doi: 10.1177/1591019918754380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh PK, Marzo A, Howard B, et al. Effects of smoking and hypertension on wall shear stress and oscillatory shear index at the site of intracranial aneurysm formation. Clin Neurol Neurosurg. 2010;112(4):306-313. doi: 10.1016/j.clineuro.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 26.Skodvin TØ, Evju Ø, Helland CA, Isaksen JG. Rupture prediction of intracranial aneurysms: a nationwide matched case-control study of hemodynamics at the time of diagnosis. J Neurosurg. 2018;129(4):854-860. doi: 10.3171/2017.5.jns17195 [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bijlenga P, Gondar R, Schilling Sabine, et al. PHASES score for the management of intracranial aneurysm. Stroke. 2017;48(8):2105-2112. doi: 10.1161/strokeaha.117.017391 [DOI] [PubMed] [Google Scholar]
- 29.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index?. Psychol Methods. 2006;11(2):193-206. doi: 10.1037/1082-989x.11.2.193 [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol. 2011;64(12):1294-1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson DA, Heung M, Deol A, et al. Cerebral aneurysms in autosomal dominant polycystic kidney disease: a comparison of management approaches. Neurosurgery. 2019;84(6):E352-E361. doi: 10.1093/neuros/nyy336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozenfeld MN, Ansari SA, Shaibani A, Russell EJ, Mohan P, Hurley MC. Should patients with autosomal dominant polycystic kidney disease be screened for cerebral aneurysms? AJNR Am J Neuroradiol. 2014;35(1):3-9. doi: 10.3174/ajnr.a3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann HPH, Malinoc A, Bacher J, et al. Characteristics of intracranial aneurysms in the else kröner-fresenius registry of autosomal dominant polycystic kidney disease. Cerebrovasc Dis Extra. 2012;2(1):71-79. doi: 10.1159/000342620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gieteling EW, Rinkel GJE. Characteristics of intracranial aneurysms and subarachnoid haemorrhage in patients with polycystic kidney disease. J Neurol. 2003;250(4):418-423. doi: 10.1007/s00415-003-0997-0 [DOI] [PubMed] [Google Scholar]
- 35.Rozenfeld MN, Ansari SA, Mohan P, Shaibani A, Russell EJ, Hurley MC. Autosomal dominant polycystic kidney disease and intracranial aneurysms: is there an increased risk of treatment? AJNR Am J Neuroradiol. 2016;37(2):290-293. doi: 10.3174/ajnr.a4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Detmer FJ, Hadad S, Chung BJ, et al. Extending statistical learning for aneurysm rupture assessment to Finnish and Japanese populations using morphology, hemodynamics, and patient characteristics. Neurosurg Focus. 2019;47(1):E16. doi: 10.3171/2019.4.focus19145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Detmer FJ, Chung BJ, Jimenez C, et al. Associations of hemodynamics, morphology, and patient characteristics with aneurysm rupture stratified by aneurysm location. Neuroradiology. 2019;61(3):275-284. doi: 10.1007/s00234-018-2135-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frösen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. 2019;47(1):E21. doi: 10.3171/2019.5.focus19234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Can A, Du R. Association of hemodynamic factors with intracranial aneurysm formation and rupture: systematic review and meta-analysis. Neurosurgery. 2016;78(4):510-520. doi: 10.1227/neu.0000000000001083 [DOI] [PubMed] [Google Scholar]
- 40.Niemczyk M, Pilecki T, Gradzik M, Bujko M, Niemczyk S, Pączek L. Blood pressure and intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Blood Press Res. 2014;39(6):630-635. doi: 10.1159/000368475 [DOI] [PubMed] [Google Scholar]
- 41.Diagbouga MR, Morel S, Cayron AF, et al. Primary cilia control endothelial permeability by regulating expression and location of junction proteins. Cardiovasc Res. 2022;118(6):1583-1596. doi: 10.1093/cvr/cvab165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flahault A, Joly D. Screening for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2019;14(8):1242-1244. doi: 10.2215/CJN.02100219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu HW, Yu SQ, Mei CL, Li MH. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke. 2011;42(1):204-206. doi: 10.1161/strokeaha.110.578740 [DOI] [PubMed] [Google Scholar]
- 44.Flahault A, Trystram D, Nataf F, et al. Screening for intracranial aneurysms in autosomal dominant polycystic kidney disease is cost-effective. Kidney Int. 2018;93(3):716-726. doi: 10.1016/j.kint.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 45.Barili F, Parolari A, Kappetein PA, Freemantle N. Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interactive CardioVascular Thorac Surg. 2018;27(3):317-321. doi: 10.1093/icvts/ivy163 [DOI] [PubMed] [Google Scholar]
- 46.Gradzik M, Niemczyk M, Gołębiowski M, Pączek L. Diagnostic imaging of autosomal dominant polycystic kidney disease. Pol J Radiol. 2015;81:441-453. doi: 10.12659/pjr.894482 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.