Introduction
Over the past decade, artificial intelligence (AI) through machine learning (ML) has been increasingly used in health care applications. The use of AI/ML to enhance the provision of continuous kidney replacement therapy (CKRT) is an area of active investigation because of the growing clinical needs of critically ill patients and the abundance of multimodal data from the electronic health records (EHRs) and the CKRT machine itself. Importantly, best clinical practices of CKRT have not been sufficiently standardized, so there is considerable heterogeneity in utilization and deliverables. In this context, AI/ML-based tools could facilitate informed decisions to enhance CKRT goal-oriented deliverables and resource allocation and improve outcomes relevant to patients. In this brief review, we discuss potential applications of AI/ML in CKRT.
Potential Applications of AI/ML in CKRT
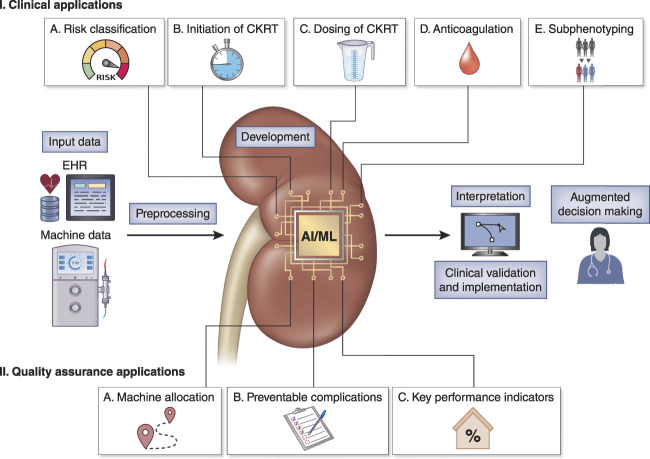
When contrasting with other fields in medicine, there has been limited research in the development, validation, utility testing, and implementation of AI/ML-based applications to enhance CKRT. Herein, we highlight areas of potential development (Figure 1).
Figure 1.
Clinical and quality assurance applications of artificial intelligence (AI) through machine learning (ML) to enhance the provision of continuous kidney replacement therapy (CKRT). Most of these applications are in early stages of development with little research on their prospective clinical validation and implementation. Importantly, the systematic access to big temporal data from the electronic health record (EHR) and the CKRT machine provide a unique infrastructure for the development of AI/ML-based tools in CKRT.
Risk Classification
Approximately two thirds of critically ill patients with AKI requiring CKRT die during hospitalization, and survivors are at risk of health-related complications. The dynamic AI/ML-based prediction of mortality and kidney recovery in these patients could directly augment capacity of bedside decisions, including informed prognostic conversations with family members and more effective resource allocation and postdischarge care.1
Initiation of CKRT
The decision to initiate CKRT at the optimal time could affect relevant outcomes. However, the optimal time of CKRT initiation is still unknown and may require patient-level individualization.
Therefore, it remains to be investigated in which patients one should wait to initiate CKRT and which ones may benefit from an early strategy of CKRT initiation. One should also recognize that inappropriate CKRT initiation might harm some patients, especially those not in the life-threatening stage. A recent scoping review evaluating AI/ML-based tools for CKRT reported two of ten studies focusing on CKRT initiation as a relevant outcome for prediction, which highlights the importance of this aspect of CKRT care in clinical practice.2 In this context, AI/ML models could be used as dynamic forecasting tools to augment decisions about the timing of CKRT initiation, which should also account for ethical considerations. Specifically, a clinician could be more proactive about CKRT initiation according to individualized goal-driven indications and prognostic information.
Dosing of CKRT
The delivered effluent dose of CKRT does not always match the prescribed dose because of treatment interruptions. Although guidelines recommend an average delivered effluent dose of 20–25 ml/kg per hour for patients with AKI receiving CKRT, the effluent dose is sometimes adjusted according to the change in patient's clinical status and needs. Therefore, AI/ML could assist clinicians in adhering to guidelines or personalizing decisions of CKRT dosing when indicated. A similar concept could be applied for fluid management during CKRT, although these areas of research are still limited in CKRT.
Anticoagulation Management
The use of regional citrate anticoagulation prolongs filter life and reduces the risk of bleeding. However, the use of regional citrate anticoagulation requires dose and electrolyte monitoring to prevent complications of overdose. AI/ML models have been used for predicting citrate overdose and demonstrated feasibility and good performance, with relevant features being citrate dose and the pH of the replacement fluid, among others.3 Another study used AI/ML-predicted filter lifespan to guide use versus not use of anticoagulation.4
Subphenotyping
Given the heterogeneity of AKI and critical illness, subphenotyping according to underlying biological processes could substantially promote precision medicine. For example, subphenotypes of hyperinflammation in sepsis-associated AKI and acute respiratory distress syndrome exhibit differential responses to therapies and higher mortality risks. Clustering AI/ML algorithms group patients by computing patient-wise differences on the basis of multiple clinical variables. Although patients with similar features are grouped into the same cluster (i.e., subphenotype), the optimal number of clusters and the exact type and/or number of features to define a cluster are still subject of investigation.
Quality Assurance
AI/ML has great potential to ensure quality of CKRT delivery by informing bedside clinical decisions by providers and also operational decisions by medical directors. For example, one study evaluated an automated AI/ML tool for optimizing CKRT machine utilization and allocation during the coronavirus disease pandemic,5 whereas other studies used AI/ML to predict and/or monitor key performance indicators of CKRT such as filter lifespan, alarms, and downtime.4,6
Considerations of AI/ML Applied to CKRT
Access to Quality Data
The combination of low-resolution clinical data (e.g., vital signs, laboratory, medications, etc.) from the EHR with high-resolution machine-centered programmatic (e.g., filter/circuit life, time on machine/treatment lost, catheter complications, clotting) and therapy (e.g., dose prescribed, dose delivered, net ultrafiltration rate, etc.) data from the CKRT machine provides a unique multimodal data infrastructure for tool development. For example, multimodal data could feed AI/ML tools for prediction of filter failure and assist with recognition patient and/or CKRT characteristics associated with filter failure.4 Importantly, data quality management should focus on variable definition, selection, and preprocessing, which includes management of missingness, alignment of feature frequencies, and data harmonization.
Model Evaluation
Performance of binary classification models is evaluated by discrimination metrics (e.g., C-statistic) to assess the model's ability to differentiate patients at higher versus lower risk of the outcome. Other metrics (e.g., accuracy, precision, sensitivity, specificity, F1 score, etc.) are used to evaluate the model prediction capability by setting various thresholds for different predicted risks. There are different methods to assess generalizability: (1) by combining multicenter data into a single set and then performing cross-validation, (2) by training a model using data from one of the centers and evaluating the model using data from the other centers, and (3) by using federated learning to validate and improve the cross-institutional performance.7 In the latter, data are kept at each center, which generates a local model and sends the model parameters to a central server. Then, the center server generates an aggregated model with the local models from different sites, and finally, the aggregated model is sent back to each local site for further training or testing.
Model Interpretation and Utility
Model-agnostic methods can assist with interpretation. For example, the SHapley Additive exPlanations model analyzes the changes in the prediction by tweaking the input data, and data values can be rationalized as a fair or reasonable allocation of the importance of the parameters given the model output.8 In addition, decision curve analysis could assist with the examination of the clinical utility of different models at different prediction thresholds. Choosing an optimal prediction threshold is a critical step for clinical models. This results in balanced true-positive and false-positive rates. Moreover, the trade-off between sensitivity (true-positive rate) and specificity (1–false-positive rate) should be considered when selecting the threshold for specific tasks/diseases.9
Algorithm Bias and Ethical Considerations
One should be cautious about the potential bias and unfairness of AI/ML models for risk classification and subphenotyping. Biases could occur at the level of data processing, development/validation, and/or utility testing/implementation.10 A biased model could favor one group of patients with specific characteristics while falling on predictions for the other groups. In the case of CKRT, practices are heterogeneous when comparing high- versus low- and middle-income countries. Therefore, evaluating the context in which a model was trained could help recognize and mitigate biases. Specifically, it is important to note that insufficient or disproportionate data on racial, ethnic, and gender minorities may lead to biases in models, extending unfairness and injustice in the social structure. In addition to the researchers who develop and test the AI/ML models, health care professionals who interpret and clinically apply these models should recognize the existence of variables that may contribute to discrimination and inequality, including information technology resources deployed for the implementation of the tools.11
In conclusion, despite recent advances of AI/ML-based health care applications, limited research has focused on enhancing the provision of CKRT, the second most common extracorporeal support treatment in the intensive care unit. Potential applications of AI/ML in CKRT include systematic and dynamic risk classification, subphenotyping, quality assurance as well as augmented decision-making capacity for CKRT initiation, dose adjustments, and anticoagulation management, among others. One should recognize that these tools could augment both bedside decisions by providers and operational decisions by medical directors. Access to quality multimodal data from both the EHR and CKRT machines, the recognition and mitigation of algorithm biases, and the careful evaluation of performance, generalizability, interpretation, and clinical utility are essential elements of AI/ML-based tool development. Importantly, the prospective validation of the utility of these tools, a pivotal step toward implementation science, has seldom been done and is an area that requires multiple stakeholder collaborations, resources, and funding.
Disclosures
J.A. Neyra reports consultancy agreements with Baxter Healthcare Inc., Biomedical Insights, and Leadiant Biosciences; serving as Section Editor for Clinical Nephrology and as Guest Editor of Critical Care Nephrology in Advances in Chronic Kidney Disease; and serving on the Editorial Boards of Advances in Chronic Kidney Disease, American Journal of Kidney Diseases, and Kidney360. All remaining authors have nothing to disclose.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases grant R56 DK126930. J.A. Neyra is currently supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK128208 and U01DK129989.
Acknowledgments
This article is part of the Artificial Intelligence and Machine Learning in Nephrology series, led by series editor Girish N. Nadkarni.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Author Contributions
Supervision: Javier A. Neyra.
Writing – original draft: Javier A. Neyra, Lucas J. Liu.
Writing – review & editing: Jin Chen, Javier A. Neyra, Tomonori Takeuchi.
References
- 1.Pattharanitima P, Vaid A, Jaladanki SK, et al. Comparison of approaches for prediction of renal replacement therapy-free survival in patients with acute kidney injury. Blood Purif. 2021;50(4-5):621–627. doi: 10.1159/000513700 [DOI] [PubMed] [Google Scholar]
- 2.Hammouda N, Neyra JA. Can artificial intelligence assist in delivering continuous renal replacement therapy? Adv Chronic Kidney Dis. 2022;29(5):439–449. doi: 10.1053/j.ackd.2022.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Ma Y, Hong N, et al. Early warning of citric acid overdose and timely adjustment of regional citrate anticoagulation based on machine learning methods. BMC Med Inform Decis Mak. 2021;21(suppl 2):126. doi: 10.1186/s12911-021-01489-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Bai M, Zhang L, et al. Development and external validation of a model for predicting sufficient filter lifespan in anticoagulation-free continuous renal replacement therapy patients. Blood Purif. 2022;51(8):668–678.doi: 10.1159/000519409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Wong A-K, Connor M. 122: using artificial intelligence to optimize RRT machine allocation during COVID-19-related RRT surge. Crit Care Med. 2021;49(1):45. doi: 10.1097/01.ccm.0000726376.93825.30 [DOI] [Google Scholar]
- 6.Zhang L, Baldwin I, Zhu G, et al. Automated electronic monitoring of circuit pressures during continuous renal replacement therapy: a technical report. Crit Care Resusc. 2015;17(1):51–4. PMID: 25702763. [PubMed] [Google Scholar]
- 7.Sarma KV, Harmon S, Sanford T, et al. Federated learning improves site performance in multicenter deep learning without data sharing. J Am Med Inform Assoc. 2021;28(6):1259–1264. doi: 10.1093/jamia/ocaa341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodríguez-Pérez R, Bajorath J. Interpretation of compound activity predictions from complex machine learning models using local approximations and shapley values. J Med Chem. 2019;63(16):8761–8777. doi: 10.1021/acs.jmedchem.9b01101 [DOI] [PubMed] [Google Scholar]
- 9.Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. 2017;318(14):1377–1384. doi: 10.1001/jama.2017.12126 [DOI] [PubMed] [Google Scholar]
- 10.de Hond AAH, Leeuwenberg AM, Hooft L, et al. Guidelines and quality criteria for artificial intelligence-based prediction models in healthcare: a scoping review. NPJ Digit Med. 2022;5(1):2. doi: 10.1038/s41746-021-00549-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesson P, Hswen Y, Valdes G, Stojanovski K, Handley MA. Risks and opportunities to ensure equity in the application of big data research in public health. Annu Rev Public Health. 2022;43(1):59–78. doi: 10.1146/annurev-publhealth-051920-110928 [DOI] [PMC free article] [PubMed] [Google Scholar]