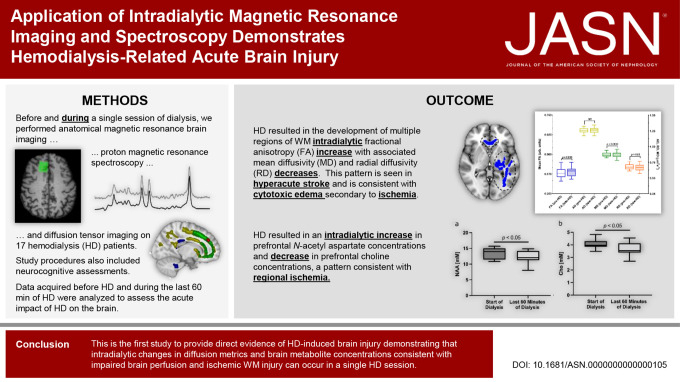
Keywords: chronic kidney disease, cytotoxic edema, diffusion tensor imaging, hemodialysis, intradialytic magnetic resonance imaging, magnetic resonance spectroscopy, neurochemistry, white matter integrity
Abstract
Significance Statement
Hemodialysis (HD) results in reduced brain blood flow, and HD-related circulatory stress and regional ischemia are associated with brain injury over time. However, studies to date have not provided definitive direct evidence of acute brain injury during a HD treatment session. Using intradialytic magnetic resonance imaging (MRI) and spectroscopy to examine HD‐associated changes in brain structure and neurochemistry, the authors found that multiple white (WM) tracts had diffusion imaging changes characteristic of cytotoxic edema, a consequence of ischemic insult and a precursor to fixed structural WM injury. Spectroscopy showed decreases in prefrontal N-acetyl aspartate (NAA) and choline concentrations consistent with energy deficit and perfusion anomaly. This suggests that one HD session can cause brain injury and that studies of interventions that mitigate this treatment's effects on the brain are warranted.
Background
Hemodialysis (HD) treatment-related hemodynamic stress results in recurrent ischemic injury to organs such as the heart and brain. Short-term reduction in brain blood flow and long-term white matter changes have been reported, but the basis of HD-induced brain injury is neither well-recognized nor understood, although progressive cognitive impairment is common.
Methods
We used neurocognitive assessments, intradialytic anatomical magnetic resonance imaging, diffusion tensor imaging, and proton magnetic resonance spectroscopy to examine the nature of acute HD-associated brain injury and associated changes in brain structure and neurochemistry relevant to ischemia. Data acquired before HD and during the last 60 minutes of HD (during maximal circulatory stress) were analyzed to assess the acute effects of HD on the brain.
Results
We studied 17 patients (mean age 63±13 years; 58.8% were male, 76.5% were White, 17.6% were Black, and 5.9% were of Indigenous ethnicity). We found intradialytic changes, including the development of multiple regions of white matter exhibiting increased fractional anisotropy with associated decreases in mean diffusivity and radial diffusivity—characteristic features of cytotoxic edema (with increase in global brain volumes). We also observed decreases in proton magnetic resonance spectroscopy–measured N-acetyl aspartate and choline concentrations during HD, indicative of regional ischemia.
Conclusions
This study demonstrates for the first time that significant intradialytic changes in brain tissue volume, diffusion metrics, and brain metabolite concentrations consistent with ischemic injury occur in a single dialysis session. These findings raise the possibility that HD might have long-term neurological consequences. Further study is needed to establish an association between intradialytic magnetic resonance imaging findings of brain injury and cognitive impairment and to understand the chronic effects of HD-induced brain injury.
Clinical Trials Information:
Introduction
Three million people worldwide require dialysis to manage CKD, with up to 90% receiving HD. HD is a lifesaving treatment, but is not without its harms. HD is known to contribute directly to multiorgan ischemic injury by creating additional hemodynamic and osmotic stress.1 Specifically, HD causes episodic hypotension and impaired organ perfusion to multiple vascular beds, including the heart and brain,2–8 ultimately resulting in low quality of life9 and progressive cognitive impairment.10
The problem of HD-induced brain injury is not well recognized or understood and is not the focus of care, despite some form of brain injury being evident in almost all patients receiving HD. The most common form of injury is leukoaraiosis, a rarefaction of brain WM associated with cognitive impairment in HD patients,11,12 with the severity of impairment proportional to the amount of WM injury.12 Cognitive deficits are seen in the domains of attention, memory, and executive function,13,14 with the latter appearing early after starting dialysis,15 resulting in executive function deficits being almost universal in patients receiving HD.15–17
The physiological mechanisms responsible for HD-related cognitive impairment remain unclear, but may be related to reduced brain blood flow. Reductions in regional brain blood flow during dialysis have been previously demonstrated using intradialytic transcranial Doppler, optical imaging, and positron emission tomography.6–8 Our group has demonstrated that HD-related circulatory stress is associated with brain injury,18 with the extent of injury determined by the degree of BP instability during HD.19,20 We have also demonstrated that the injury may be amenable to HD-based intervention, with intradialytic intervention (cooling) reducing the development of brain WM structural defects over a 12-month period.18
If we accept that cognitive impairment is related to reduced brain blood flow, it is possible that ischemia is an underlying mechanism of injury. As such, approaches that have been used to study the brain in hyperacute stroke may be useful in studying HD-related brain injury. In the stroke literature, hyperacute changes in brain WM structure after a stroke have been studied with diffusion tensor imaging (DTI), with studies showing a pattern of regional decreases in diffusivity measures with increases in fractional anisotropy (FA), a pattern attributed to cytotoxic edema that results from ischemia.21,22 Similarly, intradialytic changes in the brain's WM microstructure could be assessed with DTI,23 which may shed insight into the potential role of HD underlying cerebral WM injury, especially in WM regions linked to cognition. In stroke, complementary information about the neurochemistry of cerebral ischemia has been obtained using proton magnetic resonance spectroscopy (1H-MRS), with studies showing a reduction in NAA after the onset of cerebral infarction.24 This technique could be similarly applied to study HD-related brain injury.
Thus, the primary aim of this study was to use intradialytic brain MRI, DTI, and 1H-MRS to examine the nature of acute HD-associated brain injury and the associated changes in brain structure and neurochemistry relevant to ischemia. The secondary aim was to provide direct evidence of acute brain injury associated with a HD session to confirm the central role that HD plays in cognitive decline.
Methods
Study Design
This study is a report of the effects of HD on the brain in a cohort of patients who consented to participate in a randomized parallel-arm controlled trial investigating the application of remote ischemic preconditioning (RIPC) before HD in patients with CKD to prevent HD-induced acute ischemic brain injury. Patients were randomized 1:1 to either RIPC or sham intervention using a computer-generated randomization list. Intradialytic brain imaging, neurocognitive assessments, and laboratory blood testing were performed at baseline and repeated after 12 months of either RIPC or sham intervention.
In this study, the baseline data were analyzed to describe the acute effects of HD on brain structure and neurochemistry. Brain imaging was acquired twice, before the HD treatment (pre-HD) and within the last 60 minutes of dialysis (late-HD) to capture acute brain changes at maximal circulatory stress.20 Intradialytic brain imaging was performed during dialysis using an MRI-compatible dialysis system consisting of a Fresenius 5008 monitor and a FX800 dialyzer (Fresenius Medical Care, Bad Hombourg, Germany), as previously described.4
Blood samples were collected before HD, within the last 60 minutes of dialysis, and at the end of HD to obtain the complete blood count, urea, electrolytes, dialysate composition (sodium, potassium, calcium, magnesium, and glucose), clotting, and cardiac (troponin T) and inflammatory (C-reactive protein [CRP]) factors.
BP was also measured pre-HD, at late-HD, and at the end of HD. Intradialytic hypotension was determined as per K/DOQI 2005 guidelines25 and the nadir criteria of ≥20 mm Hg decline in pre-HD systolic BP and/or systolic BP of ≤ 100 mm Hg with accompanying clinical symptoms and/or intervention.
This study was approved by the Western University Health Sciences Research Ethics Board (Protocol no.: 109413, ClinicalTrials.gov Identifier: NCT03342183) and conducted in accordance with the Declaration of Helsinki ethical standards.
Participants
Twenty-one patients 18 years or older who had received HD treatment at least three times per week for a minimum of 90 days were recruited from the London Health Sciences Renal Program (London, ON, Canada) to participate in this study. All patients gave informed consent before enrollment in the study. Patients who received dialysis using lower limb vascular access, patients with severe cognitive impairment defined as having a score of <18 on the Montreal Cognitive Assessment (MoCA), patients with a diagnosis of dementia or stroke, or patients with a medication history of drugs that blunt response to RIPC (e.g., cyclosporin or ATP-sensitive potassium channel-directed drugs) were excluded. Although lower limb vascular access was an exclusion criterion due to the RIPC protocol involving a BP cuff applied to the lower limb, no patients were excluded because of this criterion. Demographic information collected from patients included age, biological sex, and ethnicity as well as other CKD-specific patient characteristics as summarized in Table 1. Age, biological sex, and ethnicity information were self-reported and collected to fully characterize the patient cohort being studied.
Table 1.
A summary of patient cohort (N=17) demographics
| Characteristics | Prevalence | Mean (SD) |
|---|---|---|
| Age, yr | 63 (13) | |
| Men, n (%) | 10 (58.8) | |
| Ethnicity, n (%) | ||
| Black | 3 (17.6) | |
| Indigenous | 1 (5.9) | |
| White | 13 (76.5) | |
| Dialysis vintage, mo | 50 (61) | |
| Comorbidities, n (%) | ||
| Diabetes, type 1/type 2 | 1/7 (5.9/41.2) | |
| Hypertension | 12 (70.6) | |
| Coronary artery disease | 5 (29.4) | |
| Arrhythmia | 3 (17.6) | |
| History of myocardial infarction | 2 (11.8) | |
| Dyslipidemia | 5 (29.4) | |
| History of transient ischemic attack | 2 (11.8) | |
| Depression, anxiety, and other psychiatric disorders | 2 (11.8) | |
| Migraine | 1 (5.9) | |
| Seizure disorder | 1 (5.9) | |
| Obstructive sleep apnea | 5 (29.4) | |
| Current smoker | 2 (11.8) | |
| Etiology of kidney disease, n (%) | ||
| Diabetic nephropathy | 7 (41.2) | |
| Hypertensive nephropathy | 4 (23.5) | |
| Other | 9 (52.9) | |
| Medication history | ||
| ACEi/ARB | 3 (17.6) | |
| β-blockers | 9 (52.9) | |
| ≥2 Antihypertensive agents | 6 (35.3) | |
| Dialysis treatment data | ||
| Effective blood flow rate, ml/min | 281.41 (24.39) | |
| Ultrafiltration rate, ml/kg per hour | 6.33 (3.16) | |
| Ultrafiltration volume, L | 2045.11 (956.51) | |
| Number of IDH episodes, n (%) | 4 (24) | |
| Dialysate temperature (°C) | 36.5 (0) | |
| Dialysate sodium (mmol/L) | 139 (1.66) | |
| Dialysate potassium (mmol/L) | 2.15 (0.75) | |
| Dialysate bicarbonate (mmol/L) | 39 (2.26) | |
| Dialysate calcium (mmol/L) | 1.28 (0.08) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IDH, intradialytic hypotension.
Sample Size Estimation
No data existed to allow us to estimate the power required to detect intradialytic changes. The sample size was directed at the development of fixed WM changes in the subsequent randomized control trial (RCT) portion of this study. Our prior randomized controlled trial of dialysate cooling for prevention of progressive WM injury in patients on HD who received brain MRI on nondialysis days yielded a total evaluable sample size of 38 patients with compelling results.18 Power calculation performed using these data resulted in a sample size of eight patients required to observe differences in the brain WM microstructure using DTI metrics. Thus, our sample size of 17 patients was twice the expected sample size required to observe differences in DTI metrics in HD patients.
Neurocognitive Assessments
A set of neurocognitive tests was administered at the Kidney Clinical Research Unit before the start of dialysis and MRI scanning to determine participants' cognitive status and explore associations of cognitive performance to brain WM injury. This consisted of standard tests for assessing cognitive performance in patients with end stage kidney disease (MoCA and Trail Making Test [TMT] A and B)10 and the domain-specific Cambridge Brain Sciences (CBS) test battery (https://www.cambridgebrainsciences.com/). MoCA provides an assessment of overall cognitive performance and is an established indicator of mild cognitive impairment26,27 while TMT A and B provides an assessment of alterations in executive function.28 The CBS battery is a series of 12 nonverbal tests that assesses global cognitive functioning and three broad cognitive domains: STM (spatial span, monkey ladder, paired associates, and token search), reasoning (polygons, spatial planning, odd-one-out, and rotations), and verbal ability (grammatical reasoning, digit span, feature match, and double trouble). Participants completed the full CBS battery through the CBS online platform. All participants were administered identical versions of the CBS, MoCA, and TMT tests by the same trained individual using standardized verbal instructions.
Imaging Methods
All brain imaging and spectroscopy examinations were performed on a 3T Siemens MRI scanner (Biograph mMR, Siemens Healthineers, Erlangen, Germany) using a 16-channel phased array head (12-channel) and neck (4-channel) radiofrequency coil. A 60-minute brain MRI scan to acquire imaging and spectroscopy data was performed before dialysis (pre-HD) and repeated during the last 60 minutes of dialysis (late-HD). Imaging methods are described briefly below, with more detailed descriptions available in the Detailed Description of Imaging Methods section of the Supplemental Information.
Imaging Data Acquisition
Anatomical Brain Imaging
Whole-brain, three-dimensional T1-weighted anatomical images were acquired using a magnetization-prepared rapid gradient-echo imaging sequence.29 These images were used for volumetric analysis and for determining fractions of gray matter (GM), WM, and cerebrospinal fluid (CSF) needed during the calculation of diffusion metrics and metabolite concentrations.
DTI
Diffusion-weighted imaging (DWI) data for assessing brain WM structural integrity were acquired over 11 minutes using a single-shot echo-planar imaging sequence. To enable postacquisition DWI correction for susceptibility-induced distortions, two spin-echo images were acquired in opposite phase-encoding directions.
Magnetic Resonance Spectroscopy
Single-voxel 1H-MRS data were acquired using the semi-LASER (localization by adiabatic selective refocusing) sequence30,31 in the Centre for Magnetic Resonance Research Spectroscopy Package. The magnetic resonance spectroscopy package was developed by Gülin Öz and Dinesh Deelchand31,32 and provided by the University of Minnesota under a C2P agreement. Data were acquired from a 20×20×20 mm3 spectroscopy voxel in the right prefrontal cortex WM (Supplemental Figure 1a). This voxel was placed using anatomical landmarks visualized on the T1-weighted anatomical images.
Data Processing
Processing of Volumetric Data
Voxel-based morphometry was performed on the T1-weighted images. Simply put, this technique enabled the classification of voxels in the pre-HD and late-HD T1-weighted images as GM, WM, or CSF. With the GM, WM, and CSF segmentations in hand, differences in GM, WM, and CSF volumes between pre-HD and late-HD as well as the spatial location of those differences could be estimated.
Processing of DWI Data
To measure changes in brain WM microstructural integrity, the DW images at pre-HD and late-HD were carefully preprocessed and analyzed to produce DTI scalar maps using an in-house image analysis pipeline implemented to incorporate steps from a variety of established image processing software packages as described previously.23 The DTI scalar maps produced include maps of FA, mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD).
Quantification of Brain Metabolite Concentrations
Brain metabolite concentrations were calculated directly from the 1H-MRS spectra as previously described.33 After processing, the spectroscopy data were fitted to simulated prior knowledge templates that included a predefined set of metabolites (Supplemental Table 1) and an empirically measured macromolecule model34 (Supplemental Figure 1b), an approach previously described elsewhere.35 Metabolite concentrations were then calculated as previously described,33,36 incorporating a variety of necessary corrections.
Statistical Analyses
All statistical tests of region of interest (ROI) analysis of brain volumes, DTI metrics, and brain metabolites, as well as correlation between measurements were performed using GraphPad Prism 9.3.1 (GraphPad Software, La Jolla, CA). Statistical tests of intradialytic changes in laboratory blood measures were performed using IBM SPSS Statistics version 27 (IBM, Armonk, NY).
Analysis of Laboratory Testing Data
Intradialytic changes in blood work were analyzed with linear mixed models with the measurement time points (start of dialysis, during the last 60 minutes of dialysis, and end of dialysis) as the fixed effect. Repeated observations on the same patients were modeled with an unstructured covariance matrix term. Maximum likelihood estimation was used to produce the statistical models. If any of the fixed effects were significant, post hoc tests of simple main effects and comparisons of estimated marginal means were performed to identify statistically significant intradialytic changes. The Sidak method was used to control for multiple comparisons.
Analysis of Cognitive Testing Data
The extent of cognitive impairment was quantified for each patient by comparing their individual cognitive test performance scores with scores of a normative control group as described below. Each patient's MoCA scores were converted to standardized (z) scores by comparison with a normative cohort.26 TMT A and B scores were converted to z-scores based on normative data matched in age and education (more or less than 12 years)37 while individual raw CBS scores were converted to z-scores based on age- and sex-matched normative data.38 Domain specific z-scores capturing performance on short-term memory (STM), verbal skills, and reasoning tasks were computed by averaging across respective tests of each domain within the CBS battery.
Analysis of Volumetric Imaging Data
Paired t-tests of voxel-by-voxel changes in GM and WM volumes between pre-HD and late-HD images were performed in SPM12 (Statistical Parametric Mapping software, www.fil.ion.ucl.ac.uk/spm/) on smoothed GM and WM segmentations separately. A brain region exhibiting a significant change in GM or WM volume was identified as clusters of 500 or more voxels that showed either a positive or negative change in volume at a significance level of P < 0.01 after correction of multiple comparisons using the False Discovery Rate.
The percentage of the total intracranial volume (TIV) occupied by GM, WM, and CSF was also calculated for each participant at the pre-HD and late-HD time points. This allowed the evaluation of the global change in GM, WM, and CSF volumes from pre-HD to late-HD. A two-tailed (α=0.05) Wilcoxon matched pairs signed rank test was performed to test for differences in percent GM, WM, and CSF volumes at pre-HD versus late-HD.
Tract-Based Spatial Statistics Analysis of DTI Data
Tract-based spatial statistics (TBSS)39 was performed in FMRIB's Software Library40 on the DTI scalar maps (FA, MD, and AD) to assess changes in these DTI metrics between pre-HD and late-HD. Ultimately, TBSS analysis identifies differences in DTI metrics on a voxel-wise basis, allowing the determination of brain areas with disrupted WM integrity.
Parameters for the analysis include skeletonization of the DTI scalar maps using a threshold of 0.2 to include WM tracts common across all participants and time points and to exclude nonspecific tissues (GM and CSF). Brain areas of disrupted WM integrity during the last 60 minutes of dialysis were identified as clusters of voxels in WM tracts showing significant changes in DTI metrics (FA, MD, AD, RD) between pre-HD and late-HD at a statistical threshold of P < 0.05, corrected for multiple comparisons using family-wise error and the threshold-free cluster enhancement approach (10,000 nonparametric permutations). The anatomical location of WM clusters showing significant changes in DTI metrics was determined using the ICBM-DTI-81 white matter labels atlas and the white matter tractography atlas from Johns Hopkins University.41
The WM clusters showing significant differences in DTI metrics were then used to define functional ROIs. Within these functional ROIs, the mean FA, MD, AD, and RD values were calculated and compared between pre-HD and late-HD using a two-tailed (α=0.05) Wilcoxon matched pairs signed rank test. Importantly, comparisons were only made for the metrics not used to define the functional ROI to avoid circular analysis. For example, for a functional ROI defined by the WM clusters showing a significant difference in MD and AD, only the mean FA and RD values were calculated and compared. The changes in mean FA, MD, AD, and RD from pre-HD to late-HD within these functional ROIs were reported as percent change.
Analysis of Brain Metabolite Concentrations
First, outlier analysis was performed on the calculated brain metabolite concentrations with the robust regression and outlier removal method42 using a Q value of 1%. No outliers were identified. Second, normality was assessed using the D'Agostino-Pearson omnibus normality test. If the data were normally distributed, a paired t-test was performed for each measured brain metabolite to identify differences between metabolite concentrations at the start of dialysis (pre-HD) versus the last 60 minutes of dialysis (late-HD). Otherwise, a Wilcoxon matched pairs signed rank test was performed to determine intradialytic metabolite concentration changes. A two-tailed α of 0.05 was used for both the t- and Wilcoxon tests.
Correlational Analyses
Correlational analyses were performed using GraphPad Prism version 9.3.1 for Windows (www.graphpad.com). Outliers were detected and removed using the regression and outlier removal method and a Q threshold of 1%. Pearson correlation analysis (two-tailed at an α of 0.05) was performed to investigate associations of changes in brain WM microstructure (FA, MD, AD, and RD) to changes in brain volume and cognitive performance (MoCA, TMT A, TMT B, and three domain-specific z-scores), as well as associations between changes in brain metabolite levels and changes in measured serum markers. Correction for multiple comparisons was conducted using the Bonferroni method, with the threshold of statistical significance (α) set to . Only strong correlations (|r|>0.5) were reported.
Results
Participants
Of the 21 participants recruited and consented to the study, two participants withdrew consent before any study procedures were performed, and two participants withdrew from the study because of contraindication to MRI secondary to claustrophobia. As a result, 17 participants attended the first study visit. Of these, one participant was unable to complete the MRI scan due to HD-related complications. Due to technical difficulty, good quality 1H-MRS data were not obtained from one participant. In summary, complete volumetric and DWI was obtained from 16 participants while 1H-MRS data were obtained from 15 patients. Cognitive testing was not completed for one participant because of visual impairment. One other participant completed the MoCA, TMT A, and TMT B, but not the CBS battery. All 17 participants completed the blood work. A summary of the demographic characteristics of patients enrolled in the study is given in Table 1.
Laboratory Testing
Electrolytes measured included sodium, potassium, calcium, phosphate, chloride, and magnesium. Linear mixed modeling and post hoc testing showed the commonly expected changes in the levels of these electrolytes due to dialysis (Supplemental Figure 2a). Analyses of serum bicarbonate, urea, lactate, and creatinine also showed the expected changes due to dialysis (Supplemental Figure 2b). Analyses of tests for coagulation revealed changes in international normalized ratio and partial thromboplastin consistent with the heparinization of the dialysis lines (Supplemental Figure 2c), whereas analyses of the complete blood count revealed no significant intradialytic changes, except for an intradialytic increase in hemoglobin (Supplemental Figure 2d). A detailed description of these results is available in the Supplemental Results section of the Supplemental Information.
For markers of cardiac injury and inflammation (Figure 1), there was a significant effect of time point for both CRP (F (2, 16)=3.962, P = 0.039) and cTnT (F (2, 16)=8.154, P = 0.0035). Post hoc testing showed that CRP increased significantly from the start of the dialysis to the last 60 minutes of dialysis (P = 0.046) before normalizing back to starting levels at the end of dialysis. Post hoc testing also showed that cTnT decreased significantly from the start of dialysis to the last 60 minutes of dialysis (P = 0.043) and to the end of dialysis (P = 0.003), reflecting clearance.
Figure 1.
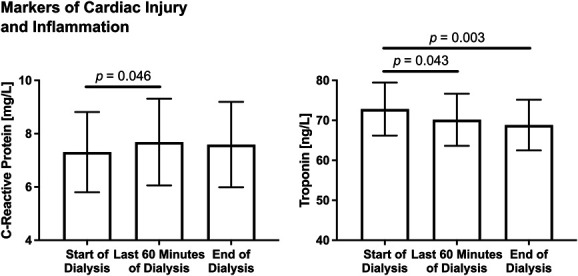
Intradialytic changes in markers of vascular injury and inflammation (CRP and troponin) are shown. For both graphs, estimated marginal means and standard errors are plotted. Linear mixed model analysis showed a significant effect of time point for both CRP (F (2, 16)=3.962, P = 0.039) and troponin (F (2, 16)=8.154, P = 0.0035). Post hoc testing showed that CRP increased significantly from the start of the dialysis to the last 60 minutes of dialysis (P = 0.046) before normalizing back to starting levels at the end of dialysis. Post hoc testing also showed that cTnT decreased significantly from the start of dialysis to the last 60 minutes of dialysis (P = 0.043) and to the end of dialysis (P = 0.003). CRP, C-reactive protein.
Cognitive Testing
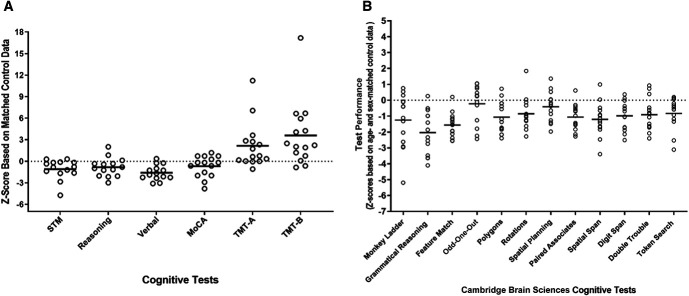
The mean MoCA score for the cohort was 25.9±3.2 (mean±SD) with five participants below the threshold score of 26 for mild cognitive impairment. On average, the patients completed TMT A and B in 55±23 and 138±59 seconds, respectively, with 60% of patients completing TMT A slower than controls while 80% completing TMT B slower than controls, after adjusting for age, sex, and years of education (Figure 2A). HD patients performed worse than age and sex-matched controls on all tests in the CBS battery, as shown in Figure 2A and illustrated along the three broad cognitive domains: STM, verbal skills, and reasoning in Figure 2B.
Figure 2.
Neurocognitive standardized (z) scores are shown. The neurocognitive standardized (z) scores of each HD patient relative to age and sex-matched controls for MoCA, TMT A and B, and CBS battery summarized along three broad cognitive domains (STM, reasoning, verbal) is shown in (A). Individual patient scores for the full CBS battery is shown in (B). TMT A and B scores were also adjusted for years of education. For all tests, except TMT A and B, a negative z-score reflects poor cognitive test performance, whereas for TMT A and B based on the speed of test completion, a positive z-score reflects poor test performance. CBS, Cambridge Brain Sciences; GM, gray matter; HD, hemodialysis; MoCA, Montreal Cognitive Assessment; TMT, Trail Making Test; WM, white matter.
Anatomical Brain Imaging
Volumetric analysis using voxel-based morphometry identified spatial regions where there were significant changes in WM and GM volumes between pre-HD and late-HD (Figure 3A and Table 2). WM volumes increased from pre-HD to late-HD most notably in the right superior parietal lobe, right middle frontal lobe, and left planum temporale encompassing the Wernicke area while GM volumes increased significantly in the left lingual gyrus, right thalamus, and left posterior cingulate gyrus.
Figure 3.
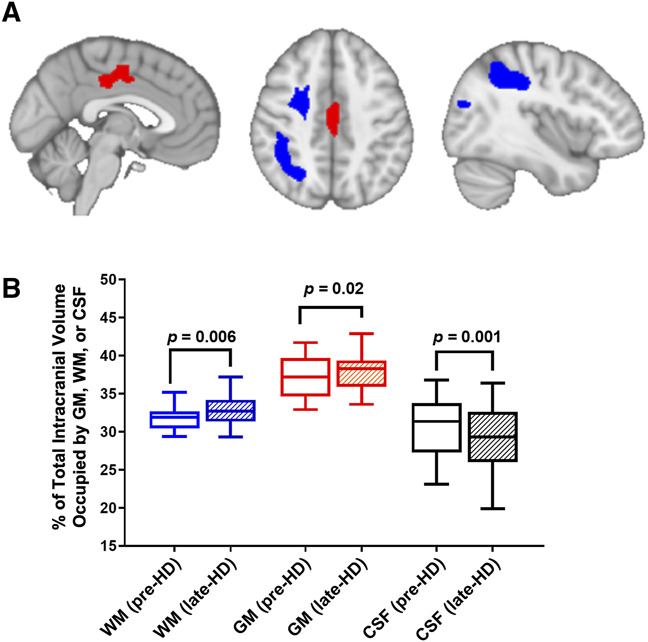
Volumetric analysis using voxel-based morphometry. (A) Voxel-based morphometry analysis shows brain areas with significant increases in WM volume (right superior parietal lobe, right middle frontal lobe, blue) and GM volume (left posterior cingulate gyrus, red) from before dialysis (pre-HD) to the last 60 minutes of dialysis (late-HD) (MNI slice coordinates: X=87, Z=114, and X=50, respectively). (B) Overall, the percentage of TIV occupied by WM and GM significantly increased from pre-HD to late-HD, whereas the percentage of TIV occupied by CSF decreased at late-HD relative to pre-HD. CSF, cerebrospinal fluid; GM, gray matter; HD, hemodialysis; TIV, total intracranial volume; WM, white matter.
Table 2.
A summary of the voxel-based morphometry results
| Cluster | Anatomical Label | MNI (X/Y/Z) Coordinate | Total Number of Voxels | t-Value | ||
|---|---|---|---|---|---|---|
| WM volumes (FDR P < 0.01, ≥500 voxels) | ||||||
| 1 | Right supramarginal | 39 | −39 | 39 | 2226 | 12.24 |
| Right posterior central | 44 | −24 | 36 | 8.20 | ||
| Unclassified | 27 | −54 | 44 | 7.24 | ||
| 2 | Right middle frontal | 28 | 1 | 47 | 1311 | 9.82 |
| Right middle frontal | 25 | 15 | 47 | 8.13 | ||
| 3 | Right lateral occipital | 33 | −73 | −5 | 726 | 7.67 |
| 4 | Left superior temporal | −49 | −8 | −5 | 819 | 7.05 |
| Left planum temporale | −56 | −20 | 8 | 7.00 | ||
| Left superior temporal | −49 | −36 | 11 | 6.15 | ||
| GM volumes (FDR P < 0.01, ≥500 voxels) | ||||||
| 1 | Right thalamus | 16 | −28 | 16 | 839 | 9.72 |
| 2 | Left cerebellum | −34 | −60 | −22 | 1619 | 8.17 |
| Left lingual gyrus | −4 | −73 | −6 | 7.45 | ||
| Left lingual gyrus | −19 | −66 | −15 | 7.02 | ||
| 3 | Left middle cingulum | −2 | −22 | 40 | 624 | 6.70 |
| Left middle cingulum | −2 | −9 | 48 | 5.80 | ||
Anatomical description of local maxima of clusters of significant change in WM and GM volume at late-HD relative to pre-HD. Coordinates are given in anatomical MNI space, and the center of mass of each ROI is shown in bold. FDR, false discovery rate; WM, white matter; GM, gray matter; HD, hemodialysis; ROI, region of interest.
Overall, the percentage of the TIV occupied by GM and WM increased significantly from pre-HD to late-HD (P < 0.05) while the percentage of the TIV occupied by the CSF decreased (P = 0.001), as shown in Figure 3B. Expressed in absolute volume, the total volume of WM and GM increased by an average of 16.9±13.4 ml and 17.3±24.3 ml, respectively, while the total CSF volume declined significantly by an average of 20±44.5 ml.
TBSS of Diffusion Metrics
Voxel-wise TBSS analysis of DTI scalar maps revealed several WM clusters distributed throughout the brain with various significant changes in FA, MD, RD, and AD from pre-HD to late-HD, as shown in Figure 4. Specifically, increased FA was observed in the body of the corpus callosum (CC), left optic radiation (OR), left inferior longitudinal fasciculus (ILF), and left inferior fronto-occipital fasciculus (IFOF) while increased MD was seen in the genu of the CC, right IFOF, and right anterior thalamic radiation (ATR). Increased RD was observed in the right cingulum and increased AD was seen in the genu of the CC, right ILF, right superior longitudinal fasciculus, right IFOF, and right OR.
Figure 4.
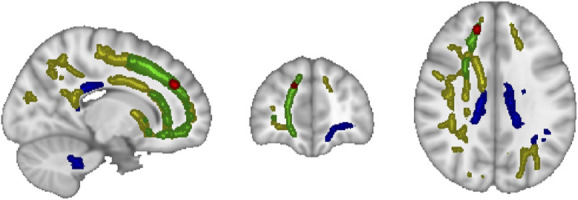
Tract-based spatial statistics analysis revealed clustered areas of white matter with increased fractional anisotropy (blue), increased axial diffusivity (yellow), increased mean diffusivity (green), and increased radial diffusivity (red) during the last 60 minutes of dialysis (late-HD) relative to before dialysis (pre-HD). MNI coordinates: X=74, Y=171, Z=102. HD, hemodialysis.
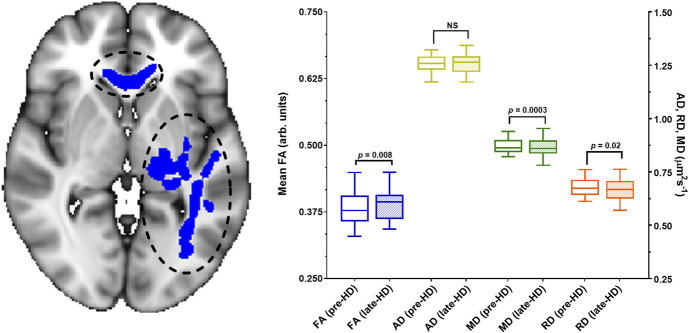
As mentioned previously, these WM clusters were used to define functional ROIs. Within the functional ROIs where FA significantly increased from pre-HD to late-HD (body of CC, left OR, left ILF, left IFOF), mean MD and RD were found to be significantly decreased (Figure 5). Specifically, percent changes in FA, MD, and RD at late-HD relative to pre-HD were 2.82±2.91% (median=3.43%), −0.86%±1.52% (median = −0.76%), and −1.85%±2% (median = −1.71%), respectively.
Figure 5.
Clusters of white matter showing significant differences in diffusion tensor imaging metrics (Figure 4) from before hemodialysis to the last 60 minutes of hemodialysis (late-HD) were used to define functional ROIs. The functional ROI where FA increased from pre-HD to late-HD (P = 0.008, blue bars) is shown here with black circles and blue fill. This functional ROI includes a portion of the corpus callosum and left inferior fronto-occipital fasciculus. Within this functional ROI, there was no significant change in AD (yellow bars) while mean diffusivity (P = 0.003, green bars) and radial diffusivity (P = 0.02, orange bars) decreased significantly from pre-HD to late-HD. Specifically, the percent changes in MD and RD at late-HD relative to pre-HD were 0.86%±1.52% (median = −0.76%) and −1.85%±2% (median = −1.71%), respectively. These changes are of a large magnitude, considering the annual rate of change in MD and RD in these regions is no more than ±0.67%.43 The MNI slice coordinate for the brain image shown is Z=72. AD, axial diffusivity; Arb, arbitrary; FA, fractional anisotropy; HD, hemodialysis; MD, mean diffusivity; NS, not statistically significant; RD, radial diffusivity; ROI, region of interest.
The mean change in FA from pre-HD to late-HD in these ROIs was significantly associated with the change in the percentage of TIV occupied by CSF (r=0.69, P = 0.002, Bonferroni-adjusted α-level of 0.008, Figure 6A), with a greater increase in FA associated with a greater increase in the percentage of TIV occupied by CSF. Within these same ROIs, a greater decrease in average MD values was observed to be associated with higher TMT B performance time (r=−0.66, P = 0.008, Figure 6B), although this association was not statistically significant after Bonferroni correction for multiple comparisons with a Bonferroni-adjusted α-level of 0.008.
Figure 6.
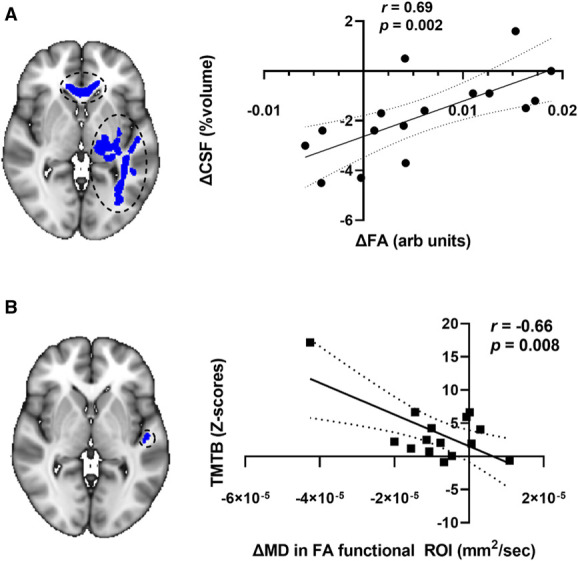
Associations of changes in diffusion tensor imaging metrics and changes in CSF volume and cognitive test performance scores in white matter regions where tract-based spatial statistics revealed changes in FA. Large strength of association between change in mean FA (corpus callosum and left inferior fronto-occipital fasciculus) and change in mean CSF volume (A) and between change in TMT B z-scores and change in average MD (left inferior fronto-occipital fasciculus) (B) are shown. Note that the correlation shown in (B) did not meet the threshold for statistical significance after Bonferroni correction. The MNI coordinate for both brain images shown is Z=72. The linear regression (solid line) with 95% confidence intervals (dotted lines) is plotted. Arb, arbitrary; CSF, cerebrospinal fluid; FA, fractional anisotropy; MD, mean diffusivity; ROI, region of interest; TMT, Trail Making Test.
Other functional ROIs also exhibited changes in DTI metrics. Within functional ROIs where mean MD significantly increased from pre-HD to late-HD (genu of CC, right IFOF, right ATR), mean AD and RD were found to be significantly decreased. Specifically, there was a median change of 1.79% (P = 0.0008) and 1.47% (P = 0.0006) in AD and RD, respectively, but no statistically significant change in FA (P = 0.78). In functional ROIs where both mean MD and AD significantly increased from pre-HD to late-HD (genu of CC and right IFOF), the mean RD increased significantly (P = 0.0002), but no change in mean FA was observed (P = 0.94). In functional ROIs where mean FA and AD increased significantly from pre-HD to late-HD, there were no significant changes in mean MD (P = 0.19) or RD (P = 0.09).
In addition, there were brain areas identified where both an increase in WM volume and an increase in FA, MD, and AD from pre-HD to late-HD were observed (left ILF, right ATR, and right superior longitudinal fasciculus, Supplemental Figure 3). There were no brain areas with concomitant change in RD and WM volume.
Brain Metabolite Concentrations
Significant intradialytic changes in the prefrontal concentration of NAA and Cho were detected. Concentrations of NAA (mean±standard error) were significantly lower at late-HD compared with pre-HD (13.5±0.5 mM versus 12.2±0.5 mM, P = 0.031, Figure 7A). Concentrations of Cho (mean±standard error) were significantly lower at late-HD compared with pre-HD (3.70±0.1 mM versus 4.06±0.1 mM, P = 0.035, Figure 7B). From pre-HD to late-HD, a greater decrease in prefrontal cortex NAA concentration was found to be significantly associated with a greater increase in serum CRP levels (r=−0.55, P = 0.043, Figure 8).
Figure 7.
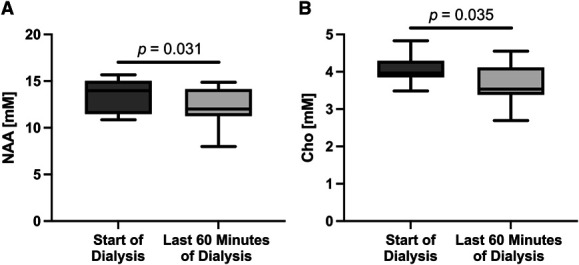
Statistically significant intradialytic metabolite concentration changes are shown. Intradialytic changes in prefrontal NAA and choline (Cho) concentrations are shown in (A) and (B), respectively. Box and whisker plots with whiskers showing minimum and maximum values are plotted. Concentrations of NAA (mean±standard error) were significantly lower during the last 60 minutes of dialysis versus the start of dialysis (13.5±0.5 mM versus 12.2±0.5 mM, P = 0.031). Concentrations of Cho (mean±standard error) were significantly lower during the last 60 minutes of dialysis versus the start of dialysis (3.70±0.1 mM versus 4.06±0.1 mM, P = 0.035). NAA, N-acetyl aspartate.
Figure 8.
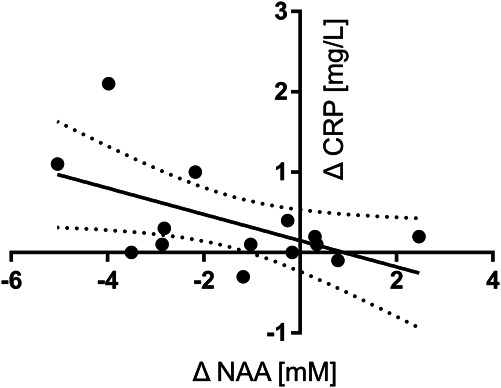
There was a statistically significant association between the change in NAA concentration with the change in serum CRP levels from the start of dialysis to the last 60 minutes of dialysis (P = 0.043). A greater decrease in NAA concentration during hemodialysis was significantly associated with a greater increase in serum CRP levels (r=−0.55). The linear regression (solid line) with 95% confidence intervals (dotted lines) is plotted. CRP, C-reactive protein; NAA, N-acetyl aspartate.
Discussion
This study has provided, for the first time, direct evidence of acute injury to the brain occurring during a single monitored HD session. There were observed intradialytic changes in brain tissue volume, diffusion metrics, and brain metabolite concentrations consistent with energy deficit, perfusion anomaly, and cytotoxic edema.
Using DTI, we observed intradialytic changes in diffusion metrics, such as increased FA, increased diffusivities (MD, AD, RD), and decreased diffusivities (MD, RD), throughout the cerebral WM of patients during HD. Specifically, we observed a pattern of decreases of MD and RD in multiple WM regions where there were FA increases from pre-HD to late-HD (Figure 5). The observed percent changes in MD and RD are of a large magnitude, considering the annual rate of change in MD and RD in these regions is no more than ±0.67%.43 This pattern has previously been demonstrated in patients undergoing HD18 and is an indication of ischemic brain injury as demonstrated in animal stroke models44,45 and in human patients with an acute ischemic stroke.21,22 Specifically, an increase in diffusion anisotropy and reduction in diffusivity is a result of the cytotoxic (intracellular) edema that follows an ischemic insult.46–48 The presence of edema was also supported by observed intradialytic increases in WM and GM volumes with a concomitant decrease in CSF volume in multiple regions of the brain (Figure 3 and Table 2). Conversely, a recent study observed reductions in FA and increases in diffusivities (MD, AD, RD) in cerebral WM of patients with end stage kidney disease immediately after HD in the absence of ischemia or neurological symptoms.49 The difference in DTI findings between that study and ours highlights the importance of investigating the effect of transient ischemic insults during HD on brain WM microstructure that may be missed after HD treatment.49
HD patients are primed for ischemic brain injury, and given the vulnerability of other vascular beds to HD-induced ischemic injury, the brain might be expected to also show evidence of such insults. Unlike those who do not have kidney disease or patients with late-stage CKD but not yet on HD, HD patients are especially susceptible to HD-induced systemic hypotension. This is a result of the characteristic impaired vascular compliance, decreased peripheral vascular resistance, and diminished brain autoregulatory reserve of dialysis patients, making maintenance of brain perfusion increasingly dependent on pressure.19 This results in an acute global and regional reduction in brain blood flow, as demonstrated previously in positron emission tomography and Doppler studies.6–8
The intradialytic changes in brain metabolite concentrations as measured using 1H-MRS are also consistent with a low-energy state as a result of reduced brain perfusion. Although NAA50,51 and choline50 have been previously reported to be lower in patients undergoing HD, our study is the first to demonstrate an intradialytic decrease in prefrontal NAA and choline concentrations (Figure 7). Intradialytic decline in prefrontal cortex NAA indicates an energy deficit in this region because NAA synthesis in the brain requires acetyl-coenzyme A (acetyl-CoA) levels exceeding neuronal metabolic requirements.52 Because the NAA decrease was observed during the last 60 minutes of dialysis (during maximal circulatory stress20), the intradialytic decline in NAA is likely to represent a consequence of decreased cerebral blood flow. Interestingly, this result is consistent with decreased NAA concentration in infarcted brain observed in acute ischemic stroke.53,54 An intradialytic decrease in estimated choline concentrations may also be a consequence of decreased cerebral blood flow because perfusion anomalies can affect free choline levels in the brain. The brain cannot synthesize free choline de novo and neurons must rely on brain perfusion for choline delivery and active transport of choline into the cells.55 In situations of energy deficit, such as poor brain perfusion, active transport is reduced and reactions that produce free choline by the breakdown of choline-containing compounds are favored. This mechanism is supported by in vitro work showing increased free choline formation secondary to phosphatidylcholine hydrolysis during energy stress.56 Free choline is open to exchange with the circulation57 and is lost through venous drainage. The measured 1H-MRS choline signal arises from both free choline and choline-containing compounds. Thus, loss of active uptake of free choline, circulatory loss of free choline, and increased breakdown of choline-containing compounds is reflected as a decrease in the 1H-MRS choline signal. Because the metabolite's estimated concentration is proportional to the amplitude of its 1H-MRS signal,36 a decreased choline signal results in lower estimated choline concentrations.
Several other consequences of HD may contribute to poor brain perfusion. In addition to cardiac stunning and injury58 and impaired autoregulatory function,59–61 HD-induced endotoxemia and systemic inflammation also contribute.62 Increase of CRP during HD (as demonstrated previously63 and in this study) is part of this physiological picture because CRP is known to rapidly increase in response to acute cardiac injury and systemic inflammation, a finding consistent with the observed association of a greater decrease in NAA concentration with a greater increase in serum CRP levels (Figure 8).
Poor brain perfusion and acute ischemic insults are precursors to permanent leukoaraiosis.64 Chronic WM injury in patients with HD has been reported in both randomized control trials and observational studies12,18 and has thus far been attributed to the high cardiovascular disease burden typical of HD patients. Because this study is the first to provide direct evidence of acute WM injury during a single HD session, there has been no confirmation until now that HD itself can acutely mediate WM injury. Interestingly, the findings of this study (intradialytic diffusivity decrease in areas of FA increase) replicate the pattern previously observed in patients after 12 months of dialysis.18 Considering that imaging for that study was performed on a nondialysis day within 24 hours of the last dialysis session, observed WM changes were likely related to the dialysis session before imaging and exacerbated over one year of HD treatment. Combined with the fact that patients on HD have evidence of fixed WM injury relative to controls,12 it appears that transient acute ischemic insults occur during HD, accumulates over multiple dialysis sessions, and develops into established brain damage, analogous to what occurs in the myocardium.3,58
This raises the possibility that the repeated delivery of HD could have a long-term effect on cognitive function. It is well known that HD patients develop cognitive impairment, especially in executive function, after starting HD treatment.13,14,16,17,65 However, whether HD-induced WM injury is associated with cognitive impairment remains an open question, unanswered by this study. Although we observed an association between increased predialysis TMT B performance time and a greater intradialytic decrease in average MD within regions with an intradialytic increase in FA (Figure 6), this association did not meet the threshold for statistical significance after Bonferroni correction. The lack of statistically significant associations between intradialytic MRI findings of brain injury and cognitive function in this study may reflect measurement of cognitive function before dialysis rather than during dialysis, inadequate power to detect such an association, or a true lack of functional significance. Nevertheless, we believe further study of the possible association between the severity of HD-induced WM injury and measures of executive function is necessary. Establishment of this association would better motivate the study of therapeutic interventions directed at the HD session itself. It has already been shown that HD-induced WM injuries are amenable to dialysis-based intervention (dialysate cooling) that prevents the development of leukoaraiotic changes over a 12-month period and may reduce long-term HD-associated cognitive impairment (when applied in patients new to HD).18 Other interventions, such as RIPC, may also provide protection against these ischemic insults with potential functional benefit to the HD patient, especially in patients with progressive injury, but have already been on HD treatment for some time. This is an avenue of active research in our laboratory.
There are some limitations to this study. First, the number of patients studied was small. Although power calculations based on a previous randomized control trial showed that our effective sample size of 16 patients was twice the expected sample size required to observe DTI metric changes, the sample size may not be big enough to provide sufficient power for other reported outcomes. The statistically significant intradialytic changes found for brain concentrations, tissue volumes, and serum measurements will need to be confirmed with a larger sample size.
Second, we have attributed observed changes to ischemic injury and perfusion anomaly on the basis of converging findings from multiple MR imaging modalities, but were unable to make any direct measurement of brain blood flow. Arterial spin labeling MR perfusion imaging was included in the original protocol, but technical challenges relating to tagging of convoluted feeding arteries and practical constraints on scan time prevented data from being suitable for analysis. Previously, we have used the mathematical analysis of continuous mean arterial BP measurement to assess intradialytic hemodynamics and organ perfusion,18 but were unable to do so as continuous mean arterial BP measurement was unavailable within the high-field MRI. In addition, no measurement of blood flow distribution or vascular anatomy was obtained; thus, the data in this study cannot be used to draw conclusions regarding the anatomical distribution of the affected brain regions. This warrants further study, but we speculate that the diffuse anatomical distribution of WM changes observed (Figure 4) is influenced by variations in vascular compliance, in vascular distribution to the cortex and deep structures, and in circle of Willis anatomy.
We also did not make intradialytic measurements of sodium or urea in the brain, which would have allowed better characterization of the etiology of observed DTI and brain volume changes. Although we attribute our observations to ischemia-mediated cytotoxic edema on the basis of the stroke literature, shifts of sodium and urea (the two principle osmotically active molecules) in the brain could result in concomitant vasogenic edema.66 Because both perfusion anomaly and osmotic shift occur during HD, the observed DTI and brain volume changes could be of mixed etiology. Measurements of brain sodium and urea would have required multinuclear MRI, acquiring signals from sodium and 13C, respectively. Technical considerations result in lengthy scan times that made this imaging technique impractical to include our protocol. This remains an area of active study within our laboratory and is the focus of ongoing studies.
This is the first study to provide direct evidence of HD-induced brain injury occurring (presumably repeatedly) within the treatment itself. This study demonstrated that intradialytic changes in brain tissue volume, diffusion metrics, and brain metabolite concentrations consistent with impaired brain perfusion and ischemic WM injury can occur in a single dialysis session. This raises the possibility that the repeated delivery of HD could affect long-term structural brain injury and cognitive function. Preventing brain injury and preserving cognitive vitality should begin to be seen as a primary responsibility of physicians and agencies providing care to HD patients. Further study of HD-induced brain injury, of associated cognitive impairment, and of HD-directed interventions designed to mitigate the effect of HD on the brain (e.g., RIPC or dialysate cooling) is an urgent imperative.
Supplementary Material
Acknowledgments
The authors are grateful for the assistance from Tanya Tamasi and Justin Dorie in recruitment and execution of the study and thank MRI technologists Heather Biernaski and John Butler for their assistance in performing the intradialytic imaging. The authors also appreciate the assistance of Sal Treesh for his expertise in facilitating the ability to administer hemodialysis in the MRI suite and thank CardioMed Supplies (Lindsay, Ontario, Canada) for manufacturing and providing study specific extension lines that enabled dialysis in the MRI suite. Finally, the authors thank the patients who volunteered and participated in the study, without whom this study would not have been possible.
Footnotes
See related editorial, “Dialysis on the Mind: The Evolution of Hemodialysis-Related Acute Brain Injury,” on pages 938–940.
DISCLOSURES
C.W. McIntyre has received research funding and speaker honoraria from Baxter Healthcare. C.W. McIntyre also reports Consultancy: Baxter, Intellomed, Sequana Medical, Spiden AG, and Vascular Dynamics; Research Funding: Sequana; Honoraria: Baxter; and Advisory or Leadership Role: Baxter, Sequana Medical, and Spiden AG. J. Théberge reports Consultancy: Siemens Healthcare Limited; and Research Funding: Multi‐Magnetics Inc. All remaining authors have nothing to disclose.
Funding
Funding was provided by the Heart and Stroke Foundation of Canada (G-17-00018311).
Author Contributions
Conceptualization: Christopher McIntyre
Data curation: Udunna Anazodo, Madeleine Dacey, Janice Gomes, Christopher McIntyre, Jarrin Penny, Stefan Poirier, Jean Théberge
Formal analysis: Udunna Anazodo, Madeleine Dacey, Michael van Ginkel, Stefan Poirier, Dickson Wong
Investigation: Udunna Anazodo, Madeleine Dacey, Janice Gomes, Christopher McIntyre, Jarrin Penny, Jean Théberge
Methodology: Udunna Anazodo, Madeleine Dacey, Christopher McIntyre, Stefan Poirier, Dickson Wong
Project administration: Christopher McIntyre, Jarrin Penny
Resources: Christopher McIntyre, Jean Théberge
Software: Udunna Anazodo, Stefan Poirier, Dickson Wong
Supervision: Christopher McIntyre, Jarrin Penny, Jean Théberge
Validation: Udunna Anazodo, Stefan Poirier, Jean Théberge, Dickson Wong
Visualization: Udunna Anazodo, Stefan Poirier, Dickson Wong
Writing – original draft: Udunna Anazodo, Michael van Ginkel, Christopher McIntyre, Stefan Poirier, Dickson Wong
Writing – review & editing: Udunna Anazodo, Janice Gomes, Christopher McIntyre, Jarrin Penny, Stefan Poirier, Jean Théberge, Dickson Wong
Data Sharing Statement
Data used in this paper cannot be shared because the data used for the present study was derived from the prerandomization visit of a subsequent RCT. All data contained within this submission relate only to the observation of the effects of HD on the brain in the entire nonrandomized cohort. Clinical trial information included in this submission is only provided for the sake of transparency and to be consistent with the NCT trial registration. The present study does not comment on the outcomes of the RCT and only demonstrates that magnetic resonance imaging techniques can measure relevant HD-related brain injury. Since the RCT results have not yet been published, data cannot be shared at this time. Data for this study will be shared on subsequent publication of the RCT results.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E32.
Detailed Description of Imaging Methods.
Supplemental Figure 1. A 20×20×20 mm3 spectroscopy voxel was placed in the right prefrontal cortex white matter according to anatomical landmarks. A representative example of voxel placement in one study participant is shown in (a). Spectroscopy data were fitted in the time-domain with simulated 3T semi-LASER prior knowledge templates that included an empirically measured macromolecule model. A representative spectrum from one study participant is shown in (b) with the fitted prior knowledge template (“Model”), the individual metabolite components, and the empirical macromolecule model (“MM”).
Supplemental Figure 2. Intradialytic changes in serum levels of electrolytes (potassium, magnesium, and phosphate) are shown in (a). Intradialytic changes in bicarbonate, urea, and creatine are shown in (b). Intradialytic changes international normalized ratio, and partial thromboplastin are shown in (c). Intradialytic changes hemoglobin levels are shown in (d). For all graphs, estimated marginal means and standard errors are plotted.
Supplemental Figure 3. White matter (WM) regions of concomitant intradialytic increase in WM volume from voxel-based morphometry (VBM) and increase in fractional anisotropy (FA, a) (MNI coordinates: Y=112, X=140, Z=72), increase in mean diffusivity (MD, b) (Montreal Neurological Institute coordinates: Y=133, X=70, Z=114), and increase in axial diffusivity (AD, c) (MNI coordinates: Y=87, X=57, Z=105) from tract-based spatial statistics (TBSS).
Supplemental Table 1. A summary of relationships between metabolite parameters in the prior knowledge template15. Peak positions (shifts) and phases of low-amplitude metabolites were linked to the shifts and phases of higheramplitude metabolites. Within metabolites, peak amplitudes, shifts, and phases were fixed relative to one another. The following metabolites were included in the prior knowledge template: NAA, Glu, Gln, GSH, GABA, Cr, Cho, Myo, Glc, Scyllo, and Tau.
Supplemental Table 2. A summary of the T1 and T2 values of metabolites and water in GM, WM, and CSF used for calculation of metabolite concentrations. All values were obtained from the literature as referenced in the table.
References
- 1.Odudu A, McIntyre CW. An update on intradialytic cardiac dysfunction. Semin Dial. 2016;29(6):435–441 doi: 10.1111/sdi.12532 [DOI] [PubMed] [Google Scholar]
- 2.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4(12):1925–1931. doi: 10.2215/CJN.04470709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CSR. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3(1):19–26. doi: 10.2215/CJN.03170707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan C, Mohammed A, Cox E, Köhler K, Canaud B, Taal MW. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol. 2017;28(4):1269–1277. doi: 10.1681/ASN.2016060686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eldehni MT, McIntyre CW. Are there neurological consequences of recurrent intradialytic hypotension? Semin Dial. 2012;25(3):253–256. doi: 10.1111/j.1525-139X.2012.01057.x [DOI] [PubMed] [Google Scholar]
- 6.Findlay MD, Dawson J, Dickie DA, Forbes KP, McGlynn D, Quinn T. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol. 2019;30(1):147–158. doi: 10.1681/ASN.2018050462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polinder-Bos HA JWJ Elting Aries MJ, et al. Changes in cerebral oxygenation and cerebral blood flow during hemodialysis—a simultaneous near-infrared spectroscopy and positron emission tomography study. J Cereb Blood Flow Metab. 2020;40(2):328–340. doi: 10.1177/0271678X18818652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polinder-Bos HA García DV Kuipers J, et al. Hemodialysis induces an acute decline in cerebral blood flow in elderly patients. J Am Soc Nephrol. 2018;29(4):1317–1325. doi: 10.1681/ASN.2017101088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayoumi M, al Harbi A, al Suwaida A, al Ghonaim M, al Wakeel J, Mishkiry A. Predictors of quality of life in hemodialysis patients. Saudi J Kidney Dis Transplant. 2013;24(2):254–259. doi: 10.4103/1319-2442.109566 [DOI] [PubMed] [Google Scholar]
- 10.Vanderlinden JA, Ross-White A, Holden R, Shamseddin MK, Day A, Boyd JG. Quantifying cognitive dysfunction across the spectrum of end-stage kidney disease: a systematic review and meta-analysis. Nephrology. 2019;24(1):5–16. doi: 10.1111/nep.13448 [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eldehni MT, Odudu A, Mcintyre CW. Brain white matter microstructure in end-stage kidney disease, cognitive impairment, and circulatory stress. Hemodial Int. 2019;23(3):356–365. doi: 10.1111/hdi.12754 [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Román S, Ostrosky-Solís F, Morales-Buenrostro LE, Nogués-Vizcaíno MG, Alberú J, McClintock SM. Neurocognitive profile of an adult sample with chronic kidney disease. J Int Neuropsychol Soc. 2011;17(1):80–90. doi: 10.1017/S1355617710001219 [DOI] [PubMed] [Google Scholar]
- 14.Tsai CF, Wang SJ, Fuh JL. Moderate chronic kidney disease is associated with reduced cognitive performance in midlife women. Kidney Int. 2010;78(6):605–610. doi: 10.1038/ki.2010.185 [DOI] [PubMed] [Google Scholar]
- 15.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52(11):1863–1869. doi: 10.1053/j.ajkd.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 16.Murray AM Tupper DE Knopman DS, et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40 [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh NT, Schiller B, Saxena AB, Thomas IC, Kurella Tamura M. Prevalence and correlates of functional dependence among maintenance dialysis patients. Hemodial Int. 2015;19(4):593–600. doi: 10.1111/hdi.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26(4):957–965. doi: 10.1681/ASN.2013101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre CW, Goldsmith DJ. Ischemic brain injury in hemodialysis patients: which is more dangerous, hypertension or intradialytic hypotension? Kidney Int. 2015;87(6):1109–1115. doi: 10.1038/ki.2015.62 [DOI] [PubMed] [Google Scholar]
- 20.Eldehni MT, Odudu A, McIntyre CW. Characterising haemodynamic stress during haemodialysis using the extrema points analysis model. Nephron Clin Pract. 2014;128(1-2):39–44. doi: 10.1159/000359958 [DOI] [PubMed] [Google Scholar]
- 21.Bhagat YA Emery DJ Shuaib A, et al. The relationship between diffusion anisotropy and time of onset after stroke. J Cereb Blood Flow Metab. 2006;26(11):1442–1450. doi: 10.1038/sj.jcbfm.9600294 [DOI] [PubMed] [Google Scholar]
- 22.Bhagat YA, Hussain MS, Stobbe RW, Butcher KS, Emery DJ, Shuaib A. Elevations of diffusion anisotropy are associated with hyper-acute stroke: a serial imaging study. Magn Reson Imaging. 2008;26(5):683–693. doi: 10.1016/j.mri.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 23.Poirier SE Kwan BYM Jurkiewicz MT, et al. 18F-FDG PET-guided diffusion tractography reveals white matter abnormalities around the epileptic focus in medically refractory epilepsy: implications for epilepsy surgical evaluation. Eur J Hybrid Imaging. 2020;4(1):10. doi: 10.1186/s41824-020-00079-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders DE. MR spectroscopy in stroke. Br Med Bull. 2000;56(2):334–345. doi: 10.1258/0007142001903256 [DOI] [PubMed] [Google Scholar]
- 25.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 suppl 3):S1–S153. doi: 10.1053/j.ajkd.2005.01.019 [DOI] [PubMed] [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27.Amatneeks TM, Hamdan AC. Montreal cognitive assessment for cognitive assessment in chronic kidney disease: a systematic review. J Bras Nefrol. 2019;41(1):112–123. doi: 10.1590/2175-8239-JBN-2018-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salthouse TA. What cognitive abilities are involved in trail-making performance? Intelligence. 2011;39(4):222–232. doi: 10.1016/j.intell.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brant-Zawadzki M, Gillan GD, Nitz WR. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence—initial experience in the brain. Radiology. 1992;182(3):769–775. doi: 10.1148/radiology.182.3.1535892 [DOI] [PubMed] [Google Scholar]
- 30.Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155–177. doi: 10.1006/jmre.2001.2340 [DOI] [PubMed] [Google Scholar]
- 31.Oz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn Reson Med. 2011;65(4):901–910. doi: 10.1002/mrm.22708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deelchand DK Berrington A Noeske R, et al. Across-vendor standardization of semi-LASER for single-voxel MRS at 3T. NMR Biomed. 2021;34(5):e4218. doi: 10.1002/nbm.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong D, Atiya S, Fogarty J, Montero-Odasso M, Pasternak SH, Brymer C. Reduced hippocampal glutamate and posterior cingulate N-acetyl aspartate in mild cognitive impairment and Alzheimer’s disease is associated with episodic memory performance and white matter integrity in the cingulum: a pilot study. J Alzheimer’s Dis. 2020;73(4):1385–1405. doi: 10.3233/JAD-190773 [DOI] [PubMed] [Google Scholar]
- 34.Birch R, Peet AC, Dehghani H, Wilson M. Influence of macromolecule baseline on 1H MR spectroscopic imaging reproducibility. Magn Reson Med. 2017;77(1):34–43. doi: 10.1002/mrm.26103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong D, Schranz AL, Bartha R. Optimized in vivo brain glutamate measurement using long-echo-time semi-LASER at 7 T. NMR Biomed. 2018;31(11):e4002. doi: 10.1002/nbm.4002 [DOI] [PubMed] [Google Scholar]
- 36.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–1226. doi: 10.1002/mrm.20901 [DOI] [PubMed] [Google Scholar]
- 37.Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 38.Hampshire A, Highfield RR, Parkin BL, Owen AM. Fractionating human intelligence. Neuron. 2012;76(6):1225–1237. doi: 10.1016/j.neuron.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 40.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 41.Mori S, Wakan S, Nagae-Poetscher LM, van Zijl PCM. MRI Atlas of Human White Matter. 1st ed. Elsevier. [Google Scholar]
- 42.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7(1):123. doi: 10.1186/1471-2105-7-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storsve AB, Fjell AM, Yendiki A, Walhovd KB. Longitudinal changes in white matter tract integrity across the adult lifespan and its relation to cortical thinning. PLoS One. 2016;11(6):e0156770. doi: 10.1371/journal.pone.0156770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitkonen M, Abo-Ramadan U, Marinkovic I, Pedrono E, Hasan KM, Strbian D. Long-term evolution of diffusion tensor indices after temporary experimental ischemic stroke in rats. Brain Res. 2012;1445:103–110. doi: 10.1016/j.brainres.2012.01.043 [DOI] [PubMed] [Google Scholar]
- 45.Hui ES, Du F, Huang S, Shen Q, Duong TQ. Spatiotemporal dynamics of diffusional kurtosis, mean diffusivity and perfusion changes in experimental stroke. Brain Res. 2012;1451:100–109. doi: 10.1016/j.brainres.2012.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Q Tress BM Barber PA, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke. 1999;30(11):2382–2390. doi: 10.1161/01.str.30.11.2382 [DOI] [PubMed] [Google Scholar]
- 47.Green HAL Peña A Price CJ, et al. Increased anisotropy in acute stroke: a possible explanation. Stroke. 2002;33(6):1517–1521. doi: 10.1161/01.str.0000016973.80180.7b [DOI] [PubMed] [Google Scholar]
- 48.Ogawa C Kidokoro H Fukasawa T, et al. Cytotoxic edema at onset in West syndrome of unknown etiology: a longitudinal diffusion tensor imaging study. Epilepsia. 2018;59(2):440–448. doi: 10.1111/epi.13988 [DOI] [PubMed] [Google Scholar]
- 49.Schaier M Wolf RC Kubera K, et al. Vasogenic brain edema during maintenance hemodialysis: preliminary results from tract-based spatial statistics and voxel-based morphometry. Clin Neuroradiol. 2021;31(1):217–224. doi: 10.1007/s00062-019-00865-2 [DOI] [PubMed] [Google Scholar]
- 50.Tryc AB Alwan G Bokemeyer M, et al. Cerebral metabolic alterations and cognitive dysfunction in chronic kidney disease. Nephrol Dial Transplant. 2011;26(8):2635–2641. doi: 10.1093/ndt/gfq729 [DOI] [PubMed] [Google Scholar]
- 51.Geissler A, Frund R, Kohler S, Eichhorn HM, Kramer BK, Feuerbach S. Cerebral metabolite patterns in dialysis patients: evaluation with H-1 MR spectroscopy. Radiology. 1995;194(3):693–697. doi: 10.1148/radiology.194.3.7862964 [DOI] [PubMed] [Google Scholar]
- 52.Ariyannur PS Moffett JR Manickam P, et al. Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res. 2010;1335:1–13. doi: 10.1016/j.brainres.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 53.Saunders DE, Howe FA, van den Boogaart AM, McLean MA, Griffiths JR, Brown MM. Continuing ischemic damage after acute middle cerebral artery infarction in humans demonstrated by short-echo proton spectroscopy. Stroke. 1995;26(6):1007–1013. doi: 10.1161/01.str.26.6.1007 [DOI] [PubMed] [Google Scholar]
- 54.Gideon P Henriksen O Sperling B, et al. Early time course of N-acetylaspartate, creatine and phosphocreatine, and compounds containing choline in the brain after acute stroke: a proton magnetic resonance spectroscopy study. Stroke. 1992;23(11):1566–1572. doi: 10.1161/01.str.23.11.1566 [DOI] [PubMed] [Google Scholar]
- 55.Wurtman RJ. Choline metabolism as a basis for the selective vulnerability of cholinergic neurons. Trends Neurosci. 1992;15(4):117–122. doi: 10.1016/0166-2236(92)90351-8 [DOI] [PubMed] [Google Scholar]
- 56.Djuricic B, Olson SR, Assaf HM, Whittingham TS, Lust WD, Drewes LR. Formation of free choline in brain tissue during in vital energy deprivation. J Cereb Blood Flow Metab. 1991;11(2):308–313. doi: 10.1038/jcbfm.1991.63 [DOI] [PubMed] [Google Scholar]
- 57.Cornford EM, Braun LD, Oldendorf WH. Carrier mediated blood-brain barrier transport of choline and certaine choline analogs. J Neurochem. 1978;30(2):299–308. doi: 10.1111/j.1471-4159.1978.tb06530.x [DOI] [PubMed] [Google Scholar]
- 58.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birns J, Jarosz J, Markus HS, Kalra L. Cerebrovascular reactivity and dynamic autoregulation in ischaemic subcortical white matter disease. J Neurol Neurosurg Psychiatry. 2009;80(10):1093–1098. doi: 10.1136/jnnp.2009.174607 [DOI] [PubMed] [Google Scholar]
- 60.Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW. Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant. 2005;20(6):1140–1147. doi: 10.1093/ndt/gfh808 [DOI] [PubMed] [Google Scholar]
- 61.Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW. Categorization of the hemodynamic response to hemodialysis: the importance of baroreflex sensitivity. Hemodial Int. 2010;14(1):18–28. doi: 10.1111/j.1542-4758.2009.00403.x [DOI] [PubMed] [Google Scholar]
- 62.McIntyre CW, Harrison LEA, Eldehni MT, Jefferies HJ, Szeto CC, John SG. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–141. doi: 10.2215/CJN.04610510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korevaar JC, van Manen JG, Dekker FW, de Waart DR, Boeschoten EW, Krediet RT. Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. J Am Soc Nephrol. 2004;15(11):2916–2922. doi: 10.1097/01.ASN.0000143744.72664.66 [DOI] [PubMed] [Google Scholar]
- 64.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28(3):652–629. doi: 10.1161/01.str.28.3.652 [DOI] [PubMed] [Google Scholar]
- 65.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. New Engl J Med. 2009;361(16):1539–1547. doi: 10.1161/01.str.28.3.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galons JP, Trouard T, Gmitro AF, Lien YHH.Hemodialysis increases apparent diffusion coefficient of brain water in nephrectomized rats measured by isotropic diffusion-weighted magnetic resonance imaging. J Clin Invest. 1996;98(3):750–755. doi: 10.1172/jci118847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this paper cannot be shared because the data used for the present study was derived from the prerandomization visit of a subsequent RCT. All data contained within this submission relate only to the observation of the effects of HD on the brain in the entire nonrandomized cohort. Clinical trial information included in this submission is only provided for the sake of transparency and to be consistent with the NCT trial registration. The present study does not comment on the outcomes of the RCT and only demonstrates that magnetic resonance imaging techniques can measure relevant HD-related brain injury. Since the RCT results have not yet been published, data cannot be shared at this time. Data for this study will be shared on subsequent publication of the RCT results.