Abstract
This study presents the first data of a Japanese nationwide multi-institutional cohort and compares them with the findings of systematic literature reviews on radiation therapies and inoperable stage III non-small cell lung cancer (NSCLC) conducted by the Lung Cancer Working Group in the Particle Beam Therapy (PBT) Committee and Subcommittee at Japanese Society for Radiation Oncology. The Lung Cancer Working Group extracted eight reports and compared their data with those of the PBT registry from May 2016 to June 2018. All the analyzed 75 patients aged ≤80 years underwent proton therapy (PT) with concurrent chemotherapy for inoperable stage III NSCLC. The median follow-up period of the surviving patients was 39.5 (range, 1.6–55.6) months. The 2- and 3-year overall survival (OS) and progression-free survival rates were 73.6%/64.7% and 28.9%/25.1%, respectively. During the follow-up period, six patients (8.0%) had adverse events of Grade ≥ 3, excluding abnormal laboratory values. These included esophagitis in four patients, dermatitis in one and pneumonitis in one. Adverse events of Grade ≥ 4 were not observed. The results of these PBT registry data in patients with inoperable stage III NSCLC suggest that the OS rate was at least equivalent to that of radiation therapy using X-rays and that the incidence of severe radiation pneumonitis was low. PT may be an effective treatment to reduce toxicities of healthy tissues, including the lungs and heart, in patients with inoperable stage III NSCLC.
Keywords: particle beam therapy, proton beam therapy, chemoradiotherapy, inoperable stage III non-small cell lung cancer
INTRODUCTION
Photon-based radiation therapy with concurrent chemotherapy is recommended for inoperable stage III non-small cell lung cancer (NSCLC) [1–4]. However, for patients with unresectable stage III NSCLC, long-term survival remains at ~30% [5, 6]. Disease recurrence, particularly local and regional, remains problematic, and more effective treatments are actively being sought. Recently, consolidation treatment with human monoclonal antibodies against programmed cell death-ligand 1 (PD-L1) has been recommended in patients without disease progression and symptomatic radiation pneumonitis after chemoradiotherapy [7–9]. One approach for improved therapeutic efficacy currently being investigated is particle beam therapy (PBT), including proton beam therapy (PT) and carbon-ion therapy, which has been shown to affect target tissue while reducing the damage to surrounding healthy organs [10–16]. Particularly with PBT, the therapeutic ratio might be improved by escalating the radiation dose to control the tumor while decreasing toxicity, even in patients with concurrent chemotherapy.
Previous studies have reported that the 2-year survival rates of PBT with concurrent chemotherapy for unresectable stage III NSCLC are ~50–60% [17–24]. The frequency of pneumonitis of Grade ≥ 3 varies from 0 to 13% [17–24]. There have been few reports of PBT with concurrent chemotherapy for unresectable stage III NSCLC. Therefore, satisfactory results of PBT with concurrent chemotherapy for unresectable stage III NSCLC have not yet been obtained.
In May 2016, a nationwide multi-institutional cohort study on PBT started in all Japanese centers providing proton or carbon-ion radiation therapy. Since then, PBT can be applied in Japan as advanced medical care to patients with NSCLC. Recently, a Lung Cancer Working Group in the Particle Beam Therapy Committee and Subcommittee at Japanese Society for Radiation Oncology (JASTRO) comprehensively analyzed PT effects in patients with lung cancer. This working group comprises radiation oncologists of the JASTRO, oncologists of the Japan Society of Clinical Oncology and biostatisticians. Herein, we present the results of the investigations on patients with unresectable stage III NSCLC based on the Japanese multi-institutional registry dataset.
MATERIALS AND METHODS
Ethical approval
The protocol of this multi-institutional prospective study was approved by the Institutional Review Board of each participating institution before study initiation. Patients were informed of the concept, methodology and rationale of the treatment, which was performed in accordance with the 1983 amendment of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Eligibility criteria
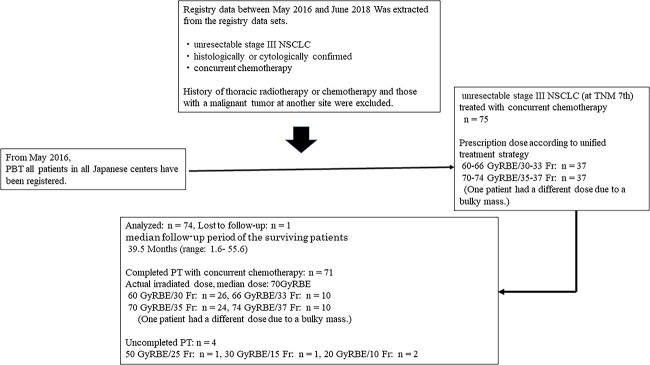
The inclusion criteria were patients with unresectable, histologically or cytologically confirmed stage III NSCLC (according to the tumor, node, metastasis [TNM] classification of tumors, 7th edition [25]). PT data have been recorded for all patients in Japan. The clinical results of consecutive patients treated with concurrent chemotherapy between May 2016 and June 2018 were analyzed in this study (Fig. 1).
Fig. 1.
The flowchart of the registry data set.
Patients who had a history of thoracic radiotherapy or chemotherapy and those with a malignant tumor at another site were excluded.
Treatments
Dose fractionation and concurrent chemotherapy were performed according to a unified treatment strategy, which was based on JASTRO-approved guidelines [26]. In addition, chemotherapy was performed based on the standard of care for inoperable stage III NSCLC. All patients were presented at multidisciplinary team in the Cancer Board meetings of each institution. Based on the joint recommendations of the multidisciplinary team in the Cancer Board meetings, patients were indicated for PT with concurrent chemotherapy. Prescription doses were selected from 60 to 66 GyRBE (standard dose) or 70 to 74 GyRBE (high dose) in two GyRBE daily fractions for each patient at each institution (Fig. 1, Table 1). The extent of irradiation to the lymph node area was determined at the discretion of the radiation oncologist in charge at each institution, since the unified treatment policy does not stipulate elective nodal irradiation (ENI) / involved field irradiation for irradiation to the lymph node areas.
Table 1.
Institutions and prescription doses
| Institutions | 60–66 GyRBE (n) | 70–74 GyRBE (n) | NA (n) |
|---|---|---|---|
| A | 0 | 1 | 1 |
| B | 0 | 10 | 0 |
| C | 2 | 0 | 0 |
| D | 0 | 19 | 0 |
| E | 17 | 0 | 0 |
| F | 4 | 5 | 0 |
| G | 0 | 2 | 0 |
| H | 14 | 0 | 0 |
Prescription doses were selected from 60 to 66 GyRBE (standard dose) or 70 to 74 GyRBE (high dose) in two GyRBE daily fractions for each patient at each institution.
Evaluation and follow-up
Overall survival (OS) was defined as the time from the first day of PT to death from any cause. Progression-free survival (PFS) was defined as the time from the first day of PT to locoregional failure, distant tumor progression or death from any cause. Primary tumor recurrence was considered an event for evaluating local control. After treatment, OS, local control and PFS were evaluated every 3–6 months. Clinical examination and imaging studies (magnetic resonance imaging, computed tomography or positron emission tomography-computed tomography) were performed as appropriate in each institution. Patients with only Karnofsky performance status (PS) in this registry data were tabulated after being converted to Eastern Cooperative Oncology Group PS.
Toxicity assessment
Acute toxicities were evaluated with the highest scores in the period from the start of PT up to 90 days according to the Common Terminology Criteria for Adverse Events, version 4.03. Late toxicities were graded 90 days after the commencement of PT according to the Common Terminology Criteria for Adverse Events, version 4.03 [27].
Systematic review of PBT for inoperable stage III NSCLC
The systematic literature review on inoperable stage III NSCLC was conducted by the Lung Cancer Working Group in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines (Fig. S1 and Table S1) [28]. PubMed was searched in 2017 and 2020 for clinical scientific reports on X-RT/X-IMRT/proton therapy/carbon ion radiation therapy and inoperable stage III NSCLC. In each team evaluating proton therapy and carbon ion radiation therapy for inoperable stage III NSCLC, two radiation oncologists independently reviewed the retrieved articles and selected potentially relevant articles based on titles and abstracts. If necessary, the two experts manually searched for other relevant articles. Afterward, full-text reviews identified studies that met the selection criteria established in each team assessing proton therapy and carbon ion radiation therapy.
Finally, five studies conducted before 2017 in patients with inoperable stage III NSCLC (5 single-center prospective studies) and three studies between 2017 and 2020 for proton therapy (2 single-center prospective studies, 1 retrospective study) were adopted [17–24].
Statistical analysis
The survival probability after commencing treatment was estimated using the Kaplan–Meier method, and the level of significance was assessed using the log-rank test. The Kaplan–Meier curves were calculated using commercial software (R, version 4.2.2). A P-value <0.05 was considered statistically significant.
RESULTS
The patients’ characteristics before treatment are summarized in Tables 2 and 3. From May 2016 to June 2018, 75 consecutive patients with inoperable stage III NSCLC (IIIA/IIIB: 46.7%/53.3%) were treated with PT and concurrent chemotherapy at multiple institutions in Japan. The median age was 66 (range, 34–80) years, and the cohort comprised 57 men and 18 women. All patients had a good PS (0 or 1). NSCLC was histologically confirmed in 74 patients, and only one patient was diagnosed cytologically.
Table 2.
Patient characteristics
| Characteristic | Value |
|---|---|
| Median age, years (range) | 66 (34–80) |
| Sex, n (%) | |
| Male:female | 57 (76):18 (24) |
| PS, n (%) | |
| 0:1 | 61 (81.3):14 (18.6) |
| Histology, n (%) | |
| Adenocarcinoma | 42 (56) |
| Squamous cell carcinoma | 27 (36) |
| Unclassified NSCLC | 5 (6.7) |
| Histology unproved | 1 (1.3) |
| UICC seventh stage, n (%) | |
| IIIA:IIIB | 35 (46.7):40 (53.3) |
| T-stage, n | |
| 0:1:2:3:4 | 3:15:22:16:19 |
| N-stage, n | |
| 0:1:2:3 | 5:7:28:35 |
| Primary site, n | |
| Upper:middle:lower | 41:5:27 |
| Others | 2 |
| Central:peripheral | 23:51 |
| Others | 1 |
| Total dose, n (%) | |
| 60–66 GyRBE/30–33 Fr | 37 (49.3) |
| 70–74 GyRBE/33–37 Fr | 37 (49.3) |
| Others | 1 (1.3) |
| Irradiation technique, n (%) | |
| Broad beam:scanning | 67 (89.3):8 (10.7) |
| Median follow-up time, months | 39.5 (range: 1.6–55.6) |
| Interstitial pneumonia, n | 5 |
| Diabetes, n | 12 |
| Smoking history, n | |
| Nonsmoker | 10 |
| Smoker (Brinkman index < 600) | 16 |
| Smoker (Brinkman index ≥ 600) | 49 |
UICC, Union for International Cancer Control [25].
Table 3.
Patient characteristics by dose
| Characteristic | Value |
|---|---|
| UICC seventh stage, n | |
| ● 60–66 GyRBE/30–33 Fr | 37 |
| IIIA:IIIB | 19:20 |
| T-stage, n | |
| X:0:1:2:3:4 | 1:2:10:12:5:7 |
| N-stage, n | |
| 0:1:2:3 | 2:1:17:17 |
| Primary site, n | |
| Upper:middle:lower:others | 17:3:15:2 |
| Central:peripheral:others | 10:26:1 |
| ● 70–74 GyRBE/33–37 Fr | 37 |
| IIIA:IIIB | 16:21 |
| T-stage, n | |
| 0:1:2:3:4 | 0:5:10:10:12 |
| N-stage, n | |
| 0:1:2:3 | 3:5:11:18 |
| Primary site, n | |
| Upper:middle:lower:others | 24:2:11:0 |
| Central:peripheral:others | 12:25:0 |
Fr, fraction, UICC, Union for International Cancer Control [25].
The selected irradiation dose in each institution was 60–66 and 70–74 gray radiobiological equivalent (GyRBE) in 37 and 37 patients, respectively. A different dose (other) was selected due to a bulky mass in one patient. Of the 75 patients, 71 completed PT. The actual irradiated dose was the median dose: 70 GyRBE, 34 patients in the high-dose group (74 GyRBE: 10 patients, 70 GyRBE: 24 patients) and 36 patients in the standard dose group (66 GyRBE: 10 patients, 60 GyRBE: 26 patients). Of four patients with uncompleted PT, the actual irradiated dose was 50, 30 and 20 GyRBE in one, one and two patients, respectively (Fig. 1).
Of the four patients who could not complete PT, two were unable to complete treatment due to deterioration of lung cancer, and the other two were unable to complete treatment due to adverse events. The remaining patients completed all the treatments. A total of 32 patients died, and one was unknown during the final follow-up. Of the 32 deceased patients, 27 died from the disease, and the other 5 died from other diseases or unknown causes. The median OS was 41.0 months.
Finally, 74 patients were analyzed, excluding the unknown case. The median follow-up period of the surviving patients was 39.5 (range, 1.6–55.6) months. The 2- and 3-year OS rates were 73.6 (95% confidence interval [CI], 63.0–86.0%) and 64.7% (95% CI, 53.3–78.4%), respectively. The median PFS was 10.9 months, and the 2- and 3-year PFS rates were 28.9 (95% CI, 19.1–43.6%) and 25.1% (95% CI, 15.9–39.6%), respectively (Fig. 2). In addition, the OS and PFS were also evaluated in terms of primary tumor stage, nodal stage, dose, occupied primary site and histology. Regarding the primary site, the PFS was significantly lower in the lower lobe compared with other primary sites (P = 0.0051). Similarly, the OS tended to be low at the lower lobe (P = 0.081). No significant difference was observed in the other analyses (Fig. 3 and Table 4).
Fig. 2.
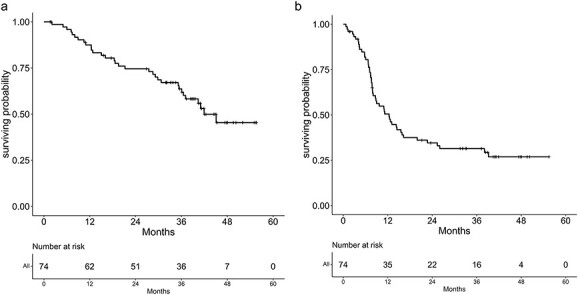
Kaplan–Meier curves of OS (a) and PFS (b) in all 74 patients.
Fig. 3.
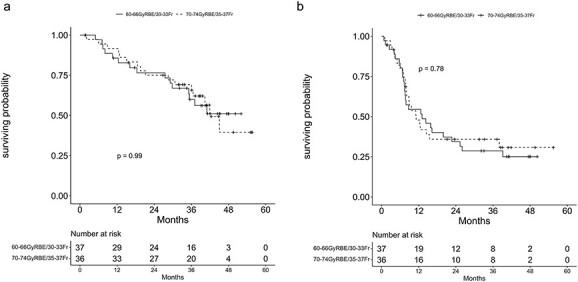
Kaplan–Meier curves of OS (a) and PFS (b) in each dose (60–66 GyRBE: 37 patients, 70–74 GyRBE: 36 patients).
Table 4.
Univariate analyses for OS and PFS
| Factors | N (%) | OS P-value | PFS P-value |
|---|---|---|---|
| Primary tumor | |||
| T0–2 | 38 (52%) | 0.14 | 0.66 |
| T3–4 | 35 (48%) | ||
| Regional lymph nodes | |||
| N0–1 | 12 (16%) | 0.68 | 0.28 |
| N2 | 27 (36%) | ||
| N3 | 35 (47%) | ||
| Primary tumor site | |||
| Lower lobe | 26 (35%) | 0.081 | 0.0051 |
| Other sites | 48 (65%) | ||
| Pathology | |||
| Adenocarcinoma | 42 (57%) | 0.95 | 0.69 |
| Squamous cell carcinoma | 26 (35%) | ||
| Other pathologies | 6 (8%) |
Six patients (8.0%) had adverse events of Grade 3. The Grade 3 esophagitis in three patients and Grade 3 pneumonitis in one patient were observed in the standard dose group, whereas in the high-dose group, Grade 3 esophagitis was observed in one patient. There was Grade 3 dermatitis in the different dose (other) patient due to a bulky mass. Adverse events of Grade ≥ 4 were not observed.
Local recurrence, regional lymph node recurrences and distant metastases are summarized in Table 5. Recurrence patterns were also examined by dose and by primary site. In all patients, there were 10 lymph node recurrences, six of which were outside the irradiation field. All isolated distant failures were observed in 29 patients, and three patients developed combined failures. Most patients who relapsed received additional therapy (48/54 patients) during the follow-up period.
Table 5.
Failure pattern
| Value | |
|---|---|
| Local recurrence, n | 15 |
| 60–66 GyRBE/30–33 Fr, n | |
| T0:T1:T2:T3:T4 | 1:1:2:3:1 |
| 70–74 GyRBE/33–37 Fr, n | |
| T0:T1:T2:T3:T4 | 0:1:1:2:3 |
| Regional lymph node recurrence, n | 10 |
| Within the irradiation field | 6 |
| Outside the irradiation field | 4 |
| Distant metastasis, n | 29 |
| Recurrence by primary site | |
| ● Lower lobe, n | 26 |
| Local recurrence | 4 |
| Regional lymph node recurrence | |
| Within the irradiation field | 5 |
| Outside the irradiation field | 2 |
| Distant metastasis | 12 |
| ● Other primary sites, n | 48 |
| Local recurrence | 11 |
| Regional lymph node recurrence | |
| Within the irradiation field | 1 |
| Outside the irradiation field | 2 |
| Distant metastasis | 17 |
Fr, fraction; T, primary tumor stage [25].
DISCUSSION
These are the first data of a Japanese nationwide multi-institutional prospective study of patients with inoperable stage III NSCLC undergoing PT. In this study, we found that PT with concurrent chemotherapy for inoperable stage III NSCLC had manageable toxicity and encouraging OS rates. In addition, there was no difference in local control and complications in both the standard dose and high-dose groups. In this study, the 3-year OS and PFS rates in the 74 patients were 64.7 (95% CI, 53.3–78.4%) and 25.1% (95% CI, 15.9–39.6%). The median OS and PFS were 41.0 and 10.9 months, respectively, and the toxicities were considered acceptable.
Treatment outcomes of systematic literature review on unresectable stage III NSCLC described the combined use with chemotherapy. In photon RT, the RTOG 0617 study, which was the best clinical data before the introduction of PD-L1 inhibitors, the median OS and PFS were 28.7 and 12.0 months, respectively [10–12]. In the PACIFIC study, in which patients were treated with consolidation durvalumab, following definitive therapy for inoperable stage III NSCLC, the subsequent 3-year OS rate was reported to be 57%, and the median PFS was 16.8 months [7–9]. In the literature, three PT studies were single-center prospective trials [17, 18, 20]. Oshiro et al. [18] reported that PT with concurrent chemotherapy for unresectable stage III NSCLC achieved favorable survival (2-year OS rate, 51%; mean, 26.7 months) with tolerable toxicities. Nguyen et al. [17] reported a 3-year OS rate of 41.0% for stage IIIA NSCLC and 44.5% for stage IIIB. Chang et al. [20] reported 5-year OS and PFS rates of 29 and 22%, respectively. Treatment outcomes of this study were at least equivalent to that of radiation therapy using X-rays and PT with concurrent chemotherapy.
Patients receiving definitive chemoradiotherapy for inoperable stage III NSCLC are at significant risk of developing treatment-related thoracic toxicities, including pneumonitis, carditis and esophagitis. Particularly, radiation pneumonitis is often described as an adverse event. In this study, six patients (8.0%) had adverse events of Grade ≥ 3, including Grade 3 pneumonitis in one patient. In systematic literature review on treatment outcomes of unresectable stage III NSCLC, which described the combined use of PBT with chemotherapy, the frequency of pneumonitis of Grade ≥ 3 varies from 0 to 13% [17–24]. It was thought that treatment-related toxicities observed in this study were mild. Similarly, the PACIFIC study reported radiation pneumonitis of Grade ≥ 3 of 4.4% in the consolidation durvalumab group. Furthermore, in the PACIFIC study, intensity-modulated radiation therapy (IMRT) was used as a modern technique that provides excellent target volume coverage and reduces high doses to organs at risk, but this technique increases the size of low-dose areas, including the lungs [10–12]. A large low-dose irradiated area, such as V5 in the lungs, has been reported to be associated with symptomatic radiation pneumonitis [29]. PT has demonstrated encouraging clinical outcomes for inoperable stage III NSCLC with less toxicity than IMRT [17, 20, 30]. It may be particularly beneficial in reducing low-dose areas, such as V5 and symptomatic radiation pneumonitis [31]. On the other hand, a randomized controlled study comparing intensity-modulated proton therapy and IMRT revealed no significant benefits regarding the occurrence of radiation pneumonitis [22]. Further examination is necessary in the future.
After chemoradiotherapy, 30–40% of patients develop locoregional tumor recurrence [10–12]. Therefore, irradiated dose escalation for inoperable stage III NSCLC has been attempted, but the results were unsatisfactory [10–12]. In this study, 15 patients (20.0%) had local recurrence; 8 and 7 in the standard dose and high-dose groups, respectively (Table 5). Furthermore, prescription doses were selected from 60 to 66 GyRBE (standard dose) or 70 to 74 GyRBE (high dose) in two GyRBE daily fractions for each patient at each institution (Table 1). Similarly, ENI/IF of lymph node irradiation was also determined for each patient at each institution. There was no difference in local control and OS rates in both groups. However, no increase in complications occurred even in the high-dose group; therefore, it is considered that the high dose using PT was safe and feasible. Considering the outcomes of systematic literature review, patients in the three studies in whom 74 GyRBE was prescribed showed no improvement in OS [17-18, 20]. Therefore, dose escalation alone may not improve treatment outcomes in unresectable stage III NSCLC, suggesting the need for multidisciplinary approaches, such as immunotherapy, including consolidation durvalumab, following definitive therapy. The need for local dose escalation seems to require further investigation in conjunction with consolidative immunotherapy. We consider it necessary to examine in future studies the optimal dose in each tissue, appropriate lymph node irradiation area and the significance of increasing the local dose and efficacy of consolidative immunotherapy, including durvalumab, following definitive therapy.
The RTOG 0617 study identified the cardiac radiation dose (heart V40) as an independent predictor of OS [10–12]. Moreover, Chang et al. [20] reported tumor location effects on OS. Their study results suggested that left-sided and right lower lobe disease was associated with worse OS, which might be related to the proximity to the heart [20]. The OS of the lower lobe primary site tended to be worse than that of other lobes in this study (Table 4). However, the present analysis did not confirm an apparent effect of the tumor location on OS. On the other hand, PFS was significantly worse in the lower lobe (Table 4). In the lower lobe primary site, there were more regional lymph node recurrences outside the irradiation field than in other primary sites (Table 5). In fact, most patients who relapsed received additional therapy (48/54 patients), which was considered a contributing factor to the lack of significance in the OS rate. Besides, no increase in severe complications was observed in the lower lobe primary site in this study. Therefore, this was thought to be due to recurrence and metastasis rather than complications. Follow-up studies should examine the association between late complications and long-term outcomes.
This study has several limitations, including its limited sample size, resulting in inevitable selection bias. In addition, dose volume histogram (DVH) parameters of the lungs, heart and esophagus were not collected as registry data in this study; therefore, it could not be evaluated. Moreover, Grade 2 complications, including symptomatic pneumonitis, could not be evaluated, though symptomatic radiation pneumonitis (Grade ≥ 2) after chemoradiotherapy was an important exclusion criterion for the administration of maintenance PD-L1 inhibitors following definitive chemoradiotherapy in the PACIFIC study [7–9]. However, this was a prospective observational study, and the treatment protocol remained unchanged. We believe that our data are sufficiently reliable and may form the basis for future prospective clinical trials regarding targeted agents and immunotherapeutic approaches. Regarding DVH and symptomatic radiation pneumonitis, future studies with larger numbers of patients and longer follow-up periods are warranted to corroborate our findings.
CONCLUSION
In conclusion PT with concurrent chemotherapy was feasible and safe in patients with inoperable stage III NSCLC undergoing definitive therapy. The results of these PBT registry data suggest that the OS rate was at least equivalent to that of radiation therapy using X-rays and that the incidence of severe radiation pneumonitis was low. PBT may be an effective treatment to reduce toxicities of healthy tissues, including the lungs and heart. Further improvement in treatment results is expected by consolidation durvalumab following definitive therapy. Therefore, it is also necessary to evaluate treatment outcomes with consolidation durvalumab following chemo-particle definitive therapy.
DATA AVAILABILITY
Participants have not consented to have their data made public, so the data is not available.
CONFLICT OF INTEREST
M. Satouchi received grants and a speaker honorarium from CHUGAI PHARMACEUTICAL CO., LTD, AstraZeneca K.K., ELI LILLY JAPAN K.K., ONO PHARMACEUTICAL CO., LTD., Bristol Myers Squibb, Merck & Co., Inc., Merck, Janssen Pharmaceutical K.K., Amgen Inc., TAIHO PHARMACEUTICAL CO., LTD., Pfizer Inc., DAIICHI SANKYO CO., LTD., Eisai Co., Ltd., Takeda Pharmaceutical Company Limited, and Novartis Pharma K.K. M Satouchi received grants from GlaxoSmithKline Consumer Healthcare Japan K.K. and Bayer Pharma Japan. H. Harada received a speaker honorarium from Hitachi, Ltd., AstraZeneca K.K., Brain Lab,Inc., Accuray Japan K.K., CHUGAI PHARMACEUTICAL CO., LTD., Eisai Co., Ltd., TAIHO PHARMACEUTICAL CO., LTD., Takeda Pharmaceutical Company Limited, Pfizer Inc., Merck & Co., Inc. and Nihon Medi-Physics Co., Ltd. K. Mori received a speaker honorarium from CHUGAI PHAEMACEURICAL CO., LTD., ONO PHARMACEUTICAL CO., LTD., DAIICHI SANKYO CO., LTD. and ELI LILLY JAPAN K.K.
FUNDING
This work was supported by Hokkaido University (Functional enhancement promotion expenses by the Ministry of Education, Culture, Sports, Science and Technology) and AMED under Grant Number JP16lm0103004.
PRESENTATION AT A CONFERENCE
None declared.
Supplementary Material
Contributor Information
Hitoshi Tatebe, Proton Therapy Center, Fukui Prefectural Hospital, 2-8-1 Yotsui, Fukui 910-0846, Japan.
Hideyuki Harada, Radiation and Proton Therapy Center, Shizuoka Cancer Center, 1007 Shimonagamubo, Nagaizumi-cho, Sunto-Gun, Shizuoka 411-8777, Japan.
Keita Mori, Department of Biostatistics, Clinical Research Center, Shizuoka Cancer Center, 1007 Shimonagamubo, Nagaizumi-cho, Sunto-Gun, Shizuoka 411-8777, Japan.
Hiromitsu Iwata, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City University West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan.
Tetsuo Akimoto, Division of Radiation Oncology and Particle Therapy, National Cancer Center Hospital East, Kashiwa 277-0882, Japan.
Masao Murakami, Southern Tohoku Proton Therapy Center, 7-172, Yatsuyamada, Fukushima, Koriyama 963-8052, Japan.
Takahiro Waki, Department of Radiology, Tsuyama Chuo Hospital, 1756 Kawasaki, Tsuyama City, Okayama 708-0841, Japan.
Takashi Ogino, Medipolis Proton Therapy and Research Center, 4423 Higashikata, Ibusuki City, Kagoshima 891-0304, Japan.
Masatoshi Nakamura, Department of Radiation Oncology, Faculty of Medicine, University of Tsukuba, 1-1-1 Tennodai, Ibaraki, Tsukuba 305-8577, Japan.
Hiroshi Taguchi, Department of Radiation Oncology, Hokkaido University Hospital, Kita 15, Nishi 7, Kita-ku, Sapporo, Hokkaido 060-0808, Japan.
Haruhiko Nakayama, Department of Thoracic Surgery, Kanagawa Cancer Center, 2-3-2 Nakao, Asahi-ku, Yokohama City, Kanagawa 241-8515, Japan.
Miyako Satouchi, Department of Thoracic Oncology, Hyogo Cancer Center, 13-70 kitaojicho, Akashi, Hyogo 673-8558, Japan.
Hidefumi Aoyama, Department of Radiation Oncology, Hokkaido University Faculty of Medicine, Hokkaido 060-0808, Japan.
References
- 1. Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stage IIIa and IIIb nonsmall cell lung cancer. A meta analysis. Cancer 1995;76:593–601. [DOI] [PubMed] [Google Scholar]
- 2. Pritchard RS, Anthony SP. Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A meta analysis. Ann Intern Med 1996;125:723–9. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Aisner DL et al. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell. Lung Cancer Version 5, JNCCN Journal of the National Comprehensive Cancer Network, 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. (01 July 2021, date last accessed). [DOI] [PubMed]
- 4. Park K, Vansteenkiste J, Lee KH et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with locally advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS SSO and TOS. Ann Oncol 2020;31:191–201. [DOI] [PubMed] [Google Scholar]
- 5. Majem M, Hernández-Hernández J, Hernando-Trancho F et al. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin Transl Oncol 2020;22:21–36. [DOI] [PubMed] [Google Scholar]
- 6. Eberhardt WE, Pöttgen C, Gauler TC et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194–201. [DOI] [PubMed] [Google Scholar]
- 7. Antonia SJ, Villegas A, Daniel D et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 8. Faivre-Finn C, Vicente D, Kurata T et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC—an update from the PACIFIC trial. J Thorac Oncol 2021;16:860–7. [DOI] [PubMed] [Google Scholar]
- 9. Gray JE, Villegas A, Daniel D et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol 2020;15:288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bradley JD, Paulus R, Komaki R et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chun SG, Hu C, Choy H et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017;35:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bradley JD, Hu C, Komaki RR et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol 2020;38:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kubo N, Saitoh JI, Shimada H et al. Dosimetric comparison of carbon ion and X-ray radiotherapy for stage IIIA non-small cell lung cancer. J Radiat Res 2016;57:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kesarwala AH, Ko CJ, Ning H et al. Intensity-modulated proton therapy for elective nodal irradiation and involved-field radiation in the definitive treatment of locally advanced non-small cell lung cancer: a dosimetric study. Clin Lung Cancer 2015;16:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohno T, Oshiro Y, Mizumoto M et al. Comparison of dose-volume histograms between proton beam and X-ray conformal radiotherapy for locally advanced non-small-cell lung cancer. J Radiat Res 2015;56:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stuschke M, Kaiser A, Pöttgen C et al. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiother Oncol 2012;104:45–51. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen QN, Ly NB, Komaki R et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol 2015;115:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oshiro Y, Okumura T, Kurishima K et al. High-dose concurrent chemo-proton therapy for stage III NSCLC: preliminary results of a phase II study. J Radiat Res 2014;55:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takahashi W, Nakajima M, Yamamoto N et al. A prospective nonrandomized phaseI/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC). Cancer 2015;121:1321–7. [DOI] [PubMed] [Google Scholar]
- 20. Chang JY, Verma V, Li M et al. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: final results of a phase 2 study. JAMA Oncol 2017;3:e172032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao Z, Lee JJ, Komaki R et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small cell lung cancer. J Clin Oncol 2018;36:1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu NY, DeWees TA, Liu C et al. Early outcomes of patients with locally advanced non-small cell lung cancer treated with intensity-modulated proton therapy versus intensity-modulated radiation therapy: the Mayo clinic experience. Adv Radiat Oncol 2019;5:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hayashi K, Yamamoto N, Nakajima M et al. Clinical outcomes of carbon-ion radiotherapy for locally advanced non-small-cell lung cancer. Cancer Sci 2019;110:734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karube M, Yamamoto N, Shioyama Y et al. Carbon-ion radiotherapy for patients with advanced stage non-small-cell lung cancer at multicenters. J Radiat Res 2017;58:761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brierley JD, Gospodarowicz MK, Wittekind C. T.N.M. Classification of Malignant Tumours, 7th edn. New York, USA: Wiley-Blackwell. [Google Scholar]
- 26. https://www.jastro.or.jp/medicalpersonnel/guideline/2020/04chest.pdf.
- 27. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. US Department of Health and Human Services. U.S. Department of Health and Human Services. National Institute of Health. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (6 August 2010, date last accessed). [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tatsuno S, Doi H, Okada W et al. Risk factors for radiation pneumonitis after rotating gantry intensity-modulated radiation therapy for lung cancer. Sci Rep 2022;12:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwata H, Akita K, Yamaba Y et al. Concurrent chemo-proton therapy using adaptive planning for unresectable stage 3 non-small cell lung cancer: a phase 2 study. Int J Radiat Oncol Biol Phys 2021;109:1359–67. [DOI] [PubMed] [Google Scholar]
- 31. Zou Z, Bowen SR, Thomas HMT et al. Scanning beam proton therapy versus photon IMRT for stage III lung cancer: comparison of dosimetry, toxicity, and outcomes. Adv Radiat Oncol 2020;5:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Participants have not consented to have their data made public, so the data is not available.