Abstract
Japanese national oncological experts convened to evaluate the efficacy and safety of particle beam therapy (PT) for pulmonary, liver and lymph node oligometastases (P-OM, L-OM and LN-OM, respectively) and to conduct a statistically comparative analysis of the local control (LC) rate and overall survival (OS) rate of PT versus those of X-ray stereotactic body radiotherapy (X-SBRT) and X-ray intensity-modulated radiotherapy (X-IMRT). They conducted [1] an analysis of the efficacy and safety of metastasis-directed therapy with PT for P-OM, L-OM and LN-OM using a Japanese nationwide multi-institutional cohort study data set; [2] a systematic review of X-ray high-precision radiotherapy (i.e. X-SBRT/X-IMRT) and PT for P-OM, L-OM and LN-OM; and [3] a statistical comparison between LC and OS of the cohort data set in PT and that of the extracted historical data set in X-SBRT/X-IMRT from the preceding systematic review. Safety was evaluated as the incidence of grade ≥ 3 adverse events, while statistical comparisons of LC and OS were conducted by estimating the incidence rate ratios (IRR) for local progression and mortality, respectively. This study demonstrated that PT provided durable LC (3-year LC rate: 72.8–83.2%) with acceptable OS (3-year OS rate: 38.5–68.1%) and risk of severe toxicity incidence of 0.8–3.5% in radical metastasis-directed therapy for P-OM, L-OM and LN-OM. Compared to LC with X-SBRT or X-IMRT, LC with PT was potentially superior for P-OM; superior for L-OM; and equivalent for LN-OM. In particular, this study demonstrated that PT may be a new treatment option for L-OM tumors measuring > 5 cm.
Keywords: particle beam therapy (PT), proton beam therapy (PBT), carbon ion radiotherapy, metastasis-directed therapy, oligometastasis
INTRODUCTION
Increasing evidence supports the concept of oligometastases [1], which was first proposed by Hellman and Weichselbaum [2], as a cancer state that is an intermediate metastatic state between localized disease and systemically metastasized disease for which local metastasis-directed therapies have the potential of prolonging survival. X-ray high-precision radiotherapy (i.e. stereotactic body radiotherapy [X-SBRT] and/or intensity-modulated radiotherapy [X-IMRT]), a local metastasis-directed therapy, is widely used, based on promising clinical evidence [3–5]. The American Society for Radiation Oncology (ASTRO) model policies indicate X-SBRT is a suitable local metastasis-directed therapy [6], and the Japanese national health insurance system has covered X-SBRT for treating oligometastatic disease since April 2020. However, limited evidence exists regarding particle beam therapy (PT), including proton beam therapy (PBT) and carbon-ion radiotherapy (C-ion RT), for treating oligometastatic disease. PT for oligometastatic disease is not indicated as a suitable treatment, except for PT of metastatic tumors of the spine [7], and it is not covered by the national health insurance system in Japan. However, PT has excellent therapeutic results, owing to the physical characteristics of the Bragg peaks [8], and it has great potential as an optimal metastasis-directed treatment for oligometastatic disease. Therefore, informative reports on the outcomes of PT for oligometastatic disease are needed to accumulate evidence on the usefulness of PT for oligometastatic disease.
In Japan, a nationwide multi-institutional cohort study on PT started in all Japanese centers with PBT and/or C-ion RT in May 2016. Several years have passed since the nationwide cohort study began, and the Japanese Society for Radiation Oncology (JASTRO) decided to conduct a comprehensive analysis of PT for pulmonary oligometastasis (P-OM), liver oligometastasis (L-OM) and lymph node oligometastasis (LN-OM). The aim of this study was (i) to analyze the efficacy and toxicity of PT by using the Japanese multi-institutional cohort study data set; (ii) to conduct a systematic review on X-SBRT/X-IMRT and PT in radical metastasis-directed therapy for P-OM, L-OM and LN-OM; and (iii) to conduct a statistical comparison between the outcomes of the cohort data in PT and the outcomes of the historical data in X-SBRT/X-IMRT, which were extracted from a previous systematic review. These studies were conducted and managed by the Oligometastatic Cancer Working Group in the Particle Beam Therapy Committee and Subcommittee at JASTRO, which involves radiation oncologists from JASTRO, oncologists of the Japan Society of Clinical Oncology, and biostatisticians. In this study, we present the results of the aforementioned investigations on P-OM, L-OM and LN-OM.
MATERIALS AND METHODS
The following three analyses were conducted individually for the analysis of P-OM, L-OM and LN-OM. This study was approved by the appropriate institutional ethics committees.
Analysis of the Japanese multi-institutional cohort study data set of PT
Among patients registered in a Japanese multi-institutional cohort study, the data of patients who received PBT or C-ion RT between May 2016 and June 2018 were reviewed to evaluate the local control (LC) rate, OS rate and grade ≥ 3 treatment-related late toxicity. The main eligibility criteria were as follows: (i) histopathological or clinical diagnosis of oligometastatic disease; (ii) number of metastatic tumors ≤3 for P-OM and L-OM, and localized metastatic region for LN-OM; (iii) absence of recurrence at the primary disease site after primary curative treatment; (iv) absence or control of other cancers and clinically detectable recurrent or metastatic diseases other than the metastatic regions; and (v) curative-intent PBT or C-ion RT to all metastatic regions. The details of the criteria are summarized in Table 1.
Table 1.
The inclusion criteria for the analyses on the Japanese multi-institutional cohort study data set of PT or C-ion RT
| Pulmonary oligometastatis |
|
| Liver oligometastasis |
|
| Lymph node oligometastasis |
|
Abbreviations: PBT, proton beam therapy; C-ion RT, carbon-ion radiotherapy; RBE, relative biological effectiveness; fr, fractions; BED10, biological effective dose using the linear-quadratic model with α/β = 10 Gy.
LC was defined as the time from the initiation of PT to the progression of treated lesions. OS was defined as the time from PT initiation to death from any cause. Treatment-related late toxicity of grade ≥ 3, defined as complications appearing more than 3 months after the end of PT, was evaluated by using the Common Terminology Criteria for Adverse Events (version 4.0) [9]. LC and OS probabilities were estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. To evaluate the impact of a prescribed dose on LC, the survival curves of PT with a prescribed dose < the median value or ≥ the median value were compared, using the log-rank test. Furthermore, among the L-OM series, the efficacy and toxicity of PT for tumors measuring > 5 cm and tumors measuring ≤5 cm were evaluated with statistical comparisons. All statistical analyses were conducted using JMP Pro16 (SAS Institute Inc., Cary, NC, USA) or SAS 9.4 (SAS Institute Inc.). All reported P-values were two sided, and P < 0.05 was considered statistically significant.
Systematic review of X-SRBT/X-IMRT and PT
Systematic literature reviews on P-OM, L-OM and LN-OM were conducted individually and in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The PubMed electronic database was searched for clinical scientific reports on X-SBRT/X-IMRT or PT for P-OM, L-OM and LN-OM published in English between January 2000 and September 2020. The search terms, selection criteria, data collection and other details of these systematic reviews are provided in Supplementary Data 1. In each review, two radiation oncologists independently reviewed the retrieved articles and selected potentially relevant articles, based on their titles and abstracts. In addition to this screening, the two experts conducted a manual search, as needed, to select other relevant articles for the full-text review. Finally, full-text reviews were conducted to identify studies that met the selection criteria.
Comparison between the cohort data for PT and the extracted historical data for X-SBRT/X-IMRT, based on the systematic review
To compare the endpoint of interest (i.e. local progression or mortality) between the cohort data of PT (i.e. PBT and C-ion RT) and historical data extracted from the systematic review of X-ray therapy (i.e. X-SBRT/X-IMRT), the incidence rate ratio (IRR) of the endpoint of interest was evaluated. 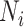 ,
, 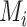 and
and 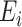 denote the number of subjects (in the case of local progression, the number of target sites), the median follow-up time and the number of events, respectively, for i = 1 and i = 2 (i = 1 represents the X-ray therapy group and i = 2 represents the particle therapy group).
denote the number of subjects (in the case of local progression, the number of target sites), the median follow-up time and the number of events, respectively, for i = 1 and i = 2 (i = 1 represents the X-ray therapy group and i = 2 represents the particle therapy group). 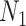 ,
, 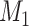 and
and 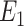 were calculated by summing the reports of all X-ray therapy articles in the analysis, assuming that they were randomly sampled from the same population. When data on the number of events were missing, they were imputed from the survival probability, under the assumption of an exponential distribution. The IRR was estimated as follows:
were calculated by summing the reports of all X-ray therapy articles in the analysis, assuming that they were randomly sampled from the same population. When data on the number of events were missing, they were imputed from the survival probability, under the assumption of an exponential distribution. The IRR was estimated as follows: 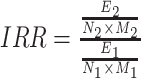 , in which IRR < 1 indicates that the incidence rate of the endpoint of interest is lower in the particle therapy group than in the X-ray therapy group. The 95% confidence interval (CI) of the IRR was estimated and tested with IRR = 1 as the null hypothesis.
, in which IRR < 1 indicates that the incidence rate of the endpoint of interest is lower in the particle therapy group than in the X-ray therapy group. The 95% confidence interval (CI) of the IRR was estimated and tested with IRR = 1 as the null hypothesis.
Analytical methods and results have varied among the selected studies on X-ray therapy (i.e. X-SBRT/X-IMRT) for P-OM, L-OM and LN-OM. Some reports focused on one specific primary cancer (specific primary cancer article), while other studies reported the outcomes of summary data on various primary cancers without revealing the individual outcomes of each primary cancer. In the current study, a statistical comparison was principally conducted between all data of PT (i.e. PBT and C-ion RT) and all data of X-ray therapy (i.e. X-SBRT/X-IMRT).
Furthermore, when possible, certain statistical comparisons were conducted between the same specific primary cancer data sets of X-ray therapy and PT. The comparisons included only data focusing on the specific primary cancer to minimize the impact of primary cancer on the values of interest (i.e. local progression rate or mortality).
RESULTS
Pulmonary oligometastasis
Analysis of the Japanese multi-institutional cohort study data set of PT
A total of 132 patients (representing 156 tumors) were eligible, based on the inclusion criteria. Among them, 85 patients received PBT at 107 sites, while 47 patients received C-ion RT at 49 sites. The median patient age was 69 years (range, 25–88 years). The patients’ and treatment characteristics are shown in Table 2 and Supplementary Data 2. The median follow-up period was 27.9 months (range, 1.6–54.7 months). The major primary disease sites were the colorectum (n = 48, 36.4%) and lungs (n = 35, 26.5%). The median biological effective dose obtained, using the linear-quadratic model with α/β = 10 Gy (biologic effective dose, BED10), was 115.2 Gy (relative biological effectiveness [RBE]; range, 84–300 Gy [RBE]).
Table 2.
Characteristics of patients and treatments
| Characteristics | P-OM 132 patients (156 tumors) |
L-OM 200 patients (266 tumors) |
LN-OM 282 patients (287 regions) |
|||||
|---|---|---|---|---|---|---|---|---|
| Age, years Median (range) |
69 (25–88) | 68 (24–90) | 66 (36–96) | |||||
| Sex, n (%) Male Female |
74 (56.1) 58 (43.9) |
125 (62.5) 75 (37.5) |
148 (52.5) 134 (47.5) |
|||||
| Performance status*, n (%) 0–1 2 unknown |
130 (98.5) 2 (1.5) 0 (0.0) |
191 (95.5) 8 (4.0) 1 (0.5) |
272 (96.5) 7 (2.5) 3 (1.0) |
|||||
| Follow-up time, months Median (range) |
27.9 (1.6–54.7) | 20.2 (1.0–55.9) | 24.2 (2.5–56.4) | |||||
| Primary disease, n (%) | Colorectal cancer Lung cancer Esophageal cancer Kidney cancer Uterine cancer Breast cancer Bone & soft tissue tumor Head & Neck cancer The others |
48 (36.4) 35 (26.5) 8 (6.1) 8 (6.1) 7 (5.3) 7 (5.3) 4 (3.0) 4 (3.0) 11 (8.3) |
Colorectal cancer Biliary tract cancer Pancreatic cancer Stomach cancer Breast cancer Esophageal cancer Lung cancer Small intestine cancer The others |
102 (51.0) 28 (14.0) 17 (8.5) 15 (7.5) 9 (4.5) 6 (3.0) 5 (2.5) 4 (2.0) 14 (7.0) |
Colorectal cancer Uterine cancer Lung cancer Esophageal cancer Pancreatic cancer Head & Neck cancer Biliary tract cancer Ovary cancer Stomach cancer Hepatocellular cancer Breast cancer Prostate cancer Kidney cancer Small intestine cancer Bladder cancer The others |
43 (15.2) 38 (13.5) 37 (13.1) 28 (9.9) 23 (8.2) 23 (8.2) 14 (5.0) 13 (4.6) 11 (3.9) 10 (3.5) 9 (3.2) 7 (2.5) 4 (1.4) 4 (1.4) 2 (0.7) 16 (5.7) |
||
| Tumor size, mm Median (range) |
15 (5-130) | 29.5 (7-170) ** | NA | |||||
| Treatment modality, n (%) PBT C-ion RT |
85 (64.4) 47 (35.6) |
151 (75.5) 49 (24.5) |
205 (72.7) 77 (27.3) |
|||||
| Total delivery dose, Gy (RBE) Median (range) |
64 (50–80) | 66 (58–76) | 60 (48–74) | |||||
| Dose per fraction, Gy (RBE) Median (range) |
8 (2–50) | 6.6 (2–58) | 2.5 (2–6.6) | |||||
| Number of treatment fractions, n Median (range) |
8 (1–35) | 10 (1–38) | 26 (10–37) | |||||
| BED10, Gy (RBE) Median (range) |
115.2 (84–300) | 109.6 (72–394.4) | 79.2 (60–109.6) | |||||
Abbreviations: P-OM, pulmonary oligometastasis; L-OM, liver oligometastasis; LN-OM, lymph node oligometastasis; RBE, relative biological effectiveness; PBT, proton beam therapy; C-ion RT, carbon-ion radiotherapy; BED10, biological effective dose using the linear-quadratic model with α/β = 10 Gy; NA, not available
*According to the Eastern Cooperative Oncology Group.
**The values were described based on the available registry data of 250 tumors.
At the last follow-up, 17 (12.9%) patients had local progression, whereas 37 (28%) patients died of any cause. In the statistical analysis of P-OM, the number of patients with local progression, but not the number of relapse lesions, was used to calculate the LC because of the difficulty in discriminating which lesion relapsed when the patient had received treatment for multiple lesions that were close to each other. In all patients, the 1-, 2- and 3-year LC rates were 94.5% (95% CI, 90.3–98.8), 83.2% (95% CI, 75.9–90.5) and 83.2% (95% CI, 75.9–90.5), respectively (Fig. 1A). The 1-, 2- and 3-year OS rates were 89% (95% CI, 83.5–94.4), 76.6% (95% CI, 68.9–84.2) and 68.1% (95% CI, 58.7–77.5), respectively (Fig. 1B). The median survival was not reached until this analysis. For LC, no significant difference existed between PBT and C-ion RT (log-rank test, P = 0.14) (Fig. 1C) and between PT series with the prescribed dose < the median value or ≥ the median value (log-rank test, P = 0.18).
Fig. 1.
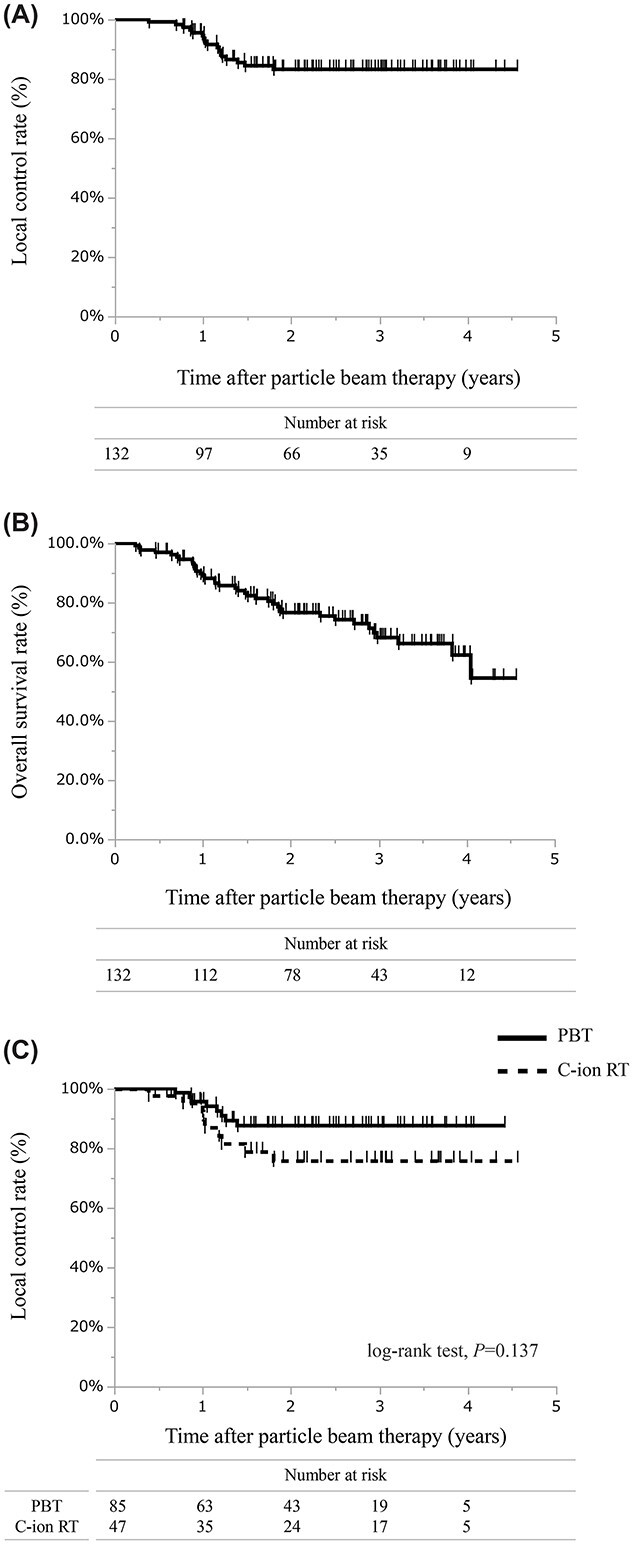
Survival curves of PT for P-OM. (A) LC rate of all data. (B) OS rate of all data. (C) LC rates of PBT and C-ion RT.
Only 1 (0.8%) treatment-related grade 3 late toxicity occurred among the 132 patients treated with PT: the patient had radiation pneumonitis. No grade ≥ 4 treatment-related late toxicity occurred.
Systematic review of X-SBRT and PT
The PRISMA flow diagram of the systematic review of X-SBRT and PT for P-OM is shown in Supplementary Data 1 (Fig. S1). In total, five relevant studies on X-SBRT [11–15] and three relevant studies on PT [16–18] were identified (Table 3). Different studies had different inclusion criteria, characteristics of patients and primary cancers, and treatment protocols. Among the five reports on X-SBRT, one study [11] was prospectively conducted, whereas one study [15] was a meta-analysis focusing on the P-OM of colorectal cancer. All reports on PT were retrospective studies with small sample sizes (< 120). The 2- and 3-year LC rates, 2- and 3-year OS rates and incidence of grade ≥ 3 toxicities were extracted from relevant reports and are listed in Table 3. The LC and OS rates were obtained from Kaplan–Meier curves, if necessary.
Table 3.
Relevant articles of the systematic review on X-SBRT and PT for P-OM
| Authors (Reported year) |
Study design |
Treatment methods |
Number of patients | Primary cancer | LC (%) | OS (%) | G ≥ 3 AEs (%) |
|---|---|---|---|---|---|---|---|
| Ricco A [11] (2017) |
M/P | X-SBRT | 447 | Various primary sites | 64 (2 Y) 59 (3 Y) |
57 (2 Y) 33 (3 Y) |
NA |
| Niibe Y [12] (2020) |
M/R | X-SBRT | 1378 | Various primary sites | NA (2 Y) 81 (3 Y) |
70 (2 Y) 60 (3 Y) |
2.2 |
| De Rose F [13] (2016) |
S/R | X-SBRT | 60 | Lung cancer | 89 (2 Y) 45 (3 Y) |
75 (2 Y) 64 (3 Y) |
3.3 |
| Yamamoto T [14] (2020) |
M/R | X-SBRT | 330 | Colorectal cancer | 67 (2 Y) 63 (3 Y) |
80 (2 Y) 65 (3 Y) |
2.0 |
| Choi HS [15] (2020) |
MA | X-SBRT | 495 | Colorectal cancer | 72 (2 Y) 56 (3 Y) |
70 (2 Y) 58 (3 Y) |
NA |
| Aibe N [16] (2021) |
M/R | PBT | 118 | Various primary sites | 86 (2 Y) 78 (3 Y) |
68 (2 Y) 60 (3 Y) |
0.8 |
| Yamamoto N [17] (2013) |
S/R | C-ion RT | 91 | Various primary sites | 92 (2 Y) 88 (3 Y) |
71 (2 Y) 62 (3 Y) |
0 |
| Takahashi W [18] (2014) |
S/R | C-ion RT | 44 | Colorectal cancer | 85 (2 Y) 85 (3 Y) |
65 (2 Y) 50 (3 Y) |
0 |
Abbreviations: PT, particle beam therapy; P-OM, pulmonary oligometastasis; X-SBRT, X-ray stereotactic body radiotherapy; PBT, proton beam therapy; C-ion RT, carbon-ion radiotherapy; S, single-institutional investigation; M, multi-institutional investigation; R, retrospective design; P, prospective design; MA, meta-analysis; LC, local control rate; OS, overall survival rate; G ≥ 3 AEs, adverse effects of grade ≥ 3; Y, years; NA, not available.
Comparison between the cohort data for PT and the extracted historical data for X-SBRT, based on the systematic review
Among the five relevant studies selected by a systematic review on X-SBRT for P-OM, the data from four studies [11–14] were used to make statistical comparisons with the Japanese cohort data of PT. However, the data of one study [15] was not used because it was a meta-analysis of X-SBRT and did not describe the number of events that were necessary for statistical comparison. The statistical comparisons were conducted in the following combinations: (i) between all data sets of X-SBRT and PT and [2] between the same specific primary cancer data sets (e.g. colorectal cancer and lung cancer) of X-SBRT and PT. The results of statistical comparison between all data sets are listed in Table 4. The results between the same specific primary cancer data sets are shown in Supplementary Data 3. The statistical comparison between all data sets revealed a statistically significant difference in LC (IRR, 0.56; 95% CI, 0.34–0.91) and in OS (IRR, 0.66; 95% CI, 0.47–0.92). However, the statistical comparisons between the same specific primary cancer data sets (e.g. colorectal cancer and lung cancer) revealed no significant difference in LC and in OS.
Table 4.
Results of comparison between the all data sets of the cohort data of PT and those of the extracted historical data of X-SBRT/X-IMRT, based on the systematic review
| Treatment modality (Number of patients / target sites) |
Local progression | Mortality | Incidence of G ≥ 3 AEs (%) |
|||
|---|---|---|---|---|---|---|
| IRR (95% CI) | P value | IRR (95% CI) | P value | |||
| P-OM | PT (132/ 156) | 0.56 (0.34–0.91) | 0.020 | 0.66 (0.47–0.92) | 0.014 | 0.8 |
| X-SBRT (2215/ 2335) | 1.5–3.3 | |||||
| L-OM | PT (200/ 266) | 0.52 (0.37–0.72) | < 0.001 | 0.97 (0.78–1.20) | 0.778 | 3.5 |
| X-SBRT (809/ 1071) | 0–9.8 | |||||
| LN-OM | PT (282/ 287) | 0.82 (0.61–1.11) | 0.200 | 1.17 (0.96–1.43) | 0.122 | 3.2 |
| X-SBRT/X-IMRT (1070/ 1267) | 0–21.1 | |||||
Abbreviations: PT, particle beam therapy; P-OM; pulmonary oligometastasis; L-OM, liver oligometastasis; LN-OM, lymph node oligometastasis; PBT, proton beam therapy; C-ion RT, carbon-ion radiotherapy; X-SBRT, X-ray stereotactic body radiotherapy; G ≥ 3 AEs, adverse effects of grade ≥ 3; IRR, incidence rate ratio; CI, confidence interval.
Liver oligometastasis
Analysis of the Japanese multi-institutional cohort study data set of PT
Two hundred patients were eligible (representing 266 tumors). Among them, 151 patients received PBT at 208 sites, while 49 patients received C-ion RT at 58 sites. The median patient age was 68 years (range, 24–90 years). The patients’ and treatment characteristics are shown in Table 2 and Supplementary Data 2. The median follow-up period was 20.2 months (range, 1.0–55.9 months). The major primary disease sites were the colorectum (n = 102, 51%), biliary tract (n = 28, 14%), pancreas (n = 17, 8.5%) and stomach (n = 17, 7.5%). The median BED10 was 109.6 Gy (RBE) [range, 72–394.4 Gy (RBE)].
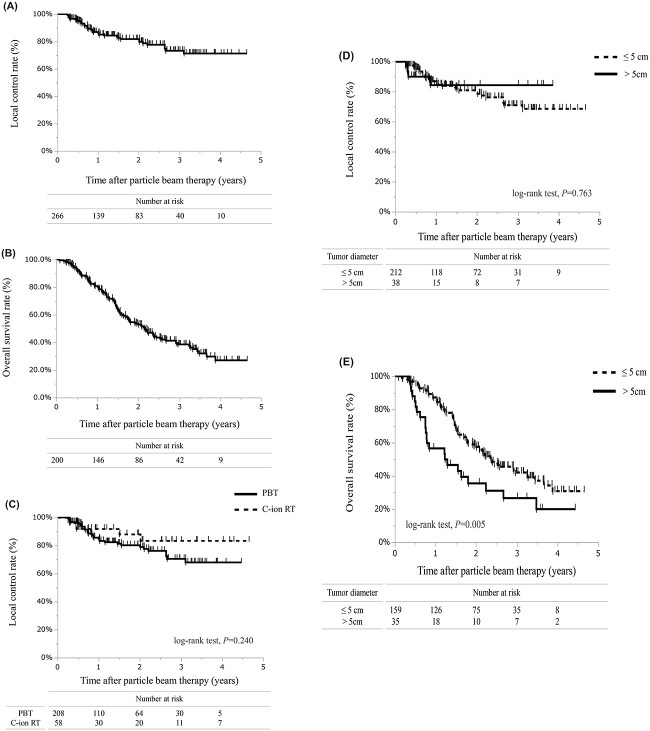
At the last follow-up, local progression occurred in 41 lesions in 266 treated sites (15.4%, 41/266), while 111/200 (55.5%) patients died of any cause. Among all irradiated liver lesions, the 1-, 2- and 3-year LC rates were 86.2% (95% CI, 81.4–91.2), 81.8% (95% CI, 75.9–87.6) and 73.2% (95% CI, 65.2–81.2), respectively (Fig. 2A). The 1-, 2- and 3-year OS rates were 80.8% (95% CI, 75.1–86.4), 52.9% (95% CI, 45.5–60.2) and 38.5% (95% CI, 30.-946.2), respectively (Fig. 2B). The median survival time was 25.9 months. For LC, no significant difference existed between PBT and C-ion RT (log-rank test, P = 0.24) (Fig. 2C) or between PT series with a prescribed dose < the median value or ≥ the median value (log-rank test, P = 0.26).
Fig. 2.
Survival curves of PT for L-OM. (A) LC rate of all data. (B) OS rate of all data. (C) LC rates of PBT and C-ion RT. (D) LC rates of PT for a target size of ≤ 5 cm and > 5 cm. (E) OS of PT for a target size of ≤ 5 cm and > 5 cm.
Seven (3.5%) of 200 patients had grade 3 treatment-related late toxicities. No grade ≥ 4 treatment-related late toxicity occurred. Grade 3 toxicities included radiation dermatitis in 3 patients, anemia in 1 patient, ascites in 1 patient, gamma-glutamyl transferase increase in 1 patient and unknown toxicity in 1 patient.
This cohort data set included available data on the maximum tumor diameter of 250 tumors in 194 patients. Among them, 159 patients with 212 lesions had tumors measuring ≤5 cm (i.e. small-size group) and 35 patients with 38 lesions had tumors measuring > 5 cm (i.e. large-size group). The 1-, 2- and 3-year LC rates of the small-size group were 86.1% (95% CI, 80.7–91.4), 80.8% (95% CI, 74.3–87.3) and 70.9% (95% CI, 61.9–80.0), respectively. The 1-, 2- and 3-year LC rates of the large-size group all had the same value at 84.5% (95% CI, 69.8–99.1). No statistical difference existed between the LC rates of the two groups (log-rank test, P = 0.76) (Fig. 2D). However, the OS rates among these groups differed significantly (log-rank test, P < 0.01) (Fig. 2E). The 1-, 2- and 3-year OS rates of the small-size group were 87.2% (95% CI, 81.8–92.6), 57.4% (95% CI, 49.2–65.6) and 42% (95% CI, 33.2–50.8), respectively. These rates in the large-size group were 56.5% (95% CI, 39.4–73.7), 35.5% (95% CI, 18.2–52.7) and 26.6% (95% CI, 9.8–43.3), respectively. The incidence of grade ≥ 3 adverse events in the small-size group was 3.9% and in the large-size group was 2.9%.
Systematic review on X-SBRT and PT
The PRISMA flow diagram of the systematic review of X-SBRT and PT for L-OM is shown in Supplementary Data 1 (Fig. S2). Overall, 11 studies [19–29] on X-SBRT and 6 studies [30–35] on PT were identified (Table 5). Different studies used different inclusion criteria, characteristics of patients and primary cancers, and treatment protocols. Among the 11 reports on X-SBRT, seven studies [19, 20, 23, 25–28] were prospectively conducted, and four studies [26–29] focused on the treatment of colorectal cancer. For PT, only one prospective study [1] existed, whereas the other studies [30, 32–35] were retrospective investigations. The LC and OS rates, median survival time and incidence of grade ≥ 3 toxicities are listed in Table 5.
Table 5.
Relevant articles of the systematic review on X-SBRT and PT for L-OM
| Authors (Reported year) |
Study design |
Treatment methods |
Number of patients | Primary cancer | LC (%) | OS (%) | G ≥ 3 AEs (%) |
|---|---|---|---|---|---|---|---|
| Kavanagh BD [19] (2006) |
S/P | X-SBRT | 21 | Various primary sites | 93 (1.5 Y) | NA | 4.8 |
| Iwata H [20] (2010) |
S/P | X-SBRT | 12 | Various primary sites | 67 (1 Y) | NA | 0 |
| Scorsetti M [21] (2013) |
S/R | X-SBRT | 61 | Various primary sites | 94 (1 Y) | MST: 19 M 83.5/65(1Y/1.5Y) |
0 |
| Yamashita H [22] (2014) |
M/R | X-SBRT | 51 | Various primary sites | 64 (2Y) | 71.9 (2Y) | 9.8 |
| Ahmed KA [23] (2016) |
S/P | X-SBRT | 22 11 |
Colorectal cancer The other primary sites |
79/59 (1Y/2Y) 100/100 (1Y/2Y) |
100/73 (1Y/2Y) 82/73(1Y/2Y) |
NA |
| Mahadevan A [24] (2018) |
M/R | X-SBRT | 427 | Various primary sites | MLCT: 51 M | MST:22 M | NA |
| Scorsetti M [25] (2018) |
S/P | X-SBRT | 61 | Various primary sites | 94/78/78 (1Y/3Y/5Y) | MST: 27.6 85.2/31.1/18 (1Y/3Y/5Y) |
1.6 |
| Comito T [26] (2014) |
S/P | X-SBRT | 41 | Colorectal cancer | 95/90/81 (1Y/2Y/3Y) | 78/61/44 (1Y/2Y/3Y) | 0 |
| Scorsetti M [27] (2015) |
S/P | X-SBRT | 42 | Colorectal cancer | 95/91/85 (1Y/2Y/3Y) | MST: 29 M 65 (2Y) |
0 |
| McPartlin A [28] (2017) |
S/P | X-SBRT | 51 | Colorectal cancer | 50/32/26 (1Y/2Y/4Y) | MST: 16 M 63/26/9 (1Y/2Y/4Y) |
0 |
| Klement RJ [29] (2019) |
M/R | X-SBRT | 255 | Colorectal cancer | NA | MST: 27.9 M | NA |
| Fukumitsu N [30] (2015) |
S/R | PBT | 133 | Various primary sites | NA | MST: 19.2 M | 1.5 |
| Makishima H [31] (2019) |
S/P | C-ion RT | 29 | Colorectal cancer | NA | MST: 65 M 78 (3Y) |
6.9 |
| Shiba S [32] (2021) |
S/R | C-ion RT | 11 | Colorectal cancer | 61 (2Y) | 100 (2Y) | 0 |
| Fukumitsu N [33] (2017) |
S/R | PBT | 9 | Stomach cancer | 71 (3Y) | 78 (3Y) | 0 |
| Yamaguchi H [34] (2020) |
S/R | PBT | 7 | Stomach cancer | 85.7 (3Y) | MST: 42 M 68.6 (3Y) |
0 |
| Fukumitsu N [35] (2017) |
S/R | PBT | 8 | Breast cancer | 73 (3 Y) | 86 (3 Y) | 0 |
Abbreviations: PT, particle beam therapy; L-OM, liver oligometasitasis; X-SBRT, X-ray stereotactic body radiotherapy; X-IMRT, x-ray intensity-modulated radiotherapy; PBT, proton beam therapy; C-ion RT, carbon-ion radiotherapy; S, single-institutional investigation; M, multi-institutional investigation; R, retrospective design; P, prospective design; LC, local control rate; OS, overall survival rate; G ≥ 3 AEs, adverse effects of grade ≥ 3; MST, median survival time; MLCT, median local control time; M, months; Y, years; NA, not available.
Comparison between the cohort data for PT and the extracted historical data for X-SBRT, based on the systematic review
Eleven X-SBRT studies [19–29] selected by a systematic review for L-OM were all used to make statistical comparisons with the Japanese cohort data of PT. The statistical comparisons were in the following combinations: (i) between all data sets of X-SBRT and PT; and (ii) between the same specific primary cancer data sets (e.g. colorectal cancer) of X-SBRT and PT. The results of the statistical comparison between all data sets and the same specific primary cancer data sets are listed in Table 4 and Supplementary Data 3, respectively. For LC, all comparative analysis in the aforementioned combinations demonstrated that PT achieved significantly lower IRR: in the statistical comparison of all data sets, the IRR was 0.52 (95% CI, 0.37–0.72). In the same specific primary cancer data sets of colorectal cancer, the IRR was 0.33 (95% CI, 0.22–0.50). For mortality, a statistical significance existed in the comparison between the colorectal cancer data sets on X-SBRT and PT (IRR, 0.69; 95% CI, 0.49–0.97), while no statistical significance existed in the statistical comparison of all data sets.
Lymph node oligometastasis
Analysis of the Japanese multi-institutional cohort study data set of PT
A total of 282 patients (representing 287 regions) were included. Among them, 205 patients received PBT in 205 regions, while 77 patients received C-ion RT in 82 regions. The median patient age was 66 years (range: 36–96 years). The patients’ and treatment characteristics are shown in Table 2 and Supplementary Data 2. The median follow-up period was 24.2 months (range: 2.5–56.4 months). The major primary disease sites were the colorectum (n = 43, 15.2%), uterus (n = 38, 13.5%), lungs (n = 37, 13.1%) and esophagus (n = 28, 9.9%). The median BED10 was 79.2 Gy (RBE) [range, 60–109.6 Gy (RBE)].
At the last follow-up, local progression occurred in 51 regions in the treated sites among the 287 metastatic lymph node regions (17.8%, 51/287). Overall, 132/282 (46.8%) patients died of any cause. Across all irradiated lymph node sites, the 1-, 2- and 3-year LC rates were 89.8% (95% CI, 85.8–96.7), 79% (95% CI, 73.2–84.9) and 72.8% (95% CI, 65.8–79.8), respectively (Fig. 3A). The 1-, 2- and 3-year OS rates were 82.5% (95% CI, 78.1–87), 62.1% (95% CI, 56.1–68) and 50.2% (95% CI, 43.7–56.7), respectively (Fig. 3B). The median survival time was 37.2 months. For LC, no significant difference existed between PBT and C-ion RT (log-rank test, P = 0.73) (Fig. 3C) or between PT series with a prescribed dose < the median value or ≥ the median value (log-rank test, P = 0.88).
Fig. 3.
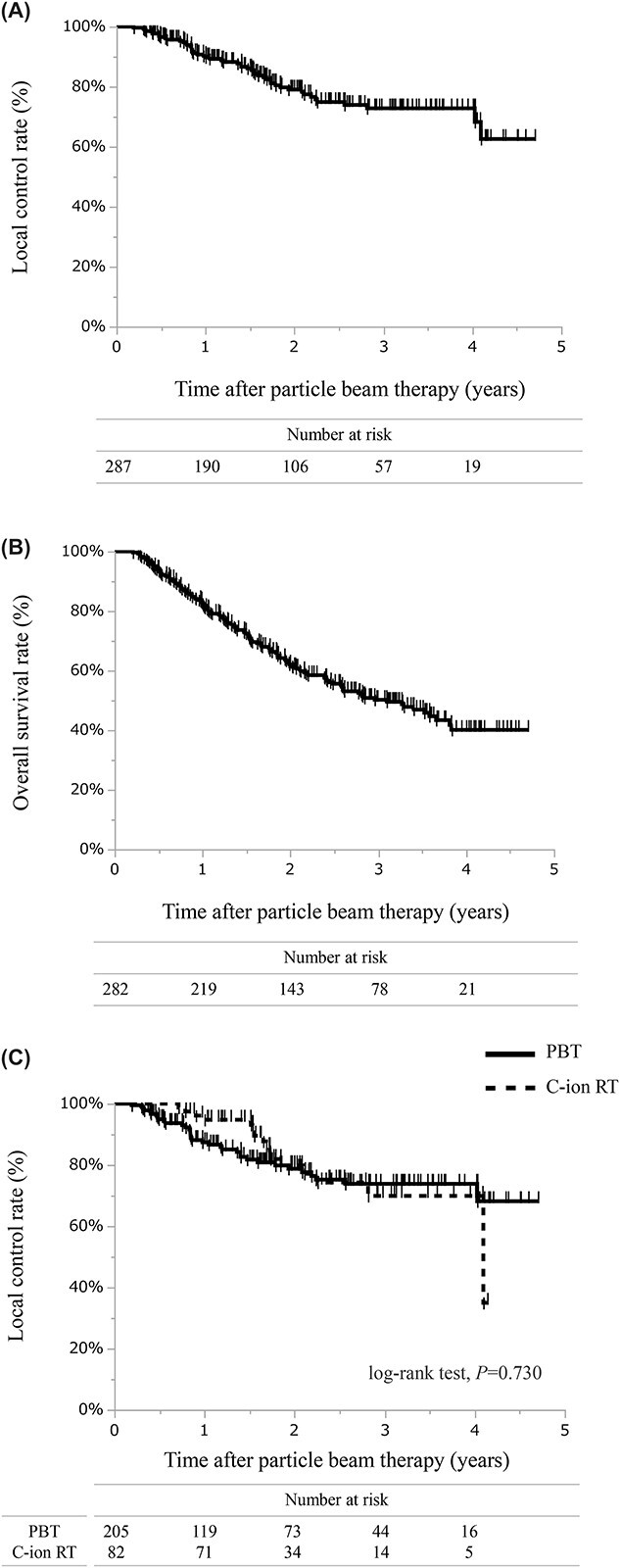
Survival curves of the PBT for LN-OM. (A) LC rate of all data. (B) OS rate of all data. (C) LC rates of PBT and C-ion RT.
Among the 282 PT patients, nine (3.2%) patients developed grade 3 treatment-related late toxicities. No grade ≥ 4 treatment-related late toxicity occurred. Grade 3 toxicities included gastroduodenal ulcer in three patients, proctitis in one patient, pelvic abscess in one patient, urinary obstruction in one patient, tracheal fistula in one patient, vertebral fracture in one patient and peripheral neuropathy in one patient.
Systematic review of X-SBRT/X-IMRT and PT
The PRISMA flow diagram of the systematic review of X-SBRT/X-IMRT and PT for LN-OM is shown in Supplementary Data 1 (Fig. S3). The review identified 17 relevant studies on X-SBRT/X-IMRT [36–52] and five relevant studies on PT [53–57], respectively (Table 6). No high-level investigations were identified. Different studies had different inclusion criteria, characteristics of patients and primary cancers, and treatment protocols. Among the 17 studies on X-SBRT/X-IMRT, two studies [41, 42] were conducted prospectively, and the other studies were conducted retrospectively. However, all studies on PT were conducted retrospectively. The 2- and 3-year LC rates, 2- and 3-year OS rates and incidence of grade ≥ 3 toxicities were extracted from the relevant reports and are listed in Table 6. The LC and OS rates were extracted from published Kaplan–Meier curves, as necessary.
Table 6.
Relevant articles of the systematic review on X-SBRT/X-IMRT and PT for LN-OM
| Authors (Reported year) |
Study design |
Treatment methods |
Number of patients | Primary cancer | LC (%) | OS (%) | G ≥ 3 AEs (%) |
|---|---|---|---|---|---|---|---|
| Aoki T [36] (2003) |
S/R | X-IMRT | 29 | Various primary sites | 58 (2 Y) 58 (3 Y) |
29 (2 Y) 18 (3 Y) |
0 |
| Jereczek-Fossa BA [37] (2014) |
S/R | X-SBRT | 69 | Various primary sites | 68 (2 Y) 64 (3 Y) |
59 (2 Y) 50 (3 Y) |
1.4 |
| Franzese C [38] (2016) |
S/R | X-SBRT | 71 | Various primary sites | 63 (2 Y) 63 (3 Y) |
77 (2 Y) 77 (3 Y) |
0 |
| Loi M [39] (2018) |
S/R | X-SBRT | 91 | Various primary sites | 78 (2 Y) 78 (3 Y) |
65 (2 Y) 52 (3 Y) |
0 |
| Ito M [40] (2020) |
M/R | X-SBRT X-IMRT (3DCRT) |
159 | Various primary sites | 60 (2 Y) NA (3 Y) |
63 (2 Y) NA (3 Y) |
9.8 |
| Franzese C [41] (2020) |
S/P | X-SBRT | 52 | Various primary sites | 82 (2 Y) 82 (3 Y) |
94 (2 Y) 84 (3 Y) |
0 |
| Franzese C [42] (2020) |
S/P | X-SBRT | 278 | Various primary sites | 77 (2 Y) 75 (3 Y) |
74 (2 Y) 67 (3 Y) |
0.4 |
| Sato A [43] (2020) |
S/R | X-IMRT | 21 | Various primary sites | 52 (2 Y) 52 (3 Y) |
89 (2 Y) 75 (3 Y) |
NA |
| Bae SH [44] (2012) |
S/R | X-SBRT | 18 | Colorectal cancer | NA (2 Y) NA (3 Y) |
NA (2 Y) NA (3 Y) |
11.1 |
| Franzese C [45] (2017) |
S/R | X-SBRT | 35 | Colorectal cancer | 75 (2 Y) 75 (3 Y) |
81 (2 Y) 81 (3 Y) |
0 |
| Ho JC [46] (2015) |
S/R | X-IMRT | 38 | Uterine cancer | NA (2 Y) NA (3 Y) |
71 (2 Y) NA (3 Y) |
21.1 |
| Park HJ [47] (2015) |
M/R | X-SBRT | 85 | Uterine cancer | 83 (2 Y) NA (3 Y) |
58 (2 Y) NA (3 Y) |
5.9 |
| Choi CW [48] (2009) |
S/R | X-SBRT (3DCRT) |
30 | Uterine cancer | 76 (2 Y) 67 (3 Y) |
71 (2 Y) 65 (3 Y) |
3.3 |
| Meng MB [49] (2015) |
S/R | X-SBRT | 33 | Lung cancer | NA (2 Y) 86 (3 Y) |
52 (2 Y) 41 (3 Y) |
6.1 |
| Manabe Y [50] (2018) |
S/R | X-SBRT | 14 | Lung cancer | 90 (2 Y) 81 (3 Y) |
40 (2 Y) 40 (3 Y) |
3.7 |
| Ost P [51] (2016) |
M/R | X-SBRT | 72 | Prostate cancer | NA (2 Y) 94 (3 Y) |
NA (2 Y) 96 (3 Y) |
0 |
| Franzese C [52] (2017) |
S/R | X-SBRT | 26 | Prostate cancer | 75 (2 Y) 75 (3 Y) |
100 (2 Y) 100 (3 Y) |
0 |
| Isozaki Y [53] (2017 |
S/R | C-ion RT | 34 | Colorectal cancer | 70 (2Y) 70 (3 Y) |
83 (2 Y) 63 (3 Y) |
0 |
| Shiba S [54] (2017) |
S/R | C-ion RT | 16 | Gynecological cancer | 94 (2 Y) 94 (3 Y) |
74 (2 Y) 74 (3 Y) |
0 |
| Isozaki Y [55] (2018) |
S/R | C-ion RT | 10 | Esophageal cancer | 92 (2 Y) 92 (3 Y) |
58 (2 Y) 58 (3 Y) |
0 |
| Okonogi N [56] (2019) |
M/R | C-ion RT | 323 | Various primary sites | 85 (2 Y) 79 (3 Y) |
87 (2 Y) 63 (3 Y) |
0.3 |
| Shirai K [57] (2019) |
S/R | C-ion RT | 15 | Lung cancer | 92 (2 Y) 92 (3 Y) |
75 (2 Y) 60 (3 Y) |
0 |
Abbreviations: PT, particle beam therapy; LN-OM, lymph node oligometastasis; X-SBRT, X-ray stereotactic body radiotherapy; X-IMRT, x-ray intensity-modulated radiotherapy; PBT, proton beam therapy; C-ion RT, carbon-ion radiotherapy; S, single-institutional investigation; M, multi-institutional investigation; R, retrospective design; P, prospective design; LC, local control rate; OS, overall survival rate; G ≥ 3 AEs, adverse effects of grade ≥ 3; 3DCRT, 3-demensional conformal radiation therapy; Y, years; NA, not available.
Comparison between the cohort data for PT and the extracted historical data for X-SBRT/X-IMRT, based on the systematic review
Among the 17 relevant studies selected by systematic review on X-SBRT/X-IMRT for LN-OM, the data of 16 articles [36–43, 45–52] were used to make a statistical comparison with the Japanese cohort data of PT. However, the data of one study [44] was not used because it did not describe the number of events necessary for statistical comparison. The statistical comparisons were conducted using the following combinations: (i) all data sets of X-SBRT/X-IMRT and PT; and (ii) between the same specific primary cancer data sets (e.g. colorectal cancer, uterine cancer and lung cancer) of X-SBRT/X-IMRT and PT. The results of statistical comparison between all data sets and the same specific primary cancer data sets are listed in Table 4 and Supplementary Data 3, respectively. For LC, the statistical comparisons showed no significant differences between all combinations. For OS, statistical comparisons revealed no significant differences between each combination, except for the combination of the same specific lung cancer data sets.
DISCUSSION
The aim of this study was: (i) to analyze the efficacy and toxicity of PT by using the Japanese multi-institutional cohort study data set; (ii) to conduct a systematic review on X-SBRT/X-IMRT and PT in radical metastasis-directed therapy for P-OM, L-OM and LN-OM; and (iii) to conduct a statistical comparison between the outcomes of the cohort data in PT and the outcomes of the historical data in X-SBRT/X-IMRT. Our study, which used Japanese nationwide cohort data sets, demonstrated that PT provided durable LC (3-year LC rate, 72.8–83.2%) with acceptable OS (3-year OS rate, 38.5–68.1%) and risk of severe toxicity (incidence, 0.8–3.5%) in radical metastasis-directed therapy for P-OM, L-OM and LN-OM. The analysis also showed the potential of PT as a promising treatment modality for L-OM tumors measuring > 5 cm. Furthermore, this study presented the results of statistical comparisons between the national cohort data sets of PT and the historical data sets of X-SBRT/X-IMRT. To the best of our knowledge, this study is the first using such comparisons between the data sets of PT and X-ray radiotherapy (i.e. X-SBRT/X-IMRT) for the radical metastasis-directed treatment of P-OM, L-OM and LN-OM.
Pulmonary oligometastasis
The analysis of the cohort study data set of PT showed durable LC (3-year LC, 83.2%) and OS (3-year OS, 68.1%) with an acceptable risk of grade ≥ 3 toxicity (incidence rate, 0.8%) in metastasis-directed therapy for P-OM. This result corresponded with the results of previous reports on PT for P-OM [16–18]. The range of the prescribed dose in this cohort series was relatively wide because the prescribed doses depended on the physician’s decisions, based on the size, number and location of a target, surrounding organs at risk (e.g. the proximity of a target to organs at risk and the tolerance of organs at risk), etc. However, the statistical analysis on the impact of the prescribed dose on LC revealed no statistical significance. The reason for this finding may be that 86.4% (114/132) of lesions in the cohort series received PT with adequate delivery dose of BED10 > 100 Gy (RBE). Some investigations have demonstrated that the delivery dose of BED10 > 100 Gy was associated with better LC [11, 14]. With regard to adverse events, the PT cohort data set demonstrated that only one patient developed grade 3 pneumonitis and no patient developed grade ≥ 4 toxicity. The incidence rate was lower than that in previous data on X-SBRT (0.8% vs 1.5–3.3%) [12–14], although both rates were acceptably low.
The statistical comparison between all data of PT and X-SBRT showed significantly better LC and OS with PT. This result implied that PT is superior to X-SBRT as the treatment modality for P-OM. However, caution is needed in interpretation because the statistical subgroup analyses on the same primary cancer data sets revealed no significant difference in LC or OS. This inconsistent result may be explained by several biases. The sample size of the PT series was smaller than that of X-SBRT series. The comparison with all data sets may unintentionally have several biases. In the analysis, no adjustment was conducted in the different parameters between the PT series and X-SBRT series because of the difficulty in appropriate adjustment. However, the sample size of the same primary cancer data sets seemed to be too small to conduct a statistical comparison with sufficient accuracy. These several factors may have caused the inconsistency. Therefore, a more accurate comparison should be conducted with a larger sample size with adjustment for several elements (e.g. patient and disease characteristics, combined treatment modality and follow-up duration).
Our analysis had some limitations, although the statistical comparisons did not demonstrate that the outcome of PT for P-OM was worse than the outcome of X-SBRT. Therefore, a conclusion is that PT for P-OM demonstrated promising outcomes with the possibility that PT is superior to X-SBRT in the radical metastasis-directed treatment for P-OM.
Liver oligometastasis
The analysis of the cohort study data set of PT showed a durable LC (3-year LC, 73.2%) with an acceptable OS (3-year OS, 38.5%) and risk of grade ≥ 3 toxicity (incidence rate, 3.5%) in metastasis-directed therapy for L-OM. This result corresponded with those of previous reports on PT for L-OM [30–35]. The prescribed dose range in this cohort series was relatively wide because the prescribed doses to treat L-OM depended on the physicians’ decisions among different facilities. However, many lesions (89.8%, 239/266) in this cohort series received PT with high prescribed doses of BED10 > 96 Gy (RBE). That factor seemed to be the reason the statistical analysis revealed no significance between PT series with prescribed dose < the median value or ≥ the median value. With regard to adverse events, the incidence rate of grade 3 adverse events was 3.5% in the PT series, and the dominant toxicity was dermatitis (3/7, 42.9%). No cases of grade ≥ 4 fatal adverse events occurred. The incidence of grade 3 adverse events in PT for L-OM was low and likely similar to the incidence in published X-SBRT data (0–9.8%).
The statistical comparison between all data sets and colorectal cancer data sets consistently showed that PT offered superior LC than did X-SBRT. In the PT for L-OM series, the median delivered BED10 was 109.6 Gy (RBE) and approximately 90% of lesions received more than 96 Gy (RBE). Previous investigations have reported that a higher delivery dose to targets is associated with better LC [22, 24]; therefore, the substantially high dose in the PT series seemed to result in better LC. For OS, the statistical comparison between all data sets of PT and X-SBRT revealed no statistically significant difference. However, the statistical comparison between the colorectal cancer data sets demonstrated that PT offered superior OS. As Klement et al. [29] suggested, metastatic disease control may potentially improve OS in patients with colorectal cancer. Therefore, the improved LC may have resulted in better OS in the cohort series of PT for L-OM. However, in general, the LC does not always have a direct impact on OS because OS is associated with many factors (e.g. patient and disease characteristics; combined treatment modality; treatment response, especially in systemic treatment; and follow-up time). Therefore, more precise validation with a larger sample size and appropriate adjustment for variations will be essential in future studies to evaluate the impact of PT for L-OM on survival benefit more appropriately.
This cohort data set of PT for L-OM included 38 (15.2%) lesions measuring > 5 cm and 30 (12%) lesions measuring > 6 cm among 250 measurable lesions. The analysis revealed that PT for large L-OM (i.e. tumor size > 5 cm) provided durable LC (3-year LC, 84.5%) with acceptable toxicity (i.e. incidence of grade ≥ 3 adverse events, 2.9%). In general, a large liver tumor measuring > 5 cm or 6 cm does not seem to be a good candidate for X-SBRT in the context of the risk of severe toxicity [19–21, 25, 26]. Thus, few studies have analyzed X-SBRT for large L-OMs. By contrast, our cohort data demonstrated that PT may be a safe treatment for L-OM tumors measuring > 5 cm, as well as for L-OM tumors measuring ≤ 5 cm, and it suggested that PT could be a promising treatment for large L-OM tumors measuring > 5 cm, which may be ineligible for X-SBRT.
Lymph node oligometastasis
The analysis of the national cohort data set showed that PT offered durable LC (3-year LC, 72.8%) with acceptable OS (3-year OS, 50.2%) and incidence of grade ≥ 3 adverse effects (3.2%) in metastasis-directed therapy for LN-OM. This result corresponded with previous findings on PT for LN-OM [53–57]. In the cohort series of PT for LN-OM, the median delivered BED10 was 79.2 Gy (RBE). Compared to the delivery doses in the series of P-OM or L-OM, the doses of LN-OM were lower, although the range of the doses was narrower. This finding may be caused by the target location in the treatment for LN-OM. In general, in the treatment for LN-OM, the target sites are often very close to bowels, which are vulnerable to radiation. In the cohort series, 44.4% (4/9) of grade 3 late toxicities was bowel toxicity.
However, the 3-year LC rate of 72.8% in the PT series for LN-OM was comparable with the reported rates of the X-SBRT series, as the statistical comparisons of all combinations demonstrated. Franzese et al. [42] in their large series (278 patients with 418 lesions) reported that a delivery dose of BED10 ≥ 75 Gy was a significant independent factor associated with better LC. Many target lesions in the cohort LN-OM series (72.8%, 209/287) were treated with a dose of BED10 ≥ 75 Gy (RBE). The relatively high-dose delivery in the cohort series of PT for LN-OM probably resulted in the durable LC. The incidence of grade ≥ 3 adverse events was low in this cohort series (3.2%), as well as in previous reports on PT for LN-OM (range, 0.0–0.3%). Therefore, we concluded that PT could provide comparable LC with acceptable toxicity as that of X-SBRT or X-IMRT.
For OS, statistical analyses had inconsistent results among the different comparative combinations. In general, OS is influenced by many factors. The population with LN-OM likely has highly heterogeneous patient and lesion characteristics and treatment history. In this study, the reports included in the comparative analysis had high heterogeneity. The correction of heterogeneity consequently was not conducted because of the difficulty in appropriate adjustment, which was likely to cause inconsistent results in our analysis. More precise validation with larger sample size and appropriate adjustment for differences will be necessary in future studies. Our analysis had some limitations, although the results demonstrated that, at any rate, PT for LN-OM provided comparable OS outcomes as those of X-SBRT/X-IMRT.
This study has several limitations. The multi-institutional cohort intrinsically includes variations between the participating institutions, which may have unintentionally resulted in a significant bias. Statistical comparisons were conducted using only the data sets extracted from reports describing the events of interest. The data sets used in the statistical comparisons may be too heterogeneous to achieve statistical results with modest bias. However, metastasis-directed therapy for oligometastatic disease intrinsically has large heterogeneity. Many factors (e.g. characteristics of the patients and disease, intensity of the metastasis-directed therapy or combined therapy, treatment history before metastasis-directed therapy) can cause heterogeneity and difficulty in making an appropriate comparison with retrospective data sets. Therefore, more precise validation with prospective comparative studies is needed.
In conclusion, PT provided durable LC with acceptable OS and risk of severe toxicity in radical metastasis-directed therapy for P-OM, L-OM and LN-OM. For LC, PT, compared to X-SBRT or X-IMRT, was potentially superior for treating P-OM; was superior for treating L-OM; and was equivalent for treating LN-OM. In particular, this study showed PT may be a new treatment option for L-OM tumors measuring > 5 cm.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Japanese Society for Radiation Oncology (Kawasaki, Japan). We wish to thank Editage (www.editage.jp) for English language editing.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
FUNDING
This work was supported by Hokkaido University and AMED [grant number JP16lm0103004] and the National Institutes for Quantum Science and Technology. Norihiro Aibe received a research grant from Japan Society for the Promotion of Science KAKENHI [grant number JP 20 K16797].
DATA AVAILABILITY
The datasets generated and/or analyzed underlying the current study are possibly available from JASTRO with proper procedures on reasonable request to the corresponding author.
Contributor Information
Norihiro Aibe, Department of Radiology, Kyoto Prefectural University of Medicine, Kyoto 602-8566, Japan.
Hiroyuki Ogino, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City University West Medical Center, Nagoya 462-8508, Japan.
Masaru Wakatsuki, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Kei Fujikawa, Department of Biostatistics, Kyoto Prefectural University of Medicine, Kyoto 602-8566, Japan.
Satoshi Teramukai, Department of Biostatistics, Kyoto Prefectural University of Medicine, Kyoto 602-8566, Japan.
Nobuyoshi Fukumitsu, Department of Radiation Oncology, Kobe Proton Center, Hyogo 650-0047, Japan.
Shintaro Shiba, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan.
Naoyoshi Yamamoto, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Akihiro Nomoto, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Takashi Ono, Department of Radiation Oncology, Yamagata University, Faculty of Medicine, Yamagata 990-9585, Japan.
Masanosuke Oguri, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City University West Medical Center, Nagoya 462-8508, Japan.
Hisashi Yamaguchi, Department of Radiology, Southern Tohoku Proton Therapy Center, Koriyama City, Fukushima 963-8052, Japan.
Haruko Numajiri, Department of Radiation Oncology, University of Tsukuba, Ibaraki 305-8550, Japan.
Kei Shibuya, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan.
Shohei Okazaki, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan.
Yuhei Miyasaka, Department of Radiation Oncology, Gunma University Graduate School of Medicine, Gunma 371-8511, Japan.
Noriyuki Okonogi, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Kazutoshi Murata, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Hitoshi Tatebe, Proton Therapy Center, Fukui Prefectural Hospital, Fukui 910-0846, Japan.
Atsushi Motegi, Department of Radiation Oncology, National Cancer Center Hospital East, Chiba 277-8577, Japan.
Tomoaki Okimoto, Department of Radiology, Hyogo Ion Beam Medical Center, Hyogo, Japan.
Takayuki Yoshino, Department of Gastrointestinal Oncology, National Cancer Center Hospital East, Chiba, Japan.
Masaki Mandai, Department of Gynecology and Obstetrics, Kyoto University Graduate School of Medicine, Kyoto 606-8501, Japan.
Norio Katoh, Department of Radiation Oncology, Hokkaido University Faculty of Medicine, Hokkaido 060-0808, Japan.
Hiroshi Tsuji, QST Hospital, National Institutes for Quantum Science and Technology, Chiba 263-8555, Japan.
Hideyuki Sakurai, Department of Radiation Oncology, University of Tsukuba, Ibaraki 305-8550, Japan.
References
- 1. Huang F, Wu G, Yang K. Oligometastasis and oligo-recurrence: more than a mirage. Radiat Oncol 2014;9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378–82. [DOI] [PubMed] [Google Scholar]
- 3. Londero F, Grossi W, Morelli A et al. Surgery versus stereotactic radiotherapy for treatment of pulmonary metastases. A systematic review of literature. Future Sci OA 2020;6:FSO471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aitken KL, Hawkins MA. Stereotactic body radiotherapy for liver metastases. Clin Oncol (R Coll Radiol) 2015;27:307–15. [DOI] [PubMed] [Google Scholar]
- 5. Matsushita H, Jingu K, Umezawa R et al. Stereotactic radiotherapy for oligometastases in lymph nodes-a review. Technol Cancer Res Treat 2018;17:153303381880359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ASTRO Model Policies Stereotactic Body Radiation Therapy . p. 1–15. https://www.astro.org/ASTRO/media/ASTRO/Daily%20Practice/PDFs/ASTROSBRTModelPolicy.pdf (26 December 2022, date last accessed).
- 7. ASTRO Model Policies Proton Beam Therapy . https://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Reimbursement/Model_Policies/Content_Pieces/ASTROPBTModelPolicy.pdf (26 December 2022, date last accessed).
- 8. Matsumoto Y, Fukumitsu N, Ishikawa H et al. A critical review of radiation therapy: from particle beam therapy (proton, carbon, and BNCT) to beyond. J Pers Med 2021;11:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Common Terminology Criteria for Adverse Events (CTCAE) . 2010. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. (2 January 2021, date last accessed).
- 10. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricco A, Davis J, Rate W et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry’s experience. Radiat Oncol 2017;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niibe Y, Yamamoto T, Onishi H et al. Pulmonary oligometastases treated by stereotactic body radiation therapy: a nationwide survey of 1,378 patients. Anticancer Res 2020;40:393–9. [DOI] [PubMed] [Google Scholar]
- 13. De Rose F, Cozzi L, Navarria P et al. Clinical outcome of stereotactic ablative body radiotherapy for lung metastatic lesions in non-small cell lung cancer oligometastatic patients. Clin Oncol (R Coll Radiol) 2016;28:13–20. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto T, Niibe Y, Matsumoto Y et al. Analyses of local control and survival after stereotactic body radiotherapy for pulmonary oligometastases from colorectal adenocarcinoma. J Radiat Res 2020;61:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi HS, Jeong BK, Kang KM et al. Tumor control and overall survival after stereotactic body radiotherapy for pulmonary oligometastases from colorectal cancer: a meta-analysis. Cancer Res Treat 2020;52:1188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aibe N, Ogino H, Teramukai S et al. Multi-institutional retrospective analysis of the outcomes of proton beam therapy for patients with 1 to 3 pulmonary oligometastases from various primary cancers. Adv Radiat Oncol 2021;6:100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamamoto N, Nakajima M, Tsujii H, Kamada T. Carbon ion radiotherapy for oligo-recurrence in the lung. Pulm Med 2013;2013:219746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takahashi W, Nakajima M, Yamamoto N et al. Carbon ion radiotherapy for oligo-recurrent lung metastases from colorectal cancer: a feasibility study. Radiat Oncol 2014;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kavanagh BD, Schefter TE, Cardenes HR et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol 2006;45:848–55. [DOI] [PubMed] [Google Scholar]
- 20. Iwata H, Shibamoto Y, Hashizume C et al. Hypofractionated stereotactic body radiotherapy for primary and metastatic liver tumors using the Novalis image-guided system: preliminary results regarding efficacy and toxicity. Technol Cancer Res Treat 2010;9:619–27. [DOI] [PubMed] [Google Scholar]
- 21. Scorsetti M, Arcangeli S, Tozzi A et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013;86:336–42. [DOI] [PubMed] [Google Scholar]
- 22. Yamashita H, Onishi H, Matsumoto Y et al. Local effect of stereotactic body radiotherapy for primary and metastatic liver tumors in 130 Japanese patients. Radiat Oncol 2014;9:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed KA, Caudell JJ, El-Haddad G et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2016;95:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahadevan A, Blanck O, Lanciano R et al. Stereotactic body radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch® patient registry. Radiat Oncol 2018;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scorsetti M, Comito T, Clerici E et al. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol 2018;13:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Comito T, Cozzi L, Clerici E et al. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer 2014;14:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scorsetti M, Comito T, Tozzi A et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol 2015;141:543–53. [DOI] [PubMed] [Google Scholar]
- 28. McPartlin A, Swaminath A, Wang R et al. Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys 2017;99:388–95. [DOI] [PubMed] [Google Scholar]
- 29. Klement RJ, Abbasi-Senger N, Adebahr S et al. The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer 2019;19:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukumitsu N, Okumura T, Takizawa D et al. Proton beam therapy for metastatic liver tumors. Radiother Oncol 2015;117:322–7. [DOI] [PubMed] [Google Scholar]
- 31. Makishima H, Yasuda S, Isozaki Y et al. Single fraction carbon ion radiotherapy for colorectal cancer liver metastasis: a dose escalation study. Cancer Sci 2019;110:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shiba S, Shibuya K, Okamoto M et al. Carbon-ion radiotherapy for oligometastatic colorectal cancer in the liver or lung. Anticancer Res 2021;41:1997–2005. [DOI] [PubMed] [Google Scholar]
- 33. Fukumitsu N, Okumura T, Takizawa D et al. Proton beam therapy for liver metastases from gastric cancer. J Radiat Res 2017;58:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamaguchi H, Honda M, Hamada K et al. The effectiveness of proton beam therapy for liver metastatic recurrence in gastric cancer patients. Jpn J Clin Oncol 2020;50:903–8. [DOI] [PubMed] [Google Scholar]
- 35. Fukumitsu N, Okumura T, Numajiri H et al. Follow-up study of liver metastasis from breast cancer treated by proton beam therapy. Mol Clin Oncol 2017;7:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aoki T, Nagata Y, Mizowaki T et al. Clinical evaluation of dynamic arc conformal radiotherapy for paraaortic lymph node metastasis. Radiother Oncol 2003;67:113–8. [DOI] [PubMed] [Google Scholar]
- 37. Jereczek-Fossa BA, Piperno G, Ronchi S et al. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am J Clin Oncol 2014;37:227–33. [DOI] [PubMed] [Google Scholar]
- 38. Franzese C, Cozzi L, Franceschini D et al. Role of stereotactic body radiation therapy with volumetric-modulated arcs and high-intensity photon beams for the treatment of abdomino-pelvic lymph-node metastases. Cancer Investig 2016;34:348–54. [DOI] [PubMed] [Google Scholar]
- 39. Loi M, Frelinghuysen M, Klass ND et al. Locoregional control and survival after lymph node SBRT in oligometastatic disease. Clin Exp Metastasis 2018;35:625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ito M, Kodaira T, Koide Y et al. Role of high-dose salvage radiotherapy for oligometastases of the localised abdominal/pelvic lymph nodes: a retrospective study. BMC Cancer 2020;20:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franzese C, Comito T, Tripoli A et al. Phase II trial of high dose stereotactic body radiation therapy for lymph node oligometastases. Clin Exp Metastasis 2020;37:565–73. [DOI] [PubMed] [Google Scholar]
- 42. Franzese C, Badalamenti M, Comito T et al. Assessing the role of stereotactic body radiation therapy in a large cohort of patients with lymph node oligometastases: does it affect systemic treatment’s intensification? Radiother Oncol 2020;150:184–90. [DOI] [PubMed] [Google Scholar]
- 43. Sato A, Omura M, Minagawa Y et al. Intensity-modulated radiation therapy for lymph node oligo-recurrence. In Vivo 2020;34:2587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bae SH, Kim MS, Cho CK et al. High dose stereotactic body radiotherapy using three fractions for colorectal oligometastases. J Surg Oncol 2012;106:138–43. [DOI] [PubMed] [Google Scholar]
- 45. Franzese C, Fogliata A, Comito T et al. Stereotactic/hypofractionated body radiation therapy as an effective treatment for lymph node metastases from colorectal cancer: an institutional retrospective analysis. Br J Radiol 2017;90:20170422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ho JC, Allen PK, Jhingran A et al. Management of nodal recurrences of endometrial cancer with IMRT. Gynecol Oncol 2015;139:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park HJ, Chang AR, Seo Y et al. Stereotactic body radiotherapy for recurrent or oligometastatic uterine cervix cancer: a cooperative study of the Korean radiation oncology group (KROG 14-11). Anticancer Res 2015;35:5103–10. [PubMed] [Google Scholar]
- 48. Choi CW, Cho CK, Yoo SY et al. Image-guided stereotactic body radiation therapy in patients with isolated Para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys 2009;74:147–53. [DOI] [PubMed] [Google Scholar]
- 49. Meng MB, Wang HH, Zaorsky NG et al. Clinical evaluation of stereotactic radiation therapy for recurrent or second primary mediastinal lymph node metastases originating from non-small cell lung cancer. Oncotarget 2015;6:15690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Manabe Y, Shibamoto Y, Baba F et al. Definitive radiotherapy for hilar and/or mediastinal lymph node metastases after stereotactic body radiotherapy or surgery for stage I non-small cell lung cancer: 5-year results. Jpn J Radiol 2018;36:719–25. [DOI] [PubMed] [Google Scholar]
- 51. Ost P, Jereczek-Fossa BA, Van As N et al. Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. Clin Oncol (R Coll Radiol) 2016;28:e115–20. [DOI] [PubMed] [Google Scholar]
- 52. Franzese C, Lopci E, Di Brina L et al. 11C-choline-PET guided stereotactic body radiation therapy for lymph node metastases in oligometastatic prostate cancer. Cancer Investig 2017;35:586–93. [DOI] [PubMed] [Google Scholar]
- 53. Isozaki Y, Yamada S, Kawashiro S et al. Carbon-ion radiotherapy for isolated Para-aortic lymph node recurrence from colorectal cancer. J Surg Oncol 2017;116:932–8. [DOI] [PubMed] [Google Scholar]
- 54. Shiba S, Okonogi N, Kato S et al. Clinical impact of re-irradiation with carbon-ion radiotherapy for lymph node recurrence of gynecological cancers. Anticancer Res 2017;37:5577–83. [DOI] [PubMed] [Google Scholar]
- 55. Isozaki Y, Yasuda S, Akutsu Y et al. Salvage carbon-ion radiotherapy for isolated lymph node recurrence following curative resection of esophageal cancer. Anticancer Res 2018;38:6453–8. [DOI] [PubMed] [Google Scholar]
- 56. Okonogi N, Kaminuma T, Okimoto T et al. Carbon-ion radiotherapy for lymph node oligo-recurrence: a multi-institutional study by the Japan carbon-ion radiation oncology study group (J-CROS). Int J Clin Oncol 2019;24:1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shirai K, Kubota Y, Ohno T et al. Carbon-ion radiotherapy for isolated lymph node metastasis after surgery or radiotherapy for lung cancer. Front Oncol 2019;9:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.