An important component of the UK's early response to the COVID-19 pandemic was increasing SARS-CoV-2 testing capacity across the National Health Service (NHS). At the time, we and others advocated for the repurposing of academic centres to deliver laboratory capacity for testing and screening of asymptomatic health-care workers, to prevent the transmission of SARS-CoV-2.1
In response to a pressing need for testing across London in March, 2020, the Francis Crick Institute rapidly repurposed its laboratory facilities in partnership with University College London Hospitals (UCLH) and the Health Services Laboratory, to create the Crick COVID Testing Pipeline (CCTP). The CCTP provided 680 602 occupational RT-PCR tests from April, 2020, to April, 2022.2 Same-day results were provided: median turnaround time was 533 min (IQR 456–654; 8·9 h [7·6–10·9]) from receipt to result reporting. All SARS-CoV-2 positive samples were reported to the NHS Test and Trace service, viral genomes were sequenced in-house and submitted to the COVID-19 Genome UK Consortium, and protocols were rapidly shared.2
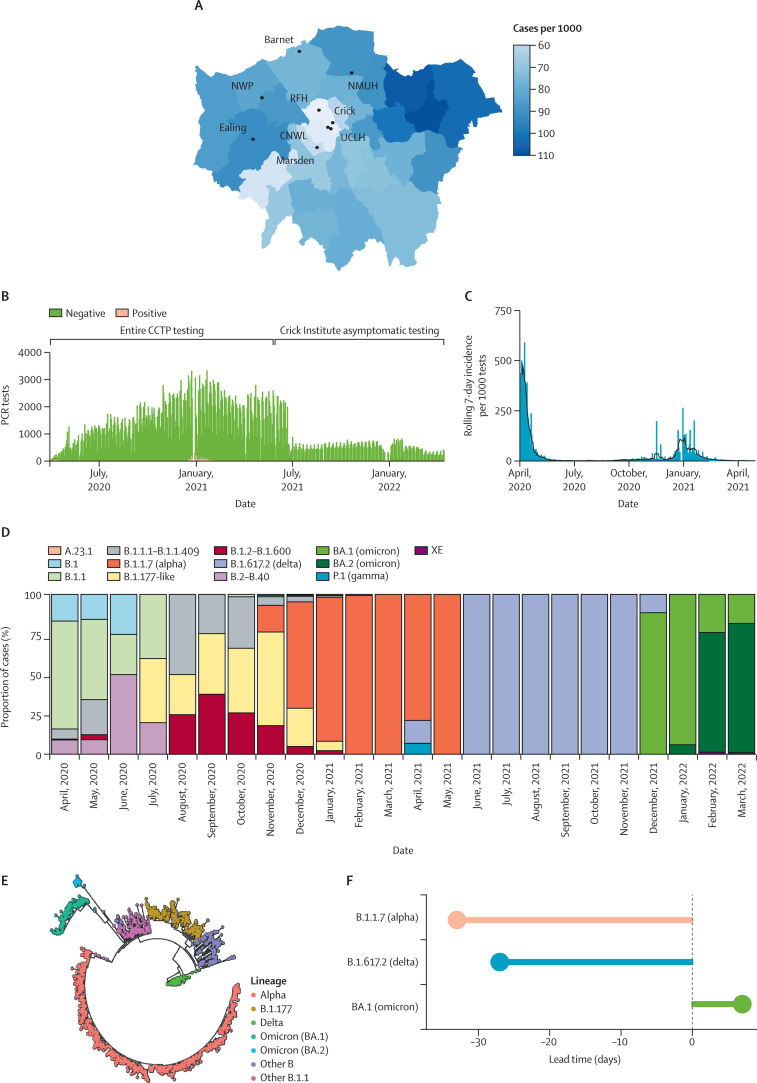
The CCTP provided both asymptomatic screening and diagnostic testing for staff in eight NHS trusts and 98 local care homes and rehabilitation facilities up until May, 2021, when NHS testing capacity was realised, covering boroughs with high rates of COVID-19 including Brent, Harrow, Barnet, and Ealing (figure A ). Testing was expanded to the Francis Crick Institute in June, 2020, to support essential clinical and research work and continued until May, 2022, under an institutional COVID-19 secure policy.2 Here, we performed an analysis of testing data with a view to understanding both the effect of hospital trust screening policies and the Francis Crick Institute's in-person work policy to inform future pandemic planning.
Figure.
The Crick COVID Testing Pipeline mirrors the dynamics of the first year of the COVID-19 pandemic in London
(A) Prevalence of all SARS-CoV-2 infections in cases per 1000 residents, by London borough from April, 2020, to May, 2021. (B) Numbers of daily tests conducted by the CCTP from April, 2020, to April, 2022. (C) Rolling 7-day incidence of SARS-CoV-2 infections across all the CCTP sites from April, 2020, to May, 2021. Vertical lines indicate peak daily positive tests, smoothed lines indicate 7-day rolling mean. (D) SARS-CoV-2 lineage positive PCR tests as a monthly proportion of total positive tests from April, 2020, to March, 2022. (E) Phylogenetic tree showing variation in all SARS-CoV-2 lineages isolated by the CCTP between 2020 and 2022, and coloured according to variants of concern. (F) Detection lead time in days for three variants of concern related to WHO designation date. CCTP=Crick COVID Testing Pipeline. Crick=Francis Crick Institute. CNWL=Camden and Northwest London NHS Trust. NMUH=North Middlesex University Hospital. NWP=Northwick Park Hospital. RFH=Barnet and Royal Free Hospitals. UCLH=University College London Hospitals.
Overall, 7316 positive infections were detected from 680 602 tests (1%) between April 1, 2020, and April 14, 2022 (figure B). Across the CCTP, the rolling 7-day incidence (7-day mean number of positive tests per 1000 tests taken per site) showed that infections in health-care workers mirrored the incidence of national trends (figure C).3 We captured variants of concern (figure D–E)—often ahead of their designation by WHO (figure F)—whereby alpha (B.1.1.7) was first detected 33 days before, delta (B.1.617.2) 27 days before, and omicron (BA.1) 5 days following its designation.
We first explored the interactions across the CCTP between positive test rates, viral loads, and different testing policies adopted by NHS trusts between 2020 and 2021 (appendix pp 10–11). We compared the 7-day rolling infection incidence between sites (appendix pp 10–11) and found, as expected, that the incidence was highest when testing was predominantly offered to symptomatic staff, including at sites managed by the London Northwest University Hospitals NHS Trust and The Royal Free Hospital NHS Trust. However, despite a low 7-day rolling infection incidence, 40·8% of all positive tests were from UCLH and Royal Marsden Hospitals; both sites offered asymptomatic testing to support the delivery of COVID-19 secure cancer and surgical services. These sites detected a substantial number of additional infections.
To investigate the differences between sites operating symptomatic and asymptomatic testing policies, we calculated an incidence for each site by adjusting for the organisation size (defined by the number of employees stated in their 2020–21 annual reports), and then calculated an incidence rate ratio (IRR) for each site over the first 4 months of testing, defined as the daily incidence of positive tests for each site, divided by the daily incidence estimates for London's population.4 We compared the IRR between primarily symptomatic and asymptomatic testing sites, including those following NHS guidelines to create COVID-19 secure clinical areas.5
During the first 4 months of testing, the incidence of cases at NHS sites (UCLH and Royal Marsden Hospitals) that offered primarily asymptomatic SARS-CoV-2 testing was 7·5 and 13 times respectively that of the London population (appendix pp 10–11) and NHS sites continued to report positive tests consistently above 5 times the background population symptomatic rate until the second week of July, 2020 (appendix pp 3–4).
Asymptomatic screening is designed to detect early infections, reducing transmission with self isolation.1 We compared the distribution of PCR cycle threshold (Ct) value between the two types of sites as a well recognised proxy measure of viral load and thus, infectiousness.6 We found the median Ct was lower (corresponding with a higher viral load) in symptomatic test sites (Ct 21·2 [IQR 8·06], n=1458), compared with asymptomatic sites (Ct 25·7 [10·1], n=3322, p<0·0001; appendix pp 3–4). Ct values typically peak just before or around symptom onset and symptomatic-only testing might be biased towards the post-peak period; therefore, asymptomatic testing might further detect a subset of people with infectious virus at or before the Ct peak.6 To capture peak Ct values and control for different Ct ranges across sites with different testing policies, we compared the cumulative occurrence of positive tests between asymptomatic and symptomatic sites in a subset of swabs with a Ct value of less than 15 (appendix pp 10–11). Within this subset, we found that asymptomatic sites identified infections at lower Ct values, suggesting enhanced detection of these highly infectious individuals.
We then analysed the Francis Crick Institute, whereby to support a rapid return to in-person working, we implemented a COVID-19 secure workplace policy.7 With an asymptomatic testing policy, entry to the institute was contingent on negative testing within the previous 8 days, internal contact tracing following positive tests, distancing, and face coverings were enforced by social compliance. The institute remained open, while the weekly test positivity remained less than 1%. Analysis of building occupancy data showed mean 7-day occupancy was maintained at over 60% of peak attendance for the overwhelming majority of days (356 [84%] of 423 days; appendix pp 10–11), reaching 75% peak attendance by September, 2020. The 1% threshold was met on Oct 22, 2020, following the spread of the EU1 variant (B.1.177), and again on Dec 8, 2021, as a result of the omicron variant (BA.1, appendix pp 5–6). The Francis Crick Institute implemented a stricter testing protocol resulting in flatter peaks of infection in employees than were otherwise observed within our local borough of Camden (appendix pp 10–11).
Taken together, we show that it was possible to repurpose laboratory facilities and integrate existing clinical and laboratory expertise to set up a comprehensive testing facility in an academic institution at pace,2 serving as a lifeboat laboratory and delivering testing for the NHS in advance of the planned national programme.
The CCTP was designed to protect both staff and patients with testing; we show important heterogeneity in the uptake of testing by hospitals. Health-care workers are susceptible to the occupational acquisition of SARS-CoV-2 despite infection control practices.8, 9 Estimates of true asymptomatic infections vary, but up to 40% of all COVID-19 infections might be asymptomatic.10 Although our study is restricted by its observational design, our data suggest that asymptomatic testing strategies captured a considerable number of additional infections, particularly in the early phase of the pandemic, and supports NHS England Infection Prevention and Control advice on the use of asymptomatic testing with non-pharmaceutical interventions to maintain green sites to provide care for clinically susceptible patients.11
Our data also provide a unique insight into workplace exposure risk and screening strategies outside of hospital settings, with most studies focusing on higher risk settings such as homeless shelters, prisons, schools, and cruise ships. UK Government recommendations on non-clinical workplace safety during COVID-19 centred on ventilation, social distancing, encouraging vaccination, and self isolation. The Francis Crick Institute's enforced testing policy supported a safe workplace, potentially minimising infection peaks during the delta wave in summer 2021. In the absence of data from similar organisations, our study suggests that asymptomatic screening with non-pharmaceutical interventions are an important addition to guidelines on workplace safety.
Overall, this study provides a blueprint for future NHS–academic partnerships to follow. With active planning underway on national preparedness for the next potential pandemic, our work highlights the importance of prioritising testing—including regular asymptomatic testing of key workers including NHS and care home staff—during the first phase of the pandemic response.
Sequencing data for all positive samples are publicly available through COG-UK resources.
CB, TS, HT, JGo, RGi, JN, DLVB, and ECW accessed and verified the data. CB, ECW, DLVB, SGan, and CSw were responsible for the decision to submit the Correspondence for publication. CB and TS were responsible for formal analysis, investigation, methodology, visualisation, writing the original draft, and conceptualisation. HT was involved in the investigation, methodology, visualisation, manuscript review and editing, and conceptualisation. JGo was responsible for software, methodology, formal analysis, and data curation. JRMB performed formal analysis and validation. JGan was responsible for the methodology, resources, data curation, and project administration. GY performed formal analysis. RGo was responsible for software, resources, and data curation. ASF, SW, DJJ, LC, VD, OO'N, MC, DS, MF, AE, JP-L, AR, JA, NO'R, SC, MYW, PAW, and CSa were involved with the methodology, resources, data curation, and project administration. EJC was involved with software and project administration. SH, JF, and KA helped with supervision, software, and methodology. MHo performed project administration and supervision. AJ was responsible for methodology, resources, data curation, and project administration. CH, EN, MHu, RM, DH, PP, TC, RGi, JM, NVA, ST, RB, ML, and SB managed resources, data curation, and project administration. BW and SGam handled funding acquisition and project administration with SGam also providing supervision. JN was involved with project administration, supervision, and methodology. SGan performed supervision, funding acquisition, methodology, project administration, writing, review, and editing. DLVB was responsible for supervision, methodology, formal analysis, visualisation, conceptualisation, and writing the original draft. ECW performed supervision, investigation, data curation, conceptualisation, writing, review, and editing. CSw was responsible for supervision, funding acquisition, conceptualisation, project administration, writing, review, and editing. This research was funded in whole, or in part, by the Wellcome Trust (FC011104, FC011233, FC001030, FC001159, FC001827, FC001078, FC001099, and FC001169). TS is supported by a Sir Henry Wellcome Postdoctoral Fellowship from the Wellcome Trust grant 210918/Z/18/Z. Unrelated to this Correspondence, CSw reports grants from BMS, Ono-Pharmaceuticals, Boehringer Ingelheim, Roche-Ventana, Pfizer, and Archer Dx; personal fees from Genentech, Sarah Canon Research Institute, Medicxi, Metabomed, Bicycle Therapeutics, GRAIL, Amgen, AstraZeneca, BMS, Illumina, GlaxoSmithKline, MSD, and Roche-Ventana; and stock options from Apogen Biotech, Epic Biosciences, GRAIL, Achilles Therapeutics, and Bicycle Therapeutics. We thank Sir Paul Nurse, Jules Marczack, Bobbi Clayton, Gita Mistry, and all the research staff who volunteered to work on the COVID-19 testing pipeline at the Francis Crick Institute. We also thank the staff of the National Institute for Health and Care Research Clinical Research Facility at University College London Hospitals NHS Foundation Trust including Dr Mike Brown, Martin Bruce, Kirsty Adams, Miguel Alvarez, Marivic Ricamara, and Dr Mike Gandy at the Health Services Laboratory.
Supplementary Material
References
- 1.Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken J, Ambrose K, Barrell S, et al. Scalable and robust SARS-CoV-2 testing in an academic center. Nat Biotechnol. 2020;38:927–931. doi: 10.1038/s41587-020-0588-y. [DOI] [PubMed] [Google Scholar]
- 3.Vöhringer HS, Sanderson T, Sinnott M, et al. Genomic reconstruction of the SARS-CoV-2 epidemic in England. Nature. 2021;600:506–511. doi: 10.1038/s41586-021-04069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greater London Authority Coronavirus (COVID-19) weekly update. https://data.london.gov.uk/dataset/coronavirus--covid-19--cases
- 5.UK Health Security Agency Infection prevention and control for seasonal respiratory infections in health and care settings (including SARS-CoV-2) for winter 2021 to 2022. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/covid-19-guidance-for-maintaining-services-within-health-and-care-settings-infection-prevention-and-control-recommendations
- 6.Killingley B, Mann AJ, Kalinova M, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28:1031–1041. doi: 10.1038/s41591-022-01780-9. [DOI] [PubMed] [Google Scholar]
- 7.de Quetteville H. How Sir Paul Nurse got 1200 scientists safely back to work with the ‘easy’ test and trace method. Aug 10, 2020. https://www.telegraph.co.uk/health-fitness/mind/sir-paul-nurse-got-1200-scientists-safely-back-work-easy-test/
- 8.Houlihan CF, Vora N, Byrne T, et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396:e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20:1263–1271. doi: 10.1016/S1473-3099(20)30562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Q, Liu J, Liu Q, et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Health Security Agency National infection prevention and control manual (NIPCM) for England. 2021. https://www.england.nhs.uk/national-infection-prevention-and-control-manual-nipcm-for-england/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.